Abstract
Advances in cancer treatment are producing a growing number of cancer survivors; therefore, issues surrounding quality of life during and following cancer treatment have become increasingly important. Chemotherapy-related cognitive impairment (CRCI) is a problem that is commonly reported following the administration of chemotherapy treatment in patients with cancer. Research suggests that CRCI can persist for months to years after completing treatment, which has implications for the trajectory of normal and pathologic cognitive aging for the growing number of long-term cancer survivors. These problems are particularly relevant for older individuals given that cancer is largely a disease of older age, and the number of patients with cancer who are age 65 years or older will increase dramatically over the coming decades. This review will briefly summarize empirical findings related to CRCI, discuss CRCI in older patients with cancer, potential causative hypotheses, and provide a canonical patient case to illustrate how CRCI presents clinically. Finally, potential intervention strategies for CRCI will be highlighted and issues to consider when evaluating older patients with a history of cancer will be discussed.
Keywords: chemotherapy-related cognitive impairment, chemotherapy, cognitive impairment, subjective cognitive decline
1. Introduction
Advances in cancer treatment are producing a growing number of cancer survivors; therefore, issues surrounding quality of life during and following cancer treatment have become increasingly important. Chemotherapy-related cognitive impairment (CRCI) is one such quality of life issue that is commonly reported following the administration of chemotherapy in patients with cancer.1 Although studies reporting cognitive impairments associated with chemotherapy have been reported in patients with non-central nervous system (non-CNS) cancers since the 1980s,2 the phenomenon commonly referred to as ‘chemo brain’ or ‘chemo fog’ is poorly understood and, until relatively recently, was largely unacknowledged.3 Research suggests that CRCI can persist for months to years after finishing treatment,4 which may have implications for the trajectory of cognitive aging for the growing number of long-term cancer survivors.5 These implications are particularly relevant for older individuals as risk for not only cancer, but cognitive impairment increases with age. As of January 2016, 62% of cancer survivors (9.61 million) are currently 65 years or older,6 and this number is expected to increase dramatically over the coming decades.7 Therefore, as the number of older cancer survivors who have will have to cope with CRCI is likely to increase, it is crucial to understand how CRCI presents clinically and to screen for symptoms of cognitive impairment. This article will briefly provide an overview of CRCI, discuss risk factors for CRCI, and will highlight potential intervention strategies and therapeutic targets for CRCI. A typical patient case summary is included to illustrate how CRCI often presents clinically, as well as to provide context for how current research can inform clinical practice.
Case Summary
The patient is a 68-year-old, married woman employed as a teacher at a high school with a history of breast cancer. At 65-years of age, she presented with an abnormal routine mammogram screening; subsequent diagnostic imaging revealed a suspicious abnormality in her right breast. She underwent lumpectomy with axillary lymph node dissection, and pathology revealed stage IIa (pT1c, pN1, cM0), estrogen receptor-positive and progesterone receptor-positive (ER+/PR+), human epidermal growth factor receptor 2-positive (HER-2/neu+), invasive ductal carcinoma. The patient received chemotherapy and targeted therapy consisting of 6 cycles of taxotere, carboplatin, and trastuzumab, as well as radiotherapy to the right breast and underarm. Chemotherapy and radiotherapy were followed by adjuvant endocrine therapy with letrozole and completion of one year of maintenance trastuzumab to reduce risk of breast cancer recurrence. During chemotherapy, the patient reported worsening fatigue and cognitive complaints, including greater difficulty with memory, attention, concentration, and ability to multitask. The patient was advised that cognitive complaints during chemotherapy are not uncommon and would likely improve following completion of chemotherapy.
Follow-up with the patient 2-years post-chemotherapy revealed that the patient’s cognitive complaints have not improved. She has stated that her symptoms have negatively impacted her job as a teacher. She denies receiving complaints about her work performance, but suspects that her coworkers are aware of changes. Upon questioning, she states that she has difficulty with word finding, needs reminders and notes to complete tasks, frequently loses paperwork, reports being easily distracted, has forgotten events and conversations, has trouble learning new work-related tasks, and that it takes greater cognitive effort to complete work-related tasks. She denies trouble recognizing faces, getting lost while navigating in familiar places, or any change in ability to manage household chores. She ambulates, dresses, bathes, drives, and shops independently. She states that she has been less interested in socializing, due to anxiety and distress surrounding her cognitive functioning.
She stated that at a breast cancer support group, the topic of “chemobrain” was discussed, prompting her concerns that the changes in her cognitive functioning were a consequence of her breast cancer treatment. She was referred for neuropsychological evaluation by her primary care physician. Neuropsychological testing revealed that she performs below expectations for age and education level on measures of memory, executive function, and attention, however her performance was within the normative range. Despite performance within the normative range on objective cognitive testing, subjective cognitive (self-report) measures reveal endorsements of cognitive complaints.
2. Overview of Chemotherapy-Related Cognitive Impairment (CRCI)
The American Cancer Society defines CRCI as: increased forgetfulness, trouble concentrating and remembering details, difficulty with multi-tasking word finding, and taking longer to finish tasks.8 Although changes across various domains on objective testing have been reported for CRCI, effects have been reported most prominently in the domains of attention, working memory, executive function, and processing speed.5 To date, the majority of CRCI research has involved women with breast cancer,1,9 who (as of January 2016) represent approximately 23% (3.6 million) of the 15.5 million cancer survivors in the US alone.10 Although it is likely that patients who receive chemotherapy for any type of cancer may experience CRCI, much of the literature in populations other than breast cancer is preliminary.11 However, research in patients with other types of cancer reveal similar results.4,12–17 Estimates of the prevalence of CRCI in cancer patients vary widely across studies.11 Current longitudinal studies suggest that approximately 40% of breast cancer patients have evidence of cognitive impairment prior to cancer treatment, up to 75% exhibit cognitive decline during treatment, and 35–60% exhibit cognitive decline following completion of chemotherapy.11 Severity of CRCI is typically mild to moderate in nature, such that impairments experienced would not typically qualify for a diagnosis of mild cognitive impairment (MCI)18 or dementia, however even subtle impairments in cognitive functioning can greatly influence quality of life.11
3. Risk Factors for CRCI
Research suggests that the causes of CRCI are likely multifactorial and a number of biological mechanisms have been suggested to play a role in the development of CRCI, including blood brain barrier (BBB) damage, neurotoxic cytokines, changes in hormones, DNA damage, oxidative stress, reduced synaptic plasticity, altered growth factor levels, and impaired hippocampal neurogenesis.19–21 Additionally, certain alleles in the Apolipoprotein E (APOE) and Catechol-O-methyltransferase (COMT) genes have been associated with increased risk for CRCI.22,23 Neuroimaging studies in patients with cancer have revealed white and gray matter loss, altered white matter integrity, altered resting state connectivity changes and brain activation during tasks.24. The question of why some cancer patients continue to experience CRCI for years following completion of chemotherapy has led to the examination of additional risk factors for CRCI, discussed below.
Aging
Aging is the most significant risk factor for developing cancer.25 Although the mechanisms that underlie the increased risk for cancer that accompanies increased age are not fully understood, there is considerable overlap in common biological changes that occur in the development of cancer, normal aging, and following chemotherapy treatment. Aging is associated with increased cell senescence, DNA damage, oxidative stress, inflammation, mitochondrial dysfunction, and decreased telomere length.26–31 Chemotherapy has been similarly associated with increased cell senescence,32 DNA damage,33,34 oxidative stress,35 inflammation,36–42 mitochondrial dysfunction,43 and decreased telomere length.35,44,45 It is important to note that all of the processes mentioned above, including age, have also been implicated as risk factors in cognitive decline and the development of neurodegenerative diseases, such as Alzheimer’s disease (AD).19,29,35,46–51 There is also overlap in alleles in the APOE gene that have been associated with both increased risk for CRCI and AD.22 Further, neuroimaging studies have revealed similar changes observed following chemotherapy treatment and in normal aging, including gray and white matter loss, altered white matter connectivity, altered resting state connectivity changes and brain activation during tasks.19,24,52–57 Together, this research suggests that the biological processes that underlie normal aging, brain response to chemotherapy, cognitive decline, and neurodegeneration overlap, leading to the hypothesis that chemotherapy may modify the normal aging trajectory.3,5,35,52
Aging is also associated with increased risk for cognitive impairment. Increasing evidence suggests that older patients are more susceptible to cognitive decline associated with chemotherapy and adjuvant endocrine therapies for breast cancer than younger patients.58,59 Additionally, age appears to interact with cognitive reserve, a predictor of future cognitive decline, to increase risk for cognitive decline following chemotherapy.58 A study by Ahles et al demonstrated that older patients with lower cognitive reserve prior to chemotherapy treatment showed reduced performance on measures of processing speed.58 This point is illustrated in Figure 1a where the effect of chemotherapy on cognitive performance may differ depending on level of pretreatment cognitive reserve.3 Investigators have proposed several models illustrating how cancer treatment may modify the trajectory of normal cognitive aging (Figure 1b; for a more extensive review, see Mandelblatt et al., 2013).5 Briefly, the phase shift hypothesis postulates that cancer patients treated with chemotherapy will experience greater decline in cognitive function compared to non-cancer/chemotherapy treated persons, and that the trajectory of decline will parallel normal aging and will remain constant over time.3 Alternatively, the accelerated aging hypothesis proposes that treatment with chemotherapy may accelerate the normal aging process.35 This model predicts that the slope of cognitive decline will be steeper for cancer patients treated with chemotherapy compared to non-cancer/chemotherapy treated patients. It is important to note that the phase shift and accelerated aging hypotheses are not mutually exclusive hypotheses.60 It is likely that some cancer patients may experience decline that follows the phase shift trajectory, while other cancer survivors may experience decline that follows the accelerated aging trajectory.
Figure 1. Interaction of chemotherapy with factors that affect normal cognitive aging.
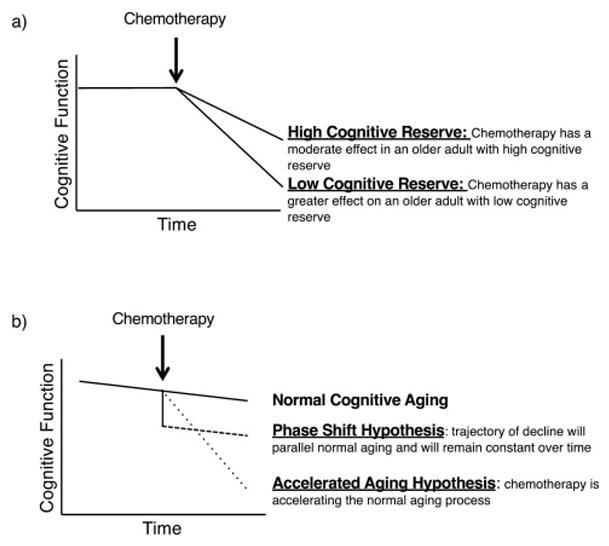
a) Effect of chemotherapy on cognitive performance may differ depending on pretreatment level of cognitive reserve. b) Possible trajectories of cognitive decline based on theories how chemotherapy interacts with normal cognitive aging. Figures adapted from Ahles et al. 2012.3
Whether or not cognitive decline associated with cancer treatments are similar to and/or increase the risk for MCI or dementia is a common concern voiced by older cancer patients. However, this issue appears to be complex given evidence that there may be an inverse relationship between risk of cancer and risk of developing dementia.61 In a prospective study of over 62,000 older women with breast cancer, no significant association between chemotherapy and drug-induced dementia or “other cognitive disorders” and in fact a significant reduction in the incidence of AD and vascular dementia was observed,62 however this finding should be further evaluated.
Pre-Morbid Cognitive Functioning
A common challenge for clinicians evaluating patients for CRCI is that, as in the case example, in normal clinical practice, cancer patients rarely receive cognitive assessment or neuropsychological testing prior to the initiation of chemotherapy. The importance of obtaining pretreatment neuropsychological assessment has been demonstrated by studies that have found that up to 41% of breast cancer patients perform below expectations for age and education prior to receiving chemotherapy,63–65 even when controlling for psychological factors, such as depression or anxiety, fatigue, or surgical factors.63 Of additional importance, without pretreatment assessment for comparison, declines in cognitive functioning that occurred during and following completion of chemotherapy treatment may go unnoticed. This is of particular importance when interpreting the test results of individuals with greater pretreatment cognitive reserve, such as individuals with high education levels.20 Individuals with high pre-morbid cognitive functioning prior to chemotherapy may also be more likely to express subjective cognitive complaints before objective cognitive measures can detect impairment,66 as may be the situation for the above case example. That is, due to the case example’s high pre-morbid cognitive functioning, her lower than expected performance may reflect a change for a previously high to mid-range normal individual; therefore, the patient could be accurately perceiving an alteration in her perceived cognitive abilities, which is reflected as cognitive complaints on subjective (self-report) measures. fMRI studies have demonstrated the potential for compensatory activation after chemotherapy, which may maintain normal performance on neuropsychological testing, but reflect a change in resource utilization, similar to what is seen in normal aging.54,67–69 Such findings suggest that the patient’s neuropsychological testing may fall in the normal range despite being associated with additional resource utilization and experienced as more effortful by patients. Further, there is increasing evidence that subjective (self-report) cognitive complaints, even with normal performance on objective neuropsychological tests, is associated with an increased risk for developing late-life cognitive decline and AD.18,66
Pre-Existing Cognitive Impairment
As our population ages, increasing numbers of patients with pre-existing MCI or dementia will be diagnosed with cancer, which represents a challenge in studying the role that cancer diagnosis and cancer treatment may play in the exacerbation of cognitive impairment in older adults.70 SEER Medicare studies suggest that the estimated prevalence of dementia in cancer patients age 65 and over ranges from 3.8 to 7%, 71–73 although these estimates may be lower than true prevalence due to lack of reporting of these diagnoses within Medicare claims.70 Few studies have examined how a prior diagnosis of MCI or dementia specifically impacts treatment decision-making for cancer and what percentage of these patients are offered various types of cancer therapies, such as surgery or chemotherapy.70 Gupta and Lamont found that colon cancer patients with a pre-existing dementia diagnosis were more likely to be diagnosed without biopsies and less likely to be treated with curative intent, compared to non-dementia colon cancer patients.71 Chemotherapy and radiation are administered less frequently to breast cancer patients with a preexisting dementia diagnosis compared to non-dementia breast cancer patients.72 Raji et al found that presence of a preexisting dementia diagnosis was associated with decreased survival after a diagnosis of breast, colon, or prostate cancer, increased mortality from cancer and from non-cancer causes, and increased odds of being diagnosed at an unknown stage of cancer.73 The issue of providing cancer treatment to patients with pre-existing dementia is a complex balancing act in terms of considering quality of life vs. quantity of life for the patient, and certainly stage of dementia at the time of cancer diagnosis should be taken into consideration. However, further research on the overall benefits, risks, and tolerance of cancer treatment in dementia patients at different stages of cancer is needed to better inform treatment decision making for such patients and to better inform the role of health care professionals involved in the care of cancer patients with preexisting dementia.
Effects of Endocrine Therapy on CRCI
While the majority of evidence for cognitive difficulties in cancer patients and survivors is attributed to chemotherapy, there is growing evidence to suggest that adjuvant endocrine therapy for hormone-receptor positive (HR+) breast cancer, which account for approximately 70–75% of breast cancers,74 may impact cognitive function, either alone or in combination with chemotherapy.75–81 However, such effects observed may not occur equally with all endocrine therapies.5 Adjuvant endocrine therapies for HR+ breast cancer act by blocking or lowering hormonal levels in patients with ER/PR+ tumors and include: 1) selective estrogen receptor modulators (SERMs), such as tamoxifen and 2) aromatase inhibitors (AIs), such as letrozole, which our case example received. Typically, studies provide evidence that tamoxifen adversely affects cognitive functioning,79,82,83 but have yielded inconclusive results with respect to AIs.77,79,83,84 However, it is important to note that breast cancer patients are often maintained on endocrine therapy for extended periods of time; the current American Society of Clinical Oncology guidelines now recommend 10 years total duration.85 Therefore, it is possible that CRCI associated with endocrine therapy for breast cancer may develop over time as patients age, although more research is needed.
Another area of growing concern is the effect of androgen-deprivation therapy (ADT) in men with prostate cancer. As of January 2016, there are more than 3.3 million men estimated to be living with prostate cancer in the United States, with the majority (64%) of these prostate cancer survivors over the age of 70 years.10 ADT is used to lower male androgens in order to treat prostate cancer and is a mainstay of treatment for both metastatic and localized disease.86,87 ADT can produce effects, such as depression and fatigue,88,89 that may indirectly affect cognitive functioning, and may also directly affect cognitive functioning as studies suggest that lower testosterone levels are associated with worse cognitive functioning in healthy older men90. In addition, both low testosterone levels and ADT increase the risk of cardiovascular disease,91,92 which is a known risk factor for dementia.93 Studies examining the effects of ADT on cognitive functioning have yielded inconclusive results,94–97 however a meta-analysis of 14 studies concluded ADT in patients with prostate cancer had a significant impact on visuomotor ability,98 and ADT has been associated with increased risk for dementia.99
Effect of Targeted Therapies on Cognition
The majority of CRCI research has focused on understanding the effects of traditional chemotherapy on cognition. By contrast, there is little to no published data on the effects of newer targeted therapies on cognitive performance after treatment. Types of targeted therapies include immunotherapies, such as monoclonal antibodies and checkpoint inhibitors, and small molecule signaling pathway inhibitors, such as tyrosine kinase (TK) inhibitors. The appeal of targeted therapies is that they aim at targeting genes or proteins specific to cancer cells or activating immune mechanisms to attack cancer cells, thus reducing off-target side effects in normal tissues.
Although such strategies may be generally less cytotoxic than traditional chemotherapy drugs, targeted cancer therapies are not without risk and can have substantial, and in some cases, life-threatening side effects. Targeted therapies also have the potential to either directly affect brain function or indirectly effect cognition through peripheral extra-CNS mechanisms. For example, sunitinib, a TK inhibitor capable of crossing the BBB used to treat a number of cancers, has been shown to have negative effects on cognitive functioning, specifically in the areas of learning, memory, and executive functioning in treated cancer patients.100,101 A subsequent mouse study revealed that sunitinib impaired spatial cognition as evidenced in Morris water maze, T-maze, and a passive avoidance task, and adversely affected cortical and hippocampal neurons.102 In a study evaluating the effect of antiangiogenic targeted therapy (primarily TK inhibitors), more than 30% of patients treated with such drugs developed cognitive decline.103
As targeted therapy use becomes increasingly more common, how these drugs affect cognition will need to be addressed, especially given that they are often used in conjunction with traditional chemotherapy. In the case example, the patient received trastuzumab in conjunction with chemotherapy followed by completion of one year of maintenance trastuzumab. Trastuzumab, a monoclonal antibody, is the most commonly used targeted therapy to treat HER-2/neu+ breast cancer, however there is no published data on the effects of trastuzumab on cognition. As the role of targeted therapy expands, cognitive performance follow-up will become increasingly important.
4. Intervention
Non-Pharmacological Interventions
There is some evidence that suggests that nonpharmacological interventions such as cognitive behavioral therapy, cognitive brain training, mindfulness based stress reduction, and physical activity may be beneficial for patients with patients with CRCI.20,104 Two pilot studies examining cognitive behavioral therapy in breast cancer patients demonstrated improvement on both objective and subjective (self-report) measures of cognitive function.105,106 Computerized cognitive brain-training studies suggest improvement in executive functioning,107 and yoga may reduce subjective memory complaints.108 The application of non-pharmacological interventions may be promising and should be tailored to each individual patient.
Pharmacological Interventions
Currently, there is no pharmacological treatment that is specific for CRCI. Most pharmacological treatment studies of cancer patients and survivors have centered on treating side effects of chemotherapy such as fatigue109–112 and anemia,113,114 and have largely not focused on treating cognitive symptoms associated with chemotherapy. Studies evaluating the efficacy of stimulants, such as methylphenidate, dexmethylphenidate, and modafinil, for the treatment of CRCI have yielded mixed results with respect to cognition, therefore it remains unclear whether these medications are useful in treating CRCI.109–114 Other pharmacologic treatment studies have evaluated donepezil, an acetylcholinesterase inhibitor, approved to treat mild to severe AD.115,116 Both open-label and placebo controlled studies in glioma patients suggested statistically significant improvements in cognitive performance.115,116 Additionally, a study in breast cancer survivors suggested improved verbal memory in those who had poorer cognitive functioning at baseline.117 Cholinesterase inhibitor studies provide support for the cholinergic system as a therapeutic target for improving cognitive functioning in CRCI.115–117 More selective cholinergic stimulation may potentially be useful for certain cognitive symptoms. For example, a randomized, placebo-controlled study evaluating the use of transdermal nicotine treatment as a therapeutic strategy for persistent CRCI is currently underway (NCT02312934).
6. Factors to Consider When Evaluating Older Cancer Survivors for CRCI
Depression
Depressive symptoms have been found to occur in up to 15% to 25% of cancer patients.118,119 Although depression is frequently associated with cancer, the role of depression as a risk factor for CRCI is not fully understood. Differentiation between cause and effect is particularly challenging when assessing older patients with depression for CRCI since depression by itself is associated with a number of cognitive deficits, including difficulty concentrating, distractibility, forgetfulness, reduced reaction time, memory loss, and indecisiveness120. For patients with a history of cancer, a cognitive assessment at the time the patient begins treatment for their depression may be valuable. If cognitive impairment continues, even with successful treatment of depression, a search for other causes can be commenced and separate treatment may be warranted.
Subjective Cognitive Decline (SCD)
In the AD literature, greater emphasis on early detection and diagnosis of cognitive impairment has led to the conceptualization of ‘subjective cognitive decline’ (SCD), in which individuals perceive changes in their cognitive abilities but perform within normal limits on cognitive tests, as in the case example.18,121 There is increasing evidence that subjective cognitive complaints, even with normal performance on objective tests, is associated with an increased risk for developing late-life cognitive decline and AD.18,66 This is of particular importance to older cancer patients due to the age-associated increase in the risk for dementia.
SCD, like CRCI, is not a discrete syndrome and could potentially reflect numerous conditions such as normal aging, psychiatric conditions, neurologic and medical disorders, substance use, and medication effects. Current evidence suggests that the following factors increase the likelihood of future cognitive decline in older individuals with SCD: 1) subjective decline in memory, rather than other domains of cognition, 2) new onset of SCD within the last 5 years, and 3) age at onset of SCD ≥60 years.121 Therefore, if patients with CRCI show additional factors that increase risk for future cognitive decline, periodic follow-up and testing is warranted.18
Mild Cognitive Impairment (MCI) & Dementia
MCI is a syndrome defined as subjective and objective decline in cognition and function greater than expected for an individual’s age and education level that does not meet criteria for a diagnosis of dementia.18 Elderly patients with MCI constitute a high-risk population for developing dementia, in particular AD. The concept of MCI originally evolved out of the effort to characterize the pre-dementia phase of cognitive impairment for which, at the time, there was no clinical definition. Older patients that meet criteria for MCI (for a more extensive review, see Vega and Newhouse, 201418) should be evaluated as to the potential cause. Comprehensive neuropsychological testing, screening for depression, brain imaging (MRI and/or PET), and neurological examination will assist in differentiation from CRCI18. Patients with predominantly amnestic-MCI or evidence for AD pathology may be considered for cholinergic or investigational therapies as well as nonpharmacological interventions, such as cognitive training or aerobic exercise.18 Regardless of treatment, longitudinal follow-up is indicated.
Structured Assessments
Older patients with a cancer history should be routinely asked about their cognitive functioning. If available, informants should be asked to confirm any reports of cognitive decline experienced by the patient. Clinicians should consider using a structured instrument that examines diverse aspects of functional abilities, psychiatric signs and symptoms, and cognitive functioning, such as the Older Adult Self-Report (OSAR) and the informant-based Older Adults Behavior Checklist (OABCL) which have been validated to correlate with evaluations of cognitive impairment.122 The Functional Assessment of Cancer Therapy-Cognitive Function (FACT-Cog)123 is an assessment tool that was developed to assess subjective cognitive function specifically in cancer patients. The FACT-Cog has been used to monitor change in CRCI subjective complaints in a number of studies, can be administered repeatedly, and therefore may be useful in a clinical setting.124–126 This 37-item questionnaire is a self-report measure of cognitive function that aims to evaluate the “real world” impact of CRCI. The scale evaluates subjective memory, concentration, mental acuity, verbal fluency, functional interference, and multitasking ability. Reports from patients and their family members and/or care-givers about subjective changes in cognitive functioning should prompt more focused clinical evaluation and structured assessment instruments will be valuable in initial and longitudinal assessment.
7. Conclusion
CRCI is a complex and evolving concept. While there is now general agreement that chemotherapy and other cancer treatments may induce cognitive impairments, current knowledge is limited by the lack of consistent findings in the identification of risk and progression factors, specific pathological and clinical markers, as well as difficulties in finding effective treatments. Although the phenomenon of CRCI is better recognized among patients and has been widely publicized in the popular press, patient reports suggest that the cognitive effects of cancer treatments are not routinely discussed and cognitive assessments are not routinely incorporated into the evaluation and management of older patients with cancer. Therefore, as in our case example, primary care physicians and/or psychiatrists have an opportunity to discuss, diagnose, and offer intervention strategies for patients with CRCI. For clinicians, making the distinction between cognitive impairment that may indicate future cognitive decline and changes in cognition related to either psychiatric illness (e.g., depression) or normal aging can be challenging. However, CRCI may be of particular importance for older patients with a history of cancer due to the age-associated increase in the risk for dementia. CRCI may have numerous contributing factors, such as interaction with normal aging, co-development of dementia, endocrine therapy, and targeted therapy. Several brief cognitive assessment tools, including the FACT-Cog and OASR/OABCL, can be used in the evaluation of older adults with cancer. Older patients with a cancer history should be routinely asked about their cognitive functioning and assessment for CRCI should be assessed at regular intervals. Interventions are still under investigation but may include specific pharmacologic and non-pharmacologic approaches.
Acknowledgments
Grant Support
Preparation of this work was supported by 1 R01 AG047992-01A1 to PN and Vanderbilt CTSA Grant the Vanderbilt CTSA award (UL1TR000445) from the National Center for Advancing Translational Sciences to JNV.
The authors would like to acknowledge Dr. Ingrid Mayer for her assistance in editing the case summary.
Footnotes
Conflicts of Interest:
JNV reports grants from National Institutes on Aging, grants from Vanderbilt CTSA Grant, during the conduct of the study; Dr. Newhouse reports grants from National Institute on Aging, during the conduct of the study; Dr. Dumas reports grants from National Institute on Aging, during the conduct of the study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Janelsins MC, Kohli S, Mohile SG, Usuki K, Ahles TA, Morrow GR. An update on cancer- and chemotherapy-related cognitive dysfunction: current status. Semin Oncol. 2011;38(3):431–8. doi: 10.1053/j.seminoncol.2011.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silberfarb PM. Chemotherapy and cognitive defects in cancer patients. Annu Rev Med. 1983;34:35–46. doi: 10.1146/annurev.me.34.020183.000343. [DOI] [PubMed] [Google Scholar]
- 3.Ahles TA. Brain vulnerability to chemotherapy toxicities. Psychooncology. 2012;21(11):1141–8. doi: 10.1002/pon.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahles TA, Saykin AJ, Furstenberg CT, et al. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J Clin Oncol. 2002;20(2):485–93. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- 5.Mandelblatt JS, Hurria A, McDonald BC, et al. Cognitive effects of cancer and its treatments at the intersection of aging: what do we know; what do we need to know? Semin Oncol. 2013;40(6):709–25. doi: 10.1053/j.seminoncol.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the "Silver Tsunami": Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev. 2016;25(7):1029–36. doi: 10.1158/1055-9965.EPI-16-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colby SL, Ortman JM. The Baby Boom Cohort in the United States: 2012 to 2060 Population Estimates and Projections. 2014 https://www.census.gov/prod/2014pubs/p25-1141.pdf.
- 8.Craig CD, Monk BJ, Farley JH, Chase DM. Cognitive impairment in gynecologic cancers: a systematic review of current approaches to diagnosis and treatment. Support Care Cancer. 2014;22(1):279–87. doi: 10.1007/s00520-013-2029-7. [DOI] [PubMed] [Google Scholar]
- 9.Wefel JS, Schagen SB. Chemotherapy-related cognitive dysfunction. Curr Neurol Neurosci Rep. 2012;12(3):267–75. doi: 10.1007/s11910-012-0264-9. [DOI] [PubMed] [Google Scholar]
- 10.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016 May; doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 11.Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin. 2015;65(2):123–38. doi: 10.3322/caac.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pedersen AD, Rossen P, Mehlsen MY, Pedersen CG, Zachariae R, von der Maase H. Long-term cognitive function following chemotherapy in patients with testicular cancer. J Int Neuropsychol Soc. 2009;15(2):296–301. doi: 10.1017/S1355617709090316. [DOI] [PubMed] [Google Scholar]
- 13.Wefel JS, Vidrine DJ, Marani SK, et al. A prospective study of cognitive function in men with non-seminomatous germ cell tumors. Psychooncology. 2014;23(6):626–33. doi: 10.1002/pon.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones D, Vichaya EG, Wang XS, Sailors MH, Cleeland CS, Wefel JS. Acute cognitive impairment in patients with multiple myeloma undergoing autologous hematopoietic stem cell transplant. Cancer. 2013;119(23):4188–95. doi: 10.1002/cncr.28323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vardy J, Tannock I. Cognitive function after chemotherapy in adults with solid tumours. Crit Rev Oncol Hematol. 2007;63(3):183–202. doi: 10.1016/j.critrevonc.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 16.Correa DD, Zhou Q, Thaler HT, Maziarz M, Hurley K, Hensley ML. Cognitive functions in long-term survivors of ovarian cancer. Gynecol Oncol. 2010;119(2):366–9. doi: 10.1016/j.ygyno.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 17.Chao HH, Uchio E, Zhang S, et al. Effects of androgen deprivation on brain function in prostate cancer patients - a prospective observational cohort analysis. BMC Cancer. 2012;12:371. doi: 10.1186/1471-2407-12-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vega JN, Newhouse PA. Mild cognitive impairment: diagnosis, longitudinal course, and emerging treatments. Curr Psychiatry Rep. 2014;16(10):490. doi: 10.1007/s11920-014-0490-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer. 2007;7(3):192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry. 2014;26(1):102–113. doi: 10.3109/09540261.2013.864260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loh KP, Janelsins MC, Mohile SG, Holmes HM, Hsu T, Inouye SK, Karuturi MS, Kimmick GG, Lichtman SM, Magnuson A, Whitehead MI, Wong MLAT. Chemotherapy-related cognitive impairment in older patients with cancer. J Geriatr Oncol. 2016 doi: 10.1016/j.jgo.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahles TA, Saykin AJ, Noll WW, et al. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology. 2003;12(6):612–9. doi: 10.1002/pon.742. [DOI] [PubMed] [Google Scholar]
- 23.Small BJ, Rawson KS, Walsh E, et al. Catechol-O-methyltransferase genotype modulates cancer treatment-related cognitive deficits in breast cancer survivors. Cancer. 2011;117(7):1369–76. doi: 10.1002/cncr.25685. [DOI] [PubMed] [Google Scholar]
- 24.Mandelblatt JS, Stern RA, Luta G, et al. Cognitive impairment in older patients with breast cancer before systemic therapy: is there an interaction between cancer and comorbidity? J Clin Oncol. 2014;32(18):1909–18. doi: 10.1200/JCO.2013.54.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Mortality - All COD, Aggregated With State, Total U.S. (1969–2010) <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, Surveillance Re.
- 26.Irminger-Finger I. Science of cancer and aging. J Clin Oncol. 2007;25(14):1844–51. doi: 10.1200/JCO.2007.10.8928. [DOI] [PubMed] [Google Scholar]
- 27.Campisi J, Yaswen P. Aging and cancer cell biology, 2009. Aging Cell. 2009;8(3):221–5. doi: 10.1111/j.1474-9726.2009.00475.x. [DOI] [PubMed] [Google Scholar]
- 28.Ramsey MR, Sharpless NE. ROS as a tumour suppressor? Nat Cell Biol. 2006;8(11):1213–5. doi: 10.1038/ncb1106-1213. [DOI] [PubMed] [Google Scholar]
- 29.von Zglinicki T, Martin-Ruiz CM. Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med. 2005;5(2):197–203. doi: 10.2174/1566524053586545. [DOI] [PubMed] [Google Scholar]
- 30.Singh KK. Mitochondria damage checkpoint, aging, and cancer. Ann N Y Acad Sci. 2006;1067:182–90. doi: 10.1196/annals.1354.022. [DOI] [PubMed] [Google Scholar]
- 31.Kowald A. The mitochondrial theory of aging: do damaged mitochondria accumulate by delayed degradation? Exp Gerontol. 1999;34(5):605–12. doi: 10.1016/s0531-5565(99)00011-x. [DOI] [PubMed] [Google Scholar]
- 32.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–40. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 33.Blasiak J, Arabski M, Krupa R, et al. Basal, oxidative and alkylative DNA damage, DNA repair efficacy and mutagen sensitivity in breast cancer. Mutat Res. 2004;554(1–2):139–48. doi: 10.1016/j.mrfmmm.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Nadin SB, Vargas-Roig LM, Drago G, Ibarra J, Ciocca DR. DNA damage and repair in peripheral blood lymphocytes from healthy individuals and cancer patients: a pilot study on the implications in the clinical response to chemotherapy. Cancer Lett. 2006;239(1):84–97. doi: 10.1016/j.canlet.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 35.Maccormick RE. Possible acceleration of aging by adjuvant chemotherapy: a cause of early onset frailty? Med Hypotheses. 2006;67(2):212–5. doi: 10.1016/j.mehy.2006.01.045. [DOI] [PubMed] [Google Scholar]
- 36.Tsavaris N, Kosmas C, Vadiaka M, Kanelopoulos P, Boulamatsis D. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br J Cancer. 2002;87(1):21–7. doi: 10.1038/sj.bjc.6600347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pusztai L, Mendoza TR, Reuben JM, et al. Changes in plasma levels of inflammatory cytokines in response to paclitaxel chemotherapy. Cytokine. 2004;25(3):94–102. doi: 10.1016/j.cyto.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Penson RT, Kronish K, Duan Z, et al. Cytokines IL-1beta, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNFalpha in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. Int J Gynecol Cancer. 2000;10(1):33–41. doi: 10.1046/j.1525-1438.2000.00003.x. [DOI] [PubMed] [Google Scholar]
- 39.Collado-Hidalgo A, Bower JE, Ganz PA, Cole SW, Irwin MR. Inflammatory biomarkers for persistent fatigue in breast cancer survivors. Clin Cancer Res. 2006;12(9):2759–66. doi: 10.1158/1078-0432.CCR-05-2398. [DOI] [PubMed] [Google Scholar]
- 40.Bower JE, Ganz PA, Aziz N, Fahey JL. Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med. 64(4):604–11. doi: 10.1097/00006842-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Ganz PA, Bower JE, Kwan L, et al. Does tumor necrosis factor-alpha (TNF-α) play a role in post-chemotherapy cerebral dysfunction? Brain Behav Immun. 2013;30(Suppl):S99–108. doi: 10.1016/j.bbi.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kesler S, Janelsins M, Koovakkattu D, et al. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain Behav Immun. 2013;30(Suppl):S109–16. doi: 10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wardell TM, Ferguson E, Chinnery PF, et al. Changes in the human mitochondrial genome after treatment of malignant disease. Mutat Res. 2003;525(1–2):19–27. doi: 10.1016/s0027-5107(02)00313-5. [DOI] [PubMed] [Google Scholar]
- 44.Schröder CP, Wisman GB, de Jong S, et al. Telomere length in breast cancer patients before and after chemotherapy with or without stem cell transplantation. Br J Cancer. 2001;84(10):1348–53. doi: 10.1054/bjoc.2001.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lahav M, Uziel O, Kestenbaum M, et al. Nonmyeloablative conditioning does not prevent telomere shortening after allogeneic stem cell transplantation. Transplantation. 2005;80(7):969–76. doi: 10.1097/01.tp.0000173649.99261.df. [DOI] [PubMed] [Google Scholar]
- 46.Migliore L, Fontana I, Trippi F, et al. Oxidative DNA damage in peripheral leukocytes of mild cognitive impairment and AD patients. Neurobiol Aging. 2005;26(5):567–73. doi: 10.1016/j.neurobiolaging.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 47.Keller JN, Schmitt FA, Scheff SW, et al. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64(7):1152–6. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- 48.Mariani E, Polidori MC, Cherubini A, Mecocci P. Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827(1):65–75. doi: 10.1016/j.jchromb.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 49.Tonelli LH, Postolache TT, Sternberg EM. Inflammatory genes and neural activity: involvement of immune genes in synaptic function and behavior. Front Biosci. 2005;10:675–80. doi: 10.2741/1562. [DOI] [PubMed] [Google Scholar]
- 50.Tan ZS, Beiser AS, Vasan RS, et al. Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. Neurology. 2007;68(22):1902–8. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 51.Wang X, Wang W, Li L, Perry G, Lee H-G, Zhu X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim Biophys Acta. 2013 Nov; doi: 10.1016/j.bbadis.2013.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol. 2012;30(30):3675–86. doi: 10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McDonald BC, Conroy SK, Smith DJ, West JD, Saykin AJ. Frontal gray matter reduction after breast cancer chemotherapy and association with executive symptoms: a replication and extension study. Brain Behav Immun. 2013;30(Suppl):S117–25. doi: 10.1016/j.bbi.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Alterations in brain activation during working memory processing associated with breast cancer and treatment: a prospective functional magnetic resonance imaging study. J Clin Oncol. 2012;30(20):2500–8. doi: 10.1200/JCO.2011.38.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McDonald BC, Conroy SK, Ahles TA, West JD, Saykin AJ. Gray matter reduction associated with systemic chemotherapy for breast cancer: a prospective MRI study. Breast Cancer Res Treat. 2010;123(3):819–28. doi: 10.1007/s10549-010-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koppelmans V, de Ruiter MB, van der Lijn F, et al. Global and focal brain volume in long-term breast cancer survivors exposed to adjuvant chemotherapy. Breast Cancer Res Treat. 2012;132(3):1099–106. doi: 10.1007/s10549-011-1888-1. [DOI] [PubMed] [Google Scholar]
- 57.Silverman DHS, Dy CJ, Castellon SA, et al. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res Treat. 2007;103(3):303–11. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- 58.Ahles TA, Saykin AJ, McDonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol. 2010;28(29):4434–40. doi: 10.1200/JCO.2009.27.0827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schilder CM, Seynaeve C, Beex LV, et al. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. J Clin Oncol. 2010;28(8):1294–300. doi: 10.1200/JCO.2008.21.3553. [DOI] [PubMed] [Google Scholar]
- 60.Mandelblatt JS, Jacobsen PB, Ahles T. Cognitive effects of cancer systemic therapy: implications for the care of older patients and survivors. J Clin Oncol. 2014;32(24):2617–26. doi: 10.1200/JCO.2014.55.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ganguli M. Cancer and Dementia: It’s Complicated. Alzheimer Dis Assoc Disord. 2015;29(2):177–82. doi: 10.1097/WAD.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Du XL, Xia R, Hardy D. Relationship between chemotherapy use and cognitive impairments in older women with breast cancer: findings from a large population-based cohort. Am J Clin Oncol. 2010;33(6):533–43. doi: 10.1097/COC.0b013e3181b9cf1b. [DOI] [PubMed] [Google Scholar]
- 63.Ahles TA, Saykin AJ, McDonald BC, et al. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat. 2008;110(1):143–52. doi: 10.1007/s10549-007-9686-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wefel JS, Lenzi R, Theriault R, Buzdar AU, Cruickshank S, Meyers CA. “Chemobrain” in breast carcinoma?: a prologue. Cancer. 2004;101(3):466–75. doi: 10.1002/cncr.20393. [DOI] [PubMed] [Google Scholar]
- 65.Lange M, Giffard B, Noal S, et al. Baseline cognitive functions among elderly patients with localised breast cancer. Eur J Cancer. 2014;50(13):2181–9. doi: 10.1016/j.ejca.2014.05.026. [DOI] [PubMed] [Google Scholar]
- 66.Saykin AJ, Wishart HA, Rabin LA, et al. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67(5):834–42. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferguson RJ, McDonald BC, Saykin AJ, Ahles TA. Brain structure and function differences in monozygotic twins: possible effects of breast cancer chemotherapy. J Clin Oncol. 2007;25(25):3866–70. doi: 10.1200/JCO.2007.10.8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kesler SR, Bennett FC, Mahaffey ML, Spiegel D. Regional brain activation during verbal declarative memory in metastatic breast cancer. Clin Cancer Res. 2009;15(21):6665–73. doi: 10.1158/1078-0432.CCR-09-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kesler SR, Kent JS, O’Hara R. Prefrontal cortex and executive function impairments in primary breast cancer. Arch Neurol. 2011;68(11):1447–53. doi: 10.1001/archneurol.2011.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Magnuson A, Mohile S, Janelsins M. Cognition and Cognitive Impairment in Older Adults with Cancer. Curr Geriatr reports. 2016;5(3):213–219. doi: 10.1007/s13670-016-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gupta SK, Lamont EB. Patterns of Presentation, Diagnosis, and Treatment in Older Patients with Colon Cancer and Comorbid Dementia. J Am Geriatr Soc. 2004;52(10):1681–1687. doi: 10.1111/j.1532-5415.2004.52461.x. [DOI] [PubMed] [Google Scholar]
- 72.Gorin SS, Heck JE, Albert S, Hershman D. Treatment for Breast Cancer in Patients with Alzheimer’s Disease. J Am Geriatr Soc. 2005;53(11):1897–1904. doi: 10.1111/j.1532-5415.2005.00467.x. [DOI] [PubMed] [Google Scholar]
- 73.Raji MA, Kuo Y-F, Freeman JL, Goodwin JS. Effect of a Dementia Diagnosis on Survival of Older Patients After a Diagnosis of Breast, Colon, or Prostate Cancer. Arch Intern Med. 2008;168(18):2033. doi: 10.1001/archinte.168.18.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17(5):1474–81. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 75.Castellon SA, Ganz PA, Bower JE, Petersen L, Abraham L, Greendale GA. Neurocognitive performance in breast cancer survivors exposed to adjuvant chemotherapy and tamoxifen. J Clin Exp Neuropsychol. 2004;26(7):955–69. doi: 10.1080/13803390490510905. [DOI] [PubMed] [Google Scholar]
- 76.Schilder CM, Seynaeve C, Beex LV, et al. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. J Clin Oncol. 2010;28(8):1294–300. doi: 10.1200/JCO.2008.21.3553. [DOI] [PubMed] [Google Scholar]
- 77.Jenkins VA, Ambroisine LM, Atkins L, Cuzick J, Howell A, Fallowfield LJ. Effects of anastrozole on cognitive performance in postmenopausal women: a randomised, double-blind chemoprevention trial (IBIS II) Lancet Oncol. 2008;9(10):953–61. doi: 10.1016/S1470-2045(08)70207-9. [DOI] [PubMed] [Google Scholar]
- 78.Bender CM, Sereika SM, Berga SL, et al. Cognitive impairment associated with adjuvant therapy in breast cancer. Psychooncology. 2006;15(5):422–30. doi: 10.1002/pon.964. [DOI] [PubMed] [Google Scholar]
- 79.Collins B, Mackenzie J, Stewart A, Bielajew C, Verma S. Cognitive effects of hormonal therapy in early stage breast cancer patients: a prospective study. Psychooncology. 2009;18(8):811–21. doi: 10.1002/pon.1453. [DOI] [PubMed] [Google Scholar]
- 80.Schilder CM, Eggens PC, Seynaeve C, et al. Neuropsychological functioning in postmenopausal breast cancer patients treated with tamoxifen or exemestane after AC-chemotherapy: cross-sectional findings from the neuropsychological TEAM-side study. Acta Oncol. 2009;48(1):76–85. doi: 10.1080/02841860802314738. [DOI] [PubMed] [Google Scholar]
- 81.Palmer JL, Trotter T, Joy AA, Carlson LE. Cognitive effects of Tamoxifen in pre-menopausal women with breast cancer compared to healthy controls. J Cancer Surviv. 2008;2(4):275–82. doi: 10.1007/s11764-008-0070-1. [DOI] [PubMed] [Google Scholar]
- 82.Paganini-Hill A, Clark LJ. Preliminary assessment of cognitive function in breast cancer patients treated with tamoxifen. Breast Cancer Res Treat. 2000;64(2):165–76. doi: 10.1023/a:1006426132338. [DOI] [PubMed] [Google Scholar]
- 83.Jenkins V, Shilling V, Fallowfield L, Howell A, Hutton S. Does hormone therapy for the treatment of breast cancer have a detrimental effect on memory and cognition? A pilot study. Psychooncology. 2004;13(1):61–6. doi: 10.1002/pon.709. [DOI] [PubMed] [Google Scholar]
- 84.Bender CM, Sereika SM, Brufsky AM, et al. Memory impairments with adjuvant anastrozole versus tamoxifen in women with early-stage breast cancer. Menopause. 14(6):995–8. doi: 10.1097/gme.0b013e318148b28b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: american society of clinical oncology clinical practice guideline focused update. J Clin Oncol. 2014;32(21):2255–69. doi: 10.1200/JCO.2013.54.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and Short-Term Androgen Deprivation for Localized Prostate Cancer. N Engl J Med. 2011;365(2):107–118. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]
- 87.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet (London, England) 2002;360(9327):103–6. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 88.Lee M, Jim HS, Fishman M, et al. Depressive symptomatology in men receiving androgen deprivation therapy for prostate cancer: a controlled comparison. Psychooncology. 2015;24(4):472–477. doi: 10.1002/pon.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Storey DJ, McLaren DB, Atkinson MA, et al. Clinically relevant fatigue in men with hormone-sensitive prostate cancer on long-term androgen deprivation therapy. Ann Oncol. 2012;23(6):1542–1549. doi: 10.1093/annonc/mdr447. [DOI] [PubMed] [Google Scholar]
- 90.Holland J, Bandelow S, Hogervorst E. Testosterone levels and cognition in elderly men: a review. Maturitas. 2011;69(4):322–37. doi: 10.1016/j.maturitas.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 91.Keating NL, O’Malley AJ, Freedland SJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102(1):39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tsai HK, D’Amico AV, Sadetsky N, Chen M-H, Carroll PR. Androgen Deprivation Therapy for Localized Prostate Cancer and the Risk of Cardiovascular Mortality. JNCI J Natl Cancer Inst. 2007;99(20):1516–1524. doi: 10.1093/jnci/djm168. [DOI] [PubMed] [Google Scholar]
- 93.Justin BN, Turek M, Hakim AM. Heart disease as a risk factor for dementia. Clin Epidemiol. 2013;5:135–45. doi: 10.2147/CLEP.S30621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gonzalez BD, Jim HSL, Booth-Jones M, et al. Course and Predictors of Cognitive Function in Patients With Prostate Cancer Receiving Androgen-Deprivation Therapy: A Controlled Comparison. J Clin Oncol. 2015;33(18):2021–2027. doi: 10.1200/JCO.2014.60.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jim HSL, Small BJ, Patterson S, Salup R, Jacobsen PB. Cognitive impairment in men treated with luteinizing hormone-releasing hormone agonists for prostate cancer: a controlled comparison. Support Care Cancer. 2010;18(1):21–7. doi: 10.1007/s00520-009-0625-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Joly F, Alibhai SMH, Galica J, et al. Impact of androgen deprivation therapy on physical and cognitive function, as well as quality of life of patients with nonmetastatic prostate cancer. J Urol. 2006;176(6 Pt 1):2443–7. doi: 10.1016/j.juro.2006.07.151. [DOI] [PubMed] [Google Scholar]
- 97.Alibhai SMH, Breunis H, Timilshina N, et al. Impact of androgen-deprivation therapy on cognitive function in men with nonmetastatic prostate cancer. J Clin Oncol. 2010;28(34):5030–7. doi: 10.1200/JCO.2010.30.8742. [DOI] [PubMed] [Google Scholar]
- 98.McGinty HL, Phillips KM, Jim HSL, et al. Cognitive functioning in men receiving androgen deprivation therapy for prostate cancer: a systematic review and meta-analysis. Support Care Cancer. 2014;22(8):2271–80. doi: 10.1007/s00520-014-2285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nead KT, Gaskin G, Chester C, Swisher-McClure S, Leeper NJ, Shah NH. Association Between Androgen Deprivation Therapy and Risk of Dementia. JAMA Oncol. 2017;3(1):49. doi: 10.1001/jamaoncol.2016.3662. [DOI] [PubMed] [Google Scholar]
- 100.Mulder SF, Bertens D, Desar IME, et al. Impairment of cognitive functioning during Sunitinib or Sorafenib treatment in cancer patients: a cross sectional study. BMC Cancer. 2014;14:219. doi: 10.1186/1471-2407-14-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.van der Veldt AAM, van den Eertwegh AJM, Hoekman K, Barkhof F, Boven E. Reversible cognitive disorders after sunitinib for advanced renal cell cancer in patients with preexisting arteriosclerotic leukoencephalopathy. Ann Oncol Off J Eur Soc Med Oncol. 2007;18(10):1747–50. doi: 10.1093/annonc/mdm455. [DOI] [PubMed] [Google Scholar]
- 102.Abdel-Aziz AK, Mantawy EM, Said RS, Helwa R. The tyrosine kinase inhibitor, sunitinib malate, induces cognitive impairment in vivo via dysregulating VEGFR signaling, apoptotic and autophagic machineries. Exp Neurol. 2016;283:129–141. doi: 10.1016/j.expneurol.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 103.Joly F, Heutte N, Duclos B, et al. Prospective Evaluation of the Impact of Antiangiogenic Treatment on Cognitive Functions in Metastatic Renal Cancer. Eur Urol Focus. 2016 May; doi: 10.1016/j.euf.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 104.Joly F, Giffard B, Rigal O, et al. Impact of Cancer and Its Treatments on Cognitive Function: Advances in Research From the Paris International Cognition and Cancer Task Force Symposium and Update Since 2012. J Pain Symptom Manage. 2015;50(6):830–841. doi: 10.1016/j.jpainsymman.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 105.Ferguson RJ, Ahles TA, Saykin AJ, et al. Cognitive-behavioral management of chemotherapy-related cognitive change. Psychooncology. 2007;16(8):772–777. doi: 10.1002/pon.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ferguson RJ, McDonald BC, Rocque MA, et al. Development of CBT for chemotherapy-related cognitive change: results of a waitlist control trial. Psychooncology. 2012;21(2):176–86. doi: 10.1002/pon.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kesler S, Hadi Hosseini SM, Heckler C, et al. Cognitive training for improving executive function in chemotherapy-treated breast cancer survivors. Clin Breast Cancer. 2013;13(4):299–306. doi: 10.1016/j.clbc.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Janelsins MC, Peppone LJ, Heckler CE, et al. YOCAS©® Yoga Reduces Self-reported Memory Difficulty in Cancer Survivors in a Nationwide Randomized Clinical Trial: Investigating Relationships Between Memory and Sleep. Integr Cancer Ther. 2015 Nov; doi: 10.1177/1534735415617021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lower EE, Fleishman S, Cooper A, et al. Efficacy of dexmethylphenidate for the treatment of fatigue after cancer chemotherapy: a randomized clinical trial. J Pain Symptom Manage. 2009;38(5):650–62. doi: 10.1016/j.jpainsymman.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 110.Mar Fan HG, Clemons M, Xu W, et al. A randomised, placebo-controlled, double-blind trial of the effects of d-methylphenidate on fatigue and cognitive dysfunction in women undergoing adjuvant chemotherapy for breast cancer. Support Care Cancer. 2008;16(6):577–83. doi: 10.1007/s00520-007-0341-9. [DOI] [PubMed] [Google Scholar]
- 111.Kohli S, Fisher SG, Tra Y, et al. The effect of modafinil on cognitive function in breast cancer survivors. Cancer. 2009;115(12):2605–16. doi: 10.1002/cncr.24287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lundorff LE, Jønsson BH, Sjøgren P. Modafinil for attentional and psychomotor dysfunction in advanced cancer: a double-blind, randomised, cross-over trial. Palliat Med. 2009;23(8):731–8. doi: 10.1177/0269216309106872. [DOI] [PubMed] [Google Scholar]
- 113.O’Shaughnessy JA. Effects of epoetin alfa on cognitive function, mood, asthenia, and quality of life in women with breast cancer undergoing adjuvant chemotherapy. Clin Breast Cancer. 2002;3(Suppl 3):S116–20. doi: 10.3816/cbc.2002.s.022. [DOI] [PubMed] [Google Scholar]
- 114.Fan HGM, Park A, Xu W, et al. The influence of erythropoietin on cognitive function in women following chemotherapy for breast cancer. Psychooncology. 2009;18(2):156–61. doi: 10.1002/pon.1372. [DOI] [PubMed] [Google Scholar]
- 115.Shaw EG, Rosdhal R, D’Agostino RB, et al. Phase II study of donepezil in irradiated brain tumor patients: effect on cognitive function, mood, and quality of life. J Clin Oncol. 2006;24(9):1415–20. doi: 10.1200/JCO.2005.03.3001. [DOI] [PubMed] [Google Scholar]
- 116.Castellino SM, Tooze JA, Flowers L, et al. Toxicity and efficacy of the acetylcholinesterase (AChe) inhibitor donepezil in childhood brain tumor survivors: a pilot study. Pediatr Blood Cancer. 2012;59(3):540–7. doi: 10.1002/pbc.24078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lawrence JA, Griffin L, Balcueva EP, et al. A study of donepezil in female breast cancer survivors with self-reported cognitive dysfunction 1 to 5 years following adjuvant chemotherapy. J Cancer Surviv. 2016;10(1):176–84. doi: 10.1007/s11764-015-0463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Derogatis LR, Morrow GR, Fetting J, et al. The prevalence of psychiatric disorders among cancer patients. JAMA. 1983;249(6):751–7. doi: 10.1001/jama.249.6.751. [DOI] [PubMed] [Google Scholar]
- 119.Lloyd-Williams M, Friedman T. Depression in palliative care patients--a prospective study. Eur J Cancer Care (Engl) 2001;10(4):270–4. doi: 10.1046/j.1365-2354.2001.00290.x. [DOI] [PubMed] [Google Scholar]
- 120.Foland-Ross LC, Gotlib IH. Cognitive and neural aspects of information processing in major depressive disorder: an integrative perspective. Front Psychol. 2012;3:489. doi: 10.3389/fpsyg.2012.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 2014 May; doi: 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brigidi BD, Achenbach TM, Dumenci L, Newhouse PA. Broad spectrum assessment of psychopathology and adaptive functioning with the Older Adult Behavior Checklist: a validation and diagnostic discrimination study. Int J Geriatr Psychiatry. 2010;25(11):1177–1185. doi: 10.1002/gps.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Jacobs SR, Jacobsen PB, Booth-Jones M, Wagner LI, Anasetti C. Evaluation of the functional assessment of cancer therapy cognitive scale with hematopoietic stem cell transplant patients. J Pain Symptom Manage. 2007;33(1):13–23. doi: 10.1016/j.jpainsymman.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 124.Sanford SD, Beaumont JL, Butt Z, Sweet JJ, Cella D, Wagner LI. Prospective longitudinal evaluation of a symptom cluster in breast cancer. J Pain Symptom Manage. 2014;47(4):721–30. doi: 10.1016/j.jpainsymman.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 125.Lai J-S, Butt Z, Wagner L, et al. Evaluating the dimensionality of perceived cognitive function. J Pain Symptom Manage. 2009;37(6):982–95. doi: 10.1016/j.jpainsymman.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wagner LI, Sweet J, Butt Z, Lai J-S, Cella D. Measuring patient self-reported cognitive function: Development of the Functional Assessment of Cancer Therapy-Cognitive Function instrument. J Support Oncol. 2009;7(6):W32–W39. [Google Scholar]


