Abstract
Introduction
Diabetic foot ulcers (DFUs) are complex chronic wounds which have a major long-term impact on the morbidity, mortality and quality of patients. The objective of this study was to assess the efficacy and time sensitivity of human amnion/chorion membrane treatment in patients with chronic DFUs.
Methods
The Cochrane Library, PubMed, Embase and Web of Science databases were systematically searched to identify relevant articles up to 10 April 2017. All randomized controlled trials (RCTs) comparing human amnion/chorion membrane + standard therapy and standard therapy alone in patients with DFUs were included in the analysis. Eligible studies were reviewed and data extracted into standard form. The Cochrane Collaboration’s tool for assessing the risk of bias was used. Review manager version 5.3 software was used for statistical analysis. Data were analyzed using a random effect model.
Results
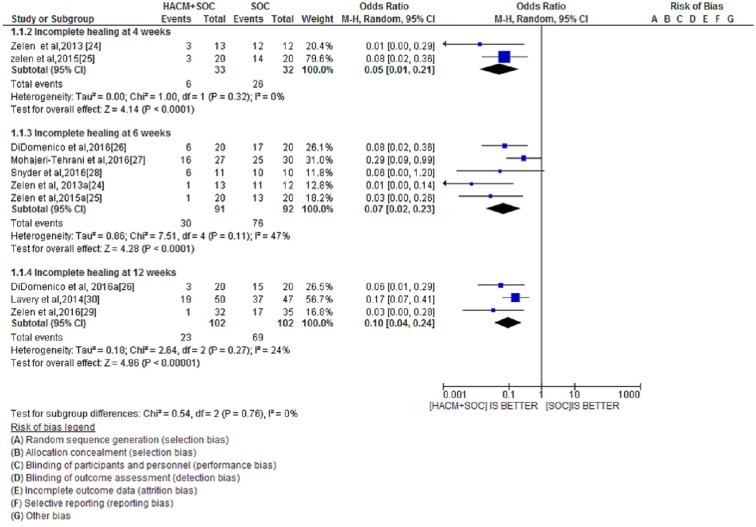
Overall, the initial search of the four databases identified 352 published studies; of these, seven RCTS were ultimately included in the meta-analysis. The overall test effect in the group assessed at 4 weeks was Z = 4.14 [P < 0.0001; odds ratio (OR) 0.05; 95% confidence interval (CI) 0.01–0.21]. The overall test effect in the group assessed at 6 weeks was Z = 4.28 (P < 0.0001; OR 0.07; 95% CI 0.02–0.23). The overall effect in the group assessed at 12 weeks was Z = 4.96 (P < 0.00001; OR 0.10; 95% CI 0.04–0.24. The results showed that patients receiving amniotic membrane + standard therapy had far fewer incomplete healing wounds than those receiving standard of care alone. Assessment of the wound healing state at 4 and 6 weeks revealed that the wound healing state was almost the same, but there was a net difference of wound healing state at 12 weeks.
Conclusion
Human amnion/chorion membrane + standard of care treatment heals DFUs significantly faster than standard of care alone. When using the amnion in patients with DFUs, the optimal times to assess progress in wound healing should be 4 and 12 weeks.
Electronic supplementary material
The online version of this article (doi:10.1007/s13300-017-0298-8) contains supplementary material, which is available to authorized users.
Keywords: Amniotic membrane, Diabetic foot ulcers, Meta-analysis, Standard therapy, Systematic review
Introduction
The prevalence of diabetes continues to rise each year. In 2011, it was estimated that approximately 366 million people—7% of the world’s population—had diabetes, with approximately 80% of these people living in developing countries [1]. The expectation is that these numbers will increase to 552 million adults (8.3% of the world’s population) by 2030 [1]. Complications due to diabetes are also a growing public health problem. One of the most common complications of diabetes is diabetic foot ulcers (DFUs). DFUs are complex chronic wounds that have a major long-term impact on the morbidity, mortality and quality of patients’ lives [2, 3]. Individuals who develop DFUs are at greater risk of premature death, myocardial infarction and fatal stroke than those without a history of this complication [4]. Approximately 25% of people with diabetes will develop a lower extremity ulcer over time [5, 6]. The treatment of these wounds remains challenging as they are often slow to heal and frequently reoccur. The poor prognosis of DFUs is attributed to those conditions often associated with diabetes, such as peripheral vascular diseases, neuropathy and poor blood glucose control. The delayed healing of ulcers increases the risk for severe wound infection and amputation [7, 8]; approximately half of all people undergoing non-traumatic amputations are diagnosed with diabetes [9], with studies showing that up to 88% of all diabetes-related amputations are preceded by a foot ulcer [10, 11]. Amputation can in turn increase morbidity and healthcare costs while at the same time reduce an individual’s productivity and quality of life. Moreover, the mortality following amputation increases with level of amputation [12]. A 5-year mortality rate is very high among patients with any amputation (major and minor combined), ranging from 53 to 100%, and in patients with major amputation, it ranges from 52 to 80% [13]. Mortality following amputation is comparable to many types of cancer [10]. Therefore, any treatment that can reduce amputation rates is more than welcome. The main goal of treating DFUs is to promote a rapid and complete healing in order to reduce the risk of infection, amputation and other form of related complications.
Moist dressings, debridement, wound offloading and infection control are standard therapy or standard of care (SOC) in the management of DFUs. Standard therapy is commonly used in many clinic centers across the world. The Wound Healing Society guidelines recommend consideration of advanced wound therapies if the diabetic ulcer does not decrease in size by 40% or more after 4 weeks of standard therapy [14]. However, the guidelines of the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine recommend adjunctive wound therapy if DFUs fail to improve (reduction of >50% wound area) after a minimum of 4 weeks of standard wound therapy [15].
Processed human amnion/chorion membrane (cryopreserved, dehydrated or acellular) is considered to be an adjunctive wound therapy. Several studies have recently shown its effectiveness in diabetic wound healing [16, 17]. It promotes wound closure, resulting in a more consistent and faster healing of chronic DFUs when compared with standard therapy alone [18]. In fact, many studies have shown human amniotic membrane has properties that enhance healing. The PURION® processed dehydrated human amnion/chorion membrane (dHACM) retains biologically active growth factors and regulatory factors that are in part responsible for its clinical effectiveness in wound healing [19]. dHACM can stimulate diabetic adipose-derived stem cells (ADSCs) to migrate, proliferate and alter cytokine expression, suggesting that ADSCs may respond to dHACM to accelerate diabetic wound healing [20]. Cell-seeded acellular amniotic membrane has the potential to deliver autologous or allogeneic cells to treat a variety of conditions, including DFUs, corneal defect and severe skin burns [21]. Cryopreserved human amniotic membrane retains all of its native human amniotic membrane components, including viable endogenous cells that can enhance angiogenic activity in chronic wounds [22]. Amnion products are now available and can be found in different forms according to their components or processing methods [23].
To further evaluate the efficacy and time sensitivity of human amnion/chorion membrane (HACM) allografts in patients with chronic DFUs, we performed a systematic review and meta-analysis to compare HACM + SOC with SOC alone (HACM + SOC vs. SOC alone), since there has never been any systematic review and meta-analysis of this topic.
Methods
Study Selection and Data Extraction
The PubMed, Embase, Web of Science and Cochrane Library databases were systematically searched for relevant papers up to and including 10 April 2017. To identify all relevant studies, we used the search terms “diabetic foot ulcers” OR “diabetic foot” AND “amniotic membrane” OR “amnion” OR “bioimplant dressing” OR “Grafix” OR “EpiFix” AND “standard therapy “or “standard of care.”
The eligibility criteria were: (1) prospective randomized control trials (RCTs); (2) studies comparing human amniotic membrane + standard therapy versus standard therapy in patients with DFUs; (3) studies in which an assessment of wound healing rates was conducted at least within a period of no less than 4 weeks and no more than 12 weeks; (4) studies published in English; (5) studies with complete outcome. Studies were excluded when they did not fulfill these inclusion criteria.
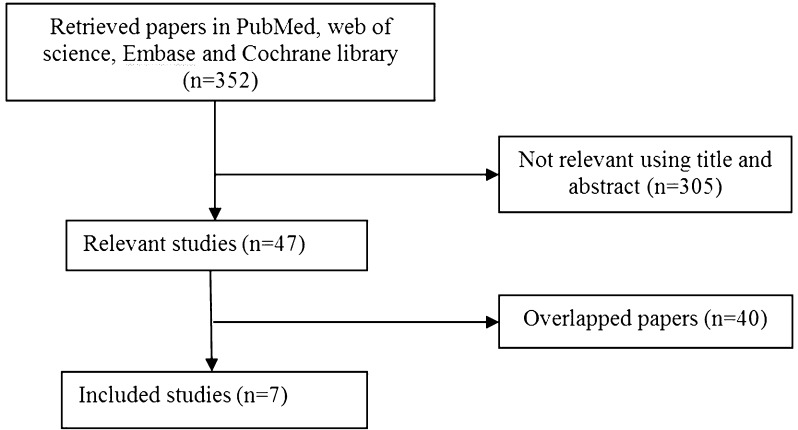
A flowchart of the study selection is shown in Fig. 1.
Fig. 1.
Flowchart of study selection
To be able to focus on the efficacy and time sensitivity of HACM + SOC treatment, the outcomes of the seven studies included in our analysis were subdivided into three groups: (1) assessment at 4 weeks [24, 25]; (2) assessment at 6 weeks [24–28]; (3) assessment at 12 weeks [26, 29, 30].
The three groups of assessment time points with respect to incomplete healing are shown in a forest plot in Fig. 2. In each group, we compared unhealed ulcers using two interventions (HACM + SOC and SOC alone).
Fig. 2.
Forest plot of incomplete healing at the different assessment time points comparing combined HACM + SOC treatment versus SOC alone. HACM Human amnion/chorion membrane, SOC standard of care/standard therapy, CI confidence interval
Study Quality and Risk of Bias Assessment
All of the authors worked independently to search for and assess studies for their methodological quality. The Cochrane Collaboration’s tool for assessing the risk of bias was used. This tool includes seven entries: random sequence generation, allocation concealment, blinding of participants, blinding of personnel, blinding of outcome assessor, incomplete outcome data and selective reporting. If one study had more than two “high-risk” entries, it was considered to be of low quality; otherwise, it was considered to be of high quality [31]. Any disagreement in the assessment of studies was resolved by consensus and, if necessary, Qing Feng Cheng and Qi Fu Li were consulted.
Statistical Analysis
Odds ratios (ORs) with 95% of confidence interval (CIs) were calculated to assess the effect of dichotomous data. I 2 and P values were calculated to assess the heterogeneity among studies (I 2 > 50% and/or P < 0.1 were considered to be statistically significant). The ORs were pooled using only a random effects model to calculate a more conservative result. When ORs were <1, the intervention was associated with lower risk on outcome; when ORs were >1, the intervention was more associated with higher risk on outcome. Subgroup analyses were performed according to the time of wound healing assessment. The subgroup differences were also tested. Sensitivity analyses were performed on the high-quality studies. P < 0.05 was considered to indicate a statistically significant difference in outcome between HACM + SOC and SOC alone. Review Manager version 5.3 software was employed for the statistical analyses.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Results
A total of 352 published studies were retrieved from the four databases, among which 47 were potentially relevant. Further study of these 47 potentially relevant papers revealed that 40 were overlapping studies and subsequently excluded from the analysis. Seven studies were ultimately were included in the meta-analysis [24–30] (Fig. 1), of which six were conducted in USA and one was conducted in Iran. The studies and the properties of the commercial amniotic membrane products are summarized in Tables 1 and 2.
Table 1.
Details on the seven studies included in the meta-analysis
| References | Study design, region and year | Period of enrollment and target population | Treatment groups | Definition of ulcers | n |
|---|---|---|---|---|---|
| Zelen et al. [24] | Single-center nonblinded RCT in southwest Virginia (USA); 2013 | Patients with DFUs | dHACM (EpiFix; MiMedx, Marietta, GA) + SOC vs. SOC |
Ulcer size >1 and <25 cm2 Ulcer duration of >4 weeks No clinical sign of infection |
25 |
| Mohajeri-Tehrani et al. [27] | RCT in Tehran, Iran; 2016 | November 2010 till March 2012; patients with DFUs | AHAM + SOC vs. SOC |
Ulcer size >2 cm2 Control group (IW = 16) Intervention group (IW = 11) |
57 |
| Lavery et al. [30] | Multicenter, single- blinded RCT, in USA; 2016 | May 2012 to April 2013; patients with DFUs | Cryopreserved human amniotic membrane (Grafix; Osiris Therapeutics, Inc., Columbus, MD) + SOC vs. SOC |
Ulcer size >1 and <15 cm2 Ulcer duration of >4 weeks and <52 weeks No active infection |
97 |
| Zelen et al. [29] | Nonblinded RCT in USA; 2016 | Patients with DFUs | EpiFix + SOC vs. SOC |
Ulcer size ≥1 and <25 cm2 Ulcer duration ≥4 weeks No clinical signs of infection |
67 |
| Zelen et al. [25] | Multicenter, nonblinded RCT in Virginia (USA);; 2015 | Patients with DFUs | EpiFix + SOC vs. SOC |
Ulcer size ≥1 and <25 cm2 Ulcer duration ≥4 weeks No clinical signs of infection |
40 |
|
DiDomenico et al. [26] |
Multicenter RCT in USA; 2016 | 23 March 2015 to 23 March 2016; patients with DFUs | dHACM + SOC vs. SOC |
Ulcer size >1 cm2 Ulcer duration ≥4 weeks No signs of infections |
40 |
| Snyder et al. [28] | Multicenter, nonblinded RCT in USA; 2016 | Patients with DFUs | DAMA + SOC vs. SOC |
Ulcer size >1 and <25 cm2 Ulcer duration ≥1 month No clinical sign of infection or osteomyelitis |
21 (PP) |
AHAM Acellular human amniotic membrane, DAMA Dehydrated amniotic membrane allograft, DFUs diabetic foot ulcers, dHACM dehydrated human amnion/chorion membrane, IW infected wounds, PP per protocol RCT randomized control trial, SOC standard of care/standard therapy
Table 2.
Characteristics of the commercial amniotic membrane products used in the seven studies included in the meta-analysis
| Amniotic membrane products | Manufacturer | Components | Processing method | Application |
|---|---|---|---|---|
| Amnioexcel [28] | Derma Sciences | Amnion | Dehydration | Patch |
| Epifix [24, 25, 29] | MiMedx | Amnion and chorion | Dehydration | Patch |
| Grafix [30] | Osiris Therapeutics | Amnion and chorion | Cryopreservation | Plastic applicator |
| AmnioBand [26] | Musculoskeletal Transplant Foundation | Amnion and chorion | Dehydration | Graft application |
| Life Patch [27] | International Bioimplant Co., Tehran, Iran | Amnion | Decellularization | Patch |
Study Characteristics
In all studies, minimum management consisted of standard care of the wound (SOC). In the intervention groups, treatment with amniotic membrane was added to the therapeutic regimen. As result, data comparing amniotic membrane + standard therapy versus standard therapy alone were considered (Table 3). As in the RCT of Snyder et al. [28], we considered per protocol subjects because eight patients were withdrawn at an early stage (4 in each group).
Table 3.
Illustration of intervention group versus control group
| Author | Management of Intervention group | Management of control group |
|---|---|---|
| Mohajeri-Teherani et al. [27] | Use of bio-implant dressing (acellular human amniotic collagen membrane) weekly + sterile gauze and adhesive tape daily (n = 27) | Use of sterile gauze and adhesive tape daily (n = 30) |
| Zelen et al. [24] | Debridement, Epifix (dehydrated human amniotic membrane allograft), non-adherent dressing, compression dressing and offloading (n = 13) | Debridement, moist dressing, compression dressing and offloading (n = 12) |
| zelen et al. [25] | Epifix, debridement, moist dressing, compressive dressing, ulcer measurement and photography, non-adherent dressing and offloading (n = 20) | Debridement, collagen alginate and gauze dressing, ulcer measurement, photography and offloading (n = 20) |
| Zelen et al. [29] | Epifix, debridement, cleansing with normal sterile saline solution, ulcer measurement and photography, non-adherent dressing, offloading (n = 32) | Debridement, cleansing with normal saline solution, collagen-alginate and gauze dressing, ulcer measurement and photography, offloading (n = 35) |
| DiDomenico et al. [26] | Offloading, appropriate debridement, moist dressing, dHACM (n = 20) | Offloading, appropriate debridement, moist dressing [n = 20] |
| Lavery et al. [30] | Standard wound care (debridement, offloading, non-adherent dressing, saline moistened gauze, Grafix (cryopreserved human amniotic membrane) (n = 50) | Standard wound care (debridement, offloading, non-adherent dressing, saline moistened gauze) (n = 47) |
| Snyder et al. [28] | Debridement, moist dressing, offloading, infection surveillance and management, DAMA (PP, n = 11) | Debridement, moist dressing, offloading, infection surveillance and management (PP, n = 10) |
Patients Characteristics
The baseline characteristics regarding age, gender, body mass index, percentage glycated hemoglobin, ankle brachial index, ulcer type, ulcer location, ulcer etiology, wound surface area, wound duration, infection and Wagner grade of patients were comparable between the intervention group (HACM + SOC) and control group (SOC). There were no statistically significant differences at baseline between the intervention group and control group in all studies.
Risk of Bias
Risk of bias assessment for each study is summarized in Table 4. All included studies were of high quality.
Table 4.
Risk of bias of the studies
| Author | Entry | Judgment | Support judgment |
|---|---|---|---|
| Zelen et al. [24] | Random sequence | Low risk | Patients were randomly assigned |
| Allocation concealment | Unclear | Not stated | |
| Blinding personnel | High risk | No blinding | |
| Blinding participant | High risk | No blinding | |
| Blinding outcome | Unclear | Not stated | |
| Incomplete outcome | Low risk | All participants were followed until the end | |
| Selective reporting | Low risk | The endpoint was expected | |
| Zelen et al. [25] | Random sequence | Low risk | Patients were randomly assigned and divided into 2 groups |
| Allocation concealment | Low risk | An envelope was randomly shuffled and labeled | |
| Blinding personnel | High risk | Physicians were aware of treatment | |
| Blinding participant | High risk | No blinding because of different handling requirements | |
| Blinding outcome | Low risk | Experts of wounds were blinded | |
| Incomplete outcome | Low risk | All randomized patients were followed until the end | |
| Selective reporting | Low risk | The endpoint was expected | |
| Zelen et al. [29] | Random sequence | Low risk | Patients were randomly assigned |
| Allocation concealment | Low risk | Placing each sheet of paper in an envelope and sealing it | |
| Blinding personnel | High risk | No blinding | |
| Blinding participant | High risk | No blinding | |
| Blinding outcome | Unclear | Not stated | |
| Incomplete outcome | Low risk | All randomized patients were followed until the end of study | |
| Selective reporting | Low risk | The endpoint was expected | |
| Lavery et al. [30] | Random sequence | Low risk | Patients were randomly assigned |
| Allocation concealment | Unclear | Not stated | |
| Blinding participant | Low risk | Single blinded study | |
| Blinding personnel | High risk | No blinding | |
| Blinding Outcome | Low risk | Two blinded wound care experts | |
| Incomplete outcome | Low risk | All randomized patients were followed until the end | |
| Selective reporting | Low risk | Expected endpoint | |
| Mohajeri-Tehrani et al. [27] | Random sequence | Low risk | Randomly assigned |
| Allocation concealment | Unclear | Not stated | |
| Blinding participant | High risk | Patients were informed about the study | |
| Blinding personnel | Unclear | Not stated | |
| Incomplete outcome | Low risk | All randomized patients were followed until the end | |
| Blinding outcome | Unclear | Not stated | |
| Selective reporting | Low risk | The endpoint was expected | |
| Snyder et al. [28] | Random sequence | Low risk | Patients were randomly assigned |
| Allocation concealment | Unclear | Not stated | |
| Blinding participant | High risk | Open-label study | |
| Blinding personnel | High risk | Open-label study | |
| Incomplete outcome | Low risk | All PP randomized patients were followed until the end | |
| Blinding outcome | Unclear | Not stated | |
| Selective reporting | Low risk | Expected endpoint | |
| DiDomenico et al. [26] | Random sequence | Low risk | Patients were randomly assigned |
| Allocation concealment | Low risk | Placing each sheet of paper in envelope and sealing it | |
| Blinding personnel | Low risk | Site investigators were not aware of methods used | |
| Blinding patients | Unclear | Not stated | |
| Blinding outcome | Low risk | Independent physicians were blinded | |
| Incomplete outcome | Low risk | All randomized patients were followed until the end | |
| Selective reporting | Low risk | Expected endpoint |
Outcome
There were far fewer unhealed wounds in patients receiving amniotic membrane + SOC treatment than in those receiving SOC alone. The overall effect in the group assessed at 4 weeks was Z = 4.14 (P < 0.0001; OR 0.05; 95% CI 0.01–0.21). The overall effect in the group assessed at 6 weeks was Z = 4.28 (P < 0.0001; OR 0.07; 95% CI 0.02–0.23). The overall effect in the group assessed at 12 weeks was Z = 4.96 (P < 0.00001; OR 0.10; 95% CI 0.04–0.24.
The heterogeneity in each subgroup was not statistically significant. In the subgroup assessed at 4 weeks, I 2 = 0% (P = 0.32); in the subgroup assessed at 6 weeks, I 2 = 47% (P = 0.11); in the subgroup assessed at 12 weeks, I 2 = 24% (P = 0.27).
In the test for subgroup differences, the heterogeneity was also not statistically significant. For the subgroup differences, I 2 = 0% (P = 0.76)
Discussion
The results of this systematic review and meta-analysis show that amniotic membrane is beneficial for treating chronic DFUs when it is combined with the SOC. Several earlier studies have already shown the great efficacy of the combined treatment amniotic membrane + SOC in enhancing the healing of complicated wounds. Our results enrich these previous findings.
In all subgroups assessed, the efficacy of treatment with amniotic membrane was remarkable. The overall effect of incomplete wound healing between the intervention group and control group at 4, 6 and 12 weeks was Z = 4.14 (P < 0.0001; OR 0.05; 95% CI 0.01–0.21), Z = 4.28 (P < 0.0001; OR 0.07; 95% CI 0.02–0.23) and Z = 4.96 (P < 0.00001; OR 0.10; 95% CI 0.04–0.24), respectively. In all three subgroups, the odds ratio and their standard deviation were <1, indicating that the incomplete healing outcomes are less associated with amniotic membrane + SOC. In addition, in all the groups, the P value was <0.05. There is statistically significant difference between the intervention group (HACM + SOC) and the control group (SOC). Those results support previous findings that the amniotic membrane + SOC treatment has a greater efficacy for wound healing than SOC alone. This combined treatment strategy allows wound closure faster than the SOC, which is in turn beneficial for the treatment of DFUs. In fact, the main goal of DFU treatment is to promote rapid and complete healing in order to reduce the risk for infection, amputation and other forms of related complications.
The use of placental membranes for wound healing was first reported towards the middle of the last century [32]. Initially, the amnion was predominantly viewed as a treatment for burns, and reports on its use in the treatment of chronic ulcers was limited to a few cases and studies [33–35]. However, more recently it has been shown that the human amnion/chorion membrane is beneficial for treating and healing DFUs [24–30].
The intrinsic properties of the amnion explain its efficacy in enhancing the healing of chronic DFUs. HACM has the potential to positively affect four distinct and pivotal physiological processes closely involved in wound healing, namely cell proliferation, inflammation, metalloproteinase activity and recruitment of progenitor cells [19]. HACM also has the potential to promote revascularization and tissue healing within poorly vascularized, non-healing wounds as it contains angiogenic growth factor that retains biological activity [36]. The low immunogenicity of HACM also permits its allogeneic use. It acts as a physical barrier against bacterial contamination and also creates the moist environment required for healing. Furthermore, it reduces pain and has anti-inflammatory, anti-fibrotic, and anti-microbial activities that are beneficial to wound healing [22, 37].
Our assessment of unhealed wounds showed no statistical difference in unhealed wounds at the 4-week and 6-week time points (Z = 4.14; P < 0.0001; OR 0.05, 95% CI 0.01–0.21 vs. Z = 4.28; P < 0.0001; OR 0.07; 95% CI 0.02–0.23, respectively). There was a statistical difference at 12 weeks (Z = 4.96; P < 0.00001; OR 0.10; 95% CI 0.04–0.24. Thus, the sensitivity to the intervention at 4 weeks was almost the same as that at 6 weeks but it was statistically different from that at 12 weeks. Our analysis reveals that when using HACM as adjuvant therapy in patients with DFUs, the optimal times to assess healing should be 4 weeks and 12 weeks. This result correlates positively with previous findings. It should be noted that when SOC alone is used as treatment, a wound that does not decrease in area by ≥40% at 4 weeks has very little chance of healing at 12 weeks [14]; therefore, other treatments should be considered. Early decrease in size indicates a good prognosis. In fact, 4 weeks of observation is a robust predictor of healing at 12 weeks [38].
In addition, amniotic membrane products are relatively inexpensive and even more effective [29]. As such, such products should be useful in low-income countries.
The studies included in the meta-analysis have a number of limitations. Although we observed a great efficacy of amniotic membrane treatment in these studies, it cannot be used alone. The SOC must be accompanied by at least debridement and dressing changes. Secondly, most of our studies assessed the amniotic membrane effect in clinically uninfected wounds, with the only exception being the study of Mohajeri-Tehrani et al. [27] in which some clinically infected wounds were included. As HACM has antibacterial properties, more RCTs are needed to assess the efficacy of this treatment in clinically infected wounds. It is not always straightforward to rule out infections in initially clinically uninfected wounds of diabetic patients. Finally, the number of studies and the sample sizes were not sufficiently large, which can increase biases.
Conclusions
This systematic review and meta-analysis included studies conducted in different countries. The final results demonstrate that human amnion/chorion membrane + standard of care heals DFUs significantly faster than SOC alone. Based on the current evidence of this meta-analysis and previous findings, we suggest that when HACM is used to treat patients with DFUs, the optimal times to assess healing should be 4 weeks and 12 weeks. Further, large-sized studies or RCTs are still needed to verify our findings and assess healing in infected DFUs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
No funding or sponsorship was received for this study or publication of this article. The article processing charges were funded by the authors. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for integrity of the work as whole, and have given approval for the version to be published. We thank Dr Zhang Fan, Mrs Shu Hua Deng, Mr John Belly and Mr. Libere Godwill for their fruitful advices. Irakoze Laurent contributed to the study design, researched the data, contributed to the discussion, and wrote and edited the manuscript. Astère Manirakiza contributed to the study design and discussion, and reviewed and edited the manuscript. Kan Ran Wang researched the data, contributed to the discussion and reviewed the manuscript. Qing Feng Cheng and Qi Fu Li are guarantors of this work and then have full access to all data in the study and they take responsibility for the integrity of the data and the accuracy of the data analysis. All authors approved the final version.
Disclosures
Irakoze Laurent, Manirakiza Astère, Kan Ran Wang, Qing-feng Cheng and Qi Fu Li have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies of human or animal subjects performed by any of the authors.
Data Availability
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Enhanced content
To view enhanced content for this article go to http://www.medengine.com/Redeem/D8FBF0607D370E56.
References
- 1.Bakker K, Schaper NC. The development of global consensus guidelines on the management and prevention of the diabetic foot 2011. Diabetes Metab Res Rev. 2012;28(Suppl 1):116–118. doi: 10.1002/dmrr.2254. [DOI] [PubMed] [Google Scholar]
- 2.Haria JM, Singh VK, Jain SK. Life with diabetic foot ulcer—a cross sectional study. Int J Sci Study. 2014;1(6):33–35. [Google Scholar]
- 3.Centre for Clinical Practice at National Institute for Health and Clinical Excellence (2011) Guidance. Diabetic foot problems: inpatient management of diabetic foot problems. National Institute for Health and Clinical Excellence, London. [PubMed]
- 4.Brownrigg JR, Davey J, Holt PJ, Davis WA, Thompson MM, Ray KK, Hinchliffe RJ. The association of ulceration of the foot with cardiovascular and all-cause mortality in patients with diabetes: a meta-analysis. Diabetologia. 2012;55(11):2906–2912. doi: 10.1007/s00125-012-2673-3. [DOI] [PubMed] [Google Scholar]
- 5.Kirsner RS, Sabolinski ML, Parsons NB, Skornicki M, Marston WA. Comparative effectiveness of a bioengineered living cellular construct vs. a dehydrated human amniotic membrane allograft for the treatment of diabetic foot ulcers in a real world setting. Wound Repair Regen. 2015;23(5):737–744. doi: 10.1111/wrr.12332. [DOI] [PubMed] [Google Scholar]
- 6.Metelko Z, Brkljacic Crkvencic N. Prevention of diabetic foot. Acta medica Croatica: casopis Hravatske akademije medicinskih znanosti. 2013;67(Suppl 1):35–44. [PubMed] [Google Scholar]
- 7.Lipsky BA, Berendt AR, Cornia PB, Pile JC, Peters EJ, Armstrong DG, Deery HG, Embil JM, Joseph WS, Karchmer AW, Pinzur MS, Senneville E. Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54(12):e132–e173. doi: 10.1093/cid/cis346. [DOI] [PubMed] [Google Scholar]
- 8.Mulder G, Tenenhaus M, D’Souza GF. Reduction of diabetic foot ulcer healing times through use of advanced treatment modalities. Int J Low Extrem Wounds. 2014;13(4):335–346. doi: 10.1177/1534734614557925. [DOI] [PubMed] [Google Scholar]
- 9.Kvitkina T, Narres M, Claessen H, Droste S, Morbach S, Kuss O, Icks A. Incidence of lower extremity amputation in the diabetic compared to the non-diabetic population: a systematic review protocol. Syst Rev. 2015;4:74. doi: 10.1186/s13643-015-0064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilbek TE, Jansen RB, Jorgensen B, Svendsen OL. The diabetic foot in a multidisciplinary team setting. Number of amputations below ankle level and mortality. Exp Clin Endocrinol Diabetes. 2016;124(9):535–540. doi: 10.1055/s-0042-109260. [DOI] [PubMed] [Google Scholar]
- 11.Alvarsson A, Sandgren B, Wendel C, Alvarsson M, Brismar K. A retrospective analysis of amputation rates in diabetic patients: can lower extremity amputations be further prevented? Cardiovasc Diabetol. 2012;11:18. doi: 10.1186/1475-2840-11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown ML, Tang W, Patel A, Baumhauer JF. Partial foot amputation in patients with diabetic foot ulcers. Foot Ankle Int. 2012;33(9):707–716. doi: 10.3113/FAI.2012.0707. [DOI] [PubMed] [Google Scholar]
- 13.Thorud JC, Plemmons B, Buckley CJ, Shibuya N, Jupiter DC. Mortality after nontraumatic major amputation among patients with diabetes and peripheral vascular disease: a systematic review. J Foot Ankle Surg. 2016;55(3):591–599. doi: 10.1053/j.jfas.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 14.Steed DL, Attinger C, Colaizzi T, Crossland M, Franz M, Harkless L, Johnson A, Moosa H, Robson M, Serena T, Sheehan P, Veves A, Wiersma-Bryant L. Guidelines for the treatment of diabetic ulcers. Wound Repair Regen. 2006;14(6):680–692. doi: 10.1111/j.1524-475X.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- 15.Hingorani A, LaMuraglia GM, Henke P, Meissner MH, Loretz L, Zinszer KM, Driver VR, Frykberg R, Carman TL, Marston W, Mills JL, Sr, Murad MH. The management of diabetic foot: a clinical practice guideline by the Society for Vascular Surgery in collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J Vasc Surg. 2016;63(2 Suppl):3s–21s. doi: 10.1016/j.jvs.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Abdo RJ. Treatment of diabetic foot ulcers with dehydrated amniotic membrane allograft: a prospective case series. J Wound Care. 2016;25(Suppl 7):S4–S9. doi: 10.12968/jowc.2016.25.Sup7.S4. [DOI] [PubMed] [Google Scholar]
- 17.Hawkins B. The use of micronized dehydrated human amnion/chorion membrane allograft for the treatment of diabetic foot ulcers: a case series. Wounds Compend Clin Res Pract. 2016;28(5):152–157. [PubMed] [Google Scholar]
- 18.Rosenblum BI. A retrospective case series of a dehydrated amniotic membrane allograft for treatment of unresolved diabetic foot ulcers. J Am Podiatr Med Assoc. 2016;106(5):328–337. doi: 10.7547/15-139. [DOI] [PubMed] [Google Scholar]
- 19.Koob TJ, Rennert R, Zabek N, Massee M, Lim JJ, Temenoff JS, Li WW, Gurtner G. Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing. Int Wound J. 2013;10(5):493–500. doi: 10.1111/iwj.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massee M, Chinn K, Lim JJ, Godwin L, Young CS, Koob TJ. Type I and II diabetic adipose-derived stem cells respond in vitro to dehydrated human amnion/chorion membrane allograft treatment by increasing proliferation, migration, and altering cytokine secretion. Adv Wound Care. 2016;5(2):43–54. doi: 10.1089/wound.2015.0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilshaw SP, Kearney J, Fisher J, Ingham E. Biocompatibility and potential of acellular human amniotic membrane to support the attachment and proliferation of allogeneic cells. Tissue Eng Part A. 2008;14(4):463–472. doi: 10.1089/tea.2007.0145. [DOI] [PubMed] [Google Scholar]
- 22.Duan-Arnold Y, Uveges TE, Gyurdieva A, Johnson A, Danilkovitch A. Angiogenic potential of cryopreserved amniotic membrane is enhanced through retention of all tissue components in their native state. Adv Wound Care. 2015;4(9):513–522. doi: 10.1089/wound.2015.0638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brantley JN, Verla TD. Use of placental membranes for the treatment of chronic diabetic foot ulcers. Adv Wound Care. 2015;4(9):545–559. doi: 10.1089/wound.2015.0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zelen CM, Serena TE, Denoziere G, Fetterolf DE. A prospective randomised comparative parallel study of amniotic membrane wound graft in the management of diabetic foot ulcers. Int Wound J. 2013;10(5):502–507. doi: 10.1111/iwj.12097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zelen CM, Gould L, Serena TE, Carter MJ, Keller J, Li WW. A prospective, randomised, controlled, multi-centre comparative effectiveness study of healing using dehydrated human amnion/chorion membrane allograft, bioengineered skin substitute or standard of care for treatment of chronic lower extremity diabetic ulcers. Int Wound J. 2015;12(6):724–732. doi: 10.1111/iwj.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiDomenico LA, Orgill DP, Galiano RD, Serena TE, Carter MJ, Kaufman JP, Young NJ, Zelen CM. Aseptically processed placental membrane improves healing of diabetic foot ulcerations: prospective, randomized clinical trial. Plast Reconstr Surg Glob Open. 2016;4(10):e1095. doi: 10.1097/GOX.0000000000001095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohajeri-Tehrani MR, Variji Z, Mohseni S, Firuz A, Annabestani Z, Zartab H, Rad MA, Tootee A, Dowlati Y, Larijani B. Comparison of a bioimplant dressing with a wet dressing for the treatment of diabetic foot ulcers: a randomized, controlled clinical trial. Wounds Compend Clin Res Pract. 2016;28(7):248–254. [PubMed] [Google Scholar]
- 28.Snyder RJ, Shimozaki K, Tallis A, Kerzner M, Reyzelman A, Lintzeris D, Bell D, Rutan RL, Rosenblum B. A prospective, randomized, multicenter, controlled evaluation of the use of dehydrated amniotic membrane allograft compared to standard of care for the closure of chronic diabetic foot ulcer. Wounds Compend Clin Res Pract. 2016;28(3):70–77. [PubMed] [Google Scholar]
- 29.Zelen CM, Serena TE, Gould L, Le L, Carter MJ, Keller J, Li WW. Treatment of chronic diabetic lower extremity ulcers with advanced therapies: a prospective, randomised, controlled, multi-centre comparative study examining clinical efficacy and cost. Int Wound J. 2016;13(2):272–282. doi: 10.1111/iwj.12566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lavery LA, Fulmer J, Shebetka KA, Regulski M, Vayser D, Fried D, Kashefsky H, Owings TM, Nadarajah J. The efficacy and safety of Grafix ((R)) for the treatment of chronic diabetic foot ulcers: results of a multi-centre, controlled, randomised, blinded, clinical trial. Int Wound J. 2014;11(5):554–560. doi: 10.1111/iwj.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Qi XS, Bao YX, Bai M, Xu WD, Dai JN, Guo XZ. Nonselective beta-blockers in cirrhotic patients with no or small varices: a meta-analysis. World J Gastroenterol. 2015;21(10):3100–3108. doi: 10.3748/wjg.v21.i10.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorsby A, Symons HM. Amniotic membrane grafts in caustic burns of eye: (Burns of the second degree) Br J Ophthalmol. 1946;30(6):337–345. doi: 10.1136/bjo.30.6.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kesting MR, Wolff KD, Hohlweg-Majert B, Steinstraesser L. The role of allogenic amniotic membrane in burn treatment. J Burn Care Res. 2008;29(6):907–916. doi: 10.1097/BCR.0b013e31818b9e40. [DOI] [PubMed] [Google Scholar]
- 34.Gruss JS, Jirsch DW. Human amniotic membrane: a versatile wound dressing. Can Med Assoc J. 1978;118(10):1237–1246. [PMC free article] [PubMed] [Google Scholar]
- 35.Singh R, Chouhan US, Purohit S, Gupta P, Kumar P, Kumar A, Chacharkar MP, Kachhawa D, Ghiya BC. Radiation processed amniotic membranes in the treatment of non-healing ulcers of different etiologies. Cell Tissue Bank. 2004;5(2):129–134. doi: 10.1023/B:CATB.0000034077.05000.29. [DOI] [PubMed] [Google Scholar]
- 36.Koob TJ, Lim JJ, Massee M, Zabek N, Rennert R, Gurtner G, Li WW. Angiogenic properties of dehydrated human amnion/chorion allografts: therapeutic potential for soft tissue repair and regeneration. Vasc Cell. 2014;6:10. doi: 10.1186/2045-824X-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mao Y, Hoffman T, Johnson A, Duan-Arnold Y, Danilkovitch A, Kohn J. Human cryopreserved viable amniotic membrane inhibits the growth of bacteria associated with chronic wounds. J Diabet Foot Compl. 2016;8(2):23–30. [Google Scholar]
- 38.Sheehan P, Jones P, Giurini JM, Caselli A, Veves A. Percent change in wound area of diabetic foot ulcers over a 4-week period is a robust predictor of complete healing in a 12-week prospective trial. Plast Reconstr Surg. 2006;117(7 Suppl):239s–244s. doi: 10.1097/01.prs.0000222891.74489.33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.