Abstract
Mechanosensitivity is a fundamental physiological capacity, which pertains to all life forms. Progress has been made with regard to understanding mechanosensitivity in bacteria, flies, and worms. In vertebrates, however, the molecular identity of mechanotransducers in somatic and neuronal cells has only started to appear. The Piezo family of mechanogated ion channels marks a pivotal milestone in understanding mechanosensitivity. Piezo1 and Piezo2 have now been shown to participate in a number of processes, ranging from arterial modeling to sensing muscle stretch. In this review, we focus on Piezo2 and its role in mediating mechanosensation and proprioception in vertebrates.
1. INTRODUCTION
Piezo2 was identified by homology with Piezo1, a mechanogated ion channel cloned by the Ardem Patapoutian group from the neuroblastoma N2A cells (Coste et al., 2010). The two molecules are the only known members of an evolutionary conserved family of proteins and are among the largest ion channels cloned to date. Mouse Piezo1 and Piezo2 are ~2500 and 2800 amino acids long nonselective cation channels with ~42% identity. Though structural information on Piezo2 is not available, predictions based on functional analysis and the cryo-EM structure of Piezo1 suggest that functional Piezo2 is formed by three subunits with 16–18 transmembrane helixes (Fig. 1) (Coste et al., 2015; Ge et al., 2015; Zhao et al., 2016).
Figure 1. Hypothetical topology of Piezo2.

Shown is a hypothetical transmembrane topology diagram of mouse Piezo2 based on the cryo-EM (Ge et al., 2015) and functional studies of mouse Piezo1 (Coste et al., 2015; Zhao et al., 2016). AD, anchor domain; CED, C-terminal extracellular domain; CTD, C-terminal domain; IH, inner helix; OH, outer helix. E2416 in the anchor domain alters pore properties of mouse Piezo2 (Coste et al., 2015). E2797 in the C-terminal domain is homologous to E2727 in human Piezo2. The deletion of E2727 in hPiezo2 prolongs kinetics of MA current inactivation in vitro (Dubin et al., 2012).
Piezo homologs are found in plants, unicellular eukaryotes (but not yeast), and invertebrates, but the distinction between Piezo1 and Piezo2 begins in vertebrates, where the two proteins carry out a number of mechanosensory functions in various cells and tissues. Known Piezo1 functions pertain to mechanotransduction in different types of somatic cells and has been extensively reviewed elsewhere (Bagriantsev, Gracheva, & Gallagher, 2014; Glokowska & Gallagher, 2015; Honore, Martins, Penton, Patel, & Demolombe, 2015; Ranade, Syeda, & Patapoutian, 2015; Volkers, Mechioukhi, & Coste, 2015; Wu, Lewis, & Grandl, 2016). Similar to Piezo1, Piezo2 is widely expressed in somatic cells: chondrocytes (Lee et al., 2014), odontoblasts (Khatibi Shahidi et al., 2015), endothelial cells (Ferrari, Bogen, Green, & Levine, 2015), and astrocytes (Choi, Sun, & Jakobs, 2015). In contrast to Piezo1, Piezo2 is also expressed in Merkel cells (Ikeda et al., 2014; Maksimovic et al., 2014; Woo et al., 2014), outer hair cells (Wu, Grillet, et al., 2016), endothelial cells in the brain (Wu, Lewis, et al., 2016), enterochromaffin cells of the gut (Wang et al., 2016), and in the neurons of the somatosensory ganglia, where it plays a key role in mechanosensation and proprioception.
Somatosensory ganglia house a very diverse array of somatosensory neurons, which project to the skin of the face (trigeminal ganglia, TG) or body (dorsal root ganglia, DRG) to detect the principal components of cutaneous senses: temperature, chemical irritants, painful touch, and light touch (Le Pichon & Chesler, 2014). In addition, the DRG houses proprioceptive neurons innervating muscle. Proprioceptors are absent from TG and reside in the mesencephalic ganglion in the brainstem (Lazarov, 2007). In most vertebrates, the vast majority of TG and DRG neurons are nonmyelinated (C-type) nociceptors and thermoreceptors, which detect temperature and painful chemical and mechanical stimuli. Light touch is detected by low-threshold mechanoreceptors (LTMRs). Most LTMRs are myelinated Aβ-fibers, but there are also thinly myelinated Aδ- and nonmyelinated C-fibers (Zimmerman, Bai, & Ginty, 2014). Aβ-LTMRs can be further segregated by the firing pattern they produce in response to mechanical stimulation: rapidly adapting mechanoreceptors (RA-LTMRs), which only fire with onset and offset of a stimulus; and slowly adapting mechanoreceptors (SA-LTMRs), which fire for the duration of a mechanical stimulus (Fleming & Luo, 2013). Mechanosensitive channels in the nerve terminals of LTMRs trigger action potential generation in these cells (Ranade et al., 2015; Sharif-Naeini, 2015). Recent studies determined that Piezo2 is a key mechanotransducer in a subset of LTMRs and in proprioceptors. Here, we review the role of Piezo2 in cutaneous and proprioceptive mechanotransduction of vertebrates.
2. SOMATOSENSORY NEURONS
2.1 Piezo2 and fast mechanoactivated current in mouse dorsal root ganglia neurons
Understanding mechanosensation in the different types of somatosensory end organs (Merkel celle–neurite complexes, Meissner and Pacinian corpuscles and others; see Zimmerman et al., 2014) requires the identification of the molecular mechanism that translates tissue deformation into action potential firing in the corresponding LTMR. This necessitates knowledge of the molecules that mediate mechanoactivated current, modulate kinetics of inactivation, generate action potentials, and shape the pattern of firing adaptation. In addition, this requires understanding the contribution from the somatic components of the end organs, such as Merkel cells in Merkel celle–neurite complexes or lamellar cells in Pacinian corpuscles. Recently, a great breakthrough was made with regard to the role of Piezo2 in Merkel celle–neurite complexes (Ikeda et al., 2014; Maksimovic et al., 2014; Woo et al., 2014), but by and large the molecular basis of peripheral neuronal mechanosensitivity remains poorly understood.
Somatosensory neurons are innately mechanosensitive, i.e., they can convert mechanical stimuli into excitatory current and mechanoactivated action potentials even in the absence of other tissue components. In culture, mechanosensitivity of dissociated TG and DRG neurons is often studied by patch clamp recording in the voltage clamp mode (McCarter, Reichling, & Levine, 1999). Stimulation with a blunt glass probe evokes mechanoactivated excitatory current (MA current, Fig. 2), a necessary prerequisite for the generation of action potentials.
Figure 2. Mechanoactivated currents in mouse trigeminal neurons.
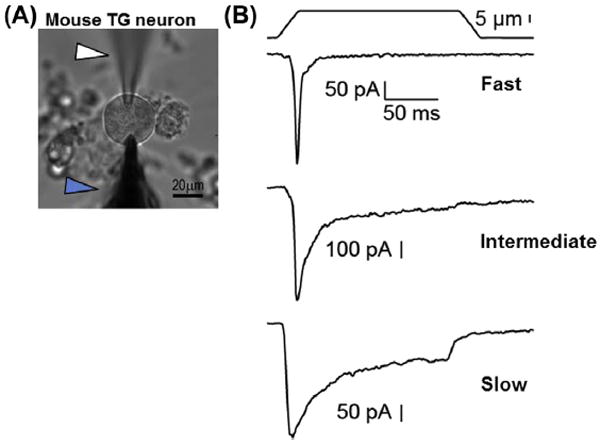
(A) An image of a mouse trigeminal (TG) neuron with an electrode (white arrowhead) and blunt probe (blue arrowhead) in the working position to record MA current. (B) Representative whole cell currents from mouse trigeminal neurons showing exemplar fast, intermediate, and slow MA currents based on the rate of exponential current decay (τ). Currents were obtained from a holding potential of −60 mV, using a probe moving at 800 μm/s velocity. (For experimental details, see Schneider et al., 2014).
Different neurons produce specific types of MA currents, which are thought to be mediated by more than one mechanogated ion channel, judging from ion selectivity, pharmacological and kinetic characteristics (Coste, Crest, & Delmas, 2007; Drew et al., 2007; Drew & Wood, 2007; Hu & Lewin, 2006; McCarter & Levine, 2006; Poole, Herget, Lapatsina, Ngo, & Lewin, 2014; Qi et al., 2015). A salient feature of all MA currents is the time constant of near-exponential decay (τ), which occurs immediately after initial channel opening. A detailed analysis of the decay kinetics reveals a complex underlying mechanism, which involves adaptation and inactivation (Hao & Delmas, 2010; Rugiero, Drew, & Wood, 2010). Traditionally, most studies classify neuronal MA currents as fast (or rapid, τ < 10 ms), intermediate (10 ms <τ < 30 ms), and slow (τ > 30 ms) adapting (Fig. 2B). Here, we will use the term “inactivation” with regard to MA current (fast, intermediately, or slowly inactivating) and “adaptation” to refer to the pattern of action potential firing (rapidly or slowly adapting).
Piezo2 was the first ion channel firmly linked to an MA current in somatosensory neurons. Piezo2 is expressed in 20–50% of mammalian DRG and TG neurons, including LTMRs and nociceptors (Alamri, Bron, Brock, & Ivanusic, 2015; Bron, Wood, Brock, & Ivanusic, 2014; Coste et al., 2010; Lou, Duan, Vong, Lowell, & Ma, 2013; Ranade et al., 2014). In one neuronal subset, C-type LTMRs, Piezo2 expression was shown to rely on the transcriptional factor Runx1 (Lou et al., 2013). Fast MA current generated by a subset of mouse DRG neurons is remarkably similar to the MA current produced by Piezo2 in HEK293T cells (Fig. 3), both in terms of kinetics and pharmacology.
Figure 3. Mouse Piezo2 generates fast inactivating MA current in HEK293 cells.
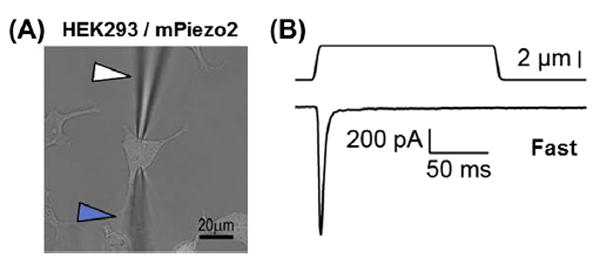
(A) An image of an HEK293 cell expressing mouse Piezo2 during voltage clamp recording to obtain MA current in response to mechanical stimulation with a glass probe. White arrowhead: electrode; blue arrowhead: mechanical probe. (B) Representative fast MA current (τ < 10 ms) trace from mPiezo2 in HEK293 cell obtained in the whole cell configuration from a holding potential of −60 mV. Currents were obtained from a holding potential of −80 mV, using a probe moving at 800 μm/s velocity. Solutions used (mM): bath, 140 NaCl, 5 KCl, 2.5 CaCl2, 1 MgCl2, 10 glucose, 10 HEPES/NaOH pH 7.4; pipette: 133CsCl, 5 EGTA, 1 CaCl2, 1 MgCl2, 4 MgATP, 0.4 Na2GTP, 10 HEPES/CsOH pH 7.3.
Small interfering RNA-mediated knockdown of Piezo2 in cultured DRG neurons leads to a specific reduction of most of fast MA current, demonstrating that it is Piezo2 dependent (Coste et al., 2010; Lou et al., 2013). These data are corroborated through DRG-specific Piezo2 knockouts (Ranade et al., 2014; Woo et al., 2015). Thus, Piezo2 mediates most, if not all, fast MA current in mouse DRG neurons (Coste et al., 2010; Ranade et al., 2014; Woo et al., 2015), but the molecular identity of intermediate and slow MA current is yet to be established.
2.2 Possible role of Piezo2 in slow mechanoactivated current
Functional studies suggested that fast MA current is largely (but not exclusively) present in mechanoreceptors, while slow MA current is present in nociceptors (Coste et al., 2007; Drew et al., 2007; Lechner, Frenzel, Wang, & Lewin, 2009; Poole et al., 2014). Overall, Aδ- and Aβ-type LTMRs are rare cells in rodent DRG, where 60–70% of neurons are C-type nociceptors and thermoreceptors (Kobayashi et al., 2005; Le Pichon & Chesler, 2014). Some LTMR subtypes account for only a few percent of the total neuronal population, which may obscure their identification in vitro by electrophysiological analyses. For example, RA-LTMRs account for mere 6% of all mouse thoracic DRG neurons (Li et al., 2011), yet they innervate a host of mechanosensitive end organs, including lanceolate endings in the hairy skin, Meissner and Pacinian corpuscles (Zimmerman et al., 2014). It is therefore plausible that such rare neurons will avoid a definitive classification in an en masse electrophysiological analysis of dissociated DRG neurons, leaving the possibility that some types of LTMRs express mechanotransducers with intermediate or slow kinetics of inactivation. In support of this, studies of cat mesenteric Pacinian corpuscles showed that mechanical stimulation of the corresponding LTMRs that have had the somatic components of the end organ manually removed evokes a slowly decaying receptor potential, indicating the presence of a mechanotransducer with slow MA current (Loewenstein & Mendelson, 1965; Mendelson & Lowenstein, 1964). In duck TG, sensory ganglia with an unusually high population of LTMRs innervating Pacinian-like corpuscles in the bill, most neurons express Piezo2 and exhibit slow MA current (Schneider et al., 2014). Thus, it is possible that RA-LTMRs innervating Pacinian corpuscles are subserved by mechanotransducers with slow MA current, possibly mediated by Piezo2. In support of this, a recent study showed that people carrying non-functional Piezo2 alleles display general losses in vibration detection in the frequency range perceived by Pacinian corpuscles (Chesler et al., 2016). So far, however, Piezo2 has been associated only with fast MA current, though it is expressed in various LTMRs, including RA-LTMRs innervating Meissner corpuscles (Ranade et al., 2014). Whether RA-LTMRs innervating Pacinian and Meissner corpuscles are subserved by different mechanotransducer channels or by the same channel with modified inactivation kinetics is unknown. These considerations provide strong rationale to identify novel slow-type mechanotransducers in nociceptors and LTMRs, as well as and molecules and pathways that prolong inactivation kinetics of Piezo2.
2.3 Functional regulation of Piezo2 in mouse dorsal root ganglia neurons
While the quest for slow mechanotransducer(s) is ongoing, recent studies have revealed mechanisms shaping Piezo2 function. In vitro, the deletion of E2727 in the distal C-terminal region of human Piezo2 (E2797 in mouse Piezo2, Fig. 1) causes a twofold prolongation of inactivation (Coste et al., 2013). Interestingly, this and other Piezo2 mutations were found to be linked to distal arthrogryposis type 5, suggesting that Piezo2 channelopathy could lead to severe developmental malformations (Coste et al., 2013; McMillin et al., 2014; Okubo et al., 2015). Piezo2 inactivation can also be reversibly prolonged by osmotic swelling and pharmacological activation of the protein kinase A (PKA)/protein kinase C (PKC) pathway in neurons and heterologous cells (Dubin et al., 2012; Jia, Ikeda, Ling, Viatchenko-Karpinski, & Gu, 2016), establishing that Piezo2 kinetics are modulated by intracellular factors. Most likely, these and other mechanisms are involved in sustained prolongation of Piezo2 kinetics in neuronal mechanoreceptor subtypes as discussed earlier, warranting further research.
The mechanism of Piezo2 fine-tuning in neurons has only started to emerge. Piezo2 activity is regulated by G-protein signaling. The inclusion of GTP in the recording pipette leads to a slow run-up of Piezo2 current in neurons and HEK293 cells (Jia, Ikeda, Ling, & Gu, 2013). Part of the mechanism probably includes the activation the PKA/PKC pathway, which can be engaged directly by GTP, through the bradykinin receptor b2 (Dubin et al., 2012), or cAMP sensor Epac1, leading to potentiation of Piezo2 current (Eijkelkamp et al., 2013; Singhmar et al., 2016). Given that the PKA/PKC pathway can be engaged through a host of receptors, Piezo2 activity is expected to respond to a number of signaling molecules. These may converge onto Piezo2 directly, e.g., via phosphorylation, or indirectly, by changing the molecular composition and physical properties of the plasma membrane. Indeed, Piezo2 was shown to interact with STOML3 (Poole et al., 2014), an important regulator of mechanosensitivity (Wetzel et al., 2007). STOML3 expression potentiates Piezo2 activity via a mechanism that leads to cholesterol-dependent stiffening of the plasma membrane (Qi et al., 2015). In support of this, it was shown that an increase in membrane tension caused by intracellular hypotonicity-induced cell swelling reversibly activates Piezo2 (Jia et al., 2016). Conversely, phosphoinositide depletion from the plasma membrane leads to suppression of Piezo2 (Borbiro, Badheka, & Rohacs, 2015). Thus, Piezo2 activity is tightly regulated in neurons by various cellular factors and pathways, including components of the plasma membrane, most of which have yet to be elucidated (Narayanan et al., 2016).
3. LIGHT TOUCH
3.1 Insights from mice
A transgenic mouse expressing Piezo2–GFP showed that not only is Piezo2 protein present in the cell bodies of DRG neurons but it is also found in the nerve terminal in the skin (Ranade et al., 2014). In particular, Piezo2 was identified in LTMRs innervating the hair follicles, Merkel cells, Meissner corpuscles (Ranade et al., 2014), in proprioceptive muscle spindles, and Golgi tendon organs (Woo et al., 2015). Conditional knockout of Piezo2 in DRG neurons yields a reduction in the total number of mechanosensitive myelinated Aβ-fibers as tested through an ex vivo skin nerve preparation (Ranade et al., 2014). The remaining mechanosensitive Aβ-fibers required more force to elicit firing and had reduced firing frequency compared to control. SA-LTMRs exhibit the most striking spiking deficits in the absence of Piezo2, with a dramatic reduction in spikes on both the onset (dynamic phase) and hold (static phase) of the mechanical stimulus. Both RA- and SA-LTMRs displayed deficits in velocity detection in the absence of Piezo2. This deficit was not seen in C-fibers that detect noxious heat, cold, and mechanical stimulation (Ranade et al., 2014). Mice lacking Piezo2 in sensory neurons demonstrate light touch deficits compared to WT, as assayed by stimulation by von Frey filaments, response to a piece of tape affixed to the back, and a two-plate vibration preference test (Ranade et al., 2014). Both ambient and noxious temperature sensation, in addition to noxious mechanosensation, were not impaired in these knockout mice (Ranade et al., 2014), indicating that Piezo2 is not a key component of nociceptor function.
Accumulated evidence shows that Piezo2 is a major but not the only mechanotransducer in murine DRG. Indeed, some fast MA current remains in the neurons even after knockout (Ranade et al., 2014; Woo et al., 2015). This could either be explained by an incomplete Cre-mediated excision or the presence of other mechanotransducer(s). The deletion of Piezo2 does not appear to affect the percentage of neurons with intermediate and slow MA currents. While most of the remaining MA currents probably arise from nociceptors, electrophysiological recordings in dissociated DRG as well other considerations discussed earlier suggest that some LTMR subtypes could also express slow MA current (Coste et al., 2007; Drew et al., 2007; Lechner et al., 2009; Poole et al., 2014). Further, there is also a possibility that some ion channels might not be expressed in neuronal soma in dissociated system, and thus mechanotransducers observed in DRG neurons in culture may not fully reflect the multitude of MA current in LTMR endings in vivo. Indeed, while the deletion of Piezo2 profoundly suppresses both LTMR spiking and behavioral responses to light touch, it does not abrogate either process, warranting further research in this area.
3.2 Insight from other vertebrates
Extensive studies of the role of Piezo2 in mouse have established that the channel is a key component in light touch detection. Other studies have aimed to understand if the role of Piezo2 is conserved in other vertebrates. In zebraflsh, a homolog of Piezo2 called piezo2b, likely the result of genome duplication, was identified in the somatosensory Rohan–Beard cells (Faucherre, Nargeot, Mangoni, & Jopling, 2013). Morpholino oligonucleotide knockdown of piezo2b in zebrafish embryos significantly reduced light touch responses in these embryos compared to wild type, but had no effect on noxious mechanical or chemical responses (Faucherre et al., 2013). A test of the mechanosensitivity of piezo2b via overexpression in cell culture followed by mechanical stimulation in voltage clamp would determine if this conserved phenotype is due to Piezo2 mechanosensitivity or some other mechanism.
Tactile specialist organisms provide a unique perspective to studying mechanosensation (Schneider, Gracheva, & Bagriantsev, 2016). Some species of ducks, including the domesticated Pekin duck, are tactile-guided foragers (Zweers, 1977). The glabrous skin covering both dorsal and ventral surfaces of the duck bill is rich in Grandry (Meissner) and Herbst (Pacinian) corpuscles–the principal detectors of the lightest forms of touch and vibration (Berkhoudt, 1980; Saxod, 1996). The corpuscles are innervated by trigeminal RA-LTMRs (Arends & Dubbeldam, 1984; Dubbeldam, Brauch, & Don, 1981; Gottschaldt, 1974; Gregory, 1973; Leitner & Roumy, 1974). Histological analysis showed that TG of several species of tactile-foraging duck are rich in large-diameter neurons expressing Piezo2, where 84% of TG neurons stain positive for Piezo2 mRNA. This is a far greater proportion of Piezo2-expressing neurons that the typical 20–30% of Piezo2 mRNA-expressing neurons found using the same method in duck DRG, mouse TG or DRG, or TG of visually foraging birds (Fig. 4) (Schneider et al., 2014). Together with the exceptionally high density (>100 mm2) of Meissner- and Pacinian-like corpuscles in the duck bill (Berkhoudt, 1980), these data suggest that duck TG contains high proportion of Piezo2+ RA-LTMRs, providing a molecular basis for the tactile-driven foraging behavior.
Figure 4. Piezo2 expression in trigeminal ganglia (TG) and dorsal root ganglia (DRG) of rodents and birds.
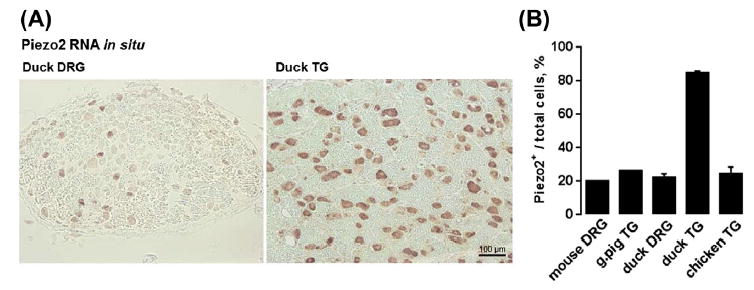
(A) Representative RNA in situ hybridization images from duck TG and DRG using Piezo2 antisense probe. (B) Quantification of Piezo2 mRNA-expressing neurons from TG and DRG of mice (Coste et al., 2010), guinea pig (Bron et al., 2014) and birds (Schneider et al., 2014).
Electrophysiological analysis showed that 80% of duck TG neurons are mechanosensitive in vitro. Unexpectedly, even though >80% of duck TG neurons express Piezo2, all three types of MA current (fast, intermediate and slow) are present in, respectively, 20%, 20%, and 60% of the neurons. Possibly, some neurons express factors that modulate Piezo2 inactivation kinetics, or Piezo2 coexists with unidentified mechanotransducers with slow MA current (Schneider et al., 2014). The duck TG is a particularly interesting system for studying mechanosensitivity because of its rich population of RA-LTMRs innervating Meissner- and Pacinian-like corpuscles in the glabrous skin of the duck bill. Further study of the role of Piezo2 and other proteins in this system could provide insight on the basis of mechanosensi-tivity of Meissner and Pacinian corpuscles, which are innervated by RA-LTMRs that are very rare in mouse DRG.
4. PROPRIOCEPTION
A subset of mechanosensitive DRG neurons are proprioceptors marked by the expression of parvalbumin (Pvalb) (Arber, Ladle, Lin, Frank, & Jessell, 2000). Proprioceptors are SA-LTMRs that innervate muscle and the Golgi tendon organ and provide information on body and limb position (Proske & Gandevia, 2012). A recent study showed that Piezo2 is the main mechanotrans-ducer in mouse proprioceptors (Woo et al., 2015). Woo et al. used two conditional Piezo2 knockout mouse strains obtained by crossing a Piezo2fl/fl strain to Pvalb-Cre, resulting in Piezo2 knockout in parvalbumin-expressing proprio-ceptors and some cutaneous mechanoreceptors; and HoxB8-Cre to target Piezo2 in a broader population of DRG neurons, inclusive of proprioceptors, in the caudal (lower thoracic, lumbar) body segments (Woo et al., 2015).
Piezo2cKO mice retained the wild-type number of proprioceptors in DRG but exhibited profoundly impaired coordination in both pairs of limbs in Pvalb-Cre; Piezo2cKO and in hind limbs only in HoxB8-Cre; Piezo2cKO, consistent with the caudal bias of HoxB8-Cre activity. Comparison of muscle spindle firing properties between WT and Piezo2cKO mice in an ex vivo muscle stretch preparation revealed a profound impairment of baseline and stretch-activated spiking in muscle-innervating afferents in Piezo2cKO mice, demonstrating that Piezo2 is vital for stretch-induced pro-prioceptor activity (Woo et al., 2015).
Dissociation of DRG neurons from Pvalb-Cre; TdTomato allowed for identification of a proprioceptor-rich population in vitro. Mechanical stimulation of TdTomato-positive neurons predominately yields fast MA currents in 89.5% of the cells, similar to the kinetics of Piezo2 in HEK293 (Fig. 3B). In contrast, only 8.3% of fluorescent DRG neurons from Pvalb-Cre; TdTomato; Piezo2cKO mice exhibited fast MA current. Interestingly, a small subset of these neurons has intermediate MA current, and the proportion of these neurons increases with Piezo2 conditional knockout (Woo et al., 2015). This suggests that another mechanotransducer ion channel could exist in this subset of neurons, although the fact that some Pvalb+ neurons are cutaneous mechanoreceptors means that neurons with intermediate MA current are not necessarily proprioceptors. Nevertheless, considering the severe behavioral phenotypes of proprioceptor-specific Piezo2 knockout, the channel appears to be the principal mechanotransducer in proprioceptors. Recently, an elegant study by the Ana Gomis group corroborated this conclusion using proprioceptive neurons from the mesencephalic ganglion, which contains a rather homogeneous population of proprioceptors (Florez-Paz, Bali, Kuner, & Gomis, 2016).
Interestingly, even though proprioceptors express Piezo2-dependent MA current with fast kinetics of inactivation, they exhibit a slowly adapting pattern of afferent discharge (Proske & Gandevia, 2012; Woo et al., 2015). The discontinuity between MA current inactivation and firing adaptation rates is puzzling. It is possible that the fast MA current measured in a dissociated soma does not fully reflect the situation in the afferent ending, where the kinetics of inactivation could be prolonged by unknown factors. Alternatively, the geometry of the muscle spindle could lead to a sequential engagement and disengagement of Piezo2 molecules, leading to repetitive generation of MA currents. It is also possible that slow adaptation requires a contribution from the somatic components surrounding the afferent ending in the muscle spindle, which are yet to be identified. The latter scenario has been shown to exist in the Merkel cell–neurite complexes.
5. MERKEL CELLS
Static mechanical stimuli, rough surface textures, as well as guard hair and whisker defiections are detected by Merkel cell–neurite complexes. The complex is composed of a modified mechanosensitive epithelial cell (Merkel cell) and a slowly adapting type I LTMR (SAI-LTMR). Recent works demonstrated that both the somatic and neural components of the complex are mechanosensitive and depend on the expression of Piezo2 (Ikeda et al., 2014; Maksimovic et al., 2014; Woo et al., 2014).
Voltage clamp recording from Merkel cells in situ in the mouse whisker follicles revealed that the cells are mechanosensitive, exhibiting fast MA current (Ikeda et al., 2014). A similar current was detected in vitro in Merkel cells isolated from cutaneous touch domes (Maksimovic et al., 2014; Woo et al., 2014). The MA current closely resembles Piezo2 activity in cultured cell lines both in terms of fast kinetics of inactivation and inhibition by Gd3+ and ruthenium red (Coste et al., 2010). The injection of an antibody or shRNA against Piezo2 into the whisker follicle suppressed MA current in Merkel cells in situ (Ikeda et al., 2014), whereas skin-specific knockout of Piezo2 in mice (Krt14-Cre;Piezo2fl/fl;Atoh1GFP) abolished MA current of isolated Merkel cells in vitro (Woo et al., 2014).
Interestingly, these studies also revealed that mechanical stimulation of mouse Merkel cells in current clamp mode yielded sustained depolarization and in some cases, Ca2+-dependent action potentials (Ikeda et al., 2014; Woo et al., 2014). This mechanically evoked depolarization is absent in Krt14-Cre and Piezo2fl/fl mice (Woo et al., 2014). Recordings from SAI-LTMRs ex vivo showed that conditional knockout of Piezo2 in Merkel cells greatly reduced the number of spikes fired in the static phase of the stimulation, while firing in the dynamic phase stayed mostly intact (Ikeda et al., 2014; Maksimovic et al., 2014; Woo et al., 2014). Rather than firing for the duration of the stimulus, Piezo2 CKO fibers only fire for part of the static phase, thus displaying intermediate adaptation to mechanical stimulation (Maksimovic et al., 2014; Woo et al., 2014). This suggests that Piezo2-mediated activation of Merkel cells is not necessary for SAI-LTMR firing, but contributes to sustained nerve firing in the static phase of stimulation. To better probe the role of Merkel cell excitation in SAI-LTMR firing, Maksimovic et al. made a mouse that expresses channelrhodopsin in Merkel cells. Light-induced activation of channelrhodopsin-expressing Merkel cells ex vivo gave rise to sustained afferent firing with an impaired dynamic phase (Maksimovic et al., 2014). This suggests that afferent stimulation is important for dynamic firing, while Merkel cells are most important for sustained firing during the static phase. As discussed earlier, Piezo2 is present in SAI terminals (Ranade et al., 2014), so it is likely that Piezo2 underlies SAI-LTMR mechanosensitivity during the dynamic phase.
Skin-specific Merkel cell Piezo2 deficiency also causes behavioral light touch deficits. Piezo2 CKO mice exhibit decreased sensitivity to von Frey filament stimulation of 1.5 g and lower (Woo et al., 2014). Mice injected with capsaicin in the facial region demonstrate nocifensive responses to low-speed whisker stimulation, while follicular injection of shRNA against Piezo2 significantly reduces this nocifensive behavior (Ikeda et al., 2014). This impairment is strikingly similar to that caused by the injection of the voltage-gated calcium channel blockers Cd2+, felodipine, and ω-conotoxin MVIIC (Ikeda et al., 2014), indicating that inhibition of Merkel cell activation can lead to behavioral light touch deficits. Together, these data support the idea that Piezo2 is a principal mechanotransducer in Merkel cells that helps the detection of light touch and whisker deflection in vivo.
6. NOCICEPTION
RNA in situ hybridization revealed that Piezo2 expression is not restricted to A- and C-type LTMRs. The channel is found in 60% of peripherin-expressing neurons, a group encompassing both peptidergic and nonpeptidergic C-fibers (Goldstein, House, & Gainer, 1991), and in 24% of TRPV1+ polymodal peptidergic nociceptors (Coste et al., 2010). This colocalization with TRPV1 is also observed on the functional level. In dissociated mouse DRG culture, small diameter neurons can be identified that display both fast, Piezo2-like MA currents and currents induced by capsaicin, a specific agonist of TRPV1 (Borbiro et al., 2015; Caterina et al., 2000). In such neurons, activation of TRPV1 by capsaicin almost completely eliminates fast MA current, an effect that can be reproduced in HEK293 cells coexpressing TRPV1 and Piezo2 (Borbiro et al., 2015). The mechanism of this inhibition is based on Ca2+-dependent depletion of phospholipids in the plasma membrane. Capsaicin is known to cause local analgesia, including numbing of mechanosensory perception, which follows the initial sensation of burning. The inhibition of Piezo2-mediated MA current in response to TRPV1 activation in nociceptors provides a molecular explanation for the analgesic effect of capsaicin (Borbiro et al., 2015).
Despite the expression of Piezo2 in murine nociceptors, it does not seem to be necessary for noxious mechanical sensation under normal conditions, as Piezo2 CKO in DRG neurons does not significantly change behavioral responses to pain (Ranade et al., 2014). However, Piezo2 appears to play a role in mechanical allodynia, a specific facet of pain sensitization whereby normally innocuous stimuli are perceived as painful as a result of tissue damage or inflammation. Nerve dissection leads to the production of cAMP, which contributes to mechanical allodynia via a mechanism involving activation of the cAMP sensor Epac1. Mechanical allodynia can be directly induced by the injection of a cAMP analog and Epac1 agonist 8-pCPT (Eijkelkamp et al., 2013). In dissociated large diameter DRG neurons, 8-pCPT is capable of potentiating fast MA current, a trend that holds true in HEK293T cells cotransfected with human Piezo2 and Epac1. Consistently, intrathecal injection of oligodeoxynucleotides against Piezo2 in mice partially rescues Epac1-mediated reduction in threshold to mechanical stimulation by von Frey filaments, both in 8-pCPT and injury models (Eijkelkamp et al., 2013).
The mechanism of the Epac1ePiezo2 cross talk is probably indirect and involves the activation of the Ras-like GTPase Rap1 via GDP to GTP exchange. Rap1, in turn, activates a host of signaling cascades, including PKC (Breckler et al., 2011), which was shown to facilitate Piezo2 current in neurons (Dubin et al., 2012). These results are also in line with the reported positive effect of GTP on Piezo2 current (Jia et al., 2013). A recent study determined that the Epac2–Piezo2 pathway is modulated by the phosphokinase GRK2, which phosphorylates Epac1, inhibiting the ensuing GDP–GTP exchange in Rap1 (Singhmar et al., 2016).
Interestingly, the Epac1-mediated allodynia persists in animals with a genomic ablation of Nav1.8, a voltage-gated ion channel critical for the generation of action potential in the majority of nociceptors (Akopian, Sivilotti, & Wood, 1996; Renganathan, Cummins, & Waxman, 2001; Sangameswaran et al., 1996; Shields et al., 2012). Thus the phenomenon appears to occur in a very specific subset of neurons, which await identification. A recent study detected Piezo2 expression in vascular endothelial cells. The injection of oligodeoxynucleotides against Piezo2 in the skin protects against endothelin- and oxaliplatin-induced pain, supporting a role for the channel in hyperalgesia (Ferrari et al., 2015). Overall, the involvement of Piezo2 in nociception has only started to emerge and requires further study.
While recent studies have revealed a likely role of Piezo2 in various types of painful responses, the channel is not the principal mechanotransducer of high-threshold mechanoreceptors (HTMRs). First, the deletion of Piezo2 in DRG neurons does not significantly alter pain responses (Ranade et al., 2014). Second, HTMRs are probably subserved by mechanotransducers with a Piezo2-independent slowly inactivating MA current (Hao & Delmas, 2010; Lechner et al., 2009; Rugiero et al., 2010), which exhibits properties different from the fast MA current mediated by Piezo2 (Coste et al., 2010; Hu & Lewin, 2006; Ranade et al., 2014). The identity of this molecule remains to be determined.
7. CONCLUSIONS AND PERSPECTIVES
Cutaneous and proprioceptive mechanosensation is dependent on mechanogated ion channels present in the neural and somatic components of mechanosensory end-organs. Evidence show that Piezo2 is expressed in SAI- and RAI-LTMRs, nociceptors, and in somatic Merkel cells. Additionally, knockout studies in mice show that Piezo2 is critical for many aspects of light touch and proprioception. In agreement with the mouse data, humans carrying non-functional Piezo2 display profound mechanosensory and proprioceptive deficits (Chesler et al., 2016; Delle Vedove et al., 2016; Mahmud et al., 2016). Knockdown experiments also indicate that it may play a role in the development of hyperalgesia. However, electrophysiological and behavioral studies demonstrate that Piezo2 is not the sole somatosensory mechanotransducer. It is firmly established that Piezo2 mediates fast inactivating MA current in neuronal mechanoreceptors, but the transducers of intermediate and slow inactivating MA current (Hong et al., 2016), and their physiological specialization in innocuous and noxious mechanosensation require further investigation.
The identification of neuronal mechanotransducers will help understand the relationship between the kinetics of MA current inactivation in the afferent ending and pattern of firing adaptation. This probably involves cross talk between mechanotransducers, voltage-gated ion channels that generate the action potential, and somatic components of the mechanoreceptive end organs, such as Merkel cells. Understanding the molecular identity and functional relationship between these components will reveal the mechanism of cutaneous receptor fine-tuning, which allows the end organs to detect mechanical stimuli of specific force, duration, and frequency. Further research using standard laboratory rodents and tactile specialists is needed to understand mechanosensation, which remains the least well-understood sense at the cellular and molecular level.
Footnotes
This work was supported by grants from National Science Foundation (1453167), National Institutes of Health (1R01NS097547-01A1) and American Heart Association (14SDG17880015) to S.N.B. E.R.S. was partially supported by a training grant from National Institutes of Health T32HD007094 and a postdoctoral fellowship from the Arnold and Mabel Beckman Foundation. E.O.A. is a fellow of The Gruber Foundation and an Edward L. Tatum Fellow.
References
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- Alamri A, Bron R, Brock JA, Ivanusic JJ. Transient receptor potential cation channel subfamily V member 1 expressing corneal sensory neurons can be subdivided into at least three subpopulations. Frontiers in Neuroanatomy. 2015;9:71. doi: 10.3389/fnana.2015.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S, Ladle DR, Lin JH, Frank E, Jessell TM. ETS gene Er81 controls the formation of functional connections between group Ia sensory afferents and motor neurons. Cell. 2000;101:485–498. doi: 10.1016/s0092-8674(00)80859-4. [DOI] [PubMed] [Google Scholar]
- Arends JJ, Dubbeldam JL. The subnuclei and primary afferents of the descending trigeminal system in the mallard (Anas platyrhynchos L.) Neuroscience. 1984;13:781–795. doi: 10.1016/0306-4522(84)90096-4. [DOI] [PubMed] [Google Scholar]
- Bagriantsev SN, Gracheva EO, Gallagher PG. Piezo proteins: Regulators of mechanosensation and other cellular processes. Journal of Biological Chemistry. 2014;289:31673–31681. doi: 10.1074/jbc.R114.612697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkhoudt H. The morphology and distribution of cutaneous mechanoreceptors (Herbst and Grandry corpuscles) in bill and tongue of the mallard (Anas platyrhynchos L.) Netherlands Journal of Zoology. 1980;30:1–34. [Google Scholar]
- Borbiro I, Badheka D, Rohacs T. Activation of TRPV1 channels inhibits mechanosensitive Piezo channel activity by depleting membrane phosphoinositides. Science Signaling. 2015;8:ra15. doi: 10.1126/scisignal.2005667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckler M, Berthouze M, Laurent AC, Crozatier B, Morel E, Lezoualc’h F. Rap-linked cAMP signaling Epac proteins: Compartmentation, functioning and disease implications. Cellular Signalling. 2011;23:1257–1266. doi: 10.1016/j.cellsig.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Bron R, Wood RJ, Brock JA, Ivanusic JJ. Piezo2 expression in corneal afferent neurons. Journal of Comparative Neurology. 2014;522 doi: 10.1002/cne.23560. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Julius D, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Chesler AT, Szczot M, Bharucha-Goebel D, Ceko M, Donkervoort S, Laubacher C, Bönnemann CG, et al. The role of PIEZO2 in human mechanosensation. The New England Journal of Medicine. 2016;375:1355–1364. doi: 10.1056/NEJMoa1602812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Sun D, Jakobs TC. Astrocytes in the optic nerve head express putative mechanosensitive channels. Molecular Vision. 2015;21:749–766. [PMC free article] [PubMed] [Google Scholar]
- Coste B, Crest M, Delmas P. Pharmacological dissection and distribution of NaN/Nav1.9, T-type Ca2+ currents, and mechanically activated cation currents in different populations of DRG neurons. Journal of General Physiology. 2007;129:57–77. doi: 10.1085/jgp.200609665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Houge G, Murray MF, Stitziel N, Bandell M, Giovanni MA, Patapoutian A, et al. Gain-of-function mutations in the mechanically activated ion channel Piezo2 cause a subtype of distal arthrogryposis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4667–4672. doi: 10.1073/pnas.1221400110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Patapoutian A, et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science. 2010;330:55–60. doi: 10.1126/science.1193270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Murthy SE, Mathur J, Schmidt M, Mechioukhi Y, Delmas P, Patapoutian A. Piezo1 ion channel pore properties are dictated by C-terminal region. Nature Communications. 2015;6:7223. doi: 10.1038/ncomms8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delle Vedove A, Storbeck M, Heller R, Hölker I, Hebbar M, Shukla A, Wirth B, et al. Biallelic loss of proprioception-related PIEZO2 causes muscular atrophy with perinatal respiratory distress, arthrogryposis, and scoliosis. The American Journal of Human Genetics. 2016;99:1206–1216. doi: 10.1016/j.ajhg.2016.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Rugiero F, Cesare P, Gale JE, Abrahamsen B, Bowden S, Wood JN, et al. High-threshold mechanosensitive ion channels blocked by a novel conopeptide mediate pressure-evoked pain. PLoS One. 2007;2:e515. doi: 10.1371/journal.pone.0000515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew LJ, Wood JN. FM1-43 is a permeant blocker of mechanosensitive ion channels in sensory neurons and inhibits behavioural responses to mechanical stimuli. Molecular Pain. 2007;3:1. doi: 10.1186/1744-8069-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubbeldam JL, Brauch CS, Don A. Studies on the somatotopy of the trigeminal system in the mallard, Anas platyrhynchos L. III. Afferents and organization of the nucleus basalis. Journal of Comparative Neurology. 1981;196:391–405. doi: 10.1002/cne.901960304. [DOI] [PubMed] [Google Scholar]
- Dubin AE, Schmidt M, Mathur J, Petrus MJ, Xiao B, Coste B, Patapoutian A. Inflammatory signals enhance piezo2-mediated mechanosensitive currents. Cell Reports. 2012;2:511–517. doi: 10.1016/j.celrep.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eijkelkamp N, Linley JE, Torres JM, Bee L, Dickenson AH, Gringhuis M, Wood JN, et al. A role for Piezo2 in EPAC1-dependent mechanical allodynia. Nature Communications. 2013;4:1682. doi: 10.1038/ncomms2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucherre A, Nargeot J, Mangoni ME, Jopling C. piezo2b regulates vertebrate light touch response. Journal of Neuroscience. 2013;33:17089–17094. doi: 10.1523/JNEUROSCI.0522-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari LF, Bogen O, Green P, Levine JD. Contribution of Piezo2 to endothelium-dependent pain. Molecular Pain. 2015;11:65. doi: 10.1186/s12990-015-0068-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming MS, Luo W. The anatomy, function, and development of mammalian Abeta low-threshold mechanoreceptors. Frontiers in Biology. 2013;8 doi: 10.1007/s11515-013-1271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florez-Paz D, Bali KK, Kuner R, Gomis A. A critical role for Piezo2 channels in the mechanotransduction of mouse proprioceptive neurons. Scientific Reports. 2016;6:25923. doi: 10.1038/srep25923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J, Li W, Zhao Q, Li N, Chen M, Zhi P, Yang M, et al. Architecture of the mammalian mechanosensitive Piezo1 channel. Nature. 2015;527 doi: 10.1038/nature15247. [DOI] [PubMed] [Google Scholar]
- Glokowska E, Gallagher PG. Disorders of erythrocyte volume homeostasis. International Journal of Laboratory Hematology. 2015;37(Suppl. 1):85–91. doi: 10.1111/ijlh.12357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein ME, House SB, Gainer H. NF-L and peripherin immunoreactivities define distinct classes of rat sensory ganglion cells. Journal of Neuroscience Research. 1991;30:92–104. doi: 10.1002/jnr.490300111. [DOI] [PubMed] [Google Scholar]
- Gottschaldt KM. The physiological basis of tactile sensibility in the beak of geese. Journal of Comparative Physiology. 1974;95:29–47. [Google Scholar]
- Gregory JE. An electrophysiological investigation of the receptor apparatus of the duck’s bill. Journal of Physiology. 1973;229:151–164. doi: 10.1113/jphysiol.1973.sp010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Delmas P. Multiple desensitization mechanisms of mechanotransducer channels shape firing of mechanosensory neurons. Journal of Neuroscience. 2010;30:13384–13395. doi: 10.1523/JNEUROSCI.2926-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong G-S, Lee B, Wee J, Chun H, Kim H, Jung J, Oh U, et al. Tentonin 3/TMEM150c confers distinct mechanosensitive currents in dorsal-root ganglion neurons with proprioceptive function. Neuron. 2016;91:107–118. doi: 10.1016/j.neuron.2016.05.029. [DOI] [PubMed] [Google Scholar]
- Honore E, Martins JR, Penton D, Patel A, Demolombe S. The piezo mechanosensitive ion channels: May the force be with you! Reviews of Physiology. Biochemistry and Pharmacology. 2015;169:25–41. doi: 10.1007/112_2015_26. [DOI] [PubMed] [Google Scholar]
- Hu J, Lewin GR. Mechanosensitive currents in the neurites of cultured mouse sensory neurones. Journal of Physiology. 2006;577:815–828. doi: 10.1113/jphysiol.2006.117648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda R, Cha M, Ling J, Jia Z, Coyle D, Gu JG. Merkel cells transduce and encode tactile stimuli to drive abeta-afferent impulses. Cell. 2014;157:664–675. doi: 10.1016/j.cell.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Ikeda R, Ling J, Gu JG. GTP-dependent run-up of Piezo2-type mechanically activated currents in rat dorsal root ganglion neurons. Molecular Brain. 2013;6:57. doi: 10.1186/1756-6606-6-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Ikeda R, Ling J, Viatchenko-Karpinski V, Gu JG. Regulation of Piezo2 mechanotransduction by static plasma membrane tension in primary afferent neurons. Journal of Biological Chemistry. 2016;291:9087–9104. doi: 10.1074/jbc.M115.692384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatibi Shahidi M, Krivanek J, Kaukua N, Ernfors P, Hladik L, Kostal V, Fried K, et al. Three-dimensional imaging reveals new compartments and structural adaptations in odontoblasts. Journal of Dental Research. 2015;94 doi: 10.1177/0022034515580796. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. Journal of Comparative Neurology. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- Lazarov NE. Neurobiology of orofacial proprioception. Brain Research Reviews. 2007;56:362–383. doi: 10.1016/j.brainresrev.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Le Pichon CE, Chesler AT. The functional and anatomical dissection of somatosensory subpopulations using mouse genetics. Frontiers in Neuroanatomy. 2014;8:21. doi: 10.3389/fnana.2014.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner SG, Frenzel H, Wang R, Lewin GR. Developmental waves of mechanosensitivity acquisition in sensory neuron subtypes during embryonic development. EMBO Journal. 2009;28:1479–1491. doi: 10.1038/emboj.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Leddy HA, Chen Y, Lee SH, Zelenski NA, McNulty AL, Liedtke WB, et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E5114–E5122. doi: 10.1073/pnas.1414298111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner LM, Roumy M. Mechanosensitive units in the upper bill and in the tongue of the domestic duck. Pflügers Archiv. 1974;346:141–150. doi: 10.1007/BF00587013. [DOI] [PubMed] [Google Scholar]
- Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Ginty DD, et al. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell. 2011;147:1615–1627. doi: 10.1016/j.cell.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein WR, Mendelson M. Components of receptor adaptation in a Pacinian corpuscle. Journal of Physiology. 1965;177:377–397. doi: 10.1113/jphysiol.1965.sp007598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou S, Duan B, Vong L, Lowell BB, Ma Q. Runx1 controls terminal morphology and mechanosensitivity of VGLUT3-expressing C-mechanoreceptors. Journal of Neuroscience. 2013;33:870–882. doi: 10.1523/JNEUROSCI.3942-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmud AA, Nahid NA, Nassif C, Sayeed MS, Ahmed MU, Parveen M, Michaud JL, et al. Loss of the proprioception and touch sensation channel PIEZO2 in siblings with a progressive form of contractures. Clinical genetics. 2016 doi: 10.1111/cge.12850. [Epub 09/09/2016] [DOI] [PubMed] [Google Scholar]
- Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, Lumpkin EA, et al. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature. 2014;509:617–621. doi: 10.1038/nature13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter GC, Levine JD. Ionic basis of a mechanotransduction current in adult rat dorsal root ganglion neurons. Molecular Pain. 2006;2:28. doi: 10.1186/1744-8069-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter GC, Reichling DB, Levine JD. Mechanical transduction by rat dorsal root ganglion neurons in vitro. Neuroscience Letters. 1999;273:179–182. doi: 10.1016/s0304-3940(99)00665-5. [DOI] [PubMed] [Google Scholar]
- McMillin MJ, Beck AE, Chong JX, Shively KM, Buckingham KJ, Gildersleeve HI, Bamshad MJ, et al. Mutations in Piezo2 cause Gordon syndrome, Marden-Walker syndrome, and distal arthrogryposis type 5. American Journal of Human Genetics. 2014;94:734–744. doi: 10.1016/j.ajhg.2014.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson M, Lowenstein WR. Mechanisms of receptor adaptation. Science. 1964;144:554–555. doi: 10.1126/science.144.3618.554. [DOI] [PubMed] [Google Scholar]
- Narayanan P, Sondermann J, Rouwette T, Karaca S, Urlaub H, Mitkovski M, Schmidt M, et al. Native Piezo2 interactomics identi3es Pericen-trin as a novel regulator of Piezo2 in somatosensory neurons. Journal of Proteome Research. 2016;15:2676–2687. doi: 10.1021/acs.jproteome.6b00235. [DOI] [PubMed] [Google Scholar]
- Okubo M, Fujita A, Saito Y, Komaki H, Ishiyama A, Takeshita E, Sasaki M, et al. A family of distal arthrogryposis type 5 due to a novel Piezo2 mutation. American Journal of Medical Genetics A. 2015;(5) doi: 10.1002/ajmg.a.36881. [DOI] [PubMed] [Google Scholar]
- Poole K, Herget R, Lapatsina L, Ngo HD, Lewin GR. Tuning Piezo ion channels to detect molecular-scale movements relevant for 3ne touch. Nature Communications. 2014;5:3520. doi: 10.1038/ncomms4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proske U, Gandevia SC. The proprioceptive senses: Their roles in signaling body shape, body position and movement, and muscle force. Physiological Reviews. 2012;92:1651–1697. doi: 10.1152/physrev.00048.2011. [DOI] [PubMed] [Google Scholar]
- Qi Y, Andolfi L, Frattini F, Mayer F, Lazzarino M, Hu J. Membrane stiffening by STOML3 facilitates mechanosensation in sensory neurons. Nature Communications. 2015;6:8512. doi: 10.1038/ncomms9512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade SS, Syeda R, Patapoutian A. Mechanically activated ion channels. Neuron. 2015;87:1162–1179. doi: 10.1016/j.neuron.2015.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Patapoutian A, et al. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature. 2014;516:121–125. doi: 10.1038/nature13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renganathan M, Cummins TR, Waxman SG. Contribution of Na(v)1.8 sodium channels to action potential electrogenesis in DRG neurons. Journal of Neurophysiology. 2001;86:629–640. doi: 10.1152/jn.2001.86.2.629. [DOI] [PubMed] [Google Scholar]
- Rugiero F, Drew LJ, Wood JN. Kinetic properties of mechanically activated currents in spinal sensory neurons. Journal of Physiology. 2010;588:301–314. doi: 10.1113/jphysiol.2009.182360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangameswaran L, Delgado SG, Fish LM, Koch BD, Jakeman LB, Stewart GR, Herman RC, et al. Structure and function of a novel voltage-gated, tetrodotoxin-resistant sodium channel specific to sensory neurons. Journal of Biological Chemistry. 1996;271:5953–5956. doi: 10.1074/jbc.271.11.5953. [DOI] [PubMed] [Google Scholar]
- Saxod R. Ontogeny of the cutaneous sensory organs. Microscopy Research and Technique. 1996;34:313–333. doi: 10.1002/(SICI)1097-0029(19960701)34:4<313::AID-JEMT4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Schneider ER, Gracheva EO, Bagriantsev SN. Evolutionary specialization of tactile perception in vertebrates. Physiology. 2016;31:193–200. doi: 10.1152/physiol.00036.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ER, Mastrotto M, Laursen WJ, Schulz VP, Goodman JB, Funk OH, Bagriantsev SN, et al. Neuronal mechanism for acute mechanosen-sitivity in tactile-foraging waterfowl. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:14941–14946. doi: 10.1073/pnas.1413656111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif-Naeini R. Contribution of mechanosensitive ion channels to somatosensation. Progress in Molecular Biology and Translational Science. 2015;131:53–71. doi: 10.1016/bs.pmbts.2014.11.011. [DOI] [PubMed] [Google Scholar]
- Shields SD, Ahn HS, Yang Y, Han C, Seal RP, Wood JN, Dib-Hajj SD, et al. Nav1.8 expression is not restricted to nociceptors in mouse peripheral nervous system. Pain. 2012;153:2017–2030. doi: 10.1016/j.pain.2012.04.022. [DOI] [PubMed] [Google Scholar]
- Singhmar P, Huo X, Eijkelkamp N, Berciano SR, Baameur F, Mei FC, Kavelaars A, et al. Critical role for Epac1 in in3ammatory pain controlled by GRK2-mediated phosphorylation of Epac1. Proceedings of the National Academy of Sciences of the United States of America. 2016;113 doi: 10.1073/pnas.1516036113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkers L, Mechioukhi Y, Coste B. Piezo channels: From structure to function. Pflügers Archiv. 2015;467:95–99. doi: 10.1007/s00424-014-1578-z. [DOI] [PubMed] [Google Scholar]
- Wang F, Knutson K, Alcaino C, Linden DR, Gibbons SJ, Kashyap P, Beyder A, et al. Mechanosensitive ion channel Piezo2 is important for enterochromaf3n cell response to mechanical forces. Journal of Physiology. 2016 doi: 10.1113/JP272718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel C, Hu J, Riethmacher D, Benckendorff A, Harder L, Eilers A, Lewin GR, et al. A stomatin-domain protein essential for touch sensation in the mouse. Nature. 2007;445:206–209. doi: 10.1038/nature05394. [DOI] [PubMed] [Google Scholar]
- Woo SH, Lukacs V, de Nooij JC, Zaytseva D, Criddle CR, Francisco A, Patapoutian A, et al. Piezo2 is the principal mechanotransduction channel for proprioception. Nature Neuroscience. 2015;18 doi: 10.1038/nn.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Patapoutian A, et al. Piezo2 is required for Merkel-cell mechanotransduction. Nature. 2014;509:622–626. doi: 10.1038/nature13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Grillet N, Zhao B, Cunningham C, Harkins-Perry S, Coste B, Ulrich M, et al. Mechanosensory hair cells express two molecularly distinct mechanotransduction channels. Nature Neuroscience. 2016 doi: 10.1038/nn.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Lewis AH, Grandl J. Touch, tension, and transduction - The function and regulation of Piezo ion channels. Trends in Biochemical Sciences. 2016 doi: 10.1016/j.tibs.2016.09.004. [Epub 10/17/2016] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Wu K, Geng J, Chi S, Wang Y, Zhi P, Xiao B, et al. Ion permeation and mechanotransduction mechanisms of mechanosensitive piezo channels. Neuron. 2016;89:1248–1263. doi: 10.1016/j.neuron.2016.01.046. [DOI] [PubMed] [Google Scholar]
- Zimmerman A, Bai L, Ginty DD. The gentle touch receptors of mammalian skin. Science. 2014;346:950–954. doi: 10.1126/science.1254229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweers GA. Mechanics of the feeding of the mallard (Anas Platyrhynchos, L; Aves, Anseriformes) 1. S Karger Pub; 1977. [Google Scholar]


