Abstract
Osteoarthritis (OA) is a widespread chronic joint disease characterized by articular cartilage destruction and accompanied by pain and disability. In this study, we found that the expression of Insulin-like Growth Factor II (IGF-II) was reduced in articular cartilage in human OA patients as well as in the murine experimental OA model of destabilization of the medial meniscus (DMM). In primary human articular chondrocytes, ectopic expression of lentiviral IGF-II inhibited pro-inflammatory cytokine IL-1β-induced NF-κB activation as well as catabolic gene expression. Interestingly, IGF-II did not significantly alter the phosphorylation states of ERK1/2 or Akt, which are kinases typically activated by IGF-I. Instead, it induced the activity of phospholipase C (PLC) and a PLC inhibitor blocked the inhibitory activity of IGF-II against IL-1β, suggesting that this activity is mediated through PLC. Furthermore, IGF-II increased cartilage matrix levels and decreased MMP13 protein expression in explanted human OA cartilage cultures in vitro. In the in vivo DMM model, intraarticular injection of lentiviral IGF-II led to enhanced cartilage matrix levels and decreased MMP13 protein expression, as well as reduced osteophyte formation and subchondral bone sclerosis. Therefore, our results suggest that IGF-II can promote cartilage integrity and halt knee joint destruction in OA.
Keywords: IGF-II, OSTEOARTHRITIS (OA), IL-1β, NF-κB, CARTILAGE, KNEE JOINT, DMM
Osteoarthritis (OA) is a widespread chronic joint disease that leads to long-term disability [Goldring, 2012; Loeser et al., 2012]. Characteristics of OA include pathological changes in joint cartilage such as matrix loss and chondrocyte cell death, changes in the synovium such as synoviocyte hyperproliferation and inflammatory cell infiltration (synovitis), as well as changes in the subchondral bone such as increased thickness of the subchondral bone plate (sclerosis) and the formation of ectopic bone along joint margins (osteophytes) [Loeser et al., 2012; Kim et al., 2014; Thysen et al., 2015]. Despite awareness of these structural changes in the joint, current treatments for OA primarily focus on pain relief, and there is no satisfactory therapy available to protect the integrity of cartilage and other joint structures.
IGF-I and IGF-II are insulin-like growth factors that regulate cartilage gene expression [Starkman et al., 2005; Hamamura et al., 2008]. IGF-I is a known anabolic factor for chondrocytes and regulates skeletal development in the embryo [Starkman et al., 2005; Tahimic et al., 2013]. To determine its effect on cartilage matrix destruction in vitro, several groups have treated chondrocytes with pro-inflammatory cytokine IL-1β, which induces catabolic changes in chondrocytes [Daheshia and Yao, 2008; Jotanovic et al., 2012; Burleigh et al., 2012; Furman et al., 2014]. Indeed, IGF-I has the ability to reduce IL-1β-induced cartilage matrix reduction in vitro and exhibits a synergistic effect with other factors [Montaseri et al., 2011]. However, the effects of IGF-I on articular cartilage in vivo in OA have not been reported. This might be related to the fact that IGF-I has been shown to be upregulated rather than downregulated in the synovial fluid and articular cartilage in OA [Matsumoto et al., 1996], implying that supplying additional IGF-I into the OA joint may not be an effective treatment method.
IGF-II is also expressed in cartilage; however, unlike IGF-I, the role of IGF-II on cartilage matrix maintenance under inflammatory and OA conditions has not been widely explored. In this report, we show that the expression of IGF-II, but not IGF-I, is reduced in human OA articular cartilage where the expression of endogenous IL-1β is increased. Lentiviral IGF-II administration in primary articular chondrocytes inhibits IL-1β-induced NF-κB activation and catabolic gene expression. Additionally, the inhibitory effect of IGF-II on catabolic genes is dependent on phospholipase C (PLC) activity. Furthermore, IGF-II enhances matrix levels in human OA cartilage explant cultures and intraarticular injection of lentiviral-IGF-II promotes articular cartilage integrity and prevents osteophyte formation and subchondral bone sclerosis in the destabilization of the medial meniscus (DMM) murine OA model.
MATERIALS AND METHODS
ISOLATION OF NORMAL AND OA HUMAN CARTILAGE SPECIMENS
Normal human articular cartilage slices were isolated from the tibial plateau of cadaveric joints (National Disease Research Interchange and Articular Engineering age/sex: 84/M, 75/M, 65/F). Human OA tibial plateau was obtained from patients undergoing total knee replacement surgery at Tufts Medical Center (age/sex: 53/F, 63/F, 65/F, 73/F, 72/M). Each specimen was divided into two groups with three samples for each group. One group was subjected to histological analysis to confirm the integrity of the articular cartilage using the Mankin scoring system [van der Sluijs et al., 1992]. The other group was subjected to gene expression analysis by RT-PCR (see details below).
IN VITRO CULTURING OF HUMAN ARTICULAR CHONDROCYTES AND CARTILAGE EXPLANTS
For in vitro culturing of normal human articular chondrocytes, primary normal human articular chondrocytes (nHAC) derived from the knee joints of three donors (age/sex: 34M, 49M, and 38M) were purchased from Lonza. Before use, nHAC were re-differentiated per manufacturer instructions. Briefly, nHAC were encapsulated in 1.2% alginate beads at a density of 8 × 105 cells/ml and cultured in chondrocyte differentiation media (CDM) containing TGF-β1 (Lonza) supplemented with ascorbic acid for 3 to 4 weeks. Alginate beads were dissolved in 55 mM EDTA supplemented with 10 mM HEPES to collect re-differentiated nHAC. Cell viability was confirmed by MTT assay (Sigma). Usually more than 80% cell viability was obtained. Re-differentiation of chondrocytes was confirmed by elevated Col-II mRNA expression and alcian blue staining before proceeding. For viral infection, re-differentiated nHAC were infected with lentiviral-human IGF-II or GFP (Open Biosystems, titer 108 IFU/ml) for 2 days and then cultured in CDM media (Lonza) supplemented with ascorbic acid. For IL-1β treatment, 1 ng/ml IL-1β (Peprotech) was added. In the phospholipase C (PLC) study, PLC inhibitor U73122 and its inactive analog U73343 (Tocris) were used at the concentration of 1 μM. For NF-κB inhibition, the NF-κB inhibitor JSH-23 (Calbiochem) was used at the concentration of 10 μM. For Western blot analysis, cells were lysed after 1hr of IL-1β treatment. For RT-PCR analysis, cells were lysed after 4 days of IL-1β treatment.
For in vitro culturing of OA patient-derived primary articular chondrocytes (OA-HAC), chondrocytes from three of the five OA donors described above were used (53/F, 63/F, and 65/F). These chondrocytes were isolated by enzymatic digestion with 1 h pronase treatment followed by overnight collagenase P digestion (Roche) [Otero et al., 2012]. After the digestion, OA-HAC at passage 0 were immediately seeded and cultured in the absence or presence of IGF-II (1 and 10 ng/ml, Peprotech) for 4 days. Culture medium consists of DMEM supplemented with 10% FBS (Hyclone) and 1% Antibiotic-Antimycotic (Invitrogen). Triplicate experiments were performed for each condition and experiments were repeated for all three donors.
For ex vivo culturing of OA cartilage slices, OA cartilage pieces were prepared from three of the five OA donors described above (53/F, 63/F, 65/F). Cartilage slices were cut into 2 × 3 × 2 mm3 and cultured in the presence or absence of 10 ng/ml IGF-II (Peprotech) for 3 weeks with media changes every 3 days. Explant cultures were performed in triplicate for each donor, and repeated for all three donors.
ELISA ASSAY FOR IGF-II PROTEIN LEVELS AFTER LENTIVIRUS INFECTION
Conditioned media was harvested from lenti-IGF-II and lenti-GFP-infected nHAC at 3 days post-infection and IGF-II protein in the media was measured by an ELISA detection kit (R&D). Briefly, samples were centrifuged at 1,000 rpm to remove cell debris before the assay. IGF-II protein was captured by goat anti-IGF-II antibody (R&D) and detected by rabbit anti-IGF-II antibody (Abcam) followed by incubation with biotin-conjugated anti-rabbit antibody (Vector lab). Samples were incubated with the provided substrate (R&D) and developed with Streptavidin-HRP (R&D). A standard curve was generated with human recombinant IGF-II protein (Peprotech) and the concentration of IGF-II protein was determined.
EXPERIMENTAL ANIMALS AND IN VIVO MANIPULATIONS
All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee at Tufts University. Wild-type CD1 male mice were purchased from Charles River Laboratories. Mice were caged in groups under the standard conditions with a 12 h light/dark cycle and standard chow diet. Destabilization of the medial meniscus (DMM) surgery was performed on 7-week-old CD1 male mice according to the established protocol [Glasson et al., 2007]. Briefly, under isoflurane anesthesia, an incision was made along the medial border of the patellar ligament to open the right knee joints (i.e., the stifle joints in mice), and the anterior medial meniscotibial ligament (MMTL) was severed. For sham surgery, MMTL from the left knee joints was visualized but not severed. Mice were monitored for pain and infection post-surgery and allowed unrestricted movement. At least six mice were used for each group. Five microliters of lentiviral-IGF-II or lentiviral-GFP (Open Biosystems, titer 108 IFU/ml) were injected intraarticularly into the mouse knees at two time points: at 1 and 2 weeks post-surgery. All mice were sacrificed at 7 weeks post-surgery.
HISTOLOGICAL ANALYSIS
Mouse knee joints were fixed with 4% PFA overnight, decalcified with 0.33M EDTA, embedded in paraffin and sectioned sagittally at 5 μm thickness. Serial sections were obtained from the medial edge to the midline of knee. Six IGF-II and six GFP lentivirus-injected mice were evaluated after DMM surgery. Human cartilage slices were fixed with 4% PFA, decalcified with 0.33 M EDTA and paraffin sectioned at 5 μm thickness. All sections were stained with 0.1% Safranin O and counterstained with Hematoxylin and Fast green. After staining, bright-field images were taken under the Olympus IX71 inverted microscope and Olympus DP70 digital camera and associated software. Three to four sections of each mouse knee joint and human cartilage slice within 100 μm intervals were then evaluated.
Cartilage destruction was evaluated by using the OARSI scoring system and by analyzing percentage loss of Safranin O staining and chondrocyte cell loss at the articular surface [Glasson et al., 2010]. The software ImageJ (NIH) was used to delineate areas of cartilage and Safranin O staining loss [Singh S, 2013]. Chon-drocyte cell density (cellularity) was determined by quantifying the total number of cells per unit area based on the Hematoxylin and Fast green staining results. Lacunae with no occupancy of chondrocytes were not taken into account [Loeser et al., 2012]. ImageJ was also used to delineate osteophytes and to measure their sizes [Loeser et al., 2012]. Severity of synovitis was scored by the established method [Krenn et al., 2006; Matsukura et al., 2015]. This scoring system has the following three criteria: enlargement of the synovial cell layer (0–3 points), cellularity of resident cells (0–3 points), and inflammatory infiltration (0–3 points). When the points are totaled, a full score of nine points would indicate severe synovitis with lower scores indicating less severe synovitis. The extent of subchondral bone sclerosis was determined by the ratio of subchondral bone plate area to total subchondral bone area delineated by ImageJ [Thysen et al., 2015].
For immunohistochemistry (IHC) of collagen II and MMP13, enzymatic antigen retrieval was performed using 0.3% hyaluronidase and 0.15% trypsin (Invitrogen) at 37°C for 10 min. For NF-κB/p65, IGF-II and GFP IHC staining, heat-induced antigen retrieval in 10 mM citric acid buffer at pH 6.0 was performed. The primary antibodies are: mouse anti-collagen II (Generous gift from Dr. Linsenmayer, Tufts University), rabbit anti-IGF-II (Abcam, ab9574), mouse anti-MMP13 (Abcam, VIIIA2), chicken anti-GFP (Abcam, ab3970), rabbit anti-NF-κB/p65 (Cell Signaling, E498), and goat anti-IGF-II (R&D, AF792). The secondary antibodies are: biotin conjugated goat/horse anti-mouse/rabbit/chicken antibodies (Vector laboratories). For chromogenic staining, the Vectastain ABC Elite kit and M.O.M. kit (Vector Laboratories) were used according to the manufacturer’s instruction. IHC sections were counterstained with Methyl green.
RT-QPCR ANALYSIS
Total RNA was isolated using the RNeasy mini kit (Qiagen) and reverse transcribed using M-MLV reverse transcriptase (Invitrogen). Quantitative PCR was carried out using iTaq universal SYBR Green supermix on the iQ5 Real Time PCR Detection System (BioRad). TATA binding protein (TBP) was used as a reference gene. Primer sequences for qPCR are listed in supplemental Table S1.
WESTERN BLOT ANALYSIS
Chondrocytes were lysed using the standard RIPA buffer. Nuclear and cytoplasmic fractions were obtained using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce) according to manufacturer instructions. Protein concentrations were determined using the DC™ Protein Assay (Bio-Rad). Total protein (10 μg), cytoplasmic protein (10 μg), and nuclear protein (10 μg) were loaded. Primary and secondary antibodies were: rabbit anti-Akt (C73H10), rabbit anti-phospho-Akt (Thr308, C31E5E), rabbit anti-phospho-Akt (Ser473, D9E), rabbit anti-ERK1/2, rabbit anti-phospho-ERK1/2 (Thr202/Tyr204), rabbit anti-IGF-II (Abcam, ab9574), mouse anti-α-tubulin (DSHB, 12G10), and goat anti-rabbit or mouse IgG, (H+L) HRP conjugate (Millipore/Chemicon).
PHOSPHOLIPASE C (PLC) ACTIVITY ASSAY
PLC activity assay was performed using total protein lysate of human articular chondrocytes after 3 days of lentiviral IGF-II and lentiviral GFP infection. Two types of PLC activities were assessed. The PC-PLC activity, which releases inositol 1,4,5-trisphosphate (IP3) and 1,2-diacylglycerol (DAG), was determined using the EnzChek direct phospholipase C assay kit (Invitrogen). The PI-PLC activity, which releases phosphocholine and DAG, was assayed using an established protocol [Burg-Golani et al., 2013]. Briefly, 1 μg of total protein lysate diluted in 100 μl water was mixed with 100 μl of assay solution (0.036 g phosphatidyl-inositol in 1 ml of 58 mM sodium-cholate, 10 mM CaCl2, and 7 ml of 0.15M NaCl) and incubated at 37°C overnight. Then the photo-absorbance at 510 nm was measured as the relative PI-PLC activity. Three independent experiments were performed in triplicate.
NF-κB TRANSACTIVATION ASSAY
Seventy nanogram of a six-copy NF-κB element-driven luciferase reporter construct (generous gift from Dr. Sonenshein, Tufts University) and 10 ng of Renilla luciferase internal control were transiently co-transfected into nHAC using X-tremeGENE HP (Roche). Twenty-four hours after transfection, chondrocytes were treated with 1 ng/ml IL-1β for 16 h. Luciferase assay was conducted using the Dual Luciferase Assay kit (Promega). NF-κB luciferase activity was normalized to Renilla luciferase activity. Fold induction of normalized luciferase activity was determined based on the control sample (GFP) as a baseline. At least three independent experiments in triplicate were performed.
STATISTICAL ANALYSIS
Data are reported as mean ± standard deviation or box-and-whisker plot. For parametric data, statistical analysis was performed using a Student’s t-test or one-way analysis of variance followed by post hoc Tukey test with P-values of <0.05 considered significant. For semi-quantitative ordinal scoring systems (as with the OARSI scores), nonparametric statistical analysis was performed using the Kruskal–Wallis test followed by Mann–Whitney U test with Bonferroni correction. P-values of <0.05 are considered significant. All statistical analyses were conducted with GraphPad Prism (Graph Pad, San Diego, CA).
RESULTS
IGF-II MRNA EXPRESSION IS REDUCED IN OA CHONDROCYTES
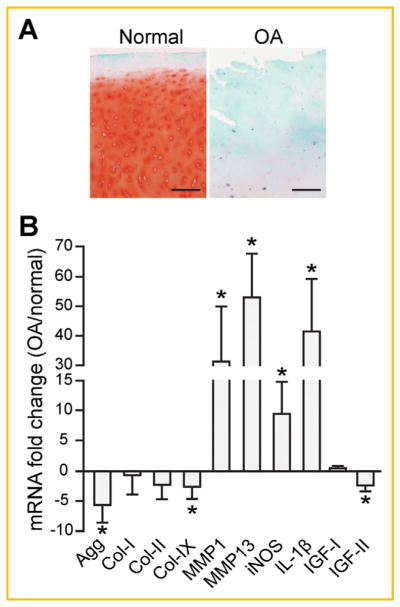
To ascertain IGF expression in OA cartilage, we performed RT-qPCR analysis using cartilage specimens obtained from the tibial plateaus of three normal healthy cadaveric donors and five OA patients undergoing total knee replacement surgery. Portions of the cartilage specimens were sectioned and stained with Safranin O to assess the integrity of the cartilage and scored using the modified Mankin scoring system (Fig. 1A). When these specimens were analyzed, it was evident that human OA chondrocytes had lower mRNA levels of cartilage matrix genes (Agg, Col-IX) and higher expression levels of cartilage matrix degradation related genes (MMPs, iNOS) (Fig. 1B). Furthermore, the level of pro-inflammatory cytokine IL-1β was also dramatically increased in OA cartilage (Fig. 1B). IGF-I expression was not significantly different between the normal and OA specimens, while IGF-II gene expression was reduced in OA cartilage (Fig. 1B).
Fig. 1.
IGF-II mRNA expression is reduced in OA cartilage. (A) Articular cartilage pieces isolated from the tibial plateau of normal and OA patients were sectioned and stained for Safranin O and counterstained with Hematoxylin and Fast green. The Mankin scores for specimens from normal donors (age/sex) are: 84/M = 1, 75/F = 2, 65/F = 3. The Mankin scores for cartilage specimens from OA donors (age/sex) are: 72/M = 8, 53/F = 6, 63/F = 7, 65/F = 9, 73/F = 7. Representative images from sections of donor 65/F (normal) and 65/F (OA) cartilage are shown. Scale bar = 200 μm. (B) RT-qPCR analysis on cartilage matrix genes and catabolic genes in cartilage specimens isolated from normal and OA patients. TATA Binding Protein (TBP) served as a reference gene. Data are reported as mean ± standard deviation from all three normal healthy donors and five OA patients. *P <0.05, healthy donors versus OA patients.
IGF-II PROMOTES CARTILAGE MATRIX GENE EXPRESSION AND INHIBITS NF-κB ACTIVATION IN NORMAL HUMAN CHONDROCYTES
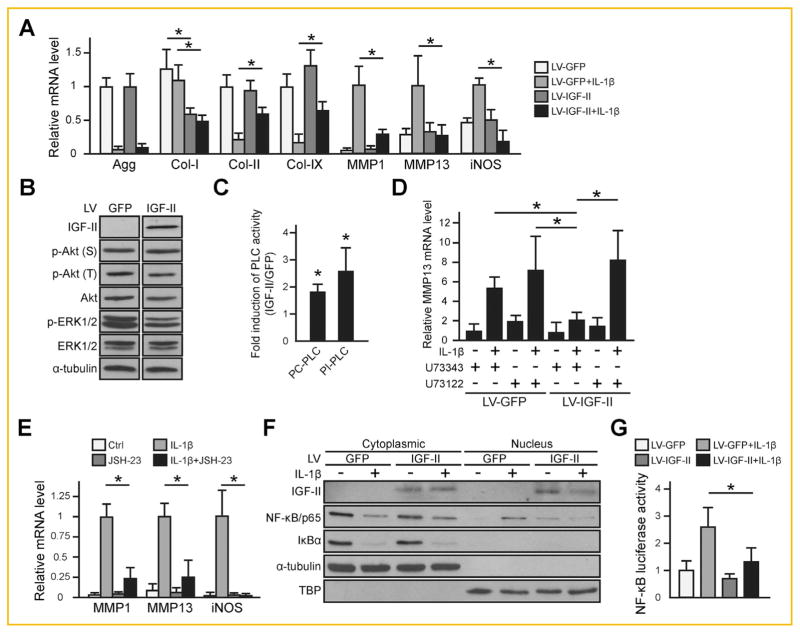
Since IL-1β mRNA expression level was increased and IGF-II mRNA expression level was reduced in OA articular cartilage, we tested whether IGF-II could antagonize IL-1β activity in primary normal human articular chondrocytes by utilizing lentiviruses encoding human IGF-II or GFP control. We first confirmed the production of IGF-II protein by performing ELISA, which showed 1–2 ng/ml of IGF-II in the media from lentiviral IGF-II-infected normal primary human articular chondrocytes, while only 0.1–0.2 ng/ml of endogenous IGF-II was detected in the medium obtained from lentiviral GFP-infected chondrocytes (data not shown). As expected, IL-1β downregulated Aggrecan (Agg), Col-II and Col-IX mRNA expression, and upregulated MMP1, MMP13 and iNOS mRNA expression. Co-treatment of IL-1β with IGF-II restored Col-II and Col-IX expression and inhibited Col-I, MMPs and iNOS induction, but did not affect Agg expression (Fig. 2A).
Fig. 2.
IGF-II promotes cartilage matrix expression and inhibits IL-1β-induced NF-κB/p65 activity in normal human articular chondrocytes (nHAC). For all experiments, nHAC were first infected with lentiviral IGF-II (LV-IGF-II) or GFP (LV-GFP) for 2 days before they were treated with PBS control or 1 ng/ml IL-1β. (A) RT-qPCR gene expression analysis after 4 days of IL-1β treatment. All samples treated with IL-1β exhibited significant differences as compared to their untreated counterpart in LV-GFP-treated samples. Statistical significances between LV-IGF-II and LV-GFP-treated samples were indicated by “*”. (B) Western blot analysis of phosphorylation status of Akt and ERK1/2. α-tubulin served as a loading control. (C) PLC activity assay. Two types of PLC activities were assessed: the PC-PLC activity, which releases inositol 1,4,5-trisphosphate (IP3) and 1,2-diacylglycerol (DAG); and the PI-PLC activity, which releases phosphocholine and DAG. Data were presented as fold induction of lentiviral IGF-II samples compared with the lentiviral GFP control. (D) Assessment of the effect of a pan-PLC inhibitor U73122 (1 μM) on MMP13 mRNA expression after IL-1β treatment for 4 days. As a control for U73122 (1 μM), an inactive analog U73343 (1 μM) was used. (E) RT-qPCR assessment of the effect of NF-κB inhibitor JSH-23 on IL-1β-induced MMP13 expression after IL-1β treatment for 4 days. (F) Western blot analysis of IκBα degradation and localization of NF-κB/p65 after IL-1β treatment for 1 h. Cytoplasmic and nuclear proteins were blotted for IκBα and NF-κB/p65. α-tubulin was used as a loading control for cytoplasmic proteins and TBP was used as a loading control for nuclear proteins. (G) NF-κB transactivation assay after IL-1β treatment for 16hr. Renilla luciferase activity served as an internal control. For quantification, NF-κB luciferase activity was normalized to Renilla luciferase activity. For all qPCR analysis, TBP was used as a reference gene. Data are reported as mean ± standard deviation from at least three independent experiments in triplicates. *P <0.05. LV = lentivirus.
To investigate the mechanisms by which IGF-II functions, we performed Western blot analysis on kinases Akt and ERK1/2 as they have been implicated in IGF-I signaling and cartilage gene expression regulation [McMahon et al., 2008; Zhang et al., 2009; Montaseri et al., 2011]. However, we did not observe any significant differences in the phosphorylation states of these kinases in IGF-II and GFP-treated samples (Fig. 2B), suggesting that IGF-II may function differently from IGF-I [Starkman et al., 2005]. Apart from those kinases, phospholipase C (PLC) has been reported to act downstream of IGF-I and IGF-II [Foncea et al., 1997; Maeng et al., 2009]. We thus assayed for PLC activity in lentiviral IGF-II and lentiviral GFP-infected chondrocytes. Two types of PLC activities were assessed: the PC-PLC activity, which releases inositol 1,4,5-trisphosphate (IP3) and 1,2-diacylglycerol (DAG) and the PI-PLC activity, which releases phosphocholine and DAG [Burg-Golani et al., 2013]. We found that IGF-II induced both PI and PC-PLC activities (Fig. 2C). In the presence of a pan-PLC inhibitor U73122 [Shen et al., 2013], IGF-II no longer prevented IL-1β-induced MMP13 expression in these human articular chondrocytes. In contrast, an inactive analog of PLC inhibitor, U73343, could not prevent IGF-II from inhibiting MMP13 mRNA expression (Fig. 2D), suggesting that PLC mediates IGF-II activity in this respect.
To determine how IL-1β signaling is perturbed by IGF-II, we investigated NF-κB activation, as NF-κB is a master mediator of this inflammatory stimulus [Marcu et al., 2010]. Indeed, we confirmed in our system that, JSH-23, an inhibitor that prevents NF-κB translocation into the nucleus, dramatically inhibited IL-1β-induced catabolic gene expression (Fig. 2E). Considering the importance of NF-κB in the signaling cascade of IL-1β, we then tested whether IGF-II inhibited IL-1β-induced NF-κB activation. We found that while IL-1β treatment reduced cytoplasmic IκBα protein and increased nuclear NF-κB subunit p65, IGF-II co-treatment dramatically inhibited IL-1β-induced NF-κB nuclear localization (Fig. 2F). To confirm that IGF-II led to a reduction in NF-κB signaling, we transfected chondrocytes with an NF-κB luciferase reporter construct. As expected, IL-1β significantly induced NF-κB luciferase activity in lentiviral GFP-infected cells, but this induction was inhibited upon lentiviral IGF-II treatment (Fig. 2G), indicating that IGF-II inhibited IL-1β-induced NF-κB activation.
IGF-II ENHANCES CARTILAGE MATRIX LEVELS IN HUMAN OA CHONDROCYTES
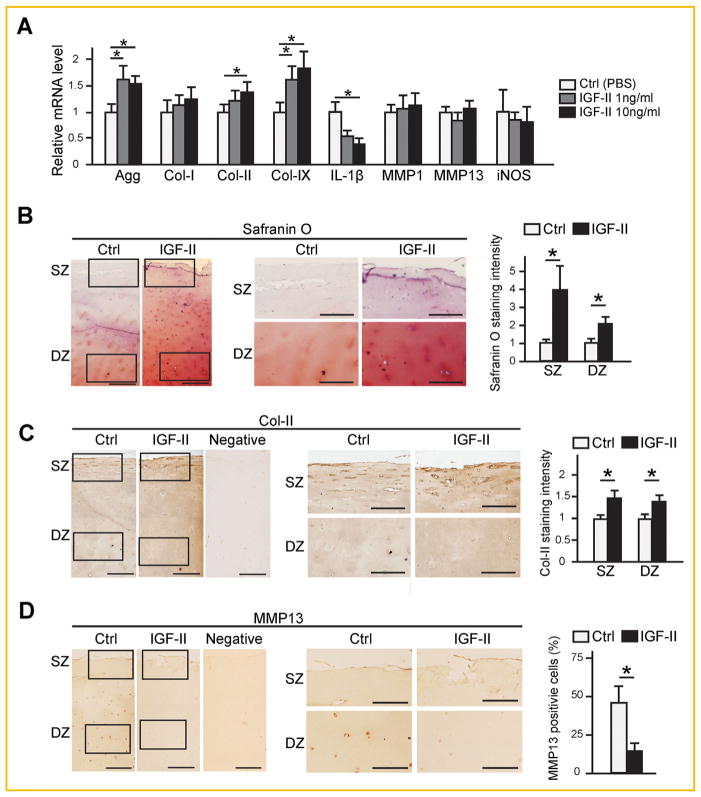
After establishing that IGF-II inhibits IL-1β-induced catabolic events in normal human chondrocytes, we investigated the effect of IGF-II on OA chondrocytes, which, as shown in Fig. 1, have reduced cartilage matrix gene expression (Col II, Col IX, Agg) and increased catabolic genes expression (IL-1β, MMPs, iNOS), compared to normal chondrocytes. We found that increasing levels of IGF-II led to elevated expression of cartilage matrix genes Agg, Col-II and Col-IX, and reduced the mRNA level of IL-1β (Fig. 3A). However, there was no effect on the expression of MMP13, MMP1 and iNOS (Fig. 3A). We considered that it is possible a longer culture time is necessary to elicit a difference in these genes. However, as these OA chondrocytes were already senescent and could not sustain a longer culture period without significant cell loss and de-differentiation, we opted to use OA cartilage in an explant culture system that allows a longer culture time, a system that has been used by other investigators, to further test the effect of IGF-II on OA chondrocytes [Sondergaard et al., 2006; Temple et al., 2006]. We found that IGF-II-treated OA cartilage explants exhibited much stronger Safranin O staining and higher levels of Col-II in both the superficial zone (SZ) and deep zone (DZ) compared to PBS controls, which was semi-quantified using ImageJ (Fig. 3B and C). Interestingly, although very little Safranin O staining was observed in these OA specimens at the beginning of culture (Fig. 1A), we did observe substantial staining after 3 weeks of culturing, suggesting that OA chondrocytes still have the capacity to secrete cartilage matrix once placed under favorable conditions. Upon examining MMP13 protein expression, we found that it was only present at very low levels in the superficial zone after 21 days of culture, but was clearly visible around chondrocytes in the deep zone in the control sample. In contrast, lower levels of MMP13 were observed in IGF-II-treated specimens (Fig. 3D). The fact that we have observed very little MMP13 staining on the superficial zone is consistent with the appearance of higher matrix levels in the superficial zone in these cultures. These results suggest that IGF-II is capable of promoting cartilage matrix maintenance in human OA cartilage cultures.
Fig. 3.
IGF-II enhances cartilage matrix production in human OA chondrocytes. (A) RT-qPCR analysis of OA articular chondrocytes after 4 days of treatment with IGF-II at 1 and 10 ng/ml. (B) Histological analysis on sections of articular cartilage explants after 3 weeks of culturing with 10 ng/ml IGF-II. Safranin O staining was counterstained with Hematoxylin and intensity of staining was quantified by ImageJ. (C) IHC analysis for Col-II and quantification of staining intensity. (D) IHC analysis for MMP13 and quantification of MMP13-positive cells. Rectangles denote areas shown in subsequent magnified images. SZ: Superficial zone. DZ: deep zone. In C and D, negative controls from IHC with no primary antibodies are shown. Scale bar = 200 μm. Data are reported as mean ±standard deviation from three independent experiments in triplicates. *P <0.05. For all qPCR analysis, TBP was used as a reference gene.
ECTOPIC IGF-II EXPRESSION INHIBITS JOINT DESTRUCTION IN A MURINE EXPERIMENTAL OA MODEL
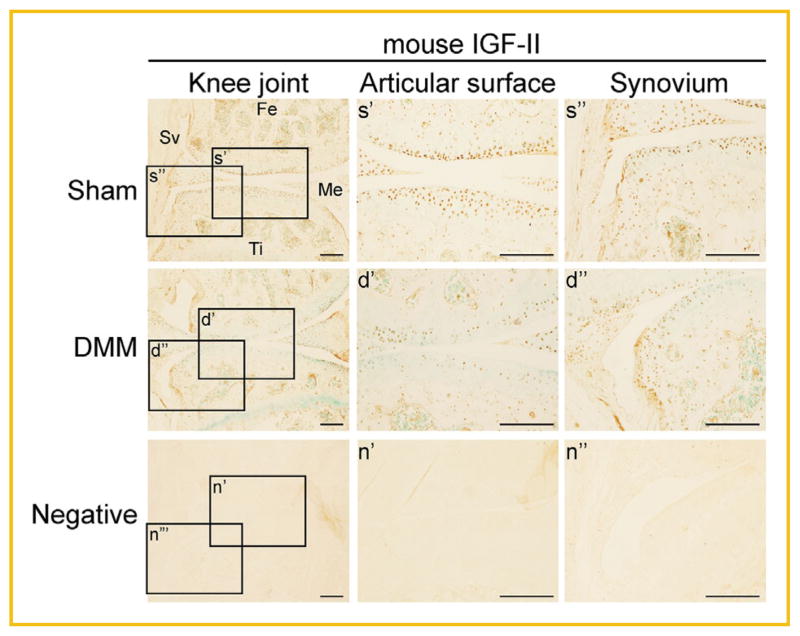
To determine the effect of IGF-II on OA joint damage in vivo, we utilized the mouse DMM (destabilization of the medial meniscus) model, which is an established OA model that mimics injury-induced OA and a system routinely used in the lab [Glasson et al., 2007; Leahy et al., 2015]. Here, the meniscotibial ligament of 7-week-old CD1 male mice was severed to destabilize the joint, causing increased mechanical stress and subsequent knee joint destruction [Glasson et al., 2007]. As a control, we performed a sham surgery on the contralateral knee in which the ligament was visualized but not severed. Normally, IGF-II protein is expressed in the articular cartilage, the meniscus and the synovium. However, upon DMM surgery, its expression seemed to be overall reduced in articular cartilage, although to a lesser degree in the synovium (Fig. 4).
Fig. 4.
Immunohistochemical analysis of endogenous IGF-II protein expression in DMM and Sham mouse knee joints. Images of IHC staining using a mouse IGF-II antibody on knee sections obtained from mice 7 weeks post-DMM or Sham surgery. Scale bar = 200 μm. Rectangles denote areas shown in higher magnification. Fe = femur, Ti = tibia, Sv = synovium, Me = meniscus. Sections incubated with no primary antibody served as a negative control for the staining.
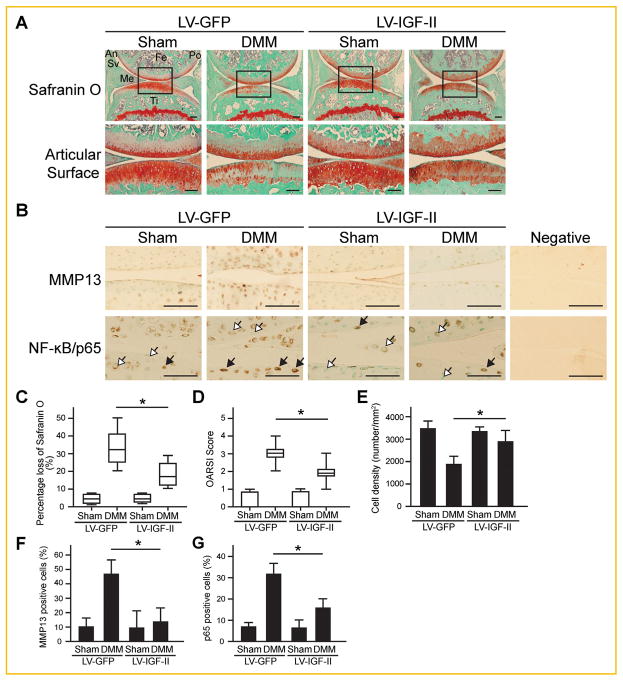
Since IGF-II expression is reduced in OA, we next determined whether ectopically introducing IGF-II into the joint was sufficient to halt joint destruction after DMM surgery. Thus, lentiviruses encoding IGF-II or GFP (control) were injected intraarticularly into Sham and DMM knee joints after the surgery. The efficiency of viral transduction was assessed by immunohistochemical (IHC) analysis (Fig. 5A and B). Lentiviral IGF-II and GFP clearly infected several layers of the articular cartilage as well as the meniscus and synovium (Fig. 5A and B). To determine the effect of ectopic IGF-II expression on articular cartilage destruction, we performed a series of histological analysis. It is evident that DMM surgery caused significant loss of Safranin O staining accompanied by elevated MMP13 and NF-κB subunit p65 expression (Fig. 6A and B). No significant differences were observed between the Sham surgery-operated knee joints injected with lentiviral-IGF-II or GFP. On the other hand, in the DMM knees, lentiviral-IGF-II injected knee joints retained more Safranin O staining and reduced MMP13 and p65 expression as compared to the joints injected with the GFP virus (Fig. 6A and B).
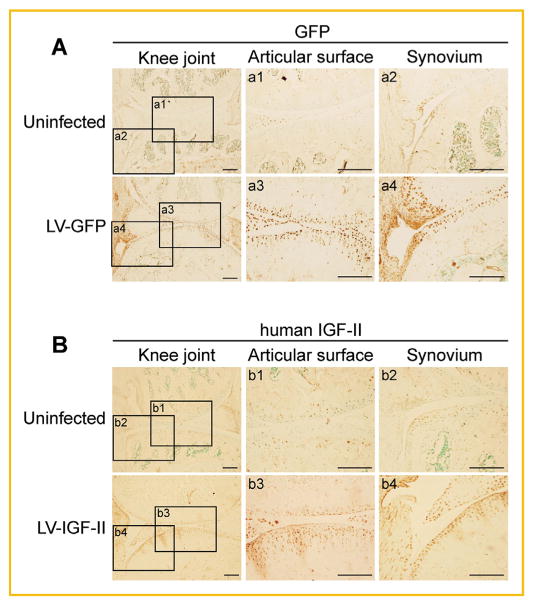
Fig. 5.
Lentiviral-IGF-II and lentiviral-GFP expression in the knee joints after intraarticular injection. Lentiviral-IGF-II and lentiviral-GFP were injected into the knee joints after sham surgery, first at 1 week and then subsequently at 2 weeks. IHC was performed at 3 weeks after the last virus injection. Images of IHC staining using (A) anti-GFP and (B) anti-human IGF-II antibodies are shown. As lentiviral-IGF-II encodes human IGF-II, an antibody of human IGF-II was used. Scale bar = 200 μm. Rectangles denote areas shown in higher magnification. LV = lentivirus.
Fig. 6.
Histological analyses of articular cartilage of post-DMM mouse knee joints injected with lentiviral-IGF-II and lentiviral-GFP. (A) Images of Safranin O staining with Hematoxylin and Fast green counterstaining. Rectangles denote areas shown in higher magnification. (B) Images of IHC analysis on the protein expression of MMP13 and NF-κB/p65. Black arrows indicate cells with high levels of nuclear NF-κB/p65 expression. Open arrows indicate cells having very low basal levels of NF-κB/p65 or showing no evidence of nuclear NF-κB/p65 staining. Scale bar = 100 μm. Fe = femur, Ti = tibia, Sv = synovium, Me = meniscus, An = anterior side, Po = posterior side. (C) Percentage of cartilage matrix loss was quantified based on the loss of Safranin O staining at the articular surface. (D) Degree of cartilage matrix destruction was examined according to the OARSI scoring system. (E) Cellularity in articular cartilage was quantified as the total number of cells per total area based on the Hematoxylin and Fast green staining results. (F) Percentage of MMP13-positive cells among the total number of chondrocytes in articular cartilage. (G) Percentage of cells with strong NF-κB/p65 staining (as cells indicated by black arrows) among the total number of chondrocytes in articular cartilage. Data are reported either by box-plot or as mean ± standard deviation. *P <0.05. LV = lentivirus.
To evaluate the effects of IGF-II on the extent of OA damage, we analyzed osteoarthritic parameters and IHC staining using the semi-quantitative evaluation systems. The DMM knee lost 30% of the matrix on average on its articular surface at 7 weeks post-surgery. Strikingly, with lentiviral-IGF-II injection, only 15% of the matrix on average was lost in the DMM knee joints (Fig. 6C). When the OARSI scoring system that is based on the staining of cartilage matrix was used, we observed similar results (Fig. 6D) [Glasson et al., 2010]. In addition to matrix loss, these histological sections demonstrated a 50% reduction in cell number in the articular cartilage of DMM knees, while IGF-II viral delivery significantly reduced chondrocyte loss in the DMM knee by half (Fig. 6E). Furthermore, we performed IHC to ascertain MMP13 and NF-κB expression, and performed a semi-quantitative assessment of cells that are positive for these OA-associated proteins. It was apparent that MMP13 and NF-κB expression was reduced by IGF-II treatment as well (Fig. 6F and G).
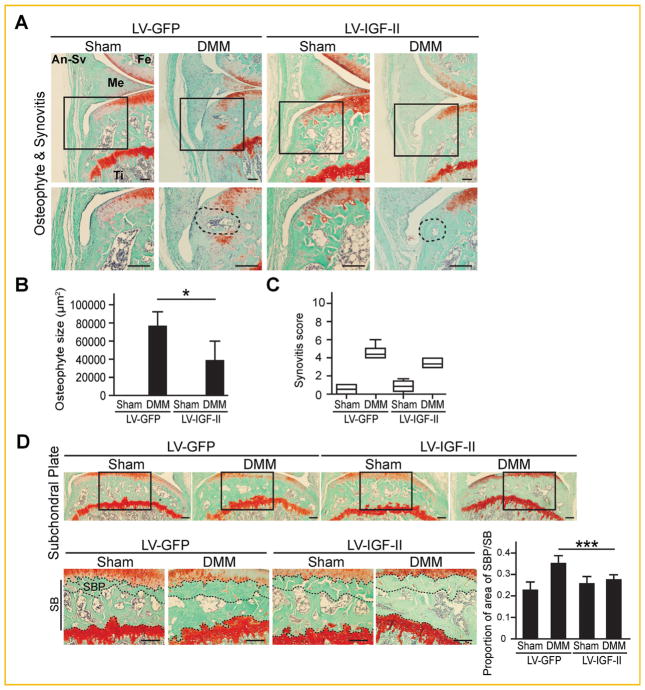
We next evaluated effects of ectopic IGF-II expression on osteophyte formation, synovitis and subchondral bone sclerosis. No osteophytes were observed in the Sham knee, while osteophyte formation in the medial tibial plateau was observed in all of the DMM knee joints assessed (Fig. 7A and B). In lentiviral-IGF-II infected knees, however, DMM surgery-induced osteophyte size was significantly reduced (Fig. 7A and B). Moderate synovitis was also observed in the DMM joint. Interestingly, lentiviral IGF-II injected knee joints, despite having significant IGF-II expression in the synovium, did not show significant reduction of the severity of synovitis, suggesting that IGF-II acts differently in the synovium (Fig. 7A and C). Finally, we examined subchondral bone sclerosis by measuring subchondral bone plate thickness of the medial tibial plateau [Thysen et al., 2015]. DMM surgery resulted in a significant increase in subchondral plate thickness compared to sham surgery in GFP knees. In contrast, the thickness of subchondral bone plate was restored to its normal level in knee joints injected with lentiviral-IGF-II (Fig. 7D). Taken together, these data indicate that IGF-II is capable of halting key events of joint knee destruction in OA including articular cartilage loss, osteophyte formation and subchondral bone sclerosis in our analysis using a murine experimental OA model.
Fig. 7.
Histological analysis on osteophyte formation, synovitis and subchondral bone sclerosis of post-DMM mouse knee joints injected with lentiviral-IGF-II and lentiviral-GFP. (A) Osteophyte and synovitis images at the medial tibial plateau of the DMM knees. Rectangles denote areas shown in higher magnification. Dashed circles indicate areas of osteophytes for quantification using ImageJ. (B) Quantification of osteophyte sizes and (C) degree of synovitis. (D) Images of subchondral bone at the medial tibial plateau of the knee joints. Rectangles denote areas shown in higher magnification. Dashed lines indicate the border of subchondral bone plate (SBP) in subchondral bone (SB). Scale bar = 200 μm. Data are reported either by box-plot or as mean ± standard deviation. *P <0.05. ***P <0.001, DMM; LV-GFP versus DMM; LV-IGF-II. LV = lentivirus. Fe = femur, Ti = tibia, Me = meniscus, An-Sv = anterior synovium.
DISCUSSION
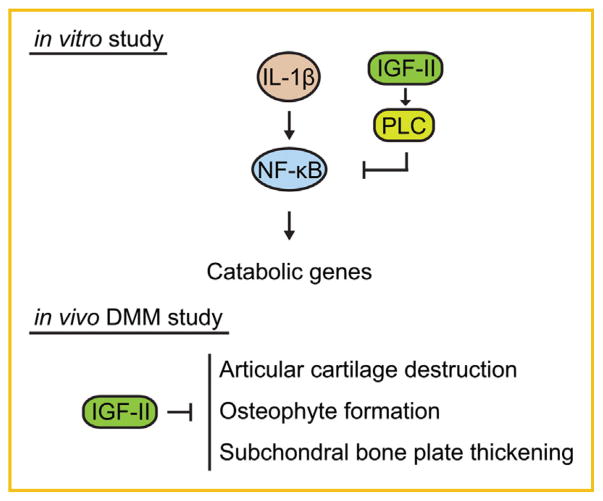
Multiple factors have been shown to regulate the process of joint damage in vivo in experimental OA, including secreted proteins (such as MMP13, ADAMTS5, TGFβ, FGF2, PRG4, and α2-macro-globulin) [Goldring, 2012; Loeser et al., 2012; Oh et al., 2012; Ruan et al., 2013], intracellular factors (such as Zinc-transporter ZIP8) [Kim et al., 2014] as well as small molecules (such as MG132 proteasome inhibitor) [Radwan et al., 2014]. Despite the large amount of work on IGF signaling in chondrocytes in vitro, most of the work was focused on IGF-I, which in fact has been reported to be upregulated in OA [Matsumoto et al., 1996]. Furthermore, the effect of IGF signaling either from IGF-I or IGF-II in OA progression in vivo has not been demonstrated. Our study suggests that IGF-II is capable of halting cartilage matrix destruction in an injury-induced OA model (DMM) in mice as well as in human chondrocyte and cartilage culture systems. In addition to matrix maintenance, we found that IGF-II can prevent loss of chondrocytes, osteophyte formation and subchondral bone sclerosis in vivo, indicating that IGF-II inhibits multiple aspects of joint degeneration in OA (Fig. 8).
Fig. 8.
Model on the chondroprotective activity of IGF-II. In vitro, IGF-II inhibits IL-1β-induced catabolic gene expression and enhances cartilage matrix gene expression in chondrocytes. Our model is that IGF-II induces PLC activity and inhibits the activation of the NF-κB pathway. In vivo, IGF-II promotes articular cartilage integrity and prevents osteophytes formation and subchondral bone sclerosis in injury-induced OA.
It is known that IGF-II is required for skeletal growth during embryogenesis [Baker et al., 1993; Liu, 1993]. After birth, however, IGF-II null mice resume growth at a normal rate, suggesting that IGF-II is primarily involved in embryonic growth, but not postnatal growth [Baker et al., 1993; Liu, 1993]. Yet, it remains possible that IGF-II is involved in biological processes other than growth in postnatal animals. Recent studies have noted that IGF-II is expressed in adult tissues such as the muscle and meniscus as well as in several tumor types [Takigawa et al., 1997; Hellio Le Graverand et al., 2001; Dynkevich et al., 2013]. Our data indicate that IGF-II protein levels are reduced in the articular cartilage of mice subjected to DMM surgery as well as in human OA cartilage, which is consistent with prior reports indicating a decline of IGF-II level in the synovial fluid and the meniscus under OA conditions [Hellio Le Graverand et al., 2001]. On the other hand, Steck et al. [2012] reported that IGF-II mRNA level increased by two-fold in OA cartilage. The discrepancy between our studies could be due to sample selection. Steck et al. [2012] selected normal donors ranging from 13–52 years of age, while OA donors ranged from 47–88 years of age. In our study, the ages of normal and OA donors were similar. In addition, while we only used tibial cartilage from the donors for RT-PCR analysis, Steck et al. [2012] used the entire joint cartilage. It would be important to include additional donors and compare tibial vs femur cartilage gene expression to address these differences.
Functionally, while IGF-II has been found to promote chondrocyte clonal growth [Vetter et al., 1986], chondrocyte survival [Loeser and Shanker, 2000] and proteoglycan synthesis [Takigawa et al., 1997; Davenport-Goodall et al., 2004], we did not observe a significant effect of IGF-II in chondrocyte behavior in these aspects in normal chondrocytes without an IL-1β challenge. This discrepancy could be due to the fact that we did not serum-starve the cells in our functional assays. On the other hand, our result is consistent with work by Bhaumick and Bala [1991] where they demonstrate that IGF-II did not promote cartilage matrix synthesis in differentiated mouse embryonic limb bud chondrocytes, while IGF-I did [Bhaumick and Bala, 1991]. Additionally, IGF-II was much more effective at promoting glucose incorporation than IGF-I [Bhaumick and Bala, 1991]. It is unclear whether IGF-II regulates glucose uptake in adult articular chondrocytes, nor is it clear whether its effect on cartilage gene expression under inflammatory or OA conditions is related to glucose metabolism.
However, in the presence of IL-1β, IGF-II significantly inhibited MMP expression and promoted cartilage matrix production in normal human chondrocytes. IGF-II also has a similar effect on OA chondrocytes, which already express a higher level of IL-1β mRNA. Therefore, our data suggests that the role of IGF-II lies more in inhibiting cartilage matrix destruction and maintaining matrix levels under adverse conditions. An earlier report examined the effect of IGF-II on IL-1β-treated equine chondrocytes and showed that although IGF-II induced GAG synthesis, IGF-II did not prevent the reduction of total amount of GAG [Davenport-Goodall et al., 2004]. The discrepancy between this study and ours is perhaps related to the shorter culture time (72 h) used for the explant culture in this prior study, as compared to the longer culture time in ours (21 days).
Toward understanding underlying mechanisms, we have demonstrated that IGF-II could inhibit IL-1β-induced NF-κB activation, a key mediator in catabolic gene expression and cartilage destruction [Marcu et al., 2010], and have shown for the first time that IGF signaling inhibits NF-κB expression in vivo under OA conditions. Interestingly, in our in vitro experiments, we did not observe that IGF-II activated any of the following kinases (Akt, ERK1/2), which are typically activated by IGF-I [McMahon et al., 2008; Zhang et al., 2009; Montaseri et al., 2011], suggesting that IGF-II may inhibit NF-κB activity through mechanisms different from IGF-I. Such differential activities may be related to the repertoire of IGF binding proteins or different receptors, which are likely associated with different signaling kinases [Bhakta et al., 2000; Siddle, 2011]. It has been shown that the direct binding of IGF-II to the IGF-II receptor, a receptor that IGF-I does not bind, stimulated proteoglycan synthesis and induced calcium influx in chondrocytes, as it occurs even in the presence of an anti-IGF-IR antibody [Sessions et al., 1987; Poiraudeau et al., 1997; Takigawa et al., 1997]. Thus, it will be interesting to determine whether IGF-II signals through the IGF-II receptor in our setting under IL-1β or OA conditions. As it has been shown that IGF-II Receptor has a G-protein binding site and phospholipase C (PLC) can act downstream of this receptor for signal transduction [Ikezu et al., 1995; Maeng et al., 2009], we have evaluated PLC activity and found it was increased by IGF-II. In addition, a PLC inhibitor completely blocked the activity of IGF-II in inhibiting IL-1β-induced MMP13 expression. This result is consistent with a prior report indicating that PLC is also required for IGF-II-mediated calcium influx in chondrocytes [Poiraudeau et al., 1997]. Our result adds to that notion indicating that PLC is also required for IGF-II’s inhibitory effects on MMP13 expression. However, additional studies are required to determine the molecular mechanisms of IGF-II signaling and how PLC mediates its activity.
It is also unclear whether the IGF-II signaling pathway interacts with other pathways (such as Wnt, Ihh, FGF, TGFβ, or BMP) that regulate cartilage destruction during OA. Both Wnt and IGF signaling are known to inhibit GSK-3 in other cell types such as muscle and endothelial cells [Devi et al., 2011; Pansters et al., 2011]. Additionally, IGF and FGF signaling pathways are known to crosstalk during embryo development [Pera et al., 2003]. Furthermore, it has been shown that TGFβ potentially mediates IGF-II activity in chondrogenesis in vitro [Hamamura et al., 2008]. Thus, it would be interesting to determine whether IGF-II signaling in the joint could alter these other pathways.
It is accepted that OA is not just a cartilage disease, but instead a “whole joint” disease with ectopic bone formation and synovium inflammation [Loeser et al., 2012]. We have found that IGF-II inhibited osteophyte formation and subchondral bone sclerosis in addition to its effect on cartilage matrix. However, it does not affect synovitis. This is consistent with other studies where not all aspects of OA pathology are changed by gene manipulations. For example, the zinc regulator Zip8 knockout mice showed a decrease in cartilage destruction without any changes in osteophyte formation upon DMM surgery [Kim et al., 2014]. Since we did not observe any significant reduction in synovitis in lentiviral IGF-II-infected knee joints even though there was ample IGF-II viral infection of the synovium, it suggests that the effect of IGF-II on joint cartilage may not act through the synovium and that IGF-II may function differently in these two tissues. On the other hand, even though there was no significant IGF-II viral infection in the subchondral bone, significant reduction of subchondral bone thickening was observed, suggesting that such an effect is not a direct effect of IGF-II on bone cells. In a similar case with Dkk-1 adenovirus intraarticular injection in the DMM model, reduced cartilage loss and subchondral bone sclerosis were both observed, even though viral expression in the bone was not noted [Oh et al., 2012]. It is possible that the effect on the subchondral bone is due to the enhanced articular cartilage integrity in these cases.
It is worth noting that this study involves the use of just one mouse strain, at one age, and one experimental OA model. It will be important to test the effect of IGF-II on other mouse strains, at older ages, and in other experimental OA models such as cruciate ligament transection, naturally occurring OA or high fat diet models [Goldring, 2012; Loeser et al., 2012; Oh et al., 2012; Ruan et al., 2013; Wang et al., 2014], as OA is a disease of heterogeneous origins with multiple subtypes [Loeser et al., 2012]. It has been reported that opposite results were obtained when testing the effect of FGF2 in the mouse and in human cartilage specimens [Li et al., 2012], highlighting the importance of testing in human cells and tissues. In our study, we observed consistent effects of IGF-II on human chondrocytes and OA cartilage slices as well as the mouse joint in vivo, which is suggestive of the translational potential of IGF-II in OA intervention.
Supplementary Material
Acknowledgments
Grant sponsor: National Institute of Health (NIH); Grant number: 1R01 AR059106-01A1; Grant sponsor: National Science Foundation; Grant number: CBET-0966920; Grant sponsor: Tufts Clinical and Translational Science Institute; Grant number: UL1TF001064.
We thank Dr. Tom Linsenmayer (Tufts University) for providing the collagen II antibody and Dr. Gail Sonenshein (Tufts University) for providing the NF-κB luciferase reporter plasmid. We thank Daisy Nakamura, Carrie Hui, and Averi Leahy for advice and comments on the manuscript. This work has been supported by grants to LZ from the NIH (AR054611), the NSF (CBET-0966920) and a grant from the Tufts Clinical Translational Science Institute (UL1TR001064).
Footnotes
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- Bhakta NR, Garcia AM, Frank EH, Grodzinsky AJ, Morales TI. The insulin-like growth factors (IGFs) I and II bind to articular cartilage via the IGF-binding proteins. J Biol Chem. 2000;275:5860–5866. doi: 10.1074/jbc.275.8.5860. [DOI] [PubMed] [Google Scholar]
- Bhaumick B, Bala RM. Differential effects of insulin-like growth factors I and II on growth, differentiation and glucoregulation in differentiating chondrocyte cells in culture. Acta Endocrinol (Copenh) 1991;125:201–211. doi: 10.1530/acta.0.1250201. [DOI] [PubMed] [Google Scholar]
- Burg-Golani T, Pozniak Y, Rabinovich L, Sigal N, Nir Paz R, Herskovits AA. Membrane chaperone SecDF plays a role in the secretion of Listeria monocytogenes major virulence factors. J Bacteriol. 2013;195:5262–5272. doi: 10.1128/JB.00697-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleigh A, Chanalaris A, Gardiner MD, Driscoll C, Boruc O, Saklatvala J, Vincent TL. Joint immobilization prevents murine osteoarthritis and reveals the highly mechanosensitive nature of protease expression in vivo. Arthritis Rheum. 2012;64:2278–2288. doi: 10.1002/art.34420. [DOI] [PubMed] [Google Scholar]
- Daheshia M, Yao JQ. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J Rheumatol. 2008;35:2306–2312. doi: 10.3899/jrheum.080346. [DOI] [PubMed] [Google Scholar]
- Davenport-Goodall CLM, Boston RC, Richardson DW. Effects of insulin-like growth factor-II on the mitogenic and metabolic activities of equine articular cartilage with and without interleukin 1-beta. Am J Vet Res. 2004;65:238–244. doi: 10.2460/ajvr.2004.65.238. [DOI] [PubMed] [Google Scholar]
- Devi TS, Singh LP, Hosoya K, Terasaki T. GSK-3beta/CREB axis mediates IGF-1-induced ECM/adhesion molecule expression, cell cycle progression and monolayer permeability in retinal capillary endothelial cells: Implications for diabetic retinopathy. Biochim Biophys Acta. 2011;1812:1080–1088. doi: 10.1016/j.bbadis.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Dynkevich Y, Rother KI, Whitford I, Qureshi S, Galiveeti S, Szulc AL, Danoff A, Breen TL, Kaviani N, Shanik MH, Leroith D, Vigneri R, Koch CA, Roth J. Tumors, IGF-2, and hypoglycemia: Insights from the clinic, the laboratory, and the historical archive. Endocr Rev. 2013;34:798–826. doi: 10.1210/er.2012-1033. [DOI] [PubMed] [Google Scholar]
- Foncea R, Andersson M, Ketterman A, Blakesley V, Sapag-Hagar M, Sugden PH, LeRoith D, Lavandero S. Insulin-like growth factor-I rapidly activates multiple signal transduction pathways in cultured rat cardiac myocytes. J Biol Chem. 1997;272:19115–19124. doi: 10.1074/jbc.272.31.19115. [DOI] [PubMed] [Google Scholar]
- Furman BD, Mangiapani DS, Zeitler E, Bailey KN, Horne PH, Huebner JL, Kraus VB, Guilak F, Olson SA. Targeting pro-inflammatory cytokines following joint injury: Acute intra-articular inhibition of interleukin-1 following knee injury prevents post-traumatic arthritis. Arthritis Res Ther. 2014;16:R134. doi: 10.1186/ar4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Glasson SS, Chambers MG, Van Den Berg WB, Little CB. The OARSI histopathology initiative - recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthritis Cartilage. 2010;18:S17–S23. doi: 10.1016/j.joca.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Goldring MB. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther Adv Musculoskelet Dis. 2012;4:269–285. doi: 10.1177/1759720X12448454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamura K, Zhang P, Yokota H. IGF2-driven PI3 kinase and TGFbeta signaling pathways in chondrogenesis. Cell Biol Int. 2008;32:1238–1246. doi: 10.1016/j.cellbi.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellio Le Graverand MP, Vignon E, Otterness IG, Hart DA. Early changes in lapine menisci during osteoarthritis development: Part I: Cellular and matrix alterations. Osteoarthritis Cartilage. 2001;9:56–64. doi: 10.1053/joca.2000.0350. [DOI] [PubMed] [Google Scholar]
- Jotanovic Z, Mihelic R, Sestan B, Dembic Z. Role of interleukin-1 inhibitors in osteoarthritis: An evidence-based review. Drugs Aging. 2012;29:343–358. doi: 10.2165/11599350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kim JH, Jeon J, Shin M, Won Y, Lee M, Kwak JS, Lee G, Rhee J, Ryu JH, Chun CH, Chun JS. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell. 2014;156:730–743. doi: 10.1016/j.cell.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Krenn V, Morawietz L, Burmester GR, Kinne RW, Mueller-Ladner U, Muller B, Haupl T. Synovitis score: Discrimination between chronic low-grade and high-grade synovitis. Histopathology. 2006;49:358–364. doi: 10.1111/j.1365-2559.2006.02508.x. [DOI] [PubMed] [Google Scholar]
- Leahy AA, Esfahani SA, Foote AT, Hui CK, Rainbow RS, Nakamura DS, Tracey BH, Mahmood U, Zeng L. Analysis of the trajectory of osteoarthritis development in a mouse model by serial near-infrared fluorescence imaging of matrix metalloproteinase activities. Arthritis Rheumatol. 2015;67:442–453. doi: 10.1002/art.38957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Ellman MB, Kroin JS, Chen D, Yan D, Mikecz K, Ranjan KC, Xiao G, Stein GS, Kim SG, Cole B, van Wijnen AJ, Im HJ. Species-specific biological effects of FGF-2 in articular cartilage: Implication for distinct roles within the FGF receptor family. J Cell Biochem. 2012;113:2532–2542. doi: 10.1002/jcb.24129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J. Mice carrying null mutations of the genes encoding insulinlike growth factor I (lgt-1) and type 1 IGF receptor (lgf1r) Cell. 1993;75:59–72. [PubMed] [Google Scholar]
- Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser RF, Shanker G. Autocrine stimulation by insulin-like growth factor 1 and insulin-like growth factor 2 mediates chondrocyte survival in vitro. Arthritis Rheum. 2000;43:1552–1559. doi: 10.1002/1529-0131(200007)43:7<1552::AID-ANR20>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Maeng YS, Choi HJ, Kwon JY, Park YW, Choi KS, Min JK, Kim YH, Suh PG, Kang KS, Won MH, Kim YM, Kwon YG. Endothelial progenitor cell homing: Prominent role of the IGF2-IGF2R-PLCbeta2 axis. Blood. 2009;113:233–243. doi: 10.1182/blood-2008-06-162891. [DOI] [PubMed] [Google Scholar]
- Marcu KB, Otero M, Olivotto E, Borzi RM, Goldring MB. NF-kappaB signaling: Multiple angles to target OA. Curr Drug Targets. 2010;11:599–613. doi: 10.2174/138945010791011938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsukura Y, Muneta T, Tsuji K, Miyatake K, Yamada J, Abula K, Koga H, Tomita M, Sekiya I. Mouse synovial mesenchymal stem cells increase in yield with knee inflammation. J Orthop Res. 2015;33:246–253. doi: 10.1002/jor.22753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Gargosky SE, Iwasaki K, Rosenfeld RG. Identification and characterization of insulin-like growth factors (IGFs), IGF-binding proteins (IGFBPs), and IGFBP proteases in human synovial fluid. J Clin Endocrinol Metab. 1996;81:150–155. doi: 10.1210/jcem.81.1.8550744. [DOI] [PubMed] [Google Scholar]
- McMahon LA, Prendergast PJ, Campbell VA. A comparison of the involvement of p38, ERK1/2 and PI3K in growth factor-induced chondrogenic differentiation of mesenchymal stem cells. Biochem Biophys Res Commun. 2008;368:990–995. doi: 10.1016/j.bbrc.2008.01.160. [DOI] [PubMed] [Google Scholar]
- Montaseri A, Busch F, Mobasheri A, Buhrmann C, Aldinger C, Rad JS, Shakibaei M. IGF-1 and PDGF-bb suppress IL-1beta-induced cartilage degradation through down-regulation of NF-kappaB signaling: Involvement of Src/PI-3K/AKT pathway. PLoS ONE. 2011;6:e28663. doi: 10.1371/journal.pone.0028663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Chun CH, Chun JS. Dkk-1 expression in chondrocytes inhibits experimental osteoarthritic cartilage destruction in mice. Arthritis Rheum. 2012;64:2568–2578. doi: 10.1002/art.34481. [DOI] [PubMed] [Google Scholar]
- Otero M, Favero M, Dragomir C, Hachem KE, Hashimoto K, Plumb DA, Goldring MB. Human chondrocyte cultures as models of cartilage-specific gene regulation. Methods Mol Biol. 2012;806:301–336. doi: 10.1007/978-1-61779-367-7_21. [DOI] [PubMed] [Google Scholar]
- Pansters NA, van der Velden JL, Kelders MC, Laeremans H, Schols AM, Langen RC. Segregation of myoblast fusion and muscle-specific gene expression by distinct ligand-dependent inactivation of GSK-3beta. Cell Mol Life Sci. 2011;68:523–535. doi: 10.1007/s00018-010-0467-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera EM, Ikeda A, Eivers E, De Robertis EM. Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev. 2003;17:3023–3028. doi: 10.1101/gad.1153603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poiraudeau S, Lieberherr M, Kergosie N, Corvol MT. Different mechanisms are involved in intracellular calcium increase by insulin-like growth factors 1 and 2 in articular chondrocytes: Voltage-gated calcium channels, and/or phospholipase C coupled to a pertussis-sensitive G-protein. Journal of cellular biochemistry. 1997;64:414–422. [PubMed] [Google Scholar]
- Radwan M, Wilkinson DJ, Hui W, Destrument AP, Charlton SH, Barter MJ, Gibson B, Coulombe J, Gray DA, Rowan AD, Young DA. Protection against murine osteoarthritis by inhibition of the 26S proteasome and lysine-48 linked ubiquitination. Ann Rheum Dis. 2014 doi: 10.1136/annrheumdis-2013-204962. [DOI] [PubMed] [Google Scholar]
- Ruan MZ, Erez A, Guse K, Dawson B, Bertin T, Chen Y, Jiang MM, Yustein J, Gannon F, Lee BH. Proteoglycan 4 expression protects against the development of osteoarthritis. Sci Transl Med. 2013;5:176ra34. doi: 10.1126/scitranslmed.3005409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessions CM, Emler CA, Schalch DS. Interaction of insulin-like growth factor II with rat chondrocytes: Receptor binding, internalization, and degradation. Endocrinology. 1987;120:2108–2116. doi: 10.1210/endo-120-5-2108. [DOI] [PubMed] [Google Scholar]
- Shen W, Martinez K, Chuang CC, McIntosh M. The phospholipase C inhibitor U73122 attenuates trans-10, cis-12 conjugated linoleic acid-mediated inflammatory signaling and insulin resistance in human adipocytes. J Nutr. 2013;143:584–590. doi: 10.3945/jn.112.173161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddle K. Signalling by insulin and IGF receptors: Supporting acts and new players. J Mol Endocrinol. 2011;47:R1–10. doi: 10.1530/JME-11-0022. [DOI] [PubMed] [Google Scholar]
- Singh SAI. A Comparison of the Histochemical and Image-Derived Proteoglycan Content of Articular Cartilage. Anatom Physiol. 2013;3:120. [Google Scholar]
- Sondergaard BC, Henriksen K, Wulf H, Oestergaard S, Schurigt U, Brauer R, Danielsen I, Christiansen C, Qvist P, Karsdal MA. Relative contribution of matrix metalloprotease and cysteine protease activities to cytokine-stimulated articular cartilage degradation. Osteoarthritis Cartilage. 2006;14:738–748. doi: 10.1016/j.joca.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Starkman BG, Cravero JD, Delcarlo M, Loeser RF. IGF-I stimulation of proteoglycan synthesis by chondrocytes requires activation of the PI 3-kinase pathway but not ERK MAPK. Biochem J. 2005;389:723–729. doi: 10.1042/BJ20041636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck E, Boeuf S, Gabler J, Werth N, Schnatzer P, Diederichs S, Richter W. Regulation of H19 and its encoded microRNA-675 in osteoarthritis and under anabolic and catabolic in vitro conditions. J Mol Med (Berl) 2012;90:1185–1195. doi: 10.1007/s00109-012-0895-y. [DOI] [PubMed] [Google Scholar]
- Tahimic CG, Wang Y, Bikle DD. Anabolic effects of IGF-1 signaling on the skeleton. Front Endocrinol (Lausanne) 2013;4:6. doi: 10.3389/fendo.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takigawa M, Okawa T, Pan H, Aoki C, Takahashi K, Zue J, Suzuki F, Kinoshita A. Insulin-like growth factors I and II are autocrine factors in stimulating proteoglycan synthesis, a marker of differentiated chondrocytes, acting through their respective receptors on a clonal human chondrosar-coma-derived chondrocyte cell line, HCS-2/8. Endocrinology. 1997;138:4390–4400. doi: 10.1210/endo.138.10.5265. [DOI] [PubMed] [Google Scholar]
- Temple MM, Xue Y, Chen MQ, Sah RL. Interleukin-1alpha induction of tensile weakening associated with collagen degradation in bovine articular cartilage. Arthritis Rheum. 2006;54:3267–3276. doi: 10.1002/art.22145. [DOI] [PubMed] [Google Scholar]
- Thysen S, Luyten FP, Lories RJ. Loss of Frzb and Sfrp1 differentially affects joint homeostasis in instability-induced osteoarthritis. Osteoarthritis Cartilage. 2015;23:275–279. doi: 10.1016/j.joca.2014.10.010. [DOI] [PubMed] [Google Scholar]
- van der Sluijs JA, Geesink RG, van der Linden AJ, Bulstra SK, Kuyer R, Drukker J. The reliability of the Mankin score for osteoarthritis. J Orthop Res. 1992;10:58–61. doi: 10.1002/jor.1100100107. [DOI] [PubMed] [Google Scholar]
- Wang S, Wei X, Zhou J, Zhang J, Li K, Chen Q, Terek R, Fleming BC, Goldring MB, Ehrlich MG, Zhang G, Wei L. Identification of alpha2-macroglobulin as a master inhibitor of cartilage-degrading factors that attenuates the progression of posttraumatic osteoarthritis. Arthritis Rheu-matol. 2014;66:1843–1853. doi: 10.1002/art.38576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Zhou Q, Liang QQ, Li CG, Holz JD, Tang D, Sheu TJ, Li TF, Shi Q, Wang YJ. IGF-1 regulation of type II collagen and MMP-13 expression in rat endplate chondrocytes via distinct signaling pathways. Osteoarthritis Cartilage. 2009;17:100–6. doi: 10.1016/j.joca.2008.05.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.