Abstract
Objective:
To cross-sectionally study subjective memory complaints (SMC) in autosomal dominant Alzheimer disease (ADAD).
Methods:
We examined self-reported and study partner–based SMC in 52 young, cognitively unimpaired individuals from a Colombian kindred with early-onset ADAD. Twenty-six carried the PSEN-1 E280A mutation, averaging 7 years of age younger than the kindred's expected clinical onset. Twenty-six were age-matched noncarriers. Participants also underwent structural MRI and cognitive testing.
Results:
Self-reported SMC were greater in carriers than noncarriers (p = 0.02). Study partner–based SMC did not differ between groups (p = 0.21), but in carriers increased with age (r = 0.66, p < 0.001) and decreased with hippocampal volume (r = −0.35, p = 0.08).
Conclusions:
Cognitively unimpaired PSEN-1 carriers have elevated SMC. Self-reported SMC may be a relatively early indicator of preclinical AD, while partner- reported SMC increases later in preclinical AD, closer to clinical onset.
Early detection of Alzheimer disease (AD) has become a priority in research, as treatments now in development must be administered as early as possible, preferably in the preclinical stage.1,2 Subjective memory complaints (SMC) are an easily acquired indicator of subtle cognitive decline in AD,3–5 which confer risk of dementia progression,6–11 and track with AD biomarkers.10,11 Still, their predictive utility could be improved by clarifying how other variables moderate SMC during the preclinical stage.4,8
Studying SMC in cognitively unimpaired individuals with autosomal dominant AD (ADAD), whose future disease course is known with relative certainty, can provide insight in this regard. In such individuals, the course of SMC across the preclinical stage and beyond can be documented precisely, as has been done with other biomarkers in ADAD.12–14 Studying ADAD has the added benefit of removing confounding sources of SMC that emerge when studying aging individuals.
We examined SMC in individuals from a Colombian kindred with high prevalence of the presenilin-1 (PSEN-1) E280A mutation.15 We compared SMC in carriers and noncarriers using both self and study partner report, and regressed SMC against age to estimate its course during the preclinical stage. Finally, we correlated SMC with hippocampal volume and cognitive performance. We hypothesized that self-reported SMC would be elevated earlier than study partner–reported SMC,4 and that study partner–based SMC would covary more closely with hippocampal volume and objective memory performance,8 indicating a closer link to variables that change proximally to clinical onset.
METHODS
Participants.
Participants were 52 volunteers from a Colombian kindred with early-onset ADAD due to the PSEN-1 280A mutation, 26 were carriers of the mutation, and 26 were noncarrier family members. Mutation carriers from this cohort have a mean onset of mild cognitive impairment (MCI) at age 44 years (95% confidence interval [CI] 43–45) and a mean onset of dementia at age 49 years (95% CI 49–50).15 The age range for carriers was 24–53 years and for noncarriers it was 24–48 years. All participants were cognitively unimpaired as defined by a score of 2 or lower on the Functional Assessment Staging Test, which ranges from 1 (normal) to 7 (severe dementia),16 which was administered by a neurologist, blind to carrier status. A score of 1 indicates no difficulties, either subjectively or objectively. A score of 2 indicates that there may be some memory concerns that are in the normal range for aging adults. A score of 3, which resulted in exclusion from the study, indicates that memory problems are affecting performance at work. All participants were unaware of their own genetic status, which is the cultural standard in this community, but all participants had a parent who was a carrier. Only participants living in the metropolitan area of the Aburra Valley, within 105 miles of the University of Antioquia, were invited to participate in the study. Potential participants were screened in advance for the presence of neurologic and psychiatric disorders, drug use, and eligibility to undergo MRI. All cognitively unimpaired carriers and noncarriers had a Mini-Mental State Examination (MMSE)17 score of at least 25/30, and all participants screened negative for depression, as defined by a score of 5 or lower on the Geriatric Depression Scale–15 (GDS).18
Standard protocol approvals, registrations, and patient consents.
The study was approved by both the institutional review board committees of the University of Antioquia in Colombia and Massachusetts General Hospital in Boston. All participants gave signed informed consent before participating.
Subjective memory complaints and other cognitive measures.
Participant/study partner dyads completed the Memory Complaint Scale in Spanish.19 The scale comprised 15 individual items concerning difficulties in daily life memory tasks, each rated on a Likert scale from 0 (no complaints) to 3 (maximal complaints). The items focus on perceived memory problems and do not compare current memory ability to ability earlier in life. For example, “Do you often forget the first or last name of known people?” and “Have you gotten lost in familiar places?” This scale conforms to the recommendations made by Rabin et al.,20 in that it is appropriate to the demographic characteristics of this sample, focuses on a single cognitive construct (memory), and combines multiple specific items rather than general ones. All participants were administered the Spanish-language version of the Consortium to Establish a Registry for Alzheimer's Disease (CERAD), which has been validated in this population including individuals in the age range used here.21
Structural MRI.
Anatomic data were acquired on a 1.5T Philips (Best, the Netherlands) Achieva MRI scanner at the Instituto de Alta Tecnología Médica in Medellin, Colombia. For each participant, 2 high-resolution T1-weighted structural MRI scans were collected to examine hippocampal volumes (3D fast field echo, repetition time 2,530 ms, echo time 3.39 ms, flip angle 7°, field of view 256 × 256, voxel size 1.0 × 1.0 × 1.0 mm, 176 slices). Automatic shimming procedures were performed. Hippocampal volumes were automatically estimated using an established algorithm available in the Freesurfer (V.4.5) data analytics package. For each participant, raw hippocampal volumes were normalized by dividing by the individual's intracranial volume and then multiplying that proportion by the mean intracranial volume of the entire sample. Left and right hippocampal volumes were averaged to create a single metric for each participant, for use in group comparisons and correlations with other variables.
Analysis.
We compared groups using t tests, and Pearson correlation coefficients (r) were used to explore the associations between ratings of SMC and other variables. In addition, to ascertain the age at which carriers began to reliably differ from noncarriers, we performed curvilinear regressions of study partner and self-reported SMC with age, using quadratic, linear, or sigmoidal curves, and determined the best fit based on Akaike criteria, as described previously.12,13 As is typical for analyses with this kindred, we used age as the only indicator of proximity to symptom onset, rather than including parental age, since all carriers had the same mutation.19
Study partner data were missing for 2 noncarriers and were replaced with multiple imputations based on a regression equation derived from the rest of the noncarrier dataset, which included self-reported SMC scores, age, education, sex, and hippocampal volume. Missing data were imputed 10 times.22 Analyses involving these multiply imputed data were done once for each imputed dataset, and in reporting these results below, we describe the mean statistic along with 95% CIs.
RESULTS
Clinical and demographic characteristics.
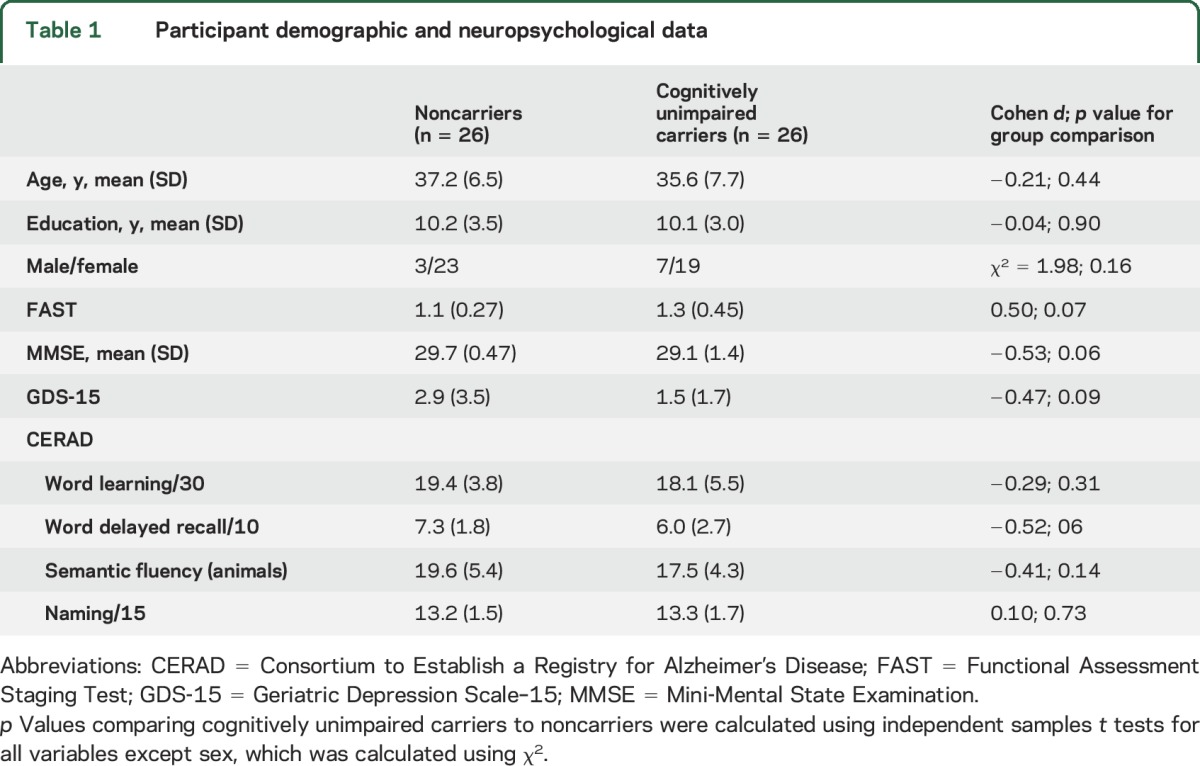
Table 1 summarizes further clinical and demographic information on the sample. Cognitively unimpaired carriers did not differ from noncarriers in terms of age, education, or ratio of men to women. Carriers and noncarriers did not differ significantly in performance on any of the clinical or cognitive tests administered, including GDS, MMSE, and CERAD subtests.
Table 1.
Participant demographic and neuropsychological data
Group differences and courses of SMC with age.
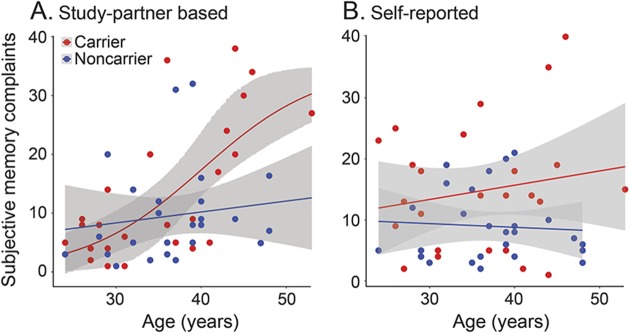
Carriers showed higher self-reported SMC scores (mean 14.7, SD 10.3) than noncarriers (mean 9.0, SD 5.8), t(39.5) = 2.5, p = 0.019, d = 0.65. For study partner–based scores, carriers showed higher SMC (mean 13.3, SD 11.6) than noncarriers (mean 9.7, 95% CI from 10 iterations of multiple imputations 9.41–9.92, SD 8.6, 95% CI 8.28–8.86), but the difference was nonsignificant, t(44.8–47.0) = 1.28 (95% CI 1.11–1.45), p = 0.21 (95% CI 0.15–0.27), d = 0.36 (95% CI 0.32–0.40). Self-reported and study partner–reported SMC as a function of age are shown in figure 1. In carriers, study partner–based SMC scores correlated with age, r(24) = 0.66, p < 0.001, while self-reported SMC scores did not, r(24) = 0.18, p = 0.39. In noncarriers, age correlated with neither self-reported, r = −0.07, p = 0.74, nor study partner–based SMC, r = 0.13 (95% CI 0.06–0.21), p = 0.54 (95% CI 0.35–0.74). In carriers, self-reported and study partner–based SMC were correlated with one another, r(24) = 0.69, p < 0.001, as they were in noncarriers to a lesser degree, r(24) = 0.44 (95% CI 0.39–0.48), p = 0.037 (95% CI 0.013–0.061).
Figure 1. Subjective memory complaints (SMC) as a function of age in autosomal dominant Alzheimer disease.
Regression lines are shown for the best fitting function out of the 3 that were tested: sigmoidal, linear, and quadratic. For carriers, study partner–based SMC were best fit by a sigmoidal function (r2 = 0.45) (A), while self-reported SMC did not vary significantly as a function of age for any of the 3 models used (r2 < 0.06) (B), and a linear model is shown. For noncarriers, none of the 3 functions fit the data significantly, and linear models are shown.
Age at reliable dissociation.
Linear, quadratic, and sigmoidal curves were fit to SMC scores in carriers and noncarriers separately, each as a function of age. The best-fitting model out of those 3 is reported below, for each group and form of SMC. For self-reported SMC, carriers did not show a relation between age and SMC for any of the 3 models (maximum r2 = 0.052). For study partner–reported SMC in carriers, the relation to age was best fit with a sigmoidal function, r2 = 0.45. In noncarriers, the relation between age and SMC was significant for neither self-report (maximum r2 = 0.004) nor study partner report (maximum r2 = 0.042, 95% CI from 10 iterations of multiple imputations analysis between 0.018 and 0.066). Since there was a significant relation between age and study partner–based SMC in carriers, but not in noncarriers, we examined the age at which carriers began to reliably dissociate from noncarriers for this metric. Carriers began to show elevated study partner–based SMC scores as compared to noncarriers at age 38.3 years, 5.7 years before the expected onset of MCI (95% CI 32.2–43.0).
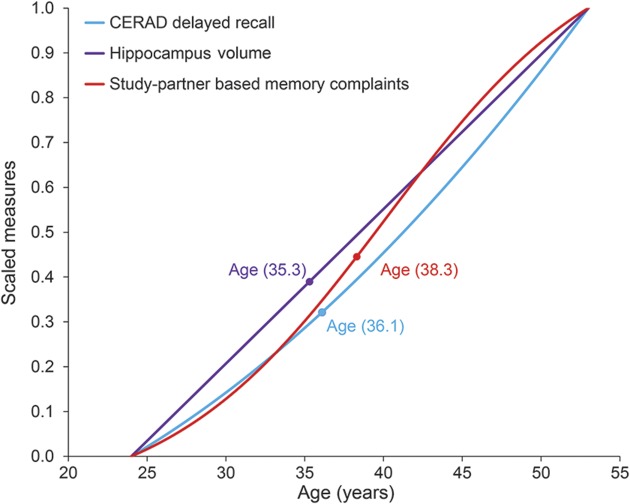
We also examined the relative courses of objective memory performance, as measured by the CERAD word delayed recall, and hippocampal volume alongside, shown in figure 2. Study partner–based SMC, as well as memory performance and hippocampal volume, began to distinguish carriers from noncarriers in the mid to late 30s, with hippocampal volume emerging first at age 35.3 years, followed by CERAD delayed recall at age 36.1 years, and then study partner–based SMC at age 38.3 years.
Figure 2. Subjective memory complaint changes relative to objective cognitive and brain structural changes.
Age-associated curves for PSEN1 mutation carriers shown for subjective memory complaints (study partner–based memory complaints), Consortium to Establish a Registry for Alzheimer's Disease (CERAD) word delayed recall scores, and hippocampal volume. Ages at which carriers and noncarriers began to reliably differ are also shown on each metric.
Relation of SMC to cognitive variables and hippocampal volume.
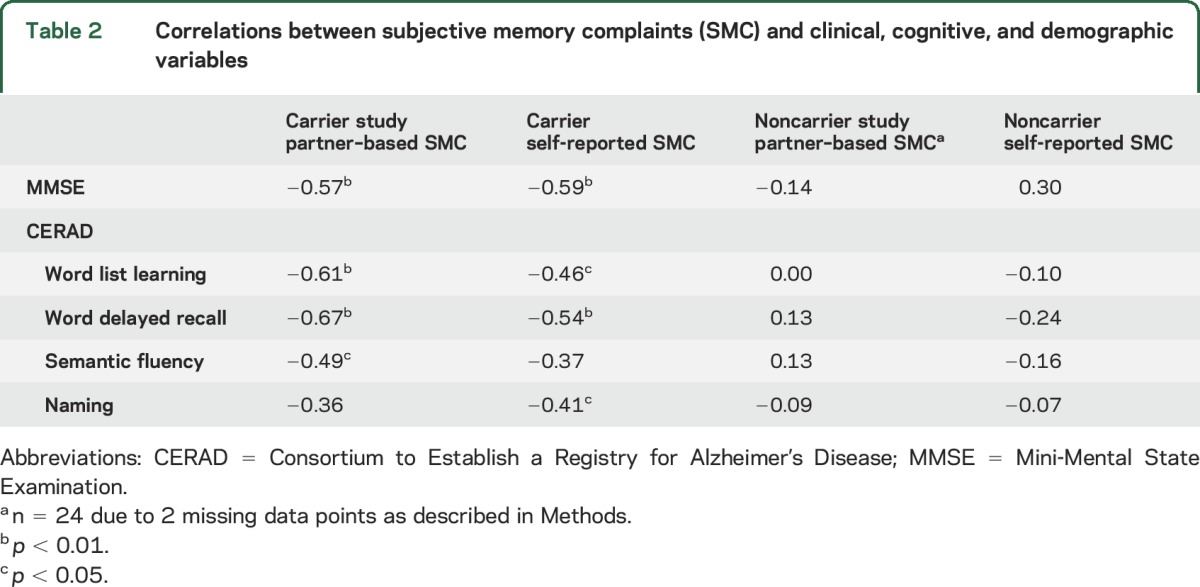
Self-reported and study partner–based SMC were related to performance on selective cognitive tests in carriers, including CERAD delayed recall and word list learning, as well as the MMSE (table 2). Carriers had smaller normalized hippocampal volumes than noncarriers (t[49.3] = 2.83, p = 0.007). The average hippocampal volume for the noncarriers was 4,195.9 mm3 (SD 388.6 mm3) and for carriers was 3,873.67 mm3 (SD 438.84 mm3). Hippocampal volume in carriers was negatively but nonsignificantly associated with study partner–reported SMC, r(24) = −0.35, p = 0.08, but less so for self-reported SMC, r(24) = −0.12, p = 0.56. In noncarriers, averaged hippocampal volume did not correlate with study partner–reported SMC, r(24) = 0.10 (95% CI 0.05–0.16), p = 0.63 (95% CI 0.46–0.80), or self-reported SMC, r(24) = −0.03, p = 0.89. Age was correlated negatively with hippocampal volume in carriers, r(24) = −0.49, p = 0.011, but not in noncarriers, r(24) = 0.042, p = 0.84, as expected.
Table 2.
Correlations between subjective memory complaints (SMC) and clinical, cognitive, and demographic variables
DISCUSSION
This study found that SMC in carriers of the PSEN-1 E280A mutation differ from those in noncarriers prior to the clinical onset of AD. Despite the cross-sectional design employed here, the homogenous nature of disease trajectory in this kindred, combined with the wide age range of the present sample, allowed an examination of the preclinical course of SMC in carriers over decades. Study partner–reported SMC progressed with age in mutation carriers, following a sigmoidal function. In contrast, self-reported SMC were significantly higher in carriers than noncarriers, but did not progress with age in either group. Regression analyses showed that study partner–reported SMC in carriers began to reliably differ from noncarriers at age 38.3 years—roughly 5.7 years before expected MCI onset and 10.7 years before expected dementia onset. In the present sample, this difference in study partner–reported SMC emerged roughly 2 years later than the reliable difference in objective memory performance (CERAD word delayed recall), and 3 years later than the reliable difference in hippocampal volume (these estimates are similar but not identical to those we reported previously13). Study partner and self-reported SMC correlated with other measures of cognition beyond memory, including naming and fluency, as well as MMSE. This is consistent with the start of a decline shown in those tests during the mid to late 30s in this kindred.23 In noncarriers, SMC of both types did not correlate with objective measures of memory performance, suggesting that the SMC scores in the noncarrier group are driven by other factors, such as perhaps neuroticism, as has been discussed in the healthy aging and sporadic AD literature.24
The differences observed between SMC in cognitively normal carriers and noncarriers can be attributed, with great confidence, to AD-related processes. The complete penetrance of the PSEN-1 E280A mutation means that virtually all of the carriers in this study will in fact develop AD in their mid-40s.15 Further, the young age of the sample removes the confound of other common age-related conditions that hinder memory and influence studies of sporadic AD in aging populations. In addition, in research with healthy aging and sporadic AD, SMC is influenced by psychological factors such as neuroticism and depression.24,25 In this sample, carriers and noncarriers did not differ in terms of depressive symptoms as measured by the GDS, meaning depression was not likely a factor in the group differences. In carriers, increased study partner–based SMC was marginally related to decreased hippocampal volume, and both study partner–based and self-reported SMC related significantly to cognitive performance. These correlations are consistent with the idea that SMC is in fact tapping subtle cognitive changes that occur during preclinical AD.
A curious point in the present results is that self-reported SMC did not significantly relate to age, linearly or otherwise, while informant-reported SMC and hippocampal volume both did. While it is difficult to address the reason for this pattern of results given the present sample size, 2 factors emerge as tentative explanations. First, in some cases, carriers' ability to accurately assess their own memory difficulties may become reduced as they get closer to the age at which symptom onset is expected in this kindred. This may have reduced SMC in some of the older carriers in this sample. Second, several of the youngest carriers in this sample—those in their mid to late 20s—displayed elevated levels of SMC, reducing the overall positive slope of SMC against age in carriers. Speculatively, if self-reported SMC were reliably elevated decades before the expected onset of MCI, it would indicate a longstanding phenotype that would be useful clinically and empirically, especially if it generalized to sporadic AD. However, at this point, lack of awareness of memory difficulties in late preclinical AD and heightened SMC very early in the preclinical stage both remain to be demonstrated in a reliable fashion.
A previous study on ADAD, which studied a different group of several mutations than the presently studied Colombian kindred, did not find any difference between cognitively unimpaired carriers and noncarriers of AD-causing mutations.5 The difference between the results of that study and the present one may arise partially from the fact that the previous study relied on a single item to define SMC, which may have hampered sensitivity, while the present study used a 15-item measure, shown to be sensitive to early memory problems.19 Another important difference between the 2 studies is that, while in the present study all participants were blind to their genetic status, an estimated 40% of those in the previous study knew their genetic status, which has been shown to interact with self-reported SMC,26 with knowledge of genetic risk increasing the harshness of self-directed memory ratings. Finally, the previous study was not limited to a single PSEN-1 mutation, and included other mutations that cause ADAD.
Limitations of the present study include a relatively small sample size and uncertainty in the extent to which our findings may be generalizable to other AD-causing mutations or sporadic late-onset AD. While our sample is relatively small compared to studies of sporadic AD, it is one of the largest of its kind in individuals with a single AD-causing mutation. There are also considerations that must be made when drawing inferences from this particular kindred to aging populations without known genetic risk factors. The main caveat here is that all individuals in the present study were aware of their high risk of developing AD, despite being unaware of their own genetic status. This puts all participants in this study on high alert for memory problems in themselves and in their relatives as they approach their early to mid-40s. To confirm this, it would have been ideal to compare the carriers and noncarriers studied here with individuals who were unrelated to the kindred, but these data were not available. The degree to which this heightened concern for memory problems in early middle age parallels the concern for memory problems that arises in late middle age in normal aging populations with no family history of ADAD is unclear. The course of SMC in preclinical ADAD also remains to be confirmed in a longitudinal sample.
The present results from cognitively unimpaired individuals with ADAD confirm that SMC are a promising early marker of AD—one that has the advantage of being intimately linked to the actual experience of affected individuals and their families. Self-reported SMC may be one of the earliest signs of cognitive changes in the preclinical stage of AD, and appear useful for identifying individuals who are at elevated risk of AD, even decades prior to clinical onset. However, self-reported SMC may not be particularly useful for identifying individuals at risk for imminent decline, given that in the present study, they did not increase with proximity to clinical onset. In contrast, increases in study partner–reported SMC over time may confer risk of clinical progression in cognitively normal individuals, which may prove useful for researchers in the process of clinical trial selection, and to clinicians in their efforts to form early diagnoses. In future studies, it will be important to compare SMC to other biomarkers, to longitudinally confirm the courses of self-reported and study partner–reported SMC, and to ascertain whether the present results from ADAD transfer to late-onset, sporadic AD.
ACKNOWLEDGMENT
The authors thank the Instituto de Alta Tecnologia Medica staff for help with MRI data acquisition and the PSEN-1 Colombian families.
GLOSSARY
- AD
Alzheimer disease
- ADAD
autosomal dominant Alzheimer disease
- CERAD
Consortium to Establish a Registry for Alzheimer's Disease
- CI
confidence interval
- GDS
Geriatric Depression Scale–15
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- SMC
subjective memory complaints
AUTHOR CONTRIBUTIONS
Drs. Norton and Quiroz had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Drs. Quiroz, Lopera, Amariglio, Reiman, and Sperling. Acquisition of data: G. Castrillon, V. Tirado, C. Munoz, and Drs. Lopera and Quiroz. Analysis or interpretation of data: Drs. Norton, Amariglio, Aguirre-Acevedo, Chen, Protas, Reiman, Lopera, Sperling, and Quiroz. Drafting of the manuscript: Drs. Norton, Amariglio, and Quiroz. Statistical analysis: Drs. Aguirre-Acevedo, Chen, Protas, and Norton. Critical revision of the manuscript for important intellectual content: All authors.
STUDY FUNDING
Supported by grants from the NIH Office of the Director (DP5OD019833 [Dr. Quiroz]), Massachusetts General Hospital ECOR (1200–228010 [Dr. Quiroz]), NIH NIA (K23AG044431 [Dr. Amariglio]), Alzheimer's Association (NIRG-12-243012 [Dr. Amariglio]), and COLCIENCIAS-Colombia (1115-408-20512, 1115-545-31651[Dr. Lopera]).
DISCLOSURE
D. Norton reports no disclosures. R. Amariglio was supported by Alzheimer's Association NIRG-12-243012 and NIH grant K23AG044431. H. Protas, K. Chen, D. Aguirre-Acevedo, B. Pulsifer, G. Castrillon, V. Tirado, and C. Munoz report no disclosures. P. Tariot reported receiving consulting fees from Abbott Laboratories, AbbVie, AC Immune, Boehringer-Ingelheim, California Pacific Medical Center, Chase Pharmaceuticals, CME Inc., Medavante, Otsuka, Sanofi-Aventis, Eli Lilly and Company, AstraZeneca, Avanir, Bristol-Myers Squibb, Cognoptix, Janssen, Merck and Company, and Roche; receiving research support from AstraZeneca, Avanir, Bristol-Myers Squibb, Cognoptix, Janssen, Merck and Company, Roche, Baxter, Functional Neuromodulation Ltd, GE Healthcare, Genentech, Pfizer, Targacept, Avid Radiopharmaceuticals, the National Institute on Aging, and the Arizona Department of Health Services; having stock options in Adamas Pharmaceuticals; and contributing to a patent for biomarkers of Alzheimer disease owned by the University of Rochester. J. Langbaum reports no disclosures. E. Reiman reports receiving research funding from Avid Radiopharmaceuticals and serving as a paid consultant for Eli Lilly and Company. F. Lopera was supported by COLCIENCIAS-Colombia (1115-408-20512, 1115-545-31651). R. Sperling receives research support for NIH grants U01 AG032438, U01 AG024904, R01 AG037497, R01 AG034556, K24 AG035007, P50 AG005134, U19 AG010483, R01 AG027435, and P01 AG036694. She is a site principal investigator or coinvestigator for Avid, Bristol-Myers Squibb, Pfizer, and Janssen Alzheimer Immunotherapy clinical trials. Y. Quiroz was supported by grants from the NIH Office of the Director (DP5OD019833) and Massachusetts General Hospital ECOR (1200-228010). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sperling R, Mormino E, Johnson K. The evolution of preclinical Alzheimer's disease: implications for prevention trials. Neuron 2014;84:608–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amariglio RE, Townsend MK, Grodstein F, Sperling RA, Rentz DM. Specific subjective memory complaints in older persons may indicate poor cognitive function. J Am Geriatr Soc 2011;59:1612–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caselli RJ, Chen K, Locke DEC, et al. Subjective cognitive decline: self and informant comparisons. Alzheimers Dement 2014;10:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laske C, Sohrabi HR, Jasielec MS, et al. Diagnostic value of subjective memory complaints assessed with a single item in dominantly inherited Alzheimer's disease: results of the DIAN study. Biomed Res Int 2015;2015:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckley RF, Maruff P, Ames D, et al. Subjective memory decline predicts greater rates of clinical progression in preclinical Alzheimer's disease. Alzheimers Dement 2016;12:796–804. [DOI] [PubMed] [Google Scholar]
- 7.Geerlings MI, Jonker C, Bouter LM, Adèr HJ, Schmand B. Association between memory complaints and incident Alzheimer's disease in elderly people with normal baseline cognition. Am J Psychiatry 1999;156:531–537. [DOI] [PubMed] [Google Scholar]
- 8.Jessen F, Amariglio RE, van Boxtel M, et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer's disease. Alzheimers Dement 2014;10:844–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmand B, Jonker C, Hooijer C, Lindeboom J. Subjective memory complaints may announce dementia. Neurology 1996;46:121–125. [DOI] [PubMed] [Google Scholar]
- 10.Waldorff FB, Siersma V, Vogel A, Waldemar G. Subjective memory complaints in general practice predicts future dementia: a 4-year follow-up study. Int J Geriatr Psychiatry 2012;27:1180–1188. [DOI] [PubMed] [Google Scholar]
- 11.Wang L, van Belle G, Crane PK, et al. Subjective memory deterioration and future dementia in people aged 65 and older. J Am Geriatr Soc 2004;52:2045–2051. [DOI] [PubMed] [Google Scholar]
- 12.Bateman RJ, Xiong C, Benzinger TLS, et al. Clinical and biomarker changes in dominantly inherited Alzheimer's disease. N Engl J Med 2012;367:795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fleisher AS, Chen K, Quiroz YT, et al. Associations between biomarkers and age in the presenilin 1 E280A autosomal dominant Alzheimer disease kindred: a cross-sectional study. JAMA Neurol 2015;72:316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quiroz YT, Schultz AP, Chen K, et al. Brain imaging and blood biomarker abnormalities in children with autosomal dominant Alzheimer disease: a cross-sectional study. JAMA Neurol 2015;72:912–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopera F, Ardilla A, Martínez A, et al. Clinical features of early-onset Alzheimer disease in a large kindred with an E280A presenilin-1 mutation. JAMA 1997;277:793–799. [PubMed] [Google Scholar]
- 16.Reisberg B. Functional Assessment Staging (FAST). Psychopharmacol Bull 1988;24:653–659. [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 18.Sheikh J, Yesavage J. Recent evidence and development of a shorter version. In: Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Hawthorn Press; 1986:165–173. [Google Scholar]
- 19.Acosta-Baena N, Sepulveda-Falla D, Lopera-Gómez CM, et al. Pre-dementia clinical stages in presenilin 1 E280A familial early-onset Alzheimer's disease: a retrospective cohort study. Lancet Neurol 2011;10:213–220. [DOI] [PubMed] [Google Scholar]
- 20.Rabin LA, Smart CM, Crane PK, et al. Subjective cognitive decline in older adults: an overview of self-report measures used across 19 International research studies. J Alzheimers Dis 2015;48(suppl 1):S63–S86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aguirre-Acevedo DC, Gómez RD, Moreno S, et al. Validity and reliability of the CERAD-Col neuropsychological battery [in Spanish]. Rev Neurol 2007;45:655–660. [PubMed] [Google Scholar]
- 22.Schafer JL. Multiple imputation: a primer. Stat Methods Med Res 1999;8:3–15. [DOI] [PubMed] [Google Scholar]
- 23.Aguirre-Acevedo DC, Lopera F, Henao E, et al. Cognitive decline in a Colombian kindred with autosomal dominant Alzheimer disease: a retrospective cohort study. JAMA Neurol 2016;73:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Comijs HC, Deeg DJ, Dik MG, Twisk JW, Jonker C. Memory complaints; the association with psycho-affective and health problems and the role of personality characteristics: a 6-year follow-up study. J Affect Disord 2002;72:157–165. [DOI] [PubMed] [Google Scholar]
- 25.Minett TSC, Da Silva RV, Ortiz KZ, Bertolucci PHF. Subjective memory complaints in an elderly sample: a cross-sectional study. Int J Geriatr Psychiatry 2008;23:49–54. [DOI] [PubMed] [Google Scholar]
- 26.Lineweaver TT, Bondi MW, Galasko D, Salmon DP. Effect of knowledge of APOE genotype on subjective and objective memory performance in healthy older adults. Am J Psychiatry 2014;171:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]