Abstract
Classic Ehlers-Danlos syndrome (EDS) patients suffer from connective tissue hyperelasticity, joint instability, skin hyperextensibility, tissue fragility, and poor wound healing due to heterozygous mutations in COL5a1 or COL5a2 genes. This study investigated the roles of collagen V in establishing structure and function in uninjured patellar tendons as well as in the injury response using a Col5a1+/− mouse, a model for classic EDS. These analyses were done comparing tendons from a classic EDS model (Col5a1+/−) with wild type controls. Tendons were subjected to mechanical testing, histological and fibril analysis before injury as well as 3 weeks and 6 weeks after injury. We found that Col5a1+/− tendons demonstrated diminished recovery of mechanical competency after injury as compared to normal wild type tendons, which recovered their pre-injury values by 6 weeks post injury. Additionally, the Col5a1+/−tendons demonstrated altered fibril morphology and diameter distributions compared to the wild type tendons. This study indicates that collagen V plays an important role in regulating collagen fibrillogenesis and the associated recovery of mechanical integrity in tendons after injury. In addition, the dysregulation with decreased collagen V expression in EDS is associated with a diminished injury response. The results presented herein have the potential to direct future targeted therapeutics for classic EDS patients.
INTRODUCTION
Classic (type I) Ehlers-Danlos syndrome (EDS) is a rare genetic disease with an estimated prevalence of 1 in 5000 live births.1 It is defined by collagen V mutations with haploinsufficiency for COL5a1 present in ~67% of affected individuals.2; 3 These patients exhibit connective tissue hyperelasticity and laxity that results in hyperextensible skin, joint hypermobility and instability. They also demonstrate tissue fragility, and poor wound healing. Mutations in COL5a1 have been linked to injury,4 performance deficiencies in sport activity,5 Achilles tendinopathy,6 and anterior cruciate ligament rupture.7 While collagen V is a quantitatively minor component in tendons and ligaments, it plays a major role in the regulation of fibrillogenesis, specifically during the early process of fibril nucleation.8; 9 Reduction of collagen V results in fewer collagen I fibrils with increased diameters in tendons, ligaments, dermis, and the cornea.9–13 This is particularly important because the ability of tendon to withstand high forces when transferring loads is often attributed to their hierarchically-organized collagen I structure, with the collagen molecules assembled into fibrils, which then bundle to form fibers, fascicles, and the full tendon proper.14; 15
To study classic EDS, we used a Col5a1+/− classic EDS mouse model that faithfully recapitulates the human clinical phenotype.9; 11 Using this model and a tendon/ligament-specific collagen V-null model, recent studies on the flexor digitorum longus (FDL) have shown altered fibril structure and deficient macroscale tensile mechanical function when collagen V is reduced.11–13 There is also evidence suggesting that the role of collagen V in establishing tendon mechanical properties is tissue-specific.16 Further, it has been shown that the unique series of dynamic re-organizations that prevent early failure in tendons were altered in the Col5a1+/− and Col5a1−/− tendons.17 EDS also is characterized by abnormal wound healing and scarring. In Col5a1+/− mice, haploinsufficiency in skin resulted in impaired wound healing.18 In addition, a significant reduction in the tensile strength of incisional wounds was observed 8 days after wounding.11
In normal tendon, the injury response involves an initial inflammatory period followed by deposition and assembly of a disorganized extracellular matrix and finally, extensive tissue and scar remodeling.19; 20 Generally, this injury response cascade does not result in a complete recovery of pre-injury functionality. Collagen V, as well as small leucine rich proteoglycans, decorin and biglycan, are known to influence collagen fibril assembly and growth during development.21; 22 The absence of decorin impairs the long-term recovery of tendon mechanical response after injury, while biglycan negatively affects early stage healing.23 The role(s) of collagen V in injured Col5a1+/− tendons have not yet been defined. Therefore, the purpose of this study was to use the Col5a1+/−haploinsufficient mouse model of classic EDS to define the regulatory roles of collagen V in the injury response by comparing uninjured and injured tendons from normal, wild type mice with the Col5a1+/− classic EDS model. Since EDS is associated with joint hypermobility and instability leading to recurrent injury, a better understanding of the pathophysiology and healing response of injured tendons will improve the ability to treat those affected as well as help to better prevent injuries. We hypothesize that collagen V haploinsufficiency will result in altered collagen fibril and tendon hierarchal structure with an associated reduction in mechanical properties and collagen fiber realignment in the repair response to injury. Compared to normal tendons, the reacquisition of structural parameters and improvement of mechanical properties in the injured Col5a1+/− tendons would be delayed and/or diminished.
METHODS
Animals and Study Design
This study was approved by the University of Pennsylvania and the University of South Florida Institutional Animal Care and Use Committees. These studies were performed on wild type mice (wild type littermates from Col5a1+/− mice) and our established classic EDS mouse model (Col5a1+/− mice in a C57/BL6 background).9; 11 Patellar tendon injury, described below, was performed on both limbs of male mice at 120 days of age from each genotype. Mice were then euthanized at 1 week, 3 weeks, or 6 weeks post injury. Tendon histology (n=4/group/time point), fibril structure (n=4/group/time point), and Col5a1 expression (n=4/group/time point) were analyzed at 1, 3, and 6 weeks post-injury. In addition, tendons at 3 and 6 weeks post-injury were assessed for changes in mechanical properties (n=15/group/time point). Uninjured tendons at 150 days of age were assessed for all properties from both genotypes to determine baseline values (n=30/group).
Surgery
All mice underwent bilateral patellar tendon injury surgery under aseptic conditions as previously described.24; 25 Briefly, mice were anesthetized and both hindlimbs were shaved and cleaned with an iodine and isopropyl alcohol mixture. The mice were placed in supine position with the knee flexed and a single skin incision was made medial to the knee to expose the patellar tendon. The tendon was isolated via longitudinal incisions adjacent to and on either side of the tendon. A rubber-coated backing was placed under the tendon to protect the underlying tissues and a 0.75 mm diameter biopsy punch was used to create a full thickness partial width (~60%) excisional injury in the midsection of the tendon. The skin wound was then closed with sutures and the mice were allowed to return to cage activity.
Immuno-blot Analysis
Immuno-blots were done as previously described.13 Briefly, patellar tendons were dissected from wild type and Col5a1+/− mice at post-natal day 30. Pooled samples with 2 tendons from different mice were analyzed (n= 2). Tendon extracts were prepared in 50 mM Tris-HCl, pH 6.8, 1% SDS lysis buffer with proteinase inhibitors (Thermal Scientific, Waltham, MA). Extracts (20 μg) were separated on 4–12% Bis-Tris gels (Thermo Fisher Scientific) and transferred onto Hybond-C membranes (GE Healthcare, Pittsburgh, PA). The membrane was hybridized with affinity purified anti-α1(V),26 and anti-β-actin antibodies (Millipore, Billerica, MA).
Real-time PCR of Col5a1 Expression
Real-time PCR was done as described.13 Briefly, patellar tendons were dissected, cut into small pieces, and total RNA was extracted using the RNeasy Micro Kit (QIAGEN, Germantown, MD). Total RNA (3 ng per well) was subjected to reverse transcription using a High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific) and real-time PCR was performed with SYBR Green PCR master mix (Thermo Fisher Scientific) on a StepOnePlus Real Time PCR system (Thermo Fisher Scientific). The primer sequences were as follows: Col5a1 forward primer: AAGCGTGGGAAACTGCTCTCCTAT, Col5a1 reverse primer: AGCAGTTGTAGGTGACGTTCTGGT, β-actin forward primer: AGATGACCCAGATCATGTTTGAGA and β-actin reverse primer: CACAGCCTGGATGGCTACGT. Each sample (n= 4 tendons (different mice)/group/time point) was run in duplicate and data were analyzed using StepOne software v2.0. β-actin was used as an internal control to standardize the amount of sample total RNA.
Mechanical Testing
Patellar tendons, along with the patella and tibia were dissected as previously described. 23 Tendon cross-sectional area was measured using a custom built device consisting of linear variable differential transformers (LVDTs), a CCD laser, and translation stages.27 Tendons were then stamped into a “dog-bone” shape to isolate the repair tissue from the uninjured struts using a custom-designed 3D printed stage and a 2mm biopsy punch. Verhoeff stain lines were placed on the tendon to differentiate regions of optical strain and alignment analysis. The first stain line was placed at the tendon insertion site on the tibia. The remaining stain lines were placed either on each side of the visualized injury site or, for the uninjured tendons, at 1 and 2 mm from the insertion. The tibia was then embedded in a custom designed 3D printed pot and secured with polymethylmethacrylate (PMMA), which was applied over the tibial plateau. The pot was inserted into a custom testing fixture and the patella was placed in a custom grip for tensile testing.
The samples were then placed in a room temperature phosphate buffered saline bath and loaded in a tensile testing system (Instron 5848, Instron, Norwood, MA) integrated with a polarized light setup consisting of a linear backlight (Dolan-Jenner, Boxborough, MA), 90 degree offset rotating polarizer sheets (Edmund Optics, Barrington, NJ) on either side of the test sample, and a digital camera (Basler, Exton, PA). The testing protocol17; 28–30 consisted of preconditioning, stress-relaxation (for 600 seconds) and frequency sweeps (0.1, 1, and 10 Hz) at 2%, 3%, and 4% peak strains, a return to zero displacement, a 60 second hold, and finally, a ramp to failure at a strain rate of 0.1%/s. Images were obtained every 5 seconds for optical strain analysis. Additionally, sets of 18 polarized light images were acquired at various points during the testing protocol and every 20 seconds during the ramp to failure as the polarizers rotated through a 135 degree range for measurement of fiber realignment during loading, as previously described.31 Images collected for optical strain were analyzed for standard tensile mechanical properties via optical tracking using custom software (Matlab, Natick, MA, USA).32–34 Polarized light images collected throughout the mechanical testing regime were analyzed with a custom program (Matlab, Natick, MA, USA) to quantify collagen fiber alignment, outputted as circular standard deviation, as previously described.31; 35
Tendon stiffness (N/mm) and maximum load at failure (N) were calculated from the force-displacement data. These data were then normalized to the tendon cross-sectional area and tendon length (gauge length, measured optically at a predetermined nominal force value of 0.007N) to obtain the tendon’s material stress-strain response and Young’s modulus (MPa) and maximum stress (MPa) were evaluated. The dynamic modulus |E*| (defined by the ratio of the amplitudes of the stress and strain sinusoids) was calculated for each strain-frequency combination from the frequency sweep data. Likewise, the phase angle δ, the peak-to-peak distance (in degrees) between curves (resulting from the time lag between stress and strain) was obtained. Outlier analysis was performed on all datasets prior to statistical analysis. Two-way ANOVAs with post-hoc Bonferroni tests were used to assess the effects of genotype and time on mechanical and structural properties.
Histology
For samples designated for histological analysis, the entire knee joint was removed by cutting the femur and the tibia at the time of sacrifice. The entire joint was flexed to 90°, placed into a cassette, fixed in formalin, and processed using standard paraffin histological techniques. Sections (7 μm) were stained with hematoxylin and eosin to assess cellularity and cell shape. These parameters were evaluated by three masked graders using a semi-quantitative method.36 Comparisons were made using Kruskal-Wallis non-parametric one-way ANOVA followed by post-hoc Dunn’s test for multiple comparisons.
Transmission Electron Microscopy
Tendons designated for analysis of fibril structure were prepared for transmission electron microscopy as previously described.15; 29 Briefly, tendons were fixed in 4% paraformaldehyde, 2.5% glutaraldehyde, 0.1 M sodium cacodylate and 8 mM CaCl2, adjusted to pH 7.4 with NaOH. They were then post-fixed with osmium tetroxide, dehydrated with ethanol, embedded in Epon 812 and polymerized at 60°C. Ultra-thin cross-sections were imaged on a JEOL 1400 transmission electron microscope (JEOL Ltd., Tokyo, Japan) equipped with a Gatan Orius widefield side mount CC Digital camera (Gatan Inc., Pleasanton, CA). Eight to ten digital images from the midsubstance of each tendon were taken from non-overlapping areas at 60,000X. Images were randomized and masked before fibril diameters were measured using an RM Biometrics-Bioquant Image Analysis System (Nashville, TN). A region of interest (ROI) of appropriate size was determined within each digital image so that a minimum of 80 fibrils could be measured using 1 or 2 ROIs. The ROIs for all samples were localized to the mid-substance of the tendon (injury-site) and the insertion site was not analyzed. All fibrils in the region of interest were measured and multiple regions of interest were used if necessary to collect at least 80 fibril diameter measurements per image. Fibril diameters were measured along the minor axis of the fibril cross-section. Fibril diameter measurements from each tendon type were pooled and graphed by size in 5 nm bins (x-axis) vs frequency (%, y-axis). All fibrils over 220nm in diameter were combined in a final bin (220+). Two-tailed t-tests were used to compare genotypes at each time point.
RESULTS
Collagen V expression
Day 30 patellar tendons demonstrated reduced collagen V expression in Col5a1+/− compared to wild type tendons using qPCR. The Col5a1+/− patellar tendons from male mice demonstrated a 37.8% reduction in Col5a1 expression compared with wild type controls (Fig. 1A). There was a 37.6% reduction in the female tendons (data not shown). In addition, an ~50% reduction was observed in Col5a1 protein content by immuno-blot analysis (Fig. 1B). These data are consistent with haploinsufficiency in Col5a1 expression in heterozygous patellar tendons.
Figure 1. Collagen V expression in the patellar tendon.
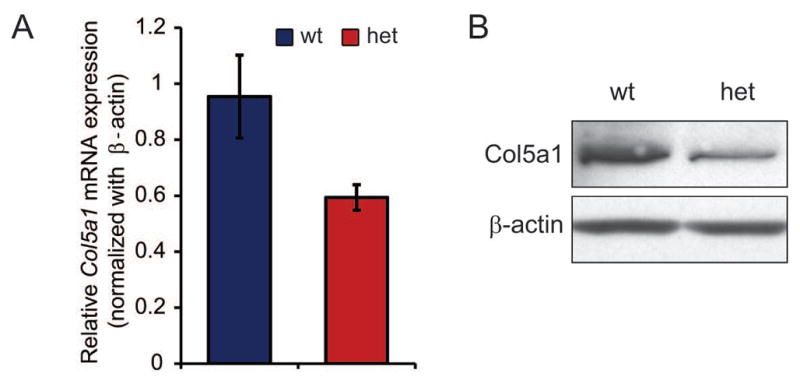
(A) Expression of Col5a1 is reduced by 37.8% in heterozygous compared to wild type tendons as determined using real-time PCR. n=4/tendons(different mice)/genotype. (B) Col5a1 protein is reduced by ~50% in heterozygous compared with wild type tendons as determined using immuno-blots. Representative immuno-blots from 2 unique pooled samples. Patellar tendons from male day 30 mice.
Col5a1 expression was analyzed in uninjured day 150 patellar tendons as well as at 1, 3 and 6 weeks post-injury. Similar to the day 30 tendons, uninjured day 150 Col5a1+/− tendons had a ~36% reduction in Col5a1 expression compared to wild type controls (Fig. 2). At 1 and 3 weeks post-injury Col5a1 expression was increased compared to the uninjured tendons in both Col5a1+/− and wild type tendons. At 6 weeks post-injury Col5a1 expression was comparable to the uninjured tendons. At 1, 3 and 6 weeks post injury Col5a1 expression was reduced by ~36%, 37% and 31% respectively in Col5a1+/− compared to wild type tendons.
Figure 2. Expression of collagen V post-injury.
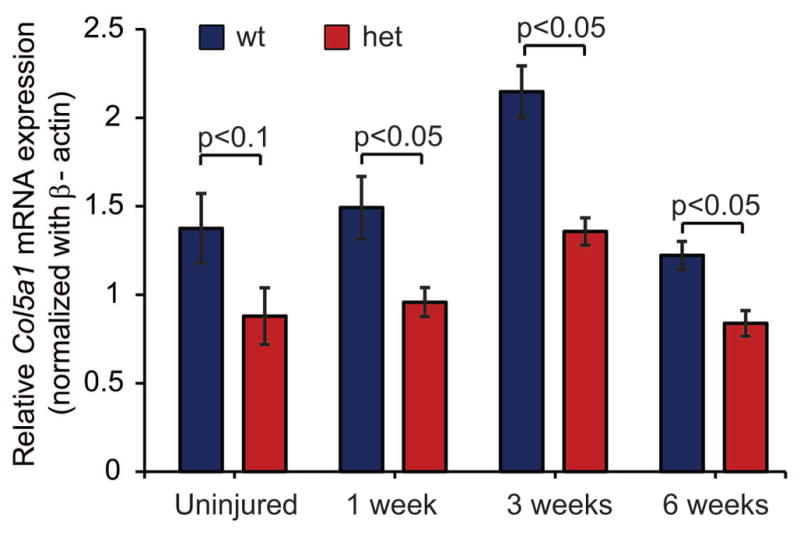
Expression of Col5a1 is decreased by 31–37% in heterozygous patellar tendons compared to wild type tendons as determined using real-time PCR in uninjured tendons and at 1, 3 and 6 weeks post injury. An increase in Col5a1 expression is observed at 1 and 3 weeks post injury, but expression is comparable to uninjured controls at 6 weeks post-injury. Mice injured at day 120, uninjured mice analyzed at day 150, n=4 tendons from 4 mice as mean of 1–3 technical replicates.
Biomechanical Properties
Average body weight of mice prior to sacrifice were: Uninjured Col5a1+/− 29.4±2.4 gm, uninjured wild type 32.3±3.8 gm, 3 weeks post-injury Col5a1+/− 29.0±2.9 gm, 3 weeks post-injury wild type 30.1±3.0 gm, 6 weeks post-injury Col5a1+/− 29.9±3.1 gm, and 6 weeks post-injury wild type 32.2±2.7 gm. Samples were excluded if measurements were noted to deviate more than two standard deviations from the mean or if issues were noted during dissection or mechanical testing. The resulting sample size for each group was: Uninjured Col5a1+/− (11/15), uninjured wild type (13/15), 3 weeks post-injury Col5a1+/− (11/15), 3 weeks post-injury wild type (12/15), 6 weeks post-injury Col5a1+/− (10/15), and 6 weeks post-injury wild type (12/15).
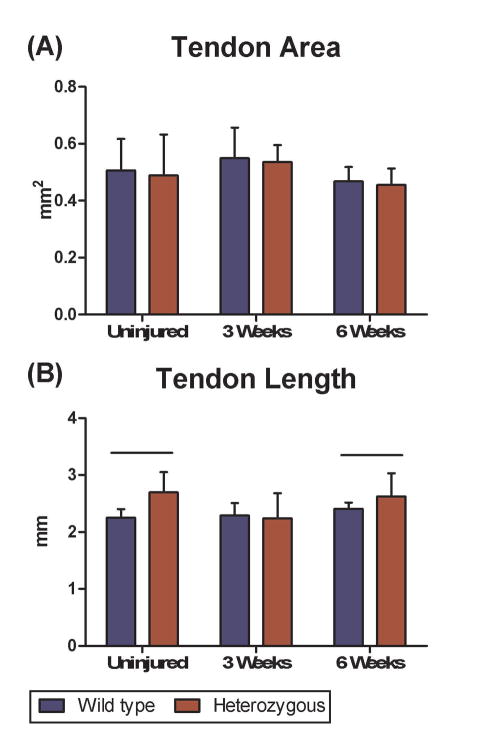
There were no differences in whole tendon cross sectional area of Col5a1+/− mice compared to wild type mice pre-injury or at any time point post-injury (Fig. 3A). However, differences were observed in tendon length with the Col5a1+/− patellar tendons significantly longer compared with wild type controls pre-injury and at 6 weeks post-injury. No significant difference was observed 3 weeks post injury (Fig. 3B). The Col5a1+/− patellar tendons had significantly lower stiffness pre-injury when compared to the wild type tendons. However, there was no difference between groups at 3 weeks post-injury (Fig. 4A). In contrast, stiffness was significantly decreased in the Col5a1+/− tendons compared to the wild type tendons at 6 weeks post-injury. No differences were observed in modulus between wild type and Col5a1+/− tendons pre-injury or at 3 weeks post-injury. However, the Col5a1+/− tendons had a significantly lower tissue modulus at 6 weeks post-injury (Fig. 4B). No differences in collagen fiber re-alignment were observed between the wild type and Col5a1+/−groups at any of the time points (data not shown).
Figure 3.
(A) No differences were observed in area between genotypes at any of the time points. (B) Significant differences were observed in length of the patellar tendons between genotypes pre-injury and 6 weeks post injury.
Figure 4.
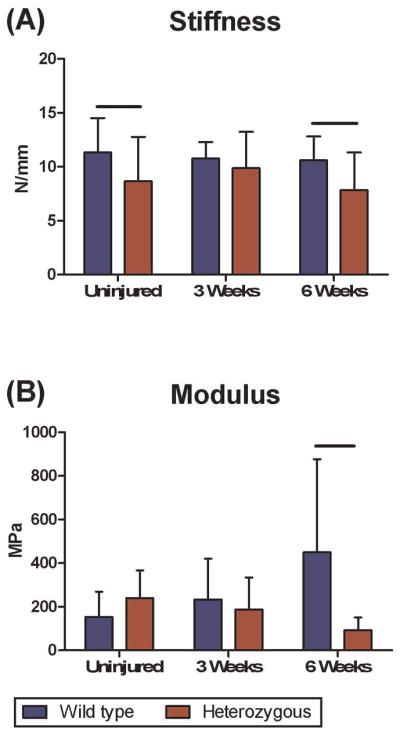
(A) Heterozygous tendons had reduced stiffness pre-injury and 6 weeks post-injury compared to wild type. (B) Wild type tendon mid-substance modulus recovered to pre-injury values while the heterozygous tendons demonstrated a diminished response.
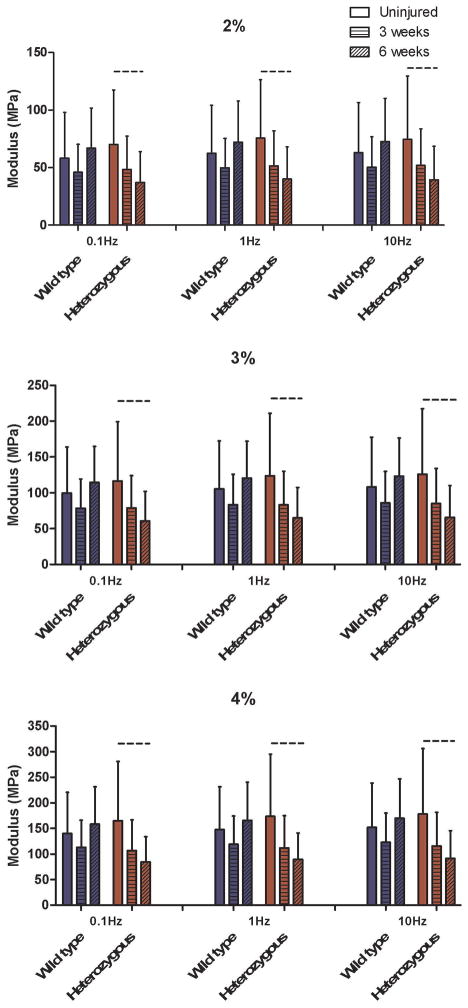
Col5a1+/−tendons had a slightly decreased dynamic modulus (trend) at 6 weeks post-injury compared to 3 weeks post-injury at each frequency (0.1, 1, and 10 Hz) and strain level (2%, 3%, and 4%), Fig. 5). There were no other differences in dynamic modulus between groups. For tan(δ), which is a measure of tendon viscosity, no differences were observed between genotype, injury status, or post-injury time point.
Figure 5.
Dynamic modulus failed to recover for the heterozygous tendons at all strain magnitudes (2%, 3%, & 4%) and frequencies (0.1Hz, 1Hz, 10Hz), with a trend for reduced values between pre-injury and 6-weeks post injury.
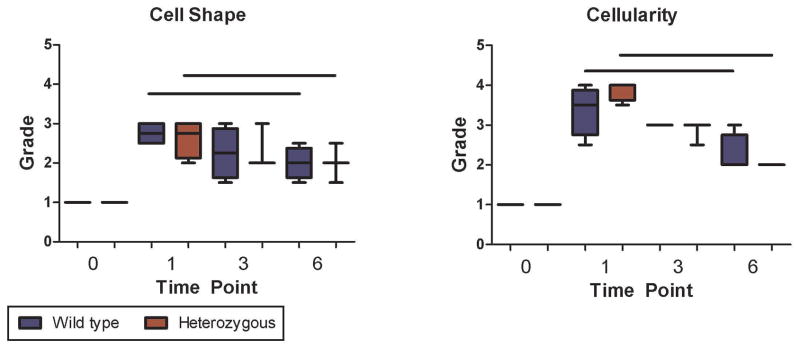
Tendon Cellularity and Shape
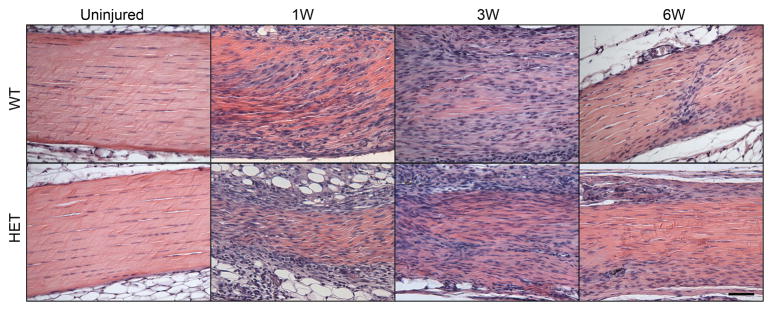
No differences in cellularity and cell shape were observed between the wild type and Col5a1+/−groups at any of the time points investigated, but as expected, cellularity and cell shape returned to baseline over time post-injury for both genotypes, with a significant reduction in cellularity and increase in spindle-like cells observed at 6 weeks as compared to 1 week post-injury (Fig. 6).
Figure 6.
(A) Uninjured tendons in both wild type and heterozygous tendons showed normal cellular activity. As expected, an increase in tenocytes was observed at all time points post-injury. (B) There were no changes between wild type and heterozygous tendons in these parameters. As expected, changes in cellularity and cell shape were observed over time post-injury for both genotypes, with a significant reduction in cellularity and increase in spindle-like cells observed at 6 weeks when compared with 1 week post injury tendons.
Collagen Fibril Structure
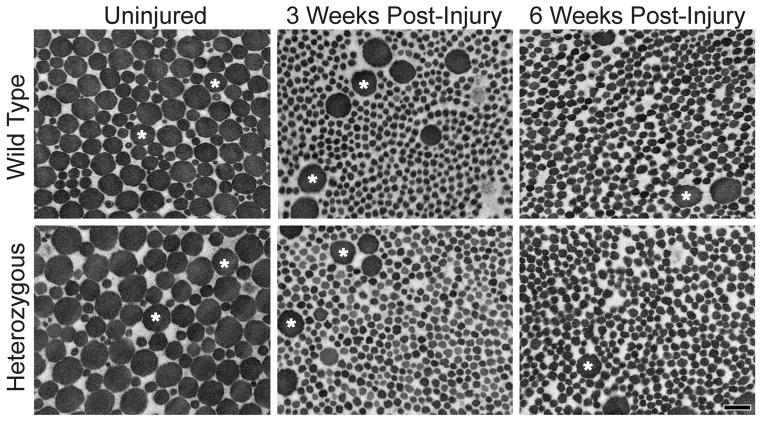
Wild type and Col5a1+/− tendons both exhibited mostly normal circular fibril cross-sectional profiles pre-injury. Qualitatively, the uninjured Col5a1+/− tendons had larger diameter fibrils when compared with the wild type tendons. Both groups demonstrated two distinct subpopulations of smaller and larger diameter fibrils. The uninjured tendons were dominated with larger diameter fibrils for both wild type and Col5a1+/−groups. At 3 weeks post-injury, both genotypes showed a substantial reduction in the quantity of larger diameter fibrils (Figs. 8, 9), with a significant increase in smaller diameter fibrils. The fibril diameter distribution was comparable in wild type and Col5a1+/−tendons at 6 weeks post-injury (Figs. 8, 9). No fibril analysis was performed for the 1-week injury time point due to cellular proliferation in both the wild type and Col5a1+/− tendons.
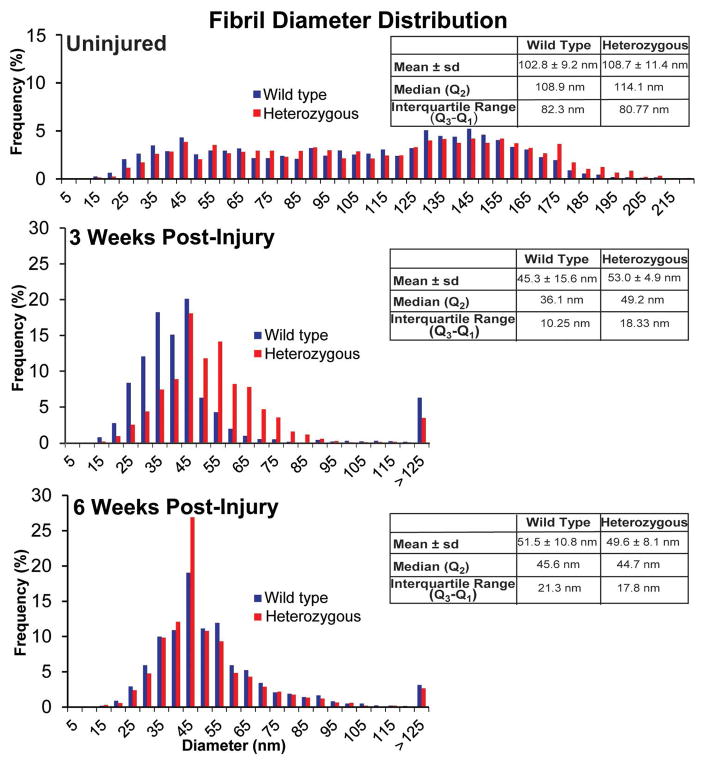
Figure 8. Collagen V expression influences fibril diameter distributions in the injury response.
Collagen fibril diameter distributions for wild type and heterozygous patellar tendons pre-injury, and 3 weeks and 6 weeks post-injury.
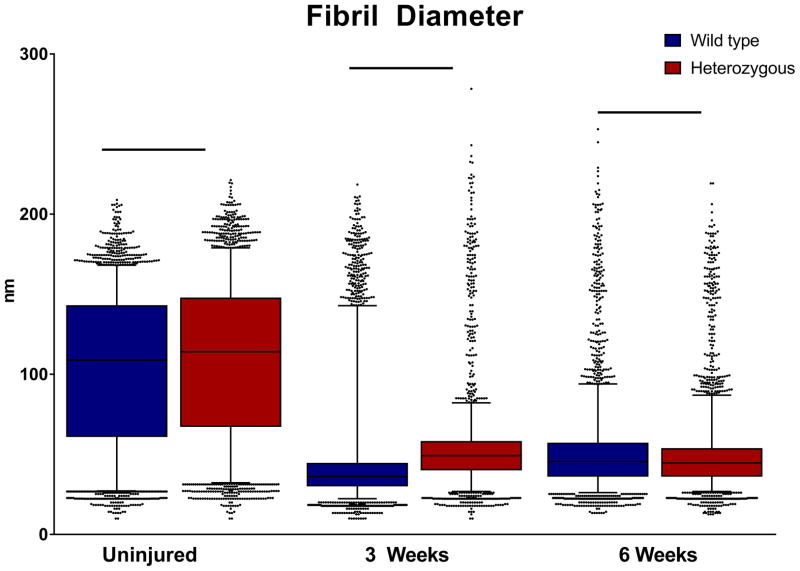
Figure 9. Analysis of fibril diameter distributions post-injury.
Collagen fibril diameters for the HET tendons were significantly larger pre-injury and at 3 weeks post injury. In contrast, the WT tendons had a larger median fibril diameter at 6 weeks post injury. Boxplot whiskers span data between the 5th and 95th percentile. Data points outside this range are shown with individual markers.
Quantitative fibril diameter analysis of the uninjured wild type and Col5a1+/−tendons demonstrated a multimodal distribution of fibril diameters with an overall shift to the right, i.e., larger diameter in the Col5a1+/− tendons. The Col5a1+/− tendons had a larger median fibril diameter of 108.2nm compared to 114.7nm for the wild type tendons (Figs. 8, 9). At 3 weeks, distinct differences were observed between wild type and Col5a1+/− diameter distributions. The wild type tendons had smaller diameter fibrils with a median of 36.1nm as compared to 49.2nm for the Col5a1+/− tendons. There also was increased heterogeneity in the Col5a1+/− tendons with the interquartile (Q3-Q1) ranges being 10.3nm and 18.3nm respectively. At 6 weeks post-injury, there was a comparable fibril diameter distribution in the wild type and Col5a1+/− tendons. The median diameters were 44.8nm and 45.6nm for the Col5a1+/− and wild type tendons respectively. In post-injury tendons, there were small numbers of large diameter (<125nm) fibrils in both genotypes consistent with the large, mature fibrils in the uninjured tendons (Fig. 5).
DISCUSSION
Multiple studies have investigated the downstream effects of Col5a1 haploinsufficiency in uninjured musculoskeletal tendinous tissues.9; 13; 16 However, given the significant role played by collagen V in tendon fibrillogenesis, 12 the primary focus of our current study was to elucidate the roles of collagen V in the tendon injury response and recovery. The Col5a1+/− tendons had a deficient mechanical response compared to wild type tendons pre-injury. After 3 and 6 weeks post-injury, the wild type tendons were able to regain most of their mechanical properties. However, the injury response of the Col5a1+/− tendons was deficient as compared to their wild type counterparts and they failed to recover their mechanical properties. Specifically, we found a trend of decreased stiffness in the pre-injury Col5a1+/− group, which has been previously observed for the FDL and supraspinatus tendons.16 Post-injury, the wild type tendons were able to regain their pre-injury stiffness by 6 weeks, which was not observed for the Col5a1+/ − patellar tendons. No difference was observed in tissue modulus between the wild type and Col5a1+/− patellar tendons pre-injury, which has been previously observed for the FDL and Achilles tendons, but for not the supraspinatus tendon or the anterior cruciate ligament. The Col5a1+/− tendons had significantly reduced modulus when compared with their wild type counterparts at 6 weeks post-injury at its mid-substance. It has been shown previously that collagen V is significantly upregulated after injury in normal tissues.37 This up-regulation is however diminished in the Col5a1+/− tendons compared to the wild type controls, which explains the observed inferior mechanical recovery at 3 and 6 weeks post injury. Analysis of tendon dynamic modulus revealed an interesting trend. At every strain, and every frequency sweep, the wild type tendons visually showed a drop at 3 weeks post-injury compared to the uninjured tendons and then a return to baseline by 6 weeks. The Col5a1+/− tendons had the same drop visually between the uninjured and at 3 weeks post-injury, however, the modulus continued to decrease and there was a trend for the 6-week post-injury tendons to have a decreased modulus compared to the uninjured tendons. These data suggest that collagen V has an important role in healing and regaining dynamic function following tendon injury. This finding is consistent with several other studies that have investigated the role of collagen V in tendon, particularly those evaluating dynamic collagen fiber re-organization and macroscale function.11; 13; 16; 17 We did not observe any differences in tan(δ) at the applied strain magnitudes and frequencies used in the analyses. Given the differences observed in dynamic modulus, one would expect similar changes with tan(δ). However, our previous study with uninjured Col5a1+/− and Col5a1−/− supraspinatus tendons only detected differences in tan(δ) between the wild type and Col5a1+/− groups at strain magnitudes higher than 4%. The inclusion of injured time points in this current study necessitated reduction of the peak strain magnitudes.
Histological analysis indicates no differences in cell shape or cellularity between genotype, but tendon cell properties did improve toward baseline uninjured conditions over time in both groups. This suggests that differences in healing properties are not caused by disparities in the recruitment of or number of tendon cells within the healing midsubstance. A rounded nuclear shape has been regarded as a sign of metabolic activation in response to matrix damage.38; 39 Our findings suggest that reduction in collagen V and the resulting alterations in collagen I fibril structure do not impact this general response mechanism.
The trend of uninjured wild type tendon fibrils to have smaller diameters as compared to Col5a1+/− tendons was expected, since we previously saw a similar trend in supraspinatus tendons, skin, cornea and the FDL.8; 16; 17; 26 The bimodal distribution of fibril diameters was lost at 3 and 6 weeks post-injury for both the wild type and Col5a1+/− tendons, with larger diameter fibrils occurring with much reduced frequency. The injured Col5a1+/− tendons continued to have larger collagen fibrils as compared to wild type tendons at 3 weeks post injury. Further, these larger fibrils in the Col5a1+/− tendons were frequently associated with irregular cross sectional morphology characteristic of collagen V-null tendons due to impaired fibrillogenesis.11 These irregularly shaped fibrils are characteristic in classic EDS patients dermis and tendon biopsies and murine dermis and supraspinatus tendons.26 Irregular collagen fibril morphology can potentially affect fibril and interfibrillar matrix interactions, which could explain the diminished dynamic mechanical response of the Col5a1+/− tendons after injury. Interestingly, at 6 weeks post-injury, Col5a1+/− tendons had smaller fibrils when compared with the wild type tendons, indicating further failure in the healing response of the Col5a1+/− tendons due to impaired collagen V regulation.
While this study provides significant insight into the injury response of wild type and Col5a1+/− tendons, it is not without limitations. We saw a higher than normal variation in tissue modulus for the 6 week post-injury wild type tendons. We did conduct outlier analysis on these data prior to statistical comparisons and believe this to be natural variation in the data. We also observed a difference in tendon gauge length between genotypes pre-injury and at 6 weeks post injury. This could be more a reflection of the method employed to measure length (at a nominal load) than an in situ change in the relative position of the patella. This study used the conventional Col5a1+/− mouse model consistent with the clinical phenotypes of classic EDS patients. However, targeted deletion of collagen V after injury could provide specificity towards defining its role in tendon injury recovery. Altered loading due to changes in joint capsule and cartilage could significantly contribute to the observed mechanical property differences in heterozygous tendons and we did not investigate these structures and tissues in the current study. Further, the microscale response of altered collagen fibrils to load, such as fibril sliding, crimp, and fibril deformation was not investigated. Our recent work has shown that uninjured Col5a1+/− supraspinatus tendons compensated for their lack in fibril strength by early realignment and increased fibril sliding. Investigating alterations in these processes can help further define tendon injury response and recovery in an altered fibrillar environment. Finally, collagen V expression may vary regionally and could affect spatial mechanical response. Although, this study evaluated healing in the mid-substance only, which is independent of any regional collagen V variation.
Overall, we found that the Col5a1+/− tendons demonstrated slower and diminished mechanical and structural recovery post injury as compared to wild type tendons. Based on the data presented, collagen V plays an important role in tendon injury recovery as well as response and may aid in developing targeted therapeutics for classic EDS patients. Future studies will investigate the specific role of collagen V in various stages of the healing process by utilizing a conditional mouse model with targeted partial and complete deletion of collagen V of tendons post-injury.
Figure 7. Changes in collagen fibril structure post-injury.
Representative micrographs of fibrils from mid-substance of the wild type and heterozygous patellar tendons for the uninjured, 3 weeks post-injury, and 6 weeks post-injury groups all containing two subpopulations of small and large diameter fibrils. Injured tendons showed a dominant population of smaller diameter fibrils indicating fibril assembly after injury. Both groups post-injury also demonstrated a second subpopulation of large diameter fibrils (*). Magnification bar = 200nm.
Acknowledgments
Grant Support: This study was supported by NIH/NIAMS AR065995 and the Penn Center for Musculoskeletal Disorders (NIH, P30 AR050950)
Footnotes
Study approved by: University of Pennsylvania IACUC, University of South Florida IACUC
Author contributions: All authors have read and approved the final version of the manuscript. Contributions are as follows: J.M.J.: Research design, acquisition of data, data analysis and interpretation, drafting and revision of paper, approval of submitted version. B.K.C.: Data analysis and interpretation, revision of paper. S.S.S.: Acquisition of data, data analysis and interpretation, drafting and revision of paper. K.A.R.: acquisition of data, revision of paper. J.H.: Acquisition of data, data analysis and interpretation, revision of paper. A.B.R.: Acquisition of data, data analysis, revision of paper. M.S.: Research design, acquisition of data, data analysis and data interpretation, paper revision. S.M.A.: Research design, data analysis and data interpretation, paper revision. D.E.B.: Research design data interpretation, paper revision, approval of submitted version. L.J.S.: Research design, data interpretation, paper revision, approval of submitted version.
References
- 1.Callewaert B, Malfait F, Loeys B, et al. Ehlers-Danlos syndromes and Marfan syndrome. Best Pract Res Clin Rheumatol. 2008;22:165–189. doi: 10.1016/j.berh.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Malfait F, Coucke P, Symoens S, et al. The molecular basis of classic Ehlers-Danlos syndrome: a comprehensive study of biochemical and molecular findings in 48 unrelated patients. Hum Mutat. 2005;25:28–37. doi: 10.1002/humu.20107. [DOI] [PubMed] [Google Scholar]
- 3.Symoens S, Syx D, Malfait F, et al. Comprehensive molecular analysis demonstrates type V collagen mutations in over 90% of patients with classic EDS and allows to refine diagnostic criteria. Hum Mutat. 2012;33:1485–1493. doi: 10.1002/humu.22137. [DOI] [PubMed] [Google Scholar]
- 4.Laguette MJ, Abrahams Y, Prince S, et al. Sequence variants within the 3′-UTR of the COL5A1 gene alters mRNA stability: implications for musculoskeletal soft tissue injuries. Matrix Biol. 2011;30:338–345. doi: 10.1016/j.matbio.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Collins M, Posthumus M. Type V collagen genotype and exercise-related phenotype relationships: a novel hypothesis. Exerc Sport Sci Rev. 2011;39:191–198. doi: 10.1097/JES.0b013e318224e853. [DOI] [PubMed] [Google Scholar]
- 6.September AV, Cook J, Handley CJ, et al. Variants within the COL5A1 gene are associated with Achilles tendinopathy in two populations. Br J Sports Med. 2009;43:357–365. doi: 10.1136/bjsm.2008.048793. [DOI] [PubMed] [Google Scholar]
- 7.Posthumus M, September AV, O’Cuinneagain D, et al. The COL5A1 gene is associated with increased risk of anterior cruciate ligament ruptures in female participants. Am J Sports Med. 2009;37:2234–2240. doi: 10.1177/0363546509338266. [DOI] [PubMed] [Google Scholar]
- 8.Sun M, Chen S, Adams SM, et al. Collagen V is a dominant regulator of collagen fibrillogenesis: dysfunctional regulation of structure and function in a corneal-stroma-specific Col5a1-null mouse model. J Cell Sci. 2011;124:4096–4105. doi: 10.1242/jcs.091363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wenstrup RJ, Florer JB, Brunskill EW, et al. Type V collagen controls the initiation of collagen fibril assembly. The Journal of biological chemistry. 2004;279:53331–53337. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]
- 10.Segev F, Heon E, Cole WG, et al. Structural abnormalities of the cornea and lid resulting from collagen V mutations. Invest Ophthalmol Vis Sci. 2006;47:565–573. doi: 10.1167/iovs.05-0771. [DOI] [PubMed] [Google Scholar]
- 11.Wenstrup RJ, Florer JB, Davidson JM, et al. Murine model of the Ehlers-Danlos syndrome. col5a1 haploinsufficiency disrupts collagen fibril assembly at multiple stages. The Journal of biological chemistry. 2006;281:12888–12895. doi: 10.1074/jbc.M511528200. [DOI] [PubMed] [Google Scholar]
- 12.Wenstrup RJ, Smith SM, Florer JB, et al. Regulation of collagen fibril nucleation and initial fibril assembly involves coordinate interactions with collagens V and XI in developing tendon. The Journal of biological chemistry. 2011;286:20455–20465. doi: 10.1074/jbc.M111.223693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun M, Connizzo BK, Adams SM, et al. Targeted deletion of collagen V in tendons and ligaments results in a classic Ehlers-Danlos syndrome joint phenotype. The American journal of pathology. 2015;185:1436–1447. doi: 10.1016/j.ajpath.2015.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birk DE, Southern JF, Zycband EI, et al. Collagen fibril bundles: a branching assembly unit in tendon morphogenesis. Development. 1989;107:437–443. doi: 10.1242/dev.107.3.437. [DOI] [PubMed] [Google Scholar]
- 15.Birk DE, Trelstad RL. Extracellular compartments in tendon morphogenesis: collagen fibril, bundle, and macroaggregate formation. J Cell Biol. 1986;103:231–240. doi: 10.1083/jcb.103.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Connizzo BK, Freedman BR, Fried JH, et al. Regulatory role of collagen V in establishing mechanical properties of tendons and ligaments is tissue dependent. J Orthop Res. 2015;33:882–888. doi: 10.1002/jor.22893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connizzo BK, Han L, Birk DE, et al. Collagen V-heterozygous and -null supraspinatus tendons exhibit altered dynamic mechanical behaviour at multiple hierarchical scales. Interface Focus. 2016;6:20150043. doi: 10.1098/rsfs.2015.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeNigris J, Yao Q, Birk EK, et al. Altered dermal fibroblast behavior in a collagen V haploinsufficient murine model of classic Ehlers-Danlos syndrome. Connect Tissue Res. 2016;57:1–9. doi: 10.3109/03008207.2015.1081901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ansorge HL, Hsu JE, Edelstein L, et al. Recapitulation of the Achilles tendon mechanical properties during neonatal development: a study of differential healing during two stages of development in a mouse model. J Orthop Res. 2012;30:448–456. doi: 10.1002/jor.21542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelberman RH, Vandeberg JS, Manske PR, et al. The early stages of flexor tendon healing: a morphologic study of the first fourteen days. J Hand Surg Am. 1985;10:776–784. doi: 10.1016/s0363-5023(85)80151-9. [DOI] [PubMed] [Google Scholar]
- 21.Zhang G, Ezura Y, Chervoneva I, et al. Decorin regulates assembly of collagen fibrils and acquisition of biomechanical properties during tendon development. J Cell Biochem. 2006;98:1436–1449. doi: 10.1002/jcb.20776. [DOI] [PubMed] [Google Scholar]
- 22.Lechner BE, Lim JH, Mercado ML, et al. Developmental regulation of biglycan expression in muscle and tendon. Muscle Nerve. 2006;34:347–355. doi: 10.1002/mus.20596. [DOI] [PubMed] [Google Scholar]
- 23.Dunkman AA, Buckley MR, Mienaltowski MJ, et al. The tendon injury response is influenced by decorin and biglycan. Ann Biomed Eng. 2013 doi: 10.1007/s10439-013-0915-2. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beason DP, Kuntz AF, Hsu JE, et al. Development and evaluation of multiple tendon injury models in the mouse. J Biomech. 2012;45:1550–1553. doi: 10.1016/j.jbiomech.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dunkman AA, Buckley MR, Mienaltowski MJ, et al. The tendon injury response is influenced by decorin and biglycan. Ann Biomed Eng. 2014;42:619–630. doi: 10.1007/s10439-013-0915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wenstrup RJ, Florer JB, Brunskill EW, et al. Type V collagen controls the initiation of collagen fibril assembly. The Journal of biological chemistry. 2004;279:53331–53337. doi: 10.1074/jbc.M409622200. [DOI] [PubMed] [Google Scholar]
- 27.Favata M. Bioengineering PhD Thesis. Philadelphia: University of Pennsylvania; 2006. Scarless healing in the fetus: Implications and strategies for postnatal tendon repair; p. 216. [Google Scholar]
- 28.Dourte LM, Pathmanathan L, Jawad AF, et al. Influence of decorin on the mechanical, compositional, and structural properties of the mouse patellar tendon. J Biomech Eng. 2012;134:031005. doi: 10.1115/1.4006200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunkman AA, Buckley MR, Mienaltowski MJ, et al. Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biol. 2013;32:3–13. doi: 10.1016/j.matbio.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lujan TJ, Underwood CJ, Jacobs NT, et al. Contribution of glycosaminoglycans to viscoelastic tensile behavior of human ligament. J Appl Physiol (1985) 2009;106:423–431. doi: 10.1152/japplphysiol.90748.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller KS, Connizzo BK, Soslowsky LJ. Collagen fiber re-alignment in a neonatal developmental mouse supraspinatus tendon model. Ann Biomed Eng. 2012;40:1102–1110. doi: 10.1007/s10439-011-0490-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Connizzo BK, Sarver JJ, Birk DE, et al. Effect of age and proteoglycan deficiency on collagen fiber re-alignment and mechanical properties in mouse supraspinatus tendon. J Biomech Eng. 2013;135:021019. doi: 10.1115/1.4023234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dourte LM, Pathmanathan L, Mienaltowski MJ, et al. Mechanical, compositional, and structural properties of the mouse patellar tendon with changes in biglycan gene expression. J Orthop Res. 2013;31:1430–1437. doi: 10.1002/jor.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ansorge HL, Adams S, Birk DE, et al. Mechanical, compositional, and structural properties of the post-natal mouse Achilles tendon. Ann Biomed Eng. 2011;39:1904–1913. doi: 10.1007/s10439-011-0299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lake SP, Miller KS, Elliott DM, et al. Effect of fiber distribution and realignment on the nonlinear and inhomogeneous mechanical properties of human supraspinatus tendon under longitudinal tensile loading. J Orthop Res. 2009;27:1596–1602. doi: 10.1002/jor.20938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loppini M, Longo UG, Niccoli G, et al. Histopathological scores for tissue-engineered, repaired and degenerated tendon: a systematic review of the literature. Curr Stem Cell Res Ther. 2014;10:43–55. doi: 10.2174/1574888x09666140710110723. [DOI] [PubMed] [Google Scholar]
- 37.Niyibizi C, Kavalkovich K, Yamaji T, et al. Type V collagen is increased during rabbit medial collateral ligament healing. Knee Surg Sports Traumatol Arthrosc. 2000;8:281–285. doi: 10.1007/s001670000134. [DOI] [PubMed] [Google Scholar]
- 38.Davidson CJ, Ganion LR, Gehlsen GM, et al. Rat tendon morphologic and functional changes resulting from soft tissue mobilization. Med Sci Sports Exerc. 1997;29:313–319. doi: 10.1097/00005768-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 39.Thorpe CT, McDermott BT, Goodship AE, et al. Ageing does not result in a decline in cell synthetic activity in an injury prone tendon. Scand J Med Sci Sports. 2016;26:684–693. doi: 10.1111/sms.12500. [DOI] [PubMed] [Google Scholar]