Abstract
Context:
Recent clinical and laboratory studies suggested that women with BRCA mutations have lower ovarian reserve and their primordial follicle oocytes may be more prone to DNA damage; however, direct proof is lacking.
Objective:
To determine whether women with germline BRCA mutations have reduced primordial follicle reserve and increased oocyte DNA damage.
Design:
A comparative laboratory study of ovarian tissue obtained from unaffected BRCA mutation carriers (BMCs) vs age-matched organ donor cadavers.
Setting:
Two academic centers.
Patients or Other Participants:
Of the 230 ovarian specimens from BMCs, 18 met the study inclusion criteria. Healthy ovaries from 12 organ donor cadavers served as controls.
Intervention:
Histology and immunohistochemical analysis on paraffin-embedded ovarian sections.
Main Outcome Measure(s):
Primordial follicle density and the percentage of DNA double-strand break (DSB)−positive primordial follicle oocytes.
Results:
Ovaries from BMCs had significantly lower primordial follicle densities than those of controls (11.2 ± 2.0 vs 44.2 ± 6.2 follicles/mm3; P = 0.0002). BRCA mutations were associated with increased DNA DSBs in primordial follicle oocytes (62% ± 5.2% vs 36% ± 3.4%; P = 0.0005). In subgroup analyses, both BRCA1 and BRCA2 mutations were associated with lower primordial follicle density (P = 0.0001 and 0.0030, respectively), and BRCA1 mutations were associated with higher DNA DSBs (P = 0.0003) than controls. The rates of follicle decline (R2 = 0.74; P = 0.0001) and DNA DSB accumulation (R2 = 0.70; P = 0.0001) appeared to be accelerated, particularly in primordial follicle oocytes of BMCs over age 30 years.
Conclusions:
We provide direct evidence of diminished ovarian reserve as well as accelerated primordial follicle loss and oocyte DNA damage in women with BRCA mutations. These findings may further our understanding of ovarian aging, and be useful when counseling BMCs.
Comparison of BRCA mutation carrier ovaries with controls showed that BRCA mutations were associated with accelerated loss of primordial follicle reserve and increased oocyte DNA damage with age.
BRCA1 and BRCA2 genes are members of the ataxia-telangiectasia-mutated (ATM)−mediated DNA damage signaling pathway and are essential for DNA double-strand break (DSB) repair (1) (2). Mutations in BRCA genes substantially increase the lifetime risk of breast, ovarian, fallopian tube, and primary peritoneal cancers (3). The current literature suggests that in addition to their increased risk for multiple malignancies, women with germline mutations in BRCA genes may have reduced ovarian reserve. Several clinical and experimental animal studies, including ours, found an association between BRCA mutations and diminished ovarian reserve and accelerated ovarian aging (4–8). Furthermore, we showed that the primordial follicle reserve of BRCA1-mutant mice was lower than that of wild-type mice, and their oocytes showed higher degrees of DNA damage with age (6).
In clinical studies, researchers evaluated serum anti-Müllerian hormone (AMH) levels as the primary outcome measure of the status of ovarian reserve. Although AMH is currently the best available serum marker of ovarian reserve, it still provides an indirect assessment of primordial follicle population (9). In addition, confounders such as oral contraceptive pill (OCP) use or the presence of polycystic ovary syndrome may distort serum AMH levels (10, 11). Hence, the assessment of ovarian reserve by AMH may still not be precise and may be subject to bias.
In this study, we aimed to directly determine whether primordial follicle reserve is reduced in the ovaries of women with BRCA mutations and whether the presence of such mutations is associated with increased DNA damage in primordial follicle oocytes. We hypothesized that in the ovaries of women with BRCA mutations, the primordial follicle reserve is lower than in ovaries of controls and that they are more prone to DNA damage because of lower efficiency of DNA repair.
Materials and Methods
Materials
The institutional review board at Brigham and Women’s Hospital/Harvard Medical School approved the histological and immunohistochemical analysis of ovarian sections from BRCA mutation carriers (BMCs). Because the samples were deidentified and the tissue blocks were archival, no consent form was required. The study of ovarian tissue of nonliving cadavers was exempt because institutional review board review applies only to living subjects and no identifiable data were used.
A total of 230 ovarian specimens from women who were BMCs and who underwent risk-reducing salpingo-oophorectomy (RRSO) between 2005 and 2012 were identified from the pathology archive of Brigham and Woman’s Hospital. Pathology reports and available clinical history were reviewed. Of these, 18 with BRCA1 (n = 13) or BRCA2 (n = 5) mutations met the study inclusion criteria of (1) age 18 to 40 years, (2) no history of cancer, (3) no ovarian pathology, and (4) no prior chemotherapy or radiation exposure.
Healthy ovaries obtained from Liveon.org (formerly NY Organ Donor Network), which were harvested from organ donor cadavers who died of nonsystemic causes meeting the same criteria, were identified and served as controls (n = 12). Information on age, parity, smoking, and OCP use was also recorded for BMCs. However, except for age, this information was not available for the control group. The archived ovarian specimens of BMCs were previously examined by the Pathology Department at Brigham and Women’s Hospital and were confirmed to be free of any malignancy or other abnormalities.
Methods
Calculation of primordial follicle density
Entire formalin-fixed and paraffin-embedded tissue blocks from each ovary were serially sectioned at 5-μm thickness followed by staining with hematoxylin and eosin (12). Because the mean diameter of a primordial follicle is 39.5 ± 7.6 μm, with a largest diameter of 49 μm, every 10th section (50 μm apart) was reviewed to determine the follicle counts (13). The counts were the average of those obtained by three independent observers who were blinded to group assignment.
A primordial follicle was defined as an oocyte surrounded by a single layer of flat pregranulosa cells (14). To avoid duplication, a primordial follicle was counted only in the section in which the nucleus was visible. The counts were performed under ×20 objective of an optical microscope. Primordial follicle density was calculated by dividing the total number of primordial follicles by the total volume of evaluated ovarian cortex and was reported as the number of follicles per millimeter cubed (follicles/mm3) (15).
Assessment of DNA DSBs
H2AX is one of the variants of the nucleosome core of histone H2A. Upon formation of DNA DSBs, H2AX is phosphorylated (and is then termed γH2AX) and forms foci at DNA DSB sites. These foci have been shown to closely reflect the extent of DNA damage when visualized by immunohistochemistry utilizing anti-γH2AX antibodies (16). To determine DNA DSBs in primordial follicles, we stained every 10th section with an anti-γH2AX antibody (IHC-00059; Bethyl Laboratories, Montgomery, TX; 1:500 dilution) followed by counterstaining with hematoxylin. Primordial follicles with oocytes that stained for γH2AX antibody were considered “γH2AX-positive” as per the methodology we previously described (6). The counts were the average of those obtained by three independent observers who were blinded to group assignment. The percentage of γH2AX-positive primordial follicles was calculated to represent the extent of DNA DSBs as previously described by us (6).
Statistical analysis
NCSS Software version 10 (NCSS, Salt Lake City, UT) and SAS Software version 9.0 (SAS Institute, Cary, NC) were used for statistical analysis. The study was powered at 90% for detecting a 25% lower primordial follicle density in the BMC group compared with the control group, which required a minimum number of 10 in each group.
For mean comparisons with data distributions consistent with normality, the Student t test was used. Because the decline in the follicle pool with age is exponential, primordial follicle density and percentage of follicles with H2AX positivity were natural log-transformed to examine correlation with age using linear regression analysis (17). The cutoff for statistical significance was P < 0.05. All linear regression and multivariate analyses were conducted on natural log-transformed data for the outcome measures of primordial follicle density and percentage of follicles with H2AX positivity.
Results
Study population
The mean age of the BMCs (36.5 ± 4.7 years; range, 24 to 40 years; n = 18) and the control group was similar (33 ± 5.8 years; range, 23 to 40 years; n = 12) (P = 0.3000). Of the BRCA carriers, 16 of 18 were parous and none were smokers. The latter information was not available from organ donor cadavers.
Primordial follicle density
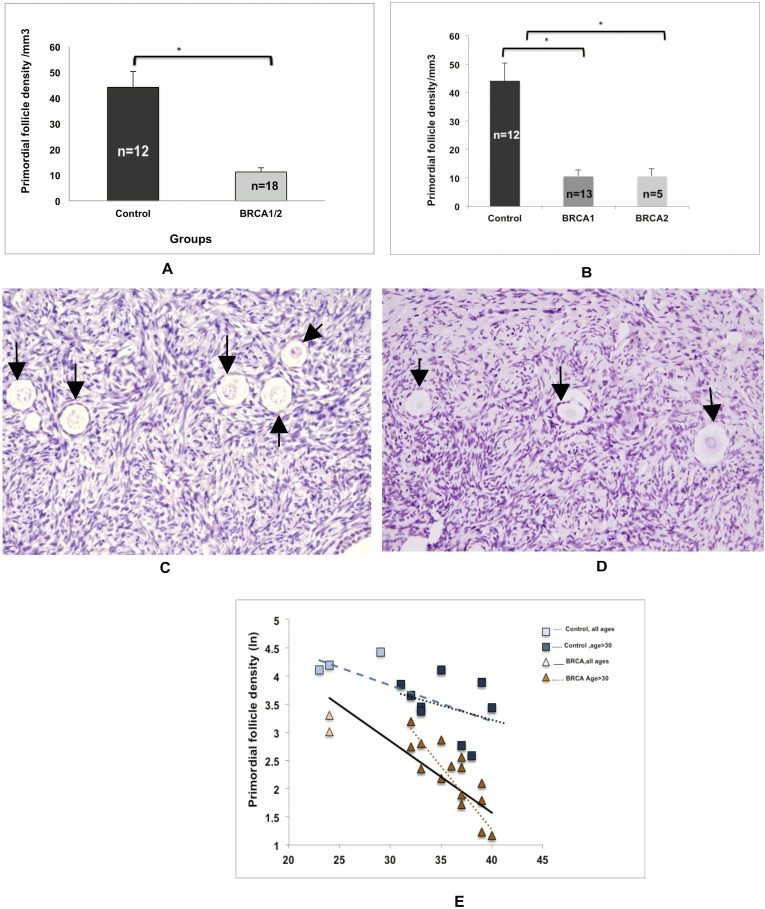
On histological analysis, ovaries from BMCs showed significantly lower mean primordial follicle density compared with those of controls (11.2 ± 6.7 vs 44.18 ± 6.1 follicles/mm3, respectively; P = 0.0002) (Fig. 1A). When the analysis was repeated on the basis of type of BRCA gene mutated, the difference in primordial follicle density remained significant both for BRCA1 (P = 0.0001) and for BRCA2 (P = 0.0003) compared with controls (Fig. 1B−1D). Comparisons between the BRCA1 and BRCA2 subgroups showed no significant difference in primordial follicle density.
Figure 1.
Effect of BRCA mutations on primordial follicle reserve. (A) The mean primordial follicle density was lower in the ovaries of BMCs than in those of controls (11.2 ± 1.59 vs 44.18 ± 6.16 follicles/mm3; P = 0.0002). (B) Both BRCA1 (P = 0.001) and BRCA2 (P = 0.0003) mutations were associated with low primordial follicle reserve. The error bars represent standard error of the mean. Statistical significance is denoted by asterisks (*). (C and D) Representative photomicrographs of primordial follicle density in (C) a control and (D) a patient with a BRCA1 mutation. Arrows show the primordial follicles. (E) Comparison of the linear regression curves by multivariate analysis indicates that there is a sharper decline of primordial follicle reserve (R2 = 0.74; P = 0.0001) in BMC ovaries (slope = −0.1270) than in control ovaries (slope = −0.0634). This difference in the slopes between BMC (slope = −0.2243) and controls (slope = −0.051) became more distinct when the linear regression was limited to those aged >30 years. The squares represent the control group, and the triangles represent the BMCs. Dotted lines represent the analyses that were limited to age >30 years in each group. Refer to the text for complete linear regression analysis results.
To establish that the rate of age-related follicle reserve decline was accelerated in BMCs, we first performed linear regression to determine the slope of decline and show the correlation of age with primordial follicle density in both groups. Linear regression showed that primordial follicle counts decreased in an exponential fashion by age in both BRCA1/2 carriers (slope = −0.1270; R2 = 0.57; P = 0.0003) and controls (slope = −0.0634; R2 = 0.38; P = 0.0321). We then compared the slopes of decline between the groups by multivariate analysis. We found that the slope of follicle decline was significantly steeper in the BMCs than in the controls (R2 = 0.74; P = 0.0001), suggesting that primordial follicle density was declining faster in BRCA1/2 mutation carriers than in controls (Fig. 1E).
When the linear regression analysis was limited to those over the age of 30 years, the slopes of age-related decline became more prominent in the BRCA1/2 carriers (slope = −0.2243; R2 = 0.65; P = 0.0002) than in controls (slope = −0.051; R2 = 0.11; P = 0.38). The comparison of these slopes by multivariate analysis again indicated significant difference in the rate of decline, suggesting faster follicle loss in the BMC group with age >30 years (R2 = 0.70; P = 0.0001).
DNA damage in primordial follicles
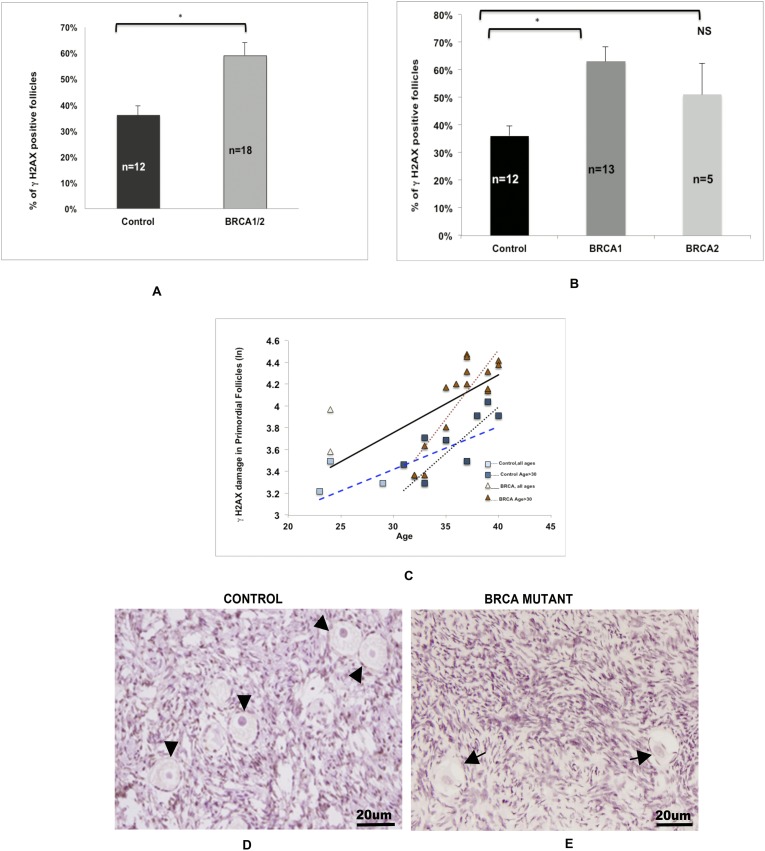
The immunohistochemical assessment of γH2AX expression revealed a significantly higher percentage of primordial follicles with DNA DSBs in the ovaries of all BMCs compared with those of controls (59% ± 5% vs 35% ± 3%; P = 0.0005) (Fig. 2A). When we repeated the analysis according to the type of BRCA gene mutated, this difference persisted for BRCA1 (P = 0.0003) but not for BRCA2 gene mutations (P = 0.2000) (Fig. 2B) compared with controls.
Figure 2.
Effect of BRCA mutations on DNA damage in primordial follicle oocytes. (A) The proportion of γH2AX-positive primordial follicle oocytes was higher in the ovaries of BMCs than in the ovaries of controls (59% ± 5% vs 36% ± 3%; P = 0.0005). (B) Compared with controls (36% ± 3%), a higher likelihood of DNA DSBs was observed in the primordial follicle oocytes of the BRCA1 subgroup (63% ± 5%; P = 0.0003) but not the BRCA2 subgroup (51% ± 11%; P = 0.24). The error bars represent standard error of the mean. Statistical significance is denoted by asterisks (*). (C) Comparison of the linear regression curves by multivariate analysis indicates that there was a faster accumulation of DNA breaks (R2 = 0.57; P = 0.002) in primordial follicle oocytes of BMC ovaries (slope = 0.0530) than in control ovaries (slope = 0.0390). This difference between the BMCs (slope = 0.1263) and controls (slope = 0.081) became more distinct when the analysis was limited to those aged >30 years. The squares represent the control group, and the triangles represent the BMCs. Dotted lines represent the analyses that were limited to age >30 years in each group. Refer to the text for linear regression results. Photomicrographic representation of γH2AX expression in (D) controls and (E) BMCs. (D) Arrowheads show the γH2AX− follicles in controls. (E) Arrows mark the γH2AX+ follicles in BMCs.
To establish that the rate of DNA damage accumulation was accelerated with age in BMCs, we first performed linear regression to determine the slope of DNA damage accumulation and show the correlation of age with γH2AX expression in primordial follicles in both groups. Linear regression showed that the percentage of γH2AX-positive primordial follicles increased in an exponential fashion by age in both BRCA1/2 carriers (slope = 0.053; R2 = 0.40; P = 0.0045) and controls (slope = 0.0390; R2 = 0.39; P = 0.0301). We then compared the slopes of decline between the groups by multivariate analysis. We found that the rate (slope) of the age-related increase in oocyte DNA damage was significantly steeper in BMCs than in controls (R2 = 0.57; P = 0.002), suggesting that primordial follicle oocytes in BMC ovaries are more prone to accumulating DNA damage (Fig. 2C).
When the linear regression analysis was limited to those over the age of 30 years, the slope of age-related accumulation of DNA damage became more prominent in the BRCA1/2 carriers (slope = 0.1263; R2 = 0.74; P = 0.0001) and controls (slope = 0.084; R2 = 0.55; P = 0.0219). The comparison of these slopes by multivariate analysis again indicated a significant difference in the rate of accumulation, showing further increased age-related liability of primordial follicle oocytes to DNA damage in the BMC ovaries from women aged >30 years (R=0.74; P = 0.0022).
Discussion
In this laboratory study, we found that the ovaries of otherwise healthy women aged 18 to 40 years with BRCA mutations had fewer primordial follicles and increased oocyte DNA damage than controls. Moreover, we found that the accumulation of DNA DSBs, as represented by γH2AX protein expression, was accelerated in the primordial follicle oocytes of women with BRCA mutations compared with controls. This is a direct demonstration of reduced primordial follicle reserve and increased oocyte DNA damage in ovaries of BMCs, indicating accelerated ovarian aging.
Age-related decreases in the numbers of ovarian follicles leading to irregularity in cycles and eventually to cessation of menses can be defined as ovarian aging. In parallel, there is a decline in oocyte quality that leads to a gradual decline in fertility (18). The growth rate and atresia of the primordial follicles are regulated by many intra- and extra-ovarian factors (19–22).
The questioning of the relationship between BRCA mutations and ovarian aging began with our earlier observations of lower response to ovarian stimulation in women with breast cancer undergoing fertility preservation with embryo and oocyte cryopreservation (4). This led us to prospectively compare serum AMH levels between women with breast cancer diagnoses who did carry BRCA mutations and those who did not (6). We found that serum AMH levels were lower in women carrying the BRCA1 but not the BRCA2 mutation. A later retrospective study reported that serum AMH concentrations of 41 healthy BMCs were similar to those of 324 noncarrier women matched for age (23). However, that study was criticized for using an ill-defined control group, inclusion of patients with polycystic ovary syndrome, and lack of adjustments for other important confounders (24).
In contrast, Wang et al. (7) demonstrated lower serum AMH levels among 89 unaffected women with a BRCA mutation compared with 54 controls. Their subgroup analysis revealed that this difference was specific to women with BRCA1 mutations (n = 62). In a very recent study including 319 BMCs, the association between carrier status and AMH concentration was analyzed by linear regression after adjustments for age, body mass index, OCP use, and smoking (8). The authors reported that BRCA1 mutation carriers had 25% lower AMH concentrations on average than noncarriers. In that study, there was no significant association between BRCA2 mutation status and serum AMH level.
Studies utilizing serum AMH levels are in keeping with several reports that showed that unaffected women with BRCA mutations experience earlier menopause (5). We compared the age at menopause of 282 unaffected BRCA carriers with that of 765 healthy controls from the SWAN study and found that on average those with BRCA mutations experienced menopause 3 years earlier than controls. Likewise, Finch et al. (25) studied 908 BMCs and found that the mean age of menopause was 1 to 1.5 years earlier than in controls. Although another study comparing the percentage of women reaching menopause in BMC and control groups initially did not find a difference, the study was limited by the fact that only 19% of the entire population reached menopause during the study. The same authors later completed an AMH analysis on the same cohort and found the levels to be 25% lower in BRCA1 carriers (8). Furthermore, a recent large, genome-wide association study found single nucleotide polymorphism in BRCA1 and related DNA DSB repair genes had strong associations with age at menopause (26).
As the foregoing discussion strongly suggests, the preponderance of clinical studies point to lower ovarian reserve among women with BRCA mutations. These observations have also been confirmed in transgenic animal models. We demonstrated that the number of superovulated oocytes, litter size, and primordial follicle density were reduced in BRCA1- but not BRCA2-mutant mice compared with wild-type mice (6). Moreover, the primordial follicles of BRCA1-mutant mouse ovaries accumulated more DNA DSBs with age.
The major strengths of our study include the utilization of risk-reducing oophorectomy samples from BMCs and the availability of rare age-matched cadaveric organ donors with no ovarian pathology to serve as controls. In a histological study of ovaries with benign and cancerous lesions in both BRCA mutation−positive and −negative women, in which ovarian specimens removed for benign ovarian conditions served as controls, primordial follicle density was found to be lower in ovaries of those with malignancy and BRCA mutations. However, because all control and mutation carrier samples contained an ovarian pathology, the difference in follicle counts could not be clearly attributed to the presence of BRCA mutations (27). Our study design ensured that any benign or malignant lesions did not confound the primordial follicle measurements. Moreover, our study demonstrated that both the rate of follicle loss and the accumulation of DNA damage were accelerated in the ovaries of women with BRCA mutations.
Another strength of our study is that the formalin-fixed and paraffin-embedded blocks of the whole ovaries were serially sectioned and examined to ensure a reliable estimation of primordial follicle reserve. Furthermore, three observers who were blinded to the source or diagnosis independently evaluated the slides.
Although this study has important implications, it also has several limitations. Because of the dearth of ovarian specimens from both BRCA mutation−positive young women and controls, the sample size was relatively limited. Although our study was sufficiently powered to assess differences in primordial follicle density between carriers and controls and the strong R2 values indicate the robustness of our results for the main outcome measures, it did not have sufficient power for subgroup comparisons. As a result, we cannot determine any differential effect of BRCA1 vs BRCA2 mutations on ovarian reserve with great certainty, though the totality of the evidence suggests that BRCA1 mutations may play a more prominent role in ovarian aging. Because we do not have information on women younger than 24 years with germline BRCA mutations, we also cannot determine whether these women had lower primordial follicle endowment to begin with or the difference was due solely to faster rate of follicle loss after the establishment of that reserve. This information may not be feasible to obtain in humans because BRCA testing or RRSO is not performed in minors and because physiological primordial follicle reserve loss begins in utero.
Furthermore, the two consequences of severe unrepaired DNA damage are cell senescence and apoptotic death (28, 29). Given the limited nature of the human material from BRCA mutation−positive ovaries, we could not study the specific mechanism of accelerated follicle loss. It is probable that both contribute to the diminished ovarian reserve in the presence of diminished DNA repair caused by BRCA mutations, and these should be addressed in future studies.
The fact that controls were not screened for BRCA mutations may also be perceived as a weakness. The likelihood of the presence of BRCA mutations in an organ donor population free of cancer is highly unlikely, as the incidence in the general population is 0.1% to 0.2% (3). However, even if there were any BMCs in the control group, this would have led only to diminished primordial follicle reserve and increased DNA DSBs in the controls, which would have reduced the effect estimate of BRCA gene mutations. The same goes for smoker status. The BMCs were nonsmokers, and the smoking status of the controls was unknown. Because smoking is associated with lower ovarian reserve (5) and increased DNA damage (30), any smoking history in the controls would have reduced the likelihood of finding differences between the BRCA and control groups. Therefore, it is possible that the observed differences are actually conservative estimates.
Finally, strong biological rationale supports the findings of the current study. Primordial follicle oocytes endure a prolonged meiotic phase arrest, which creates exposure to decades-long external insults that can lead to significant accumulated DNA damage. DNA DSBs are the most severe form of DNA damage and, when unrepaired, can lead to carcinogenesis, cellular senescence, or apoptotic cell death. To prevent the dire consequences of such serious genomic damage, several complex repair mechanisms have evolved. Homologous recombination repair is a high-fidelity repair mechanism active in cells that are in the G2/M phase and appears to be the key DNA repair mechanism in oocytes (6, 24).
Homologous recombination repair is regulated by the ATM-mediated DNA damage-signaling pathway, and BRCA1 and BRCA2 genes are key members of this pathway (2). Mutations in these genes substantially increase the lifetime risk of breast, ovarian, fallopian tube, and primary peritoneal cancers because of deficient DNA repair and accumulation of carcinogenic mutations (3). We previously showed that deficient DNA repair results in the accumulation of DNA DSBs in oocytes, in turn resulting in the activation of apoptotic cell death. This led us to hypothesize that the lower primordial follicle density in the ovaries of BMCs is due to the elimination of DNA-damaged primordial follicles via the check mechanisms built into the ATM-mediated DNA damage response pathway (6). In fact, our current finding of age-related accumulation of DNA DSBs in primordial follicle oocytes of BRCA1 carriers is consistent with this hypothesis. Because the BRCA1 gene plays a more intricate role in the ATM-mediated DNA damage response pathway than does BRCA2 and because the function of the normal BRCA1 allele declines earlier with age (4, 6, 24), the more prominent association of BRCA1 mutations with lower ovarian reserve also has a strong biological basis. However, future studies may also reveal an effect of BRCA2 mutations on ovarian aging, as our limited subgroup analysis suggests.
We recently showed that DNA DSB repair mechanisms were acutely important for oocyte survival and that the oocyte’s ability to repair DNA DSBs declined with age both in rodents and in humans. Our translational data showed that declining or inefficient activity in DNA repair mechanisms led to accelerated ovarian aging by accumulation of DNA damage, as manifested by reduced ovarian reserve and early menopause (5, 24). The findings of the current study are in accordance with these earlier results and provide strong evidence for the role of BRCA1 gene and DSB repair in ovarian aging in humans.
Conclusion
Our controlled investigation provides direct evidence of diminished ovarian reserve and increased DNA damage in healthy BMCs. In addition to furthering our understanding of ovarian aging, our findings may be useful when counseling BMCs about reproductive planning. Although our study cannot directly indicate whether the future reproductive potential of young women with BRCA mutations is lower with advancing age, given that the preponderance of the evidence suggests a smaller primordial follicle pool and increased oocyte DNA breaks in mutation carriers, these women may have a disadvantage during later reproductive age (31). Further prospective studies investigating the effect of BRCA mutations and DNA DSB repair deficiency on fertility and reproductive outcomes are needed. Furthermore, DNA repair may be an important target to explore in developing treatments for curbing oocyte aging.
Acknowledgments
We thank Dr. Enes Taylan of New York Medical College for his technical help with immunochemistry and Dr. Brooke Howitt of Brigham and Women’s Hospital for her help in identifying the RRSO specimens.
Financial Support: This study was supported by the National Institutes of Health (Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Cancer Institute) grant number RO1HD053112 (to K.O.) and Brigham and Women’s Hospital Expanding the Boundary Grant.
Author Contributions: W.L.: Conception and design, performance of experiments, data analysis and interpretation, and manuscript writing. S.T.: Performance of experiments, data analysis and interpretation, and manuscript writing. F.M.: Performance of statistical analysis and data interpretation. E.S.G.: Provision of study materials and administrative and financial support. K.O.: Conception and design, provision of study materials, administrative and financial support, manuscript writing, and data interpretation.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AMH
- anti-Müllerian hormone
- ATM
- ataxia-telangiectasia-mutated
- BMC
- BRCA mutation carrier
- DSB
- double-strand break
- OCP
- oral contraceptive pill
- RRSO
- risk-reducing salpingo-oophorectomy.
References
- 1.Sung PA, Libura J, Richardson C. Etoposide and illegitimate DNA double-strand break repair in the generation of MLL translocations: new insights and new questions. DNA Repair (Amst). 2006;5(9-10):1109–1118. [DOI] [PubMed] [Google Scholar]
- 2.Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108(2):171–182. [DOI] [PubMed] [Google Scholar]
- 3.Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A, Pasini B, Radice P, Manoukian S, Eccles DM, Tang N, Olah E, Anton-Culver H, Warner E, Lubinski J, Gronwald J, Gorski B, Tulinius H, Thorlacius S, Eerola H, Nevanlinna H, Syrjäkoski K, Kallioniemi OP, Thompson D, Evans C, Peto J, Lalloo F, Evans DG, Easton DF. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oktay K, Kim JY, Barad D, Babayev SN. Association of BRCA1 mutations with occult primary ovarian insufficiency: a possible explanation for the link between infertility and breast/ovarian cancer risks. J Clin Oncol. 2010;28(2):240–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin WT, Beattie M, Chen LM, Oktay K, Crawford SL, Gold EB, Cedars M, Rosen M. Comparison of age at natural menopause in BRCA1/2 mutation carriers with a non-clinic-based sample of women in northern California. Cancer. 2013;119(9):1652–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, Dickler M, Robson M, Moy F, Goswami S, Oktay K. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med. 2013;5(172):172ra21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang ET, Pisarska MD, Bresee C, Chen YD, Lester J, Afshar Y, Alexander C, Karlan BY. BRCA1 germline mutations may be associated with reduced ovarian reserve. Fertil Steril. 2014;102(6):1723–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Phillips KA, Regan MM, Ribi K, Francis PA, Puglisi F, Bellet M, Spazzapan S, Karlsson P, Budman DR, Zaman K, Abdi EA, Domchek SM, Feng Y, Price KN, Coates AS, Gelber RD, Maruff P, Boyle F, Forbes JF, Ahles T, Fleming GF, Bernhard J. Adjuvant ovarian function suppression and cognitive function in women with breast cancer. Br J Cancer. 2016;114(9):956–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fanchin R, Taieb J, Lozano DH, Ducot B, Frydman R, Bouyer J. High reproducibility of serum anti-Mullerian hormone measurements suggests a multi-staged follicular secretion and strengthens its role in the assessment of ovarian follicular status. Hum Reprod. 2005;20(4):923–927. [DOI] [PubMed] [Google Scholar]
- 10.Dólleman M, Faddy MJ, van Disseldorp J, van der Schouw YT, Messow CM, Leader B, Peeters PH, McConnachie A, Nelson SM, Broekmans FJ. The relationship between anti-Müllerian hormone in women receiving fertility assessments and age at menopause in subfertile women: evidence from large population studies. J Clin Endocrinol Metab. 2013;98(5):1946–1953. [DOI] [PubMed] [Google Scholar]
- 11.Iliodromiti S, Kelsey TW, Anderson RA, Nelson SM. Can anti-Mullerian hormone predict the diagnosis of polycystic ovary syndrome? A systematic review and meta-analysis of extracted data. J Clin Endocrinol Metab. 2013;98(8):3332–3340. [DOI] [PubMed] [Google Scholar]
- 12.Lass A, Silye R, Abrams DC, Krausz T, Hovatta O, Margara R, Winston RM. Follicular density in ovarian biopsy of infertile women: a novel method to assess ovarian reserve. Hum Reprod. 1997;12(5):1028–1031. [DOI] [PubMed] [Google Scholar]
- 13.Hovatta O, Silye R, Krausz T, Abir R, Margara R, Trew G, Lass A, Winston RM. Cryopreservation of human ovarian tissue using dimethylsulphoxide and propanediol-sucrose as cryoprotectants. Hum Reprod. 1996;11(6):1268–1272. [DOI] [PubMed] [Google Scholar]
- 14.Oktay K, Schenken RS, Nelson JF. Proliferating cell nuclear antigen marks the initiation of follicular growth in the rat. Biol Reprod. 1995;53(2):295–301. [DOI] [PubMed] [Google Scholar]
- 15.Tilly JL. Ovarian follicle counts--not as simple as 1, 2, 3. Reprod Biol Endocrinol. 2003;1:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowndes NF, Toh GW. DNA repair: the importance of phosphorylating histone H2AX. Curr Biol. 2005;15(3):R99–R102. [DOI] [PubMed] [Google Scholar]
- 17.Faddy MJ, Gosden RG, Oktay K, Nelson JF. Factoring in complexity and oocyte memory: can transformations and cyperpathology distort reality? Fertil Steril. 1999;71(6):1170–1172. [DOI] [PubMed] [Google Scholar]
- 18.Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev. 2009;30(5):465–493. [DOI] [PubMed] [Google Scholar]
- 19.Gougeon A. Dynamics of follicular growth in the human: a model from preliminary results. Hum Reprod. 1986;1(2):81–87. [DOI] [PubMed] [Google Scholar]
- 20.Hughesdon PE. Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called “hyperthecosis”. Obstet Gynecol Surv. 1982;37(2):59–77. [DOI] [PubMed] [Google Scholar]
- 21.Webber LJ, Stubbs S, Stark J, Trew GH, Margara R, Hardy K, Franks S. Formation and early development of follicles in the polycystic ovary. Lancet. 2003;362(9389):1017–1021. [DOI] [PubMed] [Google Scholar]
- 22.Maciel GA, Baracat EC, Benda JA, Markham SM, Hensinger K, Chang RJ, Erickson GF. Stockpiling of transitional and classic primary follicles in ovaries of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89(11):5321–5327. [DOI] [PubMed] [Google Scholar]
- 23.Michaelson-Cohen R, Mor P, Srebnik N, Beller U, Levy-Lahad E, Eldar-Geva T. BRCA mutation carriers do not have compromised ovarian reserve. Int J Gynecol Cancer. 2014;24(2):233–237. [DOI] [PubMed] [Google Scholar]
- 24.Oktay K, Turan V, Titus S, Stobezki R, Liu L. BRCA mutations, DNA repair deficiency, and ovarian aging. Biol Reprod. 2015;93(3):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finch A, Evans G, Narod SA. BRCA carriers, prophylactic salpingo-oophorectomy and menopause: clinical management considerations and recommendations. Womens Health (Lond). 2012;8(5):543–555. [DOI] [PubMed] [Google Scholar]
- 26.Day FR, Ruth KS, Thompson DJ, Lunetta KL, Pervjakova N, Chasman DI, Stolk L, Finucane HK, Sulem P, Bulik-Sullivan B, Esko T, Johnson AD, Elks CE, Franceschini N, He C, Altmaier E, Brody JA, Franke LL, Huffman JE, Keller MF, McArdle PF, Nutile T, Porcu E, Robino A, Rose LM, Schick UM, Smith JA, Teumer A, Traglia M, Vuckovic D, Yao J, Zhao W, Albrecht E, Amin N, Corre T, Hottenga JJ, Mangino M, Smith AV, Tanaka T, Abecasis G, Andrulis IL, Anton-Culver H, Antoniou AC, Arndt V, Arnold AM, Barbieri C, Beckmann MW, Beeghly-Fadiel A, Benitez J, Bernstein L, Bielinski SJ, Blomqvist C, Boerwinkle E, Bogdanova NV, Bojesen SE, Bolla MK, Borresen-Dale AL, Boutin TS, Brauch H, Brenner H, Brüning T, Burwinkel B, Campbell A, Campbell H, Chanock SJ, Chapman JR, Chen YI, Chenevix-Trench G, Couch FJ, Coviello AD, Cox A, Czene K, Darabi H, De Vivo I, Demerath EW, Dennis J, Devilee P, Dörk T, Dos-Santos-Silva I, Dunning AM, Eicher JD, Fasching PA, Faul JD, Figueroa J, Flesch-Janys D, Gandin I, Garcia ME, García-Closas M, Giles GG, Girotto GG, Goldberg MS, González-Neira A, Goodarzi MO, Grove ML, Gudbjartsson DF, Guénel P, Guo X, Haiman CA, Hall P, Hamann U, Henderson BE, Hocking LJ, Hofman A, Homuth G, Hooning MJ, Hopper JL, Hu FB, Huang J, Humphreys K, Hunter DJ, Jakubowska A, Jones SE, Kabisch M, Karasik D, Knight JA, Kolcic I, Kooperberg C, Kosma VM, Kriebel J, Kristensen V, Lambrechts D, Langenberg C, Li J, Li X, Lindström S, Liu Y, Luan J, Lubinski J, Mägi R, Mannermaa A, Manz J, Margolin S, Marten J, Martin NG, Masciullo C, Meindl A, Michailidou K, Mihailov E, Milani L, Milne RL, Müller-Nurasyid M, Nalls M, Neale BM, Nevanlinna H, Neven P, Newman AB, Nordestgaard BG, Olson JE, Padmanabhan S, Peterlongo P, Peters U, Petersmann A, Peto J, Pharoah PDP, Pirastu NN, Pirie A, Pistis G, Polasek O, Porteous D, Psaty BM, Pylkäs K, Radice P, Raffel LJ, Rivadeneira F, Rudan I, Rudolph A, Ruggiero D, Sala CF, Sanna S, Sawyer EJ, Schlessinger D, Schmidt MK, Schmidt F, Schmutzler RK, Schoemaker MJ, Scott RA, Seynaeve CM, Simard J, Sorice R, Southey MC, Stöckl D, Strauch K, Swerdlow A, Taylor KD, Thorsteinsdottir U, Toland AE, Tomlinson I, Truong T, Tryggvadottir L, Turner ST, Vozzi D, Wang Q, Wellons M, Willemsen G, Wilson JF, Winqvist R, Wolffenbuttel BBHR, Wright AF, Yannoukakos D, Zemunik T, Zheng W, Zygmunt M, Bergmann S, Boomsma DI, Buring JE, Ferrucci L, Montgomery GW, Gudnason V, Spector TD, van Duijn CM, Alizadeh BZ, Ciullo M, Crisponi L, Easton DF, Gasparini PP, Gieger C, Harris TB, Hayward C, Kardia SLR, Kraft P, McKnight B, Metspalu A, Morrison AC, Reiner AP, Ridker PM, Rotter JI, Toniolo D, Uitterlinden AG, Ulivi S, Völzke H, Wareham NJ, Weir DR, Yerges-Armstrong LM, Price AL, Stefansson K, Visser JA, Ong KK, Chang-Claude J, Murabito JM, Perry JRB, Murray A; PRACTICAL consortium; kConFab Investigators; AOCS Investigators; Generation Scotland; EPIC-InterAct Consortium; LifeLines Cohort Study . Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat Genet. 2015;47(11):1294–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pavone ME, Hirshfeld-Cytron J, Tingen C, Thomas C, Thomas J, Lowe MP, Schink JC, Woodruff TK. Human ovarian tissue cortex surrounding benign and malignant lesions. Reprod Sci. 2014;21(5):582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartkova J, Horejsí Z, Koed K, Krämer A, Tort F, Zieger K, Guldberg P, Sehested M, Nesland JM, Lukas C, Ørntoft T, Lukas J, Bartek J. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434(7035):864–870. [DOI] [PubMed] [Google Scholar]
- 29.Campisi J. Suppressing cancer: the importance of being senescent. Science. 2005;309(5736):886–887. [DOI] [PubMed] [Google Scholar]
- 30.Zenzes MT. Smoking and reproduction: gene damage to human gametes and embryos. Hum Reprod Update. 2000;6(2):122–131. [DOI] [PubMed] [Google Scholar]
- 31.Santoro N. BRCA mutations and fertility: do not push the envelope! Fertil Steril. 2013;99(6):1560. [DOI] [PubMed] [Google Scholar]