1. Introduction
The tissue graft experiments by Peter Medawar launched the basic principles of ocular immune privilege in the 1940s, with the eye being one of the special organs able to tolerate foreign antigens without rejecting the donor tissue. The eye's unique ability to manage foreign antigen exposure without mounting an inflammatory response likely evolved from the ocular surface's constant contact with the environment, the recruitment of immune cells via the dense retinal vasculature and the evolutionary pressure to preserve vision as an essential primary sense. To provide visual function, the cornea, aqueous humor, vitreous humor and the fovea are avascular in order to undergo phototransduction and convert photons into electric signals for transmission to the visual cortex in the brain. The necessity of protecting the delicate visual axis is underscored by the fact that approximately 50% of the cerebral cortex function is dedicated to visual analysis and perception [1]. Hence, a case can be made that innate, adaptive and reparative immune responses in the visual axis are among the most highly evolved. Sight threatening inflammation could be triggered by routine exposure to microbes, pathogens, irritants, neutrophils (PMN) of nocturnal tears [2] at the ocular surface, or through aberrant immune responses resulting from injury or diseases in the neural retina. However, the transparent visual axis has evolved sophisticated mechanisms to tightly control inflammation, raise the threshold for triggering immune responses, and maintain active immune tolerance. Experiments from the past few decades have established immune privilege as active surveillance where immunosuppression and regulatory cells are actively deployed to avert full-fledged innate or adaptive immune responses [3, 4]. In addition, the eye effectively minimizes immune responses through physical barriers at two sites, the corneal epithelium and the blood retinal barrier. In the eye, specialized pro-resolving mediators (SPM) lipoxin A4 (LXA4) and neuroprotectin D1 (NPD1/PD1) were first identified as endogenous lipid mediators in the immune privileged cornea [5]. Ongoing studies have identified SPM as essential endogenous factors for maintaining immune homeostasis in the visual axis, uphold an elevated basal anti-inflammatory tone, amplify wound healing, control leukocyte functional responses, downregulate inflammatory responses and drive nerve regeneration and neuroprotection [6, 7, 8, 9].
Photoreceptors have the highest DHA content and retention in comparison to all cells in the body [10], providing an abundant substrate for 5- and 15- lipoxygenase (5-, 15-LOX) to potentially generate most of the ω-3 SPM resolvins, protectins and maresins in the retina. Several lines of evidence have established protection against macular degeneration and retinopathy with DHA-enriched diets [11, 12], which underscore the importance of endogenous levels of DHA-derived SPM in the eye. SPM circuits have provided new targets to combat dysregulated ocular innate and adaptive immune responses as current treatment option are limited and aimed at broad immune suppression. Treatment with resolvin E1 (RvE1), RvE1 analogs, LXA4, NPD1/PD1 and resolvin D1 (RvD1) in murine and in vitro models of ocular diseases have established the efficacy of SPM as potential treatment options to limit inflammatory pathogenesis in the cornea and conjunctiva, prevent adaptive immune responses and protect against retinopathy due to injury or oxidative stress.
5- and 15-LOX are the rate-limiting enzymes responsible for generating SPM, especially the eicosanoid LXA4, which was the first identified SPM [13] and is generated in many tissues of the eye. A striking feature of the cornea is the high expression of 15-LOX in human and mouse corneal epithelial cells. 15-LOX-LXA4-ALX/FPR2 circuit has been identified as an important resident circuit that controls wound healing and immune responses at the ocular surface. Two 15-LOX enzymes (ALOX15, ALOX15B) have been identified in the human cornea [14, 15, 16] and the mouse homolog 12/15-LOX (Alox15) is expressed in the corneal epithelium, retinal pigment epithelium (RPE) and lens [17, 18]. Knockdown of 15-LOX (ALOX15) in the human RPE increased susceptibility to oxidative stress induced apoptosis [19]. More importantly, 12/15-LOX knockout mice exhibit a phenotype of impaired LXA4 formation and induction of cytoprotective heme oxygenase-1 (HO-1), which correlated with exacerbated inflammation and delayed wound healing [20, 21]. 5- and 15-LOX dependent formation of LXA4 and/or NPD1 has been established in the cornea, retina and ocular draining lymph nodes and 15-LOX has a key role in preventing RPE cell death during oxidative stress and pathological angiogenesis in the avascular cornea [8, 18].
SPM exert their actions through G-protein coupled receptors (GPCR) that are widely expressed in the eye. ALX (also called FPR2), the GPCR for LXA4 and its DHA-homolog RvD1, is expressed in the corneal epithelium, RPE, PMN, macrophages, T cells [22, 23, 24] and conjunctiva [25]. In humans, GPR32 has been identified as a second receptor for LXA4 and RvD1 [26] and its expression has been established in conjunctival goblet cells [27] and human retinal endothelial cells [28].
Emerging evidence has identified endogenous SPM circuits in the ocular surface, retina and draining lymph nodes of the eye. These intrinsic protective circuits have key roles in immune regulation, injury responses, infection, inflammatory neovascularization, neuropathy and autoimmunity. The concept of SPM as promising therapeutic targets has been validated in pre-clinical animal models of ocular diseases and early clinical trials with RvE1 analogs in Dry Eye Disease (DED), which have established the efficacy and broad protective action of local and systemic treatment with SPM [29, 30, 31]. This review aims to provide an overview of the endogenous role of SPM in ocular trauma and diseases (Figure 1).
Figure 1.
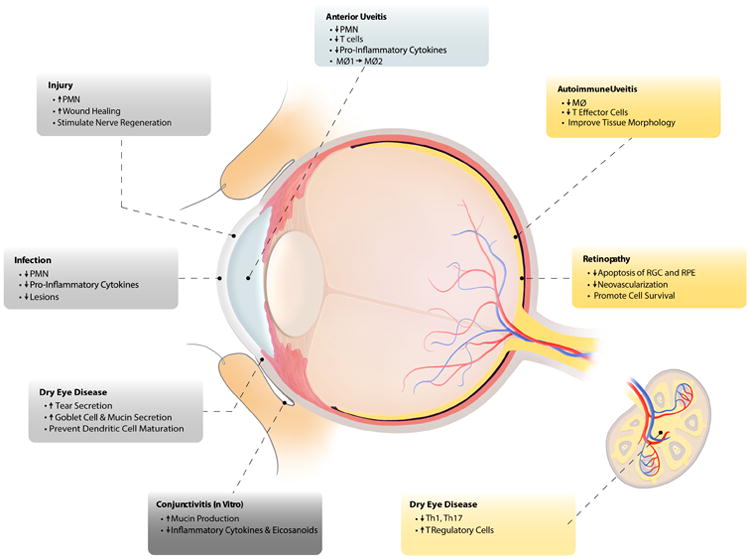
The anti-inflammatory bioactions of SPM in ocular tissues and draining lymph nodes. MØ= macrophages, Th1= T helper 1 cells, Th17= T helper 17 cells, PMN= polymorphonuclear cells, RGC= retinal ganglion cells, RPE= retinal pigmented epithelium.
2. Specialized Pro-resolving Mediators in Ocular Diseases
2.1 Corneal Injury and Wound Healing
The cornea is predominantly composed of the corneal epithelium, the stroma and a monolayer of corneal endothelium lining the inner surface. The corneal epithelium maintains tight junctions that prevent microbes, viruses and irritants from penetrating the cornea. The essential role of SPM circuits in the cornea is supported by the high expression of 12/15-LOX circuit in the corneal epithelium [14- 16], and both human and mouse corneal epithelial cells can generate LXA4. Mouse models of acute corneal injury have demonstrated that loss of epithelial cells or genetic deletion of 12/15-LOX abrogated LXA4 formation in the cornea and led to impaired epithelial wound healing, while topical treatment with LXA4, NPD1 as well as 17-HDHA, a metabolic precursor for several members of the DHA-resolvin family, accelerated re-epithelialization in acute corneal abrasions [5, 8].
Contrary to LXA4's established role of inhibiting PMN recruitment in various disease models, both topically administered and endogenously formed LXA4 and NPD1 increase PMN numbers after epithelial injury in the cornea [5]. The requirement of PMN for normal corneal wound healing and nerve regeneration in acute and self-resolving epithelial injury responses is well established [32, 33]. Multiple lines of evidence also suggest that these wound healing PMN as well as nocturnal tear PMN are a non-inflammatory phenotype [2] that is distinct from stereotypical vascular inflammatory PMN. Unlike LXA4's endogenous role in healthy and self-resolving corneal inflammatory-reparative responses, topically added or endogenous LXA4 in chronic or infectious injuries inhibit recruitment of inflammatory PMN, formation of inflammatory cytokines and chemokines, platelet activating factor and pathological angiogenesis [20, 34, 35, 36]. The protective actions of the 15-LOX-LXA4 circuit in the cornea are in part mediated by the cytoprotective hemeoxygenase-1 (HO-1) system. In a positive feedback mechanism, HO-1 expression and 15-LOX activity depend on one another to maintain an elevated anti-inflammatory tone that raises the threshold for triggering full inflammatory responses in the immune privileged cornea (20, 21). In view of endogenous formation of several SPM pathway markers and expression of their receptors (ALX, ChemR23), it is likely that in addition to NPD1 and LXA4, other SPM are formed in the ocular surface and have roles in executing healthy inflammatory and reparative responses in the cornea. A notion that is supported by a recent lipidomic analysis that document the presence of SPM in human tears, namely RvD1, RvD2, RvD5, NPD1, LXA4 and aspirin-triggered LXA4 [37].
The cause for sex-specific differences in innate and adaptive immune responses and the striking higher prevalence of autoimmune disease in females compared to males is largely unknown. A 2011 post-hoc analysis of a prospective clinical trial identified for the first time a marked sex-specific difference in the outcome of wound healing in the cornea. Namely, after correcting for confounding factors, corneal wounds in men healed two times faster than in women [38]. Employing the mouse model of self-resolving corneal injury, it was discovered that estrogen regulates the 15-LOX-LXA4-ALX circuit via the epithelial ERβ receptor [39]. Estrogen downregulated 15-LOX and ALX expression and LXA4 formation in the cornea that resulted in a female-specific phenotype of delayed re-epithelialization, reduced recruitment of wound healing PMN and an amplified inflammatory response; a phenotype that can be reversed by LXA4 treatment [39]. This study identified regulation of SPM circuits by estrogen as a potential factor in the etiology or pathogenesis of sex-specific ocular surface inflammatory diseases. Recent lipidomic analysis of human tears demonstrated that women have lower SPM levels than men [37], emphasizing the clinical relevance of animal models that have identified sex-specific regulation of SPM circuits.
The cornea is the most innervated tissue in the human body, and corneal innervation serves a vital role in maintaining homeostasis and normal injury responses. In a lamellar keratectomy model that severs corneal nerves, NPD1 treatment markedly increased nerve regeneration [40, 41, 42], a bioaction that is consistent with the originally defined role of NPD1 as a neuroprotective SPM in the retina and in mouse models of stroke [6, 43].
Several SPM have been assessed as treatment options for corneal diseases in pre-clinical animal models of infection, graft survival, inflammatory angiogenesis and keratectomy. Both NPD1 and RvE1 treatment after herpes simplex virus infection decreased pro-inflammatory cytokines, virus-induced lesions and angiogenesis [44, 45], while RvE1 and LXA4 also reduced the severity of LPS-induced stromal keratitis [20, 46]. In a mouse model of corneal transplantation RvD1 treatment prevented dendritic cell maturation by downregulating MHCII, CD40 and IL-12 expression on dendritic cells, which led to suppressed alloimmunity and enhanced graft survival [47]. Local or topical treatment with LXA4, LXA4 analogs, RvD1 or RvE1 markedly reduced pathological angiogenesis by inhibiting VEGF, VEGFR2, VEGFR3, TNFα and IL1α/IL1β [34, 48]. In a rabbit model of keratectomy, the RvE1 analog RX-10045 reduced corneal opacity after photorefractive keratectomy [49]. These in vivo studies demonstrated that consistent with established bioactions of SPM in other organ systems, SPM are promising drug targets for corneal diseases, infections and graft survival. However, regulation of SPM circuits and sex-specific differences in corneal health and diseases and cellular mechanisms of action still remain to be defined.
2.2 Conjunctivitis
The conjunctiva is a tissue composed of stratified epithelium, goblet cells, resident mast cell, lymphocytes and antigen presenting cells that covers the inner eyelids and anterior sclera. As a result of being highly vascularized, the conjunctiva is responsible for immune surveillance and cell trafficking of the ocular surface but has been largely ignored as the primary tissue for initiating ocular surface immune responses. Goblet cells serve as a physical fortification by producing mucin to keep the eye lubricated and to clear microbes and irritants. Conjunctivitis is a common ocular surface disease caused by viral or bacterial infection, allergies or autoimmune diseases. SPM actions and signaling mechanisms have been studied at length in vitro using isolated human or rat goblet cells. In vitro models that recapitulate goblet cell responses in DED and allergic conjunctivitis have demonstrated that RvD1 and RvE1 can block inflammatory eicosanoids or histamine activation of ERK and intracellular Ca2+ mobilization in goblet cells [27, 50]. LXA4 has unique actions on isolated conjunctival goblet cells as it can stimulate mucin secretion via activation of phospholipases, ERK and downstream protein kinases [51]. The in vivo expression, endogenous formation, and therapeutic actions of SPM have not been determined in the healthy or inflamed conjunctiva or animal models of conjunctivitis. However, in vitro data with goblet cells and mouse models of inflammation in other mucosal tissues (i.e. lung and colon) have established the protective therapeutic actions of SPM, inferring that treatment with SPM in general should attenuate the pathogenesis of conjunctivitis.
2.3 Retinopathy
SPM's endogenous protective roles extend beyond the ocular surface to the posterior segment of the eye, especially the retina. The neural retina is composed of densely packed photoreceptors and neurons responsible for converting light to electrical signals that are transmitted to the visual cortex, where they are decoded and interpreted to provide visual perception. Unlike the self-renewal capabilities of the corneal epithelium, cells in the retina are not equipped with regenerative mechanisms thus damage to the retina could lead to irreversible vision loss. The RPE is comprised of a monolayer of highly specialized cells that are vital in maintaining the health of retina. The RPE phagocytoses the outer segments of the photoreceptors, facilitates the visual cycle, upholds the blood-retinal barrier and expresses immunosuppressive molecules. Injury to RPE or dysregulation of homeostatic function of RPE is a key factor in the etiology of retinopathy.
NPD1 is of particular interest in retinopathy due to highly enriched levels of DHA in the retina and the pathway for NPD1 formation only requires 15-LOX. A large body of work has shown that NPD1 promotes cell survival of human RPE cells upon exposure to oxidative stress and that 15-LOX is the rate-limiting enzyme for endogenous formation [52, 53, 54]. Stereospecific binding sites for NPD1 have been identified in RPE cells and PMN [55] but the receptor remains to be identified. NPD1 mediates protection against RPE apoptosis in an autocrine fashion, suggesting a critical role for the 15-LOX-NPD1 circuit in maintaining RPE homeostasis [19]. The protective and anti-apoptotic bioactivity of RPE-released NPD1 are mediated by upregulation of anti-apoptotic Bcl-2 and Bcl-xL, as well as reduction of pro-apoptotic Bax expression and caspase-3 activation [53, 54, 56]. In addition, NPD1 promotes RPE cell survival against oxidative stress by increased phosphorylation of PI3K/Akt and mTOR/p70S6K signaling pathways [57, 58].
In addition to maintaining RPE homeostasis, NPD1 also inhibits inflammatory responses in the retina by exerting direct neuroprotective actions. In laser-induced choroidal neovascularization (CNV) that recapitulates macular degeneration, NPD1 attenuated neovascularization and shifted microglia toward a non-injury inducing phenotype in vivo [59, 60]. NPD1 also has direct actions on neuron cell survival and apoptosis. Retinal ganglion cells are neurons that relay action potentials from photoreceptors through the optic nerve, making cells along this path indispensable for processing visual information. Treatment with NPD1 after optical nerve transection in rats markedly delayed retinal ganglion cell death [61]. In vitro data has demonstrated endogenous formation of NPD1 by cone photoreceptor cell lines [62] indicating that NPD1 formation in the retina is likely not restricted to RPE cells.
Inflammation is now recognized as a prominent feature of many retinal diseases and the role of SPM in resolution of inflammation has sparked research efforts to explore their therapeutic potential in retinopathies. A leading cause of blindness is diabetic retinopathy triggered by glucose-induced inflammation of the choroid and/or the retina, characterized by dysfunction and/or abnormal growth of retinal blood vessels. Inflammatory cells are subsequently recruited and facilitate further destruction. In vitro experiments have established that RvE1and RvD1 are formed during retinal endothelial-PMN interactions and inhibit PMN transmigration and pro-inflammatory cytokines production [63]. A potential role of SPM circuits in human diabetic retinopathy is underscored by marked reduction of LXA4 in the serum and vitreous humor with a concurrent increase of IL-6 in patients with diabetic retinopathy [64]. RvD1 formation has also been demonstrated in human endothelial cells in an in vitro model of diabetic retinopathy. Endothelial formation of RvD1 is reduced in high glucose conditions and restored to normal by vasoactive intestinal peptide, suggesting that RvD1 has a role in maintaining retinal endothelial cell homeostasis [28]. A preclinical model of retinopathy of prematurity emphasizes the important role of enzymes in the formation of SPM circuits. Dietary DHA is protective in this model but inhibition of pathogenic angiogenesis by the dietary supplement required 5-LOX [65]. It is important to recognize that 5-LOX is the key enzyme for amplifying and initiating inflammation but is also the rate-limiting and required enzyme for formation of the majority of SPM. Potential roles of SPM circuits in controlling innate immune responses in the retina are suggested by in vitro experiments that demonstrated LXA4's ability to inhibit LPS induced IL-6 production in human RPE cells [64].
The retina has the highest metabolic rate, oxygen demand and DHA content of any tissue and thus evolved robust mechanisms to prevent photoreceptor and neuronal cell death. NPD1 was the first identified in vivo retinal SPM [61] and its neuroprotective actions in the retina and central nervous system after injury and oxidative stress are well established [6, 7]. Emerging evidence has identified formation and bioactions of other SPM in the retina such as RvD1 and LXA4. However, the endogenous roles of SPM in neuroprotection, retinopathy or maintaining homeostasis still remain to be explored.
2.4 Endotoxin-Induced Anterior Uveitis
Uveitis is characterized by inflammation of the uveal layer, which includes the iris, ciliary body and the choroid. Inflammation of the middle layer of the eye can be further categorized into anterior, posterior or pan uveitis depending on the tissues affected. Anterior uveitis is mediated by PMN, macrophage and T cell infiltration, modeled by endotoxin induced uveitis (EIU) in rats and is primarily an acute and self-resolving inflammatory response. Consistent with the anti-inflammatory and pro-resolving actions of SPM, treatment with LXA4 or LXA4 analogs reduced the clinical inflammation score, PMN cell counts and protein levels in the aqueous humor in rats with EIU [66]. RvD1, the structural DHA homolog of LXA4, mediates its action via the same shared receptors. Consistent with LXA4's action in EIU, tail vein injection of RvD1 upon LPS-induced uveitis led to a dose-dependent reduction of MPO activity along with PMN and T cell infiltration into the eye [67], while intravitreal injection of RvD1 decreased TNFa, chemokine MIP1-a, ubiquitin proteasome [66] and caspase 3 [68]. Macrophage profiles also shifted from the M1 phenotype to high expression of M2 upon RvD1 administration [69]. Endogenous roles for LXA4 or RvD1 in EIU have not been reported. However, the established therapeutic actions of SPM in many inflammatory diseases models and the marked inhibition of inflammation with LXA4 and RvD1 treatment in uveitis models provide strong evidence for SPM as promising treatment options for anterior uveitis.
2.5 Autoimmune Diseases: Dry Eye Disease and Posterior Uveitis
The role of eicosanoids, especially SPM, in the etiology of ocular and other autoimmune diseases is relatively unexplored. Autoimmunity occurs when the host immune cells mistakenly recognize and/or triggers T effector cell-driven responses against self-proteins and tissues. Autoimmune DED is a debilitating disease that inflicts working-age adults with unknown triggers. This T cell-driven chronic disease targets the lacrimal gland, goblet cells in the conjunctiva and ocular surface leading to impaired tear formation, inflammation and epithelial defects. DED is multifactorial and affects approximately 4 million people in the United States, and of which 3.2 million are women [70]. The cause for this striking sex-specific prevalence in women is unknown. Current primary treatment options are limited and included the long standing use of corticosteroids, the immunosuppressant cyclosporine A and artificial tears; a new treatment approach of inhibiting lymphocyte integrin/adhesion molecule interaction (LFA-1/ICAM-1) by a small molecule (lifitegrast) received FDA approval in 2016 for treating signs and symptoms of DED. A recent study identified resident LXA4 circuits in the ocular surface and draining lymph nodes of the eye, which demonstrated striking sex-specific differences and established LXA4 as a key regulator of TH1 and TH17 effector cells and T regulatory cells (Treg). In mouse models that recapitulate human T-cell driven DED, a population of regulatory PMN that highly expresses 15-LOX was identified. The regulatory PMN generate LXA4 in the lacrimal grand, lymph nodes and ocular surface. The loss of LXA4 formation in the lymph nodes upon resident regulatory PMN depletion during desiccating stress was female-specific, and caused amplified TH1 and TH17 responses and reduction of Treg. The exacerbated adaptive immune response in females was reversed by LXA4 treatment, which reduced the number of T effector cells and increased Treg in the draining lymph nodes and reduced dry eye pathogenesis [24]. This provided the first evidence that LXA4 and regulatory PMN are a resident circuit in draining lymph nodes and key factors that control TH1, TH17 and Treg. The role the LXA4 circuit is also under investigation in T cell-driven autoimmune uveitis. In a pre-clinical model of posterior uveitis that is induced by immunization with retinal interphotoreceptor retinoid-binding protein (IRBP), LXA4 treatment inhibits T effector cell infiltration and prevents retinopathy [71]. The emerging roles of the LXA4 circuit in draining lymph nodes of the eye warrant further studies to determine if sex-specific regulation of the LXA4 circuit is a factor in the etiology and striking prevalence of autoimmune diseases in women.
The therapeutic potential of amplifying endogenous SPM circuits is underscored by studies that examined the pharmacological effects of SPM treatment in experimental autoimmune DED. RvE1 increased tear production and corneal epithelium density while inhibiting macrophage infiltration in a mouse model of immune DED [72] and an analog of RvE1 applied at the initiation of DED was able to maintain goblet cell density [73]. Based on promising data form pre-clinical animal models synthetic RvE1 analogs were tested in Phase I and Phase II clinical trials for chronic DED and initial results demonstrated significant improvements [74]. New emerging data provides compelling evidence that lipoxins [24] as well as their DHA structural homologs, RvD1 and RvD2 [75] have direct actions with T cells and regulate adaptive immune responses, which warrants further research to define the cellular mechanism of action, signaling pathways and direct T cell-targeted actions.
3. Summary
The anti-inflammatory and pro-resolving therapeutic actions of SPM are well established in many organ systems and pre-clinical inflammatory diseases models. However, there is a clear gap of knowledge regarding SPM's distinct cellular mechanisms of action, their endogenous regulation and role in disease etiology and health. The unique properties of the eye, such as highly conserved primary sense with immune-privileged tissues, neural tissues that are the direct outgrowth of the central nervous system and an exceptional high metabolic rate and DHA content, have uncovered novel bioactions and insights into the endogenous roles of SPM circuits, which extend well beyond their traditional roles of regulating acute inflammation or resolution.
Enzymes for SPM formation are highly expressed in corneal and retinal epithelial cells and tissue resident leukocytes as well as receptors for LXA4, RvD1, NPD1 and RvE1. Studies in the eye have discovered that SPMs have novel intrinsic roles in driving corneal wound healing, preventing RPE and ganglion cell apoptosis, promoting survival of photoreceptors and controlling T effector cell responses in adaptive immune responses. More importantly, endogenous formation of LXA4 and NPD1 in the eye is not limited to the resolution phase of inflammation (Table 1). These SPM circuits have fundamental roles in maintaining healthy homeostasis in the cornea, retina and draining lymph nodes in the absence of acute inflammation. Of particular interest is the sex-specific regulation of the intrinsic LXA4 circuit in the ocular surface and draining lymph nodes, which has emerged as a factor in driving female-specific adaptive immune response and indicate that SPM circuits have direct actions on lymphocytes. In vitro studies and animal models have established the therapeutic potential of SPM on limiting inflammatory ocular diseases. Consistent with inflammatory disease models in other organ systems, SPM circuits are validated therapeutic targets for inflammatory diseases in the eye. However, many questions remain to be answered regarding the regulation of SPM circuits in health, disease and their role or dysregulation in the etiology of autoimmunity, retinopathy and neurodegeneration.
Table 1.
Formation of SPM in ocular tissues, tears and draining lymph node in vivo and in vitro. LXA4= lipoxin A4, NPD1= neuroprotectin D1, RvD1, 2, 5= resolvin D1, resolvin D2, resolvin D5. Grey columns depict in vivo or in vitro formation and references are in brackets.
Acknowledgments
We thank Allison Chan and Kelly Scott for their contributions in creating the artwork for this review. Cited publications from the authors were in part supported by grants from National Institutes of Health (EY022208, EY026082).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nakayama K. Mid-level vision. In: Wilson RA, Keil FC, editors. The MIT encylopedia of the cognitive sciences. Cambridge: MIT Press; 1999. [Google Scholar]
- 2.Gorbet M, et al. The Noninflammatory Phenotype of Neutrophils From the Closed-Eye Environment: A Flow Cytometry Analysis of Receptor Expression. Invest Ophthalmol Vis Sci. 2015;56(8):4582–4591. doi: 10.1167/iovs.14-15750. [DOI] [PubMed] [Google Scholar]
- 3.Stein-Streilein J, Streilein JW. Anterior chamber associated immune deviation (ACAID): regulation, biological relevance, and implications for therapy. Int Rev Immunol. 2002;21(2-3):123–152. doi: 10.1080/08830180212066. [DOI] [PubMed] [Google Scholar]
- 4.de Andrade FA, et al. The autoimmune diseases of the eyes. Autoimmun Rev. 2016;15(3):258–271. doi: 10.1016/j.autrev.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Gronert K, et al. A role for the mouse 12/15-lipoxygenase pathway in promoting epithelial wound healing and host defense. J Biol Chem. 2005;280(15):15267–15278. doi: 10.1074/jbc.M410638200. [DOI] [PubMed] [Google Scholar]
- 6.Bazan NG. Homeostatic regulation of photoreceptor cell integrity: significance of the potent mediator neuroprotectin D1 biosynthesized from docosahexaenoic acid: the Proctor Lecture. Invest Ophthalmol Vis Sci. 2007;48(11):4866–4881. doi: 10.1167/iovs.07-0918. biography 4864-4865. [DOI] [PubMed] [Google Scholar]
- 7.Bazan NG, Calandria JM, Serhan CN. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J Lipid Res. 2010;51(8):2018–2031. doi: 10.1194/jlr.R001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gronert K. Lipoxins in the eye and their role in wound healing. Prostaglandins Leukot Essent Fatty Acids. 2005;73(3-4):221–229. doi: 10.1016/j.plefa.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Kenchegowda S, Bazan HE. Significance of lipid mediators in corneal injury and repair. J Lipid Res. 2010;51(5):879–891. doi: 10.1194/jlr.R001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rice DS, et al. Adiponectin receptor 1 conserves docosahexaenoic acid and promotes photoreceptor cell survival. Nat Commun. 2015;6:6228. doi: 10.1038/ncomms7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connor KM, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tuo J, et al. A high omega-3 fatty acid diet reduces retinal lesions in a murine model of macular degeneration. Am J Pathol. 2009;175:799–807. doi: 10.2353/ajpath.2009.090089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serhan CN, et al. Lipoxins: novel series of biologically active compounds formed from arachidonic acid in human leukocytes. Proc Natl Acad Sci U S A. 1984;81(17):5335–5339. doi: 10.1073/pnas.81.17.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liminga M, Von Malmborg A, Oliw E. Lipoxygenases in human, monkey, and bovine corneal and epithelia. Ann N Y Acad Sci. 1994:744. doi: 10.1111/j.1749-6632.1994.tb52751.x. [DOI] [PubMed] [Google Scholar]
- 15.Brash AR, Boeglin WE, Chang MS. Discovery of a second 15S-lipoxygenase in humans. Proc Natl Acad Sci U S A. 1997;94:6148–6152. doi: 10.1073/pnas.94.12.6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang MS, et al. Detection and subcellular localization of two 15S-lipoxygenases in human cornea. Invest Ophthalmol Vis Sci. 2005;46:849–856. doi: 10.1167/iovs.04-1166. [DOI] [PubMed] [Google Scholar]
- 17.Liclican EL, Gronert K. Molecular circuits of resolution in the eye. ScientificWorldJournal. 2010;10:1029–1047. doi: 10.1100/tsw.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bazan NG. Cellular and molecular events mediated by docosahexaenoic acid-derived neuroprotectin D1 signaling in photoreceptor cell survival and brain protection. Prostaglandins Leukot Essent Fatty Acids. 2009;81(2-3):205–211. doi: 10.1016/j.plefa.2009.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Calandria JM, et al. Selective survival rescue in 15-lipoxygenase-1-deficient retinal pigment epithelial cells by the novel docosahexaenoic acid-derived mediator, neuroprotectin D1. J Biol Chem. 2009;284(26):17877–17882. doi: 10.1074/jbc.M109.003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biteman B, et al. Interdependence of lipoxin A4 and heme-oxygenase in counter-regulating inflammation during corneal wound healing. Faseb j. 2007;21(9):2257–2266. doi: 10.1096/fj.06-7918com. [DOI] [PubMed] [Google Scholar]
- 21.Seta F, et al. Heme oxygenase-2 is a critical determinant for execution of an acute inflammatory and reparative response. Am J Pathol. 2006;169(5):1612–1623. doi: 10.2353/ajpath.2006.060555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gronert K, et al. Identification of a human enterocyte lipoxin A4 receptor that is regulated by interleukin (IL)-13 and interferon gamma and inhibits tumor necrosis factor alphainduced IL-8 release. J Exp Med. 1998;187:1285–1294. doi: 10.1084/jem.187.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiang N, et al. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58(3):463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 24.Gao Y, et al. Female-Specific Downregulation of Tissue Polymorphonuclear Neutrophils Drives Impaired Regulatory T Cell and Amplified Effector T Cell Responses in Autoimmune Dry Eye Disease. J Immunol. 2015;195(7):3086–3099. doi: 10.4049/jimmunol.1500610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodges RR, et al. Lipoxin A4 activates ALX/FPR2 receptor to regulate conjunctival goblet cell secretion. Mucosal Immunol. 2016 doi: 10.1038/mi.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serhan CN, Chiang N. Resolution phase lipid mediators of inflammation: agonists of resolution. Curr Opin Pharmacol. 2013;13(4):632–640. doi: 10.1016/j.coph.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li D, et al. Resolvin D1 and aspirin-triggered resolvin D1 regulate histamine-stimulated conjunctival goblet cell secretion. Mucosal Immunol. 2013;6(6):1119–1130. doi: 10.1038/mi.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi H, et al. VIP protects human retinal microvascular endothelial cells against high glucose-induced increases in TNF-alpha and enhances RvD1. Prostaglandins Other Lipid Mediat. 2016;123:28–32. doi: 10.1016/j.prostaglandins.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He J, Bazan HE. Omega-3 fatty acids in dry eye and corneal nerve regeneration after refractive surgery. Prostaglandins Leukot Essent Fatty Acids. 2010;82(4-6):319–325. doi: 10.1016/j.plefa.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cortina MS, Bazan HE. Docosahexaenoic acid, protectins and dry eye. Curr Opin Clin Nutr Metab Care. 2011;14(2):132–137. doi: 10.1097/MCO.0b013e328342bb1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gronert K. Resolution, the grail for healthy ocular inflammation. Exp Eye Res. 2010;91(4):478–485. doi: 10.1016/j.exer.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Z, Burns AR, Smith CW. Two waves of neutrophil emigration in response to corneal epithelial abrasion: distinct adhesion molecule requirements. Invest Ophthalmol Vis Sci. 2006;47(5):1947–1955. doi: 10.1167/iovs.05-1193. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Burns AR, Smith CW. Lymphocyte function-associated antigen-1-dependent inhibition of corneal wound healing. Am J Pathol. 2006;169(5):1590–1600. doi: 10.2353/ajpath.2006.060415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leedom AJ, et al. Endogenous LXA4 circuits are determinants of pathological angiogenesis in response to chronic injury. Am J Pathol. 2010;176(1):74–84. doi: 10.2353/ajpath.2010.090678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kenchegowda S, et al. EGF stimulates lipoxin A4 synthesis and modulates repair in corneal epithelial cells through ERK and p38 activation. Invest Ophthalmol Vis Sci. 2011;52(5):2240–2249. doi: 10.1167/iovs.10-6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kakazu A, et al. Lipoxin A(4) inhibits platelet-activating factor inflammatory response and stimulates corneal wound healing of injuries that compromise the stroma. Exp Eye Res. 2012;103:9–16. doi: 10.1016/j.exer.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.English JT, et al. Identification and Profiling of Specialized Pro-Resolving Mediators in Human Tears by Lipid Mediator Metabolomics. Prostaglandins, Leukotrienes and Essential Fatty Acids (PLEFA), Volume. 2017;117:17–27. doi: 10.1016/j.plefa.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krishnan T, et al. Gender differences in re-epithelialisation time in fungal corneal ulcers. Br J Ophthalmol. 2012;96(1):137–138. doi: 10.1136/bjophthalmol-2011-300441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang SB, et al. Estrogen negatively regulates epithelial wound healing and protective lipid mediator circuits in the cornea. FASEB J. 2012;26(4):1506–1516. doi: 10.1096/fj.11-198036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cortina MS, et al. Neuroprotectin D1 synthesis and corneal nerve regeneration after experimental surgery and treatment with PEDF plus DHA. Invest Ophthalmol Vis Sci. 2010;51(2):804–810. doi: 10.1167/iovs.09-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cortina MS, et al. Neuroprotectin D1 restores corneal nerve integrity and function after damage from experimental surgery. Invest Ophthalmol Vis Sci. 2013;54(6):4109–4116. doi: 10.1167/iovs.13-12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kenchegowda S, et al. Involvement of pigment epithelium-derived factor, docosahexaenoic acid and neuroprotectin D1 in corneal inflammation and nerve integrity after refractive surgery. Prostaglandins Leukot Essent Fatty Acids. 2013;88(1):27–31. doi: 10.1016/j.plefa.2012.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bazan NG, Calandria JM, Gordon WC. Docosahexaenoic acid and its derivative neuroprotectin D1 display neuroprotective properties in the retina, brain and central nervous system. Nestle Nutr Inst Workshop Ser. 2013;77:121–131. doi: 10.1159/000351395. [DOI] [PubMed] [Google Scholar]
- 44.Rajasagi NK, et al. Controlling herpes simplex virus-induced ocular inflammatory lesions with the lipid-derived mediator resolvin E1. J Immunol. 2011;186(3):1735–1746. doi: 10.4049/jimmunol.1003456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rajasagi NK, et al. Neuroprotectin D1 reduces the severity of herpes simplex virus-induced corneal immunopathology. Invest Ophthalmol Vis Sci. 2013;54(9):6269–6279. doi: 10.1167/iovs.13-12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JE, et al. Inhibition of Corneal Inflammation by the Resolvin E1. Invest Ophthalmol Vis Sci. 2015;56(4):2728–2736. doi: 10.1167/iovs.14-15982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hua J, et al. The resolvin D1 analogue controls maturation of dendritic cells and suppresses alloimmunity in corneal transplantation. Invest Ophthalmol Vis Sci. 2014;55(9):5944–5951. doi: 10.1167/iovs.14-14356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jin Y, et al. Anti-angiogenesis effect of the novel anti-inflammatory and pro-resolving lipid mediators. Invest Ophthalmol Vis Sci. 2009;50(10):4743–4752. doi: 10.1167/iovs.08-2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torricelli AA, et al. Resolvin E1 analog RX-10045 0.1% reduces corneal stromal haze in rabbits when applied topically after PRK. Mol Vis. 2014;20:1710–1716. [PMC free article] [PubMed] [Google Scholar]
- 50.Dartt DA, et al. Conjunctival goblet cell secretion stimulated by leukotrienes is reduced by resolvins D1 and E1 to promote resolution of inflammation. J Immunol. 2011;186(7):4455–4466. doi: 10.4049/jimmunol.1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hodges RR, et al. Lipoxin A4 Counter-regulates Histamine-stimulated Glycoconjugate Secretion in Conjunctival Goblet Cells. Sci Rep. 2016;6:36124. doi: 10.1038/srep36124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mukherjee PK, et al. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004;101(22):8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mukherjee PK, et al. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc Natl Acad Sci U S A. 2007;104(32):13152–13157. doi: 10.1073/pnas.0705949104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calandria JM, et al. Ataxin-1 poly(Q)-induced proteotoxic stress and apoptosis are attenuated in neural cells by docosahexaenoic acid-derived neuroprotectin D1. J Biol Chem. 2012;287(28):23726–23739. doi: 10.1074/jbc.M111.287078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marcheselli VL, et al. Neuroprotectin D1/protectin D1 stereoselective and specific binding with human retinal pigment epithelial cells and neutrophils. Prostaglandins Leukot Essent Fatty Acids. 2010;82(1):27–34. doi: 10.1016/j.plefa.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Antony R, et al. Neuroprotectin D1 induces dephosphorylation of Bcl-xL in a PP2A-dependent manner during oxidative stress and promotes retinal pigment epithelial cell survival. J Biol Chem. 2010;285(24):18301–18308. doi: 10.1074/jbc.M109.095232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Faghiri Z, Bazan NG. PI3K/Akt and mTOR/p70S6K pathways mediate neuroprotectin D1-induced retinal pigment epithelial cell survival during oxidative stress-induced apoptosis. Exp Eye Res. 2010;90(6):718–725. doi: 10.1016/j.exer.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Halapin NA, Bazan NG. NPD1 induction of retinal pigment epithelial cell survival involves PI3K/Akt phosphorylation signaling. Neurochem Res. 2010;35(12):1944–1947. doi: 10.1007/s11064-010-0351-8. [DOI] [PubMed] [Google Scholar]
- 59.Sheets KG, et al. Neuroprotectin D1 attenuates laser-induced choroidal neovascularization in mouse. Mol Vis. 2010;16:320–329. [PMC free article] [PubMed] [Google Scholar]
- 60.Sheets KG, et al. Microglial ramification and redistribution concomitant with the attenuation of choroidal neovascularization by neuroprotectin D1. Mol Vis. 2013;19:1747–1759. [PMC free article] [PubMed] [Google Scholar]
- 61.Qin Q, et al. Neuroprotectin D1 inhibits retinal ganglion cell death following axotomy. Prostaglandins Leukot Essent Fatty Acids. 2008;79(6):201–207. doi: 10.1016/j.plefa.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 62.Kanan Y, et al. Neuroprotectin D1 is synthesized in the cone photoreceptor cell line 661W and elicits protection against light-induced stress. Cell Mol Neurobiol. 2015;35(2):197–204. doi: 10.1007/s10571-014-0111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tian H, et al. Resolvins E1 and D1 in choroid-retinal endothelial cells and leukocytes: biosynthesis and mechanisms of anti-inflammatory actions. Invest Ophthalmol Vis Sci. 2009;50(8):3613–3620. doi: 10.1167/iovs.08-3146. [DOI] [PubMed] [Google Scholar]
- 64.Kaviarasan K, et al. Low blood and vitreal BDNF, LXA4 and altered Th1/Th2 cytokine balance are potential risk factors for diabetic retinopathy. Metabolism. 2015;64(9):958–966. doi: 10.1016/j.metabol.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 65.Sapieha P, et al. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of omega-3 polyunsaturated fatty acids. Sci Transl Med. 2011;3(69):69ra12. doi: 10.1126/scitranslmed.3001571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karim MJ, et al. Anti-inflammatory effects of lipoxins on lipopolysaccharide-induced uveitis in rats. J Ocul Pharmacol Ther. 2009;25(6):483–486. doi: 10.1089/jop.2008.0134. [DOI] [PubMed] [Google Scholar]
- 67.Settimio R, et al. Resolvin D1 reduces the immunoinflammatory response of the rat eye following uveitis. Mediators Inflamm. 2012;2012:318621. doi: 10.1155/2012/318621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rossi S, et al. Interplay between Intravitreal RvD1 and Local Endogenous Sirtuin-1 in the Protection from Endotoxin-Induced Uveitis in Rats. Mediators Inflamm. 2015;2015:126408. doi: 10.1155/2015/126408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rossi S, et al. Protection from endotoxic uveitis by intravitreal Resolvin D1: involvement of lymphocytes, miRNAs, ubiquitin-proteasome, and M1/M2 macrophages. Mediators Inflamm. 2015;2015:149381. doi: 10.1155/2015/149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gayton JL. Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol. 2009;3:405–412. doi: 10.2147/opth.s5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei J, Ly V, Gronert K. Lipoxin A4 Regulates T cell Responses in Experimental Autoimmune Uveitis. Invest Ophthalmol Vis Sci. 2016;57(12):491. 2016. [Google Scholar]
- 72.Li N, et al. Resolvin E1 improves tear production and decreases inflammation in a dry eye mouse model. J Ocul Pharmacol Ther. 2010;26(5):431–439. doi: 10.1089/jop.2010.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.de Paiva CS, et al. Resolvin E1 (RX-10001) reduces corneal epithelial barrier disruption and protects against goblet cell loss in a murine model of dry eye. Cornea. 2012;31(11):1299–1303. doi: 10.1097/ICO.0b013e31823f789e. [DOI] [PubMed] [Google Scholar]
- 74.Hessen M, Akpek EK. Dry eye: an inflammatory ocular disease. J Ophthalmic Vis Res. 2014;9(2):240–250. [PMC free article] [PubMed] [Google Scholar]
- 75.Chiurchiu V, et al. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci Transl Med. 2016;8(353):353ra111. doi: 10.1126/scitranslmed.aaf7483. [DOI] [PMC free article] [PubMed] [Google Scholar]


