Abstract
Background
Disruption of the endothelial glycocalyx contributes to acute lung injury in experimental sepsis but has not been well studied in humans. To study glycocalyx degradation in sepsis-induced ARDS, we measured plasma levels of syndecan-1, a marker for glycocalyx degradation.
Methods
The present study is a retrospective observational study of 262 ventilated medical ICU patients at risk of ARDS due to severe sepsis and APACHE II ≥ 25. Plasma syndecan-1 was measured at study enrollment. Primary analysis focused on the association between syndecan-1 levels and the development of ARDS, other organ dysfunction (Brussels criteria), or in-hospital mortality.
Results
Overall, 135 (52%) patients developed ARDS. In patients with non-pulmonary sepsis, syndecan-1 levels were associated with ARDS (p = 0.05). Regardless of etiology of sepsis, higher syndecan-1 levels were associated with hepatic (p < 0.001), renal (p = 0.003), coagulation (p = 0.001), and circulatory (p = 0.02) failure as well as in-hospital mortality (p = 0.001), and there was a significant association between syndecan-1 levels and the number of vasopressors required in the first 24 h (p < 0.001). In addition, elevated syndecan levels were independently predictive of mortality in multivariable logistic regression adjusted for age and APACHE II score (odds ratio 1.85 per log increase in syndecan-1, 95% CI 1.056–3.241, p = 0.03).
Conclusion
The extent of endothelial glycocalyx degradation is associated with non-pulmonary organ dysfunction in subjects with sepsis and is associated with ARDS but only in the subgroup with non-pulmonary sepsis. Measurement of syndecan-1 levels in sepsis patients might be useful for identifying patients at high risk of organ dysfunction and mortality as well as those who could benefit from therapies targeted at protecting or restoring the glycocalyx.
Keywords: Syndecan-1, Glycocalyx, Sepsis, Acute respiratory distress syndrome (ARDS)
Background
The endothelial glycocalyx is a complex layer of glycoproteins, proteoglycans, and glycosaminoglycans that coats the luminal surface of the microvascular endothelium. Hydrated glycosaminoglycans form a thick and rigid endothelial surface layer (ESL) that plays a key role in limiting vascular permeability and regulating leukocyte adhesion [1, 2]. Shedding of the ESL occurs in response to a variety of insults and results in hyperpermeability, inappropriate leukocyte adhesion [3], and loss of capillary autoregulation [4].
The major endothelial cell surface proteoglycans are syndecans, which are heparan sulfate proteoglycans. Syndecan-1 is abundant in the endothelial surface layer, and circulating syndecan-1 is a marker of endothelial glycocalyx degradation [5–11]. In mice, plasma syndecan-1 levels are negatively correlated with ESL thickness and positively correlated with microvascular permeability [12]. Glycocalyx dysfunction and syndecan-1 shedding have been described in a variety of clinical pathophysiologic processes, including sepsis [13], hemorrhagic shock [14], atherosclerosis [15], acute coronary syndrome [16], renal disease [17], diabetes [10], and hypervolemia [18]. Furthermore, elevated plasma syndecan-1 levels have been associated with increased mortality in patients with trauma and sepsis [8, 11]. However, despite the known role of endothelial injury and activation in the pathogenesis of ARDS [19–23], the association between syndecan-1 and development of ARDS or other organ dysfunction in sepsis has not been well studied.
Both pulmonary and non-pulmonary sepsis can lead to ARDS with pulmonary sepsis (due to pneumonia or aspiration of gastric contents) resulting in direct injury to the lung and non-pulmonary sepsis resulting in indirect injury to the lung [22]. In both animal models and patients, direct injury to the lung is characterized by more severe lung epithelial damage, and an indirect insult to the lung is characterized by more severe systemic endothelial injury [24–26]. However, whether there is greater disruption of the glycocalyx in indirect versus direct ARDS has not been well studied. In a mouse model of non-pulmonary sepsis, glycocalyx degradation contributed to acute lung injury, and human studies have shown an association between glycocalyx degradation and development of pulmonary edema [27, 28], findings that support an association between glycocalyx degradation and ARDS. In addition, a study of 17 patients with acute respiratory failure demonstrated differences in the overall pattern of circulating glycosaminoglycans between those with direct versus indirect causes of acute respiratory failure, raising the question of whether glycocalyx degradation differs depending on the mechanism of lung injury. However, syndecan-1 was not measured in that study and it was not reported whether the patients met clinical criteria for ARDS [29].
To address these gaps in knowledge, we designed the current study to test the hypothesis that in critically ill patients with sepsis, the extent of glycocalyx disruption as measured by plasma syndecan-1 levels is associated with development of ARDS and that glycocalyx degradation is more extensive in non-pulmonary sepsis compared to pulmonary sepsis. We further hypothesized that the degree of glycocalyx degradation is associated with other organ dysfunction and adverse clinical outcomes including in-hospital mortality. Finally, we hypothesized that neutrophil activation may contribute to enzymatic cleavage of syndecan-1 from the surface of endothelial cells, and we tested this hypothesis by measuring myeloperoxidase (MPO), a circulating marker of neutrophil activation [26].
Methods
Study design and patient selection
We conducted a retrospective observational study within the validating acute lung injury biomarkers for diagnosis (VALID) study. VALID is a prospective cohort study enrolling critically ill patients in the Vanderbilt Medical, Surgical, Trauma and Cardiovascular ICUs since 2006 [30]. Patients are enrolled on the morning of ICU day 2 if they are not being transferred out of ICU on that day. At the time of enrollment, plasma is obtained for biomarker measurement. Comprehensive clinical data are collected for the first four ICU days including acute physiology and chronic health evaluation II (APACHE II) [31], daily laboratory values, hemodynamics, ventilator settings, medications, and daily phenotyping for severe sepsis, ARDS, and other organ failures. The VALID study is approved by the Vanderbilt Institutional Review Board. Informed consent is obtained from patients or their surrogates if possible; the Institutional Review Board has also granted a waiver of informed consent for this minimal risk study.
For the current study, medical ICU patients enrolled in the VALID study who met Sepsis-2 criteria for severe sepsis [32] were included if they were at high risk of ARDS based on APACHE II score ≥ 25 and need for mechanical ventilation and had citrated plasma available from enrollment day for measurement of syndecan-1. Patients with pneumonia or aspiration of gastric contents were classified as pulmonary sepsis, and the remaining patients were classified as non-pulmonary sepsis [29].
Definitions of ARDS, and non-pulmonary organ failures
ARDS and ARDS severity (mild, moderate, severe) were assessed daily using the Berlin definition [33], based on consensus review of all chest radiographs, blood gases, and clinical data by two physician investigators. If no blood gases were available, then the SpO2/FiO2 ratio was used to assess for the presence and severity of ARDS [34]. Patients who met criteria for ARDS for at least two consecutive study days were considered to have ARDS; patients who met ARDS criteria on only 1 day or on two non-consecutive days were considered to have an indeterminate ARDS status and were excluded from the study. Patients who did not meet criteria for ARDS on any of the 4 study days were included in the non-ARDS group. Patients with evidence of a primary cardiogenic cause for pulmonary edema were excluded from this study [35]. Definitions of circulatory, coagulation, hepatic and renal failures were based on the Brussels criteria [36]. Mortality analyses were conducted using in-hospital mortality as an endpoint.
Biomarker selection and assays
Because endothelial glycocalyx dysfunction has been associated with acute lung injury in animal models of sepsis [24] and syndecan-1 is a well-documented marker of endothelial injury [37], we selected syndecan-1 as our primary marker of damage to the endothelial glycocalyx.
Plasma levels of syndecan-1 and myeloperoxidase were measured in duplicate in thawed citrated plasma samples collected on the morning of ICU day 2 at the time of enrollment into the VALID study using commercially available ELISA kits (Syndecan-1 Item No. ab46506, Abcam, Cambridge, MA, USA) or electrochemiluminescent immunoassays (Myeloperoxidase, Meso Scale Discovery, Rockville, MD, USA).
Statistical analysis
Categorical variables are reported as frequencies (percentages). For clinical characteristics of the cohort, continuous variables were normally distributed and are reported as means with standard deviation, and groups were compared by T test or Chi-square analysis. For the analysis of biomarker levels which were not normally distributed, continuous variables are reported as medians (lower and upper quartiles) and groups were compared by Mann–Whitney U test. Correlation between variables was measured using Spearman’s ρ. For biomarker levels, the association between biomarker quartile and the outcome of interest was assessed by linear-by-linear association. To determine whether syndecan-1 levels were independently associated with in-hospital mortality, multivariable logistic regression was done and included age and APACHE II score, which are independent predictors of mortality, as covariates [31, 38–41]. Statistical analyses were conducted in SPSS Statistics version 24 (IBM, Armonk, NY).
Results
Patient characteristics
We studied 262 critically ill subjects with severe sepsis. Patient demographic and clinical characteristics are summarized in Table 1. Among this group, 127 (48%) had non-pulmonary sepsis, and 135 (52%) had pulmonary sepsis. Compared to patients with pulmonary sepsis, those with non-pulmonary sepsis were less likely to develop ARDS (44 vs. 59%, p = 0.03), more frequently required vasopressors (64 vs. 51%, p = 0.05) but had similar hospital mortality (38 vs. 34%, p = 0.61).
Table 1.
Clinical characteristics of 262 critically ill medical ICU patients with severe sepsis who were included in the study
| All patients N = 262 | Non-pulmonary sepsis N = 127 | Pulmonary sepsis N = 135 | p value | |
|---|---|---|---|---|
| Age (years) | 56 ± 16 | 56 ± 15 | 56 ± 17 | 0.98 |
| Male | 137 (52%) | 63 (50%) | 74 (55%) | 0.46 |
| Caucasian | 224 (86%) | 107 (84%) | 117 (87%) | 0.60 |
| Ever smoker | 149 (57%) | 68 (54%) | 82 (61%) | 0.26 |
| Alcohol abuse | 55 (21%) | 26 (21%) | 30 (22%) | 0.77 |
| APACHE II | 34 ± 6 | 34 ± 6 | 33 ± 6 | 0.06 |
| Primary site of infection | ||||
| Lung | 134 (100%) | |||
| Abdomen | 32 (25%) | |||
| Urinary tract | 23 (18%) | |||
| Skin/soft tissue/bone | 17 (13%) | |||
| CNS/sinus | 17 (13%) | |||
| Endocarditis/catheter | 25 (20%) | |||
| Other or unclear | 13 (10%) | |||
| Required vasopressors | 81 (64%) | 69 (51%) | 0.05 | |
| Developed ARDS | 135 (52%) | 56 (44%) | 79 (59%) | 0.03 |
| Ventilator-free days 150 (57%) | 14 ± 11 | 14 ± 11 | 14 ± 11 | 0.98 |
| Died in hospital | 94 (36%) | 48 (38%) | 46 (34%) | 0.61 |
Data as mean ± SD or n (%) as indicated. p values compare characteristics of groups with pulmonary versus non-pulmonary sepsis by T test or Chi-square analysis as appropriate
Syndecan-1 levels and risk of ARDS
In the entire cohort, syndecan-1 levels were not significantly associated with diagnosis of ARDS (p = 0.17 for linear-by-linear association across quartiles of plasma syndecan-1 levels). However, when only patients with non-pulmonary sepsis were considered, higher syndecan-1 levels were significantly associated with ARDS (p = 0.05, Fig. 1a). ARDS was diagnosed in 50% of patients in the highest quartile of syndecan-1 levels compared to 22% in the lowest quartile (p = 0.028). There was no significant association of syndecan-1 levels with ARDS in the group of patients with pulmonary sepsis (p = 0.72, Fig. 1b).
Fig. 1.
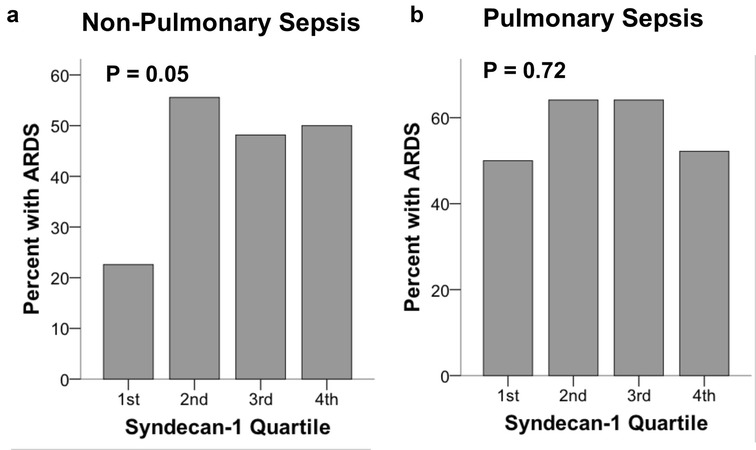
Higher plasma syndecan-1 levels by quartile were associated with development of ARDS in patients with non-pulmonary sepsis (a) but were not associated with ARDS in patients with pulmonary sepsis (b). ARDS was compared across quartiles by linear-by-linear association test
Among patients with ARDS, plasma syndecan-1 levels were significantly higher in patients with indirect ARDS due to non-pulmonary sepsis (n = 56) than in patients with direct ARDS due to pulmonary sepsis (n = 79) (p = 0.017, Fig. 2a). There was a trend toward association of higher syndecan-1 levels with greater ARDS severity (percent of patients with moderate or severe ARDS), but only in the subgroup of ARDS patients with ARDS due to non-pulmonary sepsis (Fig. 2b).
Fig. 2.
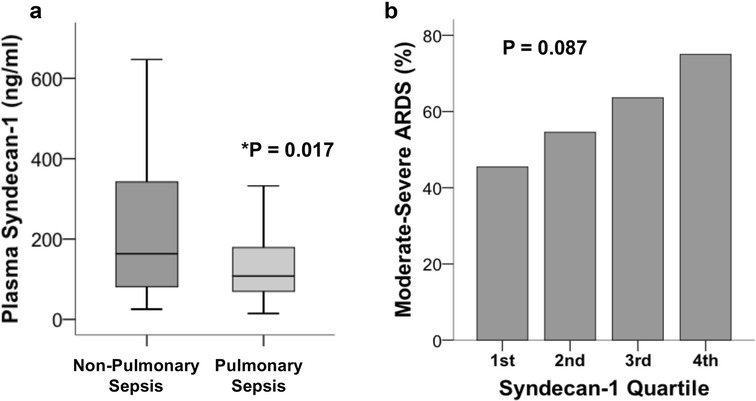
Among patients with ARDS (N = 135), plasma syndecan-1 levels were significantly higher in patients with non-pulmonary sepsis compared to those with pulmonary sepsis (a, p = 0.017 by Mann–Whitney U test). There was a trend for higher syndecan-1 levels to be associated with severity of ARDS in the subgroup (n = 56) of ARDS patients with non-pulmonary sepsis (b, p = 0.087 by linear-by-linear association test)
Syndecan-1 levels and non-pulmonary organ failures
Higher plasma syndecan-1 levels by quartile were significantly associated with the presence of circulatory failure as defined by Brussels Organ Failure Scoring (Fig. 3a, p = 0.016), number of vasopressors required (Fig. 3b, p < 0.001), or fluid balance over the first 24 h (Fig. 3c, p = 0.004). When patients were grouped by need for any vasopressor at enrollment, syndecan-1 levels were significantly higher in those requiring vasopressors (median 157, IQR 84–306 vs. median 84, IQR 52–180, p < 0.001). Higher plasma syndecan-1 levels by quartile were also significantly associated with hepatic failure (Fig. 3d, p < 0.001), renal failure (Fig. 3e, p = 0.004), and coagulation failure (Fig. 3f, p = 0.004) at study enrollment as defined by Brussels organ failure scoring. These analyses were conducted in the entire patient cohort (n = 262). By contrast, plasma lactate levels were not significantly associated with non-pulmonary organ dysfunction (data not shown), although clinically measured levels were only available in a subset of patients (n = 74). Lactate levels and syndecan-1 levels were also not significantly correlated (Spearman’s ρ = 0.181, p = 0.12).
Fig. 3.
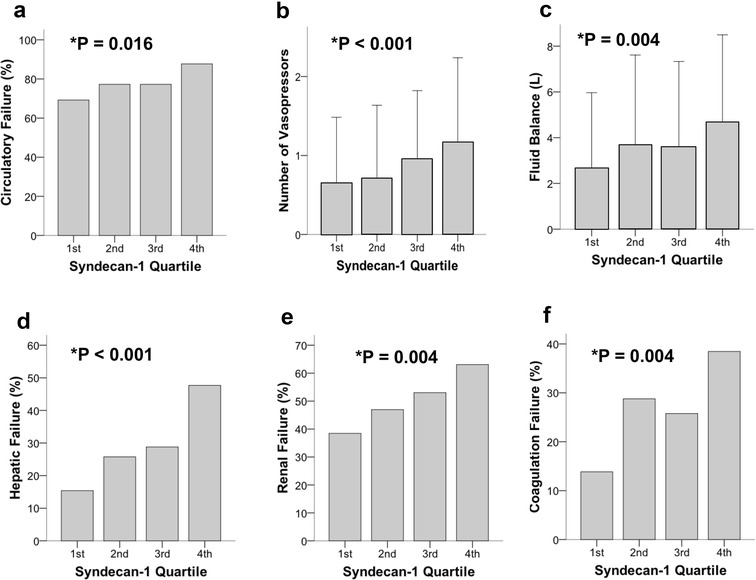
Higher plasma syndecan-1 levels by quartile were associated with non-pulmonary organ dysfunction at enrollment including a circulatory failure, b number of vasopressors, c fluid balance over the first 24 h, d hepatic failure, e renal failure, f coagulation failure. Organ failures were defined by Brussels organ failure scores at enrollment. p values by linear-by-linear association test except for number of vasopressors and fluid balance which were analyzed by linear regression
Syndecan-1 levels and clinical outcomes
In the entire cohort, patients with higher plasma syndecan-1 levels were significantly more likely to die during hospitalization (Fig. 4a, p < 0.001) and to require renal replacement therapy during hospitalization (Fig. 4b, p = 0.001). In-hospital death was much more frequent in patients in the highest quartile of syndecan-1 (48%) compared to patients in the lowest quartile (17%, p < 0.001). Higher syndecan-1 levels were also independently predictive of in-hospital mortality in a multivariable logistic regression model adjusted for age and APACHE II score (Table 2; odds ratio 1.85 per log increase in syndecan-1, 95% CI 1.056–3.241).
Fig. 4.
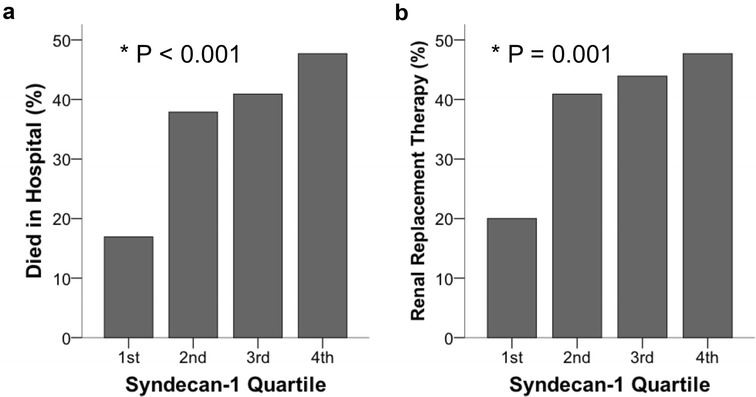
Clinical outcomes including a hospital mortality and b need for renal replacement therapy were strongly associated with increasing plasma syndecan-1 levels in 262 patients with sepsis. p values by linear-by-linear association test
Table 2.
Multivariable logistic regression model for hospital mortality in 262 sepsis patients
| Variable | OR (95% CI) | p value |
|---|---|---|
| Syndecan-1 (per log increase) | 1.850 (1.056–3.241) | 0.031 |
| Age (per year increase) | 1.009 (0.992–1.026) | 0.309 |
| APACHE II (per one point increase) | 1.035 (0.991–1.082) | 0.125 |
Syndecan-1 levels and neutrophil activation
To determine whether syndecan-1 shedding was associated with evidence of systemic neutrophil activation, we measured plasma MPO levels in all patients who had sufficient plasma available after syndecan-1 measurements (n = 247). Plasma MPO levels were only weakly associated with syndecan-1 levels (Spearman’s ρ = 0.21, p < 0.001). In patients with non-pulmonary sepsis, the association was somewhat stronger (Spearman’s ρ = 0.294, p < 0.001). Syndecan-1 levels were not associated with total white blood cell count (ρ = 0.064, p = 0.3).
Discussion
Degradation of the endothelial glycocalyx has been increasingly recognized as an important contributor to the pathophysiology of sepsis [13]. Several studies have demonstrated elevated syndecan-1 levels as a marker of glycocalyx degradation in patients with sepsis. In a study of 150 patients, Steppan et al. [9] compared patients with sepsis and patients after major abdominal surgery to controls and found highest levels of syndecan-1 and inflammatory markers in sepsis patients. Two other small observational studies of eighteen [42] and twenty [11] patients with septic shock demonstrated elevated syndecan-1 levels compared to controls. However, to our knowledge, this is the first large-scale study of syndecan-1 as a biomarker of risk of ARDS and other organ dysfunction in patients with sepsis. Similarly, it is the first study to assess the independent association of this biomarker with sepsis mortality. Our findings suggest that in patients with sepsis, the severity of glycocalyx degradation, as measured by syndecan-1, is strongly associated with organ dysfunction and mortality, but contrary to our primary hypothesis, is only associated with development of ARDS in patients with non-pulmonary sepsis.
The finding of higher syndecan-1 levels in patients with ARDS due to non-pulmonary sepsis compared to ARDS due to pulmonary sepsis suggests that degradation of the endothelial glycocalyx may be more prominent in the pathophysiology of non-pulmonary sepsis. Concordant with this finding, elevated syndecan-1 levels were only associated with development of ARDS in patients with non-pulmonary sepsis. These observations are concordant with our prior observation that other biomarkers of endothelial injury such as angiopoietin-2 and von Willebrand factor antigen are more elevated in non-pulmonary sepsis patients with indirect ARDS compared to pulmonary sepsis patients with direct ARDS [24]. Similarly, the predominance of heparan sulfate fragments in indirect respiratory failure reported by Schmidt et al. [29] likely indicates a greater degree of glycocalyx degradation in that patient group. Taken together, these findings provide strong evidence that injury to the endothelial barrier is more severe in indirect mechanisms of acute lung injury than in direct mechanisms. These findings may explain, in part, why a recent study of a large group of patients found that in contrast to direct ARDS, where age and the severity of ARDS were independent predictors of mortality, in indirect ARDS, only the number of non-pulmonary organ failures was independently associated with mortality [43].
The diagnosis of ARDS is still based on clinical criteria and does not rely on underlying pathophysiology [33]. As a result, current definitions identify a highly heterogeneous group of patients that may have different underlying mechanisms of acute lung injury and different responses to therapy. Using a latent class analysis approach, Calfee and colleagues reported that distinct subphenotypes of ARDS can be identified that respond differently to experimental therapies [44, 45]. The current findings provide further evidence of the heterogeneity of ARDS in humans as defined by the extent of endothelial glycocalyx degradation. If validated, syndecan-1 might be useful as a biomarker to help further distinguish molecular phenotypes of ARDS.
Alternatively, plasma syndecan-1 levels might be used to identify subgroups of sepsis patients for therapy targeted at protection or restoration of the endothelial glycocalyx. In patients with severe trauma and hemorrhagic shock, glycocalyx shedding causes coagulopathy and perturbations in fibrinolysis [8]. Furthermore, recent evidence suggests that transfusion of plasma reduces syndecan-1 shedding and reconstitutes the glycocalyx in hemorrhagic shock [14]. Only 22 patients in the current study received transfusion of fresh frozen plasma prior to blood sampling, and we did not find any relationship between receipt of plasma and syndecan-1 levels (data not shown); however, our power for this analysis was low. Another therapy that may affect the glycocalyx is sevoflurane, which has been shown to reduce glycocalyx shedding and leukocyte adhesion in animal models of ischemia–reperfusion injury [46–48].
This study has several strengths including the large sample size and detailed prospective phenotyping for ARDS and other organ dysfunction as part of the parent VALID cohort study. There are also some limitations. First, the observed association between syndecan-1 shedding and organ dysfunction and clinical outcomes does not prove causation, nor does it elucidate the underlying mechanisms by which glycocalyx shedding contributes to organ dysfunction. Although we hypothesized that neutrophil activation contributes to enzymatic cleavage of syndecan-1 from the endothelial cell surface, we did not find a strong correlation between MPO, a circulating marker of neutrophil activation and syndecan-1 levels. Second, we intentionally selected patients who were more severely ill and more likely to develop ARDS by requiring an APACHE II score of 25 or greater and mechanical ventilation. These inclusion criteria were selected to maximize the likelihood of studying patients with a significant degree of glycocalyx degradation. However, the findings may not be generalizable to a less severely ill patient population, nor are the findings necessarily applicable to patients at risk of ARDS from non-septic causes or to patients with surgical critical illness. Finally, it is not possible to know from the current study what fraction of elevated syndecan-1 levels is due to increased shedding of the glycocalyx, versus impaired clearance of syndecan-1. If syndecan-1 were cleared primarily in the liver or kidney, then the association between elevated levels and kidney and liver dysfunction might simply reflect impaired clearance. However, very little is known about the clearance of syndecan-1 from the circulation. In one study of patients with chronic kidney disease, plasma syndecan-1 clearance was not a function of creatinine clearance [49].
In summary, the current findings highlight the potential importance of disruption of the endothelial surface layer in the pathogenesis of organ dysfunction in sepsis. Further studies are warranted to validate the current findings in other patient populations and to determine the precise mechanisms of organ injury that occurs in association with glycocalyx degradation. In addition, this study adds to the growing body of evidence that sepsis and ARDS are not homogenous disease states. Further characterization of molecular phenotypes of organ dysfunction and acute lung injury in sepsis may help to better target therapies in the future, including therapies targeted at protection and restoration of the endothelial glycocalyx.
Conclusions
In conclusion, our study evaluated the severity of endothelial glycocalyx degradation and its impact on ARDS, non-pulmonary organ dysfunction, and mortality in a cohort of critically ill patients with sepsis. Contrary to our initial hypothesis, elevated syndecan-1 levels were associated with ARDS only in a subgroup of patients with non-pulmonary sepsis, suggesting that degradation of the glycocalyx is more severe in patients with non-pulmonary sepsis, and adding to the growing body of evidence that the mechanisms underlying direct and indirect causes of ARDS are distinct. Regardless of etiology of sepsis, elevated syndecan-1 levels were associated with non-pulmonary organ dysfunction and in-hospital mortality. Together, these findings suggest that measurement of syndecan-1 levels in patients with sepsis may be useful for identifying patients at high risk of organ dysfunction and mortality and those who may benefit from therapies targeted at protecting or restoring the glycocalyx.
Ethics approval and consent to participate: The VALID study was approved by the Vanderbilt Institutional Review Board (#051065). Informed consent was obtained from patients or their surrogates prior to enrollment. Due to the minimal risk of this observational study, a waiver of informed consent was granted by the Institutional Review Boards for patients who were unable to participate in the informed consent process and for whom no surrogate decision maker was available.
Authors’ contributions
LSM designed the study, conducted biomarker measurements, assisted with data analysis and interpretation of results, and drafted the manuscript. LBW designed the study, oversaw data analysis and interpretation of results, and edited the manuscript. NW and JBM conducted and oversaw biomarker measurements and assisted with interpretation of results. CMS and JAB assisted with design of study and interpretation of results and edited the manuscript. AKM enrolled patients in the study and assisted with interpretation of results. All authors read and approved the final manuscript.
Acknowledgements
None.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available in order to protect patient privacy.
Consent for publication
Not applicable.
Funding
This study was supported by funding to LBW and JAB from the NIH (HL103836, HL117676) and funding support to LSM from Office of Medical Student Research and Vanderbilt University School of Medicine. The funding bodies had no role in study design, in collection, analysis, or interpretation of data, or in writing of the manuscript or the decision to submit for publication.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- AECC
American-European consensus conference
- APACHE II
acute physiology and chronic health evaluation II
- ARDS
acute respiratory distress syndrome
- ELISA
enzyme-linked immunosorbent assay
- ESL
endothelial surface layer
- MPO
myeloperoxidase
- VALID
validating biomarkers of acute lung injury for diagnosis
Contributor Information
Laura S. Murphy, Email: laura.murph1@vanderbilt.edu
Nancy Wickersham, Email: nancy.wickersham@vanderbilt.edu.
J. Brennan McNeil, Email: brennan.mcneil@vanderbilt.edu.
Ciara M. Shaver, Email: Ciara.shaver@vanderbilt.edu
Addison K. May, Email: Addison.may@vanderbilt.edu
Julie A. Bastarache, Email: Julie.bastarache@vanderbilt.edu
Lorraine B. Ware, Email: Lorraine.ware@vanderbilt.edu
References
- 1.Yang Y, Schmidt EP. The endothelial glycocalyx: an important regulator of the pulmonary vascular barrier. Tissue Barriers. 2013;1(1):e23494. doi: 10.4161/tisb.23494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt EP, Lee WL, Zemans RL, Yamashita C, Downey GP. On, around, and through: neutrophil-endothelial interactions in innate immunity. Physiol (Bethesda, Md.) 2011;26(5):334–347. doi: 10.1152/physiol.00011.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curry FE, Adamson RH. Endothelial glycocalyx: permeability barrier and mechanosensor. Ann Biomed Eng. 2012;40(4):828–839. doi: 10.1007/s10439-011-0429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Florian JA, Kosky JR, Ainslie K, Pang Z, Dull RO, Tarbell JM. Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ Res. 2003;93(10):e136–e142. doi: 10.1161/01.RES.0000101744.47866.D5. [DOI] [PubMed] [Google Scholar]
- 5.Grundmann S, Fink K, Rabadzhieva L, et al. Perturbation of the endothelial glycocalyx in post cardiac arrest syndrome. Resuscitation. 2012;83(6):715–720. doi: 10.1016/j.resuscitation.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 6.Henrich M, Gruss M, Weigand MA. Sepsis-induced degradation of endothelial glycocalix. Sci World J. 2010;10:917–923. doi: 10.1100/tsw.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofmann-Kiefer KF, Knabl J, Martinoff N, et al. Increased serum concentrations of circulating glycocalyx components in HELLP syndrome compared to healthy pregnancy: an observational study. Reprod Sci (Thousand Oaks, Calif) 2013;20(3):318–325. doi: 10.1177/1933719112453508. [DOI] [PubMed] [Google Scholar]
- 8.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. 2011;254(2):194–200. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- 9.Steppan J, Hofer S, Funke B, et al. Sepsis and major abdominal surgery lead to flaking of the endothelial glycocalix. J Surg Res. 2011;165(1):136–141. doi: 10.1016/j.jss.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 10.Nieuwdorp M, Mooij HL, Kroon J, et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes. 2006;55(4):1127–1132. doi: 10.2337/diabetes.55.04.06.db05-1619. [DOI] [PubMed] [Google Scholar]
- 11.Sallisalmi M, Tenhunen J, Yang R, Oksala N, Pettila V. Vascular adhesion protein-1 and syndecan-1 in septic shock. Acta Anaesthesiol Scand. 2012;56(3):316–322. doi: 10.1111/j.1399-6576.2011.02578.x. [DOI] [PubMed] [Google Scholar]
- 12.Torres Filho IP, Torres LN, Salgado C, Dubick MA. Plasma syndecan-1 and heparan sulfate correlate with microvascular glycocalyx degradation in hemorrhaged rats after different resuscitation fluids. Am J Physiol Heart Circ Physiol. 2016;310(11):H1468–H1478. doi: 10.1152/ajpheart.00006.2016. [DOI] [PubMed] [Google Scholar]
- 13.Burke-Gaffney A, Evans TW. Lest we forget the endothelial glycocalyx in sepsis. Crit Care. 2012;16(2):121. doi: 10.1186/cc11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozar RA, Peng Z, Zhang R, et al. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth Analg. 2011;112(6):1289–1295. doi: 10.1213/ANE.0b013e318210385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cancel LM, Ebong EE, Mensah S, Hirschberg C, Tarbell JM. Endothelial glycocalyx, apoptosis and inflammation in an atherosclerotic mouse model. Atherosclerosis. 2016;252:136–146. doi: 10.1016/j.atherosclerosis.2016.07.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miranda CH, de Carvalho Borges M, Schmidt A, Marin-Neto JA, Pazin-Filho A. Evaluation of the endothelial glycocalyx damage in patients with acute coronary syndrome. Atherosclerosis. 2016;247:184–188. doi: 10.1016/j.atherosclerosis.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 17.Padberg JS, Wiesinger A, di Marco GS, et al. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis. 2014;234(2):335–343. doi: 10.1016/j.atherosclerosis.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 18.Jacob M, Saller T, Chappell D, Rehm M, Welsch U, Becker BF. Physiological levels of A-, B- and C-type natriuretic peptide shed the endothelial glycocalyx and enhance vascular permeability. Basic Res Cardiol. 2013;108(3):347. doi: 10.1007/s00395-013-0347-z. [DOI] [PubMed] [Google Scholar]
- 19.Rinaldo JE, Rogers RM. Adult respiratory-distress syndrome: changing concepts of lung injury and repair. N Engl J Med. 1982;306(15):900–909. doi: 10.1056/NEJM198204153061504. [DOI] [PubMed] [Google Scholar]
- 20.Bernard GR, Artigas A, Brigham KL, et al. The American-European consensus conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 21.Zimmerman GA, Albertine KH, Carveth HJ, et al. Endothelial activation in ARDS. Chest. 1999;116(1 Suppl):18s–24s. doi: 10.1378/chest.116.suppl_1.18S. [DOI] [PubMed] [Google Scholar]
- 22.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 23.Millar FR, Summers C, Griffiths MJ, Toshner MR, Proudfoot AG. The pulmonary endothelium in acute respiratory distress syndrome: insights and therapeutic opportunities. Thorax. 2016;71(5):462–473. doi: 10.1136/thoraxjnl-2015-207461. [DOI] [PubMed] [Google Scholar]
- 24.Calfee CS, Janz DR, Bernard GR, et al. Distinct molecular phenotypes of direct versus indirect ARDS in single-center and multicenter studies. Chest J. 2015;147(6):1539–1548. doi: 10.1378/chest.14-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menezes SL, Bozza PT, Neto HC, et al. Pulmonary and extrapulmonary acute lung injury: inflammatory and ultrastructural analyses. J Appl Physiol (Bethesda, Md : 1985) 2005;98(5):1777–1783. doi: 10.1152/japplphysiol.01182.2004. [DOI] [PubMed] [Google Scholar]
- 26.Pelosi P, D’Onofrio D, Chiumello D, et al. Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur Respir J Suppl. 2003;42:48s–56s. doi: 10.1183/09031936.03.00420803. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt EP, Yang Y, Janssen WJ, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18(8):1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Negrini D, Passi A, Moriondo A. The role of proteoglycans in pulmonary edema development. Intensive Care Med. 2008;34(4):610–618. doi: 10.1007/s00134-007-0962-y. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt EP, Li G, Li L, et al. The circulating glycosaminoglycan signature of respiratory failure in critically ill adults. J Biol Chem. 2014;289(12):8194–8202. doi: 10.1074/jbc.M113.539452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siew ED, Ware LB, Gebretsadik T, et al. Urine neutrophil gelatinase-associated lipocalin moderately predicts acute kidney injury in critically ill adults. J Am Soc Nephrol. 2009;20(8):1823–1832. doi: 10.1681/ASN.2008070673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–1655. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 33.Ranieri VM, Rubenfeld GD, Thompson BT, et al. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 34.Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132(2):410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 35.Ware LB, Matthay MA. Clinical practice. Acute pulmonary edema. N Engl J Med. 2005;353(26):2788–2796. doi: 10.1056/NEJMcp052699. [DOI] [PubMed] [Google Scholar]
- 36.Bernard G. The Brussels Score. Sepsis. 1997;1:43–44. doi: 10.1023/A:1009711301483. [DOI] [Google Scholar]
- 37.Becker BF, Jacob M, Leipert S, Salmon AH, Chappell D. Degradation of the endothelial glycocalyx in clinical settings: searching for the sheddases. Br J Clin Pharmacol. 2015;80(3):389–402. doi: 10.1111/bcp.12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nasa P, Juneja D, Singh O, Dang R, Arora V. Severe sepsis and its impact on outcome in elderly and very elderly patients admitted in intensive care unit. J Intensive Care Med. 2012;27(3):179–183. doi: 10.1177/0885066610397116. [DOI] [PubMed] [Google Scholar]
- 39.Curns AT, Holman RC, Sejvar JJ, Owings MF, Schonberger LB. Infectious disease hospitalizations among older adults in the United States from 1990 through 2002. Arch Intern Med. 2005;165(21):2514–2520. doi: 10.1001/archinte.165.21.2514. [DOI] [PubMed] [Google Scholar]
- 40.Ely EW, Angus DC, Williams MD, Bates B, Qualy R, Bernard GR. Drotrecogin alfa (activated) treatment of older patients with severe sepsis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2003;37(2):187–195. doi: 10.1086/375775. [DOI] [PubMed] [Google Scholar]
- 41.Girard TD, Opal SM, Ely EW. Insights into severe sepsis in older patients: from epidemiology to evidence-based management. Clin Infect Dis Off Publ Infect Dis Soc Am. 2005;40(5):719–727. doi: 10.1086/427876. [DOI] [PubMed] [Google Scholar]
- 42.Nelson A, Berkestedt I, Schmidtchen A, Ljunggren L, Bodelsson M. Increased levels of glycosaminoglycans during septic shock: relation to mortality and the antibacterial actions of plasma. Shock (Augusta, Ga) 2008;30(6):623–627. doi: 10.1097/SHK.0b013e3181777da3. [DOI] [PubMed] [Google Scholar]
- 43.Luo L, Shaver CM, Zhao Z, Koyama T, Calfee CS, Bastarache JA, Ware LB. Clinical predictors of hospital mortality differ between direct and indirect ARDS. Chest. 2017;151:755–763. doi: 10.1016/j.chest.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calfee CS, Delucchi K, Parsons PE, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2(8):611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Famous KR, Delucchi K, Ware LB, Kangelaris KN, Liu KD, Thompson BT, Calfee CS, ARDS Network Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med. 2017;195:331–338. doi: 10.1164/rccm.201603-0645OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chappell D, Heindl B, Jacob M, et al. Sevoflurane reduces leukocyte and platelet adhesion after ischemia-reperfusion by protecting the endothelial glycocalyx. Anesthesiology. 2011;115(3):483–491. doi: 10.1097/ALN.0b013e3182289988. [DOI] [PubMed] [Google Scholar]
- 47.Casanova J, Simon C, Vara E, Sanchez G, Rancan L, Abubakra S, Calvo A, Gonzalez FJ, Garutti I. Sevoflurane anesthetic preconditioning protects the lung endothelial glycocalyx from ischemia reperfusion injury in an experimental lung autotransplant model. J Anesth. 2016;30:755–762. doi: 10.1007/s00540-016-2195-0. [DOI] [PubMed] [Google Scholar]
- 48.Annecke T, Chappell D, Chen C, et al. Sevoflurane preserves the endothelial glycocalyx against ischaemia-reperfusion injury. Br J Anaesth. 2010;104(4):414–421. doi: 10.1093/bja/aeq019. [DOI] [PubMed] [Google Scholar]
- 49.Padberg JS, Wiesinger A, di Marco GS, et al. Damage of the endothelial glycocalyx in chronic kidney disease. Atherosclerosis. 2014;234(2):335–343. doi: 10.1016/j.atherosclerosis.2014.03.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available in order to protect patient privacy.


