Abstract
Background
Infectious diseases can appear and spread rapidly. Timely information about disease patterns and trends allows public health agencies to quickly investigate and efficiently contain those diseases. But disease case reporting to public health has traditionally been paper-based, resulting in somewhat slow, burdensome processes. Fortunately, the expanding use of electronic health records and health information exchanges has created opportunities for more rapid, complete, and easily managed case reporting and investigation. To assess how this new service might impact the efficiency and quality of a public health agency's case investigations, we compared the timeliness of usual case investigation to that of case investigations based on case report forms that were partially pre-populated with electronic data.
Intervention
Between September 2013-March 2014, chlamydia disease report forms for certain clinics in Indianapolis were electronically pre-populated with clinical, lab and patient data available through the Indiana Health Information Exchange, then provided to the patient’s doctor. Doctors could then sign the form and deliver it to public health for investigation and population-level disease tracking. Methods: We utilized a novel matched case analysis of timeliness changes in receipt and processing of communicable disease report forms. Each Chlamydia cases reported with the pre-populated form were matched to cases reported in usual ways. We assessed the time from receipt of the case at the public health agency: 1) inclusion of the case into the public health surveillance system and 2) to close to case. A hierarchical random effects model was used to compare mean difference in each outcome between the target cases and the matched cases, with random intercepts for case.
Results
Twenty-one Chlamydia cases were reported to the public health agency using the pre-populated form. Sixteen of these pre-populated form cases were matched to at least one other case, with a mean of 23 matches per case. The mean Reporting Lag for the pre-populated form cases was 2.5 days, which was 2.7 days shorter than the mean Reporting Lag for the matched controls (p = <0.001). The mean time to close a pre-populated form case was 4.7 days, which was 0.2 days shorter than time to close for the matched controls (p = 0.792).
Conclusions
Use of pre-populated forms significantly decreased the time it took for the local public health agency to begin documenting and closing chlamydia case investigations. Thoughtful use of electronic health data for case reporting may decrease the per-case workload of public health agencies, and improve the timeliness of information about the pattern and spread of disease.
Keywords: Communicable Diseases, Disease Notification, Electronic health records, Health information exchange, Public Health Surveillance
Introduction
The prompt detection of communicable and infectious disease outbreaks or trends relies on collecting, analyzing, and sharing data between the clinical health care system and local and state public health agencies (PHAs) [1]. PHAs collect morbidity data on notifiable conditions to inform their fundamental activities in investigation, prevention, control, monitoring and assessment of population-based disease. Health data are delivered to PHAs in the form of communicable disease report (CDR) forms or less formal reports from health care providers (HCPs) and laboratories. While HCPs are legally mandated to report notifiable conditions to PHAs, they consistently underreport due to workload, lack of resources or time, poor integration of CDR form completion into clinic workflow, lack of knowledge about reporting requirements, unwillingness to report or because clinics assume laboratories will report to PHAs [2-7]. When HCPs do report, the CDR forms submitted to PHAs are frequently incomplete, error-prone, and delayed [5,8]. And despite efforts to improve HCP reporting [9,10], most PHA case management, investigation and resolution, contact tracing, and cluster identification activities traditionally depend on manual, HCP-initiated reporting [11-13].
Electronic laboratory reporting (ELR) to PHAs has been demonstrated to improve timeliness of reporting [4,10] although this is likely to vary by disease [14]. However, lab reports typically do not contain the demographic and other patient information PHAs require to complete a case report, launch an investigation or close a case [5]. Thus, while ELR may increase timeliness of case reporting, little improvement may be realized when PHAs are burdened with collecting patient data that frequently is missing from lab reports. In addition, ELR increases the volume of reports [4] which can impose an additional processing burden as PHAs deal with higher volume [15], duplicate cases and reports [16], and sometimes an increase in the frequency of false positives—all of which can drain PHA investigation resources [17]. PHAs may also struggle to combine different sources, often having to use manual processes to match and merge the information, decreasing the timeliness and accuracy of their analyses of disease trends [18].
Over 95% of all US PHAs employ public health nurses or epidemiologists who comprise 18.3% of all PHA employees nationwide [19]. The majority of communicable and infectious disease case reporting and investigation is done by these employees using numerous systems which do not interoperate or exchange data [6,20]. One strategy for overcoming barriers to electronic transfer of clinical data and information between disparate information systems is the use of Health Information Exchanges (HIEs) [21]. HIEs have demonstrated savings in health care costs [22,23], improvements in patient safety [24], workflow efficiencies [25], and improved data sharing among information systems and HIE participants [26]. HIEs are also stimulating structural and business process changes for PHAs [27,28]. An HIE has the potential to link EHR and laboratory data, providing PHAs with integrated, timely, consistently organized information that alleviates many challenges PHAs face in managing case reports.
Between September 2013-March 2014, the Indiana Network for Patient Care (INPC) HIE in Indianapolis, IN implemented an intervention in which CDR forms were electronically pre-populated with clinical, lab and patient data available through the HIE's integrated EHR-ELR system. These partially completed CDRs were then delivered to the patient’s clinician for verification, signature, and delivery to the responsible PHA. The CDR form intervention was piloted at selected ambulatory care clinics (N=6), representing Family Medicine, Internal Medicine, Women's Health, Adolescent Health, and OB-GYN medical specialties. By automatically pre-populating CDR forms, this intervention aimed to reduce barriers to clinic reporting, improve timeliness and completeness of CDR forms, and reduce the need for HCPs to provide additional information to PHAs. Background and full protocols for implementation and evaluation of the pre-populated CDR form intervention are described elsewhere [29].
The focus of this intervention was to reduce HCP burden and improve their reporting timeliness and CDR form completion. However, it was uncertain whether a PHA participating in the INPC HIE could also benefit from the intervention. While it is presumed that improved reporting will improve public health surveillance [30], there is little research on how more complete capture of cases might impact the efficiency and/or quality of reporting, burden of case investigation, or assessments of community disease burden. This paper reports an assessment of the impacts of the pre-populated form process on PHAs, utilizing a novel matched case analysis of timeliness changes in receipt and processing of CDR forms and focusing on Chlamydia cases which are the condition most frequently reported to the LHD participating in the INPC HIE [31].
Methods
Ethics
This study was approved by the Indiana University Institutional Review Board with cross-institutional and concurrent IRB deferral from the University of Washington.
Setting
The Marion County Public Health Department (MCPHD) in Indianapolis participates in the INPC HIE. MCPHD utilizes SWIMSS, the Statewide Information Management Surveillance System, for recording and investigating sexually-transmitted infections (STIs) cases. Data from ELRs, faxed lab results and CDRs for STIs are entered into SWIMSS. Traditionally, paper, handwritten CDR forms reporting STI cases are faxed by clinic reporters to MCPHD and entered into SWIMSS.
Baseline (pre-intervention) Data
To describe pre-intervention reporting data was extracted from the SWIMSS surveillance systems for the time period of 01/01/2012 through 09/15/2013 for four target notifiable conditions: Chlamydia, Gonorrhea, Syphilis and Syphilis, Reactor. In addition, given Chlamydia is the most frequently reported STI [31], a sub-set analysis of Chlamydia cases in the SWIMSS baseline dataset was conducted. This allowed us, for each system and specifically for Chlamydia, to: compare CDR form receipt, processing and time to close for a 21-month baseline period to the HIE CDR form deployment (intervention) period. Fields were tabulated to determine any data anomalies; Data cleaning and quality review included removal of duplicates and generation of a missing data rate table. We also assessed potential confounders of CDR form receipt or processing, such as day of the week or reporter-specific differences. Given the focus on Reporting Lag and time close a case, outliers and anomalies regarding date were identified. Based on this analysis, it was determined that any case in which this time difference was greater than 100 calendar days was excluded as atypical of case processing. The final dataset of 39,737 records included Chlamydia (n=28018); Gonorrhea (n=7791); Syphilis (n=810); and Syphilis, Reactor (n=3118). Due to their small numbers, Syphilis disease "categories" of Early Latent, Late Latent, Neurosyphilis, Primary, Secondary, Unknown Latent were combined into the single "Syphilis" category.
The following factors were analyzed to establish the rationale for case matching criteria:
Reporting Volume: Defined as the number of cases received per month into the surveillance system—by all conditions combined and by individual condition.
"Reporting Lag": Defined as the time difference between the earliest date for activity on a case (for example, the date of a positive lab test) and the date the case was logged into the PHA’s surveillance system (SWIMSS)—by number of calendar days; by work days (i.e., Mondays through Fridays when PHAs are open, excluding holidays); and by day of the week.
"Time to Close of Investigation": Defined as the time difference between earliest date for activity on a case and the date the case was closed by public health by number of calendar days—by work days (i.e., Mondays through Fridays when PHAs are open, excluding holidays); and by day of the week.
Intervention Data
During the intervention period (09/16/2013―03/01/2014), CDR forms were automatically pre-populated with available patient information and HCP contact information before being faxed from a pilot HIE clinic to MCPHD. While the pre-populated CDR form intervention was made available to the pilot clinics, clinic. Clinic reporters were not required to use this new service and sometimes overlooked the form and used their customary reporting method. During the intervention period 23 pre-populated forms were delivered to the intervention clinics, and 21 of those were then submitted to MCPHD for Chlamydia (n=21) and Gonorrhea (n=2) cases. Only the Chlamydia pre-populated CDR forms were used for the case matched analysis.
Intervention Period Dataset
A dataset was pulled from the SWIMSS surveillance system for 08/16/2013―04/01/2014 to cover the intervention period plus and minus 30 days. After cleaning and quality review similar to preparation of the baseline dataset, the intervention period SWIMSS dataset—excluding the submitted intervention CDR forms— included 4,372 cases of which 3,165 (72%) were Chlamydia, 769 (18%) Gonorrhea and 438 (10%) were Syphilis. The chlamydia reports were received from 284 unique providers, with 38% of the providers sending a single report.
The 23 pre-populated CDR form SWIMSS cases were identified by comparing details from scans of the pre-populated CDR forms with dataset case information. Given only 2 pre-populated CDR forms for Gonorrhea, these cases were excluded from further analysis.
Case Matching and Analysis
To control for potential confounding factors [32], the 21 pre-populated CDR form target cases were matched to one or more other chlamydia cases using the following criteria:
Same reporting provider ID
Same day of the week of receipt
Receipt of matched case within 30 days of target case
Matching case received within plus or minus one week of the target time frame (i.e., between 11/10/2013—03/22/2014). This latter matching criteria was included as the total number of cases received tended to increase outside this date range.
Of the 21 cases, 16 could be matched to at least one other case. The number of matches to an individual pre-populated CDR form ranged from 1 to 72, with a mean of 23 matches per case.
Two outcomes were analyzed: 1) Reporting Lag and 2) Time to Close of Investigation. Both were measured in work days. Because different numbers of matches were associated with each pre-populated CDR form target case, a hierarchical random effects model was used to compare mean difference in each outcome between the target cases and the matched cases, with random intercepts for case [33].
Results
Here we report the results that informed the case matching method, including Reporting Lag, Time to Close of Investigation and demographic information (clinic or clinic reporter) for the SWIMSS baseline dataset.
The mean Reporting Lag by calendar days for chlamydia cases was 5.5 calendar days (see Figure 1).
Figure 1.
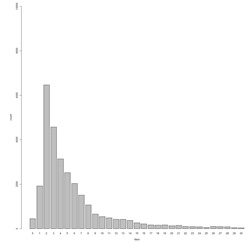
Reporting Lag: Difference in calendar days between date of earliest case activity and date case added to SWIMSS, Chlamydia only (n=28,018).
The time difference between earliest date of activity and date the case was closed (see Figure 2) was four work days or less for just over one half (53.0%) of chlamydia cases.
Figure 2.
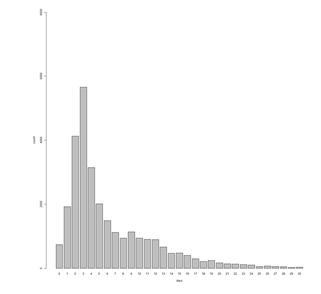
Time to Close: Time to close case in work days, Chlamydia only (n=28,018).
A systematic difference was observed between Reporting Lag of Chlamydia cases into SWIMSS and day of the week of the earliest date (see Figure 3). Monday cases took substantially less time to be delivered to public health than any other day of the week. In addition, a statistically significant relationship between day of the week of case receipt by MCPHD and time to close was identified (see Figure 4).
Figure 3.
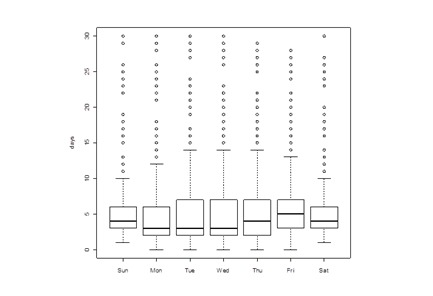
Relationship between day of the week of case receipt and Reporting Lag, in work days, Chlamydia cases (p < 0.01, Kruskall-Wallis test).
Figure 4.
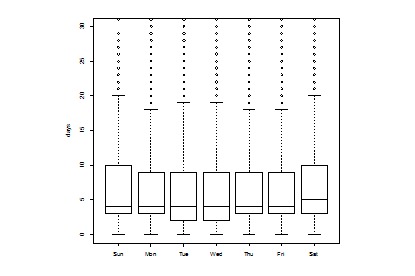
Relationship between day of the week of case receipt and time to close case, in work days, Chlamydia cases (p < 0.01, Kruskall-Wallis test).
Table 2 shows the estimated mean difference between target cases and matched controls in the number of worked days to receive a case and to close a case. Both the mean number of days to receive and case and the mean number of days to close a case were lower for the target cases than their matched controls. The mean reporting time for the pre-populated CDR form cases was 2.7 days shorter than the mean Reporting Lag for the matched controls in the intervention dataset (p = <0.001). The mean time to close a pre-populated CDR form case was 1.3 days shorter than time to close for the matched controls in the intervention dataset (p = 0.796).
Table 2. Estimated Difference in Reporting Lag and Time to Close.
| Estimated mean difference in days* | Std. Error | p-value | |
| Reporting Lag | 2.73 | 0.65 | <0.001 |
| Time to close case | 0.19 | 0.75 | 0.798 |
*Time for controls – time for cases
Discussion
The primary objective of our project was to investigate the impact of the new pre-populated form technical service being implemented within a HIE on public health reporting processes and operational outcome metrics. By collecting and sharing data across health care organizations, HIE networks are significantly transforming the work of both clinical and public health, with increased opportunity for more automated and efficient data capture [12]. The expanded adoption of HIEs promises to improve access to data and automate its extraction, making it easier to collect required data through electronic request from a single source. The pre-populated CDR form service investigated in this study is an example of an intervention that aims to reduce the burden of information-gathering with consequent quality improvements [34]. As noted earlier, ELR systems can improve timeliness of reporting to PHAs [4,10]; expanded connectivity between health record and ELR systems—as possible through HIEs—might further enhance reportable condition data capture and its delivery to PHAs.
This paper describes an effective analytical technique for investigating the impact of a technical intervention on public health agencies' reporting operations that can be utilized in future studies. A matched analysis allowed for early evaluation of the new pre-populated CDR form system, making efficient use of the cases received prior to widespread implementation. Matching to multiple cases (as opposed to matching to a single case) increases the power of the analysis by effectively reducing the variance of the mean times for matched cases, at the expense of increasing complexity in the analysis if a variable number of matches is used.
One limitation of matching is that biased estimates can be generated if a factor being matched is the result of the exposure rather than a confounding factors [35]. Of the factors matched on in this study, the only factor that could plausibly be a result of the exposure is the day of the week that the CDR form is received (which could occur if reports are batched and sent on specific days of the week); if this were the case it could plausible bias the estimate of the mean difference in time to receive a report. A separate analysis matching on the date that the test result is known rather than the date that the report is received however produced a similar time difference (mean difference 2.7 days, p < 0.001).
While constrained to a small number of target cases, we observed an improvement in the Reporting Lag when using the pre-populated CDR form intervention, and a smaller though not statistically significant improvement in the time to close cases. One possible reason for the improvement in the time to receive cases is that auto-generation of pre-populated forms may allow for processing of reportable conditions by providers as soon as the report is generated, whereas without pre-population or auto-generation it may be more efficient from the providers' view point to, again, process reports in batches every few days.
An improvement in the time to close cases might be expected if pre-population of fields on CDR forms improves data quality and/or completeness, thus requiring less public health investigator processing time. However, a high field completion rate that is still less than fully complete may not result in major time savings as missing information in a single important field would still necessitate contacting a provider to collect this information.
Limitations
One limitation of this study is that it was conducted using data from one system in one jurisdiction and examined only a single reportable condition. System factors may limit generalizability to jurisdictions that use similar systems, and differences in processing procedure between reportable conditions may limit generalizability to other conditions. A second limitation is in the ability to match for possible confounding factors. Matching is inherently limited to measured factors and it is not possible with an observational study to control the effects of unmeasured confounding factors. A third limitation is that the small numbers of cases limits the ability to rigorously determine sensitivity of the analysis procedure to the selection of factors to match on.
Acknowledgements
We wish to thank the MCPHD public health nurses and epidemiologists who reviewed baseline data analyses and provided input on PHA surveillance and investigation workflow. We also wish to thank Jennifer Williams who facilitated collection of CDR form intervention reports for this study.
IP, DR and JB designed the study. Algorithms to extract data from the surveillance systems were designed by IP, DR and PJG. IP designed and conducted all data analyses with input from DR, JB and JPG. IP and DR drafted the manuscript with review and contributions provided by JB and PJG. All authors approved the final version of this manuscript.
Abbreviations:
- Communicable disease report (CDR)
electronic laboratory reporting (ELR), health care provider (HC), health information exchange (HIE), Indiana Network for Patient Care (INPC), local health department (LHD), Marion County Public Health Department (MCPHD), Public health agency (PHA), sexually transmitted infection (STI), Statewide Information Management Surveillance System (SWIMSS)
Footnotes
Financial Disclosure: This study was conducted as part of the "Leveraging a HIE to Improve Public Health Disease Investigation" research project (RWJF Award #70338; PI: J Baseman, University of Washington). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Robert Wood Johnson Foundation.
Competing Interests: No Competing Interests.
References
- 1.Dato V, Wagner MM, Fapohunda A. 2004. How outbreaks of infectious disease are detected: a review of surveillance systems and outbreaks. Public Health Rep. 119, 464-71. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1497658/. 10.1016/j.phr.2004.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gelberg KH, Brissette IF, Cummings K. 2011. Evaluation of a communications campaign to increase physician reporting to a surveillance system. Public Health Rep. 126, 19-27. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3001818/. 10.1177/003335491112600106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McClean CM, Silk BJ, Buehler JW, Berkelman RL. 2010. Disease reporting among Georgia physicians and laboratories. J Public Health Manag Pract. 16, 535-43. 10.1097/PHH.0b013e3181cb4324 [DOI] [PubMed] [Google Scholar]
- 4.Overhage JM, Suico J, McDonald CJ. 2001. Electronic laboratory reporting: barriers, solutions and findings. J Public Health Manag Pract. 7, 60-66. 10.1097/00124784-200107060-00007 [DOI] [PubMed] [Google Scholar]
- 5.Sickbert-Bennett EE, Weber DJ, Poole C, MacDonald PDM, Maillard JM. 2011. Completeness of communicable disease reporting, North Carolina, USA, 1995–1997 and 2000–2006. Emerg Infect Dis. 17, 23-29. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3204630/. 10.3201/eid1701.100660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Staes CJ, Gesteland PH, Allison M, Mottice S, Rubin M, et al. 2009. Urgent care providers’ knowledge and attitude about public health reporting and pertussis control measures: implications for informatics. J Public Health Manag Pract. 15, 471-78. 10.1097/PHH.0b013e3181af0aab [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward LD, Spain CV, Perilla MJ, Morales KH, Linkin DR. 2008. Improving disease reporting by clinicians: The effect of an internet-based intervention. J Public Health Manag Pract. 14, 56-61. 10.1097/01.PHH.0000303414.19851.d9 [DOI] [PubMed] [Google Scholar]
- 8.Castrucci BC, Rhoades EK, Leider JP, Hearne S. 2015. What gets measured gets done: an assessment of local data uses and needs in large urban health departments. J Public Health Manag Pract. 21, S38-48. 10.1097/PHH.0000000000000169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jajosky RA, Groseclose SL. 2004. Evaluation of reporting timeliness of public health surveillance systems for infectious diseases. BMC Public Health. 4, 29. https://bmcpublichealth.biomedcentral.com/articles/10.1186/1471-2458-4-29. 10.1186/1471-2458-4-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silk BJ, Berkelman RL. 2005. A review of strategies for enhancing the completeness of notifiable disease reporting. J Public Health Manag Pract. 11, 191-200. 10.1097/00124784-200505000-00003 [DOI] [PubMed] [Google Scholar]
- 11.Birkhead GS, Klompas M, Shah NR. 2015. Uses of electronic health records for public health surveillance to advance public health. Annu Rev Public Health. 36, 345-59. 10.1146/annurev-publhealth-031914-122747 [DOI] [PubMed] [Google Scholar]
- 12.Calman N, Hauser D, Lurio J, Wu WY, Pichardo M. 2012. Strengthening public health and primary care collaboration through electronic health records. Am J Public Health. 102, e13-18. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3477979/. 10.2105/AJPH.2012.301000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomines A, Readhead H, Readhead A, Teutsch S. 2013. Applications of electronic health information in public health: uses, opportunities & barriers. EGEMS (Wash DC). 1, 1019. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4371418/. 10.13063/2327-9214.1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention (CDC) . 2008. Potential effects of electronic laboratory reporting on improving timeliness of infectious disease notification--Florida, 2002-2006. MMWR Morb Mortal Wkly Rep. 57(49), 1325-28. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5749a2.htm. [PubMed] [Google Scholar]
- 15.Allen-Dicker J, Klompas M. 2012. Comparison of electronic laboratory reports, administrative claims, and electronic health record data for acute viral hepatitis surveillance. J Public Health Manag Pract. 18, 209-14. 10.1097/PHH.0b013e31821f2d73 [DOI] [PubMed] [Google Scholar]
- 16.Lamb E, Satre J, Hurd-Kundeti G, Liscek B, Hall CJ, et al. Centers for Disease Control and Prevention (CDC) . 2015. Update on progress in electronic reporting of laboratory results to public health agencies - United States, 2014. MMWR Morb Mortal Wkly Rep. 64, 328-30. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6412a5.htm. [PMC free article] [PubMed] [Google Scholar]
- 17.Gluskin RT, Mavinkurve M, Varma JK. 2014. Government leadership in addressing public health priorities: strides and delays in electronic laboratory reporting in the United States. Am J Public Health. 104, e16-21. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3953791/. 10.2105/AJPH.2013.301753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith A. Direct ELR Import and Infectious Disease Investigation. Public Health Informatics Conf. Atlanta GA: 08/2016. [Google Scholar]
- 19.NACCHO. Local Public Health Workforce Benchmarks. Washington DC: NACCHO; 2011. Online: http://www.naccho.org/uploads/downloadable-resources/local-public-health-workforce-staffing-benchmarks.pdf
- 20.Lopez DM, Blobel BG. 2007. Semantic interoperability between clinical and public health information systems for improving public health services. Stud Health Technol Inform. 127, 256-67. [PubMed] [Google Scholar]
- 21.Health IT. gov. What is HIE (Health Information Exchange)? Washington DC: HHS; 2014. Online: http://www.healthit.gov/providers-professionals/health-information-exchange/what-hie
- 22.Shapiro JS, Mostashari F, Hripcsak G, Soulakis N, Kuperman G. 2011. Using health information exchange to improve public health. Am J Public Health. 101, 616-23. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3052326/. 10.2105/AJPH.2008.158980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eden KB, Totten AM, Kassakian SZ, Gorman PN, McDonagh MS, et al. 2016. Barriers and facilitators to exchanging health information: a systematic review. Int J Med Inform. 88, 44-51. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4778080/. 10.1016/j.ijmedinf.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frisse ME, Johnson KB, Nian H, Davison CL, Gadd CS, et al. 2012. The financial impact of health information exchange on emergency department care. J Am Med Inform Assoc. 19, 328-33. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3341788/. 10.1136/amiajnl-2011-000394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bassi J, Lau F. 2013. Measuring value for money: a scoping review on economic evaluation of health information systems. J Am Med Inform Assoc. 20, 792-801. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3721162/. 10.1136/amiajnl-2012-001422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaelber DC, Bates DW. 2007. Health information exchange and patient safety. J Biomed Inform. 40(6) (Suppl), S40-45. http://www.sciencedirect.com/science/article/pii/S1532046407000901. 10.1016/j.jbi.2007.08.011 [DOI] [PubMed] [Google Scholar]
- 27.Fontaine P, Ross SE, Zink T, Schilling LM. 2010. Systematic review of health information exchange in primary care practices. J Am Board Fam Med. 23, 655-70. http://www.jabfm.org/content/23/5/655.long. 10.3122/jabfm.2010.05.090192 [DOI] [PubMed] [Google Scholar]
- 28.Shah GH, Leider JP, Castrucci BC, Williams KS, Luo H. 2016. Characteristics of local health departments associated with implementation of electronic health records and other informatics systems. Public Health Rep. 131, 272-82. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4765976/. 10.1177/003335491613100211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dixon BE, Grannis SJ, Revere D. 2013. Measuring the impact of a health information exchange intervention on provider-based notifiable disease reporting using mixed methods: a study protocol. BMC Med Inform Decis Mak. 13, 121. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/1472-6947-13-121. 10.1186/1472-6947-13-121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klompas M, McVetta J, Lazarus R, Eggleston E, Haney G, et al. 2012. Integrating clinical practice and public health surveillance using electronic medical record systems. Am J Prev Med. 42, S154-62. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3478075/. 10.1016/j.amepre.2012.04.005 [DOI] [PubMed] [Google Scholar]
- 31.Workowski KA, Berman S, Centers for Disease Control and Prevention . 2010. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 59, 1-110. https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5912a1.htm. [PubMed] [Google Scholar]
- 32.Kupper LL, Karon JM, Kleinbaum DG, Morgenstern H, Lewis DK. 1981. Matching in epidemiologic studies: validity and efficiency considerations. Biometrics. 37, 271-91. 10.2307/2530417 [DOI] [PubMed] [Google Scholar]
- 33.Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. NY: Cambridge Univ Press;2007:235-99. [Google Scholar]
- 34.Mac Kenzie WR, Davidson AJ, Wiesenthal A, Engel JP, Turner K, et al. 2016. The promise of electronic case reporting. Public Health Rep. 131(6), 742-46. 10.1177/0033354916670871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marsh JL, Hutton JL, Binks K. 2002. Removal of radiation dose response effects: an example of over-matching. BMJ. 325(7359), 327-30. http://www.bmj.com/content/325/7359/327.long. 10.1136/bmj.325.7359.327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richards CL, Iademarco MF, Anderson TC. 2014. A new strategy for public health surveillance at CDC: improving national surveillance activities and outcomes. Public Health Rep. 129, 472-76. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4187298/. 10.1177/003335491412900603 [DOI] [PMC free article] [PubMed] [Google Scholar]


