Summary
Observations noting the presence of white blood cell infiltrates within tumors date back more than a century, however the cellular and molecular mechanisms regulating tumor immunity continue to be elucidated. The recent successful use of monoclonal antibodies to block immune regulatory pathways to enhance tumor-specific immune responses for the treatment of cancer has encouraged the identification of additional immune regulatory receptor/ligand pathways. Over the past several years, a growing body of data has identified B7-H4 (VTCN1/B7x/B7S1) as a potential therapeutic target for the treatment of cancer. The potential clinical significance of B7-H4 is supported by the high levels of B7-H4 expression found in numerous tumor tissues and correlation of the level of expression on tumor cells with adverse clinical and pathologic features, including tumor aggressiveness. The biological activity of B7-H4 has been associated with decreased inflammatory CD4+ T-cell responses and a correlation between B7-H4-expressing tumor-associated macrophages and FoxP3+ regulatory T cells (Tregs) within the tumor microenvironment. Since B7-H4 is expressed on tumor cells and tumor-associated macrophages in various cancer types, therapeutic blockade of B7-H4 could favorably alter the tumor microenvironment allowing for antigen-specific clearance tumor cells. The present review highlights the therapeutic potential of targeting B7-H4.
Keywords: B7-H4, B7-H4 receptor, cancer, CD4+ T cell, co-stimulatory/co-inhibitory molecule, regulatory T cell
1 | INTRODUCTION
The immune system of humans and other mammals is responsible for providing protection against both infection and the possibility of neoplastic transformation. Such protection is mediated both by a humoral immune response and by a cell-mediated immune response. The humoral response results in the production of antibodies and other biomolecules that are capable of recognizing and neutralizing foreign targets (antigens). In contrast, the cell-mediated immune response involves the activation of macrophages, natural killer cells, and antigen-specific cytotoxic T cells, and the release of various cytokines in response to the recognition of an antigen.1 An important goal of current research in both autoimmune disease and cancer treatment is to develop new therapies to specifically targeting immune cells. In autoimmunity, the goal is to alter T-cell receptor (TCR) and/or co-stimulatory molecule signaling to decrease deleterious inflammatory immune responses, while in cancer immunotherapies are intended to increase inflammatory immune cell function thereby allowing for increased tumor killing. The recent significant successes in cancer treatment with antibodies specific for the immune regulatory molecules programmed death-ligand 1 (anti-PD-L1), 2 programmed death-1 (anti-PD-1), 3 and cytotoxic T-lymphocyte-associated protein 4 (anti-CTLA-4) 4 has given impetus to the development of additional immunotherapeutic approaches for the cancer treatment. While the use of anti-PD-L1 and anti-CTLA-4 has been shown to increase life expectancy in patients with certain cancers, a significant proportion of treated patients do not respond, and these drugs are not useful in all cancers.2,4,5 Therefore, the rationale exists to develop alternative immune modulatory therapies that may be functional in the non-responder patient population.
The present review will focus on the biological activity of V-set domain containing T-cell activation inhibitor 1 (VTCN1/B7x/B7S1/ B7 homolog 4), commonly known as B7-H4, and its role in immune cell function. The main body of work completed to date has been a mix of studies assessing the ability of an agonistic B7-H4 immunoglobulin fusion protein (B7-H4 Ig) to be used as a therapeutic in mouse models of autoimmune disease, the use of B7-H4 knockout mice in various model systems, and the expression profile of B7-H4 within human tumors. While the summation of the data published to date all point toward B7-H4 functionally decreasing inflammatory CD4+ T cell directly and B7-H4 directly increasing the number and function of regulatory CD4+ T cells, the actual ability of B7-H4 blockade to decrease tumor burden will not be fully known until both consistent B7-H4 positive mouse tumor models as well as high quality anti-B7-H4 monoclonal antibodies are fully interrogated in a systematic manner.
2 | T-CELL ACTIVATION AND THE ROLE OF CLASSICAL B7 PROTEINS
The requirement of naive T cells to receive two signals to become fully activated was first proposed by Lafferty and Cunningham.6 This two-signal hypothesis has become the basis for many potential therapeutics for the treatment of autoimmune disease and cancer. The first signal received by a naive CD4+ T cell is from the antigen-specific TCR interacting with an antigenic peptide presented in the context of major histocompatibility complex II (MHC II) on the surface of professional antigen-presenting cells (APCs). The antigenic peptides presented by MCH II are derived from both self and non-self antigens that have been phagocytosed, processed and presented by the APC. Conversely, CD8+ T cells receive signal one via antigen-specific TCR recognition of peptides presented in the context of MHC I, and these peptides are derived from self, viral, intracellular bacterial, and tumor expressed antigens by various cells within the body.7,8 Therapies designed to regulate this first signal to CD4+ T cells have been explored via the use of non-mitogenic anti-CD3 and altered peptide ligands. 9–12 In the field of cancer immunotherapy, the use of chimeric antigen receptor (CAR) T cells has been studied to determine if tumor antigen-specific T cells will allow for lead to tumor killing and disease clearance.13,14
The second set of signals required for antigen-specific T-cell activation are delivered via co-stimulatory molecules that are expressed on the cell surface of activated APCs, and cytokines that are either produced by the APC and/or by the activated CD4+ T cell itself. Classically, B7-1 (CD80) and B7-2 (CD86) expressed on the surface of the APC interact with the co-receptor CD28 that is constitutively expressed on the surface of naive CD4+ T cells.15 The overall effect of CD28 ligation is to increase the level of proliferation and cytokine production, promote cell survival, and enhance expression of CD40 ligand (CD40L) and adhesion molecules necessary for trafficking, such as very late antigen-4 (VLA-4) (α4β1 integrin). 16 Following activation, T cells begin to express the negative regulatory protein, CTLA-4 that also interacts with CD28. The co-stimulatory molecule pairs, CD28:CD80/CD86 and CD40:CD40L, and cellular adhesion molecules, such as VLA-4, represent putative therapeutic targets for blockade of autoreactive CD4+ T-cell activation and trafficking to inflammatory sites. For example, the blockade and conversely the stimulation of CD80/CD86:CD28/ CTLA-4 interaction has been tested clinically. In autoimmune disease indications of rheumatoid arthritis, CTLA-4 Ig has been shown to inhibit CD4+ T-cell action.17 On the converse side of the CD80/ CD86:CD28/CTLA-4 interaction, a monoclonal antibody specific for CTLA-4 has been shown to enhance inflammatory T-cell function and tumor clearance.4 The efficacy of the aforementioned therapeutics provided the rationale for the development of new immune-based therapeutics that can either block or conversely stimulate the same immune regulatory pathways to treat both autoimmune and cancer, respectively.
3 | B7 SUPERFAMILY PROTEINS
The ability of the immune system to decrease peripheral activation of self-reactive T cells is dependent on the level and type of co-stimulatory molecules expressed on the surface of APCs, and/or the type of co-stimulatory molecule receptors expressed by CD4+ T cells.18 Alternatively, ligation of inhibitory molecules associated with CD4+ T cells during and/or following T-cell activation can effectively suppress T-cell responses. Over the past several decades, multiple B7/CD28-family members have been identified. CD80 is classically known to interact with both CD28 and CTLA-4, but more recent data show that CD80 also interacts with PD-L1/ B7-H1.19 To further confound the study of B7/CD28-family member proteins, the resultant functionality of a specific antibody treatment is also dependent upon the immune cell type targeted, as well as the specific antibody clone used. One such example is that cross-linking of CD80 on B cells induces a decrease in B-cell function and survival,20 while cross-linking CD80 on CD4+ T cell induces an increase in inflammatory cytokine secretion and increased CD4+ T-cell survival.21 Blockade of co-stimulatory signals, e.g. with CTLA-4 Ig to block CD28-mediated signal 2, has proven not to be the panacea for autoimmune disease therapy that was originally envisioned. However, monoclonal antibody blockade of co-inhibitory receptors, e.g. CTLA-4 and PD-1, has proven extremely useful for enhancing the immune response in cancer therapy.2,4
Further investigations into the ligands of the CD28 receptor have led to the identification and characterization of a set of related B7 molecules (the “B7 Superfamily”).22,23 There are at least eight members of the B7-family: B7-1 (CD80), B7-2 (CD86), the inducible co-stimulator ligand (ICOS-L; B7-H2), the programmed death-1 ligand (PD-L1; B7-H1), the programmed death-2 ligand (PD-L2; B7-DC), B7-H3 (B7-RP2), B7-H4,24,25 and B7-H6.26 Besides the typical co-stimulatory function of B7/CD28-family members, recent data show that some B7-family member proteins function by skewing CD4+ T-cell responses toward a specific effector phenotype. An example of an immune regulatory receptor/ligand pair is PD-1/ PD-L1. Mice deficient in PD-L1 expression show an increase in the level of myelin oligodendrocyte glycoprotein peptide (MOG35-55)-induced chronic experimental allergic encephalomyelitis (C-EAE) as compared to wildtype C57BL/6 mice, and treatment of mice with a blocking anti-PD-L1 monoclonal antibody induces an increase in the number of MOG35-55-specific CD4+ T cells-producing gamma-interferon (IFN-γ) and interleukin-17 (IL-17). 27 Also, activation of naive CD4+ T cells in the presence of beads coated with anti-CD3/ 28 plus PD-L1 Ig in the presence of CD4+ T-helper cell (Th1 cell)-or Th17 cell-promoting conditions has been shown to decrease the number of resultant IFN-γ- and IL-17-producing cells, respectively, while increasing the number of CD25+/forkhead box 3+ (Foxp3+) cells when activated in the presence of induced regulatory CD4+ T cell (iTreg cell)-promoting conditions.28
The ICOS/ICOS-L interaction has been shown to favor Th2 cell differentiation, and IL-4 production.29 In contrast to this finding, the induction of local expression of ICOS Ig within a xenograft led to increased graft survival, and potentially increased the number of CD4+CD25+Foxp3+ T cells at the periphery of the graft.30 Besides PD-L1, PD-L2 is also a ligand for PD-1,22 and PD-L2 Ig has been shown to have a two–sixfold higher affinity for PD-1 as compared to PD-L1.31 While a complete understanding of the biological significance of the differential PD-L2 and PD-L1 interaction with PD-1 remains to be determined, PD-L2 knockout mice exhibit reduced levels of IFN-γ, reduced IgG2a responses, and a decreased capacity to clear hepatic tumors. 32 Additionally, PD-L2 has been shown to interact with repulsion guidance molecule b (RGMb).33 The identification of this additional receptor for PD-L2 points to the presence of other possible non-classical receptors and receptor complexes for B7-family member proteins. In the case of PD-L2: RGMb interaction, these proteins are thought to form a large multimeric protein complex composed of the primary PD-L2-binding partner protein, i.e. RGMb, that interacts with the other proteins within a multimeric protein complex, i.e. bone morphogenetic protein (BMP) and neogenin, which serve as the signaling component of the PD-L2: RBGMb/BMP/neogenin complex.33 This finding serves as a shift away from the strict assumption of a one-to-one protein interaction for B7-family member proteins with the associated functional receptors. Therefore, while PD-L1 and PD-L2 have a shared receptor, i.e. PD-1, there are differences in function when comparing the two different ligands. This difference may be due to the higher affinity of PD-L2 for PD-1 as compared to PD-L1, and PD-L1 interacting with CD80 vs PD-L2 interacting with RBGMb/BMP/neogenin. These data support the hypothesis that some of the more novel B7-family members regulate CD4+ T-cell differentiation toward specific effector cell phenotypes, in contrast to the function of CD28 expressed on CD4+ T cells.
As mentioned previously, the resultant effect of monoclonal antibody treatment is dependent upon the clone of monoclonal antibody used. For example, the 10F.9G2 clone of anti-PD-L1 monoclonal antibody blocks both the PD-1: PD-L1 interaction and the CD80:PD-L1 interaction, while the 10F.2H11 clone only blocks the CD80:PD-L1 interaction and not the PD-1: PD-L1 interaction.34 Likewise for anti-CD80 monoclonal antibodies, the 1G10 clone blocks both the CD28:CD80 and CTLA-4: CD80 interactions, as well as the CD80:PD-1 interaction. In contrast, the 16-10A1 clone only blocks the CD28:CD80, CTLA-4: CD80 interaction,34 while also inducing direct intracellular signaling within CD80+ target cells,21 and does not inhibit the CD80:PD-1 interaction.34 Additionally, several of the B7/ CD28-family members interact with as yet unidentified receptors, and still other B7-family member proteins, like B7-H6, interact with non-classical CD28-like receptors, i.e. NKp30 expressed on the surface of NK cells.26,35,36 Therefore, the specific cell type targeted, the level of the targeted molecule and the associated ligands expressed within the tissue(s), as well as the specific monoclonal antibody used must all be considered when analyzing experimental results.
4 | B7-H4 IDENTIFICATION AND EXPRESSION
B7-H4, also known as B7x, B7S1 or VTCN1, was first identified via bioinformatics by three separate laboratories,24,37–39 and the existence of B7-H4 was later shown via expression of both B7-H4 mRNA and B7-H4 protein by human serous ovarian cancers and breast cancers, while relatively little to no B7-H4 was found to be expressed within normal tissues.40 The B7-H4 protein possesses 282 amino acid residues, which have been categorized as comprising an amino terminal extracellular domain, a large hydrophobic transmembrane domain and a very short intracellular domain (consisting of only two amino acid residues). Like other B7-family members, B7-H4 possesses a pair of Ig-like regions in its extracellular domain. The B7-H4 protein has an overall structure of a type-I transmembrane protein. The protein has minimal (about 25%) homology with other B7-family members.41 The human B7-H4 cDNA sequence has been used to identify a murine B7-H4 homolog. The level of identity between the murine and human orthologs (approximately 87%) suggests that B7-H4 is highly conserved evolutionarily.24
In contrast to other B7-family members that have tightly regulated mRNA expression patterns, B7-H4 mRNA is widely expressed. Its expression has been found in the brain, heart, kidney, liver, lung, ovary, pancreas, placenta, prostate, skeletal muscle, skin, small intestine, spleen, stomach, testis, thymus, thymus, and uterus.24,25 However, despite the widespread expression of B7-H4 mRNA, the presence of B7-H4 protein on the surface of normal cells is limited.24 For example, freshly isolated human T cells, B cells, monocytes, and dendritic cells do not express B7-H4 on their cell surfaces, however B7-H4 expression can be induced on such cells after in vitro stimulation with lipopolysaccharides (LPS), phytohemagglutinin (PHA), IFN-γ, phorbol 12-myristate 13-acetate (PMA), or ionomycin.24 Additionally, the expression of B7-H4 changes in various strains of mice with age. For example, a noticeable decrease in the expression of B7-H4 within the pancreatic islets of NOD mice is present by approximately 10 weeks of age and mice present with a significant loss of B7-H4 expression by 15 weeks of age.42 In contrast to the decreased expression of B7-H4 protein by pancreatic islet cells, the level of B7-H4 mRNA appeared to be increased within the pancreatic islets of NOD mice during this same timeframe. Therefore, there is an apparent disparity between the level of B7-H4 expression on the pancreatic islet cells and the level of B7-H4 mRNA expressed by the same population of cells. This disparity between the protein and mRNA expression profiles in the NOD was found to correlate with an increase in the level of soluble B7-H4 present within the blood. Besides B7-H4 being present on the cell surface, B7-H4 can also be cleaved from the surface of cells via the metalloproteinase nardilysin (N-arginine dibasic convertase 1 (NRD1)).42 While the immunofluorescent staining of pancreas sections shows an age-related decrease in B7-H4 expression by pancreatic islet cells, the level of NRD1 expression within the pancreatic islets is increased. In NOD mice, the loss pancreatic islet cell expressed B7-H4 may either be a genetically induced phenotype or this alteration in the level of surface B7-H4 may be induced by inflammatory immune cells infiltrating into the pancreatic islets. The loss of B7-H4 from the pancreatic islet cells did not appear to be solely due to the presence of inflammatory immune cells, as determined by the transfer of splenocytes from diabetic NOD mice into B6g7 mice.43 Therefore, it was concluded that a genetic difference between strains of mice may be responsible for this alteration in NRD1-induced cleavage of B7-H4 from the surface of pancreatic islet cells. Taken together, the expression of B7-H4 cell surface protein appears to be activation induced and may represent an immune mechanism by which immune homeostasis can be induced within sites of inflammation. Additionally, the finding of such a wide distribution of B7-H4 expression suggests that the function of B7-H4 is quite distinct from that of other inhibitory B7 molecules.41
In addition to cell surface expressed co-stimulatory molecules, the presence or absence of secreted cytokines may affect immune cell function. For example, the production of IFN-γ or IL-4 by activated CD4+ T cells, or IL-12 by APCs directs the local population of naive CD4+ T cells to differentiate toward the IFN-γ-producing Th1 cell or IL-4-producing Th2 cell phenotype, respectively.44 Recently, a third population of CD4+ effector T cells has been identified that secrete IL-17. Th17 cells secrete IL-17, IL-6, IL-22, GM-CSF, and TNF-α, and these cells have been shown to differentiate from a naive CD4+ T cells activated in the presence of TGF-β and IL-6.45 CD4+ Th17 cells are critical for the induction of experimental autoimmune encephalomyelitis (EAE). This finding runs counter to the historical hypothesis that EAE is a Th1 cell-mediated disease, but explains the findings that EAE is exacerbated in mice lacking IFN-γ or the IFN-γ receptor. Interestingly, EAE is differentially decreased in the p35 knockout and p40 knockout mice,46 i.e. the two subunits that make up IL-12, but this may partly be explained by the decrease in the level of IL-17 produced and the survival of Th17 cells due to an absence of IL-23.47
During immune homeostasis, there is a balance between the activities of pro-inflammatory and anti-inflammatory T cells such that immune surveillance is maintained. Evidence has emerged that TGF-β is a critical differentiation factor that regulates this balance dependent upon the absence or presence of IL-6. The cytokine TGF-β is a critical differentiation factor for the generation of Treg cells in the presence of IL-2. On the opposing side of this balance, if the naive CD4+ T cells are activated in the presence of both TGF-β and IL-6, the resulting cells differentiate into a Th17 cell phenotype. Therefore, the development of an immune-mediated therapy for autoimmune disease may work either alone or in combination via one of three possible mechanisms: (i) induction of energy in self-reactive CD4+ T cells; (ii) deletion of self-reactive CD4+ T cells by apoptosis; and/ or (iii) immune deviation/regulation. Similarly, the seeming counter intuitive findings may also be explained by the downstream immune regulatory role of pro-inflammatory cytokines, such as IFN-γ that induces the expression of the immune inhibitory protein PD-L1.48,49 Similarly, both recombinant and tumor microenvironment-derived IL-6 and IL-10 have been shown to stimulate monocyte/macrophage B7-H4 expression,50 while GM-CSF was found to reduce B7-H4 expression. This observation is similar to the regulatory mechanism for B7-H4 expression on myeloid dendritic cells.51 Locally produced IL-6 and IL-10 may be secreted by a combination of tumor cells, tumor-associated macrophages, and Treg cells present within the local tumor microenvironment.52,53 As will be discussed below, there is a functional connection between B7-H4 and the number/function of cells. Therefore, these data provide a potential mechanistic link by which IL-6, IL-10, B7-H4, and Treg cells present in the tumor microenvironment are functionally connected.
5 | B7-H4 EXPRESSION AND CANCER
Recent studies have demonstrated various cancer cells and tissues overexpress B7-H4 protein,54–57 and that B7-H4 protein expression within tumors correlates with various pathological and clinical characteristics. The expression level of B7-H4 protein in ovarian cancer is related to cancer types, cancer stage, the numbers of Tregs, and patient survival.58–60 For example, the data from 107 ovarian carcinoma patients show that patients with a higher level of B7-H4 expressed by tumor-associated macrophages had an increased number of Treg cells within the tumors, and these patients had a shorter life expectancy. 60 Additionally, more than 90% of endometrial and breast cancers express B7-H4 protein,61 and B7-H4 protein expression in renal cell carcinoma,62–64 melanoma,65 breast,66 lung,67,68 gastric,69 colorectal, 70 pancreatic,71 and prostate72 cancer are associated with one or more clinicopathological factors, including increased tumor size, increased primary tumor classification, increased TNM malignant tumor score, decreased survival, and decreased number of tumor-infiltrating T cells.
For example, frozen tissue samples from 259 renal carcinoma patients that had been treated with nephrectomy were stratified into 106 patients with B7-H4− tumors, and 153 patients with B7-H4+ tumors. The data show that 77 of the 153 patients with B7-H4+ tumors had a primary tumor size of 7 cm or greater, as compared to only 27 out of the 106 patients that had B7-H4− tumors.63 Similarly, from this same cohort of patients 84 of 153 (54.9%) B7-H4+ tumor patients had a primary tumor classification of pT2 or greater, and 85 of 153 (55.6%) B7-H4+ tumor patients had a TNM stage groups of II or greater. This is in contrast to the B7-H4− tumor cohort of patients where 33 of 106 (31.1%) patients had a primary tumor classification of pT2 or greater, and 37 of 106 (34.9%) with a TNM stage groups of II or greater.63 In an attempt to determine if a relationship exists between B7-H4 expression within human melanoma and disease outcome, the data show that 28 or 29 primary tumors were found to be B7-H4+ and 26 or 29 metastatic tumors were also B7-H4+. 65 Similar to the previously discussed renal carcinoma study, the authors stratified the patient samples into a B7-H4 high patient population and a B7-H4 low patient population. In doing so, the median survival of the B7-H4 high patients was only 42.2 months, as compared to 106.9 months for the B7-H4 low patient population.65 Similar to the previously mentioned study assessing changes in systemic levels of soluble B7-H4 in aging NOD mice,43 the level of soluble B7-H4 present within the sera of patients with renal cell carcinoma and gastric cancer has been reported to correlate with disease severity. Serum levels of soluble B7-H4 in gastric cancer patients were significantly higher than those of healthy control volunteers. While soluble B7-H4 is significantly increased in patients with gastric cancer, only 50 of 132 (37%) gastric cancer patients displayed increased levels. Further analysis of this patient population showed that the level of soluble B7-H4 might serve as a predictive indicator of tumor size, lymph node metastasis, depth of tumor invasion, cancer stage, and life expectancy.73
Similar to the findings that cellular activation by inflammatory mediators upregulates B7-H4 expression, in renal cell carcinoma B7-H4 mRNA and protein expression are upregulated after stimulation with IL-2, IFN-α, and IFN-γ. 62 However, the tumor microenvironment is a complicated environment characterized by infiltration of T cells, B cells, tumor-associated macrophages, mast cells and dendritic cells, and high levels of cytokines. Therefore, the cytokine network in the tumor microenvironment may have a dual function of modulating the expression of B7-H4 on both cancer cells and infiltrating immune cells. In human ovarian cancer, tumor-associated Tregs can trigger macrophages to secrete IL-6 and IL-10, and IL-6 and IL-10 in turn stimulate APCs to express B7-H4.60 In human lung cancer, tumor-associated macrophages were shown to produce IL-10, TNF-α, and a small amount of IFN-γ, that can also induce human lung cancer cells to express B7-H4.74 This finding was confirmed by data showing that the administration of IL-10 or TNF-α upregulated the expression of B7-H4 on the human Lewis lung carcinoma cell line.74 In addition to the cytokines produced by tumor-infiltrating immune cells, hypoxia is an important selective force in the pathogenesis of cancer.75 For example, the multiple myeloma bone marrow microenvironment has been shown to be hypoxic.76 A recent study demonstrated that hypoxia upregulated B7-H4 expression in primary CD138-positive multiple myeloma cells and cancer cell lines.77 While most of the discussed studies focused on the triggering factors involved in B7-H4 expression, the signaling pathways that participate in B7-H4 expression remain largely unknown. The complexity of the tumor microenvironment may significantly affect B7-H4 expression and distinct molecular mechanisms of such regulation also require further elucidation.
6 | B7-H4 AND FINDINGS ASSOCIATED WITH A PUTATIVE B7-H4 RECEPTOR
B7-H4 expression by cancer cells has been suggested to be a putative mechanism by which these cells evade anti-tumor immune responses. 68,78–80 Therefore, discussion of published findings regarding the biological function of B7-H4 in other immune models is warranted. The generation of a B7-H4 Ig-fusion protein has been the focus of multiple research groups over the past several of years, and these data have been enabled identification of the in vivo biological function of B7-H4. Besides the critical balance between Th1/Th17 cell and Treg cells during autoimmune disease models, such as EAE, published data show that alternatively activated macrophages (M2 cells) are able to suppress EAE induced by the transfer encephalitogenic T cells.81 Further, M2 cells have been suggested to have neuroprotective activity as opposed to pro-inflammatory M1 cells.82 As a potential mechanism, co-culture of CD4+ T cells with IL-10/ TGF-β-treated macrophages that express B7-H4 has been shown to decrease CD4+ T-cell proliferation and subsequently induces an increase in the number of CD4+ T cells that express FoxP3.83 B7-H4 Ig treatment has also been shown to directly modulate the level of inflammatory CD4+ T-cell function,84–86 and is currently in clinical development. The ability of B7-H4 Ig to be used therapeutically has been tested in both the NOD model of type-I diabetes and in β-islet cell transplantation. First, B7-H4 Ig treatment of prediabetic NOD mice reduced the disease incidence as compared to Control Ig-treated mice.86 Additionally, initial attempts to determine a putative mechanism of action for the B7-H4 Ig-induced decrease in the level of disease severity was found to be correlated with a decrease in the level of CD4+ T-cell proliferation ex vivo, and a transient increase in the number of Treg cells in vivo. B7-H4 also protects allografts and generates donor-specific tolerance, and prevents the development of autoimmune diabetes. More importantly, B7-H4 plays an indispensable role in alloimmunity in the absence of the classic CD28/CTLA-4: B7 pathway, suggesting a synergistic/ additive effect with other agents such as CTLA-4 on inhibition of unwanted immune responses.86
As mentioned above, B7-H4: B7-H4 receptor (B7-H4R) interactions negatively regulate inflammatory CD4+ T cells. Overexpression of a membrane-bound form of B7-H4 was found to be anti-inflammatory, 50 and lack of B7-H4 expression during both EAE and T1D is associated with increased disease severity and decreased numbers of Treg cells.87 Overexpression of a soluble form of B7-H4 increased disease severity in collagen-induced arthritis (CIA), while treatment with a B7-H4 Ig that putatively could cross-link the B7-H4R via binding of Fc receptors by the Fc portion of B7-H4 Ig decreased the level of disease severity in CIA,88 PLP139-151-induced R-EAE in SJL/J mice, MOG35-55-induced C-EAE in C57BL/6 mice,84 and type-1 diabetes in NOD mice.85,86 Additionally, B7-H4 Ig treatment of CD4+ T cell cultures is only inhibitory to the CD4+ T-cell responses if B7-H4 Ig is plate-bound, bead-bound, or added into cultures containing APCs, live or irradiated, expressing Fc receptors.84 In addition, B7-H4 Ig has the unique property of specifically blocking the differentiation of naive mouse and human CD4+ T cells into inflammatory Th1 and Th17 cells, while enhancing the numbers and suppressive function of Tregs.84 While the identity of the B7-H4R remains to be determined, these findings indicate that B7-H4 Ig functions as a receptor agonist. Additionally, the soluble B7-H4 overexpression experiments support the previously mentioned data showing that soluble B7-H4 within the blood increases with age in NOD mice and may serve as a biomarker in gastric cancer and renal cell carcinoma.
Consistent with observation that the extracellular domain of B7-H4 has only about 25% amino acid homology with other B7-family members, B7-H4 does not bind to known B7-family receptors, i.e. CTLA-4, ICOS, PD-1, or CD28. Efforts to identify a B7-H4-specific receptor have revealed that such a receptor is (i) expressed on activated T cells24; (ii) binding of B7-H4 fusion protein to its putative receptor on T cells was found to significantly inhibit T-cell proliferation and cytokine (IL-2, IFN-γ, and IL-17) production84; (iii) B7-H4-induced inhibition of T cells was found to be non-reversible by CD28 co-stimulation25; and (iv) the optimal level of B7-H4 Ig binding to activated CD4+ T cells is achieved by the inhibition of actin polymerization prior to incubation of the cells with B7-H4 Ig for flow cytometric analysis. 84 B7-H4 has been found to arrest cell cycle progression of T cells in G0/Gi phase24 suggesting that the protein mediates its inhibitory effects by arresting the cell cycle rather than by inducing apoptosis. As mentioned previously, data generated in our laboratory show that B7-H4 expression is induced on the surface of monocytes in a cytokine-dependent manner.50 Additionally, we have found that both mouse and human monocytes will bind B7-H4 Ig in a cytokine-dependent manner, and that culture of these B7-H4 Ig-binding monocytes in the presence of plate-bound B7-H4 Ig both alters monocyte morphology, as determined by an increase in cellular spreading, an increase in the level of secreted TGF-β, and a decrease in the level of secreted TNF-α as compared to monocytes cultured in the presence of plate-bound species and isotype control antibody (J.R. Podojil, M.-Y. Chiang, S.D. Miller, unpublished observation). This latter finding suggests that B7-H4 expression by monocytes within an inflammatory site may serve both a monocyte population-extrinsic immune modulatory function on activated T cells, but also a monocyte population-intrinsic function within the monocytic cells themselves.
7 | FUNCTIONAL CONNECTION BETWEEN B7-H4 AND TREGS
The association between inflammation and cancer dates back more than a century to observations noting infiltration of large numbers of white blood cells into tumors.89 Several studies have now identified two main pathways linking inflammation and cancer.90–92 First, the intrinsic pathway linking inflammation and cancer includes genetic alterations that lead to inflammation and carcinogenesis. Second, the extrinsic pathway linking inflammation and cancer is characterized by microbial/viral infections or autoimmune diseases that trigger chronic inflammation within tissues that are thereby associated with the eventual development of cancer.93 In either case, both of these pathways activate pivotal transcription factors of inflammatory mediators (e.g. NF-κB, STAT3, and HIF-1) resulting in the recruitment of inflammatory immune cells.94 One such inflammatory immune cell population is the tumor-associated macrophage that provides a link between inflammation and cancer. Macrophages are immune system cells derived from activated blood monocytes. Macrophages are primarily recognized as participating in inflammatory responses induced by pathogens or tissue damage by acting to phagocytize and remove pathogens, dead cells, cellular debris, and various components of the extracellular matrix. Macrophages have been found to constitute an important constituent in the tumor microenvironment and to represent up to 50% of the tumor mass. In addition to mediating phagocytosis, macrophages secrete pro-angiogenic growth factors and matrix-remodeling proteases, and thus play a role in the development of the vascular infrastructure, i.e. angiogenesis, required for tumor development and growth.95 While the recruitment of inflammatory macrophages into the tumor microenvironment would be expected to aid in the activation and guidance of tumor antigen-specific T-cell responses, the presence of macrophages within a tumor may also assist the growth of the tumor. A number of studies provide evidence that the presence of tumor-associated macrophages within the tumor is a negative prognostic factor of survival. 94,96,97 As has been previously discussed, the local production of inflammatory cytokines by the tumor-infiltrating macrophages, as well as by inflammatory T cells, may also induce the expression of immune regulatory proteins, such as B7-H4-promoting inhibition of the tumor antigen-specific immune response. In further support of this conclusion, B7-H4 is overexpressed in tumor-associated macrophages including those present in ovarian tumors.50,60
B7-H4 Ig binds to CD4+ T cells in an activation-dependent manner, indicating that B7-H4 may function as a co-inhibitory (negative co-stimulatory) molecule for CD4+ T cells.84–86 This is supported by our previous findings showing that B7-H4 Ig can downregulate IL-17 and IFN-γ production of mouse T cells in the absence of Treg cells.84 Similarly, B7-H4 expression by tumor cells is a putative mechanism by which these cells evade anti-tumor immune responses. In the majority of breast and ovarian cancers, B7-H4 mRNA is expressed at approximately twofold or greater than the level expressed within normal tissue,41 and B7-H4 protein is present in half of early stage and two-thirds of late stage ovarian tumors.77 Additionally, tissues from breast, uterus, ovary, colon, and pancreas tumors showed a statistically significant increase in the percentage of cells expressing B7-H4.98 In the 4T1 metastatic breast cancer model, transfer of tumor cells into B7-H4−/− mice resulted in fewer lung nodules, enhanced survival, and decreased tumor infiltration of immunosuppressive cells, as compared to wildtype mice that received the 4T1 cells.99 Based on these findings expression of B7-H4 within the tumor microenvironment, either by the tumor cells and/or by infiltrating monocytes is hypothesized to promote immune evasion. In support of this hypothesis, B7-H4 Ig treatment of mice during EAE has been shown to increase the number and function of Treg cells.84 Therefore, the expression of B7-H4 on either the tumor cells and/or tumor-infiltrating leukocytes would increase the number, function, and stability of tumor-associated Treg cells.
Treg cells can accumulate in both the peripheral blood and the cancer microenvironment and have been shown to expand during cancer progression suggesting that Treg cells may play a pivotal role in suppressing anti-tumor immunity.100 In support of this hypothesis, Treg cell depletion or functional suppression results in augmented T-cell immune responses against cancer in both mice and humans.101 As an additional link between B7-H4 and Treg cells, a study using human cervical carcinoma found that B7-H4 promoted the growth of Treg cells.99 Another study demonstrated that B7-H4 promoted cancer tolerance and may contribute to Treg cell development in colorectal cancer.70 To address the linkage between B7-H4 expression and the number/percentage of Treg cells, 4T1 metastatic breast cancer cells were transferred into both wildtype and B7-H4−/− mice. In B7-H4−/− mice, the percentage and overall number of CD4+Foxp3+ Tregs was reduced and a significantly higher ratio of effector CD4+ and CD8+ T cells was observed.98 Collectively, the above findings suggest that, B7-H4 promotes Treg cell development and inhibits effector T-cell immune responses.
APCs are a subgroup of cells that includes DCs, macrophages, and monocytes that are involved in the initiation and regulation of the T-cell response. Cheng and co-workers found that the secretion of IL-10, TNF-α, and IFN-γ by T cells is elevated after blocking B7-H4 expression on tumor-infiltrating DCs,54 and Treg cells triggered high levels of IL-10 to be secreted by APCs, thereby inducing APC B7-H4 expression and rendering these APCs immunosuppressive.51 Peripheral blood samples collected from gastric cancer patients have been found to contain increased percentages of circulating B7-H4+ monocytes, and that these monocytes inhibited the proliferation of CD4+ T cells and significantly suppressed IFN-γ production by CD4+ T cells compared with B7-H4− monocytes.102 Therefore, it has been hypothesized that B7-H4+ monocytes may be one of the critical mechanisms mediating immune evasion in gastric cancer.
Paradoxically, there is a lack of B7-H4 expression on the surface of established ovarian cancer cell lines and primary ovarian carcinoma cells obtained from patient ascites and solid neoplastic lesions103 in contrast to primary malignant cells from ovarian cancer patients that express significant amounts of B7-H4. Therefore, the limited expression of B7-H4 by tumor cell lines has been a hurdle that needs to be overcome to accurately assess the functional role of B7-H4 by tumor cells in vivo. An interesting study has been published examining the disparity of B7-H4 expression on tumor cells in vivo vs in vitro. Mice were inoculated with an ovarian cancer cell line that did not express B7-H4 on its surface, but contained cytoplasmic levels of B7-H4 detectable by immunoblotting. While the surface expression of B7-H4 on these cells increased upon in vivo passage, B7-H4 expression was rapidly downregulated following re-culturing of the tumor cells in vitro. This finding was not limited to the ovarian cancer cell line, as tumor-associated macrophage-derived factors were shown to promote the expression of B7-H4 on the surface of Lewis Lung carcinoma cells in vivo.74 Additionally, B7-H4 expression inversely correlated with the numbers of lymphocytes infiltrating renal cell cancer lesions, providing a direct link between the expression of B7-H4 on the surface of malignant cells and the presence of tumor-associated macrophage-derived cytokines.64 These results indicate that surface expression of B7-H4 on cancer cells increases in response to microenvironmental cues and may promote immune evasion.
The previous discussion regarding B7-H4 expression focused on the biological activity of B7-H4 on immune cell function, yet little is known as to how B7-H4 may function to regulate tumor growth. B7-H4 expression has the capacity to hinder the function of myeloid-derived suppressor cells, as demonstrated by 4T1 tumors in B7-H4−/− mice having increased T cell-and pro-tumor myeloid-derived suppressor cell-associated transcripts. Although the opposing effects of anti-tumor T cells and pro-tumor myeloid-derived suppressor cells led to no changes in the growth of primary 4T1 tumors, a significant alteration in the immunoediting of in vivo passaged 4T1 cells occurred. This was evident, as a secondary injection of in vivo passaged 4T1 cells from B7-H4−/− mice that were then transferred into wildtype mice resulted in slower tumor growth and greater immunogenicity relative to 4T1 tumors that were initially passaged in wildtype mice. The observed differences in tumor growth were further found to be absent if either in vivo passaged 4T1 cells from B7-H4−/− or wildtype mice were transferred into T cell-deficient or T cell-depleted mice, supporting the notion that 4T1 tumors that had grown in the absence of host B7-H4 developed reduced resistance to T cell-mediated immune attack.104
While B7-H4 overexpression on tumor cells was thought to play a dominant role, it is possible that abundant B7-H4 proteins on the surface of tumor cells can impair the effector functions of tumor-infiltrating lymphocytes akin to a molecular shield model as proposed for PD-L1.105,106 In support of this hypothesis, it has been shown that the quantity of B7-H4 on the surface of pancreatic islet cells positively correlates with their resistance to T-cell attack in murine models of type-I diabetes.87 However, the above data show that host B7-H4 contributes to differences in both pro-and anti-tumor immune components, which drives the differences observed in immunoediting between B7-H4−/− and wildtype mice. There is some evidence that B7-H4 may play immune-independent, tumor-intrinsic roles in tumorigenesis. For example, ectopic overexpression of B7-H4 in human ovarian cancer cells led to enhanced tumor growth in scid mice.40 Additionally, the knockdown of B7-H4 in human breast cancer cells rendered them more susceptible to anoikis in vitro.40 While, the relative importance of B7-H4 expression in tumor cells vs host immune cells has also been speculative and the above findings that immune cell expressed B7-H4 may alter anti-tumor T-cell immunity with little B7-H4 in tumors in the 4T1 model, B7-H4-mediated immunotherapies may need to be considered for targeting in cancers even without B7-H4 overexpression directly by the tumor.
Several lines of evidence suggest oncogenic processes and antitumor immunity can also drive B7-H4 overexpression. A small study on melanoma patients found that a high level of B7-H4 did not correlate with the degree of CD8+ T-cell infiltration.65 This may suggest that the immunosuppressive function of B7-H4 may function by inhibiting the entry of CD8+ T cells into the tumor. Additionally, data show that B7-H4 expression in other types of tumors negatively correlated with the number of tumor-infiltrating immune cells.66,68 This finding is supported by mouse model data showing that B7-H4 is not highly induced in 4T1 tumor cells under conditions in which PD-L1 and MHC class II were abundantly expressed, presumably in response to IFN-γ-producing T cells. These findings suggest that B7-H4 expression within the tumor microenvironment may inhibit the immune cell infiltration into the tumor. Also, the expression of B7-H4 by human breast cancer cell lines has been shown to be dependent on a pathway frequently altered in cancer, i.e. phosphoinositide 3-kinase/ mTOR/ p70 S6kinase signaling.107 Lastly, human B cells express a high level of B7-H4 upon EBV-mediated transformation in vitro in the absence of other inflammatory immune cells/cytokines.108 Similarly, a recently published paper shows that peripheral blood B cells have increased expression of B7-H4 in non-small cell lung cancer and colorectal cancer patients.109 Therefore, the overexpression of B7-H4 in cancer cells may also be due to oncogenic processes and may be associated with the low immunogenic nature of the developing tumor.
8 | USE OF ANTI-B7-H4 ANTIBODIES
Within the tumor microenvironment, the production of specific cytokines by immune cells has been shown to modulate the expression of co-stimulatory molecules on both tumor-infiltrating immune cells, and on the tumor cells themselves. Therefore, the activated T cell-mediated immune response potentially plays a critical role in the cancer microenvironment and cancer immunotherapy.110 Many studies indicate that B7-H1, B7-H3, B7-H4, and B7-H6 are expressed by specific human cancers and that the expression of these proteins is associated with cancer progression.111 Thus, agonistic and blocking anti-B7-H4 antibodies and soluble B7-H4 protein have been proposed for the treatment of inflammatory disorders. The expression of B7-H4 at the surface of both malignant and tumor-infiltrating immunosuppressive cells establishes a rationale for the development of therapeutic approaches based on the targeting of B7-H4. Development of an anti-B7-H4 monoclonal antibody for the treatment of cancer in preclinical studies has been completed. While a few studies have reported the use of anti-B7-H4 antibodies in vivo, it still remains to be determined if B7-H4-specific antibodies induce intracellular signaling within the B7-H4-expressing cells, or if the antibodies function via blockade of B7-H4: B7-H4R interaction. For example, anti-B7-H4 antibodies have been found to greatly increase the levels of IL-2 production by splenocytes in vitro, and to lead to a stronger immune response in vivo.25,41 It should be noted, however, that majority of the anti-B7-H4 antibodies used for identifying B7-H4 protein expression have been produced by the individual laboratories “in house” and are not available commercially, with the exception of the anti-B7-H4 antibodies used for the assessment of B7-H4 expression in the published lung,68 prostate, 72 gastric,112, and renal113 cancer studies. With this knowledge, the varied anti-B7-H4 antibody clones used by different laboratories on different tissue types make interlaboratory comparison difficult. Warnock et al. have screened several different anti-B7-H4 antibodies from commercial sources and found three anti-B7-H4 antibody clones that gave moderate expression on human pancreas tissues and mononuclear cells. Their findings show that the anti-B7-H4 antibody clone H74 provided the most consistent IHC staining for comparison of normal and disease tissues.61 Western Blot analysis using clone H74 identifies a major strong diffuse B7-H4 protein band in the 50-to 80-kd range. B7-H4 size heterogeneity is most prominently shown in a retrovirus-transduced B7-H4 overexpressing RK3E cell line compared to MCF-7 and SKBR3 breast cancer cell lines, and may be due to the variable N-linked glycosylation in the full-length B7-H4 protein.25,80
To determine the functional ability of anti-B7-H4 antibody to function as a therapeutic in cancer, a recently published study assessed the functional activity of the 1D11 clone of anti-B7-H4 antibody. The reported immunohistochemistry analyses show that membrane expressed B7-H4 was limited to the ductal epithelia of breast, acinar cells and ductal epithelium of pancreas, tubule epithelium of kidney, bile duct epithelium, and epithelia of trachea/lung, cervix, and placenta. Additionally, B7-H4 membrane staining was present in 80%–94.8%,80,114 thereby providing the rationale to assess the ability of anti-B7-H4 treatment to decrease tumor burden. To identify the subtype most useful for conducting efficacy studies, out of the 113 breast cancer samples surveyed, included TN, HR+, and HER2+ subtypes of breast cancer, all subtypes analyzed showed significant expression of B7-H4.114 Clone 1D11 has broad species specificity (human, non-human primate, and rodents) that allows for evaluation in preclinical models of both rodents and non-human primates. Anti-B7-H4 clone 1D11 has been shown to bind the Ig-V like domain of B7-H4, and use of this antibody on CIA, EAE, and tumors models was shown to not modulate disease. However, when the 1D11 clone of anti-B7-H4 antibody was used to generate a drug conjugate of monomethyl auristatin E (MMAE), linked to engineered cysteines of anti-B7-H4 (h1D11)-MC-vc-PAB-MMAE (h1D11 TDC), the data show that treatment induces durable tumor regression in both cell line and patient-derived xenograft models of triple-negative breast cancer.114
Besides classical monoclonal antibodies, generation of recombinant single-chain variable fragments (scFvs) specific for B7-H4 has been reported. The use of scFvs is suggested to allow for the targeting of selected cell populations both as naked molecules and upon conjugation to endotoxins, nanoparticles, radioisotopes, or protein domains. Via a combination of protein-and cell-based screening approaches, scFvs specific for human B7-H4 were selected based on their affinity for soluble and cell surface B7-H4, as well as on their ability to reverse T-cell inhibition. These B7-H4-specific scFvs were tested in an in vitro model system in which B7-H4 molecules expressed either in cis on APCs or cancer cells, or in trans on tumor-polarized macrophages, to determine if these anti-B7-H4 scFvs could reverse the inhibition function of B7-H4. The data show that an anti-B7-H4 scFv fully reverses the B7-H4-induced inhibition of T-cell function using recombinant B7-H4, peptide-pulsed B7-H4+ APCs, tumor-polarized B7-H4+ macrophages co-cultured with B7-H4− cancer cells, as well as B7-H4-transduced malignant cells.103
9 | CONCLUSION
The present review has highlighted the role of B7-H4 as an immune inhibitory protein, and the potential role that B7-H4 plays immune evasion and tumorigenesis. Although not yet targeted clinically, the advantage of targeting B7-H4 may be twofold as B7-H4 is expressed on both tumor cells and tumor-associated macrophages in various cancer types. For example, while the use of anti-PD-L1 and anti-CTLA-4 has been shown to significantly increase patient life expectancy, there are patients treated with anti-PD-L1 and anti-CTLA-4 that are non-responders. 2,4,5 In comparing B7-H4 to other immune regulatory molecules, such as CTLA-4 and PD-1/ PD-L1, CTLA-4 and PD-1 are expressed on activated T cells and their inhibitory effect occurs primarily in secondary lymphoid tissues.22 In contrast, both PD-L148,49,115 and B7-H451,63,66,112 may be expressed by tumor-infiltrating APCs and tumor cells within the local tumor microenvironment. Therefore, strong rationale exists for the development of alternative immune modulatory therapies for the treatment of the tumor patient population non-responsive to current check point inhibitors.
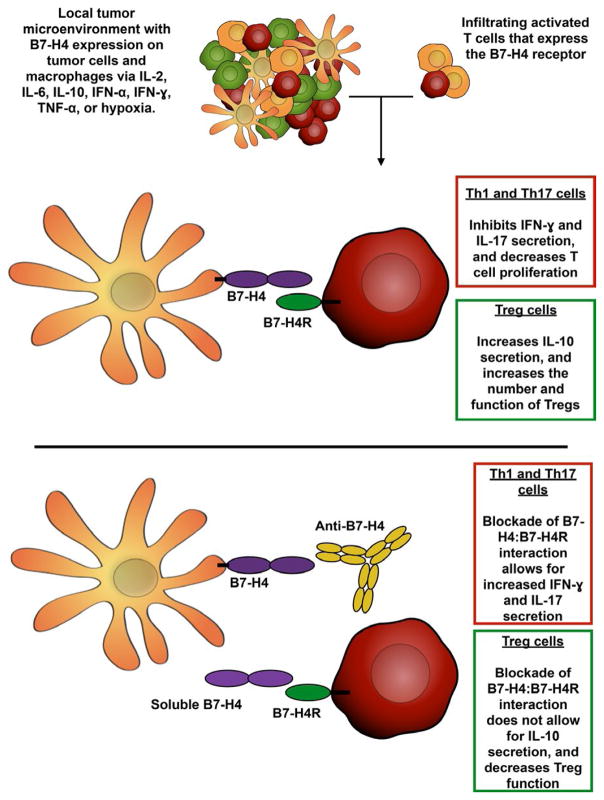
Results from several mouse in vivo and human in vitro studies utilizing various anti-B7-H4 antibodies have shown success in rescuing T-cell function in vitro,24,25,116 augmenting T-cell responses in vivo,24,25 and reducing tumor burden in a murine lung cancer model,74 demonstrating proof of concept for targeting B7-H4. As illustrated in Figure 1, while the presence of an inflammatory microenvironment within the tumor would appear to be favorable for tumor cell killing, the presence of these inflammatory cytokines and hypoxia have been shown to induce the expression of B7-H4 on the surface of both the tumor cells and monocyte/macrophages. The presence of cell surface B7-H4 would then interact with the unidentified B7-H4R that is expressed by activated T cells, thereby decreasing inflammatory T-cell responses and increasing Treg cell function within the tumor. Therefore, the blockade of B7-H4: B7-H4R interaction via anti-B7-H4 blocking antibodies or soluble B7-H4 lacking an Ig-fusion protein domain would allow for the maintenance of the inflammatory T-cell response. However, while studies of the past decade have offered insight into the functional role of B7-H4 in tumor biology and immune evasion, no anti-B7-H4-targeted therapies have been investigated clinically. The majority of the research regarding B7-H4 has focused the functional consequence of B7-H4 interaction with the putative B7-H4R on the B7-H4R-expressing cell, several papers indicate distinct intracellular effects such as decreased apoptosis, enhanced proliferation, and facilitated metastasis in B7-H4-expressing cells themselves. Besides the level of cell surface expressed B7-H4, studies assessing the level of soluble B7-H4 in serum have shown B7-H4 to be a putative biomarker for the level of disease.43,73 This, however, may be a potential confounder with the use of B7-H4-binding reagents, as B7-H4-binding reagents, i.e. anti-B7-H4 monoclonal antibody, may form immune complexes in the blood. Therefore, many opportunities still exist to determine if treatment with a B7-H4-blocking therapeutic, either alone or in combination with other therapies, will overcome the B7-H4-mediated inhibition of inflammatory T-cell function within the tumor microenvironment and can provide clinical benefit in cancer.
FIGURE 1.
Blockade of B7-H4: B7-H4R interaction. Inflammatory cytokines and other cytokines/factors within the tumor microenvironment, such as IL-2, IL-6, IL-10, IFN-α, IFN-γ, TNF-α, and hypoxia, have been shown to induce B7-H4 expression on both tumor cells and monocytes/ macrophages. Additionally, activated T cells have been shown to express the B7-H4R via specific binding of B7-H4 Ig, which also modulates T cells function via decreasing inflammatory T-cell responses while increasing Treg function. In the case of tumor and tumor-associated macrophage expressed B7-H4, the interaction of the B7-H4R-expressing T cells with B7-H4-expressing tumor cells or tumor-associated macrophages would decrease the inflammatory and proliferative response by the T cells, while increasing the number and function of the Treg cells. Therefore, the blockade of the B7-H4: B7-H4R interaction is hypothesized to allow for the maintenance of the inflammatory T-cell response within the tumor microenvironment
Acknowledgments
Funding information
National Multiple Sclerosis Society, Grant/Award Number: RG 4624A10/1; Amplimmune, Inc
This work was supported in part by NMSS grant RG 4624A10/1 and funding from Amplimmune, Inc.
Footnotes
CONFLICTS OF INTEREST
The authors do not have any conflicts of interest regarding this work.
References
- 1.Dong C, Nurieva RI, Prasad DV. Immune regulation by novel costimulatory molecules. Immunol Res. 2003;28:39–48. doi: 10.1385/IR:28:1:39. [DOI] [PubMed] [Google Scholar]
- 2.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribas A, Camacho LH, Lopez-Berestein G, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23:8968–8977. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 5.Twyman-Saint Victor C, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–377. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lafferty KJ, Cunningham AJ. A new analysis of allogeneic interactions. Aust J Exp Biol Med Sci. 1975;53:27–42. doi: 10.1038/icb.1975.3. [DOI] [PubMed] [Google Scholar]
- 7.Lustgarten J, Waks T, Eshhar Z. CD4 and CD8 accessory molecules function through interactions with major histocompatibility complex molecules which are not directly associated with the T cell receptor-antigen complex. Eur J Immunol. 1991;21:2507–2515. doi: 10.1002/eji.1830211030. [DOI] [PubMed] [Google Scholar]
- 8.Zuniga-Pflucker JC, Jones LA, Longo DL, Kruisbeek AM. CD8 is required during positive selection of CD4−/CD8+ T cells. J Exp Med. 1990;171:427–437. doi: 10.1084/jem.171.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohm AP, Williams JS, Bickford AL, et al. Treatment with nonmitogenic anti-CD3 monoclonal antibody induces CD4+ T cell unresponsiveness and functional reversal of established experimental autoimmune encephalomyelitis. J Immunol. 2005;174:4525–4534. doi: 10.4049/jimmunol.174.8.4525. [DOI] [PubMed] [Google Scholar]
- 10.O’Herrin SM, Slansky JE, Tang Q, et al. Antigen-specific blockade of T cells in vivo using dimeric MHC peptide. J Immunol. 2001;167:2555–2560. doi: 10.4049/jimmunol.167.5.2555. [DOI] [PubMed] [Google Scholar]
- 11.Masteller EL, Warner MR, Ferlin W, et al. Peptide-MHC class II dimers as therapeutics to modulate antigen-specific T cell responses in autoimmune diabetes. J Immunol. 2003;171:5587–5595. doi: 10.4049/jimmunol.171.10.5587. [DOI] [PubMed] [Google Scholar]
- 12.Chatenoud L, Bluestone JA. CD3-specific antibodies: A portal to the treatment of autoimmunity. Nat Rev. 2007;7:622–632. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
- 13.Maude SL. Future directions in chimeric antigen receptor T cell therapy. Curr Opin Pediatr. 2017;29:27–33. doi: 10.1097/MOP.0000000000000436. [DOI] [PubMed] [Google Scholar]
- 14.Oluwole OO, Davila ML. At the bedside: Clinical review of chimeric antigen receptor (CAR) T cell therapy for B cell malignancies. J Leukoc Biol. 2016;100:1265–1272. doi: 10.1189/jlb.5BT1115-524R. [DOI] [PubMed] [Google Scholar]
- 15.Damle NK, Klussman K, Linsley PS, Aruffo A, Ledbetter JA. Differential regulatory effects of intercellular adhesion molecule-1 on costimulation by the CD28 counter-receptor B7. J Immunol. 1992;149:2541–2548. [PubMed] [Google Scholar]
- 16.Harding FA, McArthur J, Gross JA, Raulet D, Allison JP. CD28 mediated signalling costimulates murine T cells and prevents induction of anergy in T cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 17.Kremer JM, Westhovens R, Leon M, et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med. 2003;349:1907–1915. doi: 10.1056/NEJMoa035075. [DOI] [PubMed] [Google Scholar]
- 18.Johnson JG, Jenkins MK. Accessory cell-derived signals required for T cell activation. Immunol Res. 1993;12:48–64. doi: 10.1007/BF02918368. [DOI] [PubMed] [Google Scholar]
- 19.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suvas S, Singh V, Sahdev S, Vohra H, Agrewala JN. Distinct role of CD80 and CD86 in the regulation of the activation of B cell and B cell lymphoma. J Biol Chem. 2002;277:7766–7775. doi: 10.1074/jbc.M105902200. [DOI] [PubMed] [Google Scholar]
- 21.Podojil JR, Kohm AP, Miller SD. CD4+ T cell expressed CD80 regulates central nervous system effector function and survival during experimental autoimmune encephalomyelitis. J Immunol. 2006;177:2948–2958. doi: 10.4049/jimmunol.177.5.2948. [DOI] [PubMed] [Google Scholar]
- 22.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 23.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 24.Sica GL, Choi IH, Zhu G, et al. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18:849–861. doi: 10.1016/s1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 25.Prasad DV, Richards S, Mai XM, Dong C. B7S1, a novel B7 family member that negatively regulates T cell activation. Immunity. 2003;18:863–873. doi: 10.1016/s1074-7613(03)00147-x. [DOI] [PubMed] [Google Scholar]
- 26.Brandt CS, Baratin M, Yi EC, et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J Exp Med. 2009;206:1495–1503. doi: 10.1084/jem.20090681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schreiner B, Bailey SL, Shin T, Chen L, Miller SD. PD-1 ligands expressed on myeloid-derived APC in the CNS regulate T-cell responses in EAE. Eur J Immunol. 2008;38:2706–2717. doi: 10.1002/eji.200838137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McAdam AJ, Chang TT, Lumelsky AE, et al. Mouse inducible costimulatory molecule (ICOS) expression is enhanced by CD28 costimulation and regulates differentiation of CD4+ T cells. J Immunol. 2000;165:5035–5040. doi: 10.4049/jimmunol.165.9.5035. [DOI] [PubMed] [Google Scholar]
- 30.Hodgson R, Christiansen D, Ziolkowski A, et al. Prolonged xenograft survival induced by inducible costimulator-Ig is associated with increased forkhead box P3(+) cells. Transplantation. 2011;91:1090–1097. doi: 10.1097/TP.0b013e31821774e0. [DOI] [PubMed] [Google Scholar]
- 31.Youngnak P, Kozono Y, Kozono H, et al. Differential binding properties of B7-H1 and B7-DC to programmed death-1. Biochem Biophys Res Commun. 2003;307:672–677. doi: 10.1016/s0006-291x(03)01257-9. [DOI] [PubMed] [Google Scholar]
- 32.Shin T, Yoshimura K, Shin T, et al. In vivo costimulatory role of B7-DC in tuning T helper cell 1 and cytotoxic T lymphocyte responses. J Exp Med. 2005;201:1531–1541. doi: 10.1084/jem.20050072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao Y, Yu S, Zhu B, et al. RGMb is a novel binding partner for PD-L2 and its engagement with PD-L2 promotes respiratory tolerance. J Exp Med. 2014;211:943–959. doi: 10.1084/jem.20130790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007;19:309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Kaifu T, Escaliere B, Gastinel LN, Vivier E, Baratin M. B7-H6/ NKp30 interaction: A mechanism of alerting NK cells against tumors. Cell Mol Life Sci. 2011;68:3531–3539. doi: 10.1007/s00018-011-0802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joyce MG, Tran P, Zhuravleva MA, Jaw J, Colonna M, Sun PD. Crystal structure of human natural cytotoxicity receptor NKp30 and identification of its ligand binding site. Proc Natl Acad Sci USA. 2011;108:6223–6228. doi: 10.1073/pnas.1100622108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi IH, Zhu G, Sica GL, et al. Genomic organization and expression analysis of B7-H4, an immune inhibitory molecule of the B7 family. J Immunol. 2003;171:4650–4654. doi: 10.4049/jimmunol.171.9.4650. [DOI] [PubMed] [Google Scholar]
- 38.Smith JB, Stashwick C, Powell DJ., Jr B7-H4 as a potential target for immunotherapy for gynecologic cancers: A closer look. Gynecol Oncol. 2014;134:181–189. doi: 10.1016/j.ygyno.2014.03.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe N, Gavrieli M, Sedy JR, et al. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat Immunol. 2003;4:670–679. doi: 10.1038/ni944. [DOI] [PubMed] [Google Scholar]
- 40.Salceda S, Tang T, Kmet M, et al. The immunomodulatory protein B7-H4 is overexpressed in breast and ovarian cancers and promotes epithelial cell transformation. Exp Cell Res. 2005;306:128–141. doi: 10.1016/j.yexcr.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 41.Zang X, Loke P, Kim J, Murphy K, Waitz R, Allison JP. B7x: A widely expressed B7 family member that inhibits T cell activation. Proc Natl Acad Sci USA. 2003;100:10388–10392. doi: 10.1073/pnas.1434299100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radichev IA, Maneva-Radicheva LV, Amatya C, et al. Nardilysin-dependent proteolysis of cell-associated VTCN1 (B7-H4) marks type 1 diabetes development. Diabetes. 2014;63:3470–3482. doi: 10.2337/db14-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radichev IA, Maneva-Radicheva LV, Amatya C, et al. Loss of peripheral protection in pancreatic islets by proteolysis-driven impairment of VTCN1 (B7-H4) presentation is associated with the development of autoimmune diabetes. J Immunol. 2016;196:1495–1506. doi: 10.4049/jimmunol.1403251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seder RA, Germain RN, Linsley PS, Paul WE. CD28-mediated costimulation of interleukin 2 (IL-2) production plays a critical role in T cell priming for IL-4 and interferon gamma production. J Exp Med. 1994;179:299–304. doi: 10.1084/jem.179.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 46.Becher B, Durell BG, Noelle RJ. Experimental autoimmune encephalitis and inflammation in the absence of interleukin-12. J Clin Invest. 2002;110:493–497. doi: 10.1172/JCI15751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen Y, Langrish CL, McKenzie B, et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wintterle S, Schreiner B, Mitsdoerffer M, et al. Expression of the B7-related molecule B7-H1 by glioma cells: A potential mechanism of immune paralysis. Cancer Res. 2003;63:7462–7467. [PubMed] [Google Scholar]
- 49.Brown JA, Dorfman DM, Ma FR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170:1257–1266. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 50.Kryczek I, Zou L, Rodriguez P, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kryczek I, Wei S, Zou L, et al. Cutting edge: Induction of B7-H4 on APCs through IL-10: Novel suppressive mode for regulatory T cells. J Immunol. 2006;177:40–44. doi: 10.4049/jimmunol.177.1.40. [DOI] [PubMed] [Google Scholar]
- 52.Zou W, Machelon V, Coulomb-L’Hermin A, et al. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7:1339–1346. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 53.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 54.Cheng C, Qu QX, Shen Y, et al. Overexpression of B7-H4 in tumor infiltrated dendritic cells. J Immunoassay Immunochem. 2011;32:353–364. doi: 10.1080/15321819.2011.578190. [DOI] [PubMed] [Google Scholar]
- 55.Zhang C, Li Y, Wang Y. Diagnostic value of serum B7-H4 for hepatocellular carcinoma. J Surg Res. 2015;197:301–306. doi: 10.1016/j.jss.2015.04.034. [DOI] [PubMed] [Google Scholar]
- 56.Maskey N, Li K, Hu M, et al. Impact of neoadjuvant chemotherapy on lymphocytes and co-inhibitory B7-H4 molecule in gastric cancer: Low B7-H4 expression associates with favorable prognosis. Tumour Biol. 2014;35:11837–11843. doi: 10.1007/s13277-014-2410-2. [DOI] [PubMed] [Google Scholar]
- 57.Zhu J, Chu BF, Yang YP, et al. B7-H4 expression is associated with cancer progression and predicts patient survival in human thyroid cancer. Asian Pac J Cancer Prev. 2013;14:3011–3015. doi: 10.7314/apjcp.2013.14.5.3011. [DOI] [PubMed] [Google Scholar]
- 58.Tringler B, Liu W, Corral L, et al. B7-H4 overexpression in ovarian tumors. Gynecol Oncol. 2006;100:44–52. doi: 10.1016/j.ygyno.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 59.Zhang LL, Shao SL, Wu Y. Expressions of osteopontin and B7-H4 in epithelial ovarian neoplasm and their significance. Chin J Cancer. 2010;29:25–29. [PubMed] [Google Scholar]
- 60.Kryczek I, Wei S, Zhu G, et al. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 2007;67:8900–8905. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- 61.Cheung SS, Ou D, Metzger DL, et al. B7-H4 expression in normal and diseased human islet beta cells. Pancreas. 2014;43:128–134. doi: 10.1097/MPA.0b013e31829695d2. [DOI] [PubMed] [Google Scholar]
- 62.Xu Y, Zhu S, Song M, et al. B7-H4 expression and its role in interleukin-2/ interferon treatment of clear cell renal cell carcinoma. Oncol Lett. 2014;7:1474–1478. doi: 10.3892/ol.2014.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krambeck AE, Thompson RH, Dong H, et al. B7-H4 expression in renal cell carcinoma and tumor vasculature: Associations with cancer progression and survival. Proc Natl Acad Sci USA. 2006;103:10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang L, Wu H, Lu D, et al. The costimulatory molecule B7-H4 promote tumor progression and cell proliferation through translocating into nucleus. Oncogene. 2013;32:5347–5358. doi: 10.1038/onc.2012.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Quandt D, Fiedler E, Boettcher D, Marsch W, Seliger B. B7-h4 expression in human melanoma: Its association with patients’ survival and antitumor immune response. Clin Cancer Res. 2011;17:3100–3111. doi: 10.1158/1078-0432.CCR-10-2268. [DOI] [PubMed] [Google Scholar]
- 66.Mugler KC, Singh M, Tringler B, et al. B7-h4 expression in a range of breast pathology: Correlation with tumor T-cell infiltration. Appl Immunohistochem Mol Morphol. 2007;15:363–370. doi: 10.1097/01.pai.0000213159.79557.71. [DOI] [PubMed] [Google Scholar]
- 67.Li ZY, Zhang XH, Chen Y, et al. Clinical significance of B7-H4 expression in matched non-small cell lung cancer brain metastases and primary tumors. Onco Targets Ther. 2013;6:869–875. doi: 10.2147/OTT.S48085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun Y, Wang Y, Zhao J, et al. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer. 2006;53:143–151. doi: 10.1016/j.lungcan.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 69.Arigami T, Uenosono Y, Ishigami S, Hagihara T, Haraguchi N, Natsugoe S. Clinical significance of the B7-H4 coregulatory molecule as a novel prognostic marker in gastric cancer. World J Surg. 2011;35:2051–2057. doi: 10.1007/s00268-011-1186-4. [DOI] [PubMed] [Google Scholar]
- 70.Zhao LW, Li C, Zhang RL, et al. B7-H1 and B7-H4 expression in colorectal carcinoma: Correlation with tumor FOXP3(+) regulatory T-cell infiltration. Acta Histochem. 2014;116:1163–1168. doi: 10.1016/j.acthis.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 71.Chen Y, Sun J, Zhao H, et al. The coexpression and clinical significance of costimulatory molecules B7-H1, B7-H3, and B7-H4 in human pancreatic cancer. Onco Targets Ther. 2014;7:1465–1472. doi: 10.2147/OTT.S66809. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 72.Zang X, Thompson RH, Al-Ahmadie HA, et al. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci USA. 2007;104:19458–19463. doi: 10.1073/pnas.0709802104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shi H, Ji M, Wu J, et al. Serum B7-H4 expression is a significant prognostic indicator for patients with gastric cancer. World J Surg Oncol. 2014;12:188. doi: 10.1186/1477-7819-12-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen C, Qu QX, Shen Y, et al. Induced expression of B7-H4 on the surface of lung cancer cell by the tumor-associated macrophages: A potential mechanism of immune escape. Cancer Lett. 2012;317:99–105. doi: 10.1016/j.canlet.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 75.Martin SK, Diamond P, Williams SA, et al. Hypoxia-inducible factor-2 is a novel regulator of aberrant CXCL12 expression in multiple myeloma plasma cells. Haematologica. 2010;95:776–784. doi: 10.3324/haematol.2009.015628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Borsi E, Terragna C, Brioli A, Tacchetti P, Martello M, Cavo M. Therapeutic targeting of hypoxia and hypoxia-inducible factor 1 alpha in multiple myeloma. Transl Res. 2015;165:641–650. doi: 10.1016/j.trsl.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 77.Jeon YK, Park SG, Choi IW, Lee SW, Lee SM, Choi I. Cancer cell-associated cytoplasmic B7-H4 is induced by hypoxia through hypoxia-inducible factor-1alpha and promotes cancer cell proliferation. Biochem Biophys Res Commun. 2015;459:277–283. doi: 10.1016/j.bbrc.2015.02.098. [DOI] [PubMed] [Google Scholar]
- 78.Ichikawa M, Chen L. Role of B7-H1 and B7-H4 molecules in down-regulating effector phase of T-cell immunity: Novel cancer escaping mechanisms. Front Biosci. 2005;10:2856–2860. doi: 10.2741/1742. [DOI] [PubMed] [Google Scholar]
- 79.Simon I, Zhuo S, Corral L, et al. B7-h4 is a novel membrane-bound protein and a candidate serum and tissue biomarker for ovarian cancer. Cancer Res. 2006;66:1570–1575. doi: 10.1158/0008-5472.CAN-04-3550. [DOI] [PubMed] [Google Scholar]
- 80.Tringler B, Zhuo S, Pilkington G, et al. B7-h4 is highly expressed in ductal and lobular breast cancer. Clin Cancer Res. 2005;11:1842–1848. doi: 10.1158/1078-0432.CCR-04-1658. [DOI] [PubMed] [Google Scholar]
- 81.Mikita J, Dubourdieu-Cassagno N, Deloire MS, et al. Altered M1/M2 activation patterns of monocytes in severe relapsing experimental rat model of multiple sclerosis. Amelioration of clinical status by M2 activated monocyte administration. Mult Scler. 2011;17:2–15. doi: 10.1177/1352458510379243. [DOI] [PubMed] [Google Scholar]
- 82.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cao Q, Wang Y, Zheng D, et al. IL-10/ TGF-beta-modified macrophages induce regulatory T cells and protect against adriamycin nephrosis. J Am Soc Nephrol. 2010;21:933–942. doi: 10.1681/ASN.2009060592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Podojil JR, Liu LN, Marshall SA, et al. B7-H4Ig inhibits mouse and human T-cell function and treats EAE via IL-10/ Treg-dependent mechanisms. J Autoimmun. 2013;44:71–81. doi: 10.1016/j.jaut.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee IF, Wang X, Hao J, et al. B7-H4. Ig inhibits the development of type 1 diabetes by regulating Th17 cells in NOD mice. Cell Immunol. 2013;282:1–8. doi: 10.1016/j.cellimm.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 86.Wang X, Hao J, Metzger DL, et al. Early treatment of NOD mice with B7-H4 reduces the incidence of autoimmune diabetes. Diabetes. 2011;60:3246–3255. doi: 10.2337/db11-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wei J, Loke P, Zang X, Allison JP. Tissue-specific expression of B7x protects from CD4 T cell-mediated autoimmunity. J Exp Med. 2011;208:1683–1694. doi: 10.1084/jem.20100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Daniel D, Gill RG, Schloot N, Wegmann D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones Isolated from NOD mice. Eur J Immunol. 1995;25:1056–1062. doi: 10.1002/eji.1830250430. [DOI] [PubMed] [Google Scholar]
- 89.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Allavena P, Garlanda C, Borrello MG, Sica A, Mantovani A. Pathways connecting inflammation and cancer. Curr Opin Genet Dev. 2008;18:3–10. doi: 10.1016/j.gde.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 91.Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 92.Porta C, Larghi P, Rimoldi M, et al. Cellular and molecular pathways linking inflammation and cancer. Immunobiology. 2009;214:761–777. doi: 10.1016/j.imbio.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 93.ElShamy WM, Sinha A, Said N. Aggressiveness niche: Can it be the foster ground for cancer metastasis precursors? Stem Cells Int. 2016;2016:4829106. doi: 10.1155/2016/4829106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- 95.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Farinha P, Masoudi H, Skinnider BF, et al. Analysis of multiple biomarkers shows that lymphoma-associated macrophage (LAM) content is an independent predictor of survival in follicular lymphoma (FL) Blood. 2005;106:2169–2174. doi: 10.1182/blood-2005-04-1565. [DOI] [PubMed] [Google Scholar]
- 97.Dave SS, Wright G, Tan B, et al. Prediction of survival in follicular lymphoma based on molecular features of tumor-infiltrating immune cells. N Engl J Med. 2004;351:2159–2169. doi: 10.1056/NEJMoa041869. [DOI] [PubMed] [Google Scholar]
- 98.Jeon H, Ohaegbulam KC, Abadi YM, Zang X. B7x and myeloid-derived suppressor cells in the tumor microenvironment: A tale of two cities. Oncoimmunology. 2013;2:e24744. doi: 10.4161/onci.24744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang X, Wang T, Xu M, et al. B7-H4 overexpression impairs the immune response of T cells in human cervical carcinomas. Hum Immunol. 2014;75:1203–1209. doi: 10.1016/j.humimm.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 100.Chang LY, Lin YC, Chiang JM, et al. Blockade of TNF-alpha signaling benefits cancer therapy by suppressing effector regulatory T cell expansion. Oncoimmunology. 2015;4:e1040215. doi: 10.1080/2162402X.2015.1040215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu X, Venkataraman G, Lin J, et al. Highly clonal regulatory T-cell population in follicular lymphoma—Inverse correlation with the diversity of CD8+ T cells. Oncoimmunology. 2015;4:e1002728. doi: 10.1080/2162402X.2014.1002728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Matsunaga T, Saito H, Ikeguchi M. Increased B7-H1 and B7-H4 expressions on circulating monocytes and tumor-associated macrophages are involved in immune evasion in patients with gastric cancer. Yonago Acta Med. 2011;54:1–10. [PMC free article] [PubMed] [Google Scholar]
- 103.Dangaj D, Lanitis E, Zhao A, et al. Novel recombinant human b7-h4 antibodies overcome tumoral immune escape to potentiate T-cell antitumor responses. Cancer Res. 2013;73:4820–4829. doi: 10.1158/0008-5472.CAN-12-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Leung J, Suh WK. Host B7-H4 regulates antitumor T cell responses through inhibition of myeloid-derived suppressor cells in a 4T1 tumor transplantation model. J Immunol. 2013;190:6651–6661. doi: 10.4049/jimmunol.1201242. [DOI] [PubMed] [Google Scholar]
- 105.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8:467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 106.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 107.Heinonen H, Nieminen A, Saarela M, et al. Deciphering downstream gene targets of PI3K/mTOR/p70S6K pathway in breast cancer. BMC Genom. 2008;9:348. doi: 10.1186/1471-2164-9-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Song H, Park G, Kim YS, et al. B7-H4 reverse signaling induces the apoptosis of EBV-transformed B cells through Fas ligand up-regulation. Cancer Lett. 2008;266:227–237. doi: 10.1016/j.canlet.2008.02.067. [DOI] [PubMed] [Google Scholar]
- 109.De Simone M, Arrigoni A, Rossetti G, et al. Transcriptional landscape of human tissue lymphocytes unveils uniqueness of tumor-infiltrating T regulatory cells. Immunity. 2016;45:1135–1147. doi: 10.1016/j.immuni.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mockler MB, Conroy MJ, Lysaght J. Targeting T cell immunometabolism for cancer immunotherapy; understanding the impact of the tumor microenvironment. Front Oncol. 2014;4:107. doi: 10.3389/fonc.2014.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhou Y, Xu Y, Chen L, Xu B, Wu C, Jiang J. B7-H6 expression correlates with cancer progression and patient’s survival in human ovarian cancer. Int J Clin Exp Pathol. 2015;8:9428–9433. [PMC free article] [PubMed] [Google Scholar]
- 112.Jiang J, Zhu Y, Wu C, et al. Tumor expression of B7-H4 predicts poor survival of patients suffering from gastric cancer. Cancer Immunol Immunother. 2010;59:1707–1714. doi: 10.1007/s00262-010-0900-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thompson RH, Zang X, Lohse CM, et al. Serum-soluble B7x is elevated in renal cell carcinoma patients and is associated with advanced stage. Cancer Res. 2008;68:6054–6058. doi: 10.1158/0008-5472.CAN-08-0869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Leong SR, Liang WC, Wu Y, et al. An anti-B7-H4 antibody-drug conjugate for the treatment of breast cancer. Mol Pharm. 2015;12:1717–1729. doi: 10.1021/mp5007745. [DOI] [PubMed] [Google Scholar]
- 115.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci USA. 2002;99:12293–12297. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Qian Y, Shen L, Cheng L, Wu Z, Yao H. B7-H4 expression in various tumors determined using a novel developed monoclonal antibody. Clin Exp Med. 2011;11:163–170. doi: 10.1007/s10238-010-0125-2. [DOI] [PubMed] [Google Scholar]