Abstract
Introduction
The optimal strategy to achieve palliation of malignant pleural effusions (MPE) is unknown. This multi-institutional, prospective, randomized trial compares 2 established methods of control for symptomatic unilateral MPE.
Methods
Patients with unilateral MPE were randomized to either tunneled catheter daily drainage (TCD) or bedside talc pleurodesis (TP). This trial is patterned a previous randomized trial that demonstrated that bedside TP was equivalent to thoracoscopic TP (CALGB 9334). The primary endpoint of the current study was combined success: consistent/reliable drainage/pleurodesis, lung expansion, and 30-day survival. A secondary endpoint, survival with effusion control, was added retrospectively.
Results
Fifty-seven patients randomized to either bedside TP or TCD, and the 2 groups were similar in terms of age (62 years), active chemotherapy (28%) and histologic diagnosis (lung 63%, breast 12%, and other/unknown cancers 25%). Combined success was higher with TCD (62%) than with TP (46%; odds ration 5.0, p=0.064). Multivariate regression analysis revealed that patients treated with TCD had better 30-day activity without dyspnea scores (8.7 versus 5.9 for TP, p=0.036), especially in the subgroup with impaired expansion (9.1 versus 4.6, p=0.042). TCD patients had better survival with effusion control (82%) at 30 days compared to TP cases (52%, p=0.024).
Conclusions
In this prospective, randomized trial, TCD achieved superior palliation of unilateral MPE than TP, particularly in patients with trapped lungs.
Keywords: Pleural effusion, palliation, lung cancer, talc pleurodesis, tunneled catheters
Introduction
Approximately 100,000 new malignant pleural effusions (MPE) occur annually in the U.S., adversely affecting quality of life (QOL) often within months of death1,2. The optimal palliative management for this multitude of symptomatic patients is still not well understood. Accordingly, this has resulted in considerable practice variation in the U.S. dependent largely on the preferences of physicians, referral patterns, and payment options for such therapies. Accordingly, inpatient or operative management dominates in some regions.
Intermittent external drainage by an indwelling catheter is gaining popularity, because it has the advantage of avoiding hospitalization and inflammatory complications caused by talc pleurodesis3. Alternatively, pleurodesis is a well-accepted and relatively brief therapy, which, if successful, yields permanent control. Talc is used commonly, because it is equivalent or better than more expensive agent4–7. We aimed to test whether tunneled catheter drainage (TCD) was equivalent or superior to talc pleurodesis (TP) to determine whether wider use of less invasive, outpatient management can be justified.
Materials and Methods
A prospective, randomized Phase III trial was initiated in the Cancer and Leukemia Group B and activated 5/15/2002 across a broad group of cooperative institutions. Central Institutional Review Board (IRB) approval was granted on 4/25/02 with local IRB approvals subsequently. From 10/15/2002 to 12/14/2004, 67 patients were registered, appropriately consented, and 57 were evaluable (Figure 1). The study was closed early due to slow accrual and this was attributed to randomization refusal because after its presentation subjects preferred in roughly equal proportions to have either the inpatient or the outpatient management. Seventeen patients were enrolled at one institution and another 20 centers enrolled between 1–7 cases.
Figure 1.
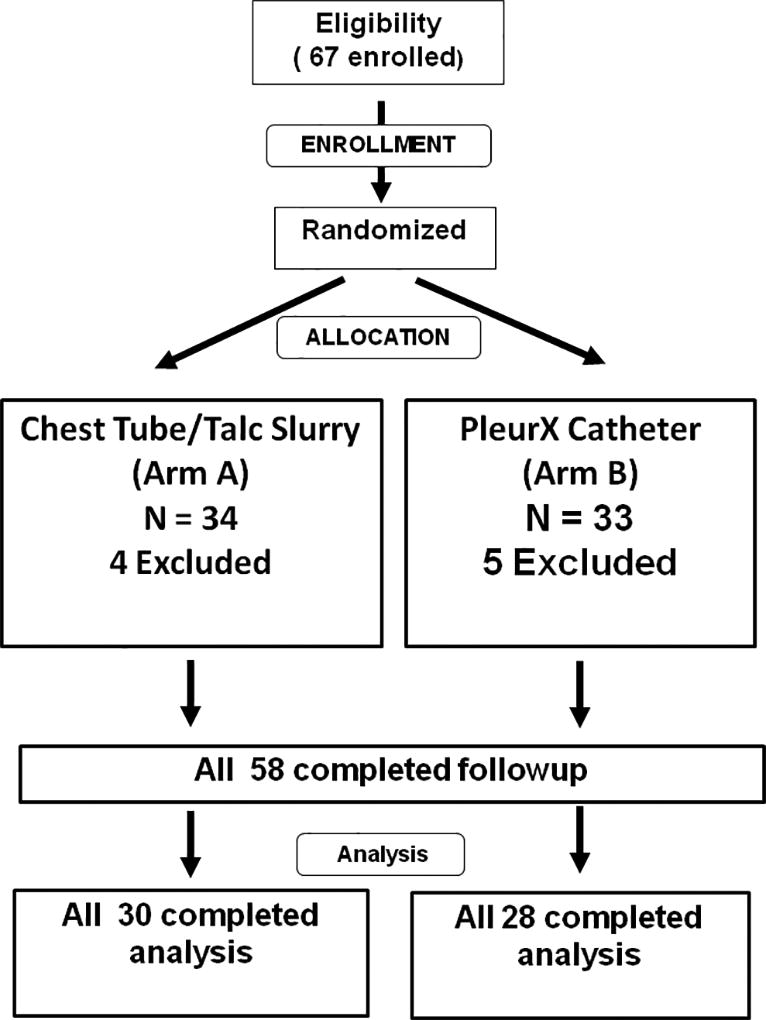
CONSORT Patient flow diagram
Eligibility and Objectives
Symptomatic patients with malignancy proven by histology or cytology were eligible for this study if there was chest roentgenogram evidence of a previously untreated, unilateral pleural effusion requiring pleurodesis or ongoing drainage. Patients were Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) 0–2 with no active pleural infection, talc allergy or other contraindication to talc usage. Investigators avoided enrolling patients with likely trapped lungs, anticipated survival <60 days, or severe co-morbid medical conditions.
The primary objective was to compare the proportion of maintained successful treatments 30 days after intervention. A combined “Success” achieved all predetermined criteria: alive (#1), no effusion recurrence (#2), lung re-expansion ≥90% after effusion drainage (#3), and completion of the intervention by 2 weeks (#4) by removal of the chest tube for TP or proper function of the TCD.
An important secondary objective was to test for differences in QOL 7 and 30 days post-treatment, including patient acceptance and satisfaction and level of symptoms and dyspnea (Memorial Symptom Assessment Scale-Condensed Form-MSAS, Dyspnea Index, and Karnofsky Self-reported Performance Rating Scale).
Chest tube with talc (TP)
A single dose of 4–5 g of sterile talc slurry within 100 cc of saline was infused into the pleural space using the chest catheter (24 F or greater) whose proper placement was confirmed by chest roentgenogram. Talc was given within 36 hours of chest tube placement, and the tube was clamped for 2 hours while patient position was changed to facilitate talc distribution. Pleurodesis was assumed when chest drainage fell to 150 cc in 24 hours leading to tube removal followed by chest roentgenogram. The management of the all patients in this arm was inpatient and replicated the talc slurry arm of the successful CALGB 9334 including chest tube size and provided guidelines for the use of sterile talc prepared locally or by pharmaceutical companies.8
PleurX™ Catheter (SCD)
The placement technique for the PleurX™ catheter (Denver Biomedical, Denver, Colorado) was described previously9. The catheters were accessed under aseptic technique and drained daily using evacuated drainage bottles. Apart from the initial drainage at the time of insertion, no more than 1000 mls of fluid was drained at a single time. A chest roentgenogram was taken within 36 hours after the initial drainage of pleural fluid. Thereafter, the catheter was drained once daily under aseptic technique by the patient, family or visiting nurse and recorded. When the drainage volume was less than 30 ml each time over 72 hours (3 consecutive days), the PleurX™ catheter was removed in the outpatient setting. TCD was generally outpatient procedure as that is how payment is structured for its use, although a few patients may have had their catheters placed as an inpatient then discharged.
Effusion control criteria
The local treating physician reviewed the chest roentgenogram (CXR) images and estimated the percent expansion of the lung beneath the effusion. While CT estimates are more accurate, CXR monitoring is standard clinical practice and was less intrusive to a frail population. Image sets were graded by the primary investigator when the local investigator was not available (5 cases) and as part of case quality review. Lung expansion was measured by CXR pre-treatment, post-drainage, last inpatient day, first clinical visit, 30 days, and 60 days.
A complete response was defined as no pleural fluid accumulation greater than that seen on the CXR after completion of MPE drainage. Recurrence was defined as accumulation of pleural fluid greater than levels measured at the time that the catheter or chest tube was permanently removed.
Statistical Considerations
CALGB 30102 was designed as a 2-arm randomized phase III study to compare TP and TCD with respect to (a) the proportion of patients with successful effusion control at 30 days (see criteria above), and (b) the quality of life scores reported by patients and physicians. Five hundred thirty (530) patients were planned to be randomized with equal proportion to the two treatment arms via permuted block randomization scheme stratified by inpatient status (yes, no), disease type (breast, lung, other) and receiving systemic chemotherapy concurrently (yes, no). For a 2-sided test conducted at the 0.10 level of significance, the study with 530 patients has approximately 90% power to detect an increase of the “success” rate from 53% in the TP arm to 66% in the TCD arm. Nine patients were excluded from analysis; 6 patients were ineligible due to the finding of bilateral effusions and 3 patients never started protocol treatment because of other complications of their disease (see Figure 1).
The 30-day and 60- day “success” rates for effusion control were compared using Fisher’s exact test and baseline prognostic factors that might affect success were evaluated further using a stepwise approach in a logistic regression model. QOL score differences at days 30 and 60 were tested by Wilcoxon rank sum test and analysis of linear regression. Lung expansion effect on success was analyzed with the Cochran-Mantel-Haenszel association test. Complete case data were used to compute the QOL scores changed over time, and the impact of the drop-outs on the estimates was investigated by imputation methods.
Statistical analyses were performed by CALGB statisticians on SAS 9.1 (SAS Institute Inc., Cary, NC, USA). All p-values are two-sided. CALGB Audit Committee and statistical staff performed regular central and on-site monitoring to insure safety, institutional protocol, and federal regulation compliance.
Post-hoc analysis
While >90% expansion was used in CALGB 9334 (from which this study was patterned) achieving that value was not incorporated into its composite endpoint quite the same way. And, more importantly, 9334 investigators were not expressly required to quantify the % expansion. We believed investigator quantification would enhance this study, particularly because the central radiologist imaging review for 9334 only validated qualitative improvement but not the local 90% expansion interpretation because of the inherent inaccuracy of plain films. Despite similar eligibility criteria, only 36% of our patients (compared to 68% in 9334) achieved >90% despite the same requirement of predicted full expansion. It became evident by chest roentgenogram review that investigators from 9334 were inclined to classify a lung expanded >90% (so the patient could be analyzed) if it largely filled the chest cavity even if that thoracic volume was reduced 10–20% by pleural restriction caused by tumor or pleurodesis effects. Accordingly, the expansion value for success was reduced to ≥70% from the original plan. With this cut-point, 65% of the cases achieved “successful” expansion normalizing the data with 9334. Also ≥70% expansion was perceived by the study team as the lowest result acceptable to most clinicians.
Furthermore, we chose to analyze patients with the same end-point as 9334, survival with maintenance of expansion, to reduce the effect of an arbitrary cut-point. Final data analysis was delayed until late 2008 for multiple reasons including the need to complete or confirm observations in a population from multiple institutions that had died or was otherwise difficult to follow, as well as fluctuations in local research and central statistical resources for this project.
Results
Efficacy Analyses
Table 1 summarizes the balanced patient demographic and baseline clinical characteristics by treatment arms. Lung (62%) or breast cancer (12%) were most common.
Table 1.
Patient Demographic and Baseline Clinical Characteristics
| Characteristics | TP (N = 29) | TCD (N = 28) | Overall (N =57) |
|---|---|---|---|
| Age | |||
| Mean / Median | 60 / 62 | 64 / 67 | 62 / 62 |
| Range | 33 – 85 | 28 – 86 | 28 – 86 |
| Gender – N (%) | |||
| Male | 16 (55) | 17 (61) | 33 (58) |
| Female | 13 (45) | 11 (39) | 24 (42) |
| Race – N (%) | |||
| White | 24 (83) | 21 (75) | 45 (79) |
| Non-White | 5 (17) | 7 (25) | 12 (21) |
| PS – N (%) | |||
| 0 / 1 | 16 (55) | 17 (63) | 33 (59) |
| 2 | 13 (45) | 10 (37)* | 23 (41) |
| Type of cancer – N (%) | |||
| Lung | 17 (59) | 19 (68) | 36 (63) |
| Breast | 4 (14) | 3 (11) | 7 (12) |
| Other types** | 8 (27) | 6 (21) | 14 (25) |
| Inpatient status – N (%)*** | |||
| Inpatient | 13 (45) | 13 (46) | 26 (46) |
| Outpatient | 16 (55) | 15 (54) | 31 (54) |
| Concurrent chemo – N (%) | |||
| Receiving | 7 (24) | 9 (32) | 16 (28) |
| Not receiving | 22 (76) | 19 (68) | 41 (72) |
| Initial drainage (ml) | |||
| Mean / Median | 1443 / 1000 | 1244 / 1150 | 1349 / 1100 |
| Range | 20 – 4000 | 192 – 2700 | 20 – 4000 |
PS – Performance Status, TCD- Tunneled Catheter Drainage
One patient’s PS data is missing.
No mesothelioma patients were in this study.
At time of randomization, see text for location of procedure.
There were no statistical differences in re-expansion between the treatment arms. The TP patients were somewhat more likely to achieve an expansion ≥70% post-drainage, (75% versus 58%, p=0.250), but maximal expansions during the entire study were similar (79% versus 73%, p=0.754). While not significant, the maximum expansion during treatment was higher than measured at pre-treatment for 96% of the TP versus 88% of the SCD (p=0.340). Similarly, there was slightly better maintenance (no lower than previous measurements) of this expansion at 30 days (58% versus 52%, p=0.761) and at 60 days (63% versus 50%, p=0.510).
Table 2 summarizes the outcomes of the two treatment procedures. The original overall combined success rate (defined above) was higher for TCD (62%) than TP (46%) but was not significant (p=0.290). Using the endpoint from 9334, TCD patients had significantly better survival without effusion recurrence (82%) within 30 days compared to TP (52%, p=0.0239).
Table 2.
Frequencies of Clinical Outcomes
| Clinical Outcomes – N / Total (%) | TP | TCD | P value1 |
|---|---|---|---|
| Overall success* | 13 / 28 (46) | 16 / 26 (62) | 0.2898 |
| Alive within 30 days of the procedure | 25 / 29 (86) | 26 / 28 (93) | 0.6701 |
| Effusion absent within 30 days | 18 / 29 (62) | 24 / 28 (86) | 0.0700 |
| Alive without recurrence within 30 days | 15 / 29 (52) | 23 /28 (82) | 0.0239 |
| Initial lung re-expansion ≥ 70% | 22 / 28 (79) | 19 / 26 (73) | 0.7540 |
| Chest tube ≤14 days** | 28 / 29 (97) | NA | NA |
| Effusion controlled within 60 days | 17 / 29 (59) | 22 / 28 (79) | 0.1550 |
| Talc / PleurX procedure completed | 24 / 30 (80) | 25 / 26 (96) | 0.1080 |
| Pleurodesis achieved | 25 / 29 (86) | 17 / 26 (65) | 0.1115 |
| Total drainage | |||
| Mean / Median | 1911 / 1480 | 5802 / 2484 | 0.07212 |
| Range | 300 – 6640 | 45 – 24895 | |
| Days drainage device in place | |||
| Mean/Median | 5 / 4 | 49 / 31 | < 0.00013 |
| Range | 1 – 32 | 2 – 286 | |
| Removal before death | |||
| N (%) | 29/29 (100) | 24/28 (86) | 0.0518 |
| Other survival – N (%) | |||
| Alive within 30 days | 25/29 (86) | 26/28 (93) | |
| Alive 31 – 90 days | 25/29 (86) | 19/28 (68) | 0.2616 |
| Alive beyond 90 days | 21/29 (72) | 17/28 (61) | |
| Median survival (KM estimates) | |||
| Median (days) | 147 | 147 | 0.51444 |
| 95% CI | 100 – 201 | 61 – 220 |
The definition of success is that a patient survived without effusion recurrence within 30 days, lung re-expansion ≥ 70% after desired effusion is drained, or chest tube removed before 14 days (TP only).
Applied to TP only.
p values were from Fisher’s exact 2-sided tests otherwise specified.
p value = 0.0721 was from Wilcoxon Rank Sum 2-sided test. P value = 0.0219 from linear regression analysis after adjusting for initial drainage, inpatient status at baseline, gender, whether receiving chemotherapy concurrently when enrolling to the study, and disease type (lung, breast, or other cancers). P value = 0.0969 of the same linear regression among lung and breast cancer patients only.
Wilcoxon Sum Rank two-sided test
Log-rank two-sided test
Logistic regression analysis modeled success rates based on arm, gender, admission status (inpatient versus outpatient), PS (0/1 versus 2), concurrent chemotherapy, good/poor expansion (see definition below in QOL section), dyspnea score, and initial drainage. The odds ratio for TCD success was five times higher than TP (95% confidence interval 1–23, two-sided p=0.064). Logistic regression also showed that good expansion patients achieve better success (Odds Ratio of 5, 95% confidence interval 1–25, p=0.053). All other covariates did not affect success significantly. This finding is should be interpreted with caution, because of the mere proximity to achieving significance and only 15 patients in TP and 18 patients in TCD had the complete data set for the logistic regression analysis. It should also be noted that we originally analyzed and presented our preliminary data including one TP-assigned patient that received the chest tube but not the talc. Both of the p-values presented above achieved statistical significance (p<0.05) until we removed this patient.
Pleurodesis occurred in 86.2% of TP patients as opposed to 68.0% of TCD cases (p=0.1883, but data missing on 3 SCD subjects). The assigned therapy could not be completed by the local investigator for TP and TCD cases, respectively, because of loculation (1 versus 2), failed lung expansion (2 versus 0), and chest tube/catheter occlusion (1 each). No patients died while the chest tube was in place, but 4 TCD deaths occurred with the catheter in place before pleurodesis was achieved.
Therapy-attributable complications from 63 treated patients (including 6 ineligible patients) were low but somewhat more frequent for the TCD group. Recurrent dyspnea was only seen in TP cases. One ARDS death occurred in the TCD group. Life-threatening (Grade 4) serious adverse events (SAE) were fatigue (1) and dyspnea (1) in the TP group and one myocardial infarction in the TCD cohort. One severe (grade 3) SAE for the Talc group (dyspnea) occurred compared to 6 for TCD (3 pain and one each of leukocytosis, wound infection, and neutropenia).
Quality of life analyses
The maximum lung expansion (best of post-drainage, last inpatient CXR and first clinical visit measurements) was correlated with dyspnea scores calculated at baseline – before randomization, 7 days, and 30 days post-treatment. There was no significant relationship between baseline dyspnea score and percent lung expansion at any of the 3 time points; however, 30 day assessments of dyspnea-free exercise and CXR lung expansion correlated significantly (r=0.322, p=0.0486). This relationship implies that those with a better dyspnea-free score had better lung expansion. Similarly, there was a trend toward better dyspnea scores for 29 patients whose maximal lung expansion at 30 days was ≥70% compared to the 9 whose values were less (7.8 versus 4.5, p=0.02, Wilcoxon)
Baseline measures of QOL were not predictors of baseline lung expansion or of 30 day CXR lung expansion. However, several QOL measures from the 30-day assessment were related to 30-day CXR expansion including Overall QOL from the Memorial Symptom Assessment Scale Condensed Form, PS, dyspnea score, physical function, social life, and overall QOL from the changes in function form. All of these are significant in a positive direction; that is, an increase in the QOL measure corresponds with an increased chance greater than 70% expansion at 30-day CXR.
To test the importance of lung trapping, the median value of early re-expansion estimates (maximum value of post-drainage, last inpatient, and first clinical visit) in TP was 80%, and 88% in TCD. “Good” and “Poor” expansion cohorts were defined by expansion above/below these medians respectively. TCD patients had better 30-day effusion control than TP after adjusting for good expansion cases (92% vs 81%) and especially poor expansion (77 vs. 33%, p=0.026, Cochran-Mantel-Haenszel association test). Reanalyzing the results using 70% expansion as the cut-point produced a similar results.
Multivariate regression analysis revealed that TCD had better dyspnea scores than TP (8.5 versus 6.1, p= 0.047) after adjusting for baseline dyspnea score, initial drainage, gender, inpatient status, and patient PS at baseline. Further analysis showed that this statistical difference was driven by scores from the poor expansion group (9.0 versus 4.9, p=0.033) but not by the good expansion group (8.6 versus 8.5, p-value = 0.949) again noting the benefits of TCD particularly for those with trapped lungs.
Discussion
Despite its limitations, this study supports TCD as an effective alternative to TP and suggests that it may be better in certain circumstances. The use of intrapleural talc has been a concern of investigators who noted problems with idiosyncratic immediate respiratory distress and the autopsy findings that patients demonstrate systemic distribution of talc after pleural administration10–18. While immediate ARDS was not associated with talc in this series, severe dyspnea was seen in two cases.
In CALGB 9334, 486 patients randomized to thoracoscopic poudrage or chest-tube talc slurry had similar success (60% versus 53%, respectively) but more respiratory complications occurred in those undergoing general anesthesia for thoracoscopy. Those patients also suffered significant 30-day mortality (17.1% versus 10.3% current), This indicates our difficulty predicting longevity in such patients as well as the pre-terminal nature of MPE. Similarly, many patients did not achieve >90% expansion (24% in 9334 versus 64% current, although the latter is probably a semantic issue, see explanation in “post-hoc” section above). Since predicting expansion is unreliable and does not influence overall relief of dyspnea and QOL, perhaps it should not be used for future studies until we develop better predictors of lung expansion.
In 9334 and other studies of pleurodesis agents, adverse events caused by sclerosant inflammation may be difficult to distinguish from the natural sequelae suffered by frail, deteriorating patients. A pleurodesis alternative (TCD) has become popular only over the past decade. In fact, TCD commonly achieves pleurodesis by maintaining apposition of pleura inflamed by catheter or tumor effects.
While one phase III randomized multicenter trial suggested equivalency between the PleurX™ catheter and chest tube drainage with doxycycline pleurodesis, no similar comparison trials between TCD and chest tube with talc pleurodesis have been reported.19 A retrospective review of inpatient and outpatient use of a pleural catheter and inpatient chest tube showed reduced short-term cost in outpatient catheter usage20. There was about a 19% incidence in complications related to use of pleural catheters, mostly related to device failure, and a 4% incidence of infection. More recently, some early device occlusive failures have been addressed by instilling fibrinolytic agents. Hospital stay data were not collected in this current study because TCD is a proven outpatient procedure but it may have been interesting to see if it speeded discharges for those randomized as inpatients.
The current study supports the findings of others that TCD is preferred for patients with complicated effusions, like those where the lung may be trapped21–24. Furthermore, it should be noted that, while the use of TCD prolongs therapy, this added duration may maintain lung expansion and QOL improvement parameters better. It is reasonable to expect that protracted evacuation therapy generates better tissue coaptation by allowing time for tissue expansion, mediastinal/diaphragmatic shifts, and sealing of the pleural space; whereas this driving force is gone in several days once the chest tube is removed for the talc group. However, some patients opt away from TCD and in situations where the lung is only partially trapped, it is reasonable to use talc as was done in this study because there is evidence that it is effective in up to half of cases. This leaves TCD as a fall-back option.
This study was limited because patients often preferred inpatient (TP) or outpatient (TCD) management making randomization difficult. This required changes in the statistical analyses (already cited), increased Type I error, reduced statistical power to detect infrequent events, and yielded a sample that might not represent the overall population. Alternatively, the data in this study are valuable because randomization tends to solve problems with retrospective bias and it is not clear that another randomized investigation of these two popular approaches will be effectuated because of the inpatient/outpatient issue. This general problem of comparing newer, less invasive (or less toxic) options standard therapies is a challenge for any traditional randomized design and calls for the adoption of alternative trial designs. An entirely outpatient study with talc delivered through a small catheter might have more popular, but there is little experience delivering talc through PleurX™ catheters While there was uneven distribution of patients over the participating centers, this probably did not affect the results as the treatment methods are well established and any practice variation would have addressed by randomization. Concerns with less experience with the newer method of TCD at the low volume centers might have been a bias against it, while the patients with trapped lungs may have biased the study in its favor. An objective method to detect trapped lungs like pleural compliance determinations, could have addressed this limitation25. On the other hand, pleural compliance measurement techniques are not performed consistently in the majority of hospitals, would have required additional invasive procedures, and never have been proven feasible or uniform enough to be used in a prospective clinical trial. Similarly, the ability to predict survival or to determine whether symptoms like dyspnea are from the MPE is very difficult to determine clinically before drainage. Accordingly, there may be a better surrogate measurement than lung expansion to assess the effectiveness of these procedures. Requiring additional procedures or objective testing for this frail population would have created additional accrual challenges that would have prevented accrual. For instance, while we would have preferred a more objective and blinded assessment of the effusion recurrence endpoint; however, we stayed with the CXR review by the treating physician proven successful in 9334 because it is the clinical standard, has low level of intrusiveness, and any institutional or investigator bias in interpretation would be handled by the randomization process.
Wide practice variability exists regarding how patients at risk for malignant effusions are evaluated and treated. The uncomfortable patient often receive an expeditious chest tube, VATS assessment, or small catheter drainage as their initial procedure based on local preferences or available resources rather than guidelines based upon high quality evidence. Furthermore, suspected histologies with better prognoses (or require more tissue for pathologic review) like breast cancer or mesothelioma could trigger different approaches using VATS.
In conclusion, clinicians should consider TCD to treat MPE not only because it avoids hospitalization and stress of pleurodesis, but also because it may more predictably relieve dyspnea. In particular, it deals better with the remarkably frequent occurrence of unsuspected trapped lung in practices that do not use objective tests like pleural compliance. Given how common and morbid MPE are, further study is warranted--perhaps with an alternative study design to improve acceptance by potential volunteers.
Table 3.
Quality of Life Results from Logistic Regression Analysis with Lung Expansion Single Predictor at 30 days
| Measure | CXR Percent Lung Expansion Log odds ratio (2 sided p-value) |
Odds ratio and its 95% confidence interval |
|---|---|---|
| Distress score* | −0.4459 (0.3313) | N/A |
| Number of symptoms* | −0.0798 (0.4642) | N/A |
| Overall QOL (MSAS)* | 0.0471 (0.0095) | 1.048 (1.012, 1.086) |
| Performance Status* | 0.0772 (0.0058) | 1.080 (1.023, 1.141) |
| Dyspnea score* | 0.2678 (0.0212) | 1.307 (1.041, 1.641) |
| Physical Function | 0.7566 (0.0687) | 2.131 (1.057, 4.813) |
| Emotional State | 0.7566 (0.0687) | 2.131 (0.944, 4.813) |
| Social Life | 1.2188 (0.0150) | 3.383 (1.268, 9.029) |
| Overall QOL (CiFF) | 1.0778 (0.0121) | 2.938 (1.267, 6.816) |
Measure was also assessed at baseline and did not have a significant association with either baseline or 30 day chest roentgenogram.
CiFF Changes in Function Form; CXR – Chest roentgenogram; MSAS-Memorial Symptom Assessment Score; N/A- not applicable; QOL- Quality of life
Acknowledgments
The research for CALGB 30102 was supported, in part, by grants from the National Cancer Institute (CA31946) to the Cancer and Leukemia Group B (Richard L. Schilsky, MD, Chairman) and to the CALGB Statistical Center (Stephen George, PhD, CA33601). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
The following institutions participated in this study:
Christiana Care Health Services, Inc. CCOP, Wilmington, DE–Stephen Grubbs, M.D., supported by CA45418
Dana-Farber Cancer Institute, Boston, MA–Harold J Burstein, M.D., Ph.D., supported by CA32291
Duke University Medical Center, Durham, NC–Jeffrey Crawford, M.D., supported by CA47577
Green Mountain Oncology Group CCOP, Bennington, VT–Herbert L. Maurer, M.D., supported by CA35091
Mount Sinai Medical Center, Miami, FL–Rogerio C. Lilenbaum, M.D., supported by CA45564
Rhode Island Hospital, Providence, RI–William Sikov, M.D., supported by CA08025
Roswell Park Cancer Institute, Buffalo, NY–Ellis Levine, M.D., supported by CA59518
State University of New York Upstate Medical University, Syracuse, NY–Stephen L. Graziano, M.D., supported by CA21060
The Ohio State University Medical Center, Columbus, OH–Clara D Bloomfield, M.D., supported by CA77658
University of Illinois MBCCOP, Chicago, IL–David J Peace, M.D., supported by CA74811
University of Iowa, Iowa City, IA–Daniel A. Vaena, M.D., supported by CA47642
University of North Carolina at Chapel Hill, Chapel Hill, NC–Thomas C. Shea, M.D., supported by CA47559
University of Vermont, Burlington, VT–Steven M Grunberg, M.D., supported by CA77406
Walter Reed Army Medical Center, Washington, DC–Brendan M Weiss, M.D., supported by CA26806
Abbreviations
- CALGB
Cancer and Leukemia Group B
- CMH
Cochran-Mantel-Haenszel
- CXR
Chest roentgenogram
- ECOG
Eastern Cooperative Oncology Group
- IRB
Institutional Review Board
- MPE
Malignant Pleural Effusion
- MSAS
Memorial Symptom Assessment Score
- PS
Performance Status
- QOL
Quality of Life
- SCD
Small catheter drainage
- TP
Talc Pleurodesis
Footnotes
Meeting Presentation: Discussed poster, ASCO 2010, June 8, Chicago, IL
| Study Design |
Analysis | Primary Clinical Investigator |
Manuscript preparation |
|
|---|---|---|---|---|
| Todd L. Demmy1 | x | x | x | |
| Lin Gu2 | x | x | x | |
| Jack E. Burkhalter3 | x | x | x | |
| Eric Toloza4 | x | x | ||
| Thomas D'Amico4 | x | x | x | |
| Susan Sutherland2 | x | x | x | |
| Xiaofei Wang2 | x | x | x | |
| Laura Archer2 | x | |||
| Linda J. Veit5 | x | |||
| Leslie Kohman5 | x | x | x |
References
- 1.Marel M, Zrustova M, Stasny B, et al. The incidence of pleural effusion in a well-defined region. Epidemiologic study in central Bohemia. Chest. 1993;104:1486–1489. doi: 10.1378/chest.104.5.1486. [DOI] [PubMed] [Google Scholar]
- 2.Putnam JB, Jr, Walsh GL, Swisher SG, et al. Outpatient management of malignant pleural effusion by a chronic indwelling pleural catheter. Ann Thorac Surg. 2000;69:369–375. doi: 10.1016/s0003-4975(99)01482-4. [DOI] [PubMed] [Google Scholar]
- 3.Putnam JB, Jr, Light RW, Rodriguez RM, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer. 1999;86:1992–1999. [PubMed] [Google Scholar]
- 4.Bresticker MA, Oba J, LoCicero J, III, et al. Optimal pleurodesis: a comparison study. Ann Thorac Surg. 1993;55:364–366. doi: 10.1016/0003-4975(93)90998-w. [DOI] [PubMed] [Google Scholar]
- 5.Dresler CM, Olak J, Herndon JE, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest. 2005;127:909–915. doi: 10.1378/chest.127.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartman DL, Gaither JM, Kesler KA, et al. Comparison of insufflated talc under thoracoscopic guidance with standard tetracycline and bleomycin pleurodesis for control of malignant pleural effusions. J Thorac Cardiovasc Surg. 1993;105:743–747. [PubMed] [Google Scholar]
- 7.Zimmer PW, Hill M, Casey K, et al. Prospective randomized trial of talc slurry vs bleomycin in pleurodesis for symptomatic malignant pleural effusions. Chest. 1997;112:430–434. doi: 10.1378/chest.112.2.430. [DOI] [PubMed] [Google Scholar]
- 8.Dresler CM, Olak J, Herndon JE, et al. Phase III intergroup study of talc poudrage vs talc slurry sclerosis for malignant pleural effusion. Chest. 2005;127:909–915. doi: 10.1378/chest.127.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Putnam JB, Jr, Walsh GL, Swisher SG, et al. Outpatient management of malignant pleural effusion by a chronic indwelling pleural catheter. Ann Thorac Surg. 2000;69:369–375. doi: 10.1016/s0003-4975(99)01482-4. [DOI] [PubMed] [Google Scholar]
- 10.Brant A, Eaton T. Serious complications with talc slurry pleurodesis. Respirology. 2001;6:181–185. doi: 10.1046/j.1440-1843.2001.00327.x. [DOI] [PubMed] [Google Scholar]
- 11.de Campos JR, Vargas FS, de Campos WE, et al. Thoracoscopy talc poudrage : a 15-year experience. Chest. 2001;119:801–806. doi: 10.1378/chest.119.3.801. [DOI] [PubMed] [Google Scholar]
- 12.Kennedy L, Harley RA, Sahn SA, et al. Talc slurry pleurodesis. Pleural fluid and histologic analysis. Chest. 1995;107:1707–1712. doi: 10.1378/chest.107.6.1707. [DOI] [PubMed] [Google Scholar]
- 13.Light RW. Talc should not be used for pleurodesis. Am J Respir Crit Care Med. 2000;162:2024–2026. doi: 10.1164/ajrccm.162.6.pc09-00b. [DOI] [PubMed] [Google Scholar]
- 14.Maskell NA, Lee YC, Gleeson FV, et al. Randomized trials describing lung inflammation after pleurodesis with talc of varying particle size. Am J Respir Crit Care Med. 2004;170:377–382. doi: 10.1164/rccm.200311-1579OC. [DOI] [PubMed] [Google Scholar]
- 15.Rossi VF, Vargas FS, Marchi E, et al. Acute inflammatory response secondary to intrapleural administration of two types of talc. Eur Respir J. 2009 doi: 10.1183/09031936.00039209. [DOI] [PubMed] [Google Scholar]
- 16.Stamatelopoulos A, Koullias G, Arnaouti M, et al. Malignant pleural effusion and talc pleurodesis. Experimental model regarding early kinetics of talc particle dissemination in the chest after experimental talc pleurodesis. J BUON. 2009;14:419–423. [PubMed] [Google Scholar]
- 17.Warren W. Talc pleurodesis for malignant pleural effusions is preferred over the pleurx catheter (contrary position) Ann Surg Oncol. 2007;14:2700–2701. doi: 10.1245/s10434-006-9158-x. [DOI] [PubMed] [Google Scholar]
- 18.Werebe EC, Pazetti R, Milanez dC, Jr, et al. Systemic distribution of talc after intrapleural administration in rats. Chest. 1999;115:190–193. doi: 10.1378/chest.115.1.190. [DOI] [PubMed] [Google Scholar]
- 19.Putnam JB, Jr, Light RW, Rodriguez RM, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer. 1999;86:1992–1999. [PubMed] [Google Scholar]
- 20.Putnam JB, Jr, Light RW, Rodriguez RM, et al. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer. 1999;86:1992–1999. [PubMed] [Google Scholar]
- 21.Efthymiou CA, Masoudi TI, Thorpe JA, et al. Malignant pleural effusion in the presence of trapped lung. Five-year experience of PleurX tunnelled catheters. Interact Cardiovasc Thorac Surg. 2009 doi: 10.1510/icvts.2009.211516. [DOI] [PubMed] [Google Scholar]
- 22.Murthy SC, Okereke I, Mason DP, et al. A simple solution for complicated pleural effusions. J Thorac Oncol. 2006;1:697–700. [PubMed] [Google Scholar]
- 23.Sioris T, Sihvo E, Salo J, et al. Long-term indwelling pleural catheter (PleurX) for malignant pleural effusion unsuitable for talc pleurodesis. Eur J Surg Oncol. 2009;35:546–551. doi: 10.1016/j.ejso.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 24.van den Toorn LM, Schaap E, Surmont VF, et al. Management of recurrent malignant pleural effusions with a chronic indwelling pleural catheter. Lung Cancer. 2005;50:123–127. doi: 10.1016/j.lungcan.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 25.Huggins JT, Sahn SA, Heidecker J, et al. Characteristics of trapped lung: pleural fluid analysis, manometry, and air-contrast chest CT. Chest. 2007;131:206–213. doi: 10.1378/chest.06-0430. [DOI] [PubMed] [Google Scholar]


