Abstract
Prolonged normothermic ex-vivo heart perfusion (NEVHP) could transform cardiac transplantation. To help identify perfusate components that might enable long-term perfusion, we evaluated the effects of cross-circulated whole blood and cross-circulated plasma from a live paracorporeal animal on donor porcine hearts preserved via NEVHP. Standard perfusion (n=40) utilized red blood cell/plasma perfusate and Langendorf technique for a goal of 12 hours. Cross-circulation groups used a similar circuit with the addition of cross-circulated venous whole blood (XC-Blood; n=6) or cross-circulated filtered plasma (XC-Plasma; n=7) between a live paracorporeal pig under anesthesia and the perfusate reservoir. Data included oxygen metabolism, vascular resistance, lactate production, left ventricular function, myocardial electrical impedance, and histopathologic injury score. All cross-circulation hearts were successfully perfused for 12 hours, compared to 22 of 40 standard perfusion hearts (55%; p=0.002). Both cross-circulation groups demonstrated higher oxygen consumption and vascular resistance than standard hearts from hours 3–12. No significant differences were seen between XC-Blood and XC-Plasma hearts in any variable, including left ventricular dP/dT after 12 hours (1478±700mmHg/s vs. 872±500; p=0.17). We conclude that cross circulation of whole blood or plasma from a live animal improves preservation of function of perfused hearts, and cross-circulated plasma performs similarly to cross-circulated whole blood.
Keywords: EVHP, heart perfusion, ex-vivo, cross-circulation, paracorporeal
Introduction
Scarce availability of donor hearts continues to be a problem in the United States; over 4,000 patients are awaiting a heart transplant, yet only 2,804 heart transplants were performed in 2015.1 One persistent issue necessitating regionalization of listing is the relatively short, 6 hour preservation time allowed by the current standard, cold preservation at 4°C.2 A solution to this problem is prolonged normothermic ex-vivo heart perfusion (NEVHP). The goal of this technology would be to extend heart preservation times well beyond the current 6-hour limitation, thereby expanding both the donor pool and the eligible recipients. Furthermore, NEVHP would allow functional assessment of a donor heart prior to transplant, and provide a window for reconditioning.
NEVHP has been studied extensively in animal models, with a sharp decline in function and structural changes when preservation times approach 12 hours.3–8 Recently, using the Organ Care System (OCS) developed by TransMedics Inc. (Andover, MA), NEVHP has also been evaluated in clinical trials showing comparable transplant outcomes when compared to standard preservation despite significantly longer preservation times, albeit still less than 6hrs.9,10 While these early successes are promising, the ideal perfusate components to sustain prolonged (>6hr) cardiac ex-vivo preservation have not been clearly defined. It has been reported that in normothermic conditions, whole blood-based perfusate provides superior cardiac functional preservation to acellular perfusate.6
We recently reported successful 12-hour preservation of porcine donor hearts using NEVHP in 22 of 40 attempts.11 The perfusate was based on donor blood. Although 12-hour perfusion was achieved, 18 hearts failed to maintain adequate function for 12 hours, suggesting that some component of the perfusate became depleted or some toxic metabolite accumulated in these hearts. To address this question, we designed experiments to evaluate the effects of a continuous supply of fresh blood or plasma to and continuous removal of potential toxins from the perfused heart. In these experiments, porcine donor hearts were perfused using our NEVHP technique, with the addition of cross circulation from a live, paracorporeal pig (PCP) under anesthesia with the perfusate reservoir. We investigated the effects of both cross-circulated whole blood and cross-circulated plasma alone. We hypothesized that cross circulation with a live PCP would improve heart preservation for 12 hours.
Materials and Methods
Standard Perfusion (SP)
We recently reported successful 12-hour perfusion in 22/40 hearts using donor blood as the basic perfusate.11 The blood perfusate was depleted of white cells and platelets to avoid a potential inflammatory response. The circuit included a perfusion chamber which drained into a cardiotomy reservoir. Perfusate was pumped from the reservoir using a peristaltic pump (Stockert Instruments Caps Roller Pump, Munich, Germany) to a combination pediatric oxygenator and heat exchanger (reservoir and oxygenator by Terumo Corp. Capiox RX05, Ann Arbor, MI) and into the aortic root in Langendorf technique12 (Figure 1A). In this report we compare hearts perfused with this standard technique to hearts similarly perfused, but with the addition of continuous cross circulation with a live animal.
Figure 1.
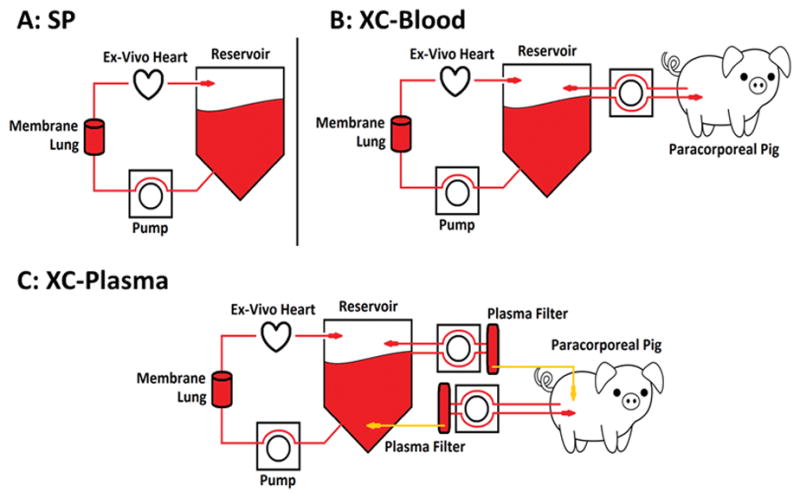
Experimental setups. A) The standard perfusion (SP) experimental setup includes a blood reservoir, from which blood is pumped by roller pump to an oxygenator/heat-exchanger, followed by retrograde aortic antegrade coronary perfusion in Langendorf fashion. Venous blood is returned to the reservoir from the right ventricle via a transpulmonic cannula. The SP setup also forms the basis for other NEVHP experiments. B) Blood cross-circulation experiments (XC-Blood) utilize the SP NEVHP setup, with the addition of a live paracorporeal pig (PCP) under anesthesia. Venous blood is drained from the femoral vein of PCP into the reservoir, and reservoir blood returned to the PCP via the external jugular vein. C) Plasma cross-circulation experiments (XC-Plasma) also utilize the SP NEVHP setup and a live PCP. Venous blood from the PCP and blood from the reservoir both are passed continuously through respective plasma filters. Plasma is then exchanged between PCP and reservoir at 1L/hr.
Cross-circulation Perfusion (XCP)
Heart Procurement
Healthy adult swine weighing 50±10kg were induced and maintained under anesthesia. Vascular access was obtained according to our established laboratory protocols.13 The animal was hydrated with 2L of Lactated Ringers, after which 500mL of venous blood was harvested to create the initial perfusate.
Lidocaine 1mg/kg IV (APP Pharma LLC, Schaumburg, IL) was administered and a midline sternotomy was performed. The superior pericardium was opened to allow for isolation of the great vessels as part of standard technique of cardiac procurement for orthotopic transplantation. The remainder of the pericardium was preserved around the heart to prevent desiccation during NEVHP. The animal was heparinized, venous return was occluded, the aortic arch clamped, and 500mL of high potassium del Nido cold cardioplegia (CAPS Inc., Detroit, MI) was infused antegrade via an aortic root cannula. Topical cooling was achieved via standard techniques and the heart with intact pericardium was removed and placed on an ice bath. Cold ischemia times did not exceed 1 hour. Additional venous blood was harvested following caval ligation.
Back Table Preparation
All cannulae used were products of Terumo (Ann Arbor, MI). A 3/8 x 3/8 in. PVC connector (Medtronic Inc., Minneapolis, MN) was secured in the aortic root for antegrade coronary perfusion. A 28Fr venous drainage cannula was placed into the right ventricle (RV) via the pulmonary artery (PA). The left ventricle (LV) was vented using a 10Fr LV vent placed across the mitral valve via the left atriotomy. A pressure-transducing apparatus consisting of a 10Fr cannula secured within a high compliance balloon was also inserted into the LV across the mitral valve via the left atriotomy. The leaflets of the mitral valve were suture approximated around this apparatus to maintain appropriate positioning.14 All open pulmonary veins were ligated. The heart was deaired and the cannulas were connected to the perfusion apparatus. The heart was then suspended by the aortic root and perfusion was initiated.
Priming Perfusate
Blood from the donor was centrifuged (Thermo IEC Centra GP8R, Rockaway, NJ) at 3,500 RPM for 15 minutes to separate blood components and remove white blood cell and platelet fragments. A total of approximately 300mL of perfuse was created by reconstituting the packed red blood cells with donor plasma. In addition, 80mg Gentamycin, 250mg Nafcillin (APP Pharma LLC, Schaumberg, IL), and 200mg Solumedrol (Pfizer, New York, NY) were added to the perfusate at the start of perfusion (hour 0), at hour 4, and at hour 8.
Heart Perfusion Circuit and Parameters
The circuit for cross-circulation perfusion was constructed in the same way as for standard perfusion, as described above. Perfusion was slowly increased from an initial aortic root pressure of 20mmHg and temperature of 20°C to goals of 40–60mmHg and 37 °C over the first hour. 100–300mL of circuit perfusate was exchanged for fresh perfusate if spontaneous contractions did not begin after 1 hour. Defibrillation with up to 20J was performed in cases of ventricular fibrillation. Perfusion was continued for 12 hours as long as the heart maintained rhythmic ventricular contraction or organized electrical activity.
Cross Circulation
Cross-circulation experiments with blood (“XC-Blood”; n=6) and plasma (“XC-Plasma”; n=7) were performed with the addition of a second healthy adult paracorporeal pig (PCP; 50±10kg) under anesthesia. 14Fr cannulas were placed into the femoral (drainage) and jugular (reinfusion) veins. Cross circulation began 1 hour after the onset of perfusion and continued for 11 hours. For XC-Blood experiments, blood was pumped by roller pump from the drainage cannula into the reservoir, and then pumped from the reservoir back to the PCP at rates from 17–150mL/min (Figure 1B). For XC-plasma experiments, blood from the PCP and from the reservoir was pumped via roller pump to respective PlasmafloTM plasma filters (Asahi Kasei Medical MT Corp., Oita, Japan). Blood effluent from the plasma filters was returned to the PCP or reservoir from which it originated, while filtered plasma was pumped into blood effluent from the opposite filter at 1L/hr (Figure 1C).
Data Collection
Aortic root pressure (coronary arterial pressure) and circuit flow were recorded every half hour and used to calculate vascular resistance. Arterial and RV blood gases were collected, and oxygen consumption calculated every hour. With LV diastolic pressure controlled to 8–12mmHg by saline-distending the pressure-transducing balloon, systolic pressure and maximum dP/dT were determined. Myocardial impedance electrodes (Ethicon, Somerville, NJ) were implanted in the left ventricle as separated by 1cm, and myocardial impedance was recorded at 1, 10, and 100kHz every hour.
After 12 hours of perfusion, hearts were disconnected from the circuit, drained, and weighed. Samples of each chamber were removed, weighed, dehydrated for 7 days, and re-weighed to calculate wet-to-dry ratios. The remainder of the heart was fixed in 10% neutral buffered formalin for histologic analysis. Routine hematoxylin and eosin (H&E) staining was performed. Myocardial injury was graded on the presence and severity of myofiber degeneration, myocardial hemorrhage, interstitial edema, and endothelial alterations. A myocardial injury grading scale with a score range of 0–4 for each category was utilized by a certified veterinary pathologist who was blinded to experimental group (Table 1).
Table 1.
Histopathologic injury scoring scheme for cardiac lesions.
| Myofiber degeneration | 0 | Absent |
|---|---|---|
| 1+ | single to multiple foci of vacuolated, shrunken, or fragmented, hypereosinophilic myofibers | |
| 2+ | more frequent multifocal foci to larger zones of vacuolated, shrunken, or fragmented hypereosinophilic myofibers | |
| 3+ | Large coalescing to regionally extensive zones of vacuolated, shrunken, or fragmented hypereosinophilic myofibers | |
| Myocardial hemorrhage | 0 | Absent |
| 1+ | Focal to multifocal mild myocardial, epicardial, or endocardial hemorrhage | |
| 2+ | Moderate multifocal to regionally extensive myocardial, epicardial, or endocardial hemorrhage | |
| 3+ | Severe regionally extensive hemorrhage involving large portions of heart section | |
| Interstitial edema | 0 | Absent |
| 1+ | Mild and multifocal separation of myofiber bundles or expansion of perivascular spaces | |
| 2+ | Moderate and multifocal separation of myofiber bundles or expansion of perivascular spaces | |
| 3+ | Marked regionally extensive or multifocal separation of myofiber bundles and perivascular spaces | |
| Endothelial changes | 0 | Absent |
| 1+ | Plump endothelial cells, separation of endothelium from underlying basal lamina | |
| 2+ | Cellular infiltration of vessel wall or disruption of layers of vessel wall | |
| 3+ | Necrosis or severe vascular cellular infiltration |
Animal Care and Treatment
All animals received humane care in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Animal protocols were approved by the University of Michigan Institutional Animal Care and Use Committee (IACUC).
Statistical Analysis
Statistical analyses were performed using Microsoft Excel 2013 (Microsoft, Redmond, WA) and SPSS v. 22.0 (IBM Corp, Armonk, NY). Continuous variables were compared using student’s t-test, while categorical variables were compared using Fisher’s Exact Test. Test results are expressed as mean ± standard deviation. A p-value < 0.05 was considered significant.
Results
Duration of Perfusion
SP hearts were successfully preserved for 12 hours in 22 of 40 attempts (55%). XCP hearts (XC-Blood and XC-Plasma) were successfully preserved for 12 hours in 13 of 13 attempts (100%; p=0.002).
Perfused Heart Function
Oxygen consumption increased over time and was significantly higher in both XC-Blood and XC-Plasma hearts than SP hearts from hours 4–12. At 12 hours, oxygen consumption was 9.9±6.9ml/min for XC-Blood hearts and 8.4±5.5 for XC-Plasma hearts (p=0.66), compared to 2.7±1.5 for SP hearts (p<0.001; Figure 2A). Vascular resistance similarly increased over time with cross-circulation, and was significantly higher with XC-Blood and XC-Plasma hearts for hours 3–12. Vascular resistance at hour 12 was 0.76±0.37mmHg/L/min for XC-Blood and 0.82±0.37 for XC-Plasma (p=0.79) compared to 0.27±0.23 for SP hearts (p<0.001; Figure 2B). The difference in lactate concentration between venous and arterial blood was recorded for cross-circulation hearts; no significant differences were seen at any time point (p>0.05 at all time points; Figure 2C).
Figure 2.
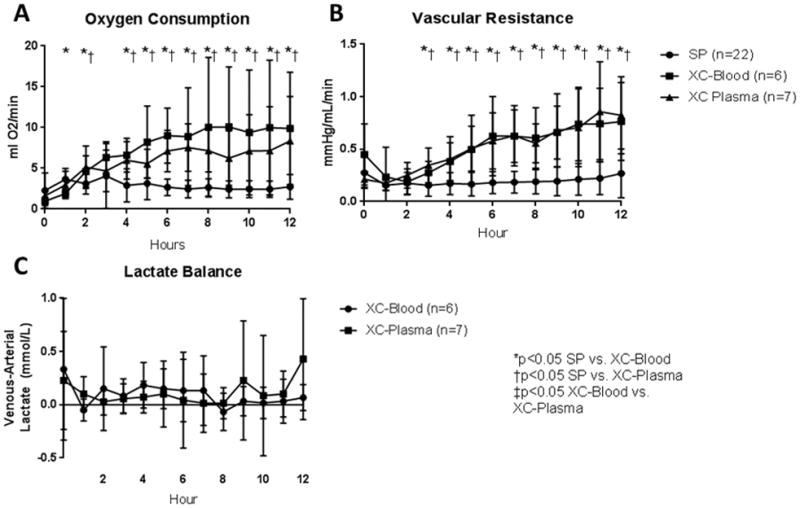
Measures of Donor Heart Metabolism. A) Oxygen consumption. Oxygen consumption were significantly higher in cross-circulation hearts than SP hearts; no differences were seen between blood and plasma. B) Vascular Resistance. Vascular resistance was significantly higher in cross-circulation hearts than SP hearts; no differences were seen between blood and plasma. C) Lactate production/consumption. Positive values reflect lactate production. Both XC-Blood and XC-Plasma hearts appeared to have slightly positive lactate production, with no differences between groups noted.
Left ventricular (LV) systolic function was compared between cross-circulation groups using a pressure-transducing balloon in a non-working model. With diastolic pressure controlled to 8–12mmHg, average LV systolic pressure over 12 hours did not significantly differ between XC-Blood and XC-Plasma (76±16mmHg vs. 67±22; p=0.66), nor did LV systolic pressure at the 12-hour time point (84±50mmHg vs. 60±21; p=0.35; Figure 3A). Similar results were seen with maximum LV dP/dT, with no significant difference seen in LV dP/dT after 12 hours between XC-Blood and XC-Plasma (1478±700mmHg/s vs. 872±500; p=0.17; Figure 3B).
Figure 3.
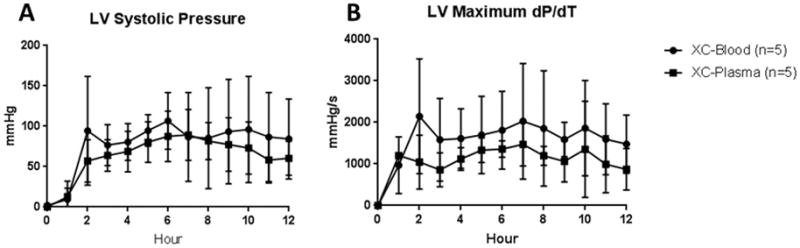
Measures of LV Function. A) LV Systolic pressure. With diastolic pressure set at 8–12mmHg by instillation of saline into the pressure-transducer, LV systolic pressure was recorded. No significant differences were observed between blood and plasma cross-circulation. B) LV dP/dT. The maximum dP/dT over a 10 second span at each time point was recorded. No significant differences between XC-blood and XC-plasma were observed.
Heart Edema and Tissue Injury
Myocardial electrical impedance, which serves to measure tissue edema and cellular injury,15–17 was recorded every hour at 1, 10, and 100kHz in cross-circulation experiments. No differences were seen at any frequency between XC-Blood and XC-Plasma Hearts (Figure 4A-C). Similarly, no difference was seen in average wet-to-dry ratio between XC-Blood and XC-Plasma at the conclusion of 12 hours (5.1±0.5 vs. 5.3±0.4; p=0.42). Wet-to-dry ratio was determined in eight of 22 successful 12 hour SP experiments, and was significantly lower than that of XC-Plasma hearts (4.4±0.8 vs. 5.3±0.4; p=0.04), though not significantly different from XC-Blood hearts (5.1±0.5; p=0.12; Figure 5).
Figure 4.
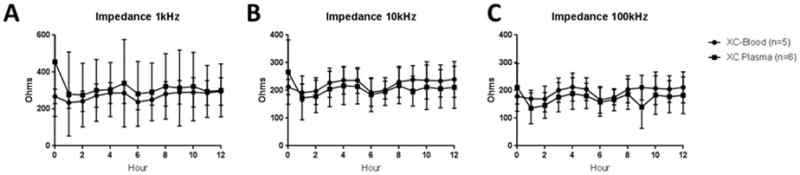
Measures of Donor Heart Edema. A-C) Impedance over time (1, 10, and 100kHz). Low frequency impedance reflects extracellular fluid volume, whereas high frequency impedance more reflects cellular damage. No differences were seen between blood and plasma cross-circulation at any frequency.
Figure 5.
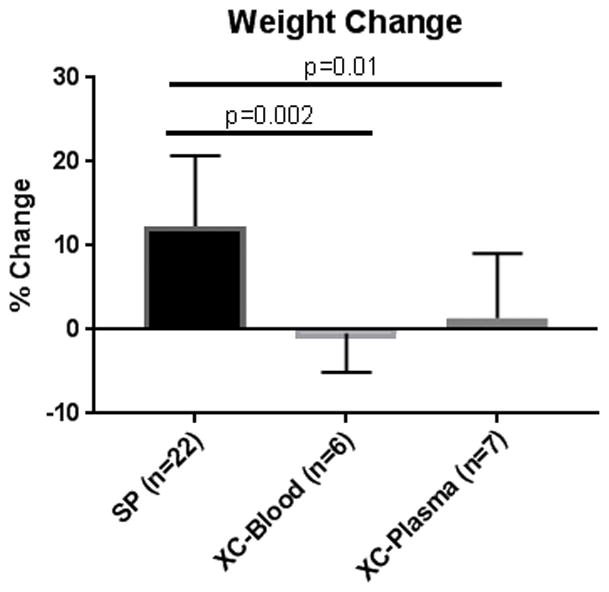
Percent weight change after 12 hours. Successful 12-hour SP hearts exhibited significantly more weight gain than both XC-Blood and XC-Plasma hearts. No significant differences were observed between XC-Blood and XC-Plasma.
On histology, successful SP hearts exhibited significantly less injury than XC-blood in every category except for edema. Average injury score in successful SP hearts was 0.9±0.6, compared to 2.0±0.4 for XC-Blood (p<0.001) and 1.7±0.2 for XC-Plasma (p=0.02). No significant differences were seen between XC-Blood and XC-Plasma in any category, including average injury score (p=0.22; Figure 6).
Figure 6.
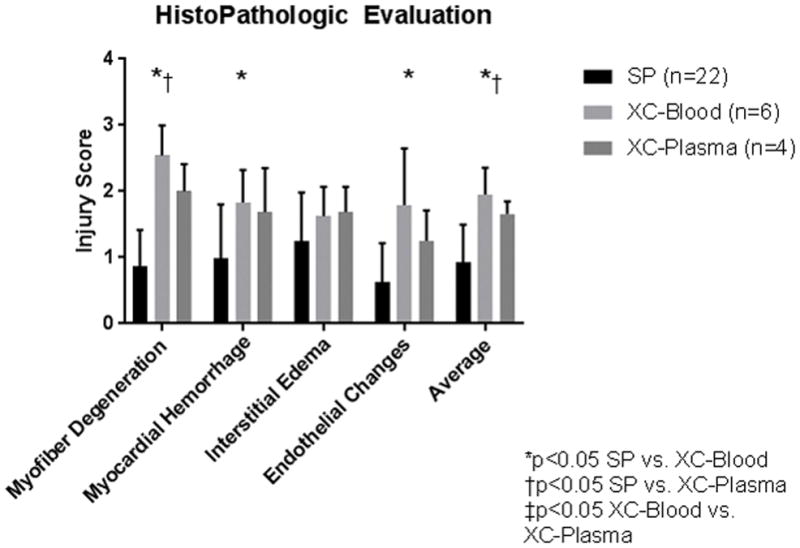
Histopathologic Injury Scores. H&E-stained slides were assessed by a pathologist blinded to experimental group and scored 0–4 in four categories: myofiber degeneration, myocardial hemorrhage, interstitial edema, and endothelial changes. SP hearts exhibited significantly less injury than XC-blood in every category except for edema. Lower myofiber degenderation and average injury scores were also seen with SP hearts compared to XC-Plasma. No significant differences were seen between XC-Blood and XC-Plasma.
Discussion
The relatively low percentage of listed patients receiving a heart for transplantation is in part due to the relatively short preservation time and regionalization that cold preservation necessitates.2 Normothermic ex-vivo heart perfusion represents a potential solution to this problem, as it could expand the donor pool by prolonging preservation times and allowing pretransplant assessment of graft function and the potential for graft reconditioning and immunomodulation. Although this has shown promise in animal and human experimentation, to the best of our knowledge, graft preservation beyond 12 hours using NEVHP has not been routinely achieved. Perfusate components that optimize hypothermic perfusion have been documented, but the ideal perfusate for NEVHP has not been identified.18 We therefore investigated the effects of cross-circulated whole blood and cross-circulated plasma from a live paracorporeal pig, not for the purpose of investigating the clinical applicability of this method, but in the interest of identifying the factors necessary to prolong cardiac graft preservation.
Using both cross-circulated blood and cross-circulated plasma, we achieved 100% success at maintaining porcine donor hearts in a functional state using NEVHP for 12 hours. This is in contrast to our SP experiments, in which successful 12-hour preservation was achieved in only 22 of 40 attempts (55%). Even when comparing only those 22 successful 12-hour SP runs to cross-circulation runs with blood or plasma, cross circulation clearly resulted in functional differences. Oxygen consumption was significantly higher in XCP hearts compared to SP hearts, indicating higher metabolic rate most likely secondary to more vigorous function. Vascular resistance also increased over time in XCP hearts, and became significantly higher than resistance in SP hearts after 3–4 hours of perfusion. Meanwhile, no differences were seen in oxygen consumption or vascular resistance between blood and plasma cross circulation. The significance of this elevated vascular resistance with cross circulation is unclear, as studies have linked elevated vascular resistance to delayed graft function in human kidneys.19 However, the vascular resistance observed in cross-circulation hearts may more closely approximate physiologic resistance while the resistance in SP preparations appears disproportionately low. Of note, graft function in this study was significantly better than SP hearts even though those hearts maintained lower vascular resistance. Regardless, we believe this vascular resistance highlights a significant difference in physiology between SP hearts and XCP hearts, as well as similar physiology between blood and plasma cross circulation. These data suggest that cross circulation with a living animal preserves ex vivo heart function, and that plasma is the vehicle by which this preservation occurs.
XC-Plasma and XC-Blood hearts exhibited similar levels of edema and cellular injury during NEVHP and after 12 hours. Percent weight change, indicative of edema, was not significantly different between XCP groups. Myocardial electrical impedance can be measured at frequent intervals during perfusion and has been shown to reflect extracellular fluid volume and cellular injury, correlating with myocardial injury, edema, and rejection in previous studies.15–17 In this study, electrical impedance at both low and high frequencies was also similar between XCP groups over 12 hours of perfusion. Myocardial injury appeared slightly less with cross-circulated plasma than blood, perhaps due to a lack of cross-circulating leukocytes with plasma. Surprisingly, despite significantly improved functional preservation, hearts in both cross-circulation groups exhibited higher injury scores than SP hearts. One working hypothesis as to the etiology of this injury is that cross-circulating leukocytes in the XC-blood experiments or cross-circulating plasma-carried antigens in the XC-plasma experiments cause immune-mediated vascular and myocardial damage. Alternatively, injury could be related to the higher vascular resistance in the XC hearts, which, while it appears to correlate with donor heart vitality, could also be elevated to a pathologic level resulting in vascular injury and edema. Details of the mechanisms of this increased injury are the subject of ongoing investigation.
The optimal perfusate for NEVHP has been the subject of substantial research, but the question remains unanswered. While blood-based perfusate is precluded during hypothermic EVHP due to issues of viscosity and oxygen exchange, studies have identified blood-based perfusate as necessary during normothermic perfusion to meet the metabolic demands of the functional heart.6,18,20 Blood perfusion alone is inadequate, however, as the isolated perfused heart tends to emulate the loss of viability and vascular tone seen in the cardiovascular collapse of brain-dead humans and animals.11,21,22 It is likely that circulating metabolic substrates and hormones are necessary to maintain organ integrity. This led Steen et al. to propose a collection of hormones which promoted hemodynamic stability in decapitated animals, including epinephrine, thyroxine, desmopressin, and cortisol; similar results were obtained by Hing et al..22,23 Keeping with these principles, the OCS includes constant infusion of TransMedics Maintenance Solution, which includes insulin, glucocorticoids, bicarbonate, adenosine, and low dose epinephrine.9,10,24 However, given that preservation time appears to remain limited, the current list of OCS perfusate ingredients must lack essential components. Our experiments suggest that some factor or factors in plasma from a live animal, and/or toxin removal by the live animal, are essential for prolonged perfusion.
Cross circulation is an old concept, first used clinically by the open-heart operations of Dr. Lillehei in the 1950s.25 In those operations, before the heart-lung machine, the donor heart and lung provided arterial blood to permit cardiopulmonary bypass and intracardiac operations in the recipient. In those patients, cross circulation in a general sense allows for sharing of collective organ function; metabolic substrates and hormones from one organism are made available for the other, while toxic metabolites produced by one organism are potentially cleared by the other. Along these lines, it is not surprising that whole blood cross-circulation would improve preservation of isolated perfused donor hearts, as this in essence represents heterotopic transplantation. In recent reports, donor pig hearts have been preserved for over 2 years in live baboons by heterotopic xenotransplantation.26 Our experiments use a perfusion apparatus, not direct donor animal perfusion. However, the limitation of cross circulation to plasma alone represents a novel experimental technique, and identifies plasma, as opposed the cellular blood components, as the vehicle by which necessary factors are delivered and/or cleared.
This finding presents two major ramifications. The first pertains to clinical application; if cross-circulated plasma from a live animal contains all necessary factors to optimize donor heart preservation, then these may also be present in fresh frozen plasma (FFP). FFP is a limited but available resource at tertiary care centers where NEVHP would be used clinically, and its use would be far more feasible than using an intermediate host as was done in these experiments. We recently reported successful isolated human limb perfusion for 36 hours, using FFP and packed red blood cells from our standard blood bank, supporting the feasibility of this strategy.27 Although these findings suggest that essential factors are supplied in the cross-circulated plasma, it is possible that clearance of toxic metabolites from the perfusate is equally important. We are planning experiments to identify the relative importance of these two variables.
The second ramification relates to further research application. With the knowledge that necessary factors for optimal heart preservation during NEVHP are contained within plasma, we can begin to elucidate the essential factors by examining the metabolomic and proteomic profiles of plasma filtered from the paracorporeal animal and plasma filtered from venous blood from the donor heart. We hope to isolate the factors which are present in plasma from a live animal that are essential to maintain prolonged perfusion.
There are numerous limitations of our study, and the first relates to the fact that data collected across experimental models was not consistent in that left ventricular function and electrical tissue impedance data were only collected in the XCP experiments and not the standard preps. This precludes comparison of ventricular function between these groups and limits comparisons of structure/injury to wet-to-dry ratios and histopathologic injury scores. The use of an anesthetized paracorporeal pig also influences our experimentation, as inhalation anesthesia effects systemic physiology, stress-response, and circulating hormones to which the donor heart is exposed.28–30 However, considering that vascular resistance was actually higher in the preps exposed to inhalational anesthesia, the hemodynamic effect of this exposure might be negligible. Our nonworking heart model using Langendorf technique also limits our assessment of cardiac function as it is not physiologic. We were able to assess left ventricular systolic function, but given that diastolic pressure is controlled in our experimentation, diastolic function and chamber compliance cannot be assessed. Although a working heart model with pressure-volume loop data has been used in other similar experiments,31,32 we believe the ultimate proof of perfused heart function is successful transplantation. We have performed one successful orthotopic transplant following 12 hours of NEVHP with plasma cross-circulation, and aim to re-demonstrate this success using our model going forward.
Future experimentation will be directed at determining the clinical feasibility of utilizing FFP and plasma exchange instead of cross circulation, and at elucidating the circulating factors that prolong and enhance cardiac organ preservation. In order to determine if FFP can be used clinically to support donor hearts during NEVHP, we plan to continue plasma cross-circulation experiments, but at lower rates of exchange, to determine the minimum necessary plasma dose required to adequately support NEVHP. To begin elucidating the list of circulating factors, we plan to determine the metabolomics and proteomic profiles of cross-circulating plasma being delivered too and filtered from the heart. The differences in these profiles should highlight candidate substances required for optimal graft preservation. Finally, we aim to prolong graft preservation beyond 24 hours using plasma cross circulation, and to continue transplanting hearts following NEVHP with plasma cross-circulation to verify their transplant viability.
Conclusion
Both blood and plasma cross circulation with a live paracorporeal animal under anesthesia consistently support donor hearts during NEVHP for 12 hours. The paracorporeal animal provided a continuous supply of plasma factors and removed any toxic metabolites from the perfused heart. Plasma exchange was as successful as whole blood perfusion. This suggests that factors in the plasma of a live animal, and perhaps in fresh frozen plasma, could permit prolonged organ perfusion.
Acknowledgments
The authors thank The Frankel Family Foundation for their generous support without which this study would not be possible. They also thank the Terumo Cardiovascular Group for contribution of vascular and perfusion materials, The University of Michigan Undergraduate Research Opportunity Program (UROP), and the Cardiovascular Center Summer Undergraduate Research Fellowship. Finally, the authors acknowledge the efforts of Cindy Cooke for review and preparation of the manuscript.
Sources of Funding: Funding courtesy of the Frankel Family Foundation.
Footnotes
Conflicts of Interest: No conflicts of interest.
References
- 1.Shanley CJ, Hirschl RB, Schumacher RE, et al. Extracorporeal life support for neonatal respiratory failure. A 20-year experience. Annals of Surgery. 1994;220(3):269–280. doi: 10.1097/00000658-199409000-00004. discussion 281–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hicks M, Hing A, Gao L, Ryan J, Macdonald PS. Organ preservation. Methods in molecular biology (Clifton, NJ) 2006;333:331–374. doi: 10.1385/1-59745-049-9:331. [DOI] [PubMed] [Google Scholar]
- 3.Iyer A, Gao L, Doyle A, et al. Normothermic ex vivo perfusion provides superior organ preservation and enables viability assessment of hearts from DCD donors. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2015;15(2):371–380. doi: 10.1111/ajt.12994. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y, Lin H, Wen Z, et al. Keeping donor hearts in completely beating status with normothermic blood perfusion for transplants. The Annals of thoracic surgery. 2013;95(6):2028–2034. doi: 10.1016/j.athoracsur.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Garbade J, Krautz C, Aupperle H, et al. Functional, metabolic, and morphological aspects of continuous, normothermic heart preservation: effects of different preparation and perfusion techniques. Tissue engineering Part C, Methods. 2009;15(2):275–283. doi: 10.1089/ten.tec.2008.0475. [DOI] [PubMed] [Google Scholar]
- 6.White CW, Hasanally D, Mundt P, et al. A whole blood-based perfusate provides superior preservation of myocardial function during ex vivo heart perfusion. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34(1):113–121. doi: 10.1016/j.healun.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 7.Garcia Saez D, Elbetanony A, Lezberg P, et al. Ex vivo heart perfusion after cardiocirculatory death; a porcine model. The Journal of surgical research. 2015;195(1):311–314. doi: 10.1016/j.jss.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 8.Hassanein WH, Zellos L, Tyrrell TA, et al. Continuous perfusion of donor hearts in the beating state extends preservation time and improves recovery of function. The Journal of thoracic and cardiovascular surgery. 1998;116(5):821–830. doi: 10.1016/S0022-5223(98)00452-8. [DOI] [PubMed] [Google Scholar]
- 9.Messer S, Ardehali A, Tsui S. Normothermic donor heart perfusion: current clinical experience and the future. Transplant international : official journal of the European Society for Organ Transplantation. 2015;28(6):634–642. doi: 10.1111/tri.12361. [DOI] [PubMed] [Google Scholar]
- 10.Ardehali A, Esmailian F, Deng M, et al. Ex-vivo perfusion of donor hearts for human heart transplantation (PROCEED II): a prospective, open-label, multicentre, randomised non-inferiority trial. Lancet (London, England) 2015;385(9987):2577–2584. doi: 10.1016/S0140-6736(15)60261-6. [DOI] [PubMed] [Google Scholar]
- 11.Trahanas JM, Witer LJ, Alghanem F, et al. Achieving 12 Hour Normothermic Ex Situ Heart Perfusion: An Experience of 40 Porcine Hearts. ASAIO journal (American Society for Artificial Internal Organs : 1992) 2016;62(4):470–476. doi: 10.1097/MAT.0000000000000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell RM, Mocanu MM, Yellon DM. Retrograde heart perfusion: the Langendorff technique of isolated heart perfusion. Journal of molecular and cellular cardiology. 2011;50(6):940–950. doi: 10.1016/j.yjmcc.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 13.Rojas-Pena A, Reoma JL, Krause E, et al. Extracorporeal support: improves donor renal graft function after cardiac death. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(6):1365–1374. doi: 10.1111/j.1600-6143.2010.03063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fallen EL, Elliott WC, Gorlin R. Apparatus for study of ventricular function and metabolism in the isolated perfused rat heart. Journal of applied physiology. 1967;22(4):836–839. doi: 10.1152/jappl.1967.22.4.836. [DOI] [PubMed] [Google Scholar]
- 15.Pfitzmann R, Muller J, Grauhan O, Hetzer R. Intramyocardial impedance measurements for diagnosis of acute cardiac allograft rejection. The Annals of thoracic surgery. 2000;70(2):527–532. doi: 10.1016/s0003-4975(00)01409-0. [DOI] [PubMed] [Google Scholar]
- 16.Cinca J, Ramos J, Garcia MA, et al. Changes in myocardial electrical impedance in human heart graft rejection. European journal of heart failure. 2008;10(6):594–600. doi: 10.1016/j.ejheart.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Grauhan O, Muller J, Knosalla C, et al. Electric myocardial impedance registration in humoral rejection after heart transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 1996;15(2):136–143. [PubMed] [Google Scholar]
- 18.Smulowitz PB, Serna DL, Beckham GE, Milliken JC. Ex vivo cardiac allograft preservation by continuous perfusion techniques. ASAIO journal (American Society for Artificial Internal Organs : 1992) 2000;46(4):389–396. doi: 10.1097/00002480-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Jochmans I, Moers C, Smits JM, et al. The prognostic value of renal resistance during hypothermic machine perfusion of deceased donor kidneys. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(10):2214–2220. doi: 10.1111/j.1600-6143.2011.03685.x. [DOI] [PubMed] [Google Scholar]
- 20.Podesser BK, Hallstrom S, Schima H, et al. The erythrocyte-perfused “working heart” model: hemodynamic and metabolic performance in comparison to crystalloid perfused hearts. Journal of pharmacological and toxicological methods. 1999;41(1):9–15. doi: 10.1016/s1056-8719(99)00018-0. [DOI] [PubMed] [Google Scholar]
- 21.Bartlett RH. Vitalin: the rationale for a hypothetical hormone. Journal of the American College of Surgeons. 2004;199(2):286–292. doi: 10.1016/j.jamcollsurg.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Steen S, Sjoberg T, Liao Q, Bozovic G, Wohlfart B. Pharmacological normalization of circulation after acute brain death. Acta anaesthesiologica Scandinavica. 2012;56(8):1006–1012. doi: 10.1111/j.1399-6576.2012.02721.x. [DOI] [PubMed] [Google Scholar]
- 23.Hing AJ, Hicks M, Garlick SR, et al. The effects of hormone resuscitation on cardiac function and hemodynamics in a porcine brain-dead organ donor model. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7(4):809–817. doi: 10.1111/j.1600-6143.2007.01735.x. [DOI] [PubMed] [Google Scholar]
- 24.Stamp NL, Shah A, Vincent V, et al. Successful Heart Transplant after Ten Hours Out-of-body Time using the TransMedics Organ Care System. Heart, lung & circulation. 2015;24(6):611–613. doi: 10.1016/j.hlc.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Moller JH, Shumway SJ, Gott VL. The first open-heart repairs using extracorporeal circulation by cross-circulation: a 53-year follow-up. The Annals of thoracic surgery. 2009;88(3):1044–1046. doi: 10.1016/j.athoracsur.2009.05.077. [DOI] [PubMed] [Google Scholar]
- 26.Mohiuddin MM, Singh AK, Corcoran PC, et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nature communications. 2016;7:11138. doi: 10.1038/ncomms11138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Werner NAF, Rakestraw S, Nicely B, Olszewksi A, Rudich S, Rojas-Pena A, Magee J, Ozer K. Ex Situ Perfusion of a Human Limb for 24 Hours. [abstract]. American Transplant Congress; 2016; Boston, MA. [Google Scholar]
- 28.Nishiyama T. Hemodynamic and catecholamine response to a rapid increase in isoflurane or sevoflurane concentration during a maintenance phase of anesthesia in humans. Journal of anesthesia. 2005;19(3):213–217. doi: 10.1007/s00540-005-0318-0. [DOI] [PubMed] [Google Scholar]
- 29.Laber-Laird K, Smith A, Swindle MM, Colwell J. Effects of isoflurane anesthesia on glucose tolerance and insulin secretion in Yucatan minipigs. Laboratory animal science. 1992;42(6):579–581. [PubMed] [Google Scholar]
- 30.Kostopanagiotou G, Kalimeris K, Christodoulaki K, et al. The differential impact of volatile and intravenous anaesthetics on stress response in the swine. Hormones (Athens, Greece) 2010;9(1):67–75. doi: 10.14310/horm.2002.1255. [DOI] [PubMed] [Google Scholar]
- 31.Colah S, Freed DH, Mundt P, et al. Ex vivo perfusion of the swine heart as a method for pre-transplant assessment. Perfusion. 2012;27(5):408–413. doi: 10.1177/0267659112449035. [DOI] [PubMed] [Google Scholar]
- 32.Abicht JM, Mayr TA, Jauch J, et al. Large-Animal Biventricular Working Heart Perfusion System with Low Priming Volume-Comparison between in vivo and ex vivo Cardiac Function. The Thoracic and cardiovascular surgeon. 2016 doi: 10.1055/s-0036-1580604. [DOI] [PubMed] [Google Scholar]


