Abstract
Context:
Previously we demonstrated, in individuals who have had type 1 diabetes (T1D) for 50 or more years (Medalists), that glycemic control was unrelated to diabetic complications, with the exception of cardiovascular disease (CVD), contrary to what has been documented in registry-based studies.
Objective:
The purpose of this study is to validate these initial findings and identify contributors to mortality on an individual basis in a large cohort.
Design:
Cross-sectional and longitudinal study.
Setting:
Joslin Diabetes Center (JDC), Boston, Massachusetts.
Patients:
50-year Medalists presenting to JDC for study participation.
Interventions:
None.
Main Outcomes Measures:
Microvascular and macrovascular complications of diabetes and mortality.
Results:
Glycemic control was not significantly associated with small-vessel complications in Medalists but was associated with CVD in the overall cohort, yet with varying effect by tertile of cohort duration. CVD was the largest contributor to mortality, whereas hemoglobin A1c was not an independent predictor of mortality either overall or substantially by diagnosis interval. Additionally, exercise mitigated mortality risk imparted by CVD.
Conclusions:
Few large populations with long duration of (T1D) have been available to examine the effects of long-term exposure to hyperglycemia. These data indicate that an association of glycemic control, complications, and mortality may change in an older population with T1D. These results suggest that careful control is still warranted in older populations with T1D.
These analyses illuminate the importance of glycemic control on large-vessel complications and provide new data on mortality patterns in type 1 diabetes and the importance of physical activity.
Diabetes duration and glycemic control are the most recognized factors associated with complications of and mortality from type 1 diabetes (T1D). This has been documented in several longitudinal studies, including the Epidemiology of Diabetes Interventions and Complications, the Pittsburgh Epidemiology of Diabetic Complications, Norwegian Cause of Death Registry, and Joslin Kidney studies (1–7). In contrast, the Joslin 50-Year Medalist Study has shown that in those with T1D duration of ≥50 years (Medalists), glycemic control did not factor significantly into the development of microvascular complications (8, 9). Subsequently, several studies and analyses based on medical record–level data, including the Scottish Registry and the Swedish National Registry studies, show that in groups diagnosed more recently, glycemic control is indeed important (10, 11). However, within these national registries, the proportion of individuals with T1D duration of ≥50 years was low.
As reported previously, the Joslin 50-Year Medalist Study found evidence of endogenous protective factors supported by uniform levels of glycemic control between those with and those without proliferative eye disease, nephropathy, and neuropathy (8, 9, 12). Additionally, the mechanisms for the tissue-specific protection have been elucidated and presented elsewhere. These include a proportion of Medalists with increased activation of proteins protecting against oxidative stress, increases in protein kinase C-δ protection, increased numbers of endothelial progenitor cells, and higher levels of larger high-density lipoprotein subparticles (12–14). Previously, we reported a minimal effect of glycemic control on the prevalence of vascular complications among Medalists, with mean (± standard deviation) hemoglobin A1c (HbA1c) at time of visit of 7.3% ± 1.0 (56 ± 10.9 mmol/mol) and duration of 56.5 ± 5.7 years (n = 351) with low levels of small- and large-vessel complication (9). Since that time, the Medalist Study has recruited >600 additional individuals diagnosed between 1925 and 1964, with a follow-up time of >4500 person-years. During this time, 123 deaths have been documented, allowing an examination of factors related to complications and contributors to mortality in this cohort. Because several factors have changed over the initial period of disease for these individuals, and subsequent years may lead to important factors related to complications and mortality, the analyses were divided into different intervals. Examination by duration tertiles was used to understand the influence of the introduction of different therapies and technologies over time (15). The analysis presented here examined the relationship between modifiable (exercise, glycemic control) and unmodifiable (age, duration, sex) factors and complication status and mortality by duration tertile and diagnosis interval in a large cohort of patients who have had T1D for >50 years. The analysis shows the varying relationship between microvascular and macrovascular complications and glycemic control, as well as mortality in strata, to understand the interplay of factors within each interval.
Methods
Participants, data sources, and measurement
Data on 952 Medalists collected between 2005 and 2015 were analyzed, and these individuals were followed until December 2015 or death. All individuals provided documentation of ≥50 years of insulin dependence since time of diagnosis and were examined at the Joslin Diabetes Center (JDC). All procedures were performed with informed consent and permission of the JDC Committee on Human Studies. Methods of the Joslin 50-Year Medalists Study have been described elsewhere (9, 12). All individuals attended the JDC for study evaluations. HbA1c at the time of first study visit was determined by high-performance liquid chromatography (Tosoh G7 and 2.2; Tosoh Bioscience, Tokyo, Japan).
Determination of complication status
Cardiovascular disease (CVD) status was based on self-reported history of coronary artery disease, angina, myocardial infarction, prior cardiac or leg angioplasty, or bypass graft surgery, as previously described, and was used as a dichotomous variable; no CVD was the reference group (9). This method of self-report has been validated by several epidemiologic studies (16–18). Additionally, electrocardiograms evaluated by the study physician (G.L.K.) were obtained at study visit and as part of standard protocol compared with patient-reported medical histories. The patient was queried if the results differed from those reported to confirm initial patient report. Estimated glomerular filtration rate (eGFR) of <45 mL/min/1.73 m2 was defined as nephropathy (19). Early Treatment Diabetic Retinopathy Study seven-field criteria were used for determination of proliferative diabetic retinopathy (PDR) (20). The Michigan Neuropathy Screening Instrument was used to assess neuropathy; scores of ≥2 were considered positive (21). Physical activity was assessed by the College Alumnus Questionnaire (22). Hypertension was considered as systolic blood pressure > 135 mm Hg or diastolic blood pressure > 85 mm Hg at the time of visit and/or current report of blood pressure medications.
Statistical analysis
Cohort tertile (tertile 1, 50 to 51 years, n = 370; tertile 2, 52 to 55 years, n = 297; tertile 3, 56 to 85 years, n = 278) analyses were done to determine which factors were associated with the development of complications during cohort duration tertiles. Glycemic control and its association with CVD, diabetic nephropathy, and PDR were the a priori exposure of interest. The absence of a complication was considered the reference group, and eGFR was considered an ascending continuous variable. All tests for significance were two-sided, with significance set P ≤ 0.05. A χ2 test and Kruskal–Wallis test were performed on nonparametric variables. Values are presented as means ± standard deviation or percentages. Logistic regression was used to adjust for potential confounders and assess effect modification; odds ratios (ORs) and 95% confidence intervals (CIs) are reported. Variables with plausible influences and those with P ≤ 0.1 at the bivariate levels were tested in the multivariable model and were included if P ≤ 0.05, had a confounding effect, or were stratified where applicable. Multiplicative interaction terms were tested in each strata and overall with the relevant covariates and main effects in the model.
Kaplan–Meier analyses were done for unadjusted analysis of survival probability by decade of diagnosis before 1940 (<1940), 1940 to 1949, 1950 to 1959, 1960+ (≥1960). Cox proportional hazards regression was used to assess the association of HbA1c with mortality risk and adjust for potential confounders; hazard ratios (HRs) and 95% CIs are presented.
Sensitivity analyses were conducted to determine whether the impact of missing data results was statistically significant. The analysis of the original data are reported. Statistical analyses were conducted by using SAS/STAT software version 9.3 (SAS Institute, Cary, NC), Stata IC 12.1 (Stata Corp., College Station, TX) and R software, version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Individuals participating in the Medalist Study between April 2005 and December 2015 were used in this analysis (n = 952). Since the inception of the study, >2000 medals have been awarded to individuals residing in the United States and Canada. Additionally, individuals who received medals between 1998 and 2003 were also sent invitations to participate. In total, 71% of US Medalists have participated, with 79% receiving care outside JDC. Medalists from all 50 states participated. Medalists declining participation cited illness, time commitment, or financial issues. The mean age and duration of participants were 65.8 years and 54.7 years, respectively. The mean HbA1c of this group was 7.2% ± 0.9% (55 ± 5.7 mmol/mol) and daily insulin dose was 0.46 ± 0.18 U/kg. Prevalence of complications was low (Supplemental Fig. 1 (248.7KB, pdf) ). Regular physical activity was reported by 79.5%. Use of a subcutaneous infusion pump was reported by 59.1% of Medalists. More than half of Medalists reported checking their blood glucose levels more than five times per day (Table 1).
Table 1.
50-Year Joslin Medalist Participants: Baseline Characteristics Overall and by Tertile of Diabetes Duration
| Characteristic | Overall: 50–85 y (n = 952) | Tertile 1: 50–51 y (n = 374) | Tertile 2: 52–55 y (n = 300) | Tertile 3: 56–85 y (n = 278) | P Value |
|---|---|---|---|---|---|
| Deceased, % (n) | 12.9 (123) | 6.5 (24) | 10.1 (30) | 24.8 (69) | <0.001 |
| Men (%) | 45.7 | 41.4 | 47.3 | 49.6 | 0.09 |
| Age (y) | 65.8 ± 7.6 | 62.9 ± 6.7 | 64.7 ± 6.5 | 71.0 ± 7.2 | <0.001 |
| Age at diagnosis (y) | 11.2 ± 6.4 | 12.4 ± 6.6 | 11.4 ± 6.5 | 9.3 ± 5.4 | <0.001 |
| Duration (y) | 54.7 ± 5.7 | 50.5 ± 0.5 | 53.2 ± 1.1 | 61.7 ± 5.9 | <0.001 |
| Insulin dose/kg (unit/kg) | 0.46 ± 0.18 | 0.45 ± 0.16 | 0.47 ± 0.2 | 0.45 ± 0.18 | 0.38 |
| HbA1c (%) | 7.2 ± 0.9 | 7.2 ± 1.0 | 7.2 ± 1.0 | 7.2 ± 0.9 | 0.49 |
| HbA1c (mmol/mol) | 54.9 ± 10.3 | 54.8 ± 10.6 | 54.7 ± 10.5 | 55.3 ± 9.6 | 0.49 |
| Total cholesterol (mmol/L) | 4.2 ± 0.8 | 4.2 ± 0.8 | 4.3 ± 0.9 | 4.1 ± 0.8 | 0.04 |
| LDL (mmol/L) | 2.1 ± 0.6 | 2.1 ± 0.6 | 2.2 ± 0.6 | 2.0 ± 0.6 | 0.09 |
| HDL cholesterol (mmol/L) | 1.7 ± 0.5 | 1.7 ± 0.5 | 1.7 ± 0.5 | 1.7 ± 0.5 | 0.21 |
| Triglycerides (mmol/L) | 0.8 ± 0.4 | 0.8 ± 0.4 | 0.9 ± 0.5 | 0.9 ± 0.4 | 0.52 |
| Body mass index (kg/m2) | 26.2 ± 4.7 | 26.6 ± 4.9 | 25.9 ± 4.5 | 25.9 ± 4.5 | 0.15 |
| eGFR (mL/min/1.73 m2) | 69.6 ± 20.3 | 74.0 ± 20.2 | 69.1 ± 19.9 | 64.2 ± 19.5 | <0.001 |
| Serum creatinine (µmol/L) | 94.6 ± 46.7 | 88.8 ± 35.4 | 95.5 ± 41.8 | 101.4 ± 61.7 | <0.001 |
| ACR (mg/mmol) | 8.1 ± 41.8 | 9.1 ± 54.2 | 7.0 ± 39.5 | 8.0 ± 18.5 | <0.001 |
| CRP (nmol/L) | 18.0 ± 44.2 | 14.1 ± 34.8 | 20.8 ± 57.3 | 20.2 ± 38.5 | <0.001 |
| Insulin pump use (%) | 59.1 | 68.0 | 60.4 | 45.6 | <0.001 |
| Regular physical activity (%) | 79.5 | 81.4 | 80.2 | 76.3 | 0.28 |
| Check blood glucose > 5 times/d (%) | 56.6 | 61.5 | 57.8 | 48.7 | 0.005 |
| MNSI ≥ 2 (%) | 69.8 | 74.3 | 66.8 | 66.8 | 0.06 |
| Hypertension medication (%) | 64.4 | 63.3 | 62.2 | 68.4 | 0.26 |
| Lipid-lowering medication (%) | 69.6 | 73.0 | 66.7 | 68.2 | 0.18 |
| Detectable random C-peptide (>0.02 nmol/L) (%) | 34.3 | 33.8 | 33.6 | 35.6 | 0.85 |
| IA2+ or GAD65+ (%) | 43.4 | 47.6 | 43.7 | 37.7 | 0.06 |
| DR3+ or DR4+ (%) | 94.9 | 96.0 | 92.5 | 96.0 | 0.09 |
Values expressed with a plus/minus sign are the mean ± standard deviation. ACR, albumin/creatinine ratio; CRP, C-reactive protein; DR, diabetic retinopathy; GAD65, glutamic acid decarboxylase 65; HDL, high-density lipoprotein; IA2, insulinoma antigen; LDL, low-density lipoprotein; MNSI, Michigan Neuropathy Screening Instrument.
Prevalence of complications across tertile of duration
The rates of microvascular complications appeared to have remained stable across duration tertiles. Additionally, HbA1c appeared to have little influence on small-vessel complications and remained stable across all intervals (Supplemental Tables 1b–c (248.7KB, pdf) ). However, the prevalence of CVD increased with duration, potentially reflecting an age-related effect or the influence of another factor (Supplemental Fig. 1 and Supplemental Table 1a (248.7KB, pdf) ).
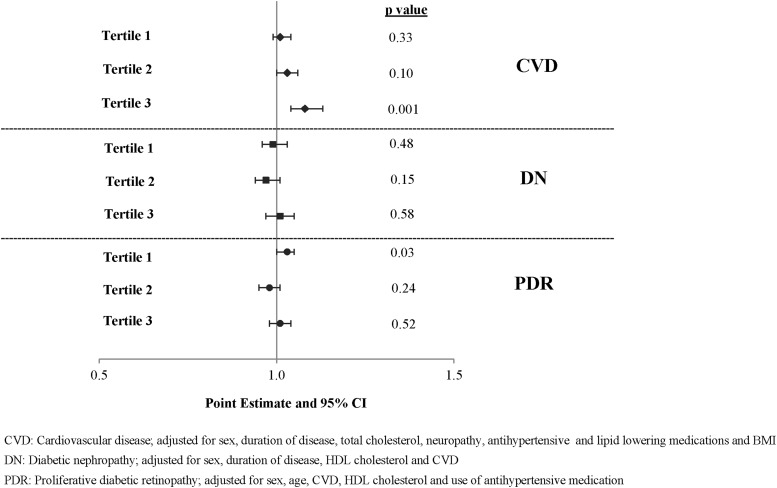
In multivariable analysis, glycemic control was associated with PDR in the shortest tertile (tertile 1) (OR, 1.03; 95% CI, 1.00 to 1.05), was of borderline significance in tertile 2 with CVD, and was significantly associated in tertile 3 (OR, 1.08; 95% CI, 1.04 to 1.13) (Fig. 1 and Supplemental Tables 2 (248.7KB, pdf) a–c).
Figure 1.
Effect of HbA1c on diabetic complications in multivariate logistic regression model. Graph showing the adjusted odds ratio on a 0.5 to 1.5 scale for a 1-mmol/mol increase in HbA1c on each complication by tertile of diabetes duration. The OR is significant for CVD in tertile 3 (P = 0.001) and for PDR in tertile 1 (P = 0.03). DN, diabetic nephropathy.
Contributions to mortality by diagnosis interval comparison
In 2015, 87% of 952 participants were living. Diagnosis intervals were broken down into temporal periods: <1940, 1940 to 1949, 1950 to 1959, and ≥1960. Of the 952 individuals enrolled in the study at the time of analysis, 4.7% (n = 45), 14.5% (n = 138), 55.0% (n = 524), and 25.7% (n = 245) had been diagnosed in each of the defined time intervals, respectively. As expected, age at study entry differed significantly between these intervals (P < 0.001), yet current HbA1c did not (P = 0.58). The prevalence of insulin infusion pump use increased across diagnosis interval (Supplemental Table 3 (248.7KB, pdf) ). In an examination of longitudinal HbA1c measurements of 99 JDC patients (10% of the study group) available back to 1995, whose clinical characteristics did not differ from those of the overall cohort, the correlation between longitudinal glycemic control and HbA1c at the time of Medalist Study visit was 0.51 by using the Pearson method, indicating that HbA1c at the time of study visit may serve as an indicator of long-term glycemic control in this cohort (Supplemental Fig. 2 (248.7KB, pdf) ).
Overall mortality
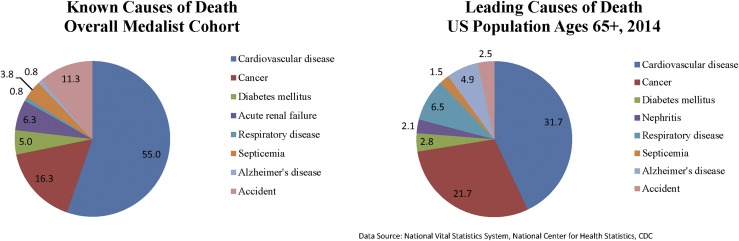
In the years of follow-up between 2005 and 2015, 123 study participants died; among these, cause of death was ascertained for 100 individuals. Mean age at presentation was 69 years and mean age at death was 73 years, with a total of 4954 person-years of follow-up. The dominant known cause of death among Medalists was CVD (55.0%), followed by cancer (16.3%) (Fig. 2). This pattern is similar to 2014 US mortality statistics: CVD as cause of death for 31.7% and cancer for 21.7% (23). More than one half to one third of Medalists with known cause of death in each diagnosis decade interval died of CVD (data not shown). This is in contrast to what is seen among those with type 2 diabetes, whose patterns of mortality show an equilibration of death from cancer and CVD with age (24). Of those who were diagnosed in earlier decades, more men were among the deceased than women. As expected, age at visit and duration of disease among the deceased declined with more recent decade of diagnosis. Levels of eGFR were lower among the deceased with more recent decade of diagnosis, as well as the prevalence of CVD (Supplemental Table 4 (248.7KB, pdf) ).
Figure 2.
Distribution of causes of death in 50-Year Medalists compared with the US population aged ≥65 years. Two pie charts show the leading causes of death in the Medalist population (55.0% CVD, 16.3% cancer, and 11.2% complications of diabetes) compared with the leading causes of death in the US population aged ≥65 years in 2014 (31.7% CVD, 21.7% cancer, and 2.8% diabetes mellitus).
Influences on survival
Table 2 shows the association of risk factors included in the analysis with mortality risk. Overall, age at study entry (HR, 1.1; 95% CI, 1.08 to 1.14) and presence of CVD (HR, 2.29; 95% CI, 1.50 to 3.49) were significantly associated with increased mortality risk. The significant and substantial association of CVD and mortality was consistent across the first three diagnosis year intervals in which there were sufficient events for stable models.
Table 2.
Fully Adjusted Model of Mortality in the Overall Cohort and by Diagnosis Interval With Main Effect of HbA1c
| Variable |
Overall |
<1940 |
1940–1949 |
1950–1959 |
>1960a |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Age (y) | 1.11 (1.08–1.14) | <0.001 | 1.12 (1.02–1.23) | 0.02 | 1.08 (1.02–1.15) | 0.01 | 1.11 (1.07–1.16) | <0.001 | 1.02 (0.85–1.21) | 0.86 |
| Sex (male vs female) | 0.81 (0.53–1.22) | 0.31 | 1.20 (0.44–3.30) | 0.72 | 0.69 (0.33–1.45) | 0.33 | 0.76 (0.40–1.46) | 0.41 | 0.1 5 (0.01–2.35) | 0.18 |
| HbA1c (mmol/mol) | 1.02 (1.00–1.04) | 0.13 | 0.98 (0.94–1.03) | 0.51 | 1.04 (1.01–1.08) | 0.02 | 1.01 (0.99–1.05) | 0.24 | 0.96 (0.83–1.12) | 0.61 |
| CVD (yes vs no) | 2.29 (1.50–3.49) | <0.001 | 3.55 1.08–11.6) | 0.04 | 2.52 (1.17–5.48) | 0.02 | 1.87 (0.99–3.51) | 0.05 | 0.26 (0.01–5.95) | 0.40 |
| eGFR (mL/min/1.73 m2) | 0.98 (0.97–0.99) | <0.001 | 1.00 (0.97–1.03) | 0.91 | 0.98 (0.96–1.00) | 0.08 | 0.97 (0.95–0.99) | 0.001 | 0.94 (0.88–0.99) | 0.02 |
| Exercise (yes vs no) | 0.54 (0.36–0.81) | 0.003 | 0.55 (0.21–1.42) | 0.22 | 0.66 (0.32–1.38) | 0.27 | 0.65 (0.32–1.30) | 0.22 | 0.36 (0.02–5.30) | 0.46 |
>1960 has four deaths.
Although sex and HbA1c were not associated with mortality in the full cohort adjusted model, glycemic control was a significant predictor of mortality among those diagnosed between 1940 and 1949, with a 4% increase in risk with every mmol/mol increase (60% increase in risk with each percentage unit increase). Of particular interest is the significant protective effect of physical activity (HR, 0.54; 95% CI, 0.36 to 0.81) (Table 2). Physical activity reduced the effect of CVD among those diagnosed in the <1940 interval from an HR of 4.4 (95% CI, 1.4 to 13.9) to an HR of 3.6 (95% CI, 1.1 to 11.6). The relationship of CVD and mortality was not altered after adjustment for lipids, lipid lowering, antihypertensive medication use, and presence of C-peptide. Subcutaneous pump use was not significant in the adjusted model.
Discussion
Recent data from the Australian, Scottish, and Swedish Registry studies document changes in life expectancy among those with T1D (10, 25, 26). As such, the Joslin 50-Year Medalists, who have ≥50 years of T1D, allow an opportunity to understand what factors will be important for the growing population of aging individuals with long-term T1D.
Longitudinal studies of patients with shorter duration of diabetes overwhelmingly suggest that glycemic control and duration are the primary factors for predicting complications and mortality (1). However, little is known regarding these outcomes in older individuals with T1D. In this study we demonstrate that the influence of modifiable and unmodifiable risk factors on complication status and mortality may depend on diabetes duration and decade of diagnosis. The variability in these influences may indicate contributions from two broad factors: the presence of endogenous protective factors against hyperglycemia enabling survival and possible improvements in glycemic control due to education and technological advances. Previous work from our group has shown the presence of these factors for small-vessel complications. However, the impending effect of long-term low-grade exposure to hyperglycemia is supported by the lack of association between HbA1c and PDR status in Medalist groups with diabetes duration ranging from 52 to 85 years (tertiles 2 and 3), but the presence of such an association in tertile 1, suggesting that the group with diabetes duration of 50 to 51 years may be vulnerable to the influence of glycemic control on PDR status. This most recently diagnosed group of Medalists might also demonstrate decreased CVD mortality due to the introduction of lipid-lowering medications, angiotensin-converting enzyme inhibitors/angiotensin-receptor blockers, and other interventions delaying the progression of substantial vascular pathology. These data suggest that the previously estimated 29-year risk window for microvascular complications development may be expanding as more people survive complications of which they would have otherwise died at an earlier age (1, 3, 15).
Examination of factors associated with complications in this cohort of >900 older individuals confirms our previous reports of a reduced association of glycemic control with microvascular complications after very long duration of T1D compared with those with shorter duration. The finding of an association of HbA1c with PDR in those with 50 to 51 years of T1D duration suggests that a more rapid development of microvascular complications related to glycemic control may be due to longer survival enabled by medication; in this cohort, there was no clear effect of different methods of diabetes management, including pump use and number of blood glucose checks. This was consistent with the findings of Klein et al. (15), who also found no effect of diabetes management tools on the development of PDR in two cohorts.
There was evidence of a significant role for glycemic control and CVD in the longest-duration Medalists. This finding is consistent with what we have previously reported (8, 9, 12). The strongest evidence for maintenance of glycemic control is the finding that for every unit increase in HbA1c, the odds of CVD increased over twofold in the longest-duration group with adjustment for potential confounders. CVD has been identified as the primary cause of death not only in our population but also in longitudinal studies and registries of younger individuals with T1D (4, 5, 10, 25). Thus, lower HbA1c reduced CVD prevalence and, ultimately, increased survival time. As clinical guidelines are loosened for this age group because of fear of hypoglycemic unawareness, attention must still be paid to this risk (27).
Despite the many characteristics that Medalists share with younger individuals with T1D, including CVD as the most common cause of death, the contrast exists that HbA1c is not the dominant contributor to death. After 27 years of follow-up, the Epidemiology of Diabetes Interventions and Complications/Diabetes Control and Complications Trial study observed a lower than expected number of deaths in the intensive control group, pointing to this disparity (5). The significant protective effect of being in the intensive control group was reflected by a reduction in mortality. Additionally, Orchard et al. (5) cite significant differences in glucose control as contributors to mortality rates in a shorter-duration group. In support of our finding of a less substantive role of HbA1c as a contributor to mortality, a meta-analysis of nine studies, in addition to several other studies, showed other factors more pertinent for development of vascular complications, including sex (28–31). Additionally, the meta-analysis of studies of younger individuals with type 1 showed no increased risk for cancer mortality, death from motor vehicle accidents or suicides, similar to our study; this is consistent with the Scottish Linkage Study, which included some longer-duration individuals (10, 25).
Because those in the Medalist population are more similar in age to those with type 2 diabetes, one may expect causes of death to be similar. However, studies comparing causes of death from those with type 2 diabetes and studies in T1D show opposing trends of mortality rates from CVD and cancer (32). Among patients with type 2 diabetes, an equilibration of cancer deaths and deaths from vascular disease is seen with age. In contrast, Harding et al. (24), in a study of >86,800 individuals with T1D and 1,102,248 people with type 2 diabetes, showed that although rates of cancer and CVD equilibrated, they remained significantly higher among those with T1D. This is consistent with what we see in the Medalists.
Despite the lack of association of glycemic control with microvascular complications in most duration tertiles and with mortality in diagnosis decades, data from the current study show a statistically significant association of glycemic control with CVD, which is a substantial contributor to mortality. Additionally, the known modifier of CVD, physical activity, was associated with a significant decrease in CVD. Although death from CVD may be inevitable, addressing the increased mortality burden from elevated levels of HbA1c may result in better quality and longer life. Several large registries have also reported the importance of the need of CVD prevention in younger populations as well, including the Australian Registry Study, which demonstrated that a significant contributor to years of life lost were mortality from circulatory disease for those ≥40 years (26).
The potential role of exercise in mediating the risk for CVD on mortality in this large cohort of aging individuals with >50 years of T1D must be considered. The difference in mortality risk for those with CVD decreases from an HR of 4.4 (95% CI, 1.4 to 13.9) to 3.6 (95% CI, 1.1 to 11.6) with adjustment for exercise. This mechanism may be through an association of a reduction of HbA1c or other risk factors associated with the cardiometabolic milieu. Long-living individuals often show a delay of aging, which is characterized by the progressive loss of cardiovascular homeostasis, along with reduced endothelial nitric oxide synthase activity, endothelial dysfunction, and impairment of tissue repair after ischemic injury (13). In previously presented work, we have demonstrated that an increase in the number of endothelial progenitor cells was associated with reported physical activity and a reduction in CVD and may explain some of this protection, yet in our cohort this was not associated with glycemic variability, in contrast to other studies (12, 13, 33).
Although clinical assessment for complication status, including retinopathy, neuropathy, and nephropathy, were done onsite for all individuals, lifestyle and previous medical history (including events defining CVD status) were collected via questionnaire. This may have prevented the detection of a relationship between glycemic control and subclinical CVD due to lower rates in reporting. However, several studies have validated self-report of CVD (16–18) against physician report throughout the United States and across age groups. This outcome has been used in several other studies for the association with other exposures (34–37). The generalizability of the findings in this cohort to those in patients with T1D across the United States, or others who have been documented to be reaching the 50-year mark, is also a potential limitation. However, examination of two other cohorts with ≥50 years of T1D show similar characteristics in terms of age at onset, mean duration, sex, presence of CVD, retinopathy, and nephropathy (38, 39). There is clearly a potential for survival bias, as we have stated previously (9), in the Joslin 50 Year Medalist Study; however, this has allowed examination of both potential protective factors and those that may cause a possible delay in the effects of hyperglycemic exposure as suggested by other investigators (15). This may help elucidate applicable factors to patients with shorter disease duration.
The current study demonstrates an evolving pattern of complications and mortality in patients aging with T1D, suggesting that careful attention be paid to modifiable variables, including glycemic control and physical activity. Early intensive glycemic control has been shown to reduce CVD prevalence and mortality and is still important in a population with >50 years of disease duration. This analysis validates our previous finding of a limited association of HbA1c with microvascular complications yet demonstrates a possible changing trend in PDR and HbA1c in those with long-term T1D. The essential clinical finding is the importance of glycemic control in older individuals with CVD and mortality. Following on this is the role of physical activity as a modifiable risk factor for the reduction in CVD prevalence in older adults with T1D. Given the potential burdens of complications and mortality risks, these results have important implications for glycemic targets in older populations with T1D.
Acknowledgments
The study would not have been possible without the staff in the Joslin Clinical Research Center and the 50-Year Medalists and their families. The authors thank Maya Khatri for her careful review of the final manuscript.
Financial Support: The 50-Year Medalist Study is funded by National Institute of Diabetes and Digestive and Kidney Disease (P30DK036836, UL1 RR025758-03, R24 DK083957-01, DP3 DK094333-01, T32DK007260), Juvenile Diabetes Research Foundation (17-2013-310), the Tom Beatson Jr. Foundation, and many Medalists. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. H.A.K. had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
Author Contributions: S.A.D., G.L.K., and H.A.K. collected the data and performed study procedures/conducted study operations. L.J.T., V.K., D.P., J.K.S., G.L.K., and H.A.K. conceived the analysis and manuscript. L.J.T., V.K., D.P., and H.A.K. performed data analysis. L.J.T., V.K., and H.A.K. prepared the manuscript. All authors reviewed/edited the manuscript.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CI
- confidence interval
- CVD
- cardiovascular disease
- eGFR
- estimated glomerular filtration rate
- HbA1c
- hemoglobin A1c
- HR
- hazard ratio
- JDC
- Joslin Diabetes Center
- OR
- odds ratio
- PDR
- proliferative diabetic retinopathy
- T1D
- type 1 diabetes.
References
- 1.The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44(8):968–983. [PubMed] [Google Scholar]
- 2.Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol. 1995;75(14):894–903. [DOI] [PubMed] [Google Scholar]
- 3.Krolewski A. Epidemiology of late complications of diabetes. In: Kahn CR, Weir GD, eds. Joslin's Diabetes Mellitus. 13th ed. Philadelphia: Lea & Febiger; 1994:605–619. [Google Scholar]
- 4.Miller RG, Secrest AM, Sharma RK, Songer TJ, Orchard TJ. Improvements in the life expectancy of type 1 diabetes: the Pittsburgh Epidemiology of Diabetes Complications study cohort. Diabetes. 2012;61(11):2987–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orchard TJ, Nathan DM, Zinman B, Cleary P, Brillon D, Backlund JY, Lachin JM; Writing Group for the DCCT/EDIC Research Group . Association between 7 years of intensive treatment of type 1 diabetes and long-term mortality. JAMA. 2015;313(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2010;53(11):2312–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gagnum V, Stene LC, Leivestad T, Joner G, Skrivarhaug T. Long-term mortality and end-stage renal disease in a type 1 diabetes population diagnosed at age 15-29 years in Norway. Diabetes Care. 2017;40(1):38–45. [DOI] [PubMed] [Google Scholar]
- 8.Keenan HA, Costacou T, Sun JK, Doria A, Cavellerano J, Coney J, Orchard TJ, Aiello LP, King GL. Clinical factors associated with resistance to microvascular complications in diabetic patients of extreme disease duration: the 50-year medalist study. Diabetes Care. 2007;30(8):1995–1997. [DOI] [PubMed] [Google Scholar]
- 9.Sun JK, Keenan HA, Cavallerano JD, Asztalos BF, Schaefer EJ, Sell DR, Strauch CM, Monnier VM, Doria A, Aiello LP, King GL. Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the Joslin 50-year Medalist Study. Diabetes Care. 2011;34(4):968–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livingstone SJ, Looker HC, Hothersall EJ, Wild SH, Lindsay RS, Chalmers J, Cleland S, Leese GP, McKnight J, Morris AD, Pearson DW, Peden NR, Petrie JR, Philip S, Sattar N, Sullivan F, Colhoun HM. Risk of cardiovascular disease and total mortality in adults with type 1 diabetes: Scottish registry linkage study. PLoS Med. 2012;9(10):e1001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrie D, Lung TW, Rawshani A, Palmer AJ, Svensson AM, Eliasson B, Clarke P. Recent trends in life expectancy for people with type 1 diabetes in Sweden. Diabetologia. 2016;59(6):1167–1176. [DOI] [PubMed] [Google Scholar]
- 12.He ZH, D’Eon SA, Tinsley LJ, Fitzgerald S, Hastings SM, Khamaisi M, Sun JK, Turek SJ, Schaefer EJ, King GL, Keenan HA. Cardiovascular disease protection in long-duration type 1 diabetes and sex differences. Diabetes Care. 2015;38(5):e73–e74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez SL, Gong JH, Chen L, Wu IH, Sun JK, Keenan HA, King GL. Characterization of circulating and endothelial progenitor cells in patients with extreme-duration type 1 diabetes. Diabetes Care. 2014;37(8):2193–2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khamaisi M, Katagiri S, Keenan H, Park K, Maeda Y, Li Q, Qi W, Thomou T, Eschuk D, Tellechea A, Veves A, Huang C, Orgill DP, Wagers A, King GL. PKCδ inhibition normalizes the wound-healing capacity of diabetic human fibroblasts. J Clin Invest. 2016;126(3):837–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115(11):1859–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergmann MM, Byers T, Freedman DS, Mokdad A. Validity of self-reported diagnoses leading to hospitalization: a comparison of self-reports with hospital records in a prospective study of American adults. Am J Epidemiol. 1998;147(10):969–977. [DOI] [PubMed] [Google Scholar]
- 17.Martin LM, Leff M, Calonge N, Garrett C, Nelson DE. Validation of self-reported chronic conditions and health services in a managed care population. Am J Prev Med. 2000;18(3):215–218. [DOI] [PubMed] [Google Scholar]
- 18.Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ. Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol. 2004;57(10):1096–1103. [DOI] [PubMed] [Google Scholar]
- 19.Grams ME, Chow EK, Segev DL, Coresh J. Lifetime incidence of CKD stages 3-5 in the United States. Am J Kidney Dis. 2013;62(2):245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Early Treatment Diabetic Retinopathy Study Research Group Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS report number 12. Ophthalmology. 1991;98(5 Suppl):823–833. [PubMed] [Google Scholar]
- 21.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17(11):1281–1289. [DOI] [PubMed] [Google Scholar]
- 22.Ainsworth BE, Leon AS, Richardson MT, Jacobs DR, Paffenbarger RS Jr. Accuracy of the College Alumnus Physical Activity Questionnaire. J Clin Epidemiol. 1993;46(12):1403–1411. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention. 10 Leading Causes of Death by Age Group, United States - 2014. 2016. Available at: https://www.cdc.gov/injury/wisqars/pdf/leading_causes_of_death_by_age_group_2014-a.pdf. Accessed 16 March 2016.
- 24.Harding JL, Shaw JE, Peeters A, Davidson S, Magliano DJ. Age-specific trends from 2000-2011 in all-cause and cause-specific mortality in type 1 and type 2 diabetes: a cohort study of more than one million people. Diabetes Care. 2016;39(6):1018–1026. [DOI] [PubMed] [Google Scholar]
- 25.Carstensen B, Read SH, Friis S, Sund R, Keskimäki I, Svensson AM, Ljung R, Wild SH, Kerssens JJ, Harding JL, Magliano DJ, Gudbjörnsdottir S; Diabetes and Cancer Research Consortium . Cancer incidence in persons with type 1 diabetes: a five-country study of 9,000 cancers in type 1 diabetic individuals. Diabetologia. 2016;59(5):980–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huo L, Harding JL, Peeters A, Shaw JE, Magliano DJ. Life expectancy of type 1 diabetic patients during 1997-2010: a national Australian registry-based cohort study. Diabetologia. 2016;59(6):1177–1185. [DOI] [PubMed] [Google Scholar]
- 27.American Diabetes Association Glycemic targets. Diabetes Targets. 2016;39(Suppl 1):S39–S46. [DOI] [PubMed] [Google Scholar]
- 28.Laing SP, Swerdlow AJ, Slater SD, Burden AC, Morris A, Waugh NR, Gatling W, Bingley PJ, Patterson CC. Mortality from heart disease in a cohort of 23,000 patients with insulin-treated diabetes. Diabetologia. 2003;46(6):760–765. [DOI] [PubMed] [Google Scholar]
- 29.Moss SE, Klein R, Klein BE. Cause-specific mortality in a population-based study of diabetes. Am J Public Health. 1991;81(9):1158–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skrivarhaug T, Bangstad HJ, Stene LC, Sandvik L, Hanssen KF, Joner G. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia. 2006;49(2):298–305. [DOI] [PubMed] [Google Scholar]
- 31.Secrest AM, Becker DJ, Kelsey SF, Laporte RE, Orchard TJ. Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes. 2010;59(12):3216–3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tancredi M, Rosengren A, Svensson AM, Kosiborod M, Pivodic A, Gudbjörnsdottir S, Wedel H, Clements M, Dahlqvist S, Lind M. Excess Mortality among Persons with Type 2 Diabetes. N Engl J Med. 2015;373(18):1720–1732. [DOI] [PubMed] [Google Scholar]
- 33.Maiorino MI, Casciano O, Della Volpe E, Bellastella G, Giugliano D, Esposito K. Reducing glucose variability with continuous subcutaneous insulin infusion increases endothelial progenitor cells in type 1 diabetes: an observational study. Endocrine. 2016;52(2):244–252. [DOI] [PubMed] [Google Scholar]
- 34.Bacon CG, Mittleman MA, Kawachi I, Giovannucci E, Glasser DB, Rimm EB. Sexual function in men older than 50 years of age: results from the health professionals follow-up study. Ann Intern Med. 2003;139(3):161–168. [DOI] [PubMed] [Google Scholar]
- 35.Thompson IM, Tangen CM, Goodman PJ, Probstfield JL, Moinpour CM, Coltman CA. Erectile dysfunction and subsequent cardiovascular disease. JAMA. 2005;294(23):2996–3002. [DOI] [PubMed] [Google Scholar]
- 36.Araujo AB, Hall SA, Ganz P, Chiu GR, Rosen RC, Kupelian V, Travison TG, McKinlay JB. Does erectile dysfunction contribute to cardiovascular disease risk prediction beyond the Framingham risk score? J Am Coll Cardiol. 2010;55(4):350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feldman HA, Goldstein I, Hatzichristou DG, Krane RJ, McKinlay JB. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol. 1994;151(1):54–61. [DOI] [PubMed] [Google Scholar]
- 38.Bain SC, Gill GV, Dyer PH, Jones AF, Murphy M, Jones KE, Smyth C, Barnett AH. Characteristics of Type 1 diabetes of over 50 years duration (the Golden Years Cohort). Diabet Med. 2003;20(10):808–811. [DOI] [PubMed] [Google Scholar]
- 39.Keenan H. Studies of those with 50 or more years with type 1 diabetes—what have we learned? Presented at: American Diabetes Association 77th Scientific Sessions; June 9–13, 2017; San Diego, California. [Google Scholar]