Abstract
Objectives
To determine the risk factors, genotype specific prevalence, and concordance of HPV infections at three anatomical sites in a cohort of high-risk Greek men.
Methods
Patients were recruited from a sexually transmitted infection and HIV clinic in Athens. Samples were obtained from oral, penile and anal sites of 294 study participants and HPV testing was conducted on the 882 samples using next-generation sequencing (NGS). Patients completed a questionnaire assessing risk factors for infection.
Results
The mean age of subjects was 33.1, 30% identified as men who have sex with men (MSM) and 21% were HIV positive. The prevalence of HPV was 49%; it was highest at anal sites (33%), compared to 23% at penile sites (p=0.008), and 4% at oral sites (p<0.001). The most common HPV types in order of frequency were 6, 44, 16, 53 and 89. The genotype concordance rate was highest between the penile and anal sites (7%), followed by 2% for anal-oral concordance. Identifying as MSM, (aOR=6.75, p<0.001) and being HIV positive (aOR=2.89, p=0.026) were significant risk factors for anal HPV infection, while alcohol use (aOR=0.45, p=0.002) were negatively associated with infection. The only significant risk factor for oral infection was an older age of sexual debut (aOR=1.32, p=0.038).
Conclusions
Nearly half of our study participants tested positive in at a least one of three anatomical sites. By using NGS we were able to identify high risk types that are not covered by the current vaccine and would be missed by traditional HPV testing kits.
Keywords: HPV, Greece, men, prevalence, high-risk, risk factors, concordance
INTRODUCTION
Human papillomavirus (HPV) is a member of the Papillomaviridae family with more than 30 subtypes that have been identified as being sexually transmitted. Some of the high-risk subtypes are well known as the primary cause of most cervical cancer in women(Muñoz et al., 2003). Recent research has also detected high-risk HPV in 26% of oropharyngeal, 48% of penile, and 72% of anal cancers in men as well (Kreimer et al., 2005, Backes et al., 2009, Hoots et al., 2009). A systematic review of worldwide HPV prevalence in men found a prevalence range between 1.3% and 72.9% (Dunne et al., 2006). A meta-analysis in Europe found that the genital HPV prevalence was 12.4% in low risk and 30.9% in high-risk male populations (Hebnes et al., 2014).
The epidemiology of HPV in Greek men is not well known as most Greek studies have focused on cervical HPV infection in women. A systematic review published by our group found that out of 50 studies on HPV prevalence and epidemiology in Greece, 20 studies have included a male population(Tsikis et al., 2015). However, the majority of these examined HPV frequency in oral and oropharyngeal cancer biopsies (n=13) while the remaining looked at HPV prevalence at anal and/or penile sites in symptomatic men. For these studies, testing was performed by commercially available test kits, and high risk subtypes may not have been well-delineated, apart from HPV types 16 and 18 (Tsikis et al., 2015). Moreover, these studies included a relatively small sample (<130), did not look at concordance at multiple sites, and did not specifically target men who may be at a higher risk for HPV infection.
It is of special interest to determine HPV frequency in high-risk male populations, such as men who have sex with men (MSM) and HIV-positive men. This subpopulation has been seriously understudied in Greece which is concerning since MSM tend to have higher rates of HPV infection than non-MSM and HIV positivity is a significant risk factor for HPV infection (Stone et al., 2002, Lissouba et al., 2013). This becomes especially important in recent times, as the HIV/AIDS prevention budget has been reduced in Greece due to the economic crisis, likely contributing to the 50% increase in the incidence rate of HIV infection in the country since 2010(Ifanti et al., 2013).
HPV in Greek men is an understudied topic and HPV-related cancer screening and vaccination are nonexistent in the Greek male population, even among those who are HIV-positive and are considered high-risk. This study aims to determine the risk factors, prevalence, and concordance of HPV genotypes in a cohort of high-risk Greek men at three anatomical sites, using next generation sequencing.
PATIENTS AND METHODS
Study design
This was a single-center cross-sectional study that was carried out between July and October 2015 in Athens, Greece. Subjects were consecutively recruited from the outpatient STI and HIV clinics of “Andreas Sygros” hospital. Greek men between the ages of 18 and 55 who were visiting these clinics were eligible to participate (n=298). Study participants were informed about the study during their regular clinic visit and informed consent was obtained prior to enrollment. The study involved the collection of oral, anal, and penile samples, and a computer-based, Greek language questionnaire. The Ethics Committee of the University of Athens approved this study (IRB 1287).
Questionnaire
The study questionnaire was self-administered in a private room as part of the study visit. The first part collected basic demographic information such as age, nationality, smoking history, and socioeconomic status. This component of the survey also collected information about income and the effects of the economic crisis on quality of life and health, using questions previously validated in a national survey (HS, 2013). The second portion of the survey, also based on previously validated questionnaires, included a detailed sexual history that asked about HIV infection status and number of sexual partners (Antonsson et al., 2014).
Sample collection and HPV testing
Samples were collected from the mouth, anus, and penis of each study participant. The oral samples were collected by means of a 20-second oral rinse of 10–15 mL isotonic saline solution. Before giving the sample, the subject was asked to gently and briefly brush their teeth with a toothbrush moistened in isotonic saline to maximize the amount of cells collected. The toothbrush was placed in the test tube used to collect the oral rinse and rotated to release any additional cells (Ong et al., 2014). For anal testing, a saline-wetted Cervex-Brush® Combi swab (Rovers Medical Devices, The Netherlands) was used to sample the perianal area and was then inserted into the anal canal up to the anal verge(Giuliano et al., 2007). The swab was rotated twice, removed and placed in the ThinPrep® (Hologic, Bedford, MA, USA) transport medium. Penile samples were similarly obtained by rubbing a saline-wetted Cervex-Brush® Combi swab over the entire surface of the penis starting with the shaft of the penis and then the glans of the penis/coronal sulcus before placing in the ThinPrep medium (Giuliano et al., 2007, Álvarez-Argüelles et al., 2013). All samples were immediately stored at 4°C prior to DNA extraction.
DNA extraction and HPV testing by Next Generation Sequencing
DNA extraction was performed on all samples within 3–5 days of collection using the QIAamp® DNA Mini Blood kit (Qiagen, Valencia, CA, USA) at the on-site laboratory of Andreas Syggros hospital, according to the manufacturer’s instructions. The DNA specimens were then shipped at −20°C in batches to the Clinical Genomics laboratory of the University of Chicago. The specimens were tested using next-generation sequencing (NGS).
Two subsequent PCR reactions were performed to generated amplicons for next-generation sequencing. First, 10 ng of genomic DNA was amplified using PGMY09/11 primers with universal sequences to amplify the HPV L1 consensus region(Gravitt et al., 2000). A subsequent PCR incorporated Illumina adapter sequences and patient-specific index sequences. Libraries were then pooled and sequenced via MiSeq® (Illumina, San Diego, CA, USA) using 2 × 152 bp paired end sequencing. HPV genotyping was obtained via a customized bioinformatics pipeline, which removed ambiguous and off-target sequences, aligned the filtered data to 179 HPV genomes from the Papillomavirus Episteme (PaVe) database (https://pave.niaid.nih.gov/), and scored each read based on its alignment to a specific HPV genome(Van Doorslaer et al., 2013). Samples from four subjects yielded insufficient DNA concentration for sequencing, and were excluded from further analysis; therefore 294 subjects were included in our final analysis.
Interpretation of NGS results
A test cohort of 29 samples with previously characterized HPV using multiplex qPCR and linear array (including HPV 16 (n=2), HPV 18 (n=2), HPV 33 (n=1), HPV 39 (n=1), HPV 45 (n=2)) and 22 specimens negative for high risk HPV (16, 18, 31, 33, 45, 52, and 58) were used to assess the assay performance and to establish parameters for classification of HPV DNA status. A Receiver Operator Characteristic (ROC) curve was created based on the HPV percent per sample. Samples with HPV percent greater than 0.04 were classified as positive for the presence of HPV DNA, samples with percent HPV at or below 0.04 were considered to be negative for HPV DNA (100% sensitive, 95% specific). We then applied this methodology to describe the 882 sequenced samples from our population of Greek men.
Forty-four Greek samples had enough left over DNA concentration following NGS testing (at least 10–20 ng of DNA), and were genotyped by a clinically validated multiplex qPCR assay that detects seven clinically relevant high-risk HPV subtypes (HPV 16, 18, 31, 33, 45, 52 and 58) using fluorescently labeled TaqMan® probes (Thermo Fischer Scientific, Waltham, MA, USA) targeting the HPV LCR/E6/E7 sequences at high analytical sensitivity, and by a previously published qPCR assay that detects HPV 6 and 11 for comparison (Mills et al., 2013). Of those, two samples failed amplification of the β-globin control gene, and were excluded from further analysis. The β-globin control gene is amplified in parallel to ensure the integrity of the specimen.
Statistical Analysis
Statistical analysis was performed using STATA Version 14.0 (StataCorp corporation, College Station, Texas, USA). For comparisons, we used the t-test for normally distributed continuous variables and the chi-squared test (or Fischer’s exact test) for categorical variables. Univariate and multivariate analysis using unconditional direct logistic regression for all variables was utilized to determine the risk factors for HPV infection. Adjusted Odds ratios (aOR), 95% confidence intervals (CI) and p values are reported. We used p<0.05 to indicate statistical significance.
RESULTS
Study population demographics
A total of 294 male patients were included in the final analysis. The detailed demographics of the participants can be found in Table 1. Patients included in the study had a mean age of 33.1 (range 18 to 55). More than half of the men were visiting the clinics for the treatment of genital warts (56%, 165/294) or STI testing (30%, 88/294). Of all included patients, 30% (89/294) of the patients reported being MSM, 21% (58/294) were HIV positive and 58% (171/294) had reported a positive past or current history of genital warts.
Table 1.
Basic demographic characteristics of participants (n=294)
| Demographic variable | Patients (%) |
|---|---|
| Age (years) | |
| <25 | 61 (20.8) |
| 25–29 | 62 (21.1) |
| 30–39 | 103 (35.0) |
| 39–55 | 68 (23.1) |
|
| |
| Birth country | |
| Greece | 245(83.3) |
| Other | 49(16.7) |
|
| |
| Educational level | |
| Elementary school | 30(10.2) |
| High school | 109(37.1) |
| University | 155(52.7) |
|
| |
| Monthly income (Euros) | |
| 0–233 | 76(25.9) |
| 234–432 | 41(14.0) |
| 433–1000 | 131(44.6) |
| >1000 | 46(15.7) |
|
| |
| Employment status | |
| Currently Employed | 195(66.3) |
| Currently Unemployed | 99(33.7) |
| Unemployed in the last 5 years | 196(66.7) |
|
| |
| Smokers | |
| Current | 170(57.8) |
| Past | 46(15.7) |
| Non-smokers | 78(26.5) |
|
| |
| HIV positive | 58(21.2) |
|
| |
| MSM | 89(30.3) |
| -MSM HIV positive | 47/89 (52.8) |
|
| |
| Mean age of sexual debut ± SEM | 17.1 ± 0.2 |
|
| |
| Median number of lifetime sexual partners (IQR) | 20 (10–50) |
Notes: IQR: Interquartile range; MSM:Men who have sex with men; HIV: Human Immunodeficiency Virus; SEM; Standard error of the mean
HPV Prevalence at the three sites
All men included in the study underwent HPV testing at penile, oral, and anal sites so 882 total specimens were tested for the presence of HPV. Nearly half of our study participants tested positive for HPV in at a least one of these sites (49%, 145/294) and 19% (28/145) had a simultaneous infection at multiple sites. HPV prevalence was highest from anal samples (33%,96/294), compared to penile samples (23%, 67/294, p=0.0076) and oral samples (4%,11/294, p<0.001). HPV prevalence varied amongst various high risk groups such as HIV positive males or those who were MSM (Table 2). Patients who identified as MSM had lower penile HPV prevalence compared to those who were non-MSM (14.6% vs. 26.3%, p=0.028). However, MSM were more likely to have an oral HPV infection compared to non-MSM (9% vs. 1.5%, p=0.004) or an anal HPV infection (65% vs. 19%, p<0.001). HIV-positive males did have higher rates of infection at oral (8.6% vs. 2.3%, p=0.038) and anal sites (71% vs. 23%, p<0.001) but the rates of HPV in penile samples did not differ based on HIV status. Finally, patients with a self-reported positive STI history had a higher prevalence of HPV at the anal site compared to those with no history (44% vs. 28%, p=0.005).
Table 2.
HPV prevalence at penile, oral and anal sites among different sub-groups
| Variable | Penile site | Oral site | Anal site | |||
|---|---|---|---|---|---|---|
|
| ||||||
| HPV positive (%) | p valuec | HPV positive (%) | p valued | HPV positive (%) | p valuec | |
| Patients (n=294) | 67 (22.8) | - | 11 (3.7) | - | 96 (32.7) | - |
|
| ||||||
| Sexual activitya | ||||||
| MSM (n=89) | 13 (14.6) | 0.028 | 8 (9.0) | 0.004 | 58 (65.2) | <0.001 |
| Non-MSM (n=205) | 54 (26.3) | 3 (1.5) | 38 (18.5) | |||
|
| ||||||
| HIV statusb | ||||||
| HIV positive (n=58) | 10 (17.2) | 0.30 | 5 (8.6) | 0.038 | 41 (70.7) | <0.001 |
| HIV negative (n=216) | 51 (23.6) | 5 (2.3) | 49 (22.7) | |||
|
| ||||||
| Genital Wartsa | ||||||
| Current (n=171) | 44 (25.7) | 0.16 | 7 (4.1) | 0.77 | 48 (28.1) | 0.048 |
| Not currently present (n=123) | 23 (18.7) | 4 (3.3) | 48 (39.0) | |||
|
| ||||||
| Other STDsa | ||||||
| Positive History (n=102) | 27 (26.5) | 0.31 | 7 (6.9) | 0.11 | 45 (44.1) | 0.005 |
| No History (n=180) | 38 (21.1) | 4 (2.2) | 50/180 (27.8) | |||
Notes: MSM:Men who have sex with men; HIV: Human Immunodeficiency Virus; STDs: sexually transmitted diseases;
Information is based on patient self-report or
file confirmation;
Determined by using chi-squared test or
Fischer’s exact test
HPV Types and anatomical site concordance
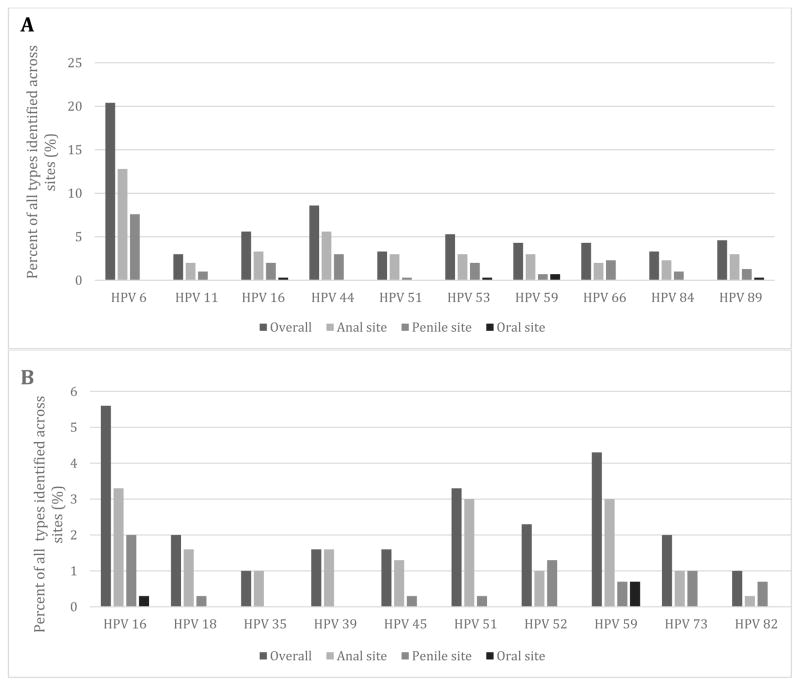
A total of 51 different types of HPV were identified across the three sites and we report the prevalence of the 10 most common types out of all HPV that was identified (Figure 1). HPV 6 was the most common low risk type found at the anal (12.8%) and penile (7.6%) sites followed by HPV 44 (Figure 1). Anal sites had the highest proportion of high-risk HPV (42%, 40/96) while penile sites (33%, 22/67) and oral sites (27%, 3/11) had a lower prevalence. The most common high-risk type at the anal site was HPV 16 (4.8%) and the second most common was HPV 59 (4.3%) (Figure 1). HPV 16 was the most prevalent high-risk type from penile samples (6.7%) while HPV 52 was the second most common (4.5%). HPV 59 and 32 were the most prevalent types from the oral site.
Figure 1.
1A. Prevalence of the ten most common types identified across the three sites 1B. Prevalence of the ten most common high-risk HPV types across the three sites
Co-infection with more than one HPV type was most common at the anal site where 46% (44/96) of positive patients had co-infection with multiple types compared to 22% (15/67) in positive penile samples and 9%(1/11) in the oral samples. Of patients infected with more than one HPV type, 61%(27/44) were co-infected with more than 2 types at the anal site, 33% (5/15) at the penile site and 100% (1/1) at the oral site. The mean number of HPV types found from anal, penile, and oral samples was 2.2 (range:1–10), 1.3 (range:1–4), and 1.2 (range:1–3), respectively.
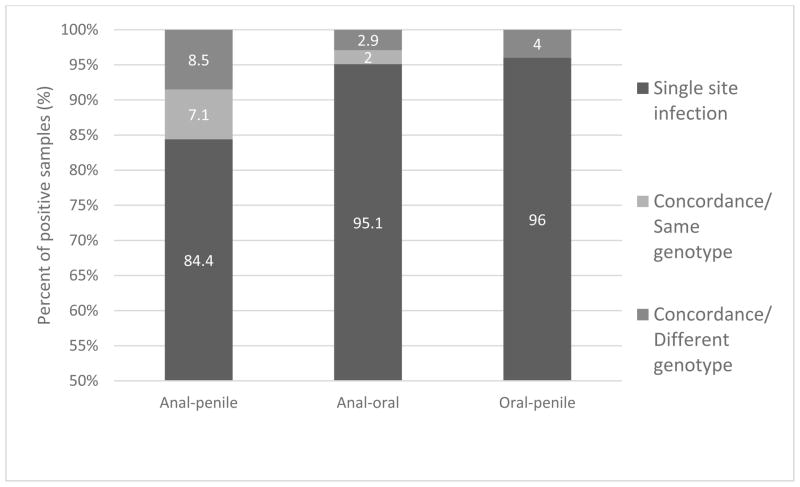
Patients had a genotype-specific concordant infection if there was at least one co-infection with the same HPV type between sites. The genotype-specific concordance rate between the penile site and anal canal was 7% (10/141) with the remaining samples either having a single infection at one of these sites or a genotype dis-concordant simultaneous infection (Figure 2). The HPV concordance rate was lower (p=0.069) between the anal and oral sites (2%, 2/102) and there was no genotype-specific concordance between oral and penile sites. One patient had a simultaneous infection at all three sites but with different genotypes at each site.
Figure 2.
Concordance of HPV infection between three pairs of anatomical sites (anal-penile, anal-oral, and oral-penile). Shown is single site infection, concordant infection with the same genotype at each of the two sites, and concordant infection but with different genotypes between the two sites.
Risk factors for infection
The risk factors for infection were determined using multivariate logistic regression analysis for each of the three sites (Table 3). For HPV infection at the anal site, identifying as a MSM was a significant risk factor (OR=6.75, 95% CI: 2.86–15.91, p<0.001) along with a history of HIV infection (OR=2.89, 95% CI:1.14–7.37, p=0.026). Interestingly, increasing alcohol use (OR=0.45, 95% CI:0.27,0.75, p=0.002) was found to be protective against anal HPV infection. A higher income may also be protective against anal HPV infection (OR=0.72, 95% CI:0.51,1.00, p=0.05) but this did not reach statistical significance.
Table 3.
Risk factors for HPV infection at three anatomical sites in high-risk Greek men in Athens (n=294)
| Risk factor | Oral site | Penile site | Anal site | |||
|---|---|---|---|---|---|---|
| Adjusted ORc [CI] | p value | Adjusted ORc [CI] | p value | Adjusted ORc [CI] | p value | |
| Current smoker (n=170) | 0.60 [0.13–2.80] | 0.52 | 1.78 [0.92–3.43] | 0.087 | 1.42 [0.72–2.79] | 0.31 |
| Alcohol usea | 1.28 [0.44–3.72] | 0.65 | 0.77 [0.49–1.21] | 0.25 | 0.45 [0.27–0.75] | 0.002 |
| Higher incomeb | 0.94 [0.48–1.85] | 0.85 | 1.05 [0.77–1.45] | 0.75 | 0.72 [0.51–1.00] | 0.05 |
| Born in Greece (n=245) | 2.68 [0.23–30.62] | 0.43 | 0.95 [0.39–2.30] | 0.91 | 2.31 [0.90–5.96] | 0.082 |
| Older age of sexual debut | 1.32 [1.02–1.73] | 0.038 | 1.11 [0.98–1.25] | 0.10 | 1.00 [0.89–1.13] | 0.96 |
| MSM( n=89) | 7.77 [0.97–62.05] | 0.053 | 0.44 [0.17–1.15] | 0.096 | 6.75 [2.86–15.91] | <0.001 |
| HIV positive (n=58) | 1.22 [0.14–10.78] | 0.86 | 1.21 [0.44–3.35] | 0.72 | 2.89 [1.14–7.37] | 0.026 |
Notes: MSM; Men who have sex with men; HIV: Human immunodeficiency virus; OR: Odds Ratio; CI: confidence internal;
Based on the following scale: 0, 1–7, 8–14, >14 drinks per week on average in the past year;
Income scale was: 0–233 euros/month, 234–432 euros/month, 433–1000 euros/month, >1000 euros/month;
Based on a multivariate logistic regression model that was also adjusted for the following variables: age, circumcision, marital status, education, condom use, number of lifetime sexual partners and presence of genital warts (all p>0.10)
An older age of sexual debut was associated with an increased risk of HPV infection at the oral site (OR=1.32, 95% CI:1.02, 1.73, p=0.038). Patients who identified as MSM may be more likely to have an oral HPV infection (OR=7.77, 95% CI: 0.97, 62.05, p=0.053). Finally, smoking may be associated with penile infection but it did not reach statistical significance (OR=1.78, 95%CI:0.92,3.43, p=0.087).
qPCR-NGS correlation
Correlation of NGS and qPCR for the Greek samples was 97% (41/42). One sample was classified as positive for HPV 31 infection by NGS, and qPCR detected a low level of HPV 18 DNA, which was not identified by NGS. We were not able to further evaluate this sample by an orthogonal method due to an insufficient DNA concentration remaining.
DISCUSSION
This study examined the genotype specific prevalence, risk factors, and concordance of HPV infection at three anatomical sites in high-risk Greek men. To our knowledge, this is the first study conducted in Greek men investigating prevalence and genotypic concordance of HPV across multiple sites. In fact, very few papers have been published worldwide to date on the topic of HPV concordance. Moreover, only one other study has investigated HPV in an asymptomatic Greek male population, that one evaluating men presenting for a routine dental exam(Lambropoulos et al., 1997).
Out of the 294 male patients included in this study, nearly half (49%) had an infection at a least one of the sites examined, with anal samples having the highest prevalence of HPV (33%). Previous studies conducted in Greece investigating male anal HPV prevalence have only looked at patients with a history of anal warts or anal carcinoma, and found HPV prevalence rates greater than 50% (Tsikis et al., 2015). The difference seen in our results could be due to the study population, as our study included asymptomatic but high risk men.
The anal HPV prevalence of Greek men who identified as MSMS was 65% in our sample which is higher than that reported in a worldwide study of HIV seronegative MSM(42.4%)(Goldstone et al., 2011). These findings are important, given that up to 72% of anal cancer may be linked to HPV infection and thus high rates of anal HPV infection implies that Greek MSM might benefit from anal cancer screening(Hoots et al., 2009). Greek MSM may also benefit from HPV vaccination, which has been shown to be a cost-effective intervention for the prevention of anal cancer (Deshmukh et al., 2014).
Similar to other published reports, we also found that MSM status and HIV positivity were independent risk factors for anal HPV infection (Stone et al., 2002, Hadjivassiliou et al., 2007, Lissouba et al., 2013). Interestingly patients who had a higher income were slightly less likely to have an anal HPV infection (OR=0.72, p=0.05). This result requires further investigation but may be significant in the Greek context given the falling income levels due to the recent economic crisis.
The prevalence of penile HPV infection was also high in our sample (23%), suggesting penile HPV infection is elevated in high-risk Greek men. A recent meta-analysis of European men found a slightly higher genital HPV prevalence of 30.9% for high-risk male populations but lower at 12.4% for the general population(Hebnes et al., 2014). The same study found that HPV 16 and 18 were the most common high-risk types in European men. While HPV 16 was also the most common high risk type in our sample of Greek men, HPV 18 had a very low prevalence and in fact HPV 52 was the second most common type. This suggests that some of the high risk types most prevalent in the Greek male population actually differ from those seen in other European countries.
Potential risk factors such as number of sexual partners, condom use and circumcision were not found to be significant for penile or anal HPV infection on multivariate analysis. A systematic review of HPV infection in men has reported these factors as being significant in other published reports (Dunne et al., 2006). It may be that these risk factors are not as significant in our specific population of high-risk Greek men.
Studying oral HPV infection in Greek men is important since smoking has been shown to be a risk factor and Greek men have one of the highest smoking rates worldwide(Bouda et al., 2000, Organization, 2015). The rate of HPV infection at oral sites was the lowest of all three anatomical locations (4%), with HPV 32 and 59 being the most common types. Our prevalence rate is lower than the reported prevalence rate of 9.5% in a study of patients from northern Greece with a normal oral mucosa(Lambropoulos et al., 1997). That study included 169 subjects, of which only 76 were males, and HPV testing was done using an older technique involving PCR and Southern blot hybridization. No other recent studies have examined HPV prevalence at the oral sites of asymptomatic Greek men and our study only had 11 patients who tested positive.
Multivariate analysis showed that an older age of sexual debut was a risk factor for oral HPV infection. One possible explanation for this could be that these patients may have been smoking for a longer period before their sexual debut causing damage to their oral epithelium, which could act as a primer for future HPV infection. This is plausible since smoking has been shown to be associated with an increased risk of oral HPV infection in other studies although we were not able to replicate this result(Bouda et al., 2000).
There was significant anal-penile genotype specific concordance (7%) and even concordance, albeit limited, between anal and oral sites (2%). As a result, patients may be at increased risk for simultaneously developing both anal and oropharyngeal cancers. These findings are in contrast to a recent study which examined paired oral and anogenital samples from 151 HIV negative MSM and found no anogenital concordance(King et al., 2015). Differences in HPV genotype detection methods as well as the specific populations examined could account for these discrepancies.
NGS is a relatively novel technique for the genotyping of HPV and it has been previously validated for use in such a setting (Arroyo et al., 2013). This technique has advantages compared to traditional kit-based testing; the NGS assay described here requires lower amounts of DNA than commercially available kits. Because several of the samples in the Greek cohort had low DNA concentration, comprehensive HPV genotyping would have not been possible by most of other commercially available methods. Further, NGS sequencing has the potential to detect all HPV subtypes including rare subtypes that may be missed by the traditional kits. For example, the Cobas® HPV Test (Roche Molecular Systems, Basel, Switzerland) is able to detect the presence of high-risk HPV types but does not distinguish between the various types beyond HPV 16 and 18. In our study, HPV 59 and 52 were the second most common high-risk types found at the anal and penile sites, respectively, and these would not be detected individually by these commercial kits. Furthermore, these high risk types are not covered by the current quadrivalent HPV vaccine which makes their detection even more important in the prevention of genital cancers in high-risk male populations. Future studies using NGS may actually show the true prevalence of these other types, with the potential to impact future vaccine design and serve as evidence for the need to expand vaccine coverage.
This study contains the largest number of Greek men, including high risk MSM and HIV positive individuals, in whom HPV has been studied to date. However, there are several important limitations. The population sample included STI clinic attendees, many of whom came for the treatment of genital warts, which is not representative of the general Greek male population. Therefore, larger studies that are more representative of the Greek male population are needed. Like many published studies, this study examined the prevalence of HPV at one point in time. However up to 75% of men are negative for HPV twelve months after testing positive(Giuliano et al., 2008). Thus, longer term or follow-up studies are needed in our study population.
In conclusion, we have found that anal and penile HPV infection are particularly prevalent in high-risk Greek men, especially amongst MSM and HIV positive individuals. HPV 6 and 16 were some of the most common subtypes identified in our sample and are covered by the current quadrivalent vaccine. Using NGS we were able to identify additional high-risk subtypes such as HPV 59 and 52 that had a high degree of prevalence. These subtypes are not currently covered by the vaccine and may not be individually detected by traditional kits. The results of our study support the creation of screening programs for high-risk Greek men and the need for expanding HPV vaccination to Greek men. To this end, future studies should focus on the epidemiology of HPV in the general Greek male population and should include longer-term follow-up of HPV persistence.
Acknowledgments
Funding
This study was funded in part by the National Institutes of Health (R21 AI118998, R01 DA033875), National Center for Advancing Translational Sciences of the National Institutes of Health (UL1 TR000430) and the University of Chicago Pritzker School of Medicine Summer Research Project fund.
We would like to express our sincere gratitude towards the nurses, patients and staff of “Andreas Sygros” hospital whose voluntary assistance and efforts made this study possible.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to report.
References
- Antonsson A, Cornford M, Perry S, Davis M, Dunne MP, Whiteman DC. Prevalence and risk factors for oral HPV infection in young Australians. PLoS One. 2014;9(3):e91761. doi: 10.1371/journal.pone.0091761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo LS, Smelov V, Bzhalava D, Eklund C, Hultin E, Dillner J. Next generation sequencing for human papillomavirus genotyping. J Clin Virol. 2013;58(2):437–442. doi: 10.1016/j.jcv.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Backes DM, Kurman RJ, Pimenta JM, Smith JS. Systematic review of human papillomavirus prevalence in invasive penile cancer. Cancer Causes Control. 2009;20(4):449–457. doi: 10.1007/s10552-008-9276-9. [DOI] [PubMed] [Google Scholar]
- Bouda M, Gorgoulis VG, Kastrinakis NG, Giannoudis A, Tsoli E, Danassi-Afentaki D, et al. “High risk” HPV types are frequently detected in potentially malignant and malignant oral lesions, but not in normal oral mucosa. Mod Pathol. 2000;13(6):644–653. doi: 10.1038/modpathol.3880113. [DOI] [PubMed] [Google Scholar]
- Deshmukh AA, Chiao EY, Das P, Cantor SB. Clinical effectiveness and cost-effectiveness of quadrivalent human papillomavirus vaccination in HIV-negative men who have sex with men to prevent recurrent high-grade anal intraepithelial neoplasia. Vaccine. 2014;32(51):6941–6947. doi: 10.1016/j.vaccine.2014.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunne EF, Nielson CM, Stone KM, Markowitz LE, Giuliano AR. Prevalence of HPV infection among men: A systematic review of the literature. J Infect Dis. 2006;194(8):1044–1057. doi: 10.1086/507432. [DOI] [PubMed] [Google Scholar]
- Giuliano AR, Lu B, Nielson CM, Flores R, Papenfuss MR, Lee JH, et al. Age-specific prevalence, incidence, and duration of human papillomavirus infections in a cohort of 290 US men. J Infect Dis. 2008;198(6):827–835. doi: 10.1086/591095. [DOI] [PubMed] [Google Scholar]
- Giuliano AR, Nielson CM, Flores R, Dunne EF, Abrahamsen M, Papenfuss MR, et al. The optimal anatomic sites for sampling heterosexual men for human papillomavirus (HPV) detection: the HPV detection in men study. J Infect Dis. 2007;196(8):1146–1152. doi: 10.1086/521629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone S, Palefsky JM, Giuliano AR, Moreira ED, Aranda C, Jessen H, et al. Prevalence of and risk factors for human papillomavirus (HPV) infection among HIV-seronegative men who have sex with men. J Infect Dis. 2011;203(1):66–74. doi: 10.1093/infdis/jiq016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravitt PE, Peyton CL, Alessi TQ, Wheeler CM, Coutlée F, Hildesheim A, et al. Improved amplification of genital human papillomaviruses. J Clin Microbiol. 2000;38(1):357–361. doi: 10.1128/jcm.38.1.357-361.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjivassiliou M, Stefanaki C, Nicolaidou E, Bethimoutis G, Anyfantakis V, Caroni C, et al. Human papillomavirus assay in genital warts--correlation with symptoms. Int J STD AIDS. 2007;18(5):329–334. doi: 10.1258/095646207780749574. [DOI] [PubMed] [Google Scholar]
- Hebnes JB, Olesen TB, Duun-Henriksen AK, Munk C, Norrild B, Kjaer SK. Prevalence of genital human papillomavirus among men in Europe: systematic review and meta-analysis. J Sex Med. 2014;11(11):2630–2644. doi: 10.1111/jsm.12652. [DOI] [PubMed] [Google Scholar]
- Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer. 2009;124(10):2375–2383. doi: 10.1002/ijc.24215. [DOI] [PubMed] [Google Scholar]
- HSA Press Release: Statistics on income and living conditions. Pireaus. ELSTAT. 2013:1–12. [Google Scholar]
- Ifanti AA, Argyriou AA, Kalofonou FH, Kalofonos HP. Financial crisis and austerity measures in Greece: their impact on health promotion policies and public health care. Health Policy. 2013;113(1–2):8–12. doi: 10.1016/j.healthpol.2013.05.017. [DOI] [PubMed] [Google Scholar]
- King EM, Gilson R, Beddows S, Soldan K, Panwar K, Young C, et al. Oral human papillomavirus (HPV) infection in men who have sex with men: prevalence and lack of anogenital concordance. Sex Transm Infect. 2015;91(4):284–286. doi: 10.1136/sextrans-2014-051955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev. 2005;14(2):467–475. doi: 10.1158/1055-9965.EPI-04-0551. [DOI] [PubMed] [Google Scholar]
- Lambropoulos AF, Dimitrakopoulos J, Frangoulides E, Katopodi R, Kotsis A, Karakasis D. Incidence of human papillomavirus 6, 11, 16, 18 and 33 in normal oral mucosa of a Greek population. Eur J Oral Sci. 1997;105(4):294–297. doi: 10.1111/j.1600-0722.1997.tb00243.x. [DOI] [PubMed] [Google Scholar]
- Lissouba P, Van de Perre P, Auvert B. Association of genital human papillomavirus infection with HIV acquisition: a systematic review and meta-analysis. Sex Transm Infect. 2013;89(5):350–356. doi: 10.1136/sextrans-2011-050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills A, Balasubramaniam R, Longacre TA, Kong CS, Pinsky BA. Laboratory-developed L1 sequencing and type-specific, real-time polymerase chain reaction for the detection and typing of human papillomaviruses in formalin-fixed, paraffin-embedded tissues. Arch Pathol Lab Med. 2013;137(1):50–54. doi: 10.5858/arpa.2011-0392-OA. [DOI] [PubMed] [Google Scholar]
- Muñoz N, Bosch FX, de Sanjosé S, Herrero R, Castellsagué X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- Ong JJ, Read T, Chen M, Walker S, Law M, Bradshaw C, et al. Improving oral human papillomavirus detection using toothbrush sampling in HIV-positive men who have sex with men. J Clin Microbiol. 2014;52(6):2206–2209. doi: 10.1128/JCM.00286-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization WH. Prevalence of tobacco smoking. 2015. [Google Scholar]
- Stone KM, Karem KL, Sternberg MR, McQuillan GM, Poon AD, Unger ER, et al. Seroprevalence of human papillomavirus type 16 infection in the United States. J Infect Dis. 2002;186(10):1396–1402. doi: 10.1086/344354. [DOI] [PubMed] [Google Scholar]
- Tsikis S, Hoefer L, Charnot-Katsikas A, Schneider JA. Human papillomavirus infection by anatomical site among Greek men and women: a systematic review. Eur J Cancer Prev. 2015 doi: 10.1097/CEJ.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doorslaer K, Tan Q, Xirasagar S, Bandaru S, Gopalan V, Mohamoud Y, et al. The Papillomavirus Episteme: a central resource for papillomavirus sequence data and analysis. Nucleic Acids Res. 2013;41:D571–578. doi: 10.1093/nar/gks984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez-Argüelles ME, Melón S, Junquera ML, Boga JA, Villa L, Pérez-Castro S, et al. Human papillomavirus infection in a male population attending a sexually transmitted infection service. PLoS One. 2013;8(1):e54375. doi: 10.1371/journal.pone.0054375. [DOI] [PMC free article] [PubMed] [Google Scholar]