Abstract
Background
Of the 50,000 men in the US who elect radical prostatectomy for prostate cancer, 24–40% will have a prostate-specific antigen (PSA) recurrence (PSA-R) within 10 years. Deciding whether to administer salvage therapy (ST) at PSA-R presents challenges, as treatment reduces risk of progression to clinical metastasis but incurs unnecessary side effects should the man die before metastasis. We develop a new harm-benefit framework using a clinical cohort to inform shared decision-making between patients and physicians at PSA-R.
Methods
Records of 1,045 Johns Hopkins University Hospital patients diagnosed in 1984–2013 who had PSA-R following radical prostatectomy were analyzed using marginal structural models to estimate the baseline risk of metastasis and the effect of ST (radiation therapy with or without hormone therapy) while accounting for selection into ST on the basis of PSA growth. The estimated model predicts harm-benefit tradeoffs of ST within patient subgroups. Benefit of ST is the absolute reduction in the risk of metastasis within 10 years; harm is the frequency of cancers that would not have metastasized in the patient's lifetime in the absence of ST (overtreatment).
Results
The adjusted hazard ratio associated with ST was 0.41 (95% CI 0.31 to 0.55). Providing ST to all men at PSA-R reduced risk of metastasis from 43% to 23% but led to 31% of men being overtreated (harm/benefit=31/(43-23)=1.6). Providing ST to men with Gleason score >7 reduced risk of metastasis from 67% to 39% with 13% of men being overtreated (harm/benefit=13/(67-39)=0.5).
Conclusions
A quantitative framework that evaluates primary harms and benefits of ST after PSA-R will facilitate informed decision-making. Immediate ST may be more appropriate in patient subgroups at elevated risk of metastasis.
Keywords: confounding, marginal structural model, overtreatment, prostate-specific antigen, prostatic neoplasms, recurrence, salvage treatment, targeted treatment, shared decision-making
Introduction
Optimal management of cancer that recurs following primary therapy can be challenging because of the need to trade off uncertain benefit against known adverse effects of secondary and salvage treatments. In the US, approximately 50,000 men are treated with radical prostatectomy (RP) for localized prostate cancer each year [1], of which an estimated 24–40% will have a prostate-specific antigen (PSA) recurrence (PSA-R) within 10 years [2]. Generally, these men have no symptoms or radiological evidence of disease progression. Men who experience PSA-R are at increased risk of metastasis and death, but many will die of other causes before incurring further prostate cancer-related morbidity. A framework for quantifying the consequences of salvage treatment (ST) to inform shared decision-making between physicians and patients at the time of PSA-R has not previously been developed.
Currently there is no consensus about how best to manage PSA-R [3] after RP. The lack of consensus relates to the type of ST—radiation therapy (RT), hormone therapy (HT), or both—to the timing of treatment, and to the selection of cases most likely to benefit. Not all PSA-R patients elect ST: a recent study of the community-based Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) database found that approximately 36% of patients received no ST [4]. Further, timing of ST relative to PSA-R varies across patients. In a cohort of 635 initially treated at Johns Hopkins University (JHU) Hospital, 155 patients (24%) received ST within two years and 83 (13%) received ST more than two years after PSA-R [5].
Regarding timing of ST, observational studies [5, 6] and a report from a recent randomized trial [7] have suggested an advantage of early salvage RT after primary RP, but other studies [8, 9] have been less positive. A recent review of early salvage HT concluded that current observational evidence lacks consensus with respect to a survival benefit for patients with PSA-R except for those at highest risk [10]. The discrepant results may be related to the cohorts, the treatments or comparison groups studied, or the analytic methods employed. Analysis of ST on the basis of observational data is challenging because the patients receiving ST represent a selective subgroup. If selection into ST is dynamic (for example, if it depends on an individual's PSA evolution), specialized methods must be used [11, 12]. In the PSA surveillance setting, it is likely that PSA changes over time impact the decision to administer ST, and variation in PSA growth may account for a significant fraction of the variability in the timing of ST in observational cohorts.
In the absence of ST, the natural history of PSA-R is lengthy, with the median time to clinical metastasis 10 years or more [13]. Thus, indiscriminate early treatment of PSA-R may lead to a significant fraction of cases being overtreated. We previously estimated that among men aged 70 years and older at the time of prostate cancer diagnosis and treated at PSA-R, more than 30% may have been overtreated [14]. Both salvage RT and HT have been associated with non-trivial side effects, such as erectile dysfunction, bowel problems, and an increase in the risks of diabetes and cardiovascular disease [15, 16]. Therefore, benefit must be weighed against harm when making informed decisions about ST.
In this study, we develop a harm-benefit framework for decision-making about immediate ST using an observational cohort of men with PSA-R treated with RP at JHU. The harm relates to the frequency of overtreatment and the benefit to the reduction in the risk of metastasis after PSA-R. We use specialized methods to address selection bias in estimating ST benefit and compare our results with standard approaches. Our objective is to provide a quantitative basis for the decision about how best to manage PSA-R after RP and to identify subgroups in whom immediate ST is likely to be of most value.
Methods
JHU PSA-R Data
The JHU PSA-R cohort has been previously described [2, 13, 17]. Data for this study consisted of records from 1,045 men who developed PSA-R after receiving RP at JHU between 1984 and 2013 inclusive. At the time of previous reports, the cohort consisted of 89% non-Hispanic White, 7% Black, and 4% other ethnicities [5]. Men who received adjuvant HT or RT prior to PSA-R were excluded. After RP, patients were followed with PSA measurements and rectal examinations every 3 months for the first year, every 6 months for the second year, and every 12 months thereafter. PSA-R was defined as a single PSA measurement of 0.2 ng/mL or higher. After PSA-R, PSA was measured approximately every 6 months. Imaging with computed tomography (CT) and radionuclide bone scan was generally performed at baseline and then annually (or sooner if symptoms developed). Men with PSA-R were given ST (RT, HT, or both) at the discretion of treating physicians, and the timing of initiating ST was not standardized. Following diagnosis, all patients were followed up on a yearly basis with JHU with a telephone interview. Time periods from surgery to PSA-R and from PSA-R to metastasis or censoring was obtained from patient records, physician of record, or self-report and recorded in the JHU RP database in whole years. Metastatic progression was defined as the presence of osseous metastases on bone scan or visceral or extra-pelvic nodal metastases on CT scan or magnetic resonance imaging. Patients were censored at death when this event occurred before metastasis, or at last follow-up. We considered all ST types together as a single group and estimated the benefit of any type of ST.
Modeling Risks of Metastasis and Death with and without ST
To accommodate potential dependence between PSA growth and selection into ST, we use a marginal structural model (MSM) [12, 18]. This approach synthetically balances baseline covariates and PSA growth within treated and non-treated groups and provides an unbiased estimate of the impact of ST given all observed characteristics, including the evolving PSA.
The MSM consists of three components: (1) a model for PSA growth after PSA-R, (2) a model for the risk of initiating ST at time t given baseline clinical characteristics and PSA level, and (3) a model to predict the risk of metastasis at time t given baseline clinical characteristics and ST status.
The PSA growth model is a linear random effects model fit to log PSA levels that incorporates all measured values from PSA-R up to ST, metastasis, or censoring, whichever comes first.
The model for initiation of ST is a Cox regression model that includes age, pathologic grade and stage, year of surgery, and time from RP to PSA-R (dichotomized as below or above the median of 3 years [8]), and the predicted PSA from the PSA growth model as a time-dependent covariate. The choice of variables that we adjusted for was based on prior studies [5, 19] and constitute known risk factors for cancer progression. As with these other studies, we do not adjust for race or cormorbidities.
The model for the time to metastasis is a Cox regression model with ST status as a time-dependent covariate and with individual weights at any given time derived from the model for initiation of ST. The weight for a given person at a specified time is given by the inverse of the predicted probability that he has not initiated ST by that time. The weights are constructed to synthetically balance the treated and untreated groups with respect to patient baseline characteristics and PSA growth. Detailed formulations are provided in the Supplementary Materials Methods I.
We compare estimates of the hazard ratio (HR) for ST from the MSM with corresponding estimates from a standard Cox regression model in which ST status is entered as a time-dependent covariate and the model adjusts for individual-specific estimates of annual PSA growth.
Combining the three components, we predict times to metastasis in the absence and presence of immediate ST following PSA-R. To obtain results in the absence of ST, we set the ST indicator to 0 and convert the estimated hazard from model (3) into a projection of the cumulative incidence of metastasis in the cohort. To obtain results after immediate ST, we repeat this exercise but set the ST indicator to 1 at the time of PSA-R.
To place our estimates in the context of those derived with methods that don't account for time-dependent confounding, model-based cumulative incidence curves for time to metastasis in the absence of ST are compared with results from two empirical approaches—one in which patients receiving ST are excluded (as in [2]) and one in which patients receiving ST are censored at the start of treatment (as in [8]).
Estimating Benefits and Harms of ST
We use simulation to project the benefits and harms of immediate ST in the JHU population overall and within important subgroups. For each analysis, we obtain 10,000 bootstrap samples from the JHU data and simulate times to clinical metastasis in the absence of ST and assuming immediate ST as described above. We also simulate times to other-cause death. Previous work has shown that prostate cancer patients who elect RP have a 60% reduced risk of other-cause death relative to the age-matched population [14]. Therefore, we use age-specific mortality rates from standard life tables [20] based on 1996 death data, multiplied by a factor of 0.4 to generate survival times to other-cause death in patients following PSA-R.
The lowered risk of metastasis due to ST is calculated as the difference in the 10-year cumulative incidence of metastasis with and without immediate ST. We use this time interval to ensure adequate follow-up for most subjects in the cohort. Overtreatment due to ST is quantified as the fraction of cases who would not reach metastasis in their lifetimes in the absence of ST. To calculate the frequency of overtreatment, we extrapolate the model-predicted cumulative incidence of metastasis in the absence of ST beyond the follow-up interval by assuming a Weibull distribution for the baseline hazard [21] as described in the Supplementary Materials Methods II.
We calculate harm-benefit tradeoffs of immediate ST overall and within subgroups defined by age, time from RP to PSA-R, and Gleason score at diagnosis. All regressions and simulations were performed using R, with the IPW package for the MSM fit [22].
Results
JHU Sample
Table 1 summarizes baseline characteristics and outcomes for 1,045 JHU PSA-R men who experienced PSA-R after undergoing primary RP for clinically localized prostate cancer at JHU stratified by receipt of ST. A version of this table stratified by type of ST is provided in Supplementary Materials eTable 1.
Table 1.
Description of Johns Hopkins cohort of 1,045 men with PSA recurrence after radical prostatectomy.
| No Salvage Treatment N=501 | Salvage Treatment N=544 | Chi-square P=value | |
|---|---|---|---|
| Age at surgery (median, IQR) | 60.0 (56.0 - 64.0) | 59.0 (55.0 - 63.0) | .81 |
| Age at PSA-R (median, IQR) | 65.0 (60.0 - 70.0) | 63.0 (58.0 - 67.0) | .023 |
| Gleason score | .055 | ||
| ≤6 | 110 (22.0%) | 98 (18.0%) | |
| 3+4 | 191 (38.1%) | 188 (34.6%) | |
| 4+3 | 93 (18.6%) | 134 (24.6%) | |
| >7 | 107 (21.4%) | 124 (22.8%) | |
| Pathology | <.001 | ||
| Organ-confined | 116 (23.2%) | 138 (25.4%) | |
| Extraprostatic extension | 221 (44.1%) | 278 (51.1%) | |
| Seminal vesicle involvement | 66 (13.2%) | 77 (14.2%) | |
| Lymph node involvement | 98 (19.6%) | 51 (9.4%) | |
| Years from RP to PSA-R | <.001 | ||
| ≤ 3 years | 239 (47.7%) | 319 (58.6%) | |
| > 3 years | 262 (52.3%) | 225 (41.4%) | |
| Year of surgery | <.001 | ||
| 1984-1989 | 53 (10.6%) | 55 (10.1%) | |
| 1990-1994 | 122 (24.4%) | 91 (16.7%) | |
| 1995-1999 | 141 (28.1%) | 126 (23.2%) | |
| 2000-2004 | 89 (17.8%) | 133 (24.4%) | |
| 2005-2010 | 76 (15.2%) | 102 (18.8%) | |
| 2010-2013 | 20 (4.0%) | 37 (6.8%) | |
| Metastasis | 165 (32.9%) | 110 (20.2%) | <.001 |
| Type of salvage treatment | <.001 | ||
| None | 501 (100.0%) | 0 (0.0%) | |
| Hormone treatment only | 0 (0.0%) | 144 (26.5%) | |
| Radiotherapy only | 0 (0.0%) | 274 (50.4%) | |
| Radiotherapy and hormone treatment | 0 (0.0%) | 126 (23.2%) | |
| Years of follow-up (median, IQR) | 4.8 (±3.9) | 7.7 (±5.2) | <.001 |
| PSA measurements after PSA-R (median, IQR) | 5.0 (3.0 - 9.0) | 3.0 (2.0 - 6.0) | <.001 |
Abbreviations: PSA=prostate specific antigen; PSA-R=PSA recurrence; RP=radical
In the overall sample, the mean time from RP to PSA-R was 3 years (IQR 1– 6 years). The median age at PSA-R was 64 years and median follow-up after PSA-R was 5 years. Approximately 52% of men had some form of salvage treatment during follow-up, consisting of HT only (14%), RT only (26%), or both (12%). Approximately 33% of men without ST and 20% with ST reached metastasis during follow-up.
Model Results
The random effects model fit to the log PSA data indicated a PSA doubling time of 1.7 years on average (95% CI 1.65–1.93). Supplemental Materials eTable 2 provides estimates of the associations between covariates and time to ST. Administration of ST before metastasis was much more likely in later years. Positive lymph nodes were associated with lower ST rates relative to organ-confined disease. Higher PSA values were associated with a modestly higher annual risk of ST initiation.
Table 2 provides estimates from the MSM Cox regression model for time to metastasis. Higher Gleason score had a strong association with the risk of metastatic disease. Men with Gleason score ≥7 had 5.5 times the annual risk relative to men with Gleason score ≤6. Longer intervals from RP to PSA-R were associated with reduced risk of metastasis. Those who developed PSA-R more than 3 years after RP surgery had hazard ratio of 0.63 relative to those who had a PSA-R within 3 years.
Table 2. Marginal Structural Cox model for time to metastasis.
| HR | 95% CI | p-value | ||
|---|---|---|---|---|
|
| ||||
| Lower Upper | ||||
| Age at PSA-R (years) | 0.98 | 0.96 | 1.00 | 0.039 |
| More than 3 years from RP to PSA-R | 0.63 | 0.48 | 0.83 | 0.001 |
| Gleason score (Reference: ≤6) | ||||
| 3+4 | 2.44 | 1.44 | 4.14 | 0.001 |
| 4+3 | 4.14 | 2.39 | 7.16 | <.001 |
| >7 | 5.46 | 3.21 | 9.31 | <.001 |
| Cancer stage (Reference: organ confined) | ||||
| Extraprostatic extension | 1.23 | 0.77 | 1.96 | 0.380 |
| Seminal vesicle invasion | 1.95 | 1.18 | 3.21 | 0.009 |
| Positive lymph nodes | 1.90 | 1.16 | 3.10 | 0.011 |
| Surgery Year (Reference: 1984-1989) | ||||
| 1990-1994 | 0.88 | 0.61 | 1.27 | 0.497 |
| 1995-1999 | 0.73 | 0.49 | 1.10 | 0.129 |
| 2000-2004 | 0.88 | 0.55 | 1.41 | 0.596 |
| 2004-2009 | 0.81 | 0.45 | 1.44 | 0.467 |
| 2010-2013 | 0.46 | 0.11 | 1.94 | 0.291 |
| Salvage treatment | 0.41 | 0.31 | 0.55 | <.001 |
Abbreviations: PSA=prostate specific antigen; PSA-R=PSA recurrence; RP=radical prostatectomy; CI=confidence interval.
The MSM estimate of ST (HR=0.41) suggested treatment was associated with a 59% lower risk of metastasis. The cumulative incidence of metastasis (CIM) after immediate ST is presented in Figure 1. At 10 years, the CIM in the presence of other-cause death is 43% in the absence of ST and 23% after immediate ST. Corresponding estimates in the absence of other-cause death were 45% and 24%, revealing the limited moderating effect of competing mortality.
Figure 1.
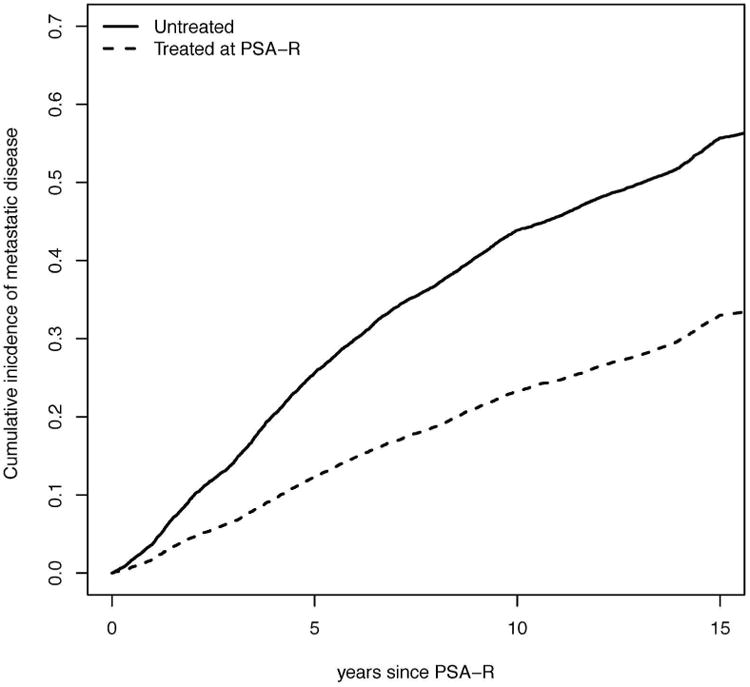
Cumulative probability of clinical metastasis in the Johns Hopkins University cohort after salvage therapy initiated at prostate-specific antigen recurrence or not at all. All estimates are in the presence of “other cause” death.
Comparison of MSM Results to Standard Approaches
Figure 2 compares the predicted time from PSA-R to metastasis in the absence of ST based on the MSM with two empirical estimates of the cumulative risk of metastasis: (a) eliminating cases who received any ST (as in [2, 13, 17]) or (b) censoring these cases at ST (as in [8]). The estimated median time to metastasis in the absence of ST is 12 years post PSA-R in the MSM model, 10 years when treated cases are removed, and 14 years based on censoring at ST. Standard Cox models for time to metastasis provided results that were qualitatively consistent with the MSM findings of a strong benefit associated with ST after PSA-R in this population (Supplemental Materials eTable 3).
Figure 2.

Cumulative incidence of metastasis after prostate-specific antigen recurrence in the Johns Hopkins University cohort using the marginal structural model, removing untreated individuals, or censoring at salvage treatment.
Benefits and Harms of Immediate ST
Table 3 provides estimates of the projected benefits and harms of immediate ST. Providing immediate ST to all cases is projected to lower the risk of metastasis at 10 years by 20% relative to not giving any ST but incur a risk of overtreatment of 31%. Men with Gleason score ≥7 have better harm-to-benefit tradeoffs (28% lower risk of metastasis and 13% risk of overtreatment), as do those with a shorter interval between RP and PSA-R. In contrast, men with Gleason score ≤6 have a worse harm-to-benefit tradeoff (8% lower risk of metastasis and 62% risk of overtreatment) as do men older than age 70 years at PSA-R (15% lower risk of metastasis and 53% risk of overtreatment). Men with combinations of low-risk features have even less favorable harm-benefit tradeoffs. For example, in men with Gleason score ≤6 and more than 3 years from RP to PSA-R, ST lowers the risk of metastasis by 6% but incurs risk of overtreatment of 71%. Pathologic stage was not a strong predictor of harms or benefits in this analysis.
Table 3.
Benefit and harm of salvage treatment at PSA recurrence by subgroups in the Johns Hopkins population. Benefit is the reduction due to immediate salvage treatment in the cumulative incidence of metastasis by 10 years after PSA recurrence; probability of overtreatment is the fraction of PSA recurrent cases that would not have reached the point of metastasis within their lifetimes. Also reported is the cumulative incidence of metastasis with and without ST at 10 years. Estimates are based on simulation from the marginal structural model.
| CIM at 10 years | Benefit at 10 years | Probability of over-treatment | ||
|---|---|---|---|---|
| No ST | ST | |||
| All | .43 | .23 | 0.20 | 0.31 |
| Gleason | ||||
| 6 | .15 | .07 | 0.08 | 0.62 |
| 3+4 | .37 | .18 | 0.19 | 0.33 |
| 4+3 | .56 | .31 | 0.25 | 0.18 |
| >7 | .67 | .39 | 0.28 | 0.13 |
| Time from RP to PSA-R | ||||
| ≤ 3 years | .55 | .30 | 0.25 | 0.19 |
| > 3 years | .30 | .14 | 0.16 | 0.45 |
| Age at PSA-R | ||||
| <70 years | .48 | .26 | 0.22 | 0.25 |
| ≥ 70 years | .29 | .14 | 0.15 | 0.53 |
| Low risk groups | ||||
| Gleason score ≤6 and >3 years from RP to PSA-R | .10 | .04 | 0.06 | 0.71 |
| Gleason score ≤6 and age ≥ 70 years | .11 | .05 | 0.06 | 0.79 |
Abbreviations: PSA=prostate specific antigen; RP=radical prostatectomy; PSA-R=PSA recurrence; CIM=cumulative incidence of metastasis
Discussion
The event of PSA recurrence can be a critical decision point for patients faced with treatment options of uncertain net benefit. In the case of prostate cancer, decision-making about salvage treatment is difficult since the side-effect profiles are not benign and the adverse event of metastasis that treatment is intended to prevent may occur more than 10 years in the future. Informed decision-making requires evidence about comparative benefits and harms of different options. Knowledge that the risk of overtreatment considerably outweighs the likelihood of benefit could help patients to make an informed decision to manage their recurrence conservatively.
This analysis presents a quantitative framework for harm-benefit tradeoffs to be used when contemplating strategies for the management of PSA-R. To quantify the benefits and harms from observational data, we used marginal structural modeling to yield a hazard ratio for ST of 0.41. Estimates of treatment efficacy that did not adjust for PSA growth estimated a hazard ratio of 0.55. This agrees with the intuition that higher-risk men are selecting into ST over time; if the elevated risk is not properly accounted for, the effect of ST will be underestimated. Marginal structural modeling has become established in the statistical literature as a method that accounts for time-dependent selection into treatment and should be generally preferred to standard survival modeling in the observational PSA-R setting.
To the extent that the MSM captures all of the confounders of the ST-metastasis association, we can conclude that this analysis provides an unbiased confirmation of a prior study of this cohort [5] that suggested a significant benefit to salvage RT with or without hormone therapy. The results also suggest a median time to metastasis without treatment that is only modestly increased over prior estimates [13]. The ability to control only for observed confounders is a known limitation of the MSM approach. Our choice of adjustment variables was based on prior studies of disease progression after PSA recurrence [5, 19] and the information we had available on the JHU sample. Our analysis included known clinical predictors of disease progression but not demographic factors or comorbidities, which may affect the propensity for treatment. However, excluding variables that relate to the propensity for treatment will only lead to bias if they also relate to the probability of cancer aggression. For example, in a more ethnically heterogeneous population, race could be a confounding variable as it may impact both the propensity to choose salvage treatment and cancer aggressiveness. We recognize, however, that there may be other unobserved confounders that we have not been able to account for.
Our assessment, based on the MSM, that ST is beneficial comports with a previous analysis of ST efficacy in the JHU cohort that used more standard methods [5]. Other MSM-based analyses of ST efficacy have been conducted in studying the effects of salvage HT [9, 11]. Garcia-Albeniz et al. [9] did not find a significant benefit in their study of salvage HT after RP or RT among men in the CaPSURE program. However, Kennedy et al. [11], in their study of salvage HT after primary RT, found a strongly beneficial effect. As we have noted, these prior studies differed in terms of patient populations as well as primary and salvage treatments. Our study adds clarity to the overall question about the benefit of ST because it confirms other, less sophisticated studies and supports significant benefit to immediate ST. Thus it bolsters the case for considering early salvage radiation treatment with or without hormones.
There are several limitations to this study. The first concerns the limited duration of follow-up in the JHU cohort. We used a Weibull distribution for the time to metastasis, which provided a close fit to 15-year follow-up data, to extrapolate beyond this horizon. Further, while our analysis provides a careful adjustment for within-cohort selection bias, it is based on a single cohort and may not generalize to a more diverse population. We also do not model treatment effect heterogeneity by timing of ST or prognostic subgroup, as was suggested in a prior analysis of ST benefit in the JHU cohort [5]. If ST is more beneficial (a hazard ratio closer to 0) for higher-risk patients than lower-risk patients, the true range of harm-benefit tradeoffs across risk strata will be even wider than that suggested by our results. Finally, the life tables that we used were based on 1996 mortality rates. Projections of overtreatment may be slightly overstated given improvements in mortality rates in the past 20 years.
There are a number of important extensions to the framework developed that will ultimately expand its utility across disease and treatment settings. These include the extension to allow for differential benefit by salvage treatment type (e.g., RT versus HT versus both) and an extension to consider dynamic treatment strategies in which a patient may opt to defer treatment until PSA growth reaches specified levels [23].
The metrics selected to quantify benefits and harms were designed to be useful for patient-physician decision-making. Clinical metastasis is a proximal event that represents a point of effectively incurable progression and, as such, is likely to be of primary concern to the PSA-recurrent patient. Our benefit metric pertains to the absolute risk of metastasis. Our harm metric pertains to the chance that ST and any associated side effects and morbidity is unnecessary. While intuitive, these metrics do not explicitly quantify the impact of the adverse side effects of treatment that may reduce quality of life or affect life expectancy [16, 24, 25].
In order for the framework to be useful in a clinical setting, we anticipate that physicians would supplement the conversation about benefit and harm, both to tailor it more closely to the health status of the particular patient and to inform him about tradeoffs of ST. For example, a physician could point out that a patient's comorbidities increase the risk of other-cause death, and this will reduce the probability of overtreatment. Further, providing the patient with information on the probability and duration of side effects after different salvage treatments [15, 26, 27] will enable him to assess if the gain in metastasis-free years of life is worth the potential side effects incurred. For example, a man with high grade disease who would typically benefit from salvage radiation, might opt to forego it if he had multiple comorbid conditions—and thus a higher risk of overtreatment--and a very low preference for erectile dysfunction, a common side effect of salvage radiation therapy.
There are potential formal extensions of the framework that explicitly and quantitatively incorporate these additional individual factors. Specifically, the framework could be extended by calculating an individual probability of overtreatment using adjusted rates of other-cause death that account for health status and comorbidities. One could also obtain quality-adjusted benefits of treatment that accounts for individual preferences using an approach similar to [28]. Here quality adjustment would involve soliciting individual patient utilities concerning side effects of salvage RT or HT and combining these with probabilities of these side effects to yield a personalized utility for post-treatment states. This utility would then be used to adjust the time to metastasis curve in the presence of salvage treatment.
There are challenges of implementing this extension of benefit, however, in a shared decision-making context. The metric requires eliciting patient preferences in a clinical setting, for example, using a time trade-off approach [29]. Alternatively, instead of incorporating individual utilities, one could use average utilities obtained in a clinical population [30]. However, there is considerable variability across men in terms of preferences [31]; quality-adjusted metrics based on population-average utilities are more useful for policy recommendations rather than individual-level decisions [32]. Finally, quality-adjusted metrics may be considerably more challenging for patients to interpret than the simpler benefit and harm metrics that we have proposed.
In conclusion, the issue of whether to administer salvage RT with or without HT after PSA-R remains a complex one in terms of risk-reward tradeoffs. Clinical practice guidelines [33] state that “physicians should offer salvage radiotherapy to patients with PSA or local recurrence after RP,” with the caveat that “offer should be interpreted as a having a detailed discussion with the patient about the risks and benefits of radiotherapy.” We hope this work will provide useful quantitative support for such discussions and facilitate informed decision-making about how best to manage recurrence after primary therapy for prostate cancer.
Supplementary Material
eFigure 1. Weibull model fit to the baseline hazard obtained after the Cox regression of time to metastasis, used to extrapolate the risk of metastasis beyond the data collection time.
eTable 1. Johns Hopkins cohort of 1,045 men with PSA recurrence after radical prostatectomy by type of salvage treatment
eTable 2. Cox model for time to salvage therapy
eTable 3. Standard Cox model for time to metastasis conditional on PSA as a time varying covariate
Acknowledgments
Funding: This work was supported by the National Cancer Institute at the National Institutes of Health (U01CA199338) as part of the Cancer Intervention and Surveillance Modeling Network (CISNET). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to report.
Preliminary results were presented at the Medical Decision Making Conference in Vancouver BC, October 2016.
References
- 1.National Cancer Institute Surveillance Research Program. 8.3.2 ed SEER*Stat software [Google Scholar]
- 2.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 3.Chen RC. Making individualized decisions in the midst of uncertainties: the case of prostate cancer and biochemical recurrence. Eur Urol. 2013;64(6):916–8. doi: 10.1016/j.eururo.2013.07.001. discussion 8-9. [DOI] [PubMed] [Google Scholar]
- 4.Cary KC, Paciorek A, Fuldeore MJ, Carroll PR, Cooperberg MR. Temporal trends and predictors of salvage cancer treatment after failure following radical prostatectomy or radiation therapy: An analysis from the CaPSURE registry. Cancer. 2014;120(4):507–12. doi: 10.1002/cncr.28446. [DOI] [PubMed] [Google Scholar]
- 5.Trock BJ, Han M, Freedland SJ, Humphreys EB, DeWeese TL, Partin AW, et al. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA. 2008;299(23):2760–9. doi: 10.1001/jama.299.23.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choueiri TK, Chen MH, D'Amico AV, Sun L, Nguyen PL, Hayes JH, et al. Impact of postoperative prostate-specific antigen disease recurrence and the use of salvage therapy on the risk of death. Cancer. 2010;116(8):1887–92. doi: 10.1002/cncr.25013. [DOI] [PubMed] [Google Scholar]
- 7.Duchesne GM, Woo HH, Bassett JK, Bowe SJ, D'Este C, Frydenberg M, et al. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): a randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol. 2016 doi: 10.1016/S1470-2045(16)00107-8. [DOI] [PubMed] [Google Scholar]
- 8.Boorjian SA, Thompson RH, Tollefson MK, Rangel LJ, Bergstralh EJ, Blute ML, et al. Long-term risk of clinical progression after biochemical recurrence following radical prostatectomy: the impact of time from surgery to recurrence. Eur Urol. 2011;59(6):893–9. doi: 10.1016/j.eururo.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Albeniz X, Chan JM, Paciorek A, Logan RW, Kenfield SA, Cooperberg MR, et al. Immediate versus deferred initiation of androgen deprivation therapy in prostate cancer patients with PSA-only relapse. An observational follow-up study. Eur J Cancer. 2015;51(7):817–24. doi: 10.1016/j.ejca.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Bergh RC, van Casteren NJ, van den Broeck T, Fordyce ER, Gietzmann WK, Stewart F, et al. Role of Hormonal Treatment in Prostate Cancer Patients with Nonmetastatic Disease Recurrence After Local Curative Treatment: A Systematic Review. Eur Urol. 2016;69(5):802–20. doi: 10.1016/j.eururo.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 11.Kennedy EH, Taylor JM, Schaubel DE, Williams S. The effect of salvage therapy on survival in a longitudinal study with treatment by indication. Stat Med. 2010;29(25):2569–80. doi: 10.1002/sim.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor JM, Shen J, Kennedy EH, Wang L, Schaubel DE. Comparison of methods for estimating the effect of salvage therapy in prostate cancer when treatment is given by indication. Stat Med. 2014;33(2):257–74. doi: 10.1002/sim.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonarakis ES, Feng Z, Trock BJ, Humphreys EB, Carducci MA, Partin AW, et al. The natural history of metastatic progression in men with prostate-specific antigen recurrence after radical prostatectomy: long-term follow-up. BJU Int. 2012;109(1):32–9. doi: 10.1111/j.1464-410X.2011.10422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia J, Trock B, Gulati A, Mallinger L, Cooperberg MR, Carroll PR, et al. Overdetection of recurrence after radical prostatectomy: Estimates based on patient and tumor characteristics. Clin Cancer Res. 2014;20(20):5302–10. doi: 10.1158/1078-0432.CCR-13-3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Resnick MJ, Koyama T, Fan KH, Albertsen PC, Goodman M, Hamilton AS, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368(5):436–45. doi: 10.1056/NEJMoa1209978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen PL, Alibhai SM, Basaria S, D'Amico AV, Kantoff PW, Keating NL, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol. 2015;67(5):825–36. doi: 10.1016/j.eururo.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 17.Freedland SJ, Humphreys EB, Mangold LA, Eisenberger M, Dorey FJ, Walsh PC, et al. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA. 2005;294(4):433–9. doi: 10.1001/jama.294.4.433. [DOI] [PubMed] [Google Scholar]
- 18.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–60. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 19.Boorjian SA, Karnes RJ, Crispen PL, Rangel LJ, Bergstralh EJ, Blute ML. Radiation therapy after radical prostatectomy: impact on metastasis and survival. J Urol. 2009;182(6):2708–14. doi: 10.1016/j.juro.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 20.National Center for Health Statistics. Vital statistics of the United States, Volume II: Mortality, part A. Washington DC: Government Printing Office various years; [Google Scholar]
- 21.Royston P. Technical Report. University, College; London: 2011. Estimating a smooth baseline hazard function for the Cox model. [Google Scholar]
- 22.van der Wal W, Geskus R. ipw: An R Package for Inverse Probability Weighting. Journal of Statistical Software. 2011;43(13):1–23. [Google Scholar]
- 23.Shen J, Wang L, Taylor JMG. Estimation of the Optimal Regime in Treatment of Prostate Cancer Recurrence from Observational Data Using Flexible Weighting Models Biometrics. 2016 doi: 10.1111/biom.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsai HK, D'Amico AV, Sadetsky N, Chen MH, Carroll PR. Androgen deprivation therapy for localized prostate cancer and the risk of cardiovascular mortality. J Natl Cancer Inst. 2007;99(20):1516–24. doi: 10.1093/jnci/djm168. [DOI] [PubMed] [Google Scholar]
- 25.Hamdy FC, Donovan JL, Lane JA, Mason M, Metcalfe C, Holding P, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med. 2016;375(15):1415–24. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 26.Zaffuto E, Gandaglia G, Fossati N, Dell'Oglio P, Moschini M, Cucchiara V, et al. Early Postoperative Radiotherapy is Associated with Worse Functional Outcomes in Patients with Prostate Cancer. J Urol. 2017;197(3 Pt 1):669–75. doi: 10.1016/j.juro.2016.09.079. [DOI] [PubMed] [Google Scholar]
- 27.Adam M, Tennstedt P, Lanwehr D, Tilki D, Steuber T, Beyer B, et al. Functional Outcomes and Quality of Life After Radical Prostatectomy Only Versus a Combination of Prostatectomy with Radiation and Hormonal Therapy. Eur Urol. 2017;71(3):330–6. doi: 10.1016/j.eururo.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Sommers BD, Beard CJ, D'Amico AV, Dahl D, Kaplan I, Richie JP, et al. Decision analysis using individual patient preferences to determine optimal treatment for localized prostate cancer. Cancer. 2007;110(10):2210–7. doi: 10.1002/cncr.23028. [DOI] [PubMed] [Google Scholar]
- 29.Albertsen P, Nease PFJ, Potosky A. Assessment of patient preferences among men with prostate cancer. J Urol. 1998;159(1):158–63. doi: 10.1016/s0022-5347(01)64043-6. [DOI] [PubMed] [Google Scholar]
- 30.Stewart ST, Lenert L, Bhatnagar V, Kaplan RM. Utilities for prostate cancer health states in men aged 60 and older. Med Care. 2005;43(4):347–55. doi: 10.1097/01.mlr.0000156862.33341.45. [DOI] [PubMed] [Google Scholar]
- 31.Sox HC. Quality of life and guidelines for PSA screening. N Engl J Med. 2012;367(7):669–71. doi: 10.1056/NEJMe1207165. [DOI] [PubMed] [Google Scholar]
- 32.Kind P, Lafata JE, Matuszewski K, Raisch D. The use of QALYs in clinical and patient decision-making: issues and prospects. Value Health. 2009;12(1):S27–30. doi: 10.1111/j.1524-4733.2009.00519.x. [DOI] [PubMed] [Google Scholar]
- 33.Freedland SJ, Rumble RB, Finelli A, Chen RC, Slovin S, Stein MN, et al. Adjuvant and salvage radiotherapy after prostatectomy: American Society of Clinical Oncology clinical practice guideline endorsement. J Clin Oncol. 2014;32(34):3892–8. doi: 10.1200/JCO.2014.58.8525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Weibull model fit to the baseline hazard obtained after the Cox regression of time to metastasis, used to extrapolate the risk of metastasis beyond the data collection time.
eTable 1. Johns Hopkins cohort of 1,045 men with PSA recurrence after radical prostatectomy by type of salvage treatment
eTable 2. Cox model for time to salvage therapy
eTable 3. Standard Cox model for time to metastasis conditional on PSA as a time varying covariate


