Abstract
IL-17A is an important proinflammatory cytokine which is frequently elevated in tumor microenvironment. However, the role of intratumoral IL-17A in solid tumors remains controversial. Herein, we conducted a meta-analysis to assess the prognostic impact of intratumoral IL-17A in patients with solid tumor. PubMed and EBSCO were searched to identify the studies evaluating the associations between intratumoral IL-17A measured by immunohistochemistry (IHC) and overall survival (OS) and disease-free survival (DFS) in solid tumors. A total of 2972 patients with solid tumor from 21 published studies were incorporated into this meta-analysis. We found that high level of intratumoral IL-17A was significantly associated with worse 3-year, 5-year OS and 1-year, 3-year DFS, but not with 1-year OS or 5-year DFS in solid tumors. In addition, in stratified analyses by cancer types, IL-17A overexpression was significantly associated with worse OS in hepatic carcinoma, but with improved OS in esophageal squamous cell carcinoma (ESCC). Furthermore, high IL-17A expression positively correlated with advanced TNM stage. In conclusion, High expression of intratumoral IL-17A leads to an unfavorable clinical outcome in majority of solid tumors, implicating IL-17A is a valuable biomarker for prognostic prediction of human solid malignances and targeting it may have a potential for effective treatment.
Keywords: intratumoral IL-17A overexpression, immunohistochemistry, worse outcome, solid tumor, meta-analysis
INTRODUCTION
Chronic inflammation is closely linked to cancer [1, 2], Recent study has demonstrated that cancer-related inflammation promotes tumor development and progression through various mechanisms, including stimulating angiogenesis, promoting cell proliferation and survival, inducing gene mutation and subverting antitumor immune responses [3, 4]. Accumulating evidence has proven that cytokines could promote carcinogenesis by both provoking inflammation [5–7] and eliciting immunosuppression [8, 9].
Interleukin-17A (IL-17A), which initially termed as cytotoxic T-lymphocyte-associated antigen (CTLA-8), is the founding member of IL-17 cytokine family [10, 11]. It is mainly produced by Th17 cells and also by γδ T, natural killer and CD8+ T cells [12, 13], relying on STAT3 activation triggered by IL-23 [14]. A large body of evidence suggests that IL-17A is an essential proinflammatory cytokine due to inducing a mass of cytokines and chemokines secretion by distinct cell types, such as mesenchymal cells and myeloid cells, which recruit monocytes and neutrophils into the site of inflammation [15]. Recently, several studies have shown that IL-17A has either a protumor or antitumor role in different cancer models [16]. IL-17A is elevated in several human tumors [17]. In previous studies, high level of IL-17A within tumor was shown to associate with poor survival of several cancers [18–21]; whereas some other studies reported opposite results [22–25]. Therefore, an in-depth assessment is warranted. Moreover, the potential of IL-17A as an effective biomarker in prognostic prediction and targeted therapy is necessary to be explored.
Here, we performed this meta-analysis to test overall survival (OS) and disease-free survival (DFS) as outcomes in patients with solid tumor with known IL-17A levels within tumor evaluated by IHC. The aim of this study was to quantitatively summarize the association between intratumoral IL-17A and clinical outcomes in cancer patients, and thereby provided more evidence on the clinical value of IL-17A as a prognostic biomarker and therapeutic target for solid malignances.
RESULTS
Search results and description of studies
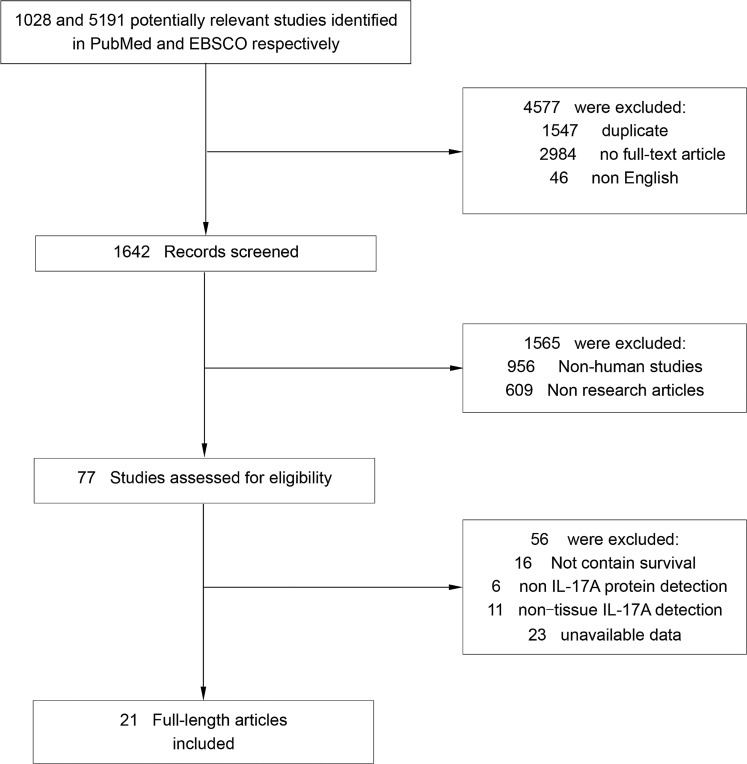
Literature searches yield 6219 records and the results are shown in Figure 1. 21 studies containing 2972 cancer patients were identified for the assessment of IL-17A expression within tumor [18–38]. All the studies were evaluated by the Newcastle–Ottawa Scale (NOS), and were in accordance with the inclusion criteria and suitable for data consolidation. Characteristics of included studies for OS, DFS and clinicopathological features such as TNM stage, tumor differentiation were shown in Table 1 and Supplementary Table 1 respectively.
Figure 1. Flow chart diagram of study selection.
Table 1. Main characteristics of the included studies.
| Study | Year | Tumor type | No. of Patients | Male/Female | median age (range) (year) | Cut off for high expression | Tumor stage | median follow-up date (months) | Survival | Quality Score (NOS) |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen WC, et al. [35] | 2013 | Breast cancer | 207 | 0/207 | 51 (23, 78) | positive cells > 90/HPF | I–III | 67.2 (7.2, 144) | OS, DFS | 8 |
| Chen X, et al. [21] | 2010 | Non-small cell lung cancer | 52 | 41/11 | ≥ 60: 40%; < 60: 60% |
positive cells > 5%/HPF | I–III | NR | OS, DFS | 7 |
| Chen JG, et al. [25] | 2011 | Gastric cancer | 192 | 129/63 | 58 (17, 85) | density of positive cells > 2.5/HPF | I–IV | 61 (0.3, 81.6) | OS | 8 |
| Cui XL, et al. [26] | 2013 | Glioblastoma | 41 | 18/23 | 47 (14, 67) | positive cells > 15% | IV | 12.9 (4, 24) | OS | 7 |
| Gu FM, et al. [28] | 2012 | Intrahepatic cholangiocarcinoma | 123 | 62/61 | 55 (18, 78) | positive cells > 111/HPF | I–III | 13 (4, 111) | OS | 7 |
| Lan CY, et al. [27] | 2013 | Ovarian cancer | 104 | 0/104 | 53 (27, 81) | positive cells > 35%/HPF | III–IV | NR | OS | 8 |
| Liao R, et al. [18] | 2013 | Hepatocellular carcinoma | 300 | 253/47 | ≤ 53: 48%; ≤ 53: 52% |
density of positive cells > 51/HPF | I–IV | NR | OS, DFS | 7 |
| Liu JK, et al. [29] | 2011 | Colorectal cancer | 52 | 31/21 | ≥ 60: 33%; < 60: 67% |
positive cells > 5% | III | NR | OS | 7 |
| Lv L, et al. [22] | 2011 | Esophageal squamous cell cancer | 181 | 141/40 | ≥ 60: 42%; < 60: 58% |
density of positive cells > 3.9/HPF | I–IV | NR | OS | 8 |
| Zhang JP, et al. [19] | 2009 | Hepatocellular carcinoma | 178 | 159/19 | NR | density of positive cells>7.8cells/0.145mm2 | I–IV | NR | OS, DFS | 7 |
| Zhang GQ, et al. [30] | 2012 | Non-small cell lung cancer | 102 | 66/36 | 65 (40, 73) | intensity of staining | I–III | 30.2 (24, 59) | OS | 7 |
| Lin Y, et al. [23] | 2014 | Colorectal cancer | 78 | 46/32 | ≥ 60: 59%; < 60: 41% |
score ≥ 3 | I–IV | NR | OS | 8 |
| Liu XS, et al. [31] | 2014 | Gastric cancer | 112 | 78/34 | 60 (33, 89) | density of positive cells/HPF | Tis, I, II, IV | 51 (39, 57) | OS | 8 |
| Gu FM, et al. [32] | 2011 | Hepatocellular carcinoma | 323 | 46/277 | > 50:48.6%; ≤ 50: 51.4% |
density of positive cells | I–III | 60 (2, 74) | OS, DFS | 7 |
| Li J, et al. [20] | 2011 | Hepatocellular carcinoma | 43 | 35/8 | > 60:18.6% ; ≤ 60:81.4% |
density of positive cells > 341/HPF | I–IV | NR | OS, DFS | 7 |
| Punt S, et al. [36] | 2015 | Squamous cervical cancer | 109 | NR | 45 (22, 87) | density of positive cells > 57/0.6mm2 | I–IV | 112.8 (73.2, 151.2) | OS | 7 |
| Zhang Y, et al. [33] | 2013 | Gallbladder carcinoma | 104 | 63/41 | 66.13 ± 11.88 | positive cells/HPF | I–IV | 39 (2, 76) | OS, DFS | 7 |
| He SB, et al. [34] | 2011 | Pancreatic adenocarcinoma | 46 | 31/15 | > 60: 63%; ≤ 60: 37% |
positive cells >5.60/HPF | I–IV | (5, 48) | OS | 6 |
| Wang B, et al. [24] | 2013 | Esophageal squamous cell cancer | 215 | 160/55 | 56 (23, 82) | density of positive cells > 10% | I–IV | 29.4 (2.2, 156.7) | OS | 7 |
| Yu Q, et al. [37] | 2014 | Cervical cancer | 153 | NR | ≥ 40: 83%; < 40: 17% |
NR | II | NR | DFS | 6 |
| Tosolini M, et al. [38] | 2011 | Colorectal cancer | 27 | NR | NR | density of positive cells > 15/mm2 | NR | NR | DFS | 6 |
Meta-analyses
Overall survival (OS)
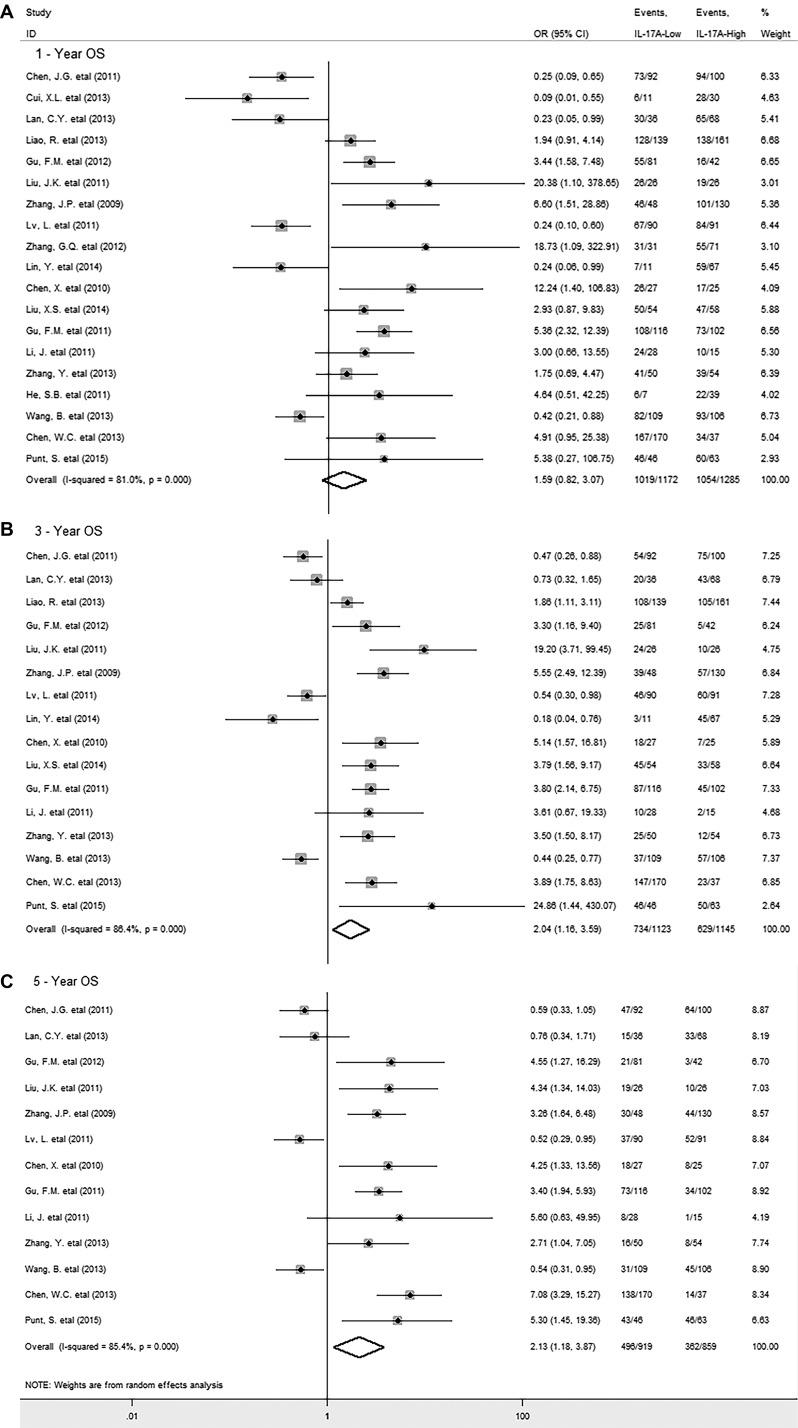
A total of 19 studies with 2562 cancer patients reported the data for OS. The meta-analysis showed that IL-17A overexpression within tumor was not associated with OS in patients with solid tumor (HR = 1.33, 95% CI 0.97 to 1.83, P = 0.079) (Supplementary Figure 1), with significant heterogeneity observed among studies (I2 = 80.2%; P = 0.000). However, it was important to find that increased level of intratumoral IL-17A was significantly associated with lower 3-year (OR = 2.04, 95% CI 1.16 to 3.59, P = 0.014) and 5-year OS rate (OR = 2.13, 95% CI 1.18 to 3.87, P = 0.012) in solid tumors (Figure 2B and 2C); whereas there was no significant association between IL-17A and 1-year OS rate (OR = 1.59, 95% CI 0.82 to 3.07, P = 0.014) (Figure 2A), with significant heterogeneity observed (I2 = 81.0%; P = 0.000; I2 = 86.4%; P = 0.000; I2 = 85.4%; P = 0.000 respectively) .
Figure 2. Forest plots describing ORs of the association between intratumoral IL-17A expression and OS at 1-year, 3-year, 5-year.
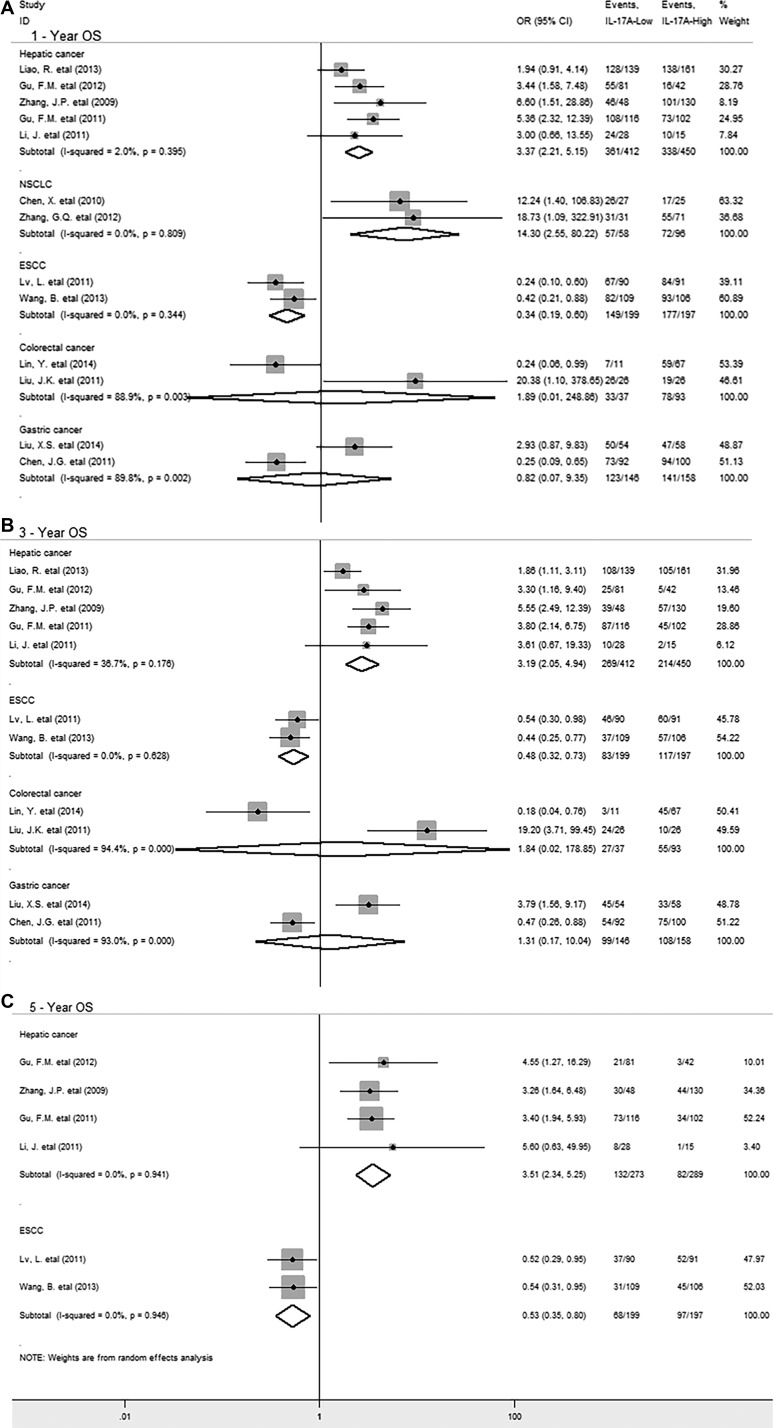
In stratified analyses by cancer types, as shown in Figure 3, pooled results showed that IL-17A overexpression was significantly associated with decreased OS at 1-year (OR = 3.37, 95% CI 2.21 to 5.15, P = 0.000), 3-year (OR = 3.19, 95% CI 2.05 to 4.94, P = 0.000) and 5-year (HR = 3.51, 95% CI 2.34 to 5.25, P = 0.000) in hepatic cancer and 1-year OS in non-small cell lung cancer (NSCLC) (OR = 14.30, 95% CI 2.55 to 80.22, P = 0.002). However, in esophageal squamous cell carcinoma (ESCC), IL-17A overexpression significantly correlated with improved 1-year (OR = 0.34, 95% CI 0.19 to 0.60, P = 0.000), 3-year (OR = 0.48, 95% CI 0.32 to 0.73, P = 0.000) and 5-year OS (OR = 0.53, 95% CI 0.35 to 0.80, P = 0.002). In addition, there was no significant association between IL-17A and OS at 1-year and 3-year in gastric or colorectal cancer. By the way, there was only one study reporting the relevant data for OS in glioblastoma, breast, ovarian, cervical cancer and gallbladder, pancreatic carcinoma respectively, thus, we couldn’t get a combined result for them.
Figure 3. Stratified analyses describing ORs of the association between intratumoral IL-17A expression and OS at 1-year, 3-year, 5-year.
Disease-free survival (DFS)
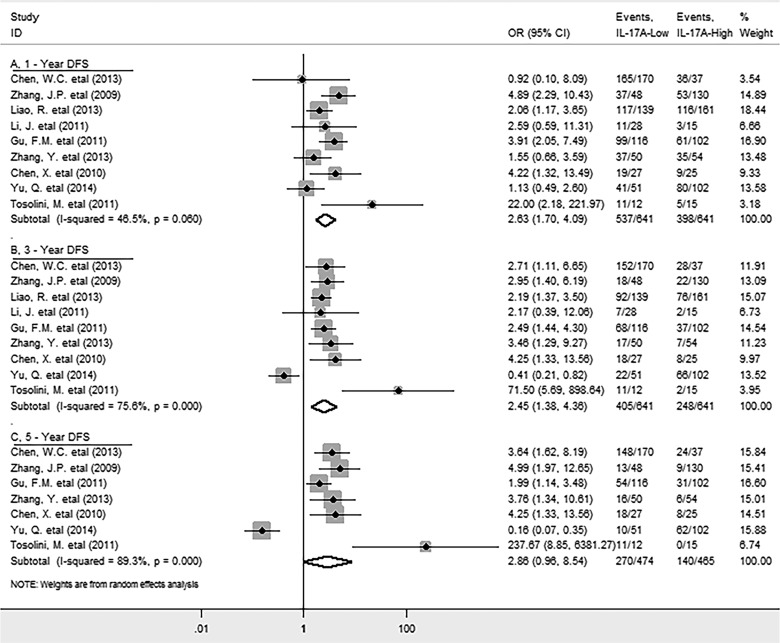
9 studies reported the data for DFS. Meta-analysis showed that high level of IL-17A within tumor was significantly associated with decreased DFS in solid tumors (HR = 1.70, 95% CI 1.17 to 2.46, P = 0.005) (Supplementary Figure 2); while significant heterogeneity was observed (I2 = 78.9%; P = 0.000). Specifically, elevated IL-17A was significantly correlated with worse 1-year (OR = 2.63, 95% CI 1.7 to 4.09, P = 0.000) and 3-year (OR = 2.45, 95% CI 1.38 to 4.36, P = 0.002), but not with 5-year (OR = 2.86, 95% CI 0.96 to 8.54, P = 0.059) DFS rate (Figure 4), with significant heterogeneity was observed in the latter two analyses (I2 = 75.6%; P = 0.000; I2 = 89.3%; P = 0.000 respectively).
Figure 4. Forest plots describing ORs of the association between intratumoral IL-17A expression and DFS at 1-year, 3-year, 5-year.
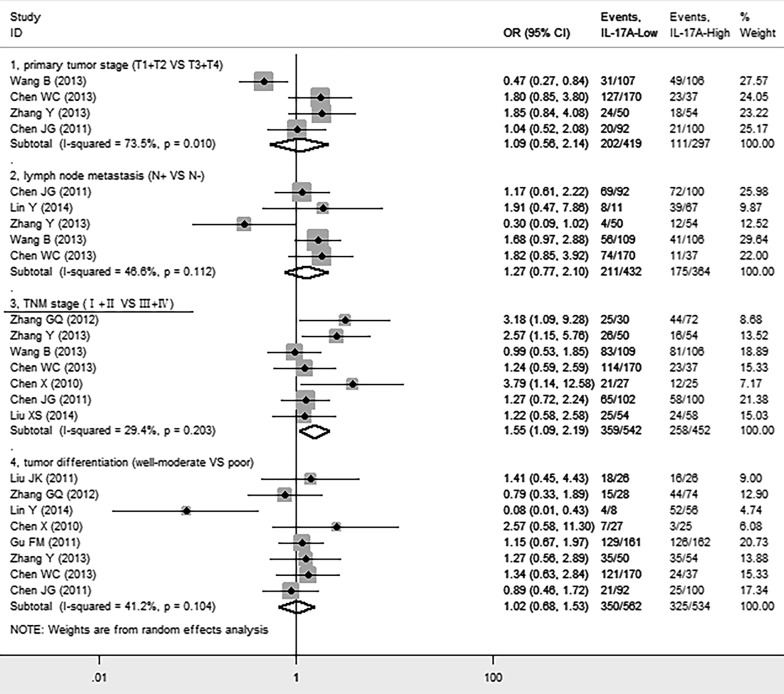
We next investigated whether intratumoral IL-17A was associated with clinicopathological features such as primary tumor (T), lymph node status (N), TNM stage, tumor differentiation of cancer patients. We found that high level of IL-17A was significantly positively correlated with advanced TNM stage (III + IV) (OR = 1.55, 95% CI 1.09 to 2.19, P = 0.014), but not with primary tumor (T) (OR = 1.09, 95% CI 0.56 to 2.14, P = 0.796), lymph node status (N) (OR = 1.27, 95% CI 0.77 to 2.10, P = 0.348), or tumor differentiation (OR = 1.02, 95% CI 0.68 to 1.47, P = 1.53) of patients (Figure 5).
Figure 5. Forest plots indicating ORs of the association between intratumoral IL-17A expression and clinicopathological features.
Sensitivity analysis
Sensitivity analyses were used to determine the influence of individual studies on the overall OR. As a result, the plot showed that all the individual studies had no important impact on the results for OS at 1, 3 and 5-year (Supplementary Figure 3).
Publication bias
Funnel plot and Egger’s test were performed to assess the publication bias of this meta-analysis. No publication bias existed between overexpression of intratumoral IL-17A and OS or DFS in solid tumors (data not shown).
DISCUSSION
IL-17A shows its bidirectional functions both in pro-tumor and anti-tumor effect. Previous meta-analyses reported that IL-17A wasn’t significantly associated with OS in cancers [39, 40]. However, the studies included in these meta-analyses reported the different sources of IL-17A involving tumor tissue, peripheral blood or peritoneal lavage and different detecting methods such as Flow Cytometry, ELISA, IHC and RT-PCR. Thus, the results were not accurate even wrong when they were from the combinations of all these studies. In this study, we found high level of intratumoral IL-17A was significantly correlated with worse 3-year, 5-year OS and 1-year, 3-year DFS, but not with 1-year OS or 5-year DFS in majority of solid tumors, which differing markedly from the previous studies [39, 40]. Furthermore, IL-17A in tumor tissue positively correlated with TNM stage. Therefore, we believe our study provides meaningful statistical evidence for the prognostic value of intratumoral IL-17A for the first time.
Actually, IL-17A, which is secreted by Th17, γδ T, Tc17 and mast cells in tumor tissue, could promote cancer-elicited inflammation and prevent tumor cells from immune surveillance [17], through subverting T cell mediated anti-tumor immune responses by polarization of myeloid-derived suppressor cells (MDSCs) [41]. In stratified analyses by cancer types, we found the IL-17A overexpression was significantly associated with decreased OS in hepatic carcinoma and NSCLC, but with improved OS in ESCC. The possible explanation might be that the number of intratumoral IL-17A-producing cells positively correlated with infiltrating effector CD8+ T and CD57+ NK cells in human ESCC as previous reported [42].
Some limitations should be noted from this meta-analysis. First, significant heterogeneity observed across studies cannot be completely accounted. The possible sources of heterogeneity were the inconsistent cut-off values for assessing IL-17A expression and the IL-17A IHC methods taken by different researchers. Therefore, we suggest researchers to use IL-17A mAbs with the same clone number and employ similar cut-off value when they assess IL-17A in the future study. In addition, most of the included studies were performed in China, and studies with negative results or small sample size may not be published, which can cause potential publication bias.
In conclusion, high level of intratumoral IL-17A leads to an unfavorable clinical outcome in majority of solid tumors, implicating IL-17A is a valuable biomarker for prognostic prediction of human solid malignances and targeting it may have a potential for effective treatment.
MATERIALS AND METHODS
Search strategy
We searched PubMed and EBSCO for studies assessing the expression of IL-17A in tumor tissue and survival in patients with solid tumor from 1996 to August 2016. The searching keywords were (“Interleukin-17A “ OR “IL-17A” OR “IL17A”) AND (“neoplasms” OR “cancer” OR “tumor” OR “carcinoma”). A total of 1028 and 5191 entries were identified in PubMed and EBSCO respectively.
Inclusion and exclusion criteria
Inclusion criteria of the study were the measurement of IL-17A by immunohistochemistry (IHC), provided the survival data (OS and /or DFS) and publication in English. Exclusion criteria included studies that evaluated IL-17A with ELISA or Flow Cytometry or RT-PCR, detected IL-17A not in tissues, and studies on animals or in lab.
Endpoints
OS was recorded as the primary endpoint, while the second endpoint was DFS. Cut-offs of intratumoral IL-17A expression level defined by individual studies classified patients with solid tumor into high- and low- groups.
Data extraction
Two authors (GM.H. and ZA.L.) independently reviewed and extracted data using predefined data abstraction forms from each eligible studies. Extracted information included first author’s name, publication year, country, type of cancer, number of patients, median age, gender, Tumor, Lymph Node, Metastasis (TNM) stage, tumor differentiation, time of follow-up, technique used to quantify IL-17A, and cut-off value to determine IL-17A positivity. OS, DFS and clinicopathological data were extracted from the text, tables, or Kaplan – Meier curves for both IL-17A-high and -low group.
Quality assessment
The studies included in the meta-analysis were cohort studies. The quality of each included study was assessed using Newcastle–Ottawa Scale (NOS) (Supplementary Table 2) by two independent authors [43]. (Supplementary Table 3) The studies with 6 scores or more were classified as high quality studies. A consensus NOS score for each item was achieved.
Statistical analysis
Extracted data were combined into a meta-analysis using STATA 12.0 analysis software (Stata Corporation, College Station, TX, USA). Statistical heterogeneity was assessed using the chi-squared based Q-test or the I2 method [44]. Data were combined according to the random-effect model in the presence of heterogeneity [45], otherwise, the fixed-effect model was performed [46]). Sensitivity analysis was employed to assess the influence of each study on the pooled result. Begg’s funnel plot and Egger’s test [47] were calculated to investigate potential publication bias. All P values were two-sided and less than 0.05 are considered statistically significant.
SUPPLEMENTARY MATERIALS FIGURES AND TABLES
Acknowledgments
We thank all the members of the departments who helped in this study.
Abbreviations
- IL-17A
interleukin-17A
- OS
overall survival
- DFS
disease-free survival
- HR
hazard rations
- OR
odds ratios
- Cl
confidence interval
- IHC
immunohistochemistry
- TNM
Tumor, Lymph Node, Metastasis
- ELISA
Enzyme-Linked Immunosorbent Assay
- RT-PCR
real-time reverse transcription polymerase chain reaction
- NR
not reported
- ESCC
esophageal squamous cell carcinoma
- NSCLC
non-small cell lung cancer
Authors’ contributions
SM.W. participated in its design and performed the statistical analysis. ZA.L. participated in the data extraction. GM.H. conceived of the study, participated in its design, extracted data, performed the statistical analysis and drafted the manuscript. All authors read and approved the final manuscript.
CONFLICTS OF INTEREST
The authors have declared that no competing interests exist.
FUNDING
None.
REFERENCES
- 1.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer cell. 2005;7:211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 4.Baniyash M. Chronic inflammation, immunosuppression and cancer: new insights and outlook. Seminars in cancer biology. 2006;16:80–88. doi: 10.1016/j.semcancer.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Chambers SK. Role of CSF-1 in progression of epithelial ovarian cancer. Future oncology. 2009;5:1429–1440. doi: 10.2217/fon.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apte RN, Voronov E. Interleukin-1—a major pleiotropic cytokine in tumor-host interactions. Seminars in cancer biology. 2002;12:277–290. doi: 10.1016/s1044-579x(02)00014-7. [DOI] [PubMed] [Google Scholar]
- 7.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 8.Waldner MJ, Neurath MF. Targeting the VEGF signaling pathway in cancer therapy. Expert opinion on therapeutic targets. 2012;16:5–13. doi: 10.1517/14728222.2011.641951. [DOI] [PubMed] [Google Scholar]
- 9.Principe DR, Doll JA, Bauer J, Jung B, Munshi HG, Bartholin L, Pasche B, Lee C, Grippo PJ. TGF-beta: duality of function between tumor prevention and carcinogenesis. Journal of the National Cancer Institute. 2014;106:djt369. doi: 10.1093/jnci/djt369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rouvier E, Luciani MF, Mattei MG, Denizot F, Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. Journal of immunology. 1993;150:5445–5456. [PubMed] [Google Scholar]
- 11.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 12.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annual review of immunology. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 13.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annual review of immunology. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 14.Cho ML, Kang JW, Moon YM, Nam HJ, Jhun JY, Heo SB, Jin HT, Min SY, Ju JH, Park KS, Cho YG, Yoon CH, Park SH, et al. STAT3 and NF-kappaB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. Journal of immunology. 2006;176:5652–5661. doi: 10.4049/jimmunol.176.9.5652. [DOI] [PubMed] [Google Scholar]
- 15.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Murugaiyan G, Saha B. Protumor vs antitumor functions of IL-17. Journal of immunology. 2009;183:4169–4175. doi: 10.4049/jimmunol.0901017. [DOI] [PubMed] [Google Scholar]
- 17.Wu D, Wu P, Huang Q, Liu Y, Ye J, Huang J. Interleukin-17: a promoter in colorectal cancer progression. Clinical & developmental immunology. 2013;2013:436307. doi: 10.1155/2013/436307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao R, Sun J, Wu H, Yi Y, Wang JX, He HW, Cai XY, Zhou J, Cheng YF, Fan J, Qiu SJ. High expression of IL-17 and IL-17RE associate with poor prognosis of hepatocellular carcinoma. Journal of experimental & clinical cancer research. 2013;32:3. doi: 10.1186/1756-9966-32-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, Wu C, Li SP, Zheng L. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. Journal of hepatology. 2009;50:980–989. doi: 10.1016/j.jhep.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Lau GK, Chen L, Dong SS, Lan HY, Huang XR, Li Y, Luk JM, Yuan YF, Guan XY. Interleukin 17A promotes hepatocellular carcinoma metastasis via NF-kB induced matrix metalloproteinases 2 and 9 expression. PLoS One. 2011;6:e21816. doi: 10.1371/journal.pone.0021816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Wan J, Liu J, Xie W, Diao X, Xu J, Zhu B, Chen Z. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung cancer. 2010;69:348–354. doi: 10.1016/j.lungcan.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Lv L, Pan K, Li XD, She KL, Zhao JJ, Wang W, Chen JG, Chen YB, Yun JP, Xia JC. The accumulation and prognosis value of tumor infiltrating IL-17 producing cells in esophageal squamous cell carcinoma. PLoS One. 2011;6:e18219. doi: 10.1371/journal.pone.0018219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Y, Xu J, Su H, Zhong W, Yuan Y, Yu Z, Fang Y, Zhou H, Li C, Huang K. Interleukin-17 is a favorable prognostic marker for colorectal cancer. Clinical & translational oncology. 2015;17:50–56. doi: 10.1007/s12094-014-1197-3. [DOI] [PubMed] [Google Scholar]
- 24.Wang B, Li L, Liao Y, Li J, Yu X, Zhang Y, Xu J, Rao H, Chen S, Zhang L, Zheng L. Mast cells expressing interleukin 17 in the muscularis propria predict a favorable prognosis in esophageal squamous cell carcinoma. Cancer immunology, immunotherapy. 2013;62:1575–1585. doi: 10.1007/s00262-013-1460-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen JG, Xia JC, Liang XT, Pan K, Wang W, Lv L, Zhao JJ, Wang QJ, Li YQ, Chen SP, He J, Huang LX, Ke ML, et al. Intratumoral expression of IL-17 and its prognostic role in gastric adenocarcinoma patients. International journal of biological sciences. 2011;7:53–60. doi: 10.7150/ijbs.7.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cui X, Xu Z, Zhao Z, Sui D, Ren X, Huang Q, Qin J, Hao L, Wang Z, Shen L, Lin S. Analysis of CD137L and IL-17 expression in tumor tissue as prognostic indicators for gliblastoma. International journal of biological sciences. 2013;9:134–141. doi: 10.7150/ijbs.4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lan C, Huang X, Lin S, Huang H, Cai Q, Lu J, Liu J. High density of IL-17-producing cells is associated with improved prognosis for advanced epithelial ovarian cancer. Cell and tissue research. 2013;352:351–359. doi: 10.1007/s00441-013-1567-0. [DOI] [PubMed] [Google Scholar]
- 28.Gu FM, Gao Q, Shi GM, Zhang X, Wang J, Jiang JH, Wang XY, Shi YH, Ding ZB, Fan J, Zhou J. Intratumoral IL-17(+) cells and neutrophils show strong prognostic significance in intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2012;19:2506–2514. doi: 10.1245/s10434-012-2268-8. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Duan Y, Cheng X, Chen X, Xie W, Long H, Lin Z, Zhu B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun. 2011;407:348–354. doi: 10.1016/j.bbrc.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Zhang GQ, Han F, Fang XZ, Ma XM. CD4+, IL17 and Foxp3 expression in different pTNM stages of operable non-small cell lung cancer and effects on disease prognosis. Asian Pacific journal of cancer prevention. 2012;13:3955–3960. doi: 10.7314/apjcp.2012.13.8.3955. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Jin H, Zhang G, Lin X, Chen C, Sun J, Zhang Y, Zhang Q, Yu J. Intratumor IL-17-positive mast cells are the major source of the IL-17 that is predictive of survival in gastric cancer patients. PLoS One. 2014;9:e106834. doi: 10.1371/journal.pone.0106834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu FM, Li QL, Gao Q, Jiang JH, Zhu K, Huang XY, Pan JF, Yan J, Hu JH, Wang Z, Dai Z, Fan J, Zhou J. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Molecular cancer. 2011;10:150. doi: 10.1186/1476-4598-10-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Huang Y, Qin M. Tumour-infiltrating FoxP3+ and IL-17-producing T cells affect the progression and prognosis of gallbladder carcinoma after surgery. Scandinavian journal of immunology. 2013;78:516–522. doi: 10.1111/sji.12109. [DOI] [PubMed] [Google Scholar]
- 34.He S, Fei M, Wu Y, Zheng D, Wan D, Wang L, Li D. Distribution and clinical significance of Th17 cells in the tumor microenvironment and peripheral blood of pancreatic cancer patients. International journal of molecular sciences. 2011;12:7424–7437. doi: 10.3390/ijms12117424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen WC, Lai YH, Chen HY, Guo HR, Su IJ, Chen HH. Interleukin-17-producing cell infiltration in the breast cancer tumour microenvironment is a poor prognostic factor. Histopathology. 2013;63:225–233. doi: 10.1111/his.12156. [DOI] [PubMed] [Google Scholar]
- 36.Punt S, Fleuren GJ, Kritikou E, Lubberts E, Trimbos JB, Jordanova ES, Gorter A. Angels and demons: Th17 cells represent a beneficial response, while neutrophil IL-17 is associated with poor prognosis in squamous cervical cancer. Oncoimmunology. 2015;4:e984539. doi: 10.4161/2162402X.2014.984539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu Q, Lou XM, He Y. Prediction of local recurrence in cervical cancer by a Cox model comprised of lymph node status, lymph-vascular space invasion, and intratumoral Th17 cell-infiltration. Medical oncology. 2014;31:795. doi: 10.1007/s12032-013-0795-1. [DOI] [PubMed] [Google Scholar]
- 38.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pages F, Galon J. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Weng W, Xu W, Wang Y, Yu W, Tang X, Ma L, Pan Q, Wang J, Sun F. Prognostic significance of interleukin 17 in cancer: a meta-analysis. International journal of clinical and experimental medicine. 2014;7:3258–3269. [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng Y, Zhang Q, Wang H, Lu M, Kong H, Zhang Y, Shi H. Prognostic significance of interleukin-17 in solid tumors: a meta-analysis. International journal of clinical and experimental medicine. 2015;8:10515–10536. [PMC free article] [PubMed] [Google Scholar]
- 41.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu L, Pan K, Zheng HX, Li JJ, Qiu HJ, Zhao JJ, Weng DS, Pan QZ, Wang DD, Jiang SS, Chang AE, Li Q, Xia JC. IL-17A promotes immune cell recruitment in human esophageal cancers and the infiltrating dendritic cells represent a positive prognostic marker for patient survival. Journal of immunotherapy. 2013;36:451–458. doi: 10.1097/CJI.0b013e3182a802cf. [DOI] [PubMed] [Google Scholar]
- 43.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European journal of epidemiology. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 44.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. British medical journal. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuritz SJ, Landis JR, Koch GG. A general overview of Mantel-Haenszel methods: applications and recent developments. Annual review of public health. 1988;9:123–160. doi: 10.1146/annurev.pu.09.050188.001011. [DOI] [PubMed] [Google Scholar]
- 46.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemporary clinical trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 47.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British medical journal. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.