Abstract
One prominent, long-standing view is that individuals with schizophrenia smoke cigarettes more than the general population to “self-medicate” cognitive deficits and other symptoms. This study tested the self-medication hypothesis by examining the effects of smoking abstinence and resumption on cognition in patients with schizophrenia. Nicotine-dependent smokers with schizophrenia (n=26) were trained on a cognitive battery and then hospitalized to achieve and maintain confirmed abstinence from smoking for ~1 week. Cognition was tested while smoking as usual (baseline), one day after smoking cessation (early abstinence), ~1 week later (extended abstinence), and within ~3 weeks of resuming smoking (resumption). The test battery included measures of processing speed, attention, conflict resolution, verbal memory, working memory, verbal fluency, and executive function to evaluate multiple cognitive domains affected by schizophrenia. Positive and negative symptoms of schizophrenia, depressive symptoms, and dyskinesia were also measured at baseline and after prolonged abstinence. There were no significant changes in global cognitive test performance with smoking cessation, abstinence, or resumption. There were small decreases in a measure of processing speed and delayed verbal recall with abstinence, but these findings failed to survive adjustments for multiple comparisons. Surprisingly, in this within subject “On- Off—Off-On” design, there were no significant effects of early or prolonged abstinence from smoking on cognitive and behavioral measures in smokers with schizophrenia. The results of this study challenge the widely held “self-medication” hypothesis of smoking and schizophrenia, question the extent of pro-cognitive effects of smoking and nicotine in schizophrenia, and support encouraging smoking cessation in schizophrenia.
Keywords: schizophrenia, cognition, smoking, nicotine, tobacco, withdrawal, abstinence
1. INTRODUCTION
The rates of tobacco use and nicotine addiction in schizophrenia are extremely high, with a recent study showing 70–80% prevalence (Hartz et al., 2014) and some studies showing near 90% prevalence (de Leon and Diaz, 2005; Hughes et al., 1986). Smokers with schizophrenia have reduced smoking cessation rates, extract more nicotine per cigarette and smoke higher numbers of stronger cigarettes, all indicators of greater nicotine dependence than other smokers (Dalack et al., 1998; de Leon and Diaz, 2005; Hughes et al., 1986). One widely-held theory about smoking behavior in schizophrenia is the “self-medication hypothesis” according to which patients smoke tobacco to attenuate positive, negative, depressive or cognitive symptoms or antipsychotic medication side effects (Kumari and Postma, 2005; Leonard et al., 2007; Wing et al., 2012). Nicotine or tobacco smoking, however, does not seem to improve positive psychotic symptoms (Smith et al., 2002), and psychotic symptoms do not worsen during nicotine withdrawal (Dalack et al., 1999; Yang et al., 2002). More research interest has been focused on the effects of nicotine on the very disabling negative symptoms and cognitive deficits of schizophrenia (Smucny et al., 2016; Yee et al., 2015), but the evidence has been mixed.
Some literature suggests that there are schizophrenia-specific beneficial effects of nicotine and nicotinic cholinergic agents on some cognitive deficits, electrophysiologic measures of early information processing, oculomotor dysfunction and affect (Dépatie et al., 2002; George et al., 2002; Olincy et al., 2000). Studies of nicotine administration and tobacco smoking on cognitive test performance in schizophrenia have yielded conflicting results with some reporting improvements in processing speed, working memory and executive function while others found no significant improvements (Boggs et al., 2013; Hahn et al., 2013; Harris et al., 2004; Smith et al., 2002; Wing et al., 2011). Some of this variability may be due to methodological differences or subject characteristics in these studies. Studies of acute, overnight, short-term (hours) withdrawal followed by resumption of smoking suggest that nicotine improves visuospatial working memory in schizophrenia (George et al., 2002; Sacco et al., 2005). Although several studies suggest that nicotine acutely improves attention in schizophrenia, whether it improves other cognitive domains, overall cognition, or confers long-term benefits is unclear (Boggs et al., 2014). Furthermore, though other nicotinic acetylcholine receptor (nAChR) ligands have been pursued for treating the cognitive deficits of schizophrenia, unfortunately standardized cognitive test batteries have failed to capture consistent improvements from these drugs (Boggs et al., 2014). If the self-medication hypothesis explained the increased smoking in schizophrenia, we would expect smokers with schizophrenia to have more cognitive gains from smoking and greater cognitive costs of smoking cessation than otherwise healthy smokers. In addition, the effects of smoking cessation and withdrawal on cognition do not seem specific to schizophrenia, which further questions the self-medication hypothesis of smoking. Instead, smoking cessation and withdrawal from nicotine has been reported to precipitate neurocognitive deficits, such as impaired working memory, in healthy smokers without psychiatric illness (Mendrek et al., 2006; Patterson et al., 2010), and, nicotine withdrawal or smoking resumption does not result in specific changes in attention in schizophrenia beyond the healthy smokers, as would be predicted by the self-medication hypothesis (AhnAllen et al., 2015; Hahn et al., 2013) However, the benefits of nicotine use for global cognition in schizophrenia during long-term abstinence, rather than acute withdrawal, is not well known. To our knowledge, there are no studies in smokers with schizophrenia assessing global cognitive test performance following a longer period of confirmed abstinence- a period that extends beyond the acute withdrawal stage. We studied the impact of confirmed early (1 day) and extended abstinence (~1 week) from smoking on cognitive test performance across several cognitive domains in smokers with schizophrenia. Furthermore, we investigated the effects of smoking resumption on cognitive test performance in the same subjects. Demonstrating that early and extended abstinence impairs, while resumption of smoking improves, global cognitive test performance might explain why smokers with schizophrenia find it harder to quit and why they are more likely to resume smoking. Given the widely held “self-medication” hypothesis of smoking and schizophrenia, we also studied the effects of smoking cessation on the core symptoms of schizophrenia, depression symptoms and dyskinesia. Finally, we studied the effects of smoking cessation on nicotine craving and nicotine withdrawal. We hypothesized early and extended smoking abstinence would be responsible for decreased global cognitive performance that would reverse during smoking resumption.
2. METHODS
2.1 Subjects
Data reported here were obtained from subjects who participated in a single- photon emission computerized tomography (SPECT) imaging study of β2*-nAChR availability in schizophrenia (D’Souza et al., 2012; Esterlis et al., 2014) that required confirmed inpatient abstinence from tobacco smoking for ~1 week (mean admission 5.8 days, SD 0.6, range 5–7 days, with reason for variability being coordinating participants’ scheduling with schedule of imaging procedures reported elsewhere). While some of the methods and the results of β2*nAChR availability are reported elsewhere (D’Souza et al., 2012; Esterlis et al., 2014), the principal findings reported here have not been published elsewhere. Eligibility criteria included: 1) age 18–70 years old; 2) schizophrenia diagnosis confirmed based on DSM-IV criteria, clinical records, clinician input, and use of the Structured Clinical Interview for DSM-IV (SCID) (First et al., 1997); 3) cigarette smokers who smoked at least 10 cigarettes a day, verified at baseline by a plasma cotinine level >150 ng/ml, urine cotinine level > 100 ng/ml (NicAlert™ by Nymox Pharmaceutical Corporation), and carbon monoxide (CO) level > 11 ppm (MicroDirect-Micro CO meter (Cat. No. MC02). Exclusion criteria included 1) any other current axis I diagnosis besides schizophrenia; 2) diagnosis of substance abuse in the past month or substance dependence in the previous 6 months (excluding nicotine and caffeine); 3) treatment with selective serotonin re-uptake inhibitors and/or tetra/tricyclic antidepressants; 4) psychiatric or medical instability. Lifetime history of and/or current substance use disorders were ascertained by psychiatric interview, chart review, SCID-I/P, 30-day Timeline Followback, and urine toxicology.
2.2 Study Procedures
2.2.1 Regulatory Approvals and Consent Process
Both the Yale University and VA Connecticut Healthcare System Institutional Review Boards approved this study. All subjects signed informed consent after the study was explained to them in detail as described elsewhere (D’Souza et al., 2012; Esterlis et al., 2014).
2.2.2 Screening
Methods for some aspects of this study have been reported previously (D’Souza et al., 2012; Esterlis et al., 2014). Subjects who met study criteria had screening assessments conducted including demographic data, clinical history, clinical ratings including the Positive and Negative Symptom Scale (PANSS) (Kay et al., 1987), Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1982), Montgomery-Asberg Depression Scale (MADRS) (Montgomery, 1979), Abnormal Involuntary Movement Scale (AIMS) (Guy, 1976b), cigarette usage, and degree of nicotine dependence measured by the Fagerström Test for Nicotine Dependence (FTND) (Heatherton et al., 1991).
2.2.3 Smoking cessation
Eligible smokers with schizophrenia were hospitalized on a smoke-free research unit to achieve and maintain abstinence from smoking for at least 5 days. Subjects were not allowed to receive any drugs that could facilitate smoking abstinence, including varenicline, bupropion, and nicotine replacement. Trained research staff counseled subjects daily to cope with withdrawal symptoms. Counseling was paired with contingency management: the latter has been shown to reduce cigarette smoking in smokers with schizophrenia in short-term studies (D’Souza et al., 2012; Roll et al., 1998; Tidey et al., 1999; Tidey et al., 2011). Subjects were paid $25 for the first day of inpatient abstinence, and payments were escalated by $25 for every inpatient day. Thus, if subjects were able to complete the entire inpatient abstinence period of at least 5 days, they were eligible to receive up to $375. Abstinence from smoking or any other nicotine products was confirmed by daily breath CO monitoring (cutoff < 8ppm) and urinary cotinine (cutoff < 50 ng/ml) [NicAlert, (Nymox)] (see Supplemental Table 1 for interpretation of assays). In addition, plasma cotinine was assayed while smoking as usual and after ~1 week of abstinence. Finally, subjects were aware that failure to abstain triggered discharge from the study.
2.2.4 Cognitive Test Battery
Multiple domains of cognitive function were tested using a battery of tests that have been shown to be sensitive to nicotine administration, withdrawal, and or abstinence in smokers with and without schizophrenia reviewed in (Boggs et al., 2014) and normal controls reviewed in (Heishman et al., 2010; Heishman et al., 1993). Furthermore, the cognitive domains tested, executive functioning, verbal working memory, processing speed, inhibition, and attention, have been shown to be consistently impaired in schizophrenia, and thus domains to monitor for sensitivity to nicotine or smoking effects to test the self-medication hypothesis of smoking in this disorder (Green et al., 2004; Westerhausen et al., 2011). To minimize practice effects and novelty of cognitive assessments, subjects were administered the cognitive battery twice on the same day ~2 weeks prior to smoking cessation (Goldberg et al., 2010; Lees et al., 2015). Executive control and cognitive inhibition were evaluated with the Stroop Color-Word Test (SCWT) (Trenerry et al., 1989). Short-term working memory was measured using the Letter Number Sequencing tasks (LNS) and Digit Span (DS) (Kaplan, 1995; Twamley et al., 2006). Processing speed was measured using the WAIS-III Digit Symbol Task (DST) and Symbol Search (SS) tests (Wechsler, 1997). Verbal fluency was measured using the Controlled Oral Word Association Test (FAS) (Benton et al., 1994). Trail Making Test (TMT) was used to measure visual tracking (Part A, TMT-A) and executive control (Part B, TMT-B) (Mitrushina et al., 2005). Immediate and delayed verbal memory were measured using the Hopkins Verbal Learning Task (HVLT) (Brandt, 1991), and alternative forms were used for each assessment on this test. Vigilance was measured using the Continuous Performance Test, Identical Pairs version (CPT-IP) (Cheung et al., 2004) (Tests are further described in the Supplemental Text and Supplement Table 2). The entire battery took under an hour to administer. The order of tests was consistent throughout and was: HVLT-immediate, DSC, SS, TMT-A, TMT-B, DS, LNS, FAS, SCWT, CPT-IP and finally HVLT-delayed.
2.2.5 Other Assessments
Positive, negative and general symptoms of psychosis were measured by the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1989) and the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1989). Depressive symptoms were assessed by the Montgomery-Asberg Depression Scale (MADRS) (Montgomery, 1979) and involuntary movements by the Abnormal Involuntary Movements Scale (AIMS) (Guy, 1976a).
2.2.6 Schedule of Assessments
The schedule of assessments is summarized in Supplementary Figure 1. Cognitive testing was conducted: 1) two weeks prior to smoking cessation twice to control for practice effects, 2) at baseline (smoking as usual state) within 1 hour of smoking, 3) one day after smoking cessation (early abstinence), 4) ~1 week of smoking abstinence (extended abstinence) and 4) 21 days +/− 3 days (~3 weeks) upon resumption of smoking within 1 hour of smoking (resumption). Cognitive testing was performed in the morning. This On-Off-Off-On design provided the opportunity to study the effects of smoking on cognitive test performance. PANSS, SANS, MADRS and AIMS testing occurred at baseline while smoking as usual and ~1 week of smoking abstinence (extended abstinence). Smoking breaks were not permitted during testing.
2.3 Statistical Analysis
Baseline raw scores on cognitive assessments were tested for normality using Shapiro-Wilk tests. Non-normally distributed data (for DS, LNS, DST, TMT-A, TMT-B, and CPT-IP number of false alarms and reaction time) were natural log- transformed to achieve a normal distribution of baseline scores. Z-scores were computed with these normally distributed baseline scores as reference, and used to calculate a global cognitive index (GCI) for each time point for each participant by averaging z scores of cognitive tests’ results (SI, DS, LNS, DST, SS, TMT-A, TMT-B, FAS, HVLT immediate). For tests where higher performance corresponds to lower numbers (SI, TMT-A, and TMT-B), z-scores signs were inverted before averaging. CPT was excluded in the GCI since 7 participants of 26 (~25%) were missing baseline data on this task for technical reasons; instead, CPT results were analyzed separately. GCI was calculated both including and excluding (HVLT-delayed) to examine the effect of using two measures from the same test (HVLT-immediate and -delayed).
GCI scores were compared over time using linear mixed models with time as a within-subjects factor. The best-fitting variance-covariance structure was selected based on information criteria. Post-hoc contrasts with paired t-tests were estimated to interpret significant main effects of time, comparing each time point to every other. Exploratory analyses included examining each cognitive measure with the same linear mixed models with time as a within-subjects factor. Behavioral assessments were compared with paired t-tests, and Bonferroni corrections were applied for multiple comparisons.
3. RESULTS
3.1 Study Participants
A total of 26 subjects (23 male) were enrolled in the study and hospitalized (Supplemental Figure 2), and their demographic data and baseline behavioral assessments are shown in Table 1. The subjects had at least moderate dependence for nicotine as assessed by the FTND. Two subjects were unable to comply with the smoking restrictions and dropped out during the inpatient phase (one subject on the 1st day and one on the 4th day). Of the remaining 24 subjects, two subjects reported remaining abstinent after discharge from the hospital and were therefore not asked to return for cognitive testing and another two subjects were lost to follow up. The remaining 20 subjects returned for repeat cognitive testing within ~3 weeks of resuming smoking (Supplementary Figure 2). Thus, 20 subjects had cognitive performance data for all 4 time points. Five subjects were not receiving antipsychotic treatment, 5 were receiving first generation antipsychotics, and 16 were receiving second-generation antipsychotics, including one receiving clozapine (Supplementary Table 3: Prescribed Antipsychotics).
Table 1.
Sample Characteristics (n=26)
| Variables | Mean | SD |
|---|---|---|
| Age | 41.7 | 11.9 |
| Years smoking | 24.6 | 12.8 |
| Cigarettes per day | 20.3 | 7.4 |
| Age of diagnosis | 23.9 | 7.5 |
| Years diagnosed | 17.3 | 10.6 |
| Fagerström Test for Nicotine Dependence | 5.5 | 2.1 |
| Minnesota Nicotine Withdrawal Questionnaire | 8.3 | 6.1 |
| Tiffany Questionnaire of Smoking Urges desire | 34.6 | 13.4 |
| Tiffany Questionnaire of Smoking Urges relief | 32.1 | 10.8 |
| Positive and Negative Syndrome Scale (PANSS) – positive | 19.1 | 4.1 |
| Positive and Negative Syndrome Scale (PANSS) – negative | 19.2 | 5.3 |
| Positive and Negative Syndrome Scale (PANSS) – total | 73.1 | 10.0 |
| Scale for the Assessment of Negative Symptoms (SANS) | 29.4 | 15.5 |
| Montgomery-Asberg Depression Rating Scale (MADRS) | 7.2 | 6.7 |
| Abnormal Involuntary Movement Scale (AIMS) | 1.7 | 2.6 |
3.2 Breath CO and urine cotinine levels
Breath CO and urine cotinine levels fell below levels consistent with smoking abstinence and remained at those levels during the inpatient hospitalization (Figure 1). Furthermore, plasma cotinine levels also confirmed abstinence from smoking. Measures of nicotine withdrawal and urges to smoke increased in early abstinence and decreased after day 2 through the week of abstinence as shown in Supplementary Figure 3.
Figure 1. Measures of Smoking Abstinence.
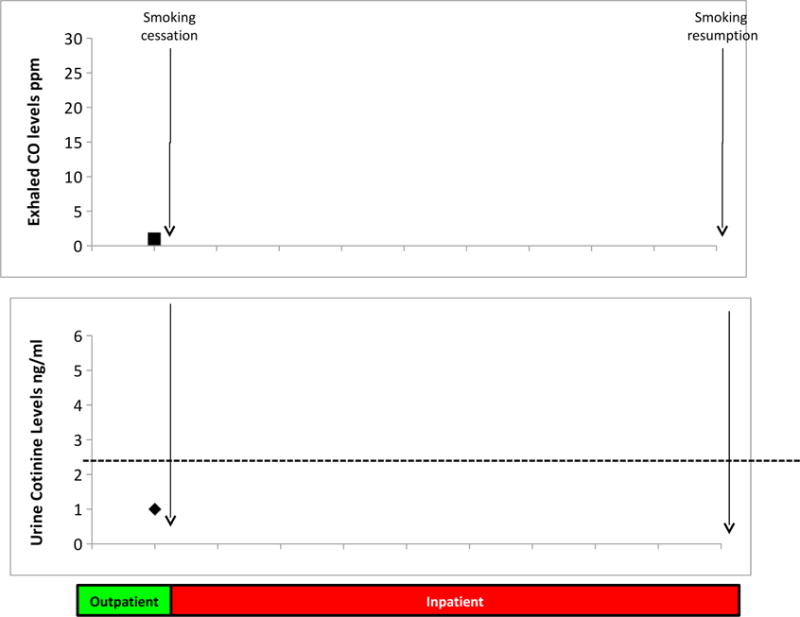
Expired carbon monoxide (CO) (top graph) and urine cotinine (bottom graph) were collected at baseline and daily during inpatient hospitalization to ensure that subjects continued to abstain from smoking cigarettes during the entire study period.
3.3 Cognitive Test Performance
Results of cognitive testing at each time point and the statistical test results are presented in Table 2. There was no main effect of time over the period including the assessments during smoking as usual, early abstinence, prolonged abstinence, and resumption of smoking on overall cognition as measured by the GCI with only immediate HVLT included (F(3,67)=1.02, p=0.39) or with both immediate and delayed HVLT included (F(3,67)=2.39, p=0.08). Despite the lack of any statistically significant effects of time, post-hoc comparisons were done to explore whether there were any possible significant differences between any two time points for the GCI; there were no significant differences between any time points except for the GCI with both HVLT measures between baseline smoking and prolonged abstinence (t=−2.3, p=0.03).
TABLE 2.
COGNITIVE TEST PERFORMANCE OVER TIME
| COGNITIVE TASKS | Baseline: Smoking as Usual (Day #1)a |
Immediate Abstinence (12–24 hours after quitting)b |
Extended Smoking Abstinence (~day 7)c |
Resumption of Smoking (~21 days)d |
Effect over time | Post hoc comparisons |
|---|---|---|---|---|---|---|
| Global cognitive index | 0 (0.641) | −0.06 (0.585) | −0.05 (0.682) | 0.04 (0.676) | F(3,67)= 1.02, p = 0.390 |
|
| SCWT interference score* | 3.68 (8.49) | 3.43 (6.07) | 3.11 (7.58) | 5.71 (7.38) | F(3,58)= 0.52, p = 0.667 |
|
| WAIS-iii DS score | 8.58 (3.11) | 8.81 (3.21) | 8.63 (3.39) | 8.50 (3.02) | F(3,67)= 0.58, p = 0.630 |
|
| WAIS-iii LNS | 8.35 (3.58) | 8.15 (3.45) | 8.00 (3.55) | 8.50 (3.36) | F(3,67)= 0.44, p = 0.723 |
|
| WAIS-iii DST | 6.92 (2.67) | 6.73 (2.65) | 6.68 (3.00) | 7.05 (2.70) | F(3,65)= 1.03, p = 0.387 |
|
| WAIS-iii SS | 7.31 (3.18) | 6.65 (2.58) | 6.86 (2.82) | 7.90 (3.37) | F(3,65)= 3.53, p = 0.019 |
d > b (p = 0.004) d > c (p = 0.013) |
| COWAT (FAS) | 35.65 (12.26) | 33.27 (12.45) | 34.08 (11.49) | 36.25 (11.28) | F(3,67) = 1.32, p = 0.277 |
|
| Trails a (sec) | 35.65 (18.89) | 34.12 (14.57) | 32.05 (18.52) | 31.75 (12.30) | F(3,64)= 0.83, p = 0.480 |
|
| Trails b (sec) | 114.77 (83.05) | 103.00 (59.92) | 102.30 (59.62) | 98.79 (84.91) | F(3,25)= 0.90, p = 0.454 |
|
| HVLT total immediate | 21.42 (5.78) | 19.31 (5.81) | 19.79 (4.86) | 20.15 (6.27) | F(3,67)= 1.67, p = 0.182 |
|
| HVLT total delayed | 5.42 (2.83) | 3.85 (3.09) | 4.13 (2.97) | 5.35 (2.89) | F(3,67)=4.4 p = 0.007 |
a > b (p =0.005) a > c (p =0.025) d > b (p =0.008) d > c (p =0.031) |
| CPT (IP) missed | 13.74 (8.05) | 16.37 (7.53) | 18.89 (9.00) | 16.56 (9.62) | F(3,49)= 2.26, p = 0.093 |
|
| CPT (IP) false alarms | 14.63 (15.22) | 14.63 (14.47) | 17 (19.10) | 18.94 (19.77) | F(3,49)= 0.54, p = 0.658 |
|
| CPT (IP) reaction time (msec) | 828 (157) | 850 (156) | 817 (138) | 807 (118) | F(3,48)= 0.36 p = 0.784 |
CPT = Continuous Performance Task, HVLT = Hopkins Verbal Learning Task, SCWT= Stroop Color-Word Test, WAIS-iii = the Wechsler Adult Intelligence Scale-iii, WAIS-iii DS = WAIS-iii Digit Span, WAIS-iii LNS = WAIS Letter Number Sequencing, WAIS-iii DST = WAIS-iii Digit Symbol Task, WAIS-iii SS = WAIS-iii Symbol Search, COWAT = Controlled Oral Word Association Test, HVLT = Hopkins Verbal Learning Test, CPT (IP) = Continuous Performance Test (Identical Pairs)
Three participants were colorblind, so they did not ever participante in the SCWT, and their CGIs at all 4 time points did not include SCWT.
All cognitive tests individually were subject to the same modeling for exploratory purposes. There was a significant effect of time on one processing speed task, the SS task (F(3,65) = 3.53, p = 0.019), as well as on HVLT-delayed recall (F(3,67) = 4.4, p = 0.007). Post-hoc testing between time points revealed that test performance was worse during early and prolonged smoking abstinence relative to both smoking as usual at baseline and after resumption of smoking. There was no main effect of time for the CPT-IP on any CPT-IP measure, though the number of misses showed a trend for being negatively affected by smoking abstinence F(3,49)=2.26, p=0.09. However, when Bonferroni corrections for multiple comparisons (13) were applied (p < 0.004), there are no main effects of time on any tests, including the SS task and HVLT-delayed recall.
3.4 Psychosis Symptoms and Antipsychotic Side Effects
As reported elsewhere (Esterlis et al., 2014) there were no significant changes between the baseline smoking-as-usual state and prolonged smoking abstinence in the PANSS total, positive symptom subscale, or negative symptom subscale scores; SANS total scores; MADRS total scores; and AIMS total scores (Table 3). The small decrease in PANSS negative symptoms from baseline to prolonged nicotine abstinence of 0.8 points out of a total of 49 did not survive adjustment for multiple comparisons.
Table 3.
Change in Behavioral and Motor Assessments from Smoking as Usual to Prolonged Smoking Abstinence
| Variable | N | Mean | Std Dev | Std Error | DF | t Value | Pr > |t| |
|---|---|---|---|---|---|---|---|
| PANSS Total Score | 24 | 1.333 | 6.041 | 1.233 | 23 | 1.08 | 0.2908 |
| PANSS Positive Symptoms score | 24 | 0.5 | 2.621 | 0.535 | 23 | 0.93 | 0.3597 |
| PANSS Negative Symptoms score | 24 | −0.792 | 1.865 | 0.381 | 23 | −2.08 | 0.0488 |
| SANS total score | 24 | 1.583 | 7.689 | 1.57 | 23 | 1.01 | 0.3236 |
| MADRS total score | 24 | −0.417 | 3.866 | 0.789 | 23 | −0.53 | 0.6026 |
| AIMS total score | 21 | 0.286 | 1.419 | 0.31 | 20 | 0.92 | 0.3672 |
PANSS = Positive and Negative Syndrome Scale; SANS = Schedule for the Assessment of Negative Symptoms; MADRS = Montgomery Asberg Depression Rating Scale; AIMs = Abnormal Involuntary Movement Scale
4. DISCUSSION
This is the first study to our knowledge to study the effects of smoking in the On – Off – Off- On design in smokers with schizophrenia. Contrary to our hypothesis, neither early nor prolonged abstinence from smoking, nor the resumption of smoking, had a significant effect on cognitive test performance in 1 pack per day heavy smokers with schizophrenia. Similarly, there were no effects on the core symptoms of schizophrenia, depression or dyskinesia. The results of this study are not consistent with the widely held view that smokers with schizophrenia smoke tobacco to “self-medicate” cognitive deficits or other symptoms associated with schizophrenia.
4.1 Cognitive Test Performance
Contrary to the study hypothesis, across a broad range of cognitive domains tested, performance did not decline significantly either 1-day or ~1 week after smoking cessation, suggesting that smoking abstinence in tobacco dependent smokers with schizophrenia, (based on self-report, FTND scores, and urine cotinine values) does not substantially worsen cognitive deficits. While performance on the 3 of the 13 measures including the SS, HVLT-delayed recall, and CPT-IP missed trials, showed worsening with abstinence and improvement with resumption of smoking, these effects were not significant (CPT-IP missed trials) or did not survive adjustment for multiple comparisons (SS and HVLT-delayed). We believe that the weak effects of smoking status we have found are non-specific and unlikely to be clinically significant. Other verbal working memory tests (LNS, DS, and most notably HVLT-immediate recall) and speed of processing tests (DSC, TMT-A) did not show this pattern of worsening performance with smoking abstinence, further suggesting that smoking status in schizophrenia may not be affecting the performance in these cognitive domains in a clinically significant manner. It is possible that delayed memory, with delay of 5-up to 60 minutes, is more sensitive to the effects of nicotine than immediate memory in some individuals with schizophrenia. Similar to our findings, Myers et al found that visuospatial delayed recognition (5–7 minutes) but not immediate recall was improved in smokers with schizophrenia by nicotine administration but this may also be an effect of reversing non-specific acute withdrawal effects, as performance was worse without nicotine in smokers with schizophrenia versus non-smokers with schizophrenia but improved only in smokers when nicotine was administered. Attention is often thought to be the domain most improved with nicotine administration and thus, the lack of any statistically significant effect of smoking abstinence or resumption on the CPT was surprising especially since some studies (Barr et al., 2007; Dépatie et al., 2002; Smith et al., 2005) but not others (Boggs et al., 2013; Sherr et al., 2002) showed that in smokers with schizophrenia CPT scores improve with nicotine administration. Studies of decreased nicotine intake in schizophrenia on CPT, however, have been mixed: Sacco et al found no effects of smoking abstinence on CPT (Sacco et al., 2005) whereas AhnAllen et al found patients smoking cigarettes with drastically decreased nicotine content worsened CPT (AhnAllen et al., 2015). Collectively, the lack of any statistically significant worsening of performance on a battery of commonly used and well-validated neuropsychological tests suggests that smoking cessation and nicotine withdrawal may have limited effects on cognition in schizophrenia.
Below herewith, several potential explanations for the discrepancy between the study findings and expectations based on the “self-medication” hypothesis are explored. First, was the level of nicotine dependence or smoking severity in this sample insufficient to have allowed for the manifestation of abstinence-related impaired cognitive test performance? The subjects were moderate to heavy smokers whose smoking status (20.3 ± 7.4 cigarettes/day and duration of smoking 24.6 ± 12.8 years) and degree of nicotine dependence (FTND scores of 5.5 ± 2.1) were confirmed by chart review, self-report and laboratory testing. Additionally, these levels of smoking in schizophrenia are also consistent with what have been found in other studies (Wing et al., 2011). Second, could subjects have failed to abstain? Abstinence from smoking was confirmed by both CO monitoring and urine cotinine assays – subjects were housed on a locked, smoke-free inpatient research unit and were paid to attain and maintain abstinence. Furthermore, subjects were not permitted any nicotine supplements or pharmacological treatments to manage smoking abstinence. Third, could floor effects in cognitive test performance explain the lack of abstinence-induced impairments? This appears unlikely given that performance was similar to cognitive test performance reported in other studies (Schretlen et al, 2007). For example, HVLT total immediate recall scores of 19–21 in this study are similar to those seen in other schizophrenia studies (Schretlen et al., 2007) – with enough room for both declines and improvements in task performance. Fourth, could practice effects on cognitive test performance have obscured abstinence-induced worsening? To minimize practice effects during the periods of abstinence and resumption of smoking, all subjects were trained on the cognitive test battery on two separate occasions prior to the baseline visit. Fifth, could the battery have been not sensitive enough to the effects of smoking abstinence? Several of the tests e.g., STROOP, CPT, and other assessments of processing speed have been shown to be sensitive to nicotine effects (Barr et al., 2007; Dépatie et al., 2002; Smith et al., 2005). Moreover, some of the tests in the battery used e.g., HVLT, are also part of the widely used MATRICS battery developed for evaluating cognitive treatments for schizophrenia (Green et al., 2004). Perhaps more proximal measures of brain function e.g., P50 gating, might have detected abstinence-related effects that could not be detected with the cognitive test battery (Boggs et al., 2014), but if the commonly used measures of cognitive test performance used in this study failed to detect the effects of abstinence, the clinical relevance of abstinence effects is questionable. Finally, while possible, could the lack of effects be explained by selection bias? It is conceivable that the patients who participated in this study were “aware” of the lack of significant cognitive impairments with abstinence and thus chose to participate. This seems unlikely since the overwhelming majority resumed smoking almost immediately after the required abstinence period.
4.2 Clinical Symptom Measures
Similar to cognition, neither 1-day nor ~1 week after smoking cessation, was associated with any worsening in the core symptoms of schizophrenia, depression or dyskinesia. Finally, as discussed earlier, it is unlikely that the level of nicotine dependence or smoking severity in this sample prevented the manifestation of abstinence-related impaired cognitive test performance.
4.3: Implications for the “Self-Medication” Hypothesis
The findings of this study are not consistent with the widely held view that schizophrenia patients smoke to “self-medicate” core symptoms. The absence of any significant abstinence-induced worsening of schizophrenia symptoms, depression symptoms, and movement disorders is consistent with the work of Dalack et al (Dalack et al., 1999). The findings are also consistent with those of Hahn et al, who reported that the subjective or objective attentional benefits are unlikely to be the primary driving force of tobacco consumption in schizophrenia (Hahn et al., 2013.) Our results also parallel the minimal effects of nicotine on cognition in a rodent neurodevelopmental model of schizophrenia (Berg et al., 2015). As reported elsewhere (D’Souza et al., 2012; Esterlis et al., 2014), the same subjects whose cognitive data are reported in this paper showed lower β2*-nAChR availability relative to smokers without schizophrenia, in striking parallel to the behavioral and imaging studies done by Berg et al., who found that smoking upregulated β2*-nAChR in both control and illness-model rodents. In both human schizophrenia and the rodent model, chronic nicotine use does not completely normalize β2*-nAChR receptor availability, nor does it substantially affect cognition differently in control and disease condition (Berg et al., 2015; Hahn et al., 2013).
One of the inadvertent negative outcomes of perpetuating the “self-medication” hypothesis is justifying continued smoking and/or discouraging quitting despite the well-characterized harmful effects of tobacco use (Chambers, 2009). Moreover, it could limit efforts to find other explanations for the high rates of tobacco smoking in schizophrenia. The “self-medication” hypothesis has also been applied to other substance misuse (e.g., cannabis and alcohol) in schizophrenia. The results of this study are also consistent with other controlled experimental studies with alcohol (D’Souza et al., 2006), THC (D’Souza et al., 2005), nicotine (Boggs et al., 2013) that failed to demonstrate clear or robust “benefits” on either positive, negative or cognitive symptoms in schizophrenia; in fact, symptom worsening was observed with alcohol and THC. Thus, existing experimental evidence does not support the “self- medication” hypothesis.
Alternatively, alterations in the neural circuits of reward in schizophrenia could predispose people with schizophrenia to a higher risk of substance abuse including tobacco smoking (Chambers et al., 2001; Krystal et al., 2006); substance abuse and schizophrenia may share common neurobiology. Evidence to support this theory is that a rodent neurodevelopmental model of schizophrenia shows increased nicotine intake and seeking without cognitive benefit from nicotine (Berg et al., 2014), and that smokers with schizophrenia choose smoking over other rewards more than control smokers do (Spring et al., 2003). Perhaps long-term smoking status enhances baseline cognitive function in individuals with schizophrenia (Wing et al., 2011). However, a cohort of non-smoking individuals with schizophrenia had statistically similar baseline functioning on the same cognitive battery (Esterlis et al., 2014).
A third possible explanation for the association between smoking and schizophrenia is that smoking itself could increase the risk of developing schizophrenia. In a large Swedish cohort, smoking in early adulthood predicted later diagnosis of schizophrenia. Furthermore, in the same study, monozygotic twins non-concordant for smoking status showed higher rates of non-affective psychosis in the smoking twins, suggesting smoking increases the risk for psychosis rather than a shared genetic link between smoking and psychosis (Kendler et al., 2015)
4.4 Strengths and Weaknesses
To our knowledge this is the only controlled study examining the effects of extended smoking abstinence (~ 1 week) on cognition in smokers with schizophrenia. A major strength of the study is the On-Off-Off-On design that provides is an ideal experimental design to study the effects of smoking abstinence and its reversal with smoking resumption. Another strength is that this was a within subject design, where each subjects served as his/her control. That subjects were hospitalized on a smoke-free inpatient unit and had nicotine abstinence confirmed using overlapping procedures and measures is another strength of the study. Given that practice effects (Goldberg et al., 2010) are known to occur with repeated administration of cognitive test batteries, training subjects during the screening period in this study minimized practice effects. The inclusion of a control group of smokers without schizophrenia who did not abstain from smoking but had the same schedule of cognitive assessments would have been a stronger control for practice effects on the cognitive battery, which could have been attenuated by abstinence. The assessment of outcomes relevant to schizophrenia including cognitive measures in multiple domains and symptoms (e.g., core symptoms (PANSS), depression (MADRS), and movement disorders) provide a more complete evaluation of possible effects of abstinence. Though the measures used were broad, other measures, such as measures of neuroplasticity or social functioning, might be affected in clinically meaningful ways by smoking abstinence, and this should be studied in the future. There was also not a dedicated measure of visuospatial working memory in this battery, though many measures depended upon this domain (CPT, SS, DSC). Additional measures of visuospatial memory would have been instructive as the literature about nicotine use on visuospatial working memory in smokers with schizophrenia is mixed (AhnAllen et al., 2015; Sacco et al., 2005). The sample size of this within- subject study was larger than or comparable to other studies of nicotine effects and/or abstinence in schizophrenia.
4.5 Conclusions and Future Directions
In this within subjects, On-Off-Off-On designed study, early and prolonged abstinence from smoking did not significantly impair cognitive test performance on a broad range of tests in 1 pack per day smokers with schizophrenia. Smoking abstinence also did not worsen core symptoms of schizophrenia, depression or movement disorders.
The study results and the growing literature of small of no effects of smoking withdrawal or prolonged abstinence on core features of schizophrenia (reviewed in Boggs et al 2014) challenge the widely held self-medication hypothesis of smoking in schizophrenia (Kumari and Postma, 2005; Leonard et al., 2007; Wing et al., 2012). Alternative hypotheses are worth pursuing, not only for understanding why people with schizophrenia smoke, but also for developing effective treatments for schizophrenia, improved strategies for smoking cessation for patients, and better ways to prevent psychosis. Most importantly, our results in the context of prior work suggest that the risks of stopping smoking in schizophrenia are small compared to the known medical risks of continued smoking (Health and Services, 2004) and provide strong support for clinicians to encourage smoking cessation in schizophrenia.
Supplementary Material
Acknowledgments
The authors wish to acknowledge support from the Department of Veterans Affairs. The authors also thank staff of the Clinical Neuroscience Research Unit of the Connecticut Mental Health Center in caring for patients while they were hospitalized to achieve abstinence from smoking, and to staff of the Schizophrenia Neuropharmacology Research Group at Yale (Maegan Krasenics, Lara Shearer and Michelle Carbuto) for their help in collecting the data.
FUNDING AND DISCLOSURE
This research was funded by R01 DA-022495 (D.C.D.) and NARSAD (I.E.). Salary support was provided by K01MH092681 (I.E.), K02DA031750 (K.C.), and VA VISN1 Career Development Award (T.S.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST: none of the authors report conflict of interest for this study.
References
- AhnAllen CG, Bidwell LC, Tidey JW. Cognitive effects of very low nicotine content cigarettes, with and without nicotine replacement, in smokers with schizophrenia and controls. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco. 2015;17(5):510–514. doi: 10.1093/ntr/ntu163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC. Negative symptoms in schizophrenia. Definition and reliability. Archives of general psychiatry. 1982;39(7):784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry. 1989;(Suppl 7):49–58. [PubMed] [Google Scholar]
- Barr RS, Culhane MA, Jubelt LE, Mufti RS, Dyer MA, Weiss AP, Deckersbach T, Kelly JF, Freudenreich O, Goff DC. The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;33(3):480–490. doi: 10.1038/sj.npp.1301423. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher KD, Sivan AB. Multilingual aphasia examination: manual of instructions. AJA Assoc 1994 [Google Scholar]
- Berg SA, Sentir AM, Bell RL, Engleman EA, Chambers RA. Nicotine effects in adolescence and adulthood on cognition and alpha(4)beta(2)-nicotinic receptors in the neonatal ventral hippocampal lesion rat model of schizophrenia. Psychopharmacology. 2015;232(10):1681–1692. doi: 10.1007/s00213-014-3800-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg SA, Sentir AM, Cooley BS, Engleman EA, Chambers RA. Nicotine is more addictive, not more cognitively therapeutic in a neurodevelopmental model of schizophrenia produced by neonatal ventral hippocampal lesions. Addiction biology. 2014;19(6):1020–1031. doi: 10.1111/adb.12082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs DL, Carlson J, Cortes-Briones J, Krystal JH, Cyril D’Souza D. Going up in Smoke? A Review of nAChRs-based Treatment Strategies for Improving Cognition in Schizophrenia. Current pharmaceutical design. 2014;20(31):5077–5092. doi: 10.2174/1381612819666131216121019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs DL, Ranganathan M, Sewell RA, Madonick S, D’Souza DC. Pilot study of intravenous nicotine effects on cognitive performance in schizophrenia. Schizophrenia research. 2013;150(1):323–324. doi: 10.1016/j.schres.2013.07.045. [DOI] [PubMed] [Google Scholar]
- Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. The Clinical neuropsychologist. 1991;5(2):125–142. [Google Scholar]
- Chambers RA. A Nicotine Challenge to the Self-Medication Hypothesis in a Neurodevelopmental Animal Model of Schizophrenia. Journal of dual diagnosis. 2009;5(2):139–148. doi: 10.1080/15504260902869808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia. Biological psychiatry. 2001;50(2):71–83. doi: 10.1016/s0006-3223(01)01134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung AM, Mitsis EM, Halperin JM. The relationship of behavioral inhibition to executive functions in young adults. Journal of clinical and experimental neuropsychology. 2004;26(3):393–404. doi: 10.1080/13803390490510103. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Abi-Saab WM, Madonick S, Forselius-Bielen K, Doersch A, Braley G, Gueorguieva R, Cooper TB, Krystal JH. Delta-9-tetrahydrocannabinol effects in schizophrenia: implications for cognition, psychosis, and addiction. Biological psychiatry. 2005;57(6):594–608. doi: 10.1016/j.biopsych.2004.12.006. [DOI] [PubMed] [Google Scholar]
- D’Souza DC, Esterlis I, Carbuto M, Krasenics M, Seibyl J, Bois F, Pittman B, Ranganathan M, Cosgrove K, Staley J. Lower Beta2*-nicotinic acetylcholine receptor availability in smokers with schizophrenia. The American journal of psychiatry. 2012;169(3):326–334. doi: 10.1176/appi.ajp.2011.11020189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza DC, Gil RB, Madonick S, Perry EB, Forselius-Bielen K, Braley G, Donahue L, Tellioglu T, Zimolo Z, Gueorguieva R, Krystal JH. Enhanced sensitivity to the euphoric effects of alcohol in schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31(12):2767–2775. doi: 10.1038/sj.npp.1301207. [DOI] [PubMed] [Google Scholar]
- Dalack GW, Becks L, Hill E, Pomerleau OF, Meador-Woodruff JH. Nicotine withdrawal and psychiatric symptoms in cigarette smokers with schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1999;21(2):195–202. doi: 10.1016/S0893-133X(98)00121-3. [DOI] [PubMed] [Google Scholar]
- Dalack GW, Healy DJ, Meador-Woodruff JH. Nicotine dependence in schizophrenia: clinical phenomena and laboratory findings. The American journal of psychiatry. 1998;155(11):1490–1501. doi: 10.1176/ajp.155.11.1490. [DOI] [PubMed] [Google Scholar]
- de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophrenia research. 2005;76(2–3):135–157. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Dépatie L, O’Driscoll GA, Holahan ALV, Atkinson V, Thavundayil JX, Kin N, Lal S. Nicotine and behavioral markers of risk for schizophrenia: a double-blind, placebo-controlled, cross-over study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;27(6):1056–1070. doi: 10.1016/S0893-133X(02)00372-X. [DOI] [PubMed] [Google Scholar]
- Esterlis I, Ranganathan M, Bois F, Pittman B, Picciotto MR, Shearer L, Anticevic A, Carlson J, Niciu MJ, Cosgrove KP, D’Souza DC. In vivo evidence for beta2 nicotinic acetylcholine receptor subunit upregulation in smokers as compared with nonsmokers with schizophrenia. Biological psychiatry. 2014;76(6):495–502. doi: 10.1016/j.biopsych.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured clinical interview for DSM-IV axis I disorders, clinician version (SCID-CV) Washington, DC: American Psychiatric Association; 1997. [Google Scholar]
- George TP, Vessicchio JC, Termine A, Sahady DM, Head CA, Pepper WT, Kosten TR, Wexler BE. Effects of smoking abstinence on visuospatial working memory function in schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;26(1):75–85. doi: 10.1016/S0893-133X(01)00296-2. [DOI] [PubMed] [Google Scholar]
- Goldberg TE, Keefe RS, Goldman RS, Robinson DG, Harvey PD. Circumstances under which practice does not make perfect: a review of the practice effect literature in schizophrenia and its relevance to clinical treatment studies. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(5):1053–1062. doi: 10.1038/npp.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, Fenton WS, Frese F, Goldberg TE, Heaton RK, Keefe RS, Kern RS, Kraemer H, Stover E, Weinberger DR, Zalcman S, Marder SR. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biological psychiatry. 2004;56(5):301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Guy W. In: ECDEU Assessment Manual for Psychopharmacology. Dept. of Health, E.a.W., editor. Washington DC: 1976a. [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76–338. US Department of Health, Education, and Welfare; Washington, D.C.: 1976b. [Google Scholar]
- Hahn B, Harvey AN, Concheiro-Guisan M, Huestis MA, Holcomb HH, Gold JM. A test of the cognitive self-medication hypothesis of tobacco smoking in schizophrenia. Biological psychiatry. 2013;74(6):436–443. doi: 10.1016/j.biopsych.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JG, Kongs S, Allensworth D, Martin L, Tregellas J, Sullivan B, Zerbe G, Freedman R. Effects of nicotine on cognitive deficits in schizophrenia. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2004;29(7):1378. doi: 10.1038/sj.npp.1300450. [DOI] [PubMed] [Google Scholar]
- Hartz SM, Pato CN, Medeiros H, Cavazos-Rehg P, Sobell JL, Knowles JA, Bierut LJ, Pato MT, Genomic Psychiatry Cohort C Comorbidity of severe psychotic disorders with measures of substance use. JAMA psychiatry. 2014;71(3):248–254. doi: 10.1001/jamapsychiatry.2013.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health, U.D.o., Services, H. The health consequences of smoking: a report of the Surgeon General. Atlanta, GA: 2004. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British journal of addiction. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology. 2010;210(4):453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Snyder FR, Henningfield JE. Performance, subjective, and physiological effects of nicotine in non-smokers. Drug and alcohol dependence. 1993;34(1):11–18. doi: 10.1016/0376-8716(93)90041-n. [DOI] [PubMed] [Google Scholar]
- Hughes J, Hatsukami D, Mitchell J, Dahlgren L. Prevalence of smoking among psychiatric outpatients. The American journal of psychiatry. 1986;143:993–997. doi: 10.1176/ajp.143.8.993. [DOI] [PubMed] [Google Scholar]
- Kaplan E. WAIS-R NI: WAIS-R as a Neuropsychological Instrument. Psychological Corporation; 1995. [Google Scholar]
- Kay SR, Flszbein A, Opfer LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia bulletin. 1987;13(2):261. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Lindenmayer JP. The Positive and Negative Syndrome Scale (PANSS): rationale and standardisation. British Journal of Psychiatry – Supplementum. 1989;(7):59–67. [PubMed] [Google Scholar]
- Kendler KS, Lonn SL, Sundquist J, Sundquist K. Smoking and schizophrenia in population cohorts of Swedish women and men: a prospective co-relative control study. The American journal of psychiatry. 2015;172(11):1092–1100. doi: 10.1176/appi.ajp.2015.15010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, D’Souza DC, Gallinat J, Driesen N, Abi-Dargham A, Petrakis I, Heinz A, Pearlson G. The vulnerability to alcohol and substance abuse in individuals diagnosed with schizophrenia. Neurotoxicity research. 2006;10(3–4):235–252. doi: 10.1007/BF03033360. [DOI] [PubMed] [Google Scholar]
- Kumari V, Postma P. Nicotine use in schizophrenia: the self medication hypotheses. Neuroscience & Biobehavioral Reviews. 2005;29(6):1021–1034. doi: 10.1016/j.neubiorev.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Lees J, Applegate E, Emsley R, Lewis S, Michalopoulou P, Collier T, Lopez-Lopez C, Kapur S, Pandina GJ, Drake RJ. Calibration and cross-validation of MCCB and CogState in schizophrenia. Psychopharmacology. 2015;232(21–22):3873–3882. doi: 10.1007/s00213-015-3960-8. [DOI] [PubMed] [Google Scholar]
- Leonard S, Mexal S, Freedman R. Smoking, Genetics and Schizophrenia: Evidence for Self Medication. Journal of dual diagnosis. 2007;3(3–4):43–59. doi: 10.1300/J374v03n03_05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrek A, Monterosso J, Simon SL, Jarvik M, Brody A, Olmstead R, Domier CP, Cohen MS, Ernst M, London ED. Working memory in cigarette smokers: comparison to non-smokers and effects of abstinence. Addictive behaviors. 2006;31(5):833–844. doi: 10.1016/j.addbeh.2005.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrushina M, Boone K, Razani J, D’Elia L. Handbook of normative data for neuropsychological assessment. 2nd. Oxford University Press; 2005. [Google Scholar]
- Montgomery SM. Depressive symptoms in acute schizophrenia. Prog Neuropsychopharmacol. 1979;3(4):429–433. doi: 10.1016/0364-7722(79)90058-4. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, 3rd, Gold JM, Goldberg T, Heaton RK, Keefe RS, Kraemer H, Mesholam-Gately R, Seidman LJ, Stover E, Weinberger DR, Young AS, Zalcman S, Marder SR. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. The American journal of psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- Olincy A, Ross RG, Harris JG, Young DA, McAndrews MA, Cawthra E, McRae KA, Sullivan B, Adler LE, Freedman R. The P50 auditory event-evoked potential in adult attention-deficit disorder: comparison with schizophrenia. Biological psychiatry. 2000;47(11):969–977. doi: 10.1016/s0006-3223(00)00239-0. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Loughead J, Perkins K, Strasser AA, Siegel S, Frey J, Gur R, Lerman C. Working memory deficits predict short-term smoking resumption following brief abstinence. Drug and alcohol dependence. 2010;106(1):61–64. doi: 10.1016/j.drugalcdep.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Steingard S, McGinley M. Use of monetary reinforcement to reduce the cigarette smoking of persons with schizophrenia: a feasibility study. Exp Clin Psychopharmacol. 1998;6(2):157–161. doi: 10.1037//1064-1297.6.2.157. [DOI] [PubMed] [Google Scholar]
- Sacco KA, Termine A, Seyal A, Dudas MM, Vessicchio JC, Krishnan-Sarin S, Jatlow PI, Wexler BE, George TP. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Archives of general psychiatry. 2005;62(6):649–659. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- Schretlen DJ, Cascella NG, Meyer SM, Kingery LR, Testa SM, Munro CA, Pulver AE, Rivkin P, Rao VA, Diaz-Asper CM, Dickerson FB, Yolken RH, Pearlson GD. Neuropsychological functioning in bipolar disorder and schizophrenia. Biological psychiatry. 2007;62(2):179–186. doi: 10.1016/j.biopsych.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr JD, Myers C, Avila MT, Elliott A, Blaxton TA, Thaker GK. The effects of nicotine on specific eye tracking measures in schizophrenia. Biological psychiatry. 2002;52(7):721–728. doi: 10.1016/s0006-3223(02)01342-2. [DOI] [PubMed] [Google Scholar]
- Smith RC, Amiaz R, Si TM, Maayan L, Jin H, Boules S, Sershen H, Li C, Ren J, Liu Y, Youseff M, Lajtha A, Guidotti A, Weiser M, Davis JM. Varenicline Effects on Smoking, Cognition, and Psychiatric Symptoms in Schizophrenia: A Double-Blind Randomized Trial. PloS one. 2016;11(1):e0143490. doi: 10.1371/journal.pone.0143490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RC, Singh A, Infante M, Khandat A, Kloos A. Effects of cigarette smoking and nicotine nasal spray on psychiatric symptoms and cognition in schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;27(3):479–497. doi: 10.1016/S0893-133X(02)00324-X. [DOI] [PubMed] [Google Scholar]
- Smith RC, Warner-Cohen J, Matute M, Butler E, Kelly E, Vaidhyanathaswamy S, Khan A. Effects of nicotine nasal spray on cognitive function in schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2005;31(3):637–643. doi: 10.1038/sj.npp.1300881. [DOI] [PubMed] [Google Scholar]
- Smucny J, Olincy A, Rojas DC, Tregellas JR. Neuronal effects of nicotine during auditory selective attention in schizophrenia. Human brain mapping. 2016;37(1):410–421. doi: 10.1002/hbm.23040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring B, Pingitore R, McChargue DE. Reward value of cigarette smoking for comparably heavy smoking schizophrenic, depressed, and nonpatient smokers. The American journal of psychiatry. 2003;160(2):316–322. doi: 10.1176/appi.ajp.160.2.316. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Higgins ST, Bickel WK, Steingard S. Effects of response requirement and the availability of an alternative reinforcer on cigarette smoking by schizophrenics. Psychopharmacology. 1999;145(1):52–60. doi: 10.1007/s002130051031. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Reid N. Effects of contingency management and bupropion on cigarette smoking in smokers with schizophrenia. Psychopharmacology. 2011;217(2):279–287. doi: 10.1007/s00213-011-2282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trenerry M, Crosson B, DeBoe J, Leber W. Psychological Assessment Resources. 1989. Stroop Neuropsychological Screening Test. [Google Scholar]
- Twamley EW, Palmer BW, Jeste DV, Taylor MJ, Heaton RK. Transient and executive function working memory in schizophrenia. Schizophrenia research. 2006;87(1–3):185–190. doi: 10.1016/j.schres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WAIS-III: Wechsler adult intelligence scale. Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Westerhausen R, Kompus K, Hugdahl K. Impaired cognitive inhibition in schizophrenia: a meta-analysis of the Stroop interference effect. Schizophrenia research. 2011;133(1–3):172–181. doi: 10.1016/j.schres.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Wing VC, Bacher I, Sacco KA, George TP. Neuropsychological performance in patients with schizophrenia and controls as a function of cigarette smoking status. Psychiatry research. 2011;188(3):320–326. doi: 10.1016/j.psychres.2011.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing VC, Wass CE, Soh DW, George TP. A review of neurobiological vulnerability factors and treatment implications for comorbid tobacco dependence in schizophrenia. Annals of the New York Academy of Sciences. 2012;1248:89–106. doi: 10.1111/j.1749-6632.2011.06261.x. [DOI] [PubMed] [Google Scholar]
- Yang YK, Nelson L, Kamaraju L, Wilson W, McEvoy JP. Nicotine decreases bradykinesia-rigidity in haloperidol-treated patients with schizophrenia. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2002;27(4):684–686. doi: 10.1016/S0893-133X(02)00325-1. [DOI] [PubMed] [Google Scholar]
- Yee A, Bt Nek Mohamed NN, Binti Hashim AH, Loh HS, Harbajan Singh MK, Ng CG, Jambunathan ST. The effect of nicotine dependence on psychopathology in patients with schizophrenia. BioMed research international. 2015;2015:730291. doi: 10.1155/2015/730291. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


