Abstract
Objectives
To test the hypothesis that variants in cerebrovascular anatomy will affect the number of patients demonstrating a plausible retrograde embolization mechanism from plaques in the descending aorta (DAo).
Methods
Thirty-five patients (63±17years) with cryptogenic stroke underwent 4D flow MRI for the assessment of aortic 3D blood flow and MR angiography for the evaluation of Circle of Willis, posterior circulation, and aortic arch architecture. In patients with proven DAo plaque, retrograde embolization was considered a potential mechanism if retrograde flow extended from the DAo to a supra-aortic vessel supplying the cerebral infarct territory.
Results
Retrograde embolization with matching cerebral infarct territory was detected in 6 (17%) patients. Circle of Willis and aortic arch variant anatomy was found in 60% of patients which led to the reclassification of retrograde embolization risk as present in 3 (9%) additional patients for a total 26% of cryptogenic stroke patients.
Conclusion
4D flow MRI demonstrated 26% concordance with infarct location on imaging with retrograde diastolic flow into the feeding vessels of the affected cerebral area, identifying a potential etiology for cryptogenic stroke. Our findings further demonstrate the importance of cerebrovascular anatomy when determining concordance of retrograde flow pathways with vascular stroke territory from DAo plaques.
Keywords: 4D flow MRI, stroke, plaque, retrograde flow, circle of Willis
INTRODUCTION
Despite comprehensive diagnostic evaluation, stroke etiology remains undetermined (cryptogenic) in 20–30% of patients1,2 and is accompanied by a high risk for recurrent events (5–10% per year3–5,6). It is well-known that complex and ≥4 mm thick plaques of the ascending aorta and arch are associated embolic stroke7. However, plaques in the descending aorta (DAo), where they occur most frequently8, were not considered a source of stroke since embolization would require reverse (upward) flow from the DAo plaque into the brain supplying supra-aortic vessels to invoke this mechanism7,9. A number of studies, however, provide compelling evidence that flow reversal in the DAo is a frequent phenomenon, even in the absence of aortic regurgitation10–12. In addition, previous 4D flow MRI13–18 studies have shown that diastolic reversal of flow from DAo plaques provided a potential mechanism for embolization to all cerebrovascular territories which constituted the only probable source in 24% of patients with cryptogenic stroke.19,20
However, previous studies did not take into account the frequently encountered inter-individual differences in the vascular anatomy of aortic arch and the Circle of Wills. As a result, the causal link between aortic retrograde flow into the supra-aortic vessel origins and the stroke territory may have been misclassified (over- or underestimated). 3D time-of flight (TOF) MR angiography (MRA) and contrast-enhanced (CE) 3D MRA provide visualization of Circle of Willis and great vessel anatomical variation, respectively, and are routinely performed in diagnostic stroke evaluation. These two techniques enable a more definitive assessment of potential connections between aortic flow reversal and the vascular supply to the region of the cerebral infarction.
The purpose of this study was to further evaluate the presence of retrograde diastolic flow from the DAo into the supra-aortic vessels as a potential mechanism for cryptogenic stroke. We hypothesized that retrograde aortic flow pathways assessed using 4D flow MRI in combination with the evaluation of cerebrovascular anatomy (e.g. Circle of Willis and aortic arch anatomy) based on 3D TOF brain MR angiography (MRA) of the head and gadolinium-enhanced 3D MRA of the neck will increase the number of patients with cryptogenic stoke in which retrograde embolization constitutes a potential mechanism.
METHODS
Study Population
Thirty-five patients (17 men, 63±17 years) with cryptogenic stroke were included via retrospective chart review as approved by the Institutional Review Board (IRB). Inclusion criteria were acute cerebral or retinal ischemic stroke with visible cerebral infarction on MRI, or retinal infarction visible on ophthalmoscopy and complete standard stroke etiologic evaluation including comprehensive brain MRI, TOF-MRA of the head, and CE-MRA of the neck, and undetermined etiology of stroke (i.e., cryptogenic stroke) using the TOAST classification21. Exclusion criteria were known or documented atrial fibrillation or other high risk cardiac source of embolism (left atrial or ventricular thrombus, left ventricular ejection fraction <35%, valvular lesions with embolic potential, patent foramen ovale with known source of venous thromboembolism, complex plaque with >4mm thickness, mobile components or superimposed thrombi in the ascending aortic arch), ipsilateral large vessel intra- or extracranial atherosclerosis causing >50% stenosis, significant atherosclerotic disease of the common carotid arteries, carotid bifurcations, cavernous carotid, and typical lacunar infarction.
MR Imaging
All MRI examinations were conducted using 1.5 or 3T MRI systems (Aera, Avanto, or Skyra, Siemens Medical Systems, Germany). All stroke patients underwent routine brain MRI including diffusion MRI, high resolution 3D time-of-flight (TOF) MRA, and conventional gadolinium-enhanced MRA of the neck. To screen for potential cardiovascular sources of cryptogenic stroke, a second routine cardiothoracic MRI exam was performed for each patient which included 2D CINE SSFP imaging of the heart, pre- and post-contrast T1 weighted fat suppressed axial and coronal imaging of the chest, and 3D high-resolution ECG-gated contrast-enhanced MR Angiography (MRA) followed by aortic 4D flow MRI. ECG and respiration synchronized 4D flow MRI (time-resolved 3D phase contrast MRI with three-directional velocity encoding) was performed to measure in-vivo time-resolved 3D blood flow with full volumetric coverage of the thoracic aorta22. Scan parameters were as follows: spatial resolution=2.9–3.4 × 2.1–2.4 × 2.5–3.2 mm3, temporal resolution=38–40ms, TE/TR/flip angle=2.2–2.4ms/4.6–4.8ms/15°, total scan time=8–15 minutes depending on heart rate and respiration control efficiency. The velocity sensitivity (venc=150–375cm/s) was individually adjusted based on the expected peak velocity in the ascending aorta as determined by 2D PC MRI performed prior to 4D flow MRI.
Brain MR imaging included high-resolution (isotropic 0.5mm3) time-of-flight (TOF) MRA. Pulse sequence parameters for 3D TOF were as follows: spatial resolution=0.5 × 0.5 × 0.5 mm3, TE/TR/flip angle=3.5ms/21.0ms/20°. Neck MRA imaging included high-resolution gadolinium-enhanced MR angiography in coronal orientation with in-line subtraction.
4D Flow Data Analysis and Evaluation of DAo Flow Reversal
All 4D flow data underwent pre-processing to correct for phase offset errors due to Maxwell terms, eddy currents and velocity aliasing using in-house software programmed in Matlab (The Mathworks, USA) as described previously22,23. Next, a 3D phase contrast (PC) MRA of the aorta was calculated from the 4D flow data. The 3D PC-MRA was used for anatomic orientation to evaluate the extent of flow reversal in the descending aorta using a 3D visualization software package (EnSight, CEI, NC, USA) as shown in figure 1A. 3D visualization of aortic 3D blood flow patterns was based on the calculation of time-resolved 3D pathlines originating from a 2D analysis plane manually positioned orthogonal to the aortic lumen (color coding = local absolute blood flow velocity). As shown in Figure 1A (mid panel), 3D pathlines originating from an emitter plane distal to the left subclavian artery at the beginning of the descending aorta were used to trace the temporal and spatial evolution of blood flow through the cardiac cycle. This case illustrates normal forward flow during systole (Figure 1B, top row) followed by marked flow reversal during diastole (Figure 1B, mid and lower row). In this example, retrograde flow originating distal to the left subclavian artery reached all supra-aortic branches (left subclavian artery (LSA), left common carotid artery (CCA), and brachiocephalic trunk (BT)) during late diastole (Figure 1B, lower row).
Fig. 1.
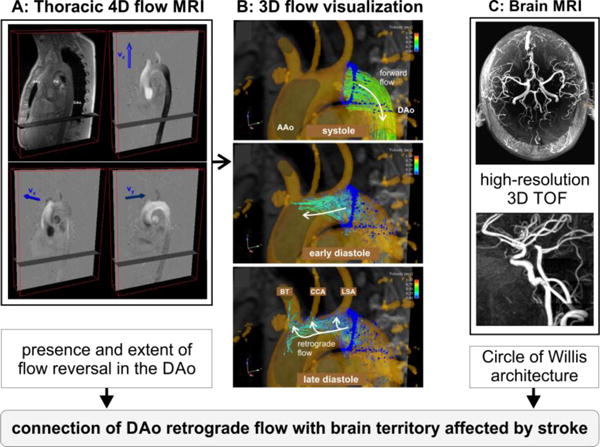
MRI protocol for the assessment of aortic 3D blood flow using thoracic 4D flow MRI (A) and the evaluation of cerebrovascular Circle of Willis architecture based on high-resolution cerebral 3D time-of-flight (TOF) MRI (B). 4D flow MRI raw data (left) and 3D blood flow visualization of aortic flow based on time-resolved pathlines (mid) illustrates diastolic flow reversal in the descending aorta (DAo) and the mechanism of retrograde embolization: marked diastolic retrograde flow originating from the DAo and reaching all brain supplying arteries. AAo = ascending aorta, BT = brachiocephalic trunk, CCA = common carotid artery, LSA = left subclavian artery.
To measure the maximum distance of diastolic flow reversal in the DAo, a series of 5 emitter planes were positioned at 10 mm intervals distal to the left subclavian artery origin as shown in Figure 2 and based on methods previously described by Harloff et al13, 14. Time-resolved 3D pathlines were emitted from all 5 planes and, for each brain supplying supra-aortic vessel origin (LSA, CCA, and BT) the maximum distance (in 10 mm intervals) of diastolic reverse flow originating in the DAo was noted.
Fig. 2.
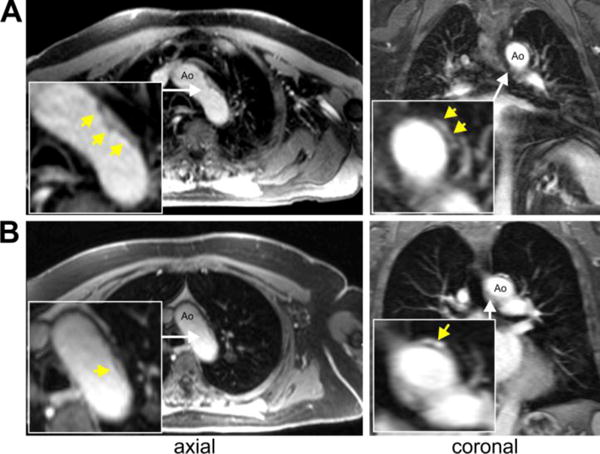
Examples for detected plaques in the descending aorta using post-contrast T1-weighted fat suppressed axial and coronal images. For both examples DAo plaques were located just distal to the outlet of the left subclavian artery with a maximum thickness of 3mm (A) and 7mm (B), respectively.
Plaques in the Descending Aorta
Plaques in the descending aorta were identified in the descending thoracic aorta by a single experienced cardiovascular radiologist blinded to stroke location. Pre- and post-contrast T1-weighted fat suppressed axial and coronal imaging was reviewed to identify regions of irregular wall thickening at least 2 mm in thickness distal to the origin of the left subclavian artery. These regions were co-localized on multi-planar review of unsubtracted 3D MRA images to identify aortic lumen irregularities at the site of the wall thickening. Based on information from T1 and MRA images, descending aortic plaques with a distance of 10–20mm distal to the left subclavian artery were characterized as present.
Cerebrovascular Architecture
To account for frequently encountered inter-individual differences in vascular anatomy of the Circle of Willis, posterior circulation, and aortic arch, an experienced neuro-radiologist analyzed all TOF MRA and MRI data to classify cerebrovascular anatomy by grading the patency of the anterior communicating artery (0=not present, 1=present), left and right posterior communicating arteries (0 = not present, 1=present), left and right vertebral artery dominance (0=not present, 1=present, 2=dominant), as well as presence and number of basilar feeding arteries. In addition, aortic arch variant origins of the great vessels were assessed.
Stroke Subtyping
The cerebral infarct topography was adjudicated by a single rate (SP) and based on diffusion weighted MRI findings. Size, vascular distribution, and number of acute ischemic lesions were noted. Vascular territory was based on presumed vascular supply of the major arterial branches in the brain (middle cerebral, anterior cerebral, posterior cerebral, internal carotid, basilar, and vertebral arteries).
Matching Descending Aortic Reverse Flow and Stroke Pattern
Retrograde embolization in a patient with proven plaque in the DAo was considered a potential mechanism if reversal of flow extended from the DAo to a great vessel origin (LSA, CCA, or BT) that could supply the cerebral infarct territory, taking into account the individual Circle of Willis architecture.
RESULTS
Baseline characteristics of the 35 stroke patients are summarized in table 1. 4D flow MRI, post-processing, and assessment of descending aortic flow reversal was successfully completed in all 35 stroke patients.
Table 1.
Baseline characteristics of the 35 stroke patients. BMI = body mass index, BP = blood pressure, EF = ejection fraction, LDL = low-density lipoprotein. ACA = anterior cerebral artery, MCA = middle cerebral artery, PCA = posterior communication artery.
| Characteristic | Value |
|---|---|
| Age [years] | 62.6 ± 16.8 |
| Male sex – no. (%) | 17 (58.8) |
| BMI [kg/m2] | 26.2 ± 4.9 |
| Heart rate [bpm] | 72.5 ± 13.3 |
| Systolic BP [mmHg] | 145.2 ± 25.8 |
| Diastolic BP [mmHg] | 74.4 ± 9.5 |
| EF [%] | 60.8 ± 8.6 |
| LDL level [mg/dl] | 105.0 ± 44.7 |
| Hypertension – no. (%) | 22 (63%) |
| Hypercoagulable state – no. (%) | none (0%) |
| Atrial fibrillation – no. (%) | none (0%) |
| Smoking history – no. (%) | 19 (54%) |
| Stroke Territory – no. (%) | |
| - left sided | 21 (60%) |
| - right sided | 11 (31%) |
| - bilateral | 3 (9%) |
| supplying brain vessels | |
| - left-sided – no. (%, vessel) | 12 (34%, 3 ACA, 5 MCA, 4 PCA) |
| - right-sided – no. (%, vessel) | 11 (31%, 3 ACA, 1 MCA, 4 PCA, 2 ACA/MCA watershed, 1 PCA/MCA watershed) |
| - bilateral & vertebrobasilar – no. (%) | 12 (34%) |
| Plaque in DAo | 22 (63%) |
Plaques in the Descending Aorta and Descending Aorta Flow Reversal
Plaques in the descending aorta (with 10–20mm distal to the left subclavian artery) were found in 22 patients (63%). Examples of plaques in the descending aorta detected by MRI are shown in figure 2. As summarized in figure 3, diastolic retrograde flow was observed in 20 patients (57%), visualized from the DAo into the brachiocephalic trunk and innominate artery in 3 subjects (9%), into the left common carotid artery (CCA) in 8 (24%) and into the left subclavian artery (LSA) in 20 (57%) stroke patients. Retrograde flow originated from DAo locations as far as 30mm distal to the LSA (emitter plane 4 in Figure 2). DAo plaques were present in 13 of the 20 cryptogenic patients with diastolic retrograde flow (65%).
Fig. 3.
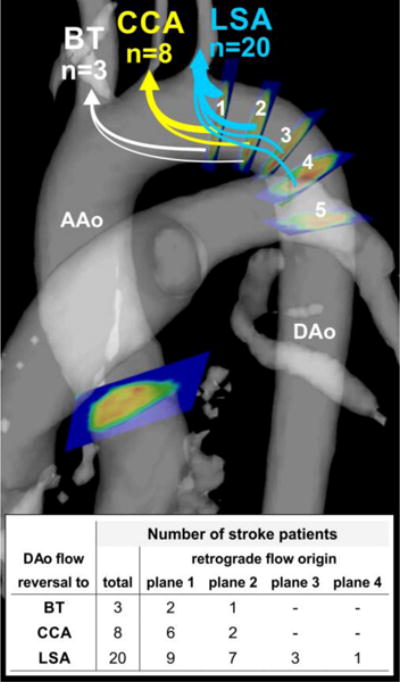
Analysis of reverse flow in the descending aorta (DAo) based on five emitter planes in the DAo. The first plane was positioned immediately distal to the left subclavian artery (LSA) and all subsequent planes 2–5 were placed downstream in 10mm intervals. Diastolic DAo flow reversal reaching the LSA, CCA, and BT was found in a total of 20, 8, and 3 stroke patients, respectively. The table provides a summary of the DAo origin for retrograde flow reaching the different supra-aortic branches. The gray shaded surface shows the aortic anatomy based on the 3D PC-MRA data. AAo = ascending aorta, BT = brachiocephalic trunk, CCA = common carotid artery.
Cerebrovascular Architecture
As summarized in table 2, variant cerebrovascular anatomy was found in a large fraction (60%) of patients: The anterior communicating artery was not present in 2 (6%) patients, posterior communicating arteries were not present on the left in 18 (51%) and right in 15 (43%) patients. In addition, both vertebral arteries were present in all subjects but 13 patients (37%) had left and 6 patients (17%) had right dominant vertebral artery; in 4 (11%) patients, the vertebral arteries did not join together to form the basilar artery. A common origin of brachiocephalic artery and left CCA was found in 12 (34%) patients.
Table 2.
Summary of the classification of cerebrovascular architecture in n=35 patients with cryptogenic stroke.
| Vascular Region | Convectional architecture | # of patients N (%) |
|---|---|---|
| Circle of Willis | presence of bilateral posterior and anterior communicating arteries | 14 (40%) |
| Aortic arch | normal arch with patent vertebral arteries and conventional branching anatomy | 23 (66%) |
| Basilar artery supply | Both vertebral arteries join together to form basilar artery | 31 (89%) |
Retrograde Embolization Mechanism
Based on the presence of DAo flow reversal alone, the retrograde embolization mechanism with existing plaque in the DAo and matching cerebral infarct territory was detected in approximately one fifth (17%, 6 of 35) of patients. The inclusion of cerebrovascular architecture findings led to the reclassification of matching flow reversal as present (rather than absent) in 5 (14%) patients (i.e. matching retrograde flow into an artery that supplied the infarct territory after accounting for variant cerebrovascular anatomy). The variant anatomy in these five patients included: left posterior communicating arteries not present (n=3), dominant left vertebral artery (n=1), dominant right vertebral artery (n=1), common origin of brachiocephalic artery and left CCA (n=3). Of these, three patients had proven DAo plaque resulting in a total 26% (9 of 35) cryptogenic stroke patients with DAo plaques, flow reversal and matching cerebral infarct territory. Interestingly, no patients were reclassified from concordant to discordant vascular supply to the infarcted territory based on variations in cerebrovascular anatomy.
DISCUSSION
The findings in this study of 35 patients with cryptogenic stroke demonstrated 26% concordance with cerebral infarct location with retrograde diastolic flow into the supplying vascular territory, identifying a potential etiology for cryptogenic stroke. Our findings further document the importance of taking into account variant anatomy of the circle of Willis, posterior circulation, and aortic arch in individual patients, which identified a vascular pathway connecting retrograde embolization to the great vessel origins with infarct location in an additional 14% of subjects.
Accurate identification of the underlying source of stroke is important to select the most optimal therapy to prevent recurrent strokes. It is well-understood that aortic stiffening can lead to atherosclerosis and subsequently the development of complex aortic plaques, which act as potential sources for embolic stroke. Complex plaques in the descending aorta, where they occur most frequently8, were traditionally not considered a source of stroke since embolization would require diastolic reversal (upward) of flow from the DAo plaque into the brain supplying supra-aortic vessels to establish a mechanism7,9.
The findings of our study confirm recent studies20,24,25 which have provided evidence that DAo flow reversal is a common epiphenomenon, even in the absence of aortic valve insufficiency. For example, a recent Doppler echocardiography study in 296 hypertension patients demonstrated that aortic stiffening causes aortic flow reversal, which was found in all subjects (reverse/forward flow ratio, 35±10%) and was positively correlated with parameters of aortic stiffness such as pulse wave velocity, independent of age, aortic diameter, and aortic pressure24.
Moreover, in two 4D flow MRI studies by Harloff et al., it has been shown that retrograde flow from complex DAo plaques can constitute a potential mechanism for embolization to all cerebrovascular territories19,20. This mechanism constituted the only probable source of retinal or cerebral infarction in 24% of patients with cryptogenic stroke. It should be noted that the prevalence of aortic plaques (14%–21%) is similar compared to the other two important causes of embolic stroke, carotid artery disease (10%–13%) and atrial fibrillation (18%–30%)7. These findings indicate that diastolic DAo reverse flow could be a frequent mechanism for otherwise cryptogenic stroke.
A number of previous studies have shown that variants in cerebrovascular are a common fining in the general population26–28. For example, A study of 300 CT angiograms of the circle of Willis (300 studies) and review of the literature27 reported the absence of the anterior communicating artery in 5% of subjects, similar to findings in our study cohort (6%). Hypoplastic posterior communication arteries and vertebral arteries were found in a large fraction of subjects (>75%) which supports our findings of frequently absent posterior communication arteries and vertebral arteries on MR angiography. Another autopsy study in 50 adult brains found that a majority of the Circles of Willis (52%) showed anomalies28 which was comparable to our study cohort.
A recent meta-analysis29 demonstrated that different classes of medications commonly used as first-line anti-hypertensive therapies (e.g. ACE-inhibitors, diuretics) can have very divergent effects on aortic stiffness. While all drugs can successfully control blood pressure and reduce stroke risk overall, ACE inhibitors were shown to affect aortic stiffness most uniformly. Conversely, many drugs in the class of diuretics have a neutral effect on aortic stiffness. This could provide the opportunity to utilize approved drugs for aortic destiffening therapy and thus reduction of aortic flow reversal as a medical therapy for acute stroke patients with a plausible retrograde embolization mechanism. Additional studies are warranted to test the impact of different medical therapies on aortic stiffness, flow reversal, and potential to reduce recurrent events in patients with DAo aortic plaques.
Study Limitations
Important limitations include the small number of patients with cryptogenic stroke with attendant biases. Nevertheless, our findings are in line with previous studies and confirm that retrograde aortic embolization based on diastolic DAo flow reversal to the great vessel origin matching a cerebral infarct territory is frequently found in patients with cryptogenic stroke and can be even more prevalent if the individual cerebrovascular anatomic variation is considered.
’A limitation of our strategy to identify risk for retrograde embolization includes that this approach can only identify associations and cannot determine causality. In addition, the 3D visualization of flow reversal into the supra-aortic branches can provide insights into which brain region maybe at risk for embolism but this approach cannot identify individual flow pathways towards a certain infarct territory. For example, reverse flow from the descending aorta to the brachiocephalic trunk, i.e. also bypassing the left common carotid artery and left subclavian artery outlets, is be associated with embolic risk for all brain territories. Further the brachiocephalic trunk supplies multiple cerebral regions (via right vertebral artery, right common carotid artery and right subclavian artery) and a direct relationship between flow reversal and variable pathways that could cause embolism cannot be derived from our current analysis technique. As a result, the reported connection between descending aorta plaque and great vessel origin in our study can only identify ‘possible’ infarcts when the supplying vessel was connected with retrograde flow.
Another drawback of our study is related to the semi-quantitative analysis of DAo flow reversal. Our study employed traditional pathlines to visualize reverse flow to individual supra-aortic branches and was thus based on a visual assessment of flow trajectories in the aorta. In addition, our analysis did not directly quantify the amount of reverse flow in the supra-aortic arteries. As a result, potential retrograde embolization was considered present if retrograde flow was observed and plaques were present in the descending aorta 10–20mm distal to the left subclavian artery. Future studies should 1) employ quantitative methods to calculate the lengths of flow trajectories and 2) emission of pathlines at the exact location of the descending aortic plaque. A potential solution might be offered by backtracking of flow pathways, i.e. placing emitter planes at the supra-aortic outlets and tracking flow pathways backwards in the cardiac cycle (from late to early diastole) to find the DAo origin of reverse flow.
’A further drawback of our study is the lack of a dedicated MR imaging technique for plque detection, which was based on standard MRI sequences (T1-weighted fat suppressed axial and coronal imaging, contrast-enhanced 3D MRA). It should be noted that the method of plaque imaging is crucial for detection of retrograde embolization as atherosclerosis of the descending aorta is very common and may be an innocent bystander and not related to stroke unless complex and vulnerable plaques are present.
Further, the patients included in this study did present with additional risk factors such as smoking history or hypertension. However, we did not separately assess the role of these factors compared to diastolic retrograde flow or control for these systemic risk factors in our analysis. Future studies should thus include larger cohorts to enable a more comprehensive analysis based on multiple regression to identify independent predictors of cryptogenic events.
Conclusions
The findings in this study demonstrated that diastolic flow reversal in the descending aorta was frequently associated with matching embolic stroke patterns in otherwise cryptogenic stroke patients. Taking into account the individual anatomy of the Circle of Willis architecture, posterior circulation, and aortic arch resulted in the identification of additional stroke patients in whom retrograde aortic embolization was a plausible stroke mechanism. Future longitudinal studies in larger cohorts are needed to investigate this mechanism further including determining recurrent stroke risk and long-term clinical outcomes.
KEY POINTS.
Retrograde embolization from descending aortic plaques constitutes a plausible etiology in cryptogenic stroke.
Common variants of cerebrovascular anatomy are important to determine retrograde embolization mechanism.
Variant cerebrovascular anatomy can link retrograde flow pathways with vascular stroke territory.
Acknowledgments
Funding:
This study has received funding by NIH NHLBI grant R21 HL132357.
Footnotes
Guarantor:
The scientific guarantor of this publication is Michael Markl.
Conflict of interest:
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry:
No complex statistical methods were necessary for this paper.
Ethical approval:
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap:
Written informed consent was waived by the Institutional Review Board.
- retrospective
- cross sectional study
- performed at one institution
References
- 1.Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Ischemic stroke subtypes: a population-based study of incidence and risk factors. Stroke. 1999;30:2513–2516. doi: 10.1161/01.str.30.12.2513. [DOI] [PubMed] [Google Scholar]
- 2.Guercini F, Acciarresi M, Agnelli G, Paciaroni M. Cryptogenic stroke: time to determine aetiology. J Thromb Haemost. 2008;6:549–554. doi: 10.1111/j.1538-7836.2008.02903.x. [DOI] [PubMed] [Google Scholar]
- 3.Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke. 2001;32:2735–2740. doi: 10.1161/hs1201.100209. [DOI] [PubMed] [Google Scholar]
- 4.Petty GW, Brown RD, Jr, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO. Ischemic stroke subtypes : a population-based study of functional outcome, survival, and recurrence. Stroke. 2000;31:1062–1068. doi: 10.1161/01.str.31.5.1062. [DOI] [PubMed] [Google Scholar]
- 5.Mohr JP, Thompson JL, Lazar RM, Levin B, Sacco RL, Furie KL, Kistler JP, Albers GW, Pettigrew LC, Adams HP, Jr, Jackson CM, Pullicino P. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001;345:1444–1451. doi: 10.1056/NEJMoa011258. [DOI] [PubMed] [Google Scholar]
- 6.Bang OY, Lee PH, Joo SY, Lee JS, Joo IS, Huh K. Frequency and mechanisms of stroke recurrence after cryptogenic stroke. Ann Neurol. 2003;54:227–234. doi: 10.1002/ana.10644. [DOI] [PubMed] [Google Scholar]
- 7.Kronzon I, Tunick PA. Aortic atherosclerotic disease and stroke. Circulation. 2006;114:63–75. doi: 10.1161/CIRCULATIONAHA.105.593418. [DOI] [PubMed] [Google Scholar]
- 8.Amarenco P, Cohen A, Tzourio C, Bertrand B, Hommel M, Besson G, Chauvel C, Touboul PJ, Bousser MG. Atherosclerotic disease of the aortic arch and the risk of ischemic stroke. N Engl J Med. 1994;331:1474–1479. doi: 10.1056/NEJM199412013312202. [DOI] [PubMed] [Google Scholar]
- 9.Reimold SC, Maier SE, Aggarwal K, Fleischmann KE, Piwnica-Worms D, Kikinis R, Lee RT. Aortic flow velocity patterns in chronic aortic regurgitation: implications for Doppler echocardiography. J Am Soc Echocardiogr. 1996;9:675–683. doi: 10.1016/s0894-7317(96)90064-4. [DOI] [PubMed] [Google Scholar]
- 10.Bogren HG, Mohiaddin RH, Kilner PJ, Jimenez-Borreguero LJ, Yang GZ, Firmin DN. Blood flow patterns in the thoracic aorta studied with three-directional MR velocity mapping: the effects of age and coronary artery disease. J Magn Reson Imaging. 1997;7:784–793. doi: 10.1002/jmri.1880070504. [DOI] [PubMed] [Google Scholar]
- 11.Bogren HG, Buonocore MH, Valente RJ. Four-dimensional magnetic resonance velocity mapping of blood flow patterns in the aorta in patients with atherosclerotic coronary artery disease compared to age-matched normal subjects. J Magn Reson Imaging. 2004;19:417–427. doi: 10.1002/jmri.20018. [DOI] [PubMed] [Google Scholar]
- 12.Harloff A, Strecker C, Frydrychowicz AP, Dudler P, Hetzel A, Geibel A, Kollum M, Weiller C, Hennig J, Markl M. Plaques in the descending aorta: A new risk factor for stroke? Visualization of potential embolization pathways by 4D MRI. J Magn Reson Imaging. 2007;26:1651–1655. doi: 10.1002/jmri.21126. [DOI] [PubMed] [Google Scholar]
- 13.Ebbers T. Flow imaging: cardiac applications of 3D cine phase-contrast MRI. Current Cardiovascular Imaging Reports. 2011;4:127–133. [Google Scholar]
- 14.Markl M, Kilner PJ, Ebbers T. Comprehensive 4D velocity mapping of the heart and great vessels by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2011;13:7. doi: 10.1186/1532-429X-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frydrychowicz A, Francois CJ, Turski PA. Four-dimensional phase contrast magnetic resonance angiography: Potential clinical applications. Eur J Radiol. 2011 doi: 10.1016/j.ejrad.2011.01.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Markl M, Frydrychowicz A, Kozerke S, Hope M, Wieben O. 4D flow MRI. J Magn Reson Imaging. 2012;36:1015–1036. doi: 10.1002/jmri.23632. [DOI] [PubMed] [Google Scholar]
- 17.Hope MD, Sedlic T, Dyverfeldt P. Cardiothoracic magnetic resonance flow imaging. J Thorac Imaging. 2013;28:217–230. doi: 10.1097/RTI.0b013e31829192a1. [DOI] [PubMed] [Google Scholar]
- 18.Stankovic Z, Allen BD, Garcia J, Jarvis KB, Markl M. 4D flow imaging with MRI. Cardiovasc Diagn Ther. 2014;4:173–192. doi: 10.3978/j.issn.2223-3652.2014.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harloff A, Strecker C, Dudler P, Nussbaumer A, Frydrychowicz A, Olschewski M, Bock J, Stalder AF, Stroh AL, Weiller C, Hennig J, Markl M. Retrograde embolism from the descending aorta: visualization by multidirectional 3D velocity mapping in cryptogenic stroke. Stroke. 2009;40:1505–1508. doi: 10.1161/STROKEAHA.108.530030. [DOI] [PubMed] [Google Scholar]
- 20.Harloff A, Simon J, Brendecke S, Assefa D, Helbing T, Frydrychowicz A, Weber J, Olschewski M, Strecker C, Hennig J, Weiller C, Markl M. Complex plaques in the proximal descending aorta: an underestimated embolic source of stroke. Stroke. 2010;41:1145–1150. doi: 10.1161/STROKEAHA.109.577775. [DOI] [PubMed] [Google Scholar]
- 21.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE., 3rd Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 22.Markl M, Harloff A, Bley TA, Zaitsev M, Jung B, Weigang E, Langer M, Hennig J, Frydrychowicz A. Time-resolved 3D MR velocity mapping at 3T: Improved navigator-gated assessment of vascular anatomy and blood flow. Journal of Magnetic Resonance Imaging. 2007;25:824–831. doi: 10.1002/jmri.20871. [DOI] [PubMed] [Google Scholar]
- 23.Bock J, Kreher B, Hennig J, Markl M. Optimized pre-processing of time-resolved 2D and 3D phase contrast MRI data. Proceedings of the 15th Annual Meeting of ISMRM; Berlin, Germany. 2007. p. 3138. [Google Scholar]
- 24.Hashimoto J, Ito S. Aortic stiffness determines diastolic blood flow reversal in the descending thoracic aorta: potential implication for retrograde embolic stroke in hypertension. Hypertension. 2013;62:542–549. doi: 10.1161/HYPERTENSIONAHA.113.01318. [DOI] [PubMed] [Google Scholar]
- 25.Wehrum T, Kams M, Strecker C, Dragonu I, Gunther F, Geibel A, Drexl J, Hennemuth A, Schumacher M, Jung B, Harloff A. Prevalence of potential retrograde embolization pathways in the proximal descending aorta in stroke patients and controls. Cerebrovasc Dis. 2014;38:410–417. doi: 10.1159/000369001. [DOI] [PubMed] [Google Scholar]
- 26.Bell R, Severson MA, 3rd, Armonda RA. Neurovascular anatomy: a practical guide. Neurosurgery clinics of North America. 2009;20:265–278. doi: 10.1016/j.nec.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Dimmick SJ, Faulder KC. Normal variants of the cerebral circulation at multidetector CT angiography. Radiographics : a review publication of the Radiological Society of North America, Inc. 2009;29:1027–1043. doi: 10.1148/rg.294085730. [DOI] [PubMed] [Google Scholar]
- 28.Iqbal S. A comprehensive study of the anatomical variations of the circle of willis in adult human brains. Journal of clinical and diagnostic research : JCDR. 2013;7:2423–2427. doi: 10.7860/JCDR/2013/6580.3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janic M, Lunder M, Sabovic M. Arterial stiffness and cardiovascular therapy. Biomed Res Int. 2014;2014:621437. doi: 10.1155/2014/621437. [DOI] [PMC free article] [PubMed] [Google Scholar]


