Abstract
Context:
Resistance to thyroid hormone-β (RTH-β) is an autosomal dominant disorder characterized by reduced sensitivity of target tissues to thyroid hormones (THs). Individuals with RTH-β have high TH levels usually due to mutations in the TH receptor-β (THRB) gene. The management of RTH-β during pregnancy is challenging, as wild-type (WT) fetuses born to RTH-β mothers have low birth weight and suppressed postnatal thyroid-stimulating hormone (TSH), due to intrauterine exposure to excess TH.
Objective:
To determine birth weight and postnatal TSH of WT fetuses carried by mothers with RTH-β whose fT4 levels were maintained below 20% of the upper limit of normal (ULN).
Design:
Retrospective chart review.
Setting:
Academic institution in collaboration with off-site hospitals and private practices.
Patients:
Thirteen women harboring THRB gene mutations were evaluated during 18 pregnancies.
Intervention:
Prenatal genetic diagnosis by amniocentesis. Women carrying WT fetuses were given the option of treatment with antithyroid medication by their treating physicians with the aim to avoid serum fT4 levels above 20% of the ULN.
Results:
No significant difference was found in birth weight corrected for gestational age and in serum TSH levels at birth between WT and RTH-β infants born to RTH-β mothers.
Conclusions:
Prenatal diagnosis may play an important role in the management of RTH-β during pregnancy. Aiming for maternal fT4 levels not above 50% of the ULN in RTH-β mothers carrying WT fetuses seems to be a prudent approach that prevents the otherwise expected low birth weight and postnatal TSH suppression.
We studied pregnant women with resistance to TH and found that antithyroid treatment in mothers with high fT4-carrying WT fetuses prevented low birth weight and suppressed postnatal TSH.
Thyroid hormones (THs) play an indispensable role in vertebrate embryogenesis, fetal development, and maturation. During early pregnancy, before the development of a functioning thyroid gland, the fetus is dependent on TH supplied by the mother. It has been well documented that maintaining a euthyroid state in utero is critical for the fetal well-being. Maternal hypothyroidism has been associated with poor pregnancy outcome, decreased birth weight, and impaired neuropsychological development of the offspring (1–3). On the other hand, maternal hyperthyroidism has been associated with increased rate of miscarriages, premature labor, and low birth weight (4). However, most studies include women with autoimmune thyroid disease, precluding differentiation of the effects of maternal thyrotoxicosis and autoimmunity from that of the direct effects of TH on the fetus.
Resistance to thyroid hormone-β (RTH-β) is a dominantly inherited disorder with a prevalence of 1:40,000 live births, caused by mutations of the TH receptor-β (THRB) gene. It is characterized by reduced responsiveness of target tissues to TH and its biochemical hallmark consists of elevated free serum T4 and often free T3 levels with normal or slightly increased thyroid-stimulating hormone (TSH) concentration. The mutant receptors have impaired function but also interfere with the activity of the normal receptors, a process termed dominant negative effect (5).
As a consequence, RTH-β is a unique condition, in that affected individuals have high TH levels but are clinically euthyroid. The increased thyroid gland activity and TH secretion are mediated by TSH rather than by thyroid stimulating antibodies (5). During pregnancy, RTH-β serves as a unique model of isolated fetal hyperthyroidism, because the wild-type (WT) fetus is exposed to excessive levels of TH while the mother remains in a clinically eumetabolic state. Furthermore, the absence of thyroid stimulating antibodies precludes the possibility of autoimmunity affecting the fetus’ thyroid status.
WT infants born to mothers with RTH-β have lower birth weight and suppressed postnatal TSH compared with infants harboring the THRB gene mutation (6). This is due to in utero exposure to high TH levels, which are incongruent with the requirements of a fetus with normal THRB genotype. The management of pregnant mothers with RTH-β remains controversial. A pregnant subject with the mutant THRB A317T, carrying a WT fetus, was treated with propylthiuracil during pregnancy and gave birth to a healthy infant of normal birth weight and TSH levels (7). Given this observation and the negative effects of high maternal TH levels on normal fetuses, some treating physicians felt it would be beneficial to lower the high maternal TH levels in women with RTH-β carrying a WT fetus.
The aim of the present report is to review the experience with in utero reduction of fetal exposure to inappropriately high TH levels provided by the mother with RTH-β. To this purpose, a prenatal determination of the fetal genotype was required to identify the embryos at risk.
Materials and Methods
Patients included 13 women harboring THRB gene mutations, during a total of 18 pregnancies. All women were managed by endocrinologists and obstetricians at different institutions within the United States or abroad, who consulted us (S.R. and R.E.W.) for prenatal diagnosis and recommendation regarding management. Upon confirmation of the pregnancy at the request of the patient and treating physician, each individual underwent either amniocentesis (n = 17) or chorionic villous sampling (n = 1). Genotyping was performed at the University of Chicago or in outside laboratories. At the University of Chicago, DNA was extracted and mutational analysis of the THRB gene was performed to determine the genotype of the fetus. After amplification by polymerase chain reaction, the presence or absence of the maternal THRB gene mutation was determined by direct sequencing or by restriction endonuclease digestion. To exclude the possibility of contamination by maternal DNA in the sample, the template was diluted to assess the relative abundance of the mutant allele (7). The mutation was also confirmed on peripheral mononuclear cells of the mother. Informed consents, approved by the Institutional Review Board of the University of Chicago, were obtained from the pregnant women.
The decision to treat or not was left to the subject and the physician responsible for her care (endocrinologist and obstetrician). Our recommendation was based on two major factors: fetus genotype and maternal thyroid function tests (TFTs). No treatment was recommended to women carrying fetuses harboring the same THRB gene mutation as their respective mother. Treatment with an antithyroid medication was recommended to women carrying WT fetuses starting early in the second trimester if their fT4 levels exceeded 20% of the upper limit of normal (ULN). Methimazole and, in some cases, propylthiouracil were used until delivery. Maternal TFTs were monitored by the local endocrinologist, and treatment was adjusted to maintain fT4 levels close to 20% of the ULN.
At delivery, the weight of the newborn and Apgar scores at 1 and 5 minutes were recorded. Serum TSH levels were measured at days of life 2 to 5, and the newborn’s genotype was confirmed.
Results were expressed either as percentages for categorical variables or as mean ± standard error of the mean (SEM) for continuous variables. The Pearson’s χ2 test was applied to categorical data and the two-tailed Student’s t test for continuous data. A P value < 0.05 was used to determine statistical significance.
Results
Maternal characteristics
The thirteen women with RTH-β carried 10 different THRB gene mutations, listed in Table 1. They were followed over the course of 18 pregnancies: subject 9 during two pregnancies, subjects 2 and 7 during three pregnancies each, and all other subjects during one pregnancy each. Subject 7 was treated with 3,5,3′-triiodothyroacetic acid prior to the onset of pregnancy; she subsequently developed silent thyroiditis and has been on treatment with levothyroxine since then (8). Regarding the obstetric history of the studied individuals, two women previously had children with RTH-β (patients 6 and 11), subject 8 reported a prior ectopic pregnancy and a pregnancy resulting in intrauterine death at 39 weeks, and subject 4 reported a history of miscarriage at 11 weeks. Subject 13 had one child with RTH-β and a history of four first-trimester miscarriages. Data on thyroid autoantibodies of the mothers with RTH-β were available in seven out of 13 subjects (see Table 2).
Table 1.
Pregnant Women With RTH-β Studied and Fetal Genotypes
| THRB Gene Mutation (Amino Acid Change) | Number of Individuals | Patient ID Number | Fetal Genotypes |
|---|---|---|---|
| L450H | 1 | 1 | 1 WTa |
| M334R | 1 | 2 (three pregnancies) | 1 WT, 2 RTH-βb |
| A317T | 3 | 3, 8, 9 (two pregnancies) | 2 WT, 2 RTH-β |
| E460K | 1 | 4 | 1 WT |
| R320C | 2 | 5, 7 (three pregnancies) | 3 WT, 1 RTH-β |
| V349M | 1 | 6 | 1 RTH-β |
| P453T | 1 | 10 | 1 WT |
| R429Q | 1 | 11 | 1 WT |
| M310L | 1 | 12 | 1 WT |
| R243Q | 1 | 13 | 1 WT |
Women are numbered in the order of their presentation.
WT indicates without a THRB gene mutation.
RTH-β indicates carriers of the maternal THRB gene mutation.
Table 2.
TFTs Prior to Pregnancy in Women With RTH-β, Gestational Period, and Newborn Weight and TSH
| Subject Number | Maternal Mutation | Infant’s Genotype | Prepregnancy Maternal TSH, mU/L | Prepregnancy Maternal fT4, %ULN | TPO/TG abs | Gestational Age at Delivery, wk | Maternal Treatment | Infant’s Sex | Infant’s Birth Weight, kg | Birth Weight Z Score | Infant’s TSH at Birth, mU/L |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | L450H | WT | 5.2 | 162 | +/− | Unknown | |||||
| 2 | M334R | WT | 1.6 | 163 | −/− | 38 | MMI | F | 2.48 | −1.28 | 1.6 |
| 3 | A317T | WT | 2.1 | 183 | −/− | 38 | Unknown | M | 2.80 | −0.98 | 4.3 |
| 4 | E460K | WT | 1.9 | 165 | −/− | Unknown | |||||
| 5 | R320C | WT | 1.9 | 158 | −/− | 37 | None | ||||
| 7 | R320C | WT | 3.0 | 129 | +/− | 39 | LT4 | F | 3.53 | +0.67 | 3.3 |
| 7 | R320C | WT | 3.0 | 129 | +/− | 40 | LT4 | F | 4.05 | +0.98 | 3.3 |
| 9 | A317T | WT | 3.0 | 219 | Unknown | 39 | PTU | F | 3.06 | −0.98 | 29.5 |
| 10 | P453T | WT | 1.1 | 236 | Unknown | 38 | PTU | M | 2.93 | −0.98 | 4.6 |
| 11 | R429Q | WT | 2.5 | 126 | Unknown | 37 | None | M | 2.30 | −1.76 | 8.0 |
| 12 | M310L | WT | Unavailable | Unknown | 38 | MMI | F | 2.92 | −0.34 | 15.0 | |
| 13 | R243Q | WT | 2.9 | 156 | Unknown | 39 | PTU | F | 3.23 | +0.34 | 4.3 |
| 2 | M334R | RTH-β | 1.6 | 163 | −/− | 37 | None | M | 2.31 | −1.76 | |
| 2 | M334R | RTH-β | 1.6 | 163 | −/− | None | F | 6.0 | |||
| 6 | V349M | RTH-β | 1.7 | 232 | −/− | None | |||||
| 7 | R320C | RTH-β | 3.0 | 129 | +/− | 39 | LT4 | F | 3.48 | +0.67 | 2.91 |
| 8 | A317T | RTH-β | 4.4 | 180 | Unknown | 40 | None | M | 3.33 | −0.34 | 4.4 |
| 9 | A317T | RTH-β | 3.0 | 219 | Unknown | 35 | None | F | 2.33 | −0.34 | 6.7 |
Abbreviations: F, female; M, male; MMI, methimazole.
Fetal characteristics
Following THRB gene sequencing, six fetuses were found to carry the maternal THRB gene mutation and twelve fetuses were WT, carrying the WT maternal allele. This was confirmed by gene sequencing of the newborn’s blood samples. The sex was known in 13 pregnancies: five male and eight female. In nine pregnancies, there were data on the mode of delivery, and these included five vaginal deliveries (four WT and one infant with a THRB gene mutation); three cesarean sections, all involving WT infants (in one case the indication for cesarean section was deceleration of the fetal heart rate); and one stillbirth. The latter (patient 2, first pregnancy) involved a fetus carrying the M334R mutation; it occurred at the 37th week of gestation, and the autopsy demonstrated fetal hypoxia and a very small placenta. Other perinatal complications included one case of postnatal respiratory distress in a newborn with RTH-β, which was attributed to pneumonia and resolved after admission to the neonatal intensive care unit and treatment with antibiotics, and one case of mild neonatal jaundice. In addition, the first (WT) newborn of subject 9 had hypothermia at birth, whereas her second child with RTH-β was reportedly tachycardic and jittery.
Of the 12 WT fetuses, the mothers of 5 of them were treated by their physicians to lower the serum fT4 with antithyroid drugs (methimazole or propylthiouracil), and in 2 cases, the mother was on LT4 therapy (subject 7). Data on maternal treatment were incomplete in five subjects (Table 2). Subjects 1, 4, 5, and 6 were excluded from statistical analysis due to lack of documented infant birth weight and postnatal TSH. No significant prematurity was noted; the mean gestational week at the time of delivery was 37.78 ± 0.32. Apgar scores at 1 and 5 minutes were 8 or above in all newborns.
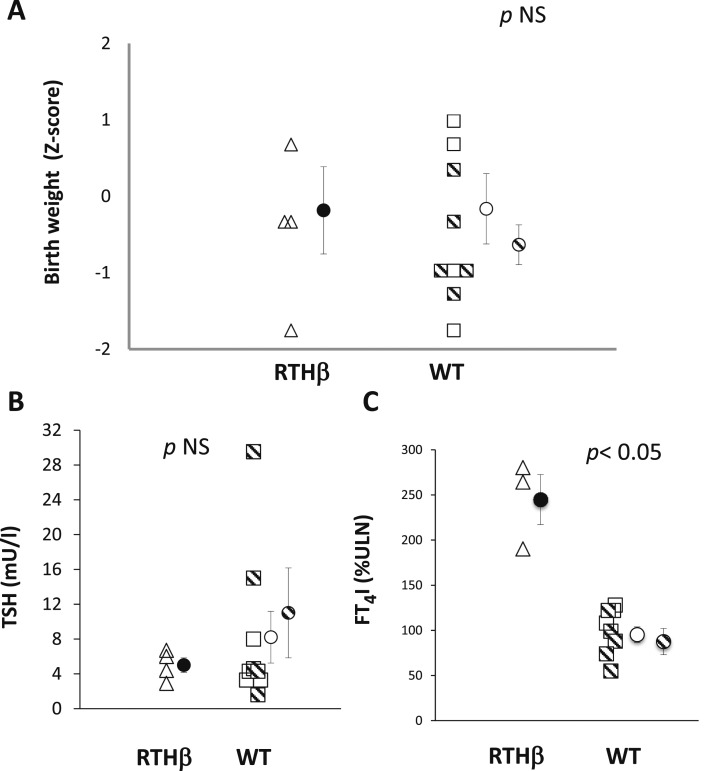
Birth weights were corrected for the gestational age at delivery and the Z score for percentiles of normal distribution was calculated (9). No significant difference was found in birth weight corrected for gestational age between RTH-β and WT newborns, whether comparisons were made with the entire group of WT infants born to mothers with RTH-β or only those whose mothers received antithyroid drugs (mean Z scores = –0.19 for RTH-β, –0.17 for all WT and –0.64 for those whose mothers were treated P = 0.98 and 0.45, respectively; Fig. 1A).
Figure 1.
(A) Birth weight of neonates born to mothers carrying a THRB gene mutation based on their genotype (RTH-β and WT). Birth weight is controlled for gestational age at delivery and expressed as Z score from the 50% percentile. Hatched symbols indicate neonates whose mothers received antithyroid treatment. (B) TSH levels at birth of neonates born to mothers carrying a THRB gene mutation based on their genotype (RTH-β and WT). (C) Free T4 index (expressed as percent of the ULN) at birth of neonates born to mothers carrying a THRB gene mutation based on their genotype (RTH-β and WT). FTI: free T4 index. Circles and vertical lines indicate, respectively, mean and SEM for the whole group of WT infants (open circles) and only those whose mothers were treated with antithyroid drugs (hatched circles).
There was no significant difference in the serum TSH levels at birth (days of life 2 to 5) between infants carrying the THRB gene mutations and either all WT infants or those whose mothers were treated (5.00 ± 0.84, 8.21 ± 2.97 and 11 ± 5.16 mU/L, P = 0.5 and 0.34, respectively). Only two infants (WT born to subjects 9 and 12) had a slight transient elevation of TSH. The temporary increase in postnatal TSH might be the result of measuring TSH concentration too soon after birth, or may be due to the effect of antithyroid treatment on the infant (Fig. 1B); maternal free T4 levels were not allowed to decline below 100% of the ULN. In 11 newborns, data on serum TH levels at birth were also available; when expressed as percent of the ULN, the free T4 index of the newborns with RTH-β was significantly higher compared with the free T4 index of WT newborns, either all or only those whose mothers were treated (244.7 ± 27.7%, 95.2 ± 8.6% and 87.5 ± 14.6% of the ULN respectively, P < 0.001 and 0.002, respectively; Fig. 1C).
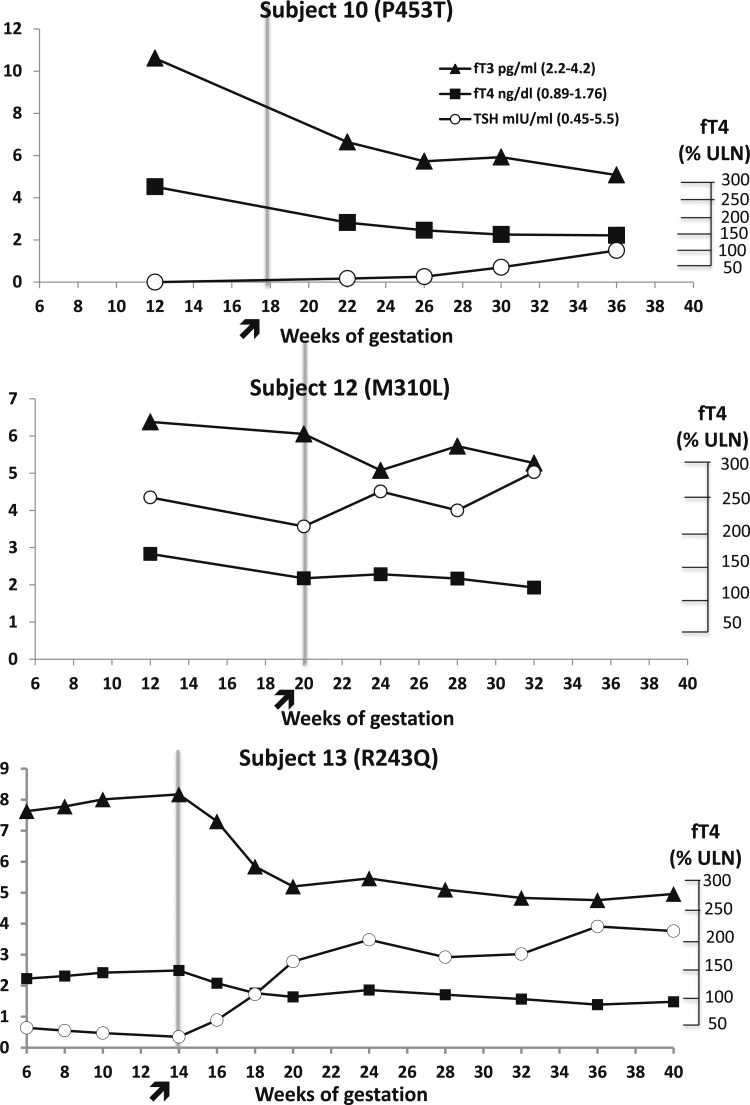
The follow-up of the pregnant women with RTH-β was performed outside our institution. Unfortunately, data collected on maternal TFTs during pregnancy was incomplete and not suitable for statistical analysis. As representative examples, the evolution of maternal TFTs is illustrated in three cases of pregnant women carrying a WT fetus (Fig. 2), whereas the prepregnancy TFTs of the studied women are shown in Table 2 along with a summary of data on the infant’s outcome.
Figure 2.
TFTs in three pregnant women with RTH-β (mutation in brackets) carrying WT fetuses. The arrow indicates initiation of antithyroid treatment. Subject 10 (P453T) received propylthiouracil requiring from 150 mg/d during the second trimester up to 450 mg/d during the third trimester. Subject 12 (M310L) received methimazole starting from 5 mg/d at gestational week 20 up to 10 mg/d during the third trimester. Subject 13 (R243Q) received propylthiouracil starting with a dose of 150 mg/d at gestational week 14, which was reduced to 100 mg/d after gestational week 20 and for the remaining gestational period.
Discussion
Review of pregnancies in women with RTH-β indicates that prenatal identification of the fetal genotype and judicious treatment of women carrying WT fetuses to reduce the high serum TH concentration can prevent the otherwise expected low birth weight and postnatal TSH suppression.
The reason why some treating physicians choose to lower TH levels with antithyroid therapy is mostly based on a study of a large Azorean family harboring the R243Q mutation. Women with RTH-β demonstrated a three- to fourfold higher miscarriage rate compared with WT women. They gave birth to a significantly higher proportion of infants with RTH-β, suggesting that the miscarriages predominantly involve WT fetuses. Moreover, WT infants born to mothers with RTH-β had significantly lower birth weight compared with infants with RTH-β and their postnatal TSH was suppressed in all cases (6). This indicated that WT fetuses, when exposed to high maternal TH levels, inappropriate to the fetus’ genotype, develop hyperthyroid features during fetal life. This is consistent with experimental data from a study in Thrb knockout mice (10). It should be noted that prenatal diagnosis would not be expected to have an impact on the miscarriage rate of mothers with RTH-β, as the miscarriages tend to occur early in the first trimester, prior to the time window for amniocentesis.
There are sparse published data on the course and outcome of pregnancy in women with RTH-β (11–13) and even fewer reports on intervention during gestation (7, 14–16). An overview of published cases of RTH-β and pregnancy outcome is presented in Table 3. Of note, in all but two pregnancies resulting in the birth of infants with normal weight and nonsuppressed TSH, maternal fT4 levels were below the 120% of ULN, and in all cases, they were below the values of 172 ± 24% (mean ± SEM; N = 18) of the ULN observed in the untreated women of the extended Azorean family (6).
Table 3.
Review of Reported Women With RTH-β During Pregnancy and Their Outcome
| Maternal Mutation | Infant’s Genotype | Maternal Treatment | Maternal fT4, %ULN | Gestational Age at Delivery, wk | Infant’s Sex | Infant’s Birth Weight, kg | Birth Weight Z Score | Infant’s TFTs | Reference |
|---|---|---|---|---|---|---|---|---|---|
| M310V | WTa | Hypo L-T3 | Low | 36 | M | 3.35 | +2.33 | (15) | |
| R383H | WT | PTU | 89 to 123 | 34 | M | 2.14 | −0.34 | “Normal” | (19) |
| R438C | WT | None | 90 to 104 | 38 | Unknown | 3.44 | +1.28 | “Normal” | (19) |
| I431M | WT | None | 104 | 39 | Unknown | 3.06 | 0.00 | “Normal” | (19) |
| R320C | WT | Hypo L-T4 | <123 | 39 | F | 3.53 | +0.98 | (8) | |
| R320C | WT | Hypo L-T4 | <123 | 40 | F | 4.05 | +1.88 | (8) | |
| Unknown | WT | None | <100 | Unknown | Unknown | 3.4 | (21) | ||
| R243W | WT | None | <100b | Unknown | F | Normal, no value | (20) | ||
| V283A | WT | None | 146 to 165 | Unknown | M | 3.15 | “Normal” | (22) | |
| V283A | WT | None | 152 to 166 | Unknown | M | 2.9 | “Normal” | (22) | |
| T239Nc | WT | D-T4 | Unknown | 35 | M | 2.8 | +1.28 | “Normal” | (16) |
| R320C | RTH-βd | Hypo L-T4 | <123 | 39 | F | 3.48 | +0.67 | (8) | |
| R243W | RTH-β | None | <100b | Unknown | M | Normal, no value | (20) | ||
| M310L | RTH-β | None | 183 | 37 | F | 2.25 | −1.65 | (11) | |
| T239Nc | RTH-β | D-T4 | Unknown | 38 | F | 2.85 | +0.34 | (16) |
Abbreviations: F, female; Hypo, hypothyroid; M, male; PTU, propylthiouracil.
WT indicates without a THRB gene mutation.
Normal values not given in reference.
Five miscarriages.
RTH-β indicates carriers of the maternal THRB gene mutation.
In the current study, prenatal testing allowed characterization of the fetal genotype and was used by the treating physicians to decide whether to lower the TH levels in the mother with RTH-β. Pregnant women with RTH-β carrying fetuses with the same mutation were monitored without intervention, because the fetuses were exposed to congruent TH levels. On the contrary, five mothers carrying WT fetuses received antithyroid medication to reduce the magnitude of the incongruent hyperiodothyroninemic intrauterine environment and improve fetal development. Indeed, birth weight corrected for gestational age and serum TSH levels at birth were similar between WT and RTH-β infants.
There are several limitations to this report that do not allow a definitive recommendation for the management of RTH-β during pregnancy. This was not a prospective randomized clinical trial, but it is not likely that such a study would be performed in pregnant women with RTH-β. The latter condition is uncommon and may not be diagnosed prior to pregnancy, as the patients are usually euthyroid with normal serum TSH concentrations. Management of women with RTH-β was performed at different locations in the United States and abroad, and not all referring physicians provided complete follow up data. Thus, information on the course of maternal TFTs during gestation was not sufficient to allow for statistical analysis and provide the degree of serum fT4 levels reduction. However, data on the biochemical course of three women carrying WT fetuses treated with antithyroid medication were available and presented in a graphic form (Fig. 2). Of note, one pregnant woman (Fig. 2, bottom) belonged to the large Azorean family with THRB R243Q, in which the low birth weight and suppressed TSH were observed in all WT offspring born to mothers with RTH-β (6). The course of this specific pregnancy, leading to the delivery of a normal-weight infant with TSH within the reference range, strengthens the validity of the suggested approach of lowering serum TH levels in such individuals. In addition, the cohort of women with RTH-β harbored 10 different THRB gene mutations constituting a heterogeneous group. The small sample size and genetic heterogeneity might explain the lack of difference in birth weight between RTH-β neonates and WT infants whose mothers did not receive antithyroid treatment. A more plausible explanation is that the severity of RTH-β was overall milder than that in the Azorean family. The latter also explains why the number of WT fetuses born to mothers with RTH-β was not lower than of infants carrying the maternal mutations. Further, this discrepancy may also be due to the fact that referring physicians who knew the genotype of the fetuses sought more often advice on management of women with RTH-β carrying normal fetuses.
Besides the three representative cases shown in Fig. 2, two other subjects had significantly high fT4 values that led to the recommendation for antithyroid therapy with subject 2 receiving methimazole and subject 9 propylthiouracil (Table 2). Of note, the maternal fT4 levels of subject 3 were significantly high, over the mean value of the Azorean cohort (6), and the WT infant was born with a normal birth weight and postnatal TSH (Table 2). Although data regarding maternal treatment in this case is lacking, we presume that the local treating physician followed our recommendation on treating the mother with antithyroid drugs. Importantly, the mean ± SEM of fT4 for women who received antithyroid drugs was 194 ± 20% of the ULN compared with 149 ± 85% of the ULN for women who were not treated or with no information (P < 0.04).
The decision to maintain fT4 levels not above 20% of the ULN in women with RTH-β carrying WT fetuses was based on the standard clinical practice for the management of maternal hyperthyroidism (usually Graves’ disease), that is, maintain fT4 levels at the high-normal range (17). Moderation and “first, do no harm” are keys in the approach of RTH-β during pregnancy. The reliability of fT4 assays during pregnancy is another factor to be considered (18, 19). Trimester- and gestational age–specific reference intervals for fT4 are available; cautious interpretation of maternal TFTs, use of laboratory established reference ranges, and consideration of fetal growth and development based on periodic ultrasounds could help circumvent this problem. Monitoring of maternal TFTs and adjustment of treatment dose in these women is even more difficult, because very little is known about thyroid physiology and evolution of TFTs during pregnancy in patients with RTH-β. Based on evidence from isolated cases harboring five different THRB gene mutations, it seems that in RTH-β, human chorionic gonadotropin still has a TSH-mimetic action leading to a transient suppression of TSH, whereas later in gestation, fT4 appears to decrease below the pregestational levels, albeit above the ULN for subjects without RTH-β (12, 20–22). However, based on the outcome of four newborns whose mothers’ fT4 values were closer to 50% above the ULN (23), and patients 10 and 12 in Fig. 2, relaxing the allowable fT4 level upward may be reasonable.
In conclusion, this study emphasizes the role of prenatal diagnosis in the management of pregnant women with RTH-β. There is a small risk of miscarriage (approximately 1%) associated with amniocentesis, which should be taken into account. However, the benefit from reducing in utero exposure to incongruently high TH levels may extend beyond postnatal life. In a recent study, WT adults born to mothers with RTH-β were found to have persistent central resistance to TH, as evidenced by reduced TSH suppression following T3 administration (24). Aiming for fT4 levels not above 50% of the ULN in women carrying WT fetuses seems to be a logical approach, which may prevent low birth weight and suppressed postnatal TSH, adverse outcomes otherwise expected based on previous evidence. Although further studies are required to shed more light on the physiologic changes and the complex fetal–maternal interaction occurring during pregnancy in a woman with RTH-β, it may be safe to delay prenatal diagnosis until the maternal fT4 surpasses 50% above the ULN for gestational age. This recommendation is based on the normal birth weight and nonsuppressed TSH of WT newborns of women with RTH-β having such fT4 values.
Acknowledgments
We thank all the referring physicians, who kindly provided clinical and laboratory data: Dr. Susan Davidson, Dr. Melissa Siegler, Dr. James Hill, Dr. Janet Schlechte, Lesley Turner, Dr. D. W. Ingram, Dr. Carol Joyce, Dr. Hamish Courtney, Dr. Evan D. Abel, Dr. Daniel Knodel, Dr. Melissa Herbst, Dr. Thomas O. Carpenter, Dr. Chantal Daumerie, Dr. Abby S. Hollander, Dr. Stanley Matthew, Dr. Peter Dureska, Dr. Elizabeth Stumpf, Dr. Paola Palma Sisto, Dr. Brandon Nathan, Dr. Catherine Bendel, Dr. Avraham Ishay, Dr. Bina C. Shah, Dr. Akshay B. Jain, and Dr. Andrew G. Gianoukakis.
Financial Support: This work was supported in part by National Institutes of Health Grant R37DK15070 and the Seymour J. Abrams and the Rabbi Morris Esformes funds for thyroid research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- RTH-β
- resistance to thyroid hormone-β
- SEM
- standard error of the mean
- TFT
- thyroid function test
- TH
- thyroid hormone
- THRB
- TH receptor-β
- TSH
- thyroid-stimulating hormone
- ULN
- upper limit of normal
- WT
- wild-type.
References
- 1.Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, O’Heir CE, Mitchell ML, Hermos RJ, Waisbren SE, Faix JD, Klein RZ. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341(8):549–555. [DOI] [PubMed] [Google Scholar]
- 2.Pop VJ, Kuijpens JL, van Baar AL, Verkerk G, van Son MM, de Vijlder JJ, Vulsma T, Wiersinga WM, Drexhage HA, Vader HL. Low maternal free thyroxine concentrations during early pregnancy are associated with impaired psychomotor development in infancy. Clin Endocrinol (Oxf). 1999;50(2):149–155. [DOI] [PubMed] [Google Scholar]
- 3.Maraka S, Ospina NM, O’Keeffe DT, Espinosa De Ycaza AE, Gionfriddo MR, Erwin PJ, Coddington CC III, Stan MN, Murad MH, Montori VM. Subclinical hypothyroidism in pregnancy: a systematic review and meta-analysis. Thyroid. 2016;26(4):580–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phoojaroenchanachai M, Sriussadaporn S, Peerapatdit T, Vannasaeng S, Nitiyanant W, Boonnamsiri V, Vichayanrat A. Effect of maternal hyperthyroidism during late pregnancy on the risk of neonatal low birth weight. Clin Endocrinol (Oxf). 2001;54(3):365–370. [DOI] [PubMed] [Google Scholar]
- 5.Dumitrescu AM, Refetoff S. Impaired sensitivity to thyroid hormone: defects of transport, metabolism and action. In: De Groot LJ, Beck-Peccoz P, Chrousos G, Dungan K, Grossman A, Hershman JM, Koch C, Korbonits M, McLachlan R, New M, Purnell J, Rebar R, Singer F, Vinik A, eds. Endotext. South Dartmouth, MA: MDText.com, Inc; 2000. [PubMed] [Google Scholar]
- 6.Anselmo J, Cao D, Karrison T, Weiss RE, Refetoff S. Fetal loss associated with excess thyroid hormone exposure. JAMA. 2004;292(6):691–695. [DOI] [PubMed] [Google Scholar]
- 7.Weiss RE, Dumitrescu A, Refetoff S. Approach to the patient with resistance to thyroid hormone and pregnancy. J Clin Endocrinol Metab. 2010;95(7):3094–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jonas C, Daumerie C. Conservative management of pregnancy in patients with resistance to thyroid hormone associated with Hashimoto’s thyroiditis. Thyroid. 2014;24(11):1656–1661. [DOI] [PubMed] [Google Scholar]
- 9.Mikolajczyk RT, Zhang J, Betran AP, Souza JP, Mori R, Gülmezoglu AM, Merialdi M. A global reference for fetal-weight and birthweight percentiles. Lancet. 2011;377(9780):1855–1861. [DOI] [PubMed] [Google Scholar]
- 10.Alonso M, Goodwin C, Liao X, Page D, Refetoff S, Weiss RE. Effects of maternal levels of thyroid hormone (TH) on the hypothalamus-pituitary-thyroid set point: studies in TH receptor beta knockout mice. Endocrinology. 2007;148(11):5305–5312. [DOI] [PubMed] [Google Scholar]
- 11.Furlanetto TW, Kopp P, Peccin S, Gu WX, Jameson JL. A novel mutation (M310L) in the thyroid hormone receptor beta causing resistance to thyroid hormone in a Brazilian kindred and a neonate. Mol Genet Metab. 2000;71(3):520–526. [DOI] [PubMed] [Google Scholar]
- 12.Anselmo J, Kay T, Dennis K, Szmulewitz R, Refetoff S, Weiss RE. Resistance to thyroid hormone does not abrogate the transient thyrotoxicosis associated with gestation: report of a case. J Clin Endocrinol Metab. 2001;86(9):4273–4275. [DOI] [PubMed] [Google Scholar]
- 13.Blair JC, Mohan U, Larcher VF, Rajanayagam O, Burrin JM, Perry LA, Grossman AB, Chatterjee VK, Savage MO. Neonatal thyrotoxicosis and maternal infertility in thyroid hormone resistance due to a mutation in the TRbeta gene (M313T). Clin Endocrinol (Oxf). 2002;57(3):405–409. [DOI] [PubMed] [Google Scholar]
- 14.Asteria C, Rajanayagam O, Collingwood TN, Persani L, Romoli R, Mannavola D, Zamperini P, Buzi F, Ciralli F, Chatterjee VK, Beck-Peccoz P. Prenatal diagnosis of thyroid hormone resistance. J Clin Endocrinol Metab. 1999;84(2):405–410. [DOI] [PubMed] [Google Scholar]
- 15.Boix E, Picó A, Zapico M, López A, Mauri M. Outcome of pregnancy in a hypothyroid woman with resistance to thyroid hormone treated with triiodothyronine. J Endocrinol Invest. 2007;30(3):253–255. [DOI] [PubMed] [Google Scholar]
- 16.Sarkissian G, Dace A, Mesmacque A, Bony-Trifunovic H, Malezet-Desmoulins C, Torresani J, Margotat A. A novel resistance to thyroid hormone associated with a new mutation (T329N) in the thyroid hormone receptor beta gene. Thyroid. 1999;9(2):165–171. [DOI] [PubMed] [Google Scholar]
- 17.Ross DS, Burch HB, Cooper DS, Greenlee MC, Laurberg P, Maia AL, Rivkees SA, Samuels M, Sosa JA, Stan MN, Walter MA. 2016 American Thyroid Association guidelines for diagnosis and management of hyperthyroidism and other causes of thyrotoxicosis. Thyroid. 2016;26(10):1343–1421. [DOI] [PubMed] [Google Scholar]
- 18.Lee RH, Spencer CA, Mestman JH, Miller EA, Petrovic I, Braverman LE, Goodwin M. Free T4 immunoassays are flawed during pregnancy. Am J Obstet Gynecol. 2009;200(3):260e1–260e6. [DOI] [PubMed] [Google Scholar]
- 19.Bliddal S, Feldt-Rasmussen U, Boas M, Faber J, Juul A, Larsen T, Precht DH. Gestational age-specific reference ranges from different laboratories misclassify pregnant women’s thyroid status: comparison of two longitudinal prospective cohort studies. Eur J Endocrinol. 2013;170(2):329–339. [DOI] [PubMed] [Google Scholar]
- 20.Dhingra S, Owen PJ, Lazarus JH, Amin P. Resistance to thyroid hormone in pregnancy. Obstet Gynecol. 2008;112(2 Pt 2):501–503. [DOI] [PubMed] [Google Scholar]
- 21.Massaad D, Poppe K, Lissens W, Velkeniers B. A case of thyroid hormone resistance: prospective follow-up during pregnancy and obstetric outcome. Eur J Intern Med. 2007;18(3):253–254. [DOI] [PubMed] [Google Scholar]
- 22.Kane S, Fox R, Close C. Thyroid hormone resistance and pregnancy. BJOG. 2003;110(6):633–634. [PubMed] [Google Scholar]
- 23.Paragliola RM, Concolino P, De Rosa A, Mello E, Zuppi C, Pontecorvi A, Capoluongo E, Corsello SM. The first case of association between postpartum thyroiditis and thyroid hormone resistance in an Italian patient showing a novel p.V283A THRB mutation. Thyroid. 2013;23(4):506–510. [DOI] [PubMed] [Google Scholar]
- 24.Srichomkwun P, Anselmo J, Liao XH, Hönes GS, Moeller LC, Alonso-Sampedro M, Weiss RE, Dumitrescu AM, Refetoff S. Fetal exposure to high maternal thyroid hormone (TH) levels causes central resistance to TH in adult humans and mice [published online ahead of print June 6, 2017]. J Clin Endocrinol Metab. doi:10.1210/jc.2017-00019. [DOI] [PMC free article] [PubMed]