Historically considered as merely cellular “powerhouse” that manufactures ATP and other metabolites, the mitochondrion is increasingly being recognized as a “sentinel” organelle capable of both detecting cellular insults and orchestrating inflammatory responses [1]. Mitochondria are complex organelles, containing their own DNA and composed of a double membrane: outer mitochondrial membrane (OMM) and inner mitochondrial membrane (IMM). This double membrane gives rise to two compartments; (1) the intermembrane space (IMS) located between OMM and IMM, and (2) the matrix (M) located in the internal space created by the IMM itself.
During oxidative phosphorylation, the electrons removed from biological fuels such as glucose and fatty acids goes through a series of electron carrier system located in IMM, which is widely known as mitochondrial electron transport chain (ETC). The passage of electrons through ETC generate energy; and ultimately these electrons reduce molecular oxygen in to water [2]. According to the chemiosmotic theory introduced by Peter Mitchell in 1961, the energy produced by electron transfer through ETC is used to establish a proton gradient across the IMM that drives mitochondrial ATP production [3]. Simply, chemiosmotic theory explains that the mechanism which propels oxidative phosphorylation is the proton gradient formed across the IMM, further coupling respiratory oxygen to ADP phosphorylation/ATP generation.
The proton gradient is established by pumping protons against their electrochemical gradient across the IMM. This process simultaneously induce a proton gradient (chemical) across the membranes which is known as a protonmotive force (ΔP) and electrical gradient known as mitochondrial membrane potential (ΔΨm) [4].
Mitochondrial Proton Leak Is the Principal, but Not the Only, Mechanism That Incompletely Couples Substrate Oxygen to ATP Generation
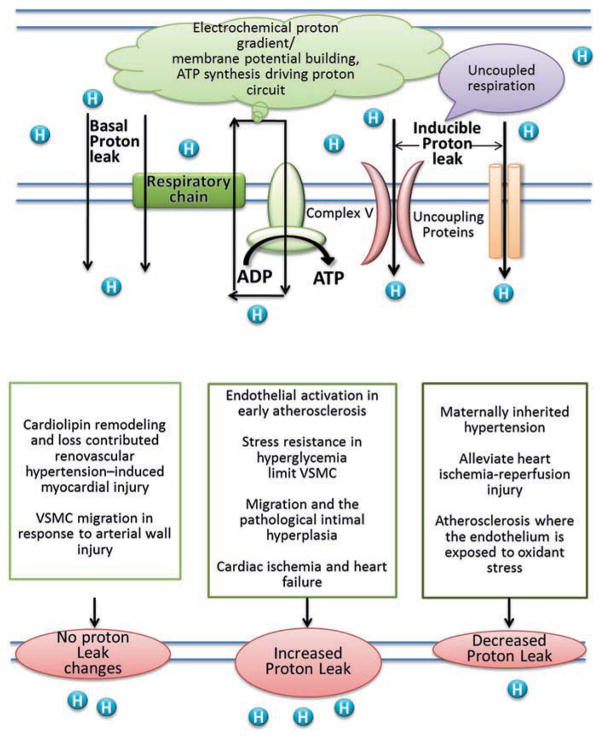
The mitochondrial ETC is comprised of a series of redox carriers named: Complex I(NADH: ubiquinone oxidoreductase), complex II (succinate dehydrogenase), complex III (ubiquinol-cytochrome c reductase) and complex IV (cytochrome c oxidase). The exergonic process of electron transport through complex I–IV create ΔP, which will drive the protons back to matrix from IMS via ATP synthase (also known as complex V), during which the ATP are generated with the aid of adenine nucleotide and phosphate carriers. However, protons can migrate to the matrix independent of ATP synthase, a process known as “proton leak”. Proton leak can also be defined as the dissipation of ΔP in the presence of ATP synthase inhibitor Oligomycin in both isolated mitochondria and intact cells [5]. As proton leak depicts the protons that migrate in to the matrix without producing ATP, it makes the coupling of substrate oxygen and ATP generation incomplete. Proton leak is the principal, but not the only, mechanism that incompletely couples substrate oxygen to ATP generation (Fig. 20.1). Even though the contribution is insignificant, a phenomenon called “electron slip” is also attributed to incomplete coupling of ATP generation and substrate oxygen as well. Electron slip refers to the process where electrons are transported via ETC without pumping protons to IMS. Therefore, electron slip results in disproportionate increase in oxygen consumption at high ΔP [6, 7].
Fig. 20.1.
Schematic representation of proton-leak and of the proposed role of UCPs in pathogenesis of cardiovascular disease
The exact mechanism of how proton leak takes place is not fully known. The physiological regulation of proton leak is categorized in to two; (1) basal/constitutive proton leak, and (2) regulated/inducible proton leak.
Basal Proton Conductance Accounts for Only 5% of Proton Leak
Basal proton conductance is generally unregulated, and largely depends on the fatty acyl composition of the inner membrane phospholipids [8, 9]. However, the proton conductance through the lipid bilayer of the inner membrane accounts for only 5% of the proton leak. Therefore, this suggests that majority of basal conductance is regulated via mitochondrial inner membrane proteins. Approximately, two-third of the basal proton conductance is correlated to the abundance of ANT (adenine nucleotide translocase), which is a type of mitochondrial anion carrier protein found in the mitochondrial inner membrane [10].
Inducible Proton Leak Is Catalyzed by Specific Mitochondrial Inner Membrane Proteins Such as Uncoupling Proteins (UCPs)
Inducible proton leak is catalyzed by specific mitochondrial inner membrane proteins such as uncoupling proteins (UCPs) [11]. UCPs are a subfamily of the mitochondrial solute carrier family proteins that mediate transport of various metabolites across the IMM. There are five types of UCPs found in mammals, named as UCP1-5. Out of the five UCPs, UCP1 was the first to be identified in brown adipose tissue and had been extensively studied. It had been implicated in converting mitochondrial energy potential to heat, thus mainly regulate adaptive thermogenesis. Even though UCP2 and UCP3 reduce the mitochondrial coupling efficiency, it is evident that their role is largely focused on regulating reactive oxygen species (ROS) levels rather that regulating thermogenesis [12, 13]. UCPs-mediated proton conductance is regulated at molecular, transcriptional, translational and proteolytic levels [14].
Electron Leak Is the Major Causative Factor for Production of Mitochondrial Superoxide
Electron leak refers to exit of electrons from the ETC before they get reduced to water at Complex IV, and is the major causative factor for production of mitochondrial superoxide. Generally, superoxide generation is attributed to Complexes I and III, however, other mitochondrial proteins such as flavin adenine dinucleotide (FAD)-linked pyruvate and α-ketoglutarate dehydrogenase complexes are also reported to generate mitochondrial superoxide [15]. Superoxide is the primary reactive oxygen species (ROS) generated by ETC. Increased level of ROS can be detrimental to overall viability of the cells, therefore, superoxide once generated get rapidly dismutated by superoxide dismutase (SOD) to hydrogen peroxide as a protective mechanism [16]. However, at low concentrations, ROS are involved as signaling molecules in various biological pathways. Under non-pathological conditions, mitochondria are the most significant ROS producers in the cells. Other than mitochondrial ROS, cell membrane bound NADPH (Nicotinamide Adenine Dinucleotide Phosphate) oxidase enzyme complex also contribute to total cellular ROS production during certain pathological conditions [17].
ROS Generation and Proton Leak
There are contradictory reports that link proton leak to ROS generation. Some reports provide evidence that the dissipation of ΔP [18–20] or presence of ADP decreases the mtROS production, while few claims that uncoupling enhances the ROS production [21, 22]. These contradictory evidence might be due to the differences in biological and experimental settings. However, the view that uncoupling decreases mitochondrial ROS production prevails. Therefore, uncoupling may play a protective role by mitigating ROS production in cells. This cyto-protective role of uncoupling was specifically observed in heart under conditions of oxidative stress such as ischemia reperfusion (IR) injury [23], aging [24] and diabetes [25]. Most prominently, UCP1 and UCP2, and also chemical uncouplers offers protection against IR injury. Similarly, it was observed that uncoupling can exert a profound protective effect against toxicity produced in the presence of hyperglycemia in endothelial cells. In addition, protective role of uncoupling against atherosclerosis was reported.
On the other hand, increased level of ROS seems to cause an increase proton leak [18]. It was observed that peroxynitrite, which is a potent inducer of lipid oxidation, increased the proton leak in isolated brain mitochondria [26]. Additionally, superoxide can also enhance the electron leak to a similar extent as peroxynitrite, and was shown to activate UCPs [27]. The UCPs and ANTs are attributed to enhanced proton conductance in the presence of ROS. Therefore, the presence of a protective feedback loop had been suggested, where increased ROS generation activates mechanisms that promote proton leak; and the enhanced proton leak in turn reduce the ROS production limiting further damage to mitochondrial function [18, 27].
“Uncoupling” in Pathogenesis of Cardiovascular Disease
The Magnitude of the Proton Leak and the Mechanism Involved in Mediating the Proton Leak Determine Whether There Is a Protective Effect in IR Injury
IR injury, atherosclerosis and diabetes-mediated cardiovascular complications are known to induce oxidative stress by elevating ROS generation including peroxynitrite, superoxide and hydrogen peroxide that adversely affect the viability of cardiomyocytes. Increased levels of ROS produced during various cardiac pathologies were shown to mediate breakdown of plasma membrane of cardiomyocytes, increased the potential for arrhythmogenesis during a post-ischemic events and also led to apoptosis of myocytes [28, 29] (see Chap. 10). Recent evidence suggests that UCPs exert a protective role against oxidative stress by suppressing ROS.
Diseased heart has a strong association with increased oxidative stress and an impairment of reserve respiratory capacity. During physiological conditions, mitochondria does not use its full bioenergetic capacity unless there is an increase in energy demand during situations where cellular repair and detoxifying ROS is required to maintain cellular function. Mitochondrial reserve respiratory capacity refers to the difference between maximum respiratory capacity and basal respiratory capacity [30]. Therefore, depletion of reserve respiratory capacity indicate the inability of the cells to meet the increased energy demand in response to cellular stress that subsequently affect adversely to the cell viability. Intact rat neonatal ventricular myocytes exhibit exhaustion of the reserve capacity with an increased proton leak when exposed to pathologically relevant concentrations of HNE (4-hydroxynonenal), which is a reactive lipid species accumulated in the heart during ischemia and heart failure [31].
Higher rate of proton leak is associated in mitochondria isolated from hearts subjected to IR [32, 33]. Furthermore, few research groups have shown that using uncoupling agents such as FCCP [carbonyl cyanide p-(tri-fluromethoxy) phenyl-hydrzone] or DNP (2,4-Dinitrophenol) protects mitochondrial and cardiomyocyte function from IR injury [34, 35]. However, this observation was reversed in mitochondria subjected to ischemic preconditioning (IPC), which is a cardioprotective strategy consisting of brief cycles of IR (3–5 min) to protect the myocardium from the damaging effects of subsequent longer episodes of IR. Surprisingly, mitochondria subjected to protective IPC had a smaller increment in proton conductance after IR compared to non-preconditioned mitochondria with higher proton conductance [32, 33]. This is contradictory to the observation that increased proton conductance exert cardioprotective against IR injury. This observation was explained by differential mechanisms that are responsible for promoting mild proton leak and intensive proton leak. It was observed that generally mild-moderate proton leak during IR injury is regulated by UCPs [33], whereas a larger proton leak is mediated by ANT, which was shown to be a part of mitochondrial permeability transition pore (MPTP) [36]. Opening of MPTP under pathological conditions rapidly dissipate ΔΨm leading mitochondrial swelling, and mediate cellular apoptosis or necrosis. The larger proton leak mediated by opening of MPTP is detrimental to mitochondria and adversely impact to the cell viability [36]. Therefore, these observations suggest that magnitude of the proton leak and the mechanism involved in mediating the proton leak determine whether there is a protective effect against IR injury.
Uncoupling by UCP2 Preserves Vascular Function in Diet-Induced Obesity Mice as Well as In Diabetes
Oxidative stress plays a central role in development of diabetes-mediated macrovascular and microvascular complications. In bovine aortic endothelial cells, overexpressed UCP1 blocked hyperglycemia-induced ROS production, indicating that uncoupling may play a role in reducing the progression of diabetes induced vascular complications [37]. Further, endothelial UCP2 also function as a sensor and negatively regulate mitochondrial ROS production in response to hyperglycemia, further validating importance of uncoupling in attenuating diabetes induced vascular diseases [38].
Interestingly, it was observed that UCP2 expression was gradually reduced with increased lipid deposition in atherosclerotic lesion in aorta in hyperlipidemic apolipoprotein E deficient (ApoE−/−) mice. Furthermore, it was observed that proinflammatory tumor necrosis factor-α (TNF-α) can downregulate expression of UCP2 in vasculature and accelerate vascular damage. Therefore, this suggests that lowering the TNF-α expression within the aorta during exposure to vascular stress factors such as hyperinsulinemia may prevent vascular damage [39]. UCP2 preserves endothelial function through increasing endothelial nitric oxide synthase (eNOS) phosphorylation secondary to the inhibition of ROS production in the endothelium of obese diabetic mice. Silencing UCP2 impairs endothelium-dependent relaxation in aorta and mesenteric arteries from diet-induced obesity mice as well as aortic rings exposed to high glucose [40].
UCP2 in lung endothelial cells was recently reported to play a significant role in progression of pulmonary hypertension. UCP2 ablation in mice causes high right ventricular pressure and right ventricular hypertrophy when subjected to intermittent hypoxia induced pulmonary hypertension. Loss of UCP2 in lung endothelial cells increases mitophagy leading to progression of pulmonary hypertension. Mitophagy refers to selective mitochondrial autophagy, a process initiated by changes in the ΔΨm. Therefore, loss of UCP2 was implicated in deficiency of mitochondria and increased apoptosis in lung endothelial cells that contribute to exacerbation of pulmonary hypertension [41].
Also, in maternally inherited hypertension, it was shown that a specific mutation in mitochondrial tRNAAla (A5665G) significantly reduced the proton leak. The changes in the mitochondrial proton conductance and bioenergetics led to increased ROS and subsequently elevated blood pressure [42].
However, Eirin and colleagues demonstrated that the use of organic peroxide tert-Butyl hydrogen peroxide (tBHP) in isolated rat cardiomyocytes can suppress the basal respiration, maximal respiration and ATP production without altering the proton conductance in mitochondria. Also, the same research group demonstrated that use of MTP (mitochondrial peptide), which protects cardiolipin from peroxidation, ameliorated this effects while keeping the proton leak still intact. Cardiolipin is an inner membrane mitochondrial protein, which tethers cytochrome C to the inner membrane, thus facilitating the electron transport from complex III to complex IV. This protein is highly vulnerable to oxidative damage and subsequent loss, therefore leading to mitochondrial loss and contributing to development of cardiovascular disorders. The authors also demonstrate that restoration of cardiolipin improves cardiac function of pigs subjected to renovascular hypertension [43]. Therefore, all these observations indicate that the response of altering proton conductance depends on the etiology of hypertension.
Proton Leak Regulates ATP Synthesis-Uncoupled Mitochondrial ROS Generation, Which Determines Pathological Activation of Endothelial Cells for Recruitment of Inflammatory Cells
Most interestingly, alterations in mitochondrial morphology were reported to alter the proton conductance as well. Mitochondria are dynamic organelles which undergo constant shape changes by fission and fusion. As the name implies, fission results in smaller mitochondria while fusion results in larger mitochondria. In addition, variations in mitochondrial morphology have been found to contribute to vascular smooth muscle cell migration in response to arterial wall injury. Silencing Drp1 (mitochondrial fission protein dynamin-like protein 1), which is the protein that regulates mitochondrial fission, limits VSMC migration through a markedly increase in proton leak, which suppresses not only ATP synthesis but also ROS production. Similarly, respiration coupling efficiency reduction was also validated in vivo with mitochondrial fission suppression, proposing an effective strategy to inhibit intimal hyperplasia in restenosis and the progression of atherosclerotic lesions [44].
Furthermore, UCP2 was shown to mitigate the atherosclerotic burden as well. Blanc and colleagues demonstrated that UCP2 is a protective mediator against early stage of atherosclerosis. The same group had shown that depletion of UCP2 in blood cells significantly increased the lesion size in thoracic aorta with more macrophage infiltration in hyperlipidemic mice. This finding was attributed to increased ROS production and inflammatory responsiveness in UCP2 deficient macrophages [45]. Similarly, another study demonstrated that UCP2 prevents progression of atherosclerosis by exerting antioxidant effect presumably by suppressing NF-κB activation in human aortic endothelial cells (HAECs). Moreover, UCP2 can attenuate apoptotic death of HAECs mediated by pro-atherogenic LPC (lysophosphatidylcholine) [46].
We recently demonstrated that LPC promotes HAEC activation, which is one of the primary steps in disease progression of atherosclerosis [47]. We demonstrated the LPC specifically induces mitochondrial ROS rather than cytosolic ROS generation. Interestingly, we observed that LPC-mediated ROS generation was accompanied by increased proton leak without affecting the overall ATP production. Albeit the proton conductance was increased in LPC treated HAECs, we could not detect any change in the net proton gradient (ΔP) in mitochondria. The fact that ΔP and ATP production was not significantly compromised in LPC-mediated endothelial cell activation indicates that proton conductance through complex V was not impaired. Also, intact ΔP in the presence of an increased proton leak implies that pro-atherogenic stimuli such as LPC may promote ETC activity. Furthermore, we demonstrated that LPC-mediated mitochondrial ROS was responsible for increasing the expression of adhesion molecules such as ICAM-1 in endothelial cells, which initiates atherosclerosis process in vasculature [48]. We have further validated this observation by using mitoTempo, which is a specific mitochondrial ROS inhibitor. Our data implies that inhibition of mitochondrial ROS generation can significantly reduce the atherosclerotic burden in aortas of hyperlipidemic mice. Therefore, the major purpose for LPC-mediated increase in proton leak may be to modulate the mitochondrial ROS generation for signal transduction that ultimately lead to endothelial cell activation, without inducing mitochondrial damage or endothelial cell death [47, 49].
Conclusions
Mitochondria account for one third of the volume of cardiomyocytes and regulate ATP production that is essential to maintain cardiomyocyte health and survival [50]. Therefore, changes in mitochondrial biogenesis, morphology and function may contribute significantly to the development of cardiovascular diseases. Increased ROS can enhance proton leak, and in turn increased proton leak reduces the ROS generation, indicating the existence of a protective feedback loop that helps to ameliorate the detrimental effects caused by ROS on biological systems. The magnitude of proton current and the mechanism of proton conductance determine the level of ROS production, extent of mitochondrial damage and cell viability during cardiac pathologies. Moreover, cellular stresses such as pro-atherogenic stimuli can enhance proton leak without affecting the overall energy efficiency in endothelial cells. This finding indicates that proton leak acts as an indirect mediator of signal transduction by fine tuning ROS production, which acts as a signaling molecule to enhance endothelial activation during early atherogenesis, and contribute to the progression of the disease. Therefore, regulation of proton leak can be a potential therapeutic target for the treatment of many cardiovascular disorders.
Contributor Information
Jiali Cheng, Department of Cardiovascular Medicine, The First Affiliate Hospital of Harbin Medical University, Harbin, Heilongjiang Province, China. Centers for Metabolic Disease Research, Cardiovascular Research, & Thrombosis Research, Department of Pharmacology, Lewis Katz School of Medicine at Temple University, 3500 North Broad Street, MERB-1059, Philadelphia, PA 19140, USA.
Gayani Nanayakkara, Centers for Metabolic Disease Research, Cardiovascular Research, & Thrombosis Research, Department of Pharmacology, Lewis Katz School of Medicine at Temple University, 3500 North Broad Street, MERB-1059, Philadelphia, PA 19140, USA.
Ying Shao, Centers for Metabolic Disease Research, Cardiovascular Research, & Thrombosis Research, Department of Pharmacology, Lewis Katz School of Medicine at Temple University, 3500 North Broad Street, MERB-1059, Philadelphia, PA 19140, USA.
Ramon Cueto, Centers for Metabolic Disease Research, Cardiovascular Research, & Thrombosis Research, Department of Pharmacology, Lewis Katz School of Medicine at Temple University, 3500 North Broad Street, MERB-1059, Philadelphia, PA 19140, USA.
Luqiao Wang, Centers for Metabolic Disease Research, Cardiovascular Research, & Thrombosis Research, Department of Pharmacology, Lewis Katz School of Medicine at Temple University, 3500 North Broad Street, MERB-1059, Philadelphia, PA 19140, USA.
William Y. Yang, Department of Cardiovascular Medicine, The First Affiliate Hospital of Harbin Medical University, Harbin, Heilongjiang Province, China
Ye Tian, Department of Cardiovascular Medicine, The First Affiliate Hospital of Harbin Medical University, Harbin, Heilongjiang Province, China.
Hong Wang, Centers for Metabolic Disease Research, Cardiovascular Research, & Thrombosis Research, Department of Pharmacology, Lewis Katz School of Medicine at Temple University, 3500 North Broad Street, MERB-1059, Philadelphia, PA 19140, USA.
Xiaofeng Yang, Centers for Metabolic Disease Research, Cardiovascular Research, & Thrombosis Research, Department of Pharmacology, Lewis Katz School of Medicine at Temple University, 3500 North Broad Street, MERB-1059, Philadelphia, PA 19140, USA.
References
- 1.Li X, Fang P, Mai J, Choi ET, Wang H, Yang XF. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J Hematol Oncol. 2013;6:19. doi: 10.1186/1756-8722-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rich PR, Marechal A. The mitochondrial respiratory chain. Essays Biochem. 2010;47:1–23. doi: 10.1042/bse0470001. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191:144–8. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 4.Noji H, Yasuda R, Yoshida M, Kinosita K., Jr Direct observation of the rotation of F1-ATPase. Nature. 1997;386:299–302. doi: 10.1038/386299a0. [DOI] [PubMed] [Google Scholar]
- 5.Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. III. The steady state. J Biol Chem. 1955;217:409–27. [PubMed] [Google Scholar]
- 6.Kadenbach B. Intrinsic and extrinsic uncoupling of oxidative phosphorylation. Biochim Biophys Acta. 2003;1604:77–94. doi: 10.1016/s0005-2728(03)00027-6. [DOI] [PubMed] [Google Scholar]
- 7.Murphy MP. Slip and leak in mitochondrial oxidative phosphorylation. Biochim Biophys Acta. 1989;977:123–41. doi: 10.1016/s0005-2728(89)80063-5. [DOI] [PubMed] [Google Scholar]
- 8.Brookes PS, Buckingham JA, Tenreiro AM, Hulbert AJ, Brand MD. The proton permeability of the inner membrane of liver mitochondria from ectothermic and endothermic vertebrates and from obese rats: correlations with standard metabolic rate and phospholipid fatty acid composition. Comp Biochem Physiol B Biochem Mol Biol. 1998;119:325–34. doi: 10.1016/s0305-0491(97)00357-x. [DOI] [PubMed] [Google Scholar]
- 9.Fontaine EM, Moussa M, Devin A, Garcia J, Ghisolfi J, Rigoulet M, Leverve XM. Effect of polyunsaturated fatty acids deficiency on oxidative phosphorylation in rat liver mitochondria. Biochim Biophys Acta. 1996;1276:181–7. doi: 10.1016/0005-2728(96)00075-8. [DOI] [PubMed] [Google Scholar]
- 10.Brand MD, Pakay JL, Ocloo A, Kokoszka J, Wallace DC, Brookes PS, Cornwall EJ. The basal proton conductance of mitochondria depends on adenine nucleotide translocase content. Biochem J. 2005;392:353–62. doi: 10.1042/BJ20050890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parker N, Vidal-Puig A, Brand MD. Stimulation of mitochondrial proton conductance by hydroxynonenal requires a high membrane potential. Biosci Rep. 2008;28:83–8. doi: 10.1042/BSR20080002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R, Bouillaud F, Richard D, Collins S, Ricquier D. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet. 2000;26:435–9. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- 13.Vidal-Puig AJ, Grujic D, Zhang CY, Hagen T, Boss O, Ido Y, Szczepanik A, Wade J, Mootha V, Cortright R, Muoio DM, Lowell BB. Energy metabolism in uncoupling protein 3 gene knockout mice. J Biol Chem. 2000;275:16258–66. doi: 10.1074/jbc.M910179199. [DOI] [PubMed] [Google Scholar]
- 14.Azzu V, Brand MD. The on-off switches of the mitochondrial uncoupling proteins. Trends Biochem Sci. 2010;35:298–307. doi: 10.1016/j.tibs.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, Beal MF. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J Neurosci. 2004;24:7779–88. doi: 10.1523/JNEUROSCI.1899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santulli G, Xie W, Reiken SR, Marks AR. Mitochondrial calcium overload is a key determinant in heart failure. Proc Natl Acad Sci U S A. 2015;112:11389–94. doi: 10.1073/pnas.1513047112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brookes PS. Mitochondrial H(+) leak and ROS generation: an odd couple. Free Radic Biol Med. 2005;38:12–23. doi: 10.1016/j.freeradbiomed.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Turrens JF, Boveris A. Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria. Biochem J. 1980;191:421–7. doi: 10.1042/bj1910421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrero A, Barja G. ADP-regulation of mitochondrial free radical production is different with complex I- or complex II-linked substrates: implications for the exercise paradox and brain hypermetabolism. J Bioenerg Biomembr. 1997;29:241–9. doi: 10.1023/a:1022458010266. [DOI] [PubMed] [Google Scholar]
- 21.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–16. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Starkov AA, Fiskum G. Regulation of brain mitochondrial H2O2 production by membrane potential and NAD(P)H redox state. J Neurochem. 2003;86:1101–7. doi: 10.1046/j.1471-4159.2003.01908.x. [DOI] [PubMed] [Google Scholar]
- 23.Ganote CE, Armstrong SC. Effects of CCCP-induced mitochondrial uncoupling and cyclosporin A on cell volume, cell injury and preconditioning protection of isolated rabbit cardiomyocytes. J Mol Cell Cardiol. 2003;35:749–59. doi: 10.1016/s0022-2828(03)00114-7. [DOI] [PubMed] [Google Scholar]
- 24.Speakman JR, Talbot DA, Selman C, Snart S, McLaren JS, Redman P, Krol E, Jackson DM, Johnson MS, Brand MD. Uncoupled and surviving: individual mice with high metabolism have greater mitochondrial uncoupling and live longer. Aging Cell. 2004;3:87–95. doi: 10.1111/j.1474-9728.2004.00097.x. [DOI] [PubMed] [Google Scholar]
- 25.Green K, Brand MD, Murphy MP. Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes. 2004;53(Suppl 1):S110–8. doi: 10.2337/diabetes.53.2007.s110. [DOI] [PubMed] [Google Scholar]
- 26.Brookes PS, Land JM, Clark JB, Heales SJ. Peroxynitrite and brain mitochondria: evidence for increased proton leak. J Neurochem. 1998;70:2195–202. doi: 10.1046/j.1471-4159.1998.70052195.x. [DOI] [PubMed] [Google Scholar]
- 27.Echtay KS, Roussel D, St-Pierre J, Jekabsons MB, Cadenas S, Stuart JA, Harper JA, Roebuck SJ, Morrison A, Pickering S, Clapham JC, Brand MD. Superoxide activates mitochondrial uncoupling proteins. Nature. 2002;415:96–9. doi: 10.1038/415096a. [DOI] [PubMed] [Google Scholar]
- 28.Zhu X, Zuo L, Cardounel AJ, Zweier JL, He G. Characterization of in vivo tissue redox status, oxygenation, and formation of reactive oxygen species in postischemic myocardium. Antioxid Redox Signal. 2007;9:447–55. doi: 10.1089/ars.2006.1389. [DOI] [PubMed] [Google Scholar]
- 29.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfleger J, He M, Abdellatif M. Mitochondrial complex II is a source of the reserve respiratory capacity that is regulated by metabolic sensors and promotes cell survival. Cell Death Dis. 2015;6:e1835. doi: 10.1038/cddis.2015.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hill BG, Dranka BP, Zou L, Chatham JC, Darley-Usmar VM. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem J. 2009;424:99–107. doi: 10.1042/BJ20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quarrie R, Cramer BM, Lee DS, Steinbaugh GE, Erdahl W, Pfeiffer DR, Zweier JL, Crestanello JA. Ischemic preconditioning decreases mitochondrial proton leak and reactive oxygen species production in the postischemic heart. J Surg Res. 2011;165:5–14. doi: 10.1016/j.jss.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nadtochiy SM, Tompkins AJ, Brookes PS. Different mechanisms of mitochondrial proton leak in ischaemia/reperfusion injury and preconditioning: implications for pathology and cardio-protection. Biochem J. 2006;395:611–8. doi: 10.1042/BJ20051927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brennan JP, Southworth R, Medina RA, Davidson SM, Duchen MR, Shattock MJ. Mitochondrial uncoupling, with low concentration FCCP, induces ROS-dependent cardioprotection independent of KATP channel activation. Cardiovasc Res. 2006;72:313–21. doi: 10.1016/j.cardiores.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 35.Minners J, van den Bos EJ, Yellon DM, Schwalb H, Opie LH, Sack MN. Dinitrophenol, cyclosporin A, and trimetazidine modulate preconditioning in the isolated rat heart: support for a mitochondrial role in cardioprotection. Cardiovasc Res. 2000;47:68–73. doi: 10.1016/s0008-6363(00)00069-9. [DOI] [PubMed] [Google Scholar]
- 36.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341(Pt 2):233–49. [PMC free article] [PubMed] [Google Scholar]
- 37.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–90. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 38.Koziel A, Sobieraj I, Jarmuszkiewicz W. Increased activity of mitochondrial uncoupling protein 2 improves stress resistance in cultured endothelial cells exposed in vitro to high glucose levels. Am J Physiol Heart Circ Physiol. 2015;309:H147–56. doi: 10.1152/ajpheart.00759.2014. [DOI] [PubMed] [Google Scholar]
- 39.Gomez-Hernandez A, Perdomo L, de las Heras N, Beneit N, Escribano O, Otero YF, Guillen C, Diaz-Castroverde S, Gozalbo-Lopez B, Cachofeiro V, Lahera V, Benito M. Antagonistic effect of TNF-alpha and insulin on uncoupling protein 2 (UCP-2) expression and vascular damage. Cardiovasc Diabetol. 2014;13:108. doi: 10.1186/s12933-014-0108-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian XY, Wong WT, Xu A, Lu Y, Zhang Y, Wang L, Cheang WS, Wang Y, Yao X, Huang Y. Uncoupling protein-2 protects endothelial function in diet-induced obese mice. Circ Res. 2012;110:1211–6. doi: 10.1161/CIRCRESAHA.111.262170. [DOI] [PubMed] [Google Scholar]
- 41.Haslip M, Dostanic I, Huang Y, Zhang Y, Russell KS, Jurczak MJ, Mannam P, Giordano F, Erzurum SC, Lee PJ. Endothelial uncoupling protein 2 regulates mitophagy and pulmonary hypertension during intermittent hypoxia. Arterioscler Thromb Vasc Biol. 2015;35:1166–78. doi: 10.1161/ATVBAHA.114.304865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang P, Wang M, Xue L, Xiao Y, Yu J, Wang H, Yao J, Liu H, Peng Y, Li H, Chen Y, Guan MX. A hypertension-associated tRNAAla mutation alters tRNA metabolism and mitochondrial function. Mol Cell Biol. 2016;36:1920–30. doi: 10.1128/MCB.00199-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eirin A, Ebrahimi B, Kwon SH, Fiala JA, Williams BJ, Woollard JR, He Q, Gupta RC, Sabbah HN, Prakash YS, Textor SC, Lerman A, Lerman LO. Restoration of mitochondrial cardiolipin attenuates cardiac damage in swine renovascular hypertension. J Am Heart Assoc. 2016;5:e003118. doi: 10.1161/JAHA.115.003118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang L, Yu T, Lee H, O’Brien DK, Sesaki H, Yoon Y. Decreasing mitochondrial fission diminishes vascular smooth muscle cell migration and ameliorates intimal hyperplasia. Cardiovasc Res. 2015;106:272–83. doi: 10.1093/cvr/cvv005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blanc J, Alves-Guerra MC, Esposito B, Rousset S, Gourdy P, Ricquier D, Tedgui A, Miroux B, Mallat Z. Protective role of uncoupling protein 2 in atherosclerosis. Circulation. 2003;107:388–90. doi: 10.1161/01.cir.0000051722.66074.60. [DOI] [PubMed] [Google Scholar]
- 46.Lee KU, Lee IK, Han J, Song DK, Kim YM, Song HS, Kim HS, Lee WJ, Koh EH, Song KH, Han SM, Kim MS, Park IS, Park JY. Effects of recombinant adenovirus-mediated uncoupling protein 2 overexpression on endothelial function and apoptosis. Circ Res. 2005;96:1200–7. doi: 10.1161/01.RES.0000170075.73039.5b. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Liu X, Bai J, Tian X, Zhao X, Liu W, Duan X, Shang W, Fan HY, Tong C. Mitoguardin regulates mitochondrial fusion through mitoPLD and is required for neuronal homeostasis. Mol Cell. 2016;61:111–24. doi: 10.1016/j.molcel.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 48.Gambardella J, Sardu C, Sacra C, Del Giudice C, Santulli G. Quit smoking to outsmart atherogenesis: molecular mechanisms underlying clinical evidence. Atherosclerosis. 2017;257:242–5. doi: 10.1016/j.atherosclerosis.2016.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Fang P, Yang WY, Chan K, Lavallee M, Xu K, Gao T, Wang H, Yang X. Mitochondrial ROS, uncoupled from ATP synthesis, determine endothelial activation for both physiological recruitment of patrolling cells and pathological recruitment of inflammatory cells. Can J Physiol Pharmacol. 2017;95(3):247–52. doi: 10.1139/cjpp-2016-0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goffart S, von Kleist-Retzow JC, Wiesner RJ. Regulation of mitochondrial proliferation in the heart: power-plant failure contributes to cardiac failure in hypertrophy. Cardiovasc Res. 2004;64:198–207. doi: 10.1016/j.cardiores.2004.06.030. [DOI] [PubMed] [Google Scholar]