Abstract
Context:
Full-term pregnancy is associated with a transient increase and life-time decrease in maternal breast cancer risk. Estrone (E1), estradiol (E2), and estriol (E3) are in high concentration during the third trimester. E1 and E2 metabolism produces carcinogenic intermediaries, and E3 metabolism does not.
Objective:
We tested the hypothesis that higher E3 in pregnancy is protective while higher E1 plus E2 increases risk.
Design:
Prospective case-cohort study (n = 620; 204 cases) nested in a 38-year follow-up of 15,528 pregnant women in the Child Health and Development Studies. We measured E1, E2, and E3 in archived third trimester serum and estimated associations with breast cancer.
Setting:
Northern California Kaiser members receiving obstetric care from 1959 to 1967.
Main Outcome Measure:
Breast cancer diagnosed through 1997.
Results:
Doubling of E1+E2 was associated with greater risk [hazard ratio (HR), 1.7; 95% confidence interval (CI), 1.2 to 2.4]. In contrast, doubling of E3 or the E3/E1+E2 ratio was associated with protection (HR, 0.7; 95% CI, 0.5 to 1.0; HR, 0.6; 95% CI, 0.4 to 0.8, respectively). Associations were stronger for diagnoses within 15 years after delivery compared with 16 to 38 years (Pinteraction = 0.0002) for gravidas >27 years at delivery vs ≤27 (Pinteraction = 0.01) and for primiparas vs multiparas (Pinteraction = 0.02).
Conclusions:
Relatively high third trimester E3 levels might protect parous women from breast cancer and E1 and E2 might enhance the risk. If findings are confirmed, third trimester pregnancy estrogens could help explain how parity affects breast cancer.
In a prospective study of third trimester pregnancy estrogens and breast cancer, we found that high E1 and E2 predicted for an increased risk and high E3 predicted for a decreased risk.
Lemon et al. (1) suggested that estriol (E3) could protect against breast cancer, proposing that the ratio of E3 to estrone (E1) plus estradiol (E2) reflects “the balance between estrogens of widely varying carcinogenicity, growth inducing activity and estrogen impeding activity in the pathogenesis of disease.” Siiteri (2) and Satten et al. (3) suggested a complementary hypothesis that the abundant E3 in the third trimester of pregnancy would help clear the breast and other organs of accumulated carcinogens via increased blood flow. This hypothesis has been supported by experimental work in sheep in which Resnik et al. (4) named E3 the “vascular estrogen,” having demonstrated E3 to be equipotent to E2 for stimulating uterine blood flow. Siiteri (2) proposed that pregnancy E3 might reduce the accumulation of E1 and E2 and their metabolic byproducts now known to be carcinogenic (5), thereby reducing the risk of breast cancer among parous women. To test this hypothesis, we measured E1, E2, and E3 in pregnancy serum collected >30 years earlier in the Child Health and Development Studies (CHDS) cohort and correlated these with the subsequent risk of maternal breast cancer.
Materials and Methods
Study population
Members of the Kaiser Permanente Health Plan, residing in the East Bay area of San Francisco, California and pregnant during the June 1959 to September 1966 period, were eligible for the CHDS (6). The CHDS included 15,528 women with observations on 20,754 pregnancies. The women were interviewed in-person as soon as possible after their first contact with the obstetrics department. Serum samples were collected at each trimester and frozen at −20°C. The subjects voluntarily participated in the CHDS, providing oral informed consent for an in-person interview, collection of blood specimens at several points during pregnancy and early postpartum, and permission for medical record access. The institutional review board of the Public Health Institute approved the present study, and we complied with all federal guidelines governing the use of human participants.
The CHDS are regularly monitored by linkage to (1) the California Department of Motor Vehicles, for a history of location and timing of residence to identify the population at risk of morbidity and mortality (7–9); (2) the California Department of Vital Statistics to identify deaths and cause; and (3) the California Cancer Registry to identify cancer diagnoses (10–14). All members of the CHDS families and all names ever used by each family member are regularly matched to these sources. The accumulation of a name and address history protects against false matches and failing to identify true matches. Surveillance efforts routinely identify one or more members of >80% of CHDS families. This information is used to calculate the person-years of exposure for the outcome of interest and to construct a censoring variable for Cox proportional hazards models. Record abstraction for cancer diagnoses to the California Cancer Registry is based primarily on pathology reports, and case identification is considered to be >99% complete after a 2-year lag (15). Breast cancer cases are censored at the year of diagnosis. Noncases are censored at the year of last contact or the year of death. More than 70% of CHDS cohort members have >15 years of follow-up and 63% have >30 years of follow-up data available.
Study design
The present study was a case-cohort design (16). In the case-cohort design, cases are enumerated for the entire eligible cohort, and accumulated person-years are estimated from a subcohort. Subcohort serum assay results can be used in multiple studies for different outcomes, saving costs and serum by reusing assay data from the subcohort (17). The subcohort for the present study was an 8% random sample of women who had a completed interview and had a singleton pregnancy with a gestation of ≥28 weeks (73% of study pregnancies), with no history of breast cancer at pregnancy (99%). For women with more than one pregnancy in the cohort, we included the serum samples and data only from their first CHDS pregnancy. From this random sample, we selected women with available data from a standardized placental examination (60%), prenatal measures of weight and blood pressure in the second and third trimesters (80%), and an available serum sample (95%), resulting in a subcohort of 432 women, including 16 breast cancer cases (details shown in Supplemental Fig. 1 (1.8MB, tif) ). As of 1997, an additional 188 cases of breast cancer had met the same criteria. The availability of maternal serum, placental morphology, and prenatal measures was not related to breast cancer incidence (10) (Supplemental Fig. 1 (1.8MB, tif) ), and these criteria were chosen to facilitate future research on the contribution of estrogens to our previously reported findings on the relation of placental and prenatal variables to the development of maternal breast cancer (10). The minimum hazard ratio (HR) detectable with 80% power was 1.46 for the comparison of the upper vs three lower quartiles grouped together (18).
Measurement of estrogens and gestational age at blood sampling
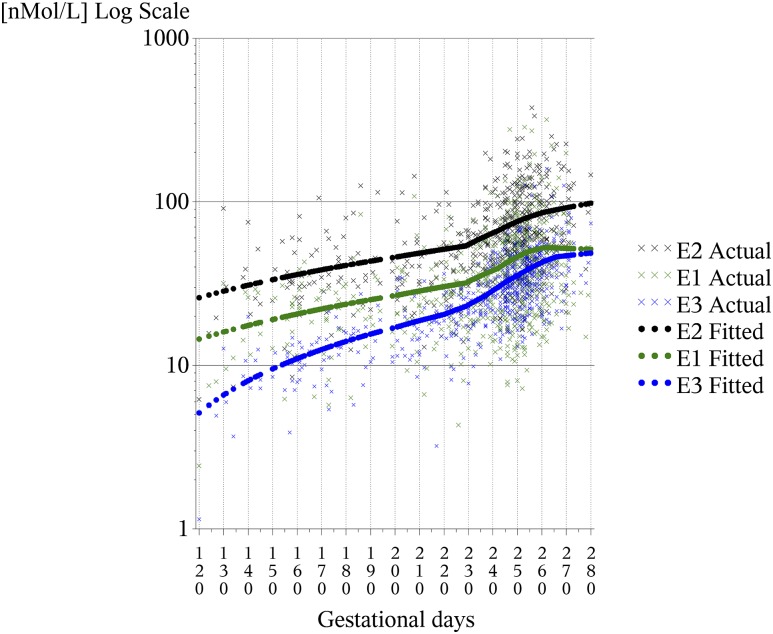
The gravida’s date of last menstrual period (LMP) was obtained from her interview conducted just before the first prenatal visit and was used to calculate the gestational age at blood sampling. Women with missing LMP dates (n = 199) were excluded (Supplemental Fig. 1 (1.8MB, tif) ). Where available, we used maternal blood samples collected after 28 weeks of gestation. Samples collected after 28 weeks of gestation were available for 90% of the subcohort and 85% of the breast cancer cases. In addition, the estrogen levels were further adjusted to compensate for the remaining variation in gestational day of blood sampling by regressing the estrogen levels on the day of blood sampling using locally weighted scatterplot smoothing regression (LOESS) (19). The predicted estrogen levels were displayed as a function of gestational day and plotted against observed values (Fig. 1). The fitted curves estimated by LOESS were similar to those reported previously for the correlation of gestation with estrogen hormone levels (20, 21), lending confidence to the validity of the curve-fitting procedure. The differences between the predicted and observed values (residuals) represent a ranking for each subject as greater than or less than the predicted value for each estrogen. To adjust individual estrogen levels to the same gestational day (day 247 of gestation, the median day of gestation for blood samples in the present study), a constant (the LOESS-fitted estrogen level observed for day 247) was added to the residual for each subject. This adjustment provided predicted estrogen levels for day 247 for each woman, thus adjusting for differences in estrogen levels attributable to variations in the timing of the blood samples. We conducted sensitivity analyses to examine the effect of excluding subjects with blood samples taken before 28 weeks of gestation and found no differences in the associations.
Figure 1.
E1, E2, and E3 stratified by day of gestation. “X” represents measured levels. Solid circles represent fitted values from LOESS regression.
One of us (B.R.H.) was an expert in steroid hormone measurement (22). He optimized all assays, including the radioimmunoassay (RIA) used for E2 by evaluating the method we used against the reference standard consisting of solvent extraction, followed by chromatography (23). Hopper based the evaluation on duplicate assays of native samples. He determined that the chromatography step was unnecessary for the measurement of E2 but was essential for the measurement of E1. Eliminating chromatography for E3, which at the time was a practical necessity owing to limited availability of tracer, yielded consistently higher values but preserved the ranking of the samples. Details of the assay methods for the estrogens reported follow.
E2 was measured by RIA after serum samples were extracted with seven volumes of hexane/ethyl acetate (3:1 ratio), which was evaporated and then reconstituted in buffer. The E2 antibody was a very specific antibody generated against estradiol-6-carboxymethyl:bovine serum albumin. No differences were found between the same samples measured after extraction only or measured after extraction and celite column chromatography. The general procedures for RIA have been described previously (22). E1 was measured by RIA as described by Anderson et al. (22), except for the following modifications. Serum samples were extracted with seven volumes of hexane/ethyl acetate (3:1), which was then evaporated and subjected to celite column chromatography. The celite column used a stationary phase of 60% propylene glycol and 40% ethylene glycol. The stationary phase was 60% (in milliliters) of the weight of celite. Samples were added to the column in 1 mL of isooctane; 5 mL of isooctane was run through the column and discarded. E1 elutes in 3.5 mL of 15% ethyl acetate in isooctane. The E1 assay used an antibody generated against estrone-3-hemisuccinate:bovine serum albumin. E3 was assayed using kits obtained from Diagnostic Systems Laboratories (Webster, TX), which uses a two-site immunoradiometric assay principle. For E3, the assay results from 19 samples using the kits were compared with those obtained after celite chromatography. The results measured directly were 16% greater than those obtained using chromatography but were highly reproducible (coefficient of variation, 8.6%). Assays using Diagnostic Systems Laboratories kits gave values in the same range as other reported E3 values (24). In addition to the standard quality control samples run by the laboratory, quality control samples derived from a pool of CHDS samples stored under conditions identical to the samples from cases and controls, blinded to the laboratory, were inserted at random among the samples to be assayed (5% of samples assayed). The coefficients of variation were 5.3% for E1, 8.5% for E2, and 4.7% for E3.
Statistical analysis
The data were analyzed using the Cox proportional hazard model in the context of a case-cohort design (25, 26). Calculations were performed using methods developed by Ichikawa and Barlow (27) with slight modifications. Tests of time dependence were based on robust variance estimators and were performed using methods implemented by Langholz and Jiao (16) in Statistical Analysis Software (SAS; SAS Institute, Cary, NC). To facilitate comparison of hormone associations and to minimize the effect of outliers on the regressions, log2-transformed estrogen concentrations were used in the models. The HRs estimated for a 1-unit change in log2(estrogen) represent the HR for a twofold increase in estrogen. Because E2 and E1 are dynamically interconverted (28), these two estrogens were summed after confirming that the direction of their association with breast cancer was consistent. We also report the results for the ratio of E3 to E1+E2 [E3/(E1+E2); E3 ratio]. This ratio was calculated using the original values of E1, E2, and E3 and then log2-transformed for analysis in the Cox proportional hazard models. We examined associations for estrogens categorized in quartiles to reduce the influence of outliers and found that the trends over the quartiles were consistent with the findings from the models in which log2(estrogen) values were entered as continuous variables.
Because pregnancy has been associated with a short-term increase in breast cancer (29), we investigated whether the interval to diagnosis modified the associations. We defined two periods, a priori, based on data from a previous study (30): diagnosis within 15 years of delivery vs diagnosis >15 years after delivery. We tested whether the main effects in our model were consistent for these two periods by adding a time-dependent interaction term to our models using a SAS macro with assistance from Langholz and Jiao (16).
We chose gravida age at delivery (age, ≤27 vs >27 years, corresponding to the median age at delivery) and parity (primipara vs multipara) as the primary covariates of interest, because both age at delivery and parity at delivery are considered likely to modify the effect of pregnancy on breast cancer development (31, 32). We tested for interactions between the estrogen variables and each of these covariates by entering a multiplicative term (e.g., one term for estrogen multiplied by parity at delivery and one term for estrogen multiplied by age at delivery). Interactions were considered statistically significant at Wald P < 0.05. We also tested a three-way interaction between estrogen, parity, and age at delivery; however, the differences were not statistically significant. Maternal height, weight, and body mass index (continuous), race/ethnicity (African American, Hispanic, Asian, other vs non-Hispanic white as reference) and alcohol (drinks of wine, beer, and liquor weekly) were investigated as covariates because these are known risk factors for breast cancer (32). Because none of these variables affected the regression coefficients by ≥10%, they were not included in the models.
Results
The baseline characteristics of the study sample are presented in Table 1. Cases were born earlier, were older at delivery, were more likely to be multiparous, and were taller. The year of blood sampling did not differ for cases and the subcohort. The median age at diagnosis for the cases was 54 years (interquartile range, 13). The median interval to diagnosis was 25 years (interquartile range, 12).
Table 1.
Study Population Characteristics
| Characteristic | Subcohort (n = 432) | Cases (n = 204) |
|---|---|---|
| Year of mother's birth | 1936 (9) | 1931 (11) |
| Age at first full-term pregnancy, y | 22 (5) | 24 (8) |
| Age at delivery, y | 27 (8) | 31 (11) |
| Year of blood sampling | 1962 (2) | 1962 (2) |
| Gestational day of blood sampling | 248 (23) | 246 (31) |
| Weight at first prenatal visit, kga | 58.5 (12.6) | 60.3 (12.6) |
| Height, ma | 1.63 (0.10) | 1.65 (0.08) |
| BMI at first prenatal visit, kg/m2a | 22.1 (4.3) | 22.6 (4.2) |
| Alcohol (sum of wine, beer, liquor), drinks/wka | 0.2 (0.9) | 0.2 (1.7) |
| Parity at blood sampling, % | ||
| 0 | 36 | 27 |
| 1 | 28 | 26 |
| 2 | 17 | 23 |
| ≥3 | 19 | 25 |
| Race, % | ||
| non-Hispanic white | 69 | 69 |
| African American | 20 | 19 |
| Hispanic | 2 | 1 |
| Asian | 6 | 7 |
| Mixed | 3 | 3 |
Data presented as median (interquartile range) or percentage.
Abbreviation: BMI, body mass index.
Sample sizes were smaller owing to missing data: subcohort: alcohol (n = 339), BMI (n = 425), height (n = 427), and weight (n = 425); cases: alcohol (n = 155), BMI (n = 200); height (n = 201); and weight (n = 201).
Figure 1 shows the distribution of serum estrogens by gestational day of blood sampling for the present study. The median day of blood sampling was 247 days, or 35 weeks of gestation. The serum levels of estrogens adjusted to day 247 of gestation are summarized in Table 2. In general, the values are similar to those previously reported for this time of gestation (21, 24).
Table 2.
Maternal Pregnancy Serum Estrogens Adjusted to Week 35 of Gestation (n = 620)
| Estrogen | Percentile | ||||
|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | |
| E3, nmol/L | 15 | 25 | 31 | 38 | 54 |
| E2, nmol/L | 22 | 44 | 59 | 85 | 148 |
| E1, nmol/L | 9 | 24 | 36 | 51 | 90 |
| E1 + E2, nmol/L | 41 | 72 | 99 | 133 | 243 |
| E3/(E1 + E2) | 0.15 | 0.24 | 0.31 | 0.40 | 0.64 |
Serum estrogens adjusted to median day of gestation for blood sampling in our study (247 days or week 35) using LOESS regression, as described in the Materials and Methods section; Fig. 1 shows the distribution by gestational day of blood sampling.
The results of the models that included estrogens, gravida age at delivery, parity, and gravida age at first full-term pregnancy stratified by the interval to diagnosis after delivery are listed in Table 3. During the entire follow-up period of 38 years, E1+E2 was associated with an increased risk of breast cancer [HR, 1.4; 95% confidence interval (CI), 1.1 to 1.8; P = 0.02 in a model that did not include E3; and HR, 1.7; 95% CI, 1.2 to 2.4; P = 0.005 in a model that did include E3]. In contrast, for the entire follow-up period of 38 years, E3 was marginally associated with a decreased risk of breast cancer (HR, 0.7; 95% CI, 0.5 to 1.0; P = 0.05) but only when E1+E2 was also included in the same model. The positive correlations among estrogens (r = 0.53 for E3 with E2 and r = 0.33 for E3 with E1) likely explain why the estimated associations for each estrogen term were stronger in the models that included both estrogen terms. The association of E1+E2 with increased risk confounds the association of E3 with decreased risk and vice versa.
Table 3.
Associations of Third Trimester Pregnancy Estrogens With Maternal Breast Cancer Stratified by Interval to Diagnosis After Delivery
| Model Specification | Interval to Diagnosis After Delivery |
P Value for Time Dependencea | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2–15 y (40 cases) |
16–38 y (164 cases) |
2–38 y (204 cases) |
||||||||
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | ||
| One estrogen term | ||||||||||
| Model A: log2(E1+E2) only | 1.4 | 0.6–2.8 | 0.43 | 1.4 | 1.1–1.8 | 0.01b | 1.4 | 1.1–1.8 | 0.02b | 0.0002b |
| Model B: log2(E3) only | 0.5 | 0.3–0.8 | 0.008b | 1.1 | 0.9–1.5 | 0.37 | 1.0 | 0.7–1.3 | 0.90 | 0.0002b |
| Two estrogen terms | ||||||||||
| Model C: mutually adjusted | 0.0002b | |||||||||
| log2(E1+E2) | 2.5 | 1.0–6.3 | 0.06 | 1.5 | 1.1–2.2 | 0.02b | 1.7 | 1.2–2.4 | 0.005b | |
| log2(E3) | 0.3 | 0.2–0.6 | 0.0002b | 0.8 | 0.6–1.3 | 0.41 | 0.7 | 0.5–1.0 | 0.05 | |
| Ratio of E3 to E1+E2 | ||||||||||
| Model D: log2[E3/(E1+E2)] only | 0.3 | 0.1–0.5 | 0.0002b | 0.7 | 0.5–1.0 | 0.07 | 0.6 | 0.4–0.8 | 0.003b | 0.0002b |
HRs for estrogen variables given for one-unit change in log2(estrogen variable), corresponding to the estimated HR for a twofold increase in the estrogen variables, a range represented in the study sample (Table 2); all models include the following covariates: gravida age at delivery, parity, and gravida age at first full-term pregnancy.
Refers to significance of difference in model fit for breast cancer diagnosed 2 to 15 years after delivery vs model fit for breast cancer diagnosed 16 to 38 years after delivery.
Statistically significant association (P < 0.05).
All estrogen associations, regardless of model specification, were significantly stronger within the first 15 years of follow-up (Ptimedependence = 0.0002; Table 3). In models in which E1+E2 and E3 were simultaneously included, the E1+E2 association was stronger for the first 15 years of follow-up (HR, 2.5; 95% CI, 1.0 to 6.3; P = 0.06) than for years 16 to 38 (HR, 1.5; 95% CI, 1.1 to 2.2; P = 0.02). The protective association for E3 was also stronger for the first 15 years of follow-up (HR, 0.3; 95% CI, 0.2 to 0.6; P = 0.0002) than for years 16 to 38 (HR, 0.8; 95% CI, 0.6 to 1.3; P = 0.41) in models in which E3 and E1+E2 were included. As noted, for the entire follow-up period, the strength of these associations in each period was generally greater in the models that included both the E1+E2 and the E3 terms compared with models in which only one estrogen term was included.
The results for the models in which the estrogens were represented as an E3 ratio are also presented in Table 3. In all follow-up periods, a greater proportion of E3 to E1+E2 was associated with a lower risk of breast cancer. However, this protective association was significantly stronger within the first 15 years of follow-up (HR, 0.3; 95% CI, 0.1 to 0.5; P = 0.0002) compared with years 16 to 38 of follow-up (HR, 0.7; 95% CI, 0.5 to 1.0; P = 0.07). This difference stratified by follow-up period was statistically significant (Ptimedependence = 0.0002). Still, during the entire 38-year follow-up period, the proportion of E3 to E1+E2 remained significantly associated with a decreased risk of breast cancer (HR, 0.6; 95% CI, 0.4 to 0.8; P = 0.003).
The results for the E3 ratio in models testing for interaction by age at delivery and parity for the entire follow-up period are presented in Table 4. The risk reduction associated with the E3 ratio was the strongest and most statistically significant for primiparas aged >27 years (HR, 0.3; 95% CI, 0.1 to 0.5; P = 0.0001) and was also observed in multiparas aged >27 years (HR, 0.6; 95% CI, 0.4 to 1.0; P = 0.03). The E3 ratio was not related to risk in gravidas aged ≤27, age regardless of parity. The interactions of parity with the E3 ratio and gravida age with the E3 ratio were statistically significant (P = 0.02 and P = 0.01, respectively).
Table 4.
Association of E3 Ratio in Third Trimester Pregnancy Serum With Maternal Breast Cancer Stratified by Gravida Age and Parity
| Gravida Age at Delivery, y | Primipara |
Multipara |
P Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | Cases, n | HR | 95% CI | P Value | Cases, n | ||
| ≤27 | 0.7 | 0.3–1.5 | 0.39 | 33 | 1.7 | 0.8–3.3 | 0.15 | 33 | |
| >27 | 0.3 | 0.1–0.5 | 0.0001a | 22 | 0.6 | 0.4–1.0b | 0.03 | 116 | |
| Interaction with age | 0.01a | ||||||||
| Interaction with parity | 0.02a | ||||||||
HRs given for one-unit change in log2[E3/(E1+E2)], corresponding to estimated effect for twofold increase in E3 ratio, a range represented in the study sample (Table 2); HRs were estimated by a single model that included log2[E3/(E1+E2)], gravida age at delivery dichotomized at the median (27 years), parity dichotomized as primipara vs multipara, gravida age at first full-term pregnancy, and product terms of log2[E3/(E1+E2)] with dichotomized gravida age at delivery and with dichotomized parity.
The three-way interaction of log2[E3/(E1+E2)] × gravida age at delivery × parity was not statistically significant (P = 0.90) in a model also containing two-way interactions of parity × gravida age at delivery, log2[E3/(E1+E2)] × parity, and log2[E3/(E1+E2)] × gravida age at delivery.
Statistically significant association (P < 0.05).
Upper bound for 95% CI was 0.96, which was rounded to 1.0.
Discussion
Previous studies of estrogens in pregnancy and subsequent breast cancer
Three previous studies reported on the relationship between naturally occurring pregnancy estrogens and subsequent maternal breast cancer (33–35). The report by Peck et al. (33), which was also based on the CHDS cohort, did not adjust the E3 associations for E1 and E2 and, thus, cannot be directly compared with our results. Peck et al. (33) found no association for third trimester E2 or E3 alone and a marginally significant positive association for E1 in models that were not simultaneously adjusted for all three estrogens. We also find no E3 association with breast cancer when E1+E2 were not in the model. This likely resulted from the positive correlations among these estrogens, which masked the protective effects of E3 on breast cancer compared with the enhanced risk associated with E1 and E2. The other two reports were on first trimester E1 and E2 in relation to breast cancer in the large Finnish registry study (34, 35). That study did not measure E3 in their first trimester samples, when levels would have been low. Thus, the findings from the Finnish study also could not be directly compared with our findings. However, the study of the Finnish pregnancy cohort is large and provides convincing evidence that first trimester E1 and E2 levels are linked to breast cancer and that the associations depend on estrogen and progesterone receptor status and age at diagnosis (35). The Finnish study had several features that differ from the present study, in addition to the timing of blood sampling. The median age at diagnosis was younger in the Finnish study (40 years vs 54 years), the follow-up period was shorter (19 years vs 38 years), and the study investigated only primiparous pregnancies that resulted in a live birth vs all gestations of ≥28 weeks in the present study. In addition, the Finnish study followed up pregnancies that occurred after 1983 compared with the present study in which the pregnancies were from 1959 to 1967. Their cohort had an older median age at observed pregnancy (30 years vs 27 years) and older age at first full-term pregnancy (30 years vs 22 years). Finally, the Finnish cohort was large enough to report on risk factors for rare breast cancers that occur before age 40 years and to study the heterogeneity of risk according to estrogen and progesterone receptor tumor status. Because of these differences in study design and the characteristics of the two cohorts, the present study can only be considered complementary to the Finnish cohort reports, rather than comparable. Taken together, however, the results from the Finnish study and the present study strongly support the hypothesis that pregnancy estrogen levels are linked to breast cancer risk. Our finding that E1 and E2 in pregnancy are linked to an increased risk of maternal breast cancer also aligns with the known increase in maternal breast cancer associated with pregnancy exposure to the synthetic estrogen, diethylstilbestrol (36) and a recent preclinical study in rats that found exposure to excess estradiol in pregnancy increased the risk of mammary cancer and altered the protective genomic changes associated with parity (37).
Significance of primiparous pregnancy and older age at pregnancy
Our finding that the E3 ratio was more strongly associated with breast cancer in primiparous pregnancies is consistent with a heightened vulnerability of the breast in primiparas, a hypothesis supported by numerous previous human pathology and animal studies (31, 38). Similarly, our finding that the E3 ratio had stronger associations with breast cancer in older gravidas (age >27 years) for both primiparas and multiparas is consistent with previous epidemiological studies that found a greater risk associated with older age at first pregnancy (39) and also older age at the second and possibly third pregnancy (40). The mechanism for these variations in breast cancer risk by age at pregnancy and parity remain poorly understood (38, 41).
Study limitations
Our study has provided unique evidence about the relation of estrogens in the third trimester of pregnancy to subsequent breast cancer but did not address estrogen exposure at other times. We did not have complete data on tumor markers and, thus, could not specify whether the estrogen associations vary by tumor subtype. We lacked data on any intervening risk factors between pregnancy and breast cancer development and could not address mediation such as subsequent parity or changes in maternal risk factors such as postpregnancy weight gain. However, even if third trimester estrogen levels are linked to breast cancer via postnatal fecundity or weight gain, this evidence would not rule out a mechanistic role for previous exposure to pregnancy estrogens. We also lacked information on family history of breast cancer. We did not have measured estrogen levels from the first or second trimester. However, the strong correlations reported by Schock et al. (42) for second and third trimester E1 and E2 in the Finnish Maternity Cohort suggest that hormone rankings according to third trimester assays are likely to be in good agreement with rankings using second trimester assays. However, Schock et al. (42) also reported that the correlations of third trimester E1 and E2 with first trimester E1 and E2 were lower than for consecutive trimesters, leaving open the possibility that assays of first trimester samples for E1 and E2 would add additional information. The report of first trimester associations for E1 and E2 with breast cancer in the Finnish registry (35) supports this conjecture. Because E3 increases dramatically in the third trimester, we would not expect E3 measurement in the first or second trimester to add considerably more information.
The sample sizes in the subgroups defined by gravida age and parity or by follow-up period were relatively small and resulted in broad CIs for some associations observed. This limited certainty regarding the magnitude of the associations we observed even when the P values were statistically significant.
The coefficients of variation for estrogen assays in the present study were not large. However, we could not rule out the possibility that the true underlying associations between estrogens and maternal breast cancer are actually greater than those observed in our study. The observed associations are likely to be lower than the true underlying associations in the setting in which hormone assays are imprecise (43). Misclassification of pregnancy estrogen levels could also occur if one measurement does not fully characterize the actual or relevant exposure. Limited information is available on this issue. Schock et al. (42) reported a correlation of E1 and E2 across gestation, showing that the correlations were stronger during consecutive trimesters, not surprising given the known increases in placental function across gestation. However, measurement of third trimester estrogens might not correctly capture the full effect of pregnancy estrogens on breast cancer.
Systematic differences in gestational age at blood sampling for the cases compared with the noncases could have contributed to the estrogen associations we observed with breast cancer. This was unlikely, however, because gestational age at blood sampling was similar for the cases and noncases. Moreover, a direct association was found with E1+E2 but an inverse association with E3. These contrasting findings are unlikely to be explained by a systematic difference in the timing of blood sampling for the cases compared with the noncases because all these estrogens increase during gestation.
Cohort enrollment predated the use of ultrasonography to estimate gestational age. Because the LMP date was used to adjust the estrogen levels for gestational age, it is possible that unreliable reporting of the LMP date could have resulted in misclassification of the gestational day. This could have been a source of bias primarily if the misclassification differed for the cases and noncases. Verburg et al. (44) reported that the LMP date classified gestations in the normal range 87% of the time compared with 92% for ultrasound assessment at 10 to 12 weeks of gestation, after excluding women with a missing LMP date, women who had used oral contraceptives, and women with irregular cycles. Their findings, which were based on a large cohort enrolled from 2002 to 2006, suggest that some error in estimation of gestational age is likely whether dating is by LMP or ultrasound assessment. Just as in the study by Verburg et al. (44), we excluded women with a missing LMP date, but we did not exclude women with irregular cycles (13% among noncases and 8% among cases) or women using oral contraceptives within 3 months of conception (n = 5 noncases and n = 3 cases). The use of oral contraceptives was rare in our population (13 women overall), because enrollment predated the wide availability and use of lower dose oral contraceptives. Although we could not exclude the possibility that misclassification of gestational age affected our findings, we have no evidence that misclassification of gestational age was systematically different for the cases vs the controls.
Our samples were assayed in 2000 and had been stored since 1959 to 1967. Although we could not rule out storage artifacts, we would expect the same artifact to occur in both case and subcohort serum assays. The year of blood sampling was nearly identical in the subcohort and cases, making it unlikely that storage could explain the estrogen associations observed with breast cancer. The levels of estrogens and patterns by day of gestation that we observed in the present study are consistent with previous reports (45). In addition, the correlation of E2 with E3 in our study was r = 0.53, comparing well with the correlation of r = 0.51 reported previously for normal pregnancies between 20 and 40 weeks of gestation (45). These comparisons lend confidence to the quality of the assays in the present study.
Holl et al. (46) examined the stability of estradiol over 25 to 34 years for first trimester samples from primiparas that had been stored at −25°C (compared with our third trimester samples stored at −20°C for 33 to 41 years for primiparas and multiparas). The data in their Figure 1 show an inconsistent and also modest difference in estradiol assay results conducted every other year for 22 years (46). The Spearman partial correlation between storage time and E2 (adjusted for maternal age, gestational day, and bench lag time) was modest (rs = 0.23). Thus, some limited data support the stability of estradiol in first trimester pregnancy samples over time. To the best of our knowledge, no similar data are available for E1, E2, or E3 from third trimester samples.
We identified breast cancer cases by linkage to the California Cancer Registry and vital status files. It is possible that we missed some cases of breast cancer. However, we had no reason to expect that incomplete surveillance would correlate with pregnancy estrogens, which would be required to bias estimates of associations. However, pregnancy estrogens might be related to other factors that correlate with incomplete surveillance and could have resulted in a selection bias.
E3 hypothesis
Differentiation of the maternal breast is completed in the third trimester of pregnancy (47) at the same time that E3 levels in the maternal compartment are increasing (48). Pregnancy protection from breast cancer requires a pregnancy duration of ≥7 months (49), suggesting an important role for third trimester events. Our results suggest that E3 might play a role in this protection.
Siiteri (2) proposed that the decreased risk of breast cancer after pregnancy could be explained by removal of tumor promoting chemicals accumulated in the breasts by the greatly increased E3 stimulated blood flow. These substances include carcinogenic metabolites of E1 formed from circulating androstenedione secreted by both the adrenal glands and the ovaries (50, 51) and potential exogenous carcinogens (11, 52). Residual vascular changes after pregnancy have been described, and these decline over time (53). This observation is consistent with greater E3 protection for cancers that occur within 15 years of pregnancy that we observed in the present study. Moreover, the pattern of cardiovascular adaptation to pregnancy, characterized by greater cardiac output, higher heart rate, and decreased systemic vascular resistance peaks in the third trimester of pregnancy, and this pattern closely matches the surge of E3 in the third trimester (54). In the CHDS, we have observed that the mean arterial blood pressure, a measure of tissue perfusion, increases rapidly during the third trimester and correlates with serum E3 levels (55). Studies of estrogen levels in breast nipple aspirates have shown that the levels of both E1 and E2 in fluids from parous women are only about one half of those found in fluids from nonparous women (56). The added protection against breast cancer afforded to pregnant women who develop higher blood pressure in pregnancy (10, 14) and preeclampsia (57) is consistent with the concept of increased perfusion of breast tissue and removal of tumor causing agents. The same cleansing mechanism might also explain the protective effect of pregnancy against the development of cancer in other organs, including the endometrium (58) and ovaries (59). The apparent protective effect of hormonal treatment, including E3, against breast tumor formation in the Huggins rat model (60) also could be explained by increased blood flow and reduced tissue levels of the administered carcinogen relative to controls. We could not rule out other mechanisms reviewed previously (38), including the potential role of cell surface G protein estrogen receptor (GPER or GPR 30), in which E3 has been reported to act as an antagonist in estrogen receptor-negative breast cancer cells (61).
The estrogen associations in the present study were stronger for breast cancer cases diagnosed within 15 years of pregnancy, for older gravidas, and for primiparas. These findings suggest that E3 might in some way protect more vulnerable gravidas from progression of an existing lesion or initiation of a quickly growing tumor due to pregnancy exposure to E2 or E1 or their carcinogenic metabolites (5). It is intriguing to speculate that the abundant production of E3 in human pregnancy might have evolved as a protective mechanism. This could be an interesting beneficial function of E3, in addition to what is known regarding its effect on pregnancy and fetal well-being.
Conclusions
A reevaluation of the role of E3 in human pregnancy suggests that its importance might have been underestimated for many years. The results from the present study suggest that relatively high late pregnancy E3 might play a role in protecting parous women from breast cancer, even as pregnancy exposure to E1 and E2 enhances that risk.
Acknowledgments
We acknowledge the late Jacob Yerushalmy, founder of the Child Health and Development Studies (CHDS), for creating this research opportunity; the late Barbara J. van den Berg, second CHDS Director, who made this work possible by safeguarding the serum and data archive in collaboration with Roberta Christianson and the late Frank Oechsli; Richard D. Cohen for statistical help with implementation of case-cohort analytics; Robert I. Sholtz for statistical advice and comments on manuscript drafts and data interpretation; Bryan Langholz for providing statistical consultation and a SAS macro for implementation of models and estimation of time dependence; and Eugene Albrecht for comments on earlier drafts. The late B.R.H., University of California, San Diego, advised on hormone assay methods and interpretation and completed hormone assays for our study. The late P.K.S., University of California, San Francisco, is responsible for the estriol hypothesis described in our report.
Financial Support: This research was sponsored by the US Department of the Army (Grant DAMD17-99-1-9358; the US Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick, Maryland 21702-5014 is the awarding and administering acquisition office). The content of our report does not necessarily reflect the position or the policy of the Government, and no official endorsement should inferred; Eunice Kennedy Shriver National Institute of Child Health and Development, National Institutes of Health, Department of Health and Human Services Contract HHSN275201100020C. The collection of cancer incidence data used in our study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute Surveillance, Epidemiology, and End Results Program under Contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, Contract HHSN261201000035C awarded to the University of Southern California, and Contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention National Program of Cancer Registries, under Agreement U58DP003862-01 awarded to the California Department of Public Health. The ideas and opinions expressed herein are those of the authors and endorsement by the State of California, Department of Public Health, National Cancer Institute, and Centers for Disease Control and Prevention or their contractors and subcontractors, or any of the funders of this research is not intended nor should be inferred.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CHDS
- Child Health and Development Studies
- CI
- confidence interval
- E1
- estrone
- E2
- estradiol
- E3
- estriol
- E3 ratio
- ratio of E3 to E1+E2
- HR
- hazard ratio
- LMP
- last menstrual period
- LOESS
- locally weighted scatterplot smoothing regression
- RIA
- radioimmunoassay
- SAS
- Statistical Analysis Software.
References
- 1.Lemon HM, Wotiz HH, Parsons L, Mozden PJ. Reduced estriol excretion in patients with breast cancer prior to endocrine therapy. JAMA. 1966;196(13):1128–1136. [PubMed] [Google Scholar]
- 2.Siiteri PK. The continuing saga of dehydroepiandrosterone (DHEA). J Clin Endocrinol Metab. 2005;90(6):3795–3796. [DOI] [PubMed] [Google Scholar]
- 3.Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A. History of aromatase: saga of an important biological mediator and therapeutic target. Endocr Rev. 2009;30(4):343–375. [DOI] [PubMed] [Google Scholar]
- 4.Resnik R, Killam AP, Battaglia FC, Makowski EL, Meschia G. The stimulation of uterine blood flow by various estrogens. Endocrinology. 1974;94(4):1192–1196. [DOI] [PubMed] [Google Scholar]
- 5.Yager JD. Mechanisms of estrogen carcinogenesis: the role of E2/E1-quinone metabolites suggests new approaches to preventive intervention—a review. Steroids. 2015;99(Pt A):56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Berg BJ. The California Child Health and Development Studies: twenty years of research. World Health Stat Q. 1979;32(4):269–286. [PubMed] [Google Scholar]
- 7.Mongraw-Chaffin ML, Cirillo PM, Cohn BA. Preeclampsia and cardiovascular disease death: prospective evidence from the child health and development studies cohort. Hypertension. 2010;56(1):166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang ET, Cirillo PM, Vittinghoff E, Bibbins-Domingo K, Cohn BA, Cedars MI. Menstrual irregularity and cardiovascular mortality. J Clin Endocrinol Metab. 2011;96(1):E114–E118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cirillo PM, Cohn BA. Pregnancy complications and cardiovascular disease death: 50-year follow-up of the Child Health and Development Studies pregnancy cohort. Circulation. 2015;132(13):1234–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohn BA, Cirillo PM, Christianson RE, van den Berg BJ, Siiteri PK. Placental characteristics and reduced risk of maternal breast cancer. J Natl Cancer Inst. 2001;93(15):1133–1140. [DOI] [PubMed] [Google Scholar]
- 11.Cohn BA, Wolff MS, Cirillo PM, Sholtz RI. DDT and breast cancer in young women: new data on the significance of age at exposure. Environ Health Perspect. 2007;115(10):1406–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whittemore AS, Cirillo PM, Feldman D, Cohn BA. Prostate specific antigen levels in young adulthood predict prostate cancer risk: results from a cohort of black and white Americans. J Urol. 2005;174(3):872–876, discussion 876. [DOI] [PubMed] [Google Scholar]
- 13.Cirillo PM, Wang ET, Cedars MI, Chen LM, Cohn BA. Irregular menses predicts ovarian cancer: prospective evidence from the Child Health and Development Studies. Int J Cancer. 2016;139(5):1009–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cirillo PM, Benz CC, Cohn BA. Comment on: “hypertensive diseases in pregnancy and breast cancer risk.” Br J Cancer. 2016;114(11):e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perkins C, Hoegh H, Wright WE, Young JL. Cancer Incidence and Mortality in California, 1988–1990. Sacramento: Cancer Surveillance Section, Department of Health Services; 1993. [Google Scholar]
- 16.Langholz B, Jiao J. Computational methods for case-cohort studies. Comput Stat Data Anal. 2007;51:3737–3748. [Google Scholar]
- 17.Wacholder S. Practical considerations in choosing between the case-cohort and nested case-control designs. Epidemiology. 1991;2(2):155–158. [DOI] [PubMed] [Google Scholar]
- 18.Hu W, Cai J, Zeng D. Sample size/power calculation for stratified case-cohort design. Stat Med. 2014;33(23):3973–3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–836. [Google Scholar]
- 20.O’Leary P, Boyne P, Flett P, Beilby J, James I. Longitudinal assessment of changes in reproductive hormones during normal pregnancy. Clin Chem. 1991;37(5):667–672. [PubMed] [Google Scholar]
- 21.Yen SSC. Endocrine-metabolic adaptations in pregnancy In: Yen SSC, Jaffe RB, eds. Reproductive Endocrinology. 3rd ed. Philadelphia: WB Saunders; 1991:936–981. [Google Scholar]
- 22.Anderson DC, Hopper BR, Lasley BL, Yen SS. A simple method for the assay of eight steroids in small volumes of plasma. Steroids. 1976;28(2):179–196. [DOI] [PubMed] [Google Scholar]
- 23.Stanczyk FZ, Jurow J, Hsing AW. Limitations of direct immunoassays for measuring circulating estradiol levels in postmenopausal women and men in epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2010;19(4):903–906. [DOI] [PubMed] [Google Scholar]
- 24.Loriaux DL, Ruder HJ, Knab DR, Lipsett MB. Estrone sulfate, estrone, estradiol and estriol plasma levels in human pregnancy. J Clin Endocrinol Metab. 1972;35(6):887–891. [DOI] [PubMed] [Google Scholar]
- 25.Prentice RL. A case-cohort design for epidemiologic cohort studies and disease prevention trials. Biometrika. 1986;73:1–11. [Google Scholar]
- 26.Barlow WE, Ichikawa L, Rosner D, Izumi S. Analysis of case-cohort designs. J Clin Epidemiol. 1999;52(12):1165–1172. [DOI] [PubMed] [Google Scholar]
- 27.Ichikawa L, Barlow W. User’s Guide to the Survival Analysis Macro With Robust Variance, Version 1.0.2. Seattle: Center for Health Studies, Group Cooperative; 1998. [Google Scholar]
- 28.Samavat H, Kurzer MS. Estrogen metabolism and breast cancer. Cancer Lett. 2015;356(2 Pt A):231–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pathak DR. Dual effect of first full term pregnancy on breast cancer risk: empirical evidence and postulated underlying biology. Cancer Causes Control. 2002;13(4):295–298. [DOI] [PubMed] [Google Scholar]
- 30.Lambe M, Hsieh C, Trichopoulos D, Ekbom A, Pavia M, Adami HO. Transient increase in the risk of breast cancer after giving birth. N Engl J Med. 1994;331(1):5–9. [DOI] [PubMed] [Google Scholar]
- 31.Russo IH, Russo J. Pregnancy-induced changes in breast cancer risk. J Mammary Gland Biol Neoplasia. 2011;16(3):221–233. [DOI] [PubMed] [Google Scholar]
- 32.Institute of Medicine Breast Cancer and the Environment: A Life Course Approach. Washington, DC: Institute of Medicine; 2012. [Google Scholar]
- 33.Peck JD, Hulka BS, Poole C, Savitz DA, Baird D, Richardson BE. Steroid hormone levels during pregnancy and incidence of maternal breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11(4):361–368. [PubMed] [Google Scholar]
- 34.Lukanova A, Surcel HM, Lundin E, Kaasila M, Lakso HA, Schock H, Husing A, Kaaks R, Koskela P, Grankvist K, Pukkala E, Zeleniuch-Jacquotte A, Lehtinen M, Toniolo P. Circulating estrogens and progesterone during primiparous pregnancies and risk of maternal breast cancer. Int J Cancer. 2012;130(4):910–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fortner RT, Schock H, Kaaks R, Lehtinen M, Pukkala E, Lakso HA, Tanner M, Kallio R, Joensuu H, Grankvist K, Zeleniuch-Jacquotte A, Toniolo P, Lundin E, Surcel HM. Early pregnancy sex steroids and maternal breast cancer: a nested case-control study. Cancer Res. 2014;74(23):6958–6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Titus-Ernstoff L, Hatch EE, Hoover RN, Palmer J, Greenberg ER, Ricker W, Kaufman R, Noller K, Herbst AL, Colton T, Hartge P. Long-term cancer risk in women given diethylstilbestrol (DES) during pregnancy. Br J Cancer. 2001;84(1):126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Assis S, Wang M, Jin L, Bouker KB, Hilakivi-Clarke LA. Exposure to excess estradiol or leptin during pregnancy increases mammary cancer risk and prevents parity-induced protective genomic changes in rats. Cancer Prev Res (Phila). 2013;6(11):1194–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meier-Abt F, Bentires-Alj M, Rochlitz C. Breast cancer prevention: lessons to be learned from mechanisms of early pregnancy-mediated breast cancer protection. Cancer Res. 2015;75(5):803–807. [DOI] [PubMed] [Google Scholar]
- 39.Kelsey JL. A review of the epidemiology of human breast cancer. Epidemiol Rev. 1979;1:74–109. [DOI] [PubMed] [Google Scholar]
- 40.Albrektsen G, Heuch I, Hansen S, Kvåle G. Breast cancer risk by age at birth, time since birth and time intervals between births: exploring interaction effects. Br J Cancer. 2005;92(1):167–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muenst S, Mechera R, Däster S, Piscuoglio S, Ng CKY, Meier-Abt F, Weber WP, Soysal SD. Pregnancy at early age is associated with a reduction of progesterone-responsive cells and epithelial Wnt signaling in human breast tissue. Oncotarget. 2017;8(14):22353–22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schock H, Zeleniuch-Jacquotte A, Lundin E, Grankvist K, Lakso H-Å, Idahl A, Lehtinen M, Surcel H-M, Fortner RT. Hormone concentrations throughout uncomplicated pregnancies: a longitudinal study. BMC Pregnancy Childbirth. 2016;16(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rosner W, Hankinson SE, Sluss PM, Vesper HW, Wierman ME. Challenges to the measurement of estradiol: an endocrine society position statement. J Clin Endocrinol Metab. 2013;98(4):1376–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verburg BO, Steegers EAP, De Ridder M, Snijders RJM, Smith E, Hofman A, Moll HA, Jaddoe VWV, Witteman JCM. New charts for ultrasound dating of pregnancy and assessment of fetal growth: longitudinal data from a population-based cohort study. Ultrasound Obstet Gynecol. 2008;31(4):388–396. [DOI] [PubMed] [Google Scholar]
- 45.Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. Plasma estradiol, estriol, and progesterone in human pregnancy. II. Clinical applications in Rh-isoimmunization disease. Am J Obstet Gynecol. 1972;113(6):766–770. [DOI] [PubMed] [Google Scholar]
- 46.Holl K, Lundin E, Kaasila M, Grankvist K, Afanasyeva Y, Hallmans G, Lehtinen M, Pukkala E, Surcel HM, Toniolo P, Zeleniuch-Jacquotte A, Koskela P, Lukanova A. Effect of long-term storage on hormone measurements in samples from pregnant women: the experience of the Finnish maternity cohort. Acta Oncol. 2008;47(3):406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russo J, Mailo D, Hu Y-F, Balogh G, Sheriff F, Russo IH. Breast differentiation and its implication in cancer prevention. Clin Cancer Res. 2005;11(2 Pt 2):931s–936s. [PubMed] [Google Scholar]
- 48.Buster JE, Chang RJ, Preston DL, Elashoff RM, Cousins LM, Abraham GE, Hobel CJ, Marshall JR. Interrelationships of circulating maternal steroid concentrations in third trimester pregnancies. II. C18 and C19 steroids: estradiol, estriol, dehydroepiandrosterone, dehydroepiandrosterone sulfate, delta 5-androstenediol, delta 4-androstenedione, testosterone, and dihydrotestosterone. J Clin Endocrinol Metab. 1979;48(1):139–142. [DOI] [PubMed] [Google Scholar]
- 49.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15(1):36–47. [DOI] [PubMed] [Google Scholar]
- 50.Cavalieri EL, Rogan EG. A unified mechanism in the initiation of cancer. Ann N Y Acad Sci. 2002;959:341–354. [DOI] [PubMed] [Google Scholar]
- 51.Cavalieri E, Chakravarti D, Guttenplan J, Hart E, Ingle J, Jankowiak R, Muti P, Rogan E, Russo J, Santen R, Sutter T. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim Biophys Acta. 2006;1766(1):63–78. [DOI] [PubMed] [Google Scholar]
- 52.Cohn BA, La Merrill M, Krigbaum NY, Yeh G, Park JS, Zimmermann L, Cirillo PM. DDT exposure in utero and breast cancer. J Clin Endocrinol Metab. 2015;100(8):2865–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morris EA, Hale SA, Badger GJ, Magness RR, Bernstein IM. Pregnancy induces persistent changes in vascular compliance in primiparous women. Am J Obstet Gynecol. 2015;212(5):633.e1–633.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Andreas M, Kuessel L, Kastl SP, Wirth S, Gruber K, Rhomberg F, Gomari-Grisar FA, Franz M, Zeisler H, Gottsauner-Wolf M. Bioimpedance cardiography in pregnancy: a longitudinal cohort study on hemodynamic pattern and outcome. BMC Pregnancy Childbirth. 2016;16(1):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siiteri PK, Cohn BA. Mechanism of Pregnancy Protection against Breast Cancer. In: Era of Hope, Department of Defense Breast Cancer Research Program Meeting; June 8–11, 2005; Philadelphia, PA. [Google Scholar]
- 56.Petrakis NL, Wrensch MR, Ernster VL, Miike R, Murai J, Simberg N, Siiteri PK. Influence of pregnancy and lactation on serum and breast fluid estrogen levels: implications for breast cancer risk. Int J Cancer. 1987;40(5):587–591. [DOI] [PubMed] [Google Scholar]
- 57.Innes KE, Byers TE. Preeclampsia and breast cancer risk. Epidemiology. 1999;10(6):722–732. [PubMed] [Google Scholar]
- 58.Ali AT. Reproductive factors and the risk of endometrial cancer. Int J Gynecol Cancer. 2014;24(3):384–393. [DOI] [PubMed] [Google Scholar]
- 59.Wentzensen N, Poole EM, Trabert B, White E, Arslan AA, Patel AV, Setiawan VW, Visvanathan K, Weiderpass E, Adami HO, Black A, Bernstein L, Brinton LA, Buring J, Butler LM, Chamosa S, Clendenen TV, Dossus L, Fortner R, Gapstur SM, Gaudet MM, Gram IT, Hartge P, Hoffman-Bolton J, Idahl A, Jones M, Kaaks R, Kirsh V, Koh WP, Lacey JV Jr, Lee IM, Lundin E, Merritt MA, Onland-Moret NC, Peters U, Poynter JN, Rinaldi S, Robien K, Rohan T, Sandler DP, Schairer C, Schouten LJ, Sjöholm LK, Sieri S, Swerdlow A, Tjonneland A, Travis R, Trichopoulou A, van den Brandt PA, Wilkens L, Wolk A, Yang HP, Zeleniuch-Jacquotte A, Tworoger SS. Ovarian cancer risk factors by histologic subtype: an analysis from the Ovarian Cancer Cohort Consortium. J Clin Oncol. 2016;34(24):2888–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huggins C, Mainzer K, Torralba Y. Hormonal influences on mammary tumors of the rat. I. Acceleration of growth of transplanted fibroadenoma in ovariectomized and hypophysectomized rats. J Exp Med. 1956;104(4):525–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lappano R, Rosano C, De Marco P, De Francesco EM, Pezzi V, Maggiolini M. Estriol acts as a GPR30 antagonist in estrogen receptor-negative breast cancer cells. Mol Cell Endocrinol. 2010;320(1-2):162–170. [DOI] [PubMed] [Google Scholar]