Abstract
Natural killer (NK) cells have long been known to mediate anti-tumor responses without prior sensitization or recognition of specific tumor antigens. However, the tumor microenvironment can suppress NK cell function resulting in tumor escape and disease progression. Despite recent advances in cytokine therapy and NK cell adoptive transfer, tumor expression of ligands to NK - expressed checkpoint receptors can still suppress NK mediated tumor lysis. This review will explore many of the checkpoint receptors tumors utilize to manipulate the NK cell response as well as some of the current and upcoming pharmacological solutions to limit tumor suppression of NK cell function. Furthermore, we will discuss the potential to use these drugs in combinational therapies with novel antibody reagents such as bi- and tri-specific killer engagers (BiKEs and TriKEs) against tumor-specific antigens to enhance NK cell-mediated tumor rejection.
Introduction
Natural killer (NK) cells were originally described in the 1970’s by their ability to recognize and destroy tumor-transformed cells without any prior sensitization to tumor antigens[1]. These innate lymphocytes are large and granular in their morphology, with the large granules present in their cytoplasm containing the cellular machinery necessary to perforate and induce apoptosis of susceptible targets[2]. The two primary molecules involved in this process are perforin and granzyme-B. These large granules, or specialized lysosomes, are pre-formed in resting, unstimulated NK cells[3]. In addition to lytic enzymes, NK cells also contain preformed stores of inflammatory cytokines (e.g., tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), etc.), which are secreted quickly upon stimulation without prior priming to induce a potent inflammatory response[4]. Due to this, unlike T cells, NK cells require little priming to respond to an appropriate target immediately upon detection. NK cells can also be classified as “serial killers”, in that they exert their effector function against subsequent targets with no refractory period [5]. Their importance in the cancer setting is highlighted in NK deficient or depleted animal models where lack of NK cells results in failure to reject tumor cells[6]. Other studies demonstrate that various NK cell functional abnormalities correlate with an increased risk of certain types of cancer[7]. Additionally, NK cells with aberrant function are frequently found in cancer patients, making NK cell function a potential biomarker for cancer[8–10]. The importance of NK cells in tumor recognition and destruction has made NK cell functional enhancement and/or adoptive transfer an enticing focus of immunotherapies. Understanding how tumors influence the NK cell compartment is integral in devising appropriate methods of restoring NK cell function through circumventing tumor immunosuppressive effects. In recent years, checkpoint blockade has been an area of great interest in cancer immunotherapy. Checkpoint blockade involves the use of antagonistic antibodies against lymphocyte-expressed receptors, or their ligands, that suppress immune function. To maximize NK cell therapy, checkpoint blockade could be utilized in conjunction with novel molecules, termed bi- and tri-specific killer engagers (BiKEs and TriKEs, respectively), that drive NK cell mediated antigen specific recognition of tumors and their killing. This review covers current understanding of how checkpoint blockade and BiKE/TriKE molecule utilization impacts NK cell biology and their translational potential.
NK cells and immune surveillance
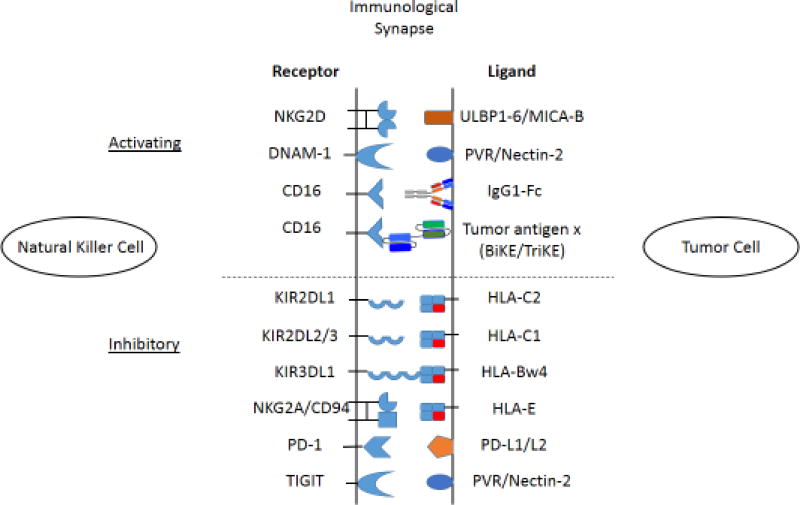
Natural killer cells constitute an important first line of defense against tumor-transformed cells. While both T and NK cells identify and kill tumor cells, the mechanisms by which they recognize their targets are substantially different. T cells recognize tumor antigens in the context of MHC class I, directly through T cell receptors, which undergo recombination during development[11]. The result of this recombination is a T cell receptor that recognizes a specific antigen to trigger the T cells cytotoxic or cytokine secreting function. NK cells, on the other hand, do not identify target cells based on the presentation of viral or tumor-specific antigens. Instead, they utilize germ-line encoded receptors that recognize ligands present on cells as a result of the cell stress or DNA damage that occurs during viral infection or tumor transformation[12–14]. Due to NK cell recognition of targets based on germ-line encoded ligands, NK responses must be tightly regulated to minimize killing of healthy cells and tissues. This regulation is controlled through a delicate balance between activating and inhibitory signals provided by the repertoire of activation and inhibitory receptors on the surface of NK cells (Figure 1). This concept of regulation, known as the ‘missing self hypothesis’, states that NK cells inhibitory receptor recognition of their cognate ligands, MHC-I molecules, provide an inhibitory signal to the NK that prevent its response to a target despite the presence of activating ligands[15]. Only when MHC-I is missing, or down-regulated on a cell is the NK cell capable of responding. NK cells also express a variety of germ-line encoded activation receptors which include the natural cytotoxicity triggering receptors (NCRs) (e.g., NKp30, NKp44, NKp46 and NKp80), the SLAM-family receptors (e.g., 2B4 and NTB-A), the c-type lectins (i.e.,NKG2D and NKG2C/CD94) and the low affinity Fc receptor, CD16 (FcγRIII)[16]. It is important to delineate the role of CD16 from the other activation receptors, as CD16 does not recognize a cell-expressed ligand. Rather, CD16 ligates the Fc portion of cell- bound IgG antibodies. Through its ligation CD16 provides a strong enough activation signal to overcome most inhibitory signals to trigger NK antibody directed cell-mediated cytotoxicity (ADCC)[17] without the need for co-ligation of other activating receptors[18]. This concept will be discussed in greater detail and in the context of immunotherapy later in this review. Of the noted receptors, the c-type lectin homodimer NKG2D[19–22] is amongst the best characterized. This receptor recognizes molecules upregulated on the surface of cells that have undergone DNA damage or cellular stress; these include the MHC-I polypeptide-related sequence A/B (MIC-A/B) and the UL16 binding proteins 1–6 (ULBP1–6)[14]. Co-ligation with a co-activation receptor like NTB-A or 2B4[23, 24] results in NK cell degranulaiton. Perforin and granzymes then create pores in the target cells and trigger caspases, resulting in apoptosis of the target[25–28]. Ligation of these activating receptors also results in the secretion of cytokines such as IFN-γ and TNF-α[29]. Secretion of IFN-γ by NK cells acts as a bridge between innate and adaptive immunity by inducing the upregulation of MHC-I on surrounding cells[30], which enhances recognition of targets by CD8 T cells as well as skews CD4 T cells towards a TH1 phenotype[31]. There are also NK cell activating receptors that recognize ligands constitutively expressed on healthy cells. DNAM-1 (CD226) recognizes the ligands CD155 (PVR) and CD112 (Nectin-2), which are expressed on many endothelial cells as well as resting CD4 and CD8 T cells[32–36]. Similarly, the NK co-activation ligand NTB-A is basally expressed on all NK, T and B cells. Tight regulation of activating pathways, particularly in settings where activating ligands are present on healthy cells, is an integral component necessary to prevent NK cell mediated autoimmunity and fratricide.
Figure 1. Activating and inhibitory receptor interactions between natural killer cells and tumor targets.
NK cell responses to tumor targets are regulated by a delicate balance of activating and inhibitory signals provided by the receptors depicted. NK activating and inhibitory receptor expression varies based on NK cell subset as well as cytokines and soluble ligands present at or near the tumor microenvironment. Ligands present on tumor cells also vary depending on tumor type and conditions in the microenvironment.
Negative regulation of NK cell function is controlled by a host of inhibitory receptors. NK inhibitory receptors fall into one of four main categories: 1) killer immunoglobulin-like receptors (KIRs), 2) c-type lectin receptors (NKG2A/CD94,), 3) the leukocyte immunoglobulin-like receptors (LILRs,), and 4) the commonly considered checkpoint receptors (PD-1, TIM-3, LAG-3 and TIGIT). The ligands for many of these inhibitory receptors are the major histocompatibility class-I (MHC-I) molecules. KIR recognition of MHC-I is highly specific in that certain KIRs have defined MHC-I molecules as their ligands. KIR2DL1 recognizes HLA-C2 molecules while KIR2DL2/3 recognize HLA-C1[37, 38]. Another inhibitory KIR, KIR3DL1, recognizes HLA-B molecules that express the Bw4 epitope[39, 40]. The c-type lectin, heterodimer, NKG2A/CD94 recognizes HLA-E molecules[41, 42]. Though LIR-1 (ILT-2/CD85j/LILRB1) has a number of binding partners, HLA-G exhibits the strongest binding characteristics[43].
Induction of inhibitory signaling via broadly expressed MHC-I ligands is a critical aspect of regulation of the NK cell response. Since healthy cells express a normal compliment of MHC-I molecules even though they may constitutively express some activating ligands, the inhibitory signal will dominate, resulting in the inhibition of NK function and maintenance of tolerance. It is important to note that NK cells as a population are highly variegated in terms of their inhibitory receptor expression[44]. Not every NK cell expresses every inhibitory receptor. For example, while CD56bright NK cells all express NKG2A/CD94, they lack expression of KIRs. Only about 50–60% of CD56dim NK cells express NKG2A/CD94 while around 70–75% express KIRs. CD56dim NK cells are further subdivided based on which KIRs they express. The stochastic expression of inhibitory receptors means that not all NK cells will be negatively regulated in the same way. On the other hand, expression of checkpoint receptors is not stochastic and much more complex in nature. The therapeutic implications of this variegation will be discussed later in this review.
NK cell dysfunction against tumor targets
Cancer cells are cells undergoing unregulated cell division. This unregulated division is a result of DNA damage in genes that control cell cycle[45–47]. Activation of the DNA damage response (DDR) occurs in many tumor cells and pre-cancerous lesions, resulting in the induction of NKG2D and DNAM-1 ligands[48–52]. Expression of these ligands should trigger NK cell lysis of the tumor. However, cancer cells can abrogate NK cell activation through a number of means. One mechanism by which tumor cells evade the NK response is through upregulation of MHC-I[53]. While many tumors down-regulate certain MHC-I molecules to evade the T cell response, the non-classical MHC-I molecule, HLA-G, is frequently upregulated on the tumor surface[54]. As mentioned before, this HLA molecule acts as a ligand for the NK- expressed inhibitory receptor LIR-1[55]. The result is a strong inhibitory signal provided to NK cells expressing LIR-1, thereby preventing them from mediating their cytotoxic function despite the presence of activating ligands[56].
Another mechanism of tumor evasion by NK cells is through the shedding of soluble NKG2D-ligands. This can occur as a result of alternative splicing, proteolytic cleavage or secretion by exosomes[57, 58]. Soluble MIC and ULBP proteins have been identified in the sera of patients with a variety of different cancers including lung, colon, breast, ovarian, glioma, neuroblastoma, melanoma and leukemia[59–64]. The effects of secreted NKG2D-ligands are numerous. One obvious effect is decreased ligand expression on the tumor surface reducing their susceptibility to NK-mediated killing. Another effect is binding of the soluble ligands to NKG2D on NK cells at proximal and distal sites from the tumor. In vitro studies using soluble ULBP-1–3 in co-culture with primary human NK cells identified a substantial decrease in NKG2D expression. NKG2D–ligand coated exosomes can crosslink NKG2D resulting in the continuous activation of the NK cells, thereby desensitizing them[65]. In fact, chronic activation of NK cells with targets expressing NKG2D-ligands results in decreased NK cell function, even resulting in decreased function mediated through other NK cell receptors. However, regardless of NKG2D–ligand shedding, the serum from cancer patients contains other immunosuppressive factors such as TGF-β, which has also been reported to down-regulate NKG2D as well as the NCR NKp30 on NK cells[66].
Immunosuppressive cells, like myeloid-derived suppressor cells (MDSCs) and regulatory T (Treg) cells residing within the tumor microenvironment can also potently deter NK cell anti-tumor function. MDSCs are generated in the bone marrow of tumor-bearing hosts and then migrate to lymphoid and tumor tissues through chemoattractants such as CCL2 and CCL5. These cells are one of the major constituents of the tumor microenvironment and have been shown to suppress T cell proliferation as well as block CD8+ T cell entry to the tumor[67]. MDSCs mediate some of their functions through secretion of the immunosuppressive cytokines IL-10 and TGF-β[68]. As mentioned previously, TGF-β down-modulates NKG2D expression on NK cells. Recent studies from our group have identified a mechanism by which MDSCs suppress NK activity via direct cell contact[69]. This will be discussed in more detail in a later section. However, high proportions of Tregs, the other culprit likely involved in cellular immunosuppression of NK cells at the tumor microenvironment, have been correlated to cancer progression[70]. These high proportions were shown to be accompanied with decreased numbers and functionality of NK cells in a number of studies[71–74]. Conversely, therapeutic treatments that induce a reduction in Treg numbers, such as low dose cyclophosphamide treatment or treatment with Lenalidomide and Pomalidomide, or direct depletion of Tregs using an IL-2 diphtheria toxin fusion protein, resulted in enhanced NK cell function[75–78]. The Tregs can mediate NK cell inhibition via cell-cell contact, membrane-bound TGF-β, and soluble TGF-β[79]. As noted TGF-β can decrease NKG2D expression on NK cells but can also help induce Treg expansion in the suppressive tumor microenvironment. Another possible mechanism by which Tregs suppress NK cell functions is by limiting the access of NK cells to IL-2 through its consumption[80]. Both MDSCs and Tregs induce a number of their immunosuppressive features via checkpoint blockade, discussed in the next section.
Breaking tolerance through checkpoint blockade
PD-1
A target of particular interest in the current field of cancer immunotherapy is the checkpoint receptor programmed death-1 (PD-1). PD-1 has emerged as one of the most important checkpoint pathways for tumor-mediated immunosuppression. This receptor modulates the duration and intensity of the immune response resulting in tolerance to tumor cells as well as mitigating damage to surrounding tissues[81, 82]. PD-1 expression is inducible on B and T cells after BCR or TCR engagement as well by stimulation with the common γ-chain cytokines (i.e., IL-2, IL-7, IL-15 and IL-21)[83]. As stated earlier, this receptor also appears to be expressed on NK cells although its expression profile is less clear. What is clear is that PD-1’s primary role is the attenuation of immune responses. PD-1 has two-well characterized ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC)[84]. While PD-L1 is expressed on a wide variety of tissues and cell types, PD-L2 is mainly restricted to antigen-presenting cells. A great deal of insight on the functional importance of the PD-1 receptor was derived from a murine model using lymphocyte choriomeningitis virus (LCMV)[85]. LCMV-specific T cells from infected animals had high levels of PD-1 expression and by 80-days post infection were mostly non-functional. When PD-1/PD-L1 interaction was blocked in these animals, T cell function was partially restored and LCMV viral load was reduced. LCMV-infected mice lacking PD-L1 entirely died from immune-related pathologies, indicating an important role for PD-1 in modulating the immune response during viral infection[85]. Well characterized in T cells, ligation of PD-1 results in the phosphorylation of SHP-2, which blocks the activation of phosphatidylinositol 3-kinase (PI3K) and its downstream target, Akt[84]. Activation of Akt results in the increased expression of glucose transporters on the plasma membrane and upregulation of glycolytic enzyme activity[84]. Inhibition of PI3K activation through PD-1 signaling prevents T cell division and effector function by blocking metabolic pathways necessary for these activities. It is important to remember that all information currently known about the function and signaling of PD-1 is primarily derived from studies on T cells. Given that PD-1 signaling is best characterized on T cells, most of the clinical immunotherapy trials on PD-1/PD-L1 blockade have focused on this cell subset, but that does not preclude function on other subsets. However, PD-1’s expression and effects on NK cells are a less clear.
A recent study by Pesce et al. proposes a link between NK expression of PD-1 and CMV-serostatus[86]. Data from this study indicates that PD-1 expression on NK cells is closely linked with CMV-seropositivity; however, a quarter of all healthy, seronegative donors tested also had PD-1+ NK cells. Furthermore, PD-1 was only found to be expressed on a small subset of NK cells in PD-1+ donors. Subsequently, data regarding PD-1 expression on NK cells has been difficult to interpret as other groups have published differing staining patterns. Studies from the Caliguiri lab have identified increased PD-1 expression on NK cells from patients with multiple myeloma[87]. PD-1 staining of these NK cells indicates that all NK cells from these patients are PD-1+, not just a subset. The use of the PD-1 blocking antibody (CT-011) in in vitro assays enhanced both target cell killing and cytokine production by NK cells in response to PD-L1 expressing primary multiple myeloma cells or myeloma cell lines.
The two currently available PD-1 immunologics for clinical use are pembrolizumab (Keytruda™), developed by Merck (Kenilworth, NJ, USA), and nivolumab (Opdivo™), developed by Bristol-Myers Squibb (Lawrence Township, NJ, USA) (Table 1). Both products are anti-PD-1 antibodies with human IgG4a Fc’s, which is important in the context of NK cells as an IgG4a is incapable of triggering NK-mediated ADCC through CD16. Keytruda™ is currently enjoying some acclaim for its use and effectiveness in the treatment of metastatic melanoma and lung cancer. While there is clear evidence that NK cells in some capacity express the PD-1 receptor, little is known about the effects of these clinical biologics on the NK cell compartment and further study is warranted. However, increased NK responses to tumor cells, especially in IFN-γ secretion, may be a double-edged sword in that IFN-γ stimulation results in the up-regulation of PD-1 ligands on many primary tumors and tumor cell lines. The interaction between NK cells and PD-L1 -expressing tumor cells may therefore instigate a negative feedback loop where NK responses to tumor targets would induce greater PD-1 ligand expression on the tumor cell further reducing NK cell mediated tumor responses.
Table 1.
Checkpoint Receptor Blockade Reagents in Clinical Trials
| Target | Drug | Manufacturer | Phase of Trial |
|---|---|---|---|
| CTLA-4 | Ipilibumab | Bristol-Myers Squibb | FDA Approved |
| PD-1 | Pembrolizumab | Merck | FDA Approved |
| PD-1 | Nivolumab | Bristol-Myers Squibb | FDA Approved |
| PD-L1 | Atezolizumab | Genentech/Roche | FDA Approved |
| PD-L1 | Durvalumab | AstraZeneca | FDA Approved |
| PD-L1 | Avelumab | Merck/Pfizer | FDA Approved |
| PD1 | PDR001 | Novartis | Phase III |
| LAG-3 | BMS-986016 | Bristol-Myers Squibb | Phase I/II |
| Tim-3 | TSR-022 | Tesaro | Phase I |
| KIR | IPH2101 | Innate Pharma | Phase II |
| KIR | 1-7F9 | Innate Pharma | Phase I |
| KIR | Lirilumab | Bristol-Myers Squibb | Phase II |
| NKG2A | IPH2201 | Innate Pharma | Phase I/II |
| TIGIT | OMP-313M32 | OncoMed | Phase I |
CTLA-4
CTLA-4, another T cell expressed checkpoint receptor that binds the co-stimulatory ligands CD80 and CD86, has been found to be expressed on activated mouse NK cells. While expression of CD80 and CD86 on tumor cells has been shown to enhance human NK cell cytotoxicity[88], little evidence supports the expression of CTLA-4 on human NK. CTLA-4 is a CD28 homologue expressed primarily on activated T cells where it interacts with its ligands CD80 and CD86 on antigen- presenting cells[89]. Ligation of CTLA-4 on T cells provides a suppressive immunomodulatory signal, playing an important role in self-tolerance[90]. Studies utilizing CTLA-4 -deficient mice show a marked development of a lymphoproliferative disorder that ultimately results in death of the animal[91]. While there is no evidence that CTLA-4 is expressed on human natural killer cells, this does not preclude an off-target effect on the ability of NK cells to respond to tumors.
In a study of head and neck cancer patients treated with the endothelial growth factor receptor (EGFR)-targeted antibody, cetuximab, there was a noted increase in CD4+FOXP3+ intratumoral Tregs expressing CTLA-4 and TGF-β[92]. These Treg’s were able to suppress cetuximab-mediated ADCC by NK cells, which was restored when Tregs were treated with anti-CTLA-4 antibody, ipilimumab (Yervoy™) (Bristol-Myers Squibb, Lawrence Township, NJ, USA). As noted earlier, TGF-β production by intratumoral Tregs might down-regulate NKG2D on tumor-infiltrating NK cells, thereby reducing their ability to mediate a cytotoxic effect against NKG2D–L expressing tumor cells. Though evidence is lacking that NK cells are involved in any way in the B7.1-CD28/CTLA-4 signaling axis directly, evidence does support a potential for indirect effects on NK functionality through the blockade of CTLA-4 signaling on other cells or through ADCC-mediated targeting and elimination of CTLA-4 expressing cells.
TIGIT
Conventional NK cells express the T-cell immunoreceptor with Ig and ITIM-domains (TIGIT), an inhibitory receptor that binds PVR and Nectin-2, the same ligands used by DNAM-1. Many tumors have been shown to overexpress the TIGIT ligand, CD155, and have been linked with enhanced tumor proliferation and migration[93, 94]. TIGIT itself has been found to be upregulated on CD8+ T cells and Tregs in many clinical tumor settings[95–97]. Blockade of TIGIT in vitro results in enhanced T cell function[95, 98]. Similarly, blockade of TIGIT on NK cells enhanced both cytokine secretion as well as cytotoxic function[35, 99].
In recent studies from our lab, when NK cells were co-cultured with MDSCs, which express both PVR and Nectin-2, decreased proliferation was observed when compared to NK co-cultured with monocytes. Moreover, NK cells had depressed degranulation and cytokine production when subsequently stimulated with anti-CD16 after co-culture with MDSCs. NK functionality was restored when the NK-MDSC culture was supplemented with a TIGIT-antagonistic antibody. Furthermore, no NK inhibition was observed when NK cells and MDSCs were separated via transwell, indicating cell-to-cell contact is necessary for the NK cell immunomodulation[69]. A recently identified subset of NK cells that arises in cytomegalovirus (CMV) infected individuals demonstrates enhanced secondary responses to previously encountered stimulus not seen in conventional NK cells. Interestingly, these ‘adaptive’ NK cells express lower levels of TIGIT than the conventional NK cells are more resistant to MDSC-mediated suppression, indicating the potential for TIGIT blockade to enhance conventional NK mediated anti-tumor responses [69].
Killer Immunoglobulin-like receptors (KIR)
Amongst commonly expressed NK cell receptors, one of the more promising checkpoint-targets for blockade to enhance anti-tumor responses is the KIR family of inhibitory receptors. KIRs come in two varieties, activating and inhibitory, but for the purposes of immunotherapy the focus is on the inhibitory KIRs. Inhibitory KIRs fall into one of two groups: 1) those that express two (KIR2DL) or 2) those that express three (KIR3DL) extracellular immunoglobulin- like domains. Both KIR2DL and KIR3DL signal through intracellular tyrosine-based inhibitory motifs (ITIM) present in their long cytoplasmic tails[100]. As previously discussed, KIR recognize MHC-I molecules as their ligands on normal, healthy cells and ligation of MHC-I by KIR inhibit NK cell function. Moreover, the interaction between KIR and their cognate MHC-I during NK cell development and homeostasis is critical for the dynamic process of NK cell ‘education’. The concept of NK cell ‘education’, ‘licensing’ or ‘tuning’ is important because NK cells only become functional and retain functionality against targets missing MHC-I if they receive tonic signals via KIR-cognate ligand interactions present within their environment[101–103]. In the context of cancer, despite the up-regulation of ligands to NK activation receptors, many tumors retain much of their complement of MHC-I molecules thereby restricting KIR-expressing NK cells from responding to, and ultimately clearing the tumor burden.
The importance of inhibitory KIR signaling in cancer has been highlighted by the allogeneic haplo-mismatched stem cell transplantation (allo-SCT). In a study of 112 patients with high-risk acute myeloid leukemia (AML) who received HLA-haploidentical transplants, patients had significantly better clinical outcomes when receiving cells from NK alloreactive donors (possessing HLA-alleles that are missing in the recipients and are thus not engaged by the donor’s KIRs) than patients who received cells from non-alloreactive donors[104]. In short, KIR-expressing, ‘educated’ NK cells from a donor put into a recipient lacking the cognate MHC-I are no longer restricted and are able to respond to the patient’s tumor due to lack of inhibitory signaling via KIR. While this method is effective, it is not realistic to do adoptive cell transplantation for every patient. As such, the utilization of a KIR-antagonistic antibody is currently being tested as a potential therapeutic.
Phase I clinical trials of the anti-KIR antibody, IPH2101 (Innate Pharma, Marselle, France) (Table 1), in AML patients in complete remission has demonstrated KIR-binding on >90% of NK cells for 2 weeks at a minimum dose of 1mg/kg. Treatment with the anti-KIR antibody resulted in some increases in serum concentrations of TNF-α and MIP-1β as well as increases in NK expression of the early activation marker, CD69. Adverse events from treatment with IPH2101 were mild and transient with grade 3–4 adverse events occurring in only a single patient[105]. A similar phase-I trial of IPH2101 was conducted on multiple myeloma (MM) patients with similar results[106]. In addition, patient-derived peripheral blood mononuclear cells taken before and after the first dose were used to evaluate NK functional activity against the MM cell line, RPMI 8226. These initial clinical studies indicated possible greater NK functionality against the MM cell line post-IPH2101 treatment. However, a Phase II study on smoldering multiple myeloma showed no therapeutic benefit[107]. A follow up study demonstrated that the limited efficacy in smoldering multiple myeloma patients was due to IPH2101-mediated trogocytosis of KIR2D receptors on NK cells[108]. While this effectively eliminated inhibitory signaling through KIR2D receptors, it also eliminated the ability of those KIR2D- cells to become ‘educated’, thus resulting in an overall net decrease in NK cell functional responses to the MM cells. This complication highlights some of the challenges of targeting checkpoint blockade in complex biologic systems.
NKG2A/CD94
The c-type lectin heterodimer NKG2A/CD94 is expressed on both natural killer cells as well as CD8+ T cells. This inhibitory receptor recognizes HLA-E molecules as its ligand. NKG2A/CD94 is expressed on a large proportion of circulating NK cells (>60%) thereby making its ligation a hugely suppressive event. Many tumor types, including solid tumors and hematological malignancies, up-regulate HLA-E expression, dampening NKG2A–expressing NK cell responses. In allogeneic and autologous hematopoietic stem cell transplants NK2G2A is expressed uniformly on newly formed NK cells, making NKG2A–HLA-E interactions one of the main deterrents in NK cell function post therapeutic transplant[109, 110]. Maturation of the NK cells in this setting is associated with a decrease in NKG2A expression and recovery of NK cell function, but blockade of NKG2A can be used to bridge functionality until full maturation[111]. This has spurred interest in NKG2A–antagonisitc antibodies as a cancer therapeutic. An anti-NKG2A monoclonal antibody (IPH2201-Monalizumab) is currently being evaluated in phase I/II clinical trials for a variety of tumor types (Table 1).
Other NK-expressed checkpoint receptors
Other notable checkpoint receptors of interest are T cell immunoglobulin- and mucin-domain containing molecule 3 (Tim-3),) and lymphocyte activation gene 3 (Lag-3). Originally identified on T cells, Tim-3 is a negative regulator of T cell mediated immune responses. Treatment of mice with anti-Tim-3 enhanced the development of spontaneous autoimmunity. Tim-3 is found to be up-regulated on peripheral NK cells in patients with advanced gastric cancer and patients with lung adenocarcinoma. It has also been identified on tumor-infiltrating NK cells in 75% of patients with gastrointestinal stromal tumors[88]. The relevance of this receptor on NK cells appears to be somewhat clouded. While functionally depressed NK cells from patients with advanced melanoma were rescued by treatment with a Tim-3 antagonist, blockade of Tim-3’s ligand, galectin-9, reduced the IFN-γ production of healthy NK cells against AML targets[112]. Tim-3 blockade is an emerging target in patients displaying resistance to PD-1 blockade, where Tim-3 expression is increased and thought to drive suppression. A Phase-I clinical trial is currently in progress in patients with advanced tumors testing combinational treatment with PD-1 blockade and a Tim-3 blocking therapeutic antibody TSR-022[113] (Table 1).
Lag-3, also originally identified on activated CD4 and CD8 T cells, is structurally similar to the CD4 co-receptor and binds MHC-II as its ligand. Lag-3 is expressed on a small proportion of NK cells (~10%). However, since NK cells do not interact with MHC-II, alternative ligands have been proposed for Lag-3 and the significance of Lag-3 expression remains unclear[114].
Breaking tolerance through activation signaling
The balance of activating and inhibitory signals is crucial to the regulation of NK effector function. While suppression of inhibitory signals can restore NK- mediated anti-tumor responses, enhancement of NK activating signals can similarly result in increased tumor killing. This can be achieved therapeutically through two primary mechanisms: 1) NK stimulation with activating cytokines or 2) antibody-directed killing through the low-affinity Fc receptor, CD16.
Overcoming inhibition through activating cytokines
NK cells express a wide array of cytokine receptors that can modulate NK effector function, development, proliferation and homeostasis. A number of cytokine receptors are constitutively expressed while others are inducible[115]. Although stimulation with individual cytokines may enhance NK function, synergistic effects from multiple cytokines can result in a more robust response than any single type alone[116]. Of interest in the context of enhancing NK mediated anti-tumor responses are the common γ-chain cytokines IL-2 and IL-15. For a more through review on clinical applications of a variety of NK-modulating cytokines, please refer to Romee et. al 2014[117]. However, for the purposes of this review, we will focus on IL-2 and IL-15. These two cytokines activate a host of downstream signaling molecules including Jak1/3, STAT3/5, PI3K, MAPK and the transcriptional factor NF-κB; the result of which is enhanced cytokine production, cytotoxic effector function, proliferation and survival [118–120]. Both IL-2 and IL-15 utilize the IL-2/15Rβ and the common gamma chain (γc) subunits for signaling. While soluble IL-2 has a high affinity for NK-expressed IL-2Rα (CD25) in picomolar concentrations[121, 122], a strong IL-15 signal is transmitted when trans-presented by IL-15Rα expressed on, or shed by, monocytes and dendritic cells[120].
IL-2 therapy has been well studied in cancer patients, but has yielded little clinical benefit as a monotherapy[123, 124]. A 2003 study of relapsed lymphoma and metastatic breast cancer patients at the University of Minnesota showed that while NK cells pre-activated with IL-2 prior to infusion enhanced NK cytokine and cytotoxic function, neither infusion of pre-activated NK nor subcutaneous infusion of IL-2 resulted in an improvement of disease outcomes or survival[125]. A drawback of IL-2 is that although it activates NK cells it also enhances function of T-regulatory cells, which can limit NK responses[126]. High dose IL-2 treatment of patients with renal cell carcinoma and metastatic melanoma only induced remission in a minority of patients but also results in substantial toxicity[127, 128]. Other studies tested low-dose IL-2 therapies, which enhanced NK cell function but similarly resulted in the expansion of regulatory T cells. Due to the undesired expansion of Tregs in IL-2 therapy, IL-15 has become a cytokine of interest in cancer immunotherapy because of its NK stimulating effects without the augmentation of Tregs.
Recombinant human IL-15, in monomeric form, is currently being investigated for use in solid tumors as well as to support NK cell persistence and activity post-adoptive transfer in patients with leukemia[129]. Pre-clinical, non-human primate and early clinical data all indicate that IL-15 can induce augmentation of NK cell numbers, which are thought to enhance immunotherapy[130]. However, as previously noted, IL-15 transpresentation via IL-15Rα is necessary to maximize IL-15 signaling and it is not entirely clear if this occurs with the monomeric form of IL-15. Altor Bioscience Corporation (Altor, Mirimar, FL) has developed the IL-15N72D/IL-15Rα-Fc super-agonist complex (ALT-803) currently being tested in vitro and in phase I clinical trials. Recent studies have shown a strong enhancement in NK cell degranulation and cytokine production against ovarian cancer cell lines both in vitro and in murine models after treatment with ALT-803. Furthermore, ALT-803 rescued function of NK cells derived from ovarian cancer patient ascites[131]. ALT-803 also enhanced in vivo CD16-triggered NK cell clearance of B-cell lymphomas when treated with an anti-CD20 monoclonal antibody[132]. In conjunction with the postulated role of γc cytokine-mediated bypass of some checkpoint inhibition pathways[133], the ability of IL-15 to prime CD16 mediated functions on NK cells makes this cytokine an attractive immunotherapeutic target.
FcγRIIIa (CD16a)
In addition to the mediation of target cell killing through the recognition of naturally expressed ligands, NK cells express the low affinity Fc receptor, CD16a, which mediates antibody directed killing. Two isoforms of CD16 exist, CD16a and CD16b[134]. These isoforms possess a distinct expression pattern[135–138]. CD16a has a transmembrane domain and is expressed on the surface of NK cells, macrophages and placental trophoblasts. CD16b is expressed on neutrophils and is anchored to the membrane via a GPI-domain. While the extracellular domains of CD16a and CD16b share greater than 90% homology, CD16b doesn’t signal and only CD16a triggering results in the killing of tumor targets[134, 135, 139, 140].
CD16a is expressed on the CD56dim subset of human NK cells, which accounts for >80% of all peripheral NK cells in healthy individuals[4]. Triggering of CD16a is mediated by engagement of the Fc-portion of IgG-antibodies, which results in signaling through the immunoreceptor tyrosine-based activation motif (ITAM) and association with adaptor molecules FcεR1γ and CD3ζ[141]. Ligation of CD16a results in potent cytokine production and degranulation. Unlike other NK activating receptors, ligation of CD16a triggers a robust response without the need for co-activation[142]. The ability to mediate a response to antibody-coated targets without the need for co-activation allows for NK responses in the natural settings of viral infection and during the early stages of tumor formation[143–145]. This NK function has been exploited in the clinic through the administration of monoclonal antibodies against tumor-specific antigens to drive ADCC[146–150]. There are different allotypes of CD16a with varying affinities for the Fc-portion of IgG antibodies. NK cells expressing CD16a with the 158VV or -VF allotypes display a lower affinity for IgG Fc portion of the anti-CD20 monoclonal Rituximab than the CD16a-158FF allotype[151]. The differences restrict positive outcomes on individuals with the 158VV or -VF allotypes in terms of standard antibody- based therapies. This observation supports the use of a new class of antibody therapeutic, known as BiKEs and TriKEs, which could negate this difference in affinity while allowing for the targeting of specific surface expressed antigens.
Bi-specific and Tri-specific Killer Engagers (BiKEs and TriKEs)
Issues with toxicity, lack of specificity and unreliable effectiveness of chemotherapy have prompted great interest in the potential of targeted cancer immunotherapies[152]. While most of the recent focus has been on the generation and success of chimeric antigen receptor (CAR) expressing T cells[153, 154], these personalized approaches are expensive, time consuming, have not shown efficacy in solid tumors, and have resulted in an unacceptable toxicity at times. Complications with the CAR T cell methodology demonstrate a clear need for a targeted off-the-shelf product that could fill this niche. Thus, the interest in BiKEs and TriKEs.
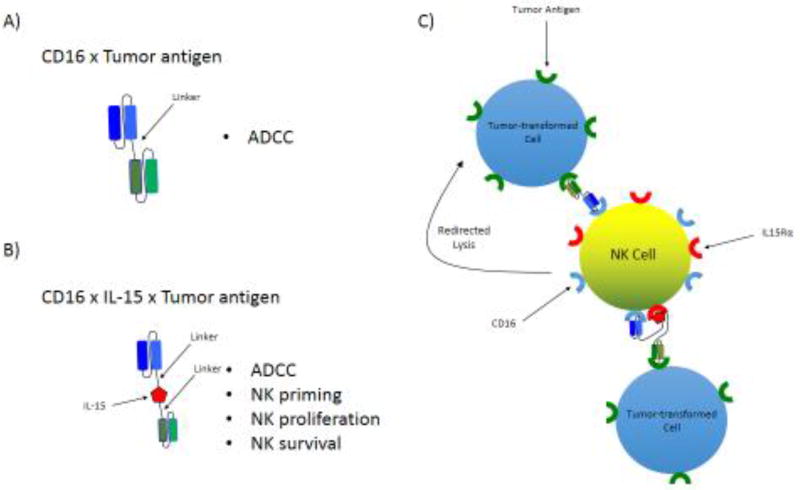
These molecules are designed to form an antigen-specific immunological synapse between NK cells and tumor cells in order to trigger NK cell-mediated killing of tumor targets. Unlike full-length bi- and tri-specific antibodies (300–450 kDa), these small molecule constructs (50–75 kDa) are composed by a single chain variable fragment (scFv) made up of a variable heavy and variable light chain (VH and VL) of an antibody connected by a short peptide[155], and linked to the scFvs of one (BiKE) or two (TriKE) other antibodies of different specificities. Alternatively, one of the scFvs can be swapped out for a cytokine in the TriKE constructs, or can be left in place with the addition of the cytokine in TetraKE constructs (Figure 2).
Figure 2. Design and function of tumor antigen-specific bi- and tri-specific killer engagers (BiKEs and TriKEs).
A) Schema of the basic design and structure of a BiKE consisting of an anti-CD16 scFV linked to an scFv specific to a tumor-expressed antigen or B) a TriKE of the same design including the cytokine, IL-15, included as a linker between the two scFv components. C) Proposed function of tumor antigen-specific BiKE and TriKE.
Considering that monoclonal antibody-driven ADCC has resulted in significant clinical success, BiKEs and TriKEs were designed with an anti-CD16 component. Furthermore, based on the previously discussed differences in CD16 allotype affinity for IgG-Fc, the use of an anti-CD16 component has the potential to negate those differences and improve NK function by instigating a stronger interaction with CD16 than the natural CD16-Fc interaction. This concept was demonstrated when comparing the affinity of CD16 for the Fc-portion of an anti-HER2 antibody versus binding of an anti-HER2 x anti-CD16 bi-specific antibody (3.4-fold increase)[156]. Additionally, BiKEs and TriKEs have several advantages including increased biodistribution compared to mAbs due to their significantly smaller size, are non-immunogenic and can be quickly engineered, thus alleviating many of the complications of their CAR counterparts[155, 157, 158].
Design of these constructs is a complicated process that begins with the selection of a target of interest. Selected targets are usually surface antigens that are highly, and possibly selectively, expressed or up-regulated on the surface of tumor cells. Once a target has been selected, a source must be identified for the gene sequence for the scFvs. Common sources include published works, hybridomas, B-cells from immunized animals or biopanning from phage display. Once the sequence of interest has been identified, a proper linker is chosen to connect the two antigen-binding domains, or scFvs. Selection of the linker is important as the linker must provide separation of the antigen-binding components while maintaining flexibility to allow binding of the two or three components of the construct to their antigens on target cells[159]. Next, an appropriate expression vector is chosen for production. Bacterial and mammalian expression systems are suitable for this process but have different characteristics. Though bacterial production is cheaper, it requires refolding which is unnecessary in mammalian systems. For use in bacterial expression, the pET vector is commonly used in conjunction with Rosetta 2(DE3) host cells (Novagen). These cells are engineered to express a “universe” set of transfer RNAs, which reduce the need for codon optimization. For mammalian expression, the pTT5 vector is used with HEK-293-E6 cells or the pcDNA3.1 vector is used with HEK293 Freestyle cells (Invitrogen). However, higher protein yields have been obtained with the HEK293-E6 system[160]. The ExpiCho expression system is another mammalian system that can be used for achieving higher protein yields. Once the vector and expression system has been chosen, cloning of the BiKE components into the vector backbone can begin. This can be achieved through a number of modalities, including restriction based cloning and the Gibson assembly method, favored by our group due to ease of use and cost[161], [162]. The vector can be chemically transduced into E. coli, where protein products are extracted from inclusion bodies, or transfected into mammalian cells where protein can be harvested from the culture supernatant. The final step is protein purification, achieved through size exclusion chromatography or column isolation with an affinity tag.
Currently, bi-specific antibody constructs have been generated to engage CD16 in conjunction with: CD20/CD19 on B cell Non-Hodgkin’s lymphomas[163–171], CD19/CD33 on mixed lineage leukemia[172], CD33 or CD33/CD123 on acute myelogenous leukemia (AML)[173–175], HLA Class II on lymphoma[176], CD30 on Hodgkin’s lymphoma[177–186], EGF-R on EGF-R+ tumors[187, 188], HER2/neu on metastatic breast cancer and other HER2 expressing tumors[156, 189, 190] and MOV19 on ovarian cancer[191].
Our group has generated and tested BiKEs and TriKEs that engage CD16 along with: CD19/CD22 on B cell Non-Hodgkin’s lymphomas[192], CD33 on AML[193] and MDS/MDSCs[194], EpCAM on prostate, breast, colon, head, and neck carcinomas[195] and CD133 on cancer stem cells or a combination of EpCAM/CD133 for a broad spectrum molecule[196, 197]. Newer generation TriKEs and TetraKEs all incorporate an IL-15 moiety that substantially enhances NK cell function (Figure 2). The TriKE/TetraKE molecules containing an IL-15 moiety demonstrate clear advantages over their BiKE predecessors in terms of cytotoxicity of targets and generation of inflammatory cytokines. These molecules also induce robust NK cell expansion as well as NK cell survival in vitro. When compared to its BiKE counterpart, one of these molecules, an anti-CD16 x IL-15 x anti-CD33 TriKE, has demonstrated better tumor control and NK cell maintenance/expansion in a preclinical AML xenogeneic mouse model containing human NK cells. It is tempting to speculate that the IL-15 moiety can also enhance a bypass of checkpoint signaling. Studies are currently under way to evaluate this question. The anti-CD16 x IL-15 x anti-CD33 TriKE is headed for the clinic (summer 2017) in a Phase I clinical trial for patients with refractory AML and high risk MDS at the University of Minnesota.
While the current generation of TriKEs and TetraKEs trigger NK responses via CD16 ligation and cytokine signaling, additional components can be added to enhance the NK response. Blocking scFvs against checkpoint receptors like KIRs, TIGIT, NKG2A or PD-1 can be included in TriKE and TetraKE constructs in order to bypass checkpoint blockade and further drive NK-mediated anti-tumor responses. Alternatively, an scFv blocking TGF-β could be included to reduce negative signaling in the tumor microenvironment. Another interesting concept is incorporation of an scFv blocking ADAM-17, a matrix metalloproteinase involved in CD16 shedding, to maximize CD16-mediated killing. Besides IL-15, a number of other cytokines that differentially modulate NK cell biology could be included[198]. This illustrates the flexibility of the BiKE/TriKE platform, which allows for a variety of interchangeable components to be custom tailored to not only mediate NK specific targeting of tumor cells, but also allow for bypass of checkpoint inhibition.
Conclusions
Despite the ability of natural killer cells to recognize and kill tumor cells, NK-expressed checkpoint receptors present a hurdle when considering NK-mediated immunotherapies. However, better understanding of the expression and function of these receptors, as well as emerging pharmacological therapeutics, offers opportunities to eliminate checkpoint receptor suppression of NK responses. Nevertheless, several caveats must be addressed when considering some of these immunotherapies. Activation of NK cells has been shown to activate the matrix-metalloprotease, ADAM-17, which is responsible for cleaving CD16 from the surface of NK cells which would potentially limit NK-mediated ADCC responses. Furthermore, NK-secreted IFN-γ can up-regulate MHC-I molecules as well as PD-1 ligands on tumor cells thus, increasing suppression of NK function. Combinational treatments, such as checkpoint blockade antibodies in conjunction with TriKEs, must therefore be considered when devising immunotherapies that utilize CD16 engagement.
It is important to note that the utilization of checkpoint blockade has the potential to enhance not only endogenous NK cell function against tumor targets but also to enhance the function of NK cells given as part of adoptive transfer therapies. With many cancers resulting in aberrant NK function or NK loss, as a result of the tumor microenvironment or lymphodepleting chemotherapy regiments, adoptive transfer of allogeneic NK cells or off-the-shelf NK cell products can replace the missing immune component. Though these infused products provide functional NK cells to support a robust anti-tumor response, they may still be limited by inhibition through checkpoint receptors and may therefore benefit from additional support through checkpoint blockade. Additionally blockade of checkpoint receptors in combination with targeted antibody therapies, like BiKEs and TriKEs, can add antigen specificity and further enhance NK responses in the immunosuppressive tumor microenvironment. The future of NK cell-based immunotherapy likely lies in a combination of these approaches rather than monotherapy.
Highlights.
Natural killer cell function is negatively regulated by the tumor microenvironment
Checkpoint receptor blockade has the potential to restore NK function
Checkpoint blockade can be used in conjunction with novel antibody therapies (BiKE/Trike) or NK adoptive transfer to enhance targeted responses
Acknowledgments
Financial support
This work was supported by the following grants: P01 CA111412 (NIH), P01 CA65493 (NIH), R35 CA197292 (NIH), T32 HL007062 (NIH), and CA150085 (U.S. Department of Defense).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity, International journal of cancer. Journal international du cancer. 1975;16(2):216–29. doi: 10.1002/ijc.2910160204. [DOI] [PubMed] [Google Scholar]
- 2.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9(5):503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 3.Timonen T, Ortaldo JR, Herberman RB. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. Journal of experimental medicine. 1981;153(3):569–82. doi: 10.1084/jem.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 5.Bhat R, Watzl C. Serial killing of tumor cells by human natural killer cells--enhancement by therapeutic antibodies. PloS one. 2007;2(3):e326. doi: 10.1371/journal.pone.0000326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci U S A. 2000;97(6):2731–6. doi: 10.1073/pnas.050588297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356(9244):1795–9. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- 8.Roder JC, Haliotis T, Klein M, Korec S, Jett JR, Ortaldo J, Heberman RB, Katz P, Fauci AS. A new immunodeficiency disorder in humans involving NK cells. Nature. 1980;284(5756):553–5. doi: 10.1038/284553a0. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan JL, Byron KS, Brewster FE, Purtilo DT. Deficient natural killer cell activity in x-linked lymphoproliferative syndrome. Science. 1980;210(4469):543–5. doi: 10.1126/science.6158759. [DOI] [PubMed] [Google Scholar]
- 10.Gineau L, Cognet C, Kara N, Lach FP, Dunne J, Veturi U, Picard C, Trouillet C, Eidenschenk C, Aoufouchi S, Alcais A, Smith O, Geissmann F, Feighery C, Abel L, Smogorzewska A, Stillman B, Vivier E, Casanova JL, Jouanguy E. Partial MCM4 deficiency in patients with growth retardation adrenal insufficiency, and natural killer cell deficiency. J Clin Invest. 2012;122(3):821–32. doi: 10.1172/JCI61014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang N, Bevan MJ. CD8(+) T cells: foot soldiers of the immune system. Immunity. 2011;35(2):161–8. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bix M, Liao NS, Zijlstra M, Loring J, Jaenisch R, Raulet D. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature. 1991;349(6307):329–31. doi: 10.1038/349329a0. [DOI] [PubMed] [Google Scholar]
- 13.Raulet DH, Guerra N. Oncogenic stress sensed by the immune system: role of natural killer cell receptors. Nat Rev Immunol. 2009;9(8):568–80. doi: 10.1038/nri2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nausch N, Cerwenka A. NKG2D ligands in tumor immunity. Oncogene. 2008;27(45):5944–58. doi: 10.1038/onc.2008.272. [DOI] [PubMed] [Google Scholar]
- 15.Ljunggren HG, Karre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunology Today. 1990;11(7):237–44. doi: 10.1016/0167-5699(90)90097-s. [DOI] [PubMed] [Google Scholar]
- 16.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol. 2008;9(5):495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anegon I, Cuturi MC, Trinchieri G, Perussia B. Interaction of Fc receptor (CD16) ligands induces transcription of interleukin 2 receptor (CD25) and lymphokine genes and expression of their products in human natural killer cells. J Exp Med. 1988;167(2):452–72. doi: 10.1084/jem.167.2.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227–58. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Upshaw JL, Arneson LN, Schoon RA, Dick CJ, Billadeau DD, Leibson PJ. NKG2D–mediated signaling requires a DAP10-bound Grb2-Vav1 intermediate and phosphatidylinositol-3-kinase in human natural killer cells. Nature immunology. 2006;7(5):524–32. doi: 10.1038/ni1325. [DOI] [PubMed] [Google Scholar]
- 20.Sutherland CL, Rabinovich B, Chalupny NJ, Brawand P, Miller R, Cosman D. ULBPs, human ligands of the NKG2D receptor, stimulate tumor immunity with enhancement by IL-15. Blood. 2006;108(4):1313–9. doi: 10.1182/blood-2005-11-011320. [DOI] [PubMed] [Google Scholar]
- 21.Smyth MJ, Swann J, Cretney E, Zerafa N, Yokoyama WM, Hayakawa Y. NKG2D function protects the host from tumor initiation. J Exp Med. 2005;202(5):583–8. doi: 10.1084/jem.20050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCann FE, Eissmann P, Onfelt B, Leung R, Davis DM. The activating NKG2D ligand MHC class I-related chain A transfers from target cells to NK cells in a manner that allows functional consequences. Journal of immunology. 2007;178(6):3418–26. doi: 10.4049/jimmunol.178.6.3418. [DOI] [PubMed] [Google Scholar]
- 23.Bryceson YT, March ME, Ljunggren HG, Long EO. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107(1):159–66. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bryceson YT, March ME, Ljunggren HG, Long EO. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev. 2006;214:73–91. doi: 10.1111/j.1600-065X.2006.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Millard PJ, Henkart MP, Reynolds CW, Henkart PA. Purification and properties of cytoplasmic granules from cytotoxic rat LGL tumors. Journal of immunology. 1984;132(6):3197–204. [PubMed] [Google Scholar]
- 26.Tschopp J, Masson D, Stanley KK. Structural/functional similarity between proteins involved in complement- and cytotoxic T-lymphocyte-mediated cytolysis. Nature. 1986;322(6082):831–4. doi: 10.1038/322831a0. [DOI] [PubMed] [Google Scholar]
- 27.Keefe D, Shi L, Feske S, Massol R, Navarro F, Kirchhausen T, Lieberman J. Perforin triggers a plasma membrane-repair response that facilitates CTL induction of apoptosis. Immunity. 2005;23(3):249–62. doi: 10.1016/j.immuni.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Metkar SS, Wang B, Aguilar-Santelises M, Raja SM, Uhlin-Hansen L, Podack E, Trapani JA, Froelich CJ. Cytotoxic cell granule-mediated apoptosis: perforin delivers granzyme B-serglycin complexes into target cells without plasma membrane pore formation. Immunity. 2002;16(3):417–28. doi: 10.1016/s1074-7613(02)00286-8. [DOI] [PubMed] [Google Scholar]
- 29.Morvan MG, Lanier LL. NK cells and cancer: you can teach innate cells new tricks. Nat Rev Cancer. 2016;16(1):7–19. doi: 10.1038/nrc.2015.5. [DOI] [PubMed] [Google Scholar]
- 30.Wallach D, Fellous M, Revel M. Preferential effect of gamma interferon on the synthesis of HLA antigens and their mRNAs in human cells. Nature. 1982;299(5886):833–6. doi: 10.1038/299833a0. [DOI] [PubMed] [Google Scholar]
- 31.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nature immunology. 2004;5(12):1260–5. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 32.Casado JG, Pawelec G, Morgado S, Sanchez-Correa B, Delgado E, Gayoso I, Duran E, Solana R, Tarazona R. Expression of adhesion molecules and ligands for activating and costimulatory receptors involved in cell-mediated cytotoxicity in a large panel of human melanoma cell lines. Cancer immunology, immunotherapy : CII. 2009;58(9):1517–26. doi: 10.1007/s00262-009-0682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levin SD, Taft DW, Brandt CS, Bucher C, Howard ED, Chadwick EM, Johnston J, Hammond A, Bontadelli K, Ardourel D, Hebb L, Wolf A, Bukowski TR, Rixon MW, Kuijper JL, Ostrander CD, West JW, Bilsborough J, Fox B, Gao Z, Xu W, Ramsdell F, Blazar BR, Lewis KE. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. Eur J Immunol. 2011;41(4):902–15. doi: 10.1002/eji.201041136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mendelsohn CL, Wimmer E, Racaniello VR. Cellular receptor for poliovirus: molecular cloning nucleotide, sequence and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56(5):855–65. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- 35.Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, Stern-Ginossar N, Tsukerman P, Jonjic S, Mandelboim O. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proc Natl Acad Sci U S A. 2009;106(42):17858–63. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, Eaton D, Grogan JL. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10(1):48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- 37.Rajagopalan S, Long EO. The direct binding of a p58 killer cell inhibitory receptor to human histocompatibility leukocyte antigen (HLA)-Cw4 exhibits peptide selectivity. The Journal of experimental medicine. 1997;185(8):1523–8. doi: 10.1084/jem.185.8.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malnati MS, Peruzzi M, Parker KC, Biddison WE, Ciccone E, Moretta A, Long EO. Peptide specificity in the recognition of MHC class I by natural killer cell clones. Science. 1995;267(5200):1016–8. doi: 10.1126/science.7863326. [DOI] [PubMed] [Google Scholar]
- 39.Wan AM, Ennis P, Parham P, Holmes N. The primary structure of HLA-A32 suggests a region involved in formation of the Bw4/Bw6 epitopes. Journal of immunology. 1986;137(11):3671–4. [PubMed] [Google Scholar]
- 40.Gumperz JE, Litwin V, Phillips JH, Lanier LL, Parham P. The Bw4 public epitope of HLA-B molecules confers reactivity with natural killer cell clones that express NKB1, a putative HLA receptor. Journal of experimental medicine. 1995;181(3):1133–44. doi: 10.1084/jem.181.3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller JD, Weber DA, Ibegbu C, Pohl J, Altman JD, Jensen PE. Analysis of HLA-E peptide-binding specificity and contact residues in bound peptide required for recognition by CD94/NKG2. Journal of immunology. 2003;171(3):1369–75. doi: 10.4049/jimmunol.171.3.1369. [DOI] [PubMed] [Google Scholar]
- 42.Petrie EJ, Clements CS, Lin J, Sullivan LC, Johnson D, Huyton T, Heroux A, Hoare HL, Beddoe T, Reid HH, Wilce MC, Brooks AG, Rossjohn J. CD94-NKG2A recognition of human leukocyte antigen (HLA)-E bound to an HLA class I leader sequence. The Journal of experimental medicine. 2008;205(3):725–35. doi: 10.1084/jem.20072525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiroishi M, Tsumoto K, Amano K, Shirakihara Y, Colonna M, Braud VM, Allan DS, Makadzange A, Rowland-Jones S, Willcox B, Jones EY, van der Merwe PA, Kumagai I, Maenaka K. Human inhibitory receptors Ig-like transcript 2 (ILT2) and ILT4 compete with CD8 for MHC class I binding and bind preferentially to HLA-G. Proc Natl Acad Sci U S A. 2003;100(15):8856–61. doi: 10.1073/pnas.1431057100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sternberg-Simon M, Brodin P, Pickman Y, Onfelt B, Karre K, Malmberg KJ, Hoglund P, Mehr R. Natural killer cell inhibitory receptor expression in humans and mice: a closer look. Front Immunol. 2013;4:65. doi: 10.3389/fimmu.2013.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, Raine K, Latimer C, Santos CR, Nohadani M, Eklund AC, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, Takaoka M, Nakagawa H, Tort F, Fugger K, Johansson F, Sehested M, Andersen CL, Dyrskjot L, Orntoft T, Lukas J, Kittas C, Helleday T, Halazonetis TD, Bartek J, Gorgoulis VG. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444(7119):633–7. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 47.Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA, Jr, Kastrinakis NG, Levy B, Kletsas D, Yoneta A, Herlyn M, Kittas C, Halazonetis TD. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434(7035):907–13. doi: 10.1038/nature03485. [DOI] [PubMed] [Google Scholar]
- 48.Vales-Gomez M, Chisholm SE, Cassady-Cain RL, Roda-Navarro P, Reyburn HT. Selective induction of expression of a ligand for the NKG2D receptor by proteasome inhibitors. Cancer Res. 2008;68(5):1546–54. doi: 10.1158/0008-5472.CAN-07-2973. [DOI] [PubMed] [Google Scholar]
- 49.Berghuis D, Schilham MW, Vos HI, Santos SJ, Kloess S, Buddingh EP, Egeler RM, Hogendoorn PC, Lankester AC. Histone deacetylase inhibitors enhance expression of NKG2D ligands in Ewing sarcoma and sensitize for natural killer cell-mediated cytolysis. Clin Sarcoma Res. 2012;2(1):8. doi: 10.1186/2045-3329-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cerboni C, Zingoni A, Cippitelli M, Piccoli M, Frati L, Santoni A. Antigen-activated human T lymphocytes express cell-surface NKG2D ligands via an ATM/ATR-dependent mechanism and become susceptible to autologous NK- cell lysis. Blood. 2007;110(2):606–15. doi: 10.1182/blood-2006-10-052720. [DOI] [PubMed] [Google Scholar]
- 51.Croxford JL, Tang ML, Pan MF, Huang CW, Kamran N, Phua CM, Chng WJ, Ng SB, Raulet DH, Gasser S. ATM-dependent spontaneous regression of early Emu-myc-induced murine B-cell leukemia depends on natural killer and T cells. Blood. 2013;121(13):2512–21. doi: 10.1182/blood-2012-08-449025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Soriani A, Zingoni A, Cerboni C, Iannitto ML, Ricciardi MR, Di Gialleonardo V, Cippitelli M, Fionda C, Petrucci MT, Guarini A, Foa R, Santoni A. ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype. Blood. 2009;113(15):3503–11. doi: 10.1182/blood-2008-08-173914. [DOI] [PubMed] [Google Scholar]
- 53.Wang B, Niu D, Lai L, Ren EC. p53 increases MHC class I expression by upregulating the endoplasmic reticulum aminopeptidase ERAP1. Nat Commun. 2013;4:2359. doi: 10.1038/ncomms3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin A, Yan WH. HLA-G expression in cancers: roles in immune evasion. metastasis and target for therapy. Mol Med. 2015 doi: 10.2119/molmed.2015.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gonzalez A, Rebmann V, LeMaoult J, Horn PA, Carosella ED, Alegre E. The immunosuppressive molecule HLA-G and its clinical implications. Crit Rev Clin Lab Sci. 2012;49(3):63–84. doi: 10.3109/10408363.2012.677947. [DOI] [PubMed] [Google Scholar]
- 56.Heidenreich S, Zu Eulenburg C, Hildebrandt Y, Stubig T, Sierich H, Badbaran A, Eiermann TH, Binder TM, Kroger N. Impact of the NK cell receptor LIR-1 (ILT-2/CD85j/LILRB1) on cytotoxicity against multiple myeloma. Clin Dev Immunol. 2012;2012:652130. doi: 10.1155/2012/652130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chitadze G, Bhat J, Lettau M, Janssen O, Kabelitz D. Generation of soluble NKG2D ligands: proteolytic cleavage, exosome secretion and functional implications. Scand J Immunol. 2013;78(2):120–9. doi: 10.1111/sji.12072. [DOI] [PubMed] [Google Scholar]
- 58.Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol. 2013;31:413–41. doi: 10.1146/annurev-immunol-032712-095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hilpert J, Grosse-Hovest L, Grunebach F, Buechele C, Nuebling T, Raum T, Steinle A, Salih HR. Comprehensive analysis of NKG2D ligand expression and release in leukemia: implications for NKG2D–mediated NK cell responses. J Immunol. 2012;189(3):1360–71. doi: 10.4049/jimmunol.1200796. [DOI] [PubMed] [Google Scholar]
- 60.Salih HR, Goehlsdorf D, Steinle A. Release of MICB molecules by tumor cells: mechanism and soluble MICB in sera of cancer patients. Hum Immunol. 2006;67(3):188–95. doi: 10.1016/j.humimm.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 61.Salih HR, Holdenrieder S, Steinle A. Soluble NKG2D ligands: prevalence, release, and functional impact. Front Biosci. 2008;13:3448–56. doi: 10.2741/2939. [DOI] [PubMed] [Google Scholar]
- 62.Yamaguchi K, Chikumi H, Shimizu A, Takata M, Kinoshita N, Hashimoto K, Nakamoto M, Matsunaga S, Kurai J, Miyake N, Matsumoto S, Watanabe M, Yamasaki A, Igishi T, Burioka N, Shimizu E. Diagnostic and prognostic impact of serum-soluble UL16-binding protein 2 in lung cancer patients. Cancer Sci. 2012;103(8):1405–13. doi: 10.1111/j.1349-7006.2012.02330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paschen A, Sucker A, Hill B, Moll I, Zapatka M, Nguyen XD, Sim GC, Gutmann I, Hassel J, Becker JC, Steinle A, Schadendorf D, Ugurel S. Differential clinical significance of individual NKG2D ligands in melanoma: soluble ULBP2 as an indicator of poor prognosis superior to S100B. Clin Cancer Res. 2009;15(16):5208–15. doi: 10.1158/1078-0432.CCR-09-0886. [DOI] [PubMed] [Google Scholar]
- 64.Nuckel H, Switala M, Sellmann L, Horn PA, Durig J, Duhrsen U, Kuppers R, Grosse-Wilde H, Rebmann V. The prognostic significance of soluble NKG2D ligands in B-cell chronic lymphocytic leukemia. Leukemia. 2010;24(6):1152–9. doi: 10.1038/leu.2010.74. [DOI] [PubMed] [Google Scholar]
- 65.Lundholm M, Schroder M, Nagaeva O, Baranov V, Widmark A, Mincheva-Nilsson L, Wikstrom P. Prostate tumor-derived exosomes down-regulate NKG2D expression on natural killer cells and CD8+ T cells: mechanism of immune evasion. PLoS One. 2014;9(9):e108925. doi: 10.1371/journal.pone.0108925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rouce RH, Shaim H, Sekine T, Weber G, Ballard B, Ku S, Barese C, Murali V, Wu MF, Liu H, Shpall EJ, Bollard CM, Rabin KR, Rezvani K. The TGF-beta/SMAD pathway is an important mechanism for NK cell immune evasion in childhood B-acute lymphoblastic leukemia. Leukemia. 2016;30(4):800–11. doi: 10.1038/leu.2015.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marvel D, Gabrilovich DI. Myeloid-derived suppressor cells in the tumor microenvironment: expect the unexpected. J Clin Invest. 2015;125(9):3356–64. doi: 10.1172/JCI80005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182(8):4499–506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sarhan D, Cichocki F, Zhang B, Yingst A, Spellman SR, Cooley S, Verneris MR, Blazar BR, Miller JS. Adaptive NK Cells with Low TIGIT Expression Are Inherently Resistant to Myeloid-Derived Suppressor Cells. Cancer Res. 2016;76(19):5696–5706. doi: 10.1158/0008-5472.CAN-16-0839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Orentas RJ, Kohler ME, Johnson BD. Suppression of anti-cancer immunity by regulatory T cells: back to the future. Semin Cancer Biol. 2006;16(2):137–49. doi: 10.1016/j.semcancer.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 71.Ghiringhelli F, Menard C, Terme M, Flament C, Taieb J, Chaput N, Puig PE, Novault S, Escudier B, Vivier E, Lecesne A, Robert C, Blay JY, Bernard J, Caillat-Zucman S, Freitas A, Tursz T, Wagner-Ballon O, Capron C, Vainchencker W, Martin F, Zitvogel L. CD4+CD25+ regulatory T cells inhibit natural killer cell functions in a transforming growth factor-beta-dependent manner. J Exp Med. 2005;202(8):1075–85. doi: 10.1084/jem.20051511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Betts G, Jones E, Junaid S, El-Shanawany T, Scurr M, Mizen P, Kumar M, Jones S, Rees B, Williams G, Gallimore A, Godkin A. Suppression of tumour-specific CD4(+) T cells by regulatory T cells is associated with progression of human colorectal cancer. Gut. 2012;61(8):1163–71. doi: 10.1136/gutjnl-2011-300970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Doubrovina ES, Doubrovin MM, Vider E, Sisson RB, O’Reilly RJ, Dupont B, Vyas YM. Evasion from NK cell immunity by MHC class I chain-related molecules expressing colon adenocarcinoma. J Immunol. 2003;171(12):6891–9. doi: 10.4049/jimmunol.171.12.6891. [DOI] [PubMed] [Google Scholar]
- 74.Wu JD, Higgins LM, Steinle A, Cosman D, Haugk K, Plymate SR. Prevalent expression of the immunostimulatory MHC class I chain-related molecule is counteracted by shedding in prostate cancer. J Clin Invest. 2004;114(4):560–8. doi: 10.1172/JCI22206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer immunology, immunotherapy : CII. 2007;56(5):641–8. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Galustian C, Meyer B, Labarthe MC, Dredge K, Klaschka D, Henry J, Todryk S, Chen R, Muller G, Stirling D, Schafer P, Bartlett JB, Dalgleish AG. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer immunology, immunotherapy : CII. 2009;58(7):1033–45. doi: 10.1007/s00262-008-0620-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, Lin B, Podar K, Gupta D, Chauhan D, Treon SP, Richardson PG, Schlossman RL, Morgan GJ, Muller GW, Stirling DI, Anderson KC. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98(1):210–6. doi: 10.1182/blood.v98.1.210. [DOI] [PubMed] [Google Scholar]
- 78.Bachanova V, Cooley S, Defor TE, Verneris MR, Zhang B, McKenna DH, Curtsinger J, Panoskaltsis-Mortari A, Lewis D, Hippen K, McGlave P, Weisdorf DJ, Blazar BR, Miller JS. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein. Blood. 2014;123(25):3855–63. doi: 10.1182/blood-2013-10-532531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pedroza-Pacheco I, Madrigal A, Saudemont A. Interaction between natural killer cells and regulatory T cells: perspectives for immunotherapy. Cell Mol Immunol. 2013;10(3):222–9. doi: 10.1038/cmi.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gasteiger G, Hemmers S, Firth MA, Le Floc’h A, Huse M, Sun JC, Rudensky AY. IL-2-dependent tuning of NK cell sensitivity for target cells is controlled by regulatory T cells. J Exp Med. 2013;210(6):1167–78. doi: 10.1084/jem.20122462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Okazaki T, Honjo T. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 2006;27(4):195–201. doi: 10.1016/j.it.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 82.Okazaki T, Wang J. PD-1/PD-L pathway and autoimmunity. Autoimmunity. 2005;38(5):353–7. doi: 10.1080/08916930500124072. [DOI] [PubMed] [Google Scholar]
- 83.Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O’Shea MA, Fauci AS. The common gamma-chain cytokines IL-2 IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181(10):6738–46. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 84.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–42. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439(7077):682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 86.Pesce S, Greppi M, Tabellini G, Rampinelli F, Parolini S, Olive D, Moretta L, Moretta A, Marcenaro E. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J Allergy Clin Immunol. 2017;139(1):335–346. doi: 10.1016/j.jaci.2016.04.025. e3. [DOI] [PubMed] [Google Scholar]
- 87.Benson DM, Jr, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, Baiocchi RA, Zhang J, Yu J, Smith MK, Greenfield CN, Porcu P, Devine SM, Rotem-Yehudar R, Lozanski G, Byrd JC, Caligiuri MA. The PD-1/ PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116(13):2286–94. doi: 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Anderson AC, Joller N, Kuchroo VK. Lag-3 Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity. 2016;44(5):989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Buchbinder EI, Desai A. CTLA-4 PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am J Clin Oncol. 2016;39(1):98–106. doi: 10.1097/COC.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fife BT, Bluestone JA. Control of peripheral T-cell tolerance and autoimmunity via the CTLA-4 and PD-1 pathways. Immunol Rev. 2008;224:166–82. doi: 10.1111/j.1600-065X.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 91.Khattri R, Auger JA, Griffin MD, Sharpe AH, Bluestone JA. Lymphoproliferative disorder in CTLA-4 knockout mice is characterized by CD28-regulated activation of Th2 responses. J Immunol. 1999;162(10):5784–91. [PubMed] [Google Scholar]
- 92.Jie HB, Schuler PJ, Lee SC, Srivastava RM, Argiris A, Ferrone S, Whiteside TL, Ferris RL. CTLA-4(+) Regulatory T Cells Increased in Cetuximab-Treated Head and Neck Cancer Patients Suppress NK Cell Cytotoxicity and Correlate with Poor Prognosis. Cancer Res. 2015;75(11):2200–10. doi: 10.1158/0008-5472.CAN-14-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sloan KE, Eustace BK, Stewart JK, Zehetmeier C, Torella C, Simeone M, Roy JE, Unger C, Louis DN, Ilag LL, Jay DG. CD155/PVR plays a key role in cell motility during tumor cell invasion and migration. BMC Cancer. 2004;4:73. doi: 10.1186/1471-2407-4-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kono T, Imai Y, Yasuda S, Ohmori K, Fukui H, Ichikawa K, Tomita S, Imura J, Kuroda Y, Ueda Y, Fujimori T. The CD155/poliovirus receptor enhances the proliferation of ras-mutated cells. International journal of cancer. Journal international du cancer. 2008;122(2):317–24. doi: 10.1002/ijc.23080. [DOI] [PubMed] [Google Scholar]
- 95.Chauvin JM, Pagliano O, Fourcade J, Sun Z, Wang H, Sander C, Kirkwood JM, Chen TH, Maurer M, Korman AJ, Zarour HM. TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients. J Clin Invest. 2015;125(5):2046–58. doi: 10.1172/JCI80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kong Y, Zhu L, Schell TD, Zhang J, Claxton DF, Ehmann WC, Rybka WB, George MR, Zeng H, Zheng H. T-Cell Immunoglobulin and ITIM Domain (TIGIT) Associates with CD8+ T-Cell Exhaustion and Poor Clinical Outcome in AML Patients. Clin Cancer Res. 2016;22(12):3057–66. doi: 10.1158/1078-0432.CCR-15-2626. [DOI] [PubMed] [Google Scholar]
- 97.Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, Eaton DL, Grogan JL. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell. 2014;26(6):923–37. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 98.Kurtulus S, Sakuishi K, Ngiow SF, Joller N, Tan DJ, Teng MW, Smyth MJ, Kuchroo VK, Anderson AC. TIGIT predominantly regulates the immune response via regulatory T cells. J Clin Invest. 2015;125(11):4053–62. doi: 10.1172/JCI81187. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 99.Stanietsky N, Rovis TL, Glasner A, Seidel E, Tsukerman P, Yamin R, Enk J, Jonjic S, Mandelboim O. Mouse TIGIT inhibits NK-cell cytotoxicity upon interaction with PVR. Eur J Immunol. 2013;43(8):2138–50. doi: 10.1002/eji.201243072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Purdy AK, Campbell KS. Natural killer cells and cancer: regulation by the killer cell Ig-like receptors (KIR) Cancer Biol Ther. 2009;8(23):2211–20. doi: 10.4161/cbt.8.23.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Anfossi N, Andre P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, Romagne F, Ugolini S, Vivier E. Human NK cell education by inhibitory receptors for MHC class I. Immunity. 2006;25(2):331–42. doi: 10.1016/j.immuni.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 102.Hoglund P, Brodin P. Current perspectives of natural killer cell education by MHC class I molecules. Nature reviews immunology. 2010;10(10):724–34. doi: 10.1038/nri2835. [DOI] [PubMed] [Google Scholar]
- 103.Elliott JM, Yokoyama WM. Unifying concepts of MHC-dependent natural killer cell education. Trends Immunol. 2011;32(8):364–72. doi: 10.1016/j.it.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Stern M, de Angelis C, Urbani E, Mancusi A, Aversa F, Velardi A, Ruggeri L. Natural killer-cell KIR repertoire reconstitution after haploidentical SCT. Bone Marrow Transplant. 2010;45(11):1607–10. doi: 10.1038/bmt.2010.19. [DOI] [PubMed] [Google Scholar]
- 105.Vey N, Bourhis JH, Boissel N, Bordessoule D, Prebet T, Charbonnier A, Etienne A, Andre P, Romagne F, Benson D, Dombret H, Olive D. A phase 1 trial of the anti-inhibitory KIR mAb IPH2101 for AML in complete remission. Blood. 2012;120(22):4317–23. doi: 10.1182/blood-2012-06-437558. [DOI] [PubMed] [Google Scholar]
- 106.Benson DM, Jr, Hofmeister CC, Padmanabhan S, Suvannasankha A, Jagannath S, Abonour R, Bakan C, Andre P, Efebera Y, Tiollier J, Caligiuri MA, Farag SS. A phase 1 trial of the anti-KIR antibody IPH2101 in patients with relapsed/refractory multiple myeloma. Blood. 2012;120(22):4324–33. doi: 10.1182/blood-2012-06-438028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Korde N, Carlsten M, Lee MJ, Minter A, Tan E, Kwok M, Manasanch E, Bhutani M, Tageja N, Roschewski M, Zingone A, Costello R, Mulquin M, Zuchlinski D, Maric I, Calvo KR, Braylan R, Tembhare P, Yuan C, Stetler-Stevenson M, Trepel J, Childs R, Landgren O. A phase II trial of pan-KIR2D blockade with IPH2101 in smoldering multiple myeloma. Haematologica. 2014;99(6):e81–3. doi: 10.3324/haematol.2013.103085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carlsten M, Korde N, Kotecha R, Reger R, Bor S, Kazandjian D, Landgren O, Childs RW. Checkpoint Inhibition of KIR2D with the Monoclonal Antibody IPH2101 Induces Contraction and Hyporesponsiveness of NK Cells in Patients with Myeloma. Clin Cancer Res. 2016;22(21):5211–5222. doi: 10.1158/1078-0432.CCR-16-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nguyen S, Dhedin N, Vernant JP, Kuentz M, Al Jijakli A, Rouas-Freiss N, Carosella ED, Boudifa A, Debre P, Vieillard V. NK-cell reconstitution after haploidentical hematopoietic stem-cell transplantations: immaturity of NK cells and inhibitory effect of NKG2A override GvL effect. Blood. 2005;105(10):4135–42. doi: 10.1182/blood-2004-10-4113. [DOI] [PubMed] [Google Scholar]
- 110.Picardi A, Mengarelli A, Marino M, Gallo E, Benevolo M, Pescarmona E, Cocco R, Fraioli R, Tremante E, Petti MC, De Fabritiis P, Giacomini P. Up-regulation of activating and inhibitory NKG2 receptors in allogeneic and autologous hematopoietic stem cell grafts. J Exp Clin Cancer Res. 2015;34:98. doi: 10.1186/s13046-015-0213-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ghasemzadeh M, Hosseini E, Schwarer AP, Pourfathollah AA. NK cell maturation to CD56(dim) subset associated with high levels of NCRs overrides the inhibitory effect of NKG2A and recovers impaired NK cell cytolytic potential after allogeneic hematopoietic stem cell transplantation. Leuk Res. 2016;43:58–65. doi: 10.1016/j.leukres.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 112.Gleason MK, Lenvik TR, McCullar V, Felices M, O’Brien MS, Cooley SA, Verneris MR, Cichocki F, Holman CJ, Panoskaltsis-Mortari A, Niki T, Hirashima M, Blazar BR, Miller JS. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood. 2012;119(13):3064–72. doi: 10.1182/blood-2011-06-360321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ, Asahina H, Jones RE, Kulkarni MM, Kuraguchi M, Palakurthi S, Fecci PE, Johnson BE, Janne PA, Engelman JA, Gangadharan SP, Costa DB, Freeman GJ, Bueno R, Hodi FS, Dranoff G, Wong KK, Hammerman PS. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Andrews LP, Marciscano AE, Drake CG, Vignali DAA. LAG3 (CD223) as a cancer immunotherapy target. Immunological Reviews. 2017;276(1):80–96. doi: 10.1111/imr.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vivier E, Nunes JA, Vely F. Natural killer cell signaling pathways. Science. 2004;306(5701):1517–9. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 116.Fehniger TA, Shah MH, Turner MJ, VanDeusen JB, Whitman SP, Cooper MA, Suzuki K, Wechser M, Goodsaid F, Caligiuri MA. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162(8):4511–20. [PubMed] [Google Scholar]
- 117.Romee R, Leong JW, Fehniger TA. Utilizing cytokines to function-enable human NK cells for the immunotherapy of cancer. Scientifica (Cairo) 2014;2014:205796. doi: 10.1155/2014/205796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97(1):14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 119.Fehniger TA, Cooper MA, Caligiuri MA. Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth Factor Rev. 2002;13(2):169–83. doi: 10.1016/s1359-6101(01)00021-1. [DOI] [PubMed] [Google Scholar]
- 120.Waldmann TA. The biology of IL-15: implications for cancer therapy and the treatment of autoimmune disorders. J Investig Dermatol Symp Proc. 2013;16(1):S28–30. doi: 10.1038/jidsymp.2013.8. [DOI] [PubMed] [Google Scholar]
- 121.Caligiuri MA, Zmuidzinas A, Manley TJ, Levine H, Smith KA, Ritz J. Functional consequences of interleukin 2 receptor expression on resting human lymphocytes. Identification of a novel natural killer cell subset with high affinity receptors. J Exp Med. 1990;171(5):1509–26. doi: 10.1084/jem.171.5.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nagler A, Lanier LL, Phillips JH. Constitutive expression of high affinity interleukin 2 receptors on human CD16-natural killer cells in vivo. J Exp Med. 1990;171(5):1527–33. doi: 10.1084/jem.171.5.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rosenberg SA. Interleukin-2 and the development of immunotherapy for the treatment of patients with cancer. Cancer J Sci Am. 2000;6(Suppl 1):S2–7. [PubMed] [Google Scholar]
- 124.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6(8):595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 125.Burns LJ, Weisdorf DJ, DeFor TE, Vesole DH, Repka TL, Blazar BR, Burger SR, Panoskaltsis-Mortari A, Keever-Taylor CA, Zhang MJ, Miller JS. IL-2-based immunotherapy after autologous transplantation for lymphoma and breast cancer induces immune activation and cytokine release: a phase I/II trial. Bone Marrow Transplant. 2003;32(2):177–86. doi: 10.1038/sj.bmt.1704086. [DOI] [PubMed] [Google Scholar]
- 126.Kennedy-Nasser AA, Ku S, Castillo-Caro P, Hazrat Y, Wu MF, Liu H, Melenhorst J, Barrett AJ, Ito S, Foster A, Savoldo B, Yvon E, Carrum G, Ramos CA, Krance RA, Leung K, Heslop HE, Brenner MK, Bollard CM. Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin Cancer Res. 2014;20(8):2215–25. doi: 10.1158/1078-0432.CCR-13-3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rosenberg SA. Interleukin 2 for patients with renal cancer. Nat Clin Pract Oncol. 2007;4(9):497. doi: 10.1038/ncponc0926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Buchbinder EI, Gunturi A, Perritt J, Dutcher J, Aung S, Kaufman HL, Ernstoff MS, Miletello GP, Curti BD, Daniels GA, Patel SP, Kirkwood JM, Hallmeyer S, Clark JI, Gonzalez R, Richart JM, Lutzky J, Morse MA, Sullivan RJ, McDermott DF. A retrospective analysis of High-Dose Interleukin-2 (HD IL-2) following Ipilimumab in metastatic melanoma. J Immunother Cancer. 2016;4:52. doi: 10.1186/s40425-016-0155-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Davis ZB, Felices M, Verneris MR, Miller JS. Natural Killer Cell Adoptive Transfer Therapy: Exploiting the First Line of Defense Against Cancer. Cancer J. 2015;21(6):486–91. doi: 10.1097/PPO.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Berger C, Berger M, Hackman RC, Gough M, Elliott C, Jensen MC, Riddell SR. Safety and immunologic effects of IL-15 administration in nonhuman primates. Blood. 2009;114(12):2417–26. doi: 10.1182/blood-2008-12-189266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Felices M, Chu S, Kodal B, Bendzick L, Ryan C, Lenvik AJ, Boylan KL, Wong HC, Skubitz AP, Miller JS, Geller MA. IL-15 super-agonist (ALT-803) enhances natural killer (NK) cell function against ovarian cancer. Gynecol Oncol. 2017 doi: 10.1016/j.ygyno.2017.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu B, Kong L, Han K, Hong H, Marcus WD, Chen X, Jeng EK, Alter S, Zhu X, Rubinstein MP, Shi S, Rhode PR, Cai W, Wong HC. A Novel Fusion of ALT-803 (Interleukin (IL)-15 Superagonist) with an Antibody Demonstrates Antigen-specific Antitumor Responses. J Biol Chem. 2016;291(46):23869–23881. doi: 10.1074/jbc.M116.733600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cheng M, Chen Y, Xiao W, Sun R, Tian Z. NK cell-based immunotherapy for malignant diseases. Cell Mol Immunol. 2013;10(3):230–52. doi: 10.1038/cmi.2013.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ravetch JV, Perussia B. Alternative membrane forms of Fc gamma RIII(CD16) on human natural killer cells and neutrophils. Cell type-specific expression of two genes that differ in single nucleotide substitutions. J Exp Med. 1989;170(2):481–97. doi: 10.1084/jem.170.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Selvaraj P, Carpen O, Hibbs ML, Springer TA. Natural killer cell and granulocyte Fc gamma receptor III (CD16) differ in membrane anchor and signal transduction. J Immunol. 1989;143(10):3283–8. [PubMed] [Google Scholar]
- 136.Perussia B, Ravetch JV. Fc gamma RIII (CD16) on human macrophages is a functional product of the Fc gamma RIII-2 gene. Eur J Immunol. 1991;21(2):425–9. doi: 10.1002/eji.1830210226. [DOI] [PubMed] [Google Scholar]
- 137.Klaassen RJ, Ouwehand WH, Huizinga TW, Engelfriet CP, von dem Borne AE. The Fc-receptor III of cultured human monocytes. Structural similarity with FcRIII of natural killer cells and role in the extracellular lysis of sensitized erythrocytes. J Immunol. 1990;144(2):599–606. [PubMed] [Google Scholar]
- 138.Nishikiori N, Koyama M, Kikuchi T, Kimura T, Ozaki M, Harada S, Saji F, Tanizawa O. Membrane-spanning Fc gamma receptor III isoform expressed on human placental trophoblasts. Am J Reprod Immunol. 1993;29(1):17–25. doi: 10.1111/j.1600-0897.1993.tb00832.x. [DOI] [PubMed] [Google Scholar]
- 139.Wirthmueller U, Kurosaki T, Murakami MS, Ravetch JV. Signal transduction by Fc gamma RIII (CD16) is mediated through the gamma chain. J Exp Med. 1992;175(5):1381–90. doi: 10.1084/jem.175.5.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lanier LL, Ruitenberg JJ, Phillips JH. Functional and biochemical analysis of CD16 antigen on natural killer cells and granulocytes. J Immunol. 1988;141(10):3478–85. [PubMed] [Google Scholar]
- 141.Vivier E, Morin P, O’Brien C, Druker B, Schlossman SF, Anderson P. Tyrosine phosphorylation of the Fc gamma RIII(CD16): zeta complex in human natural killer cells. Induction by antibody-dependent cytotoxicity but not by natural killing. J Immunol. 1991;146(1):206–10. [PubMed] [Google Scholar]
- 142.Bryceson YT, Ljunggren HG, Long EO. Minimal requirement for induction of natural cytotoxicity and intersection of activation signals by inhibitory receptors. Blood. 2009;114(13):2657–66. doi: 10.1182/blood-2009-01-201632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Laszlo A, Petri I, Ilyes M. Antibody dependent cellular cytotoxicity (ADCC)-reaction and an in vitro steroid sensitivity test of peripheral lymphocytes in children with malignant haematological and autoimmune diseases. Acta Paediatr Hung. 1986;27(1):23–9. [PubMed] [Google Scholar]
- 144.Natsume A, Niwa R, Satoh M. Improving effector functions of antibodies for cancer treatment: Enhancing ADCC and CDC. Drug Des Devel Ther. 2009;3:7–16. [PMC free article] [PubMed] [Google Scholar]
- 145.Shore SL, Nahmias AJ, Starr SE, Wood PA, McFarlin DE. Detection of cell-dependent cytotoxic antibody to cells infected with herpes simplex virus. Nature. 1974;251(5473):350–2. doi: 10.1038/251350a0. [DOI] [PubMed] [Google Scholar]
- 146.Albertini MR, Hank JA, Sondel PM. Native and genetically engineered anti-disialoganglioside monoclonal antibody treatment of melanoma. Cancer chemotherapy and biological response modifiers. 2005;22:789–97. doi: 10.1016/s0921-4410(04)22037-3. [DOI] [PubMed] [Google Scholar]
- 147.Garcia-Foncillas J, Diaz-Rubio E. Progress in metastatic colorectal cancer: growing role of cetuximab to optimize clinical outcome. Clinical & translational oncology : official publication of the Federation of Spanish Oncology Societies and of the National Cancer Institute of Mexico. 2010;12(8):533–42. doi: 10.1007/s12094-010-0551-3. [DOI] [PubMed] [Google Scholar]
- 148.Garnock-Jones KP, Keating GM, Scott LJ. Trastuzumab: A review of its use as adjuvant treatment in human epidermal growth factor receptor 2 (HER2)-positive early breast cancer. Drugs. 2010;70(2):215–39. doi: 10.2165/11203700-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 149.Navid F, Santana VM, Barfield RC. Anti-GD2 antibody therapy for GD2-expressing tumors. Current cancer drug targets. 2010;10(2):200–9. doi: 10.2174/156800910791054167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Winter MC, Hancock BW. Ten years of rituximab in NHL. Expert opinion on drug safety. 2009;8(2):223–35. doi: 10.1517/14740330902750114. [DOI] [PubMed] [Google Scholar]
- 151.Congy-Jolivet N, Bolzec A, Ternant D, Ohresser M, Watier H, Thibault G. Fc gamma RIIIa expression is not increased on natural killer cells expressing the Fc gamma RIIIa-158V allotype. Cancer Res. 2008;68(4):976–80. doi: 10.1158/0008-5472.CAN-07-6523. [DOI] [PubMed] [Google Scholar]
- 152.Masters GA, Krilov L, Bailey HH, Brose MS, Burstein H, Diller LR, Dizon DS, Fine HA, Kalemkerian GP, Moasser M, Neuss MN, O’Day SJ, Odenike O, Ryan CJ, Schilsky RL, Schwartz GK, Venook AP, Wong SL, Patel JD. Clinical cancer advances 2015: Annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2015;33(7):786–809. doi: 10.1200/JCO.2014.59.9746. [DOI] [PubMed] [Google Scholar]
- 153.Hermanson DL, Kaufman DS. Utilizing chimeric antigen receptors to direct natural killer cell activity. Front Immunol. 2015;6:195. doi: 10.3389/fimmu.2015.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Glienke W, Esser R, Priesner C, Suerth JD, Schambach A, Wels WS, Grez M, Kloess S, Arseniev L, Koehl U. Advantages and applications of CAR-expressing natural killer cells. Front Pharmacol. 2015;6:21. doi: 10.3389/fphar.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Holliger P, Hudson PJ. Engineered antibody fragments and the rise of single domains. Nat Biotechnol. 2005;23(9):1126–36. doi: 10.1038/nbt1142. [DOI] [PubMed] [Google Scholar]
- 156.Moore GL, Bautista C, Pong E, Nguyen DH, Jacinto J, Eivazi A, Muchhal US, Karki S, Chu SY, Lazar GA. A novel bispecific antibody format enables simultaneous bivalent and monovalent co-engagement of distinct target antigens. MAbs. 2011;3(6):546–57. doi: 10.4161/mabs.3.6.18123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009;157(2):220–33. doi: 10.1111/j.1476-5381.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Scott AM, Wolchok JD, Old LJ. Antibody therapy of cancer. Nat Rev Cancer. 2012;12(4):278–87. doi: 10.1038/nrc3236. [DOI] [PubMed] [Google Scholar]
- 159.Chen X, Zaro JL, Shen WC. Fusion protein linkers: property, design and functionality. Adv Drug Deliv Rev. 2013;65(10):1357–69. doi: 10.1016/j.addr.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jager V, Bussow K, Wagner A, Weber S, Hust M, Frenzel A, Schirrmann T. High level transient production of recombinant antibodies and antibody fusion proteins in HEK293 cells. BMC Biotechnol. 2013;13:52. doi: 10.1186/1472-6750-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nakayama H, Shimamoto N. Modern and simple construction of plasmid: saving time and cost. J Microbiol. 2014;52(11):891–7. doi: 10.1007/s12275-014-4501-6. [DOI] [PubMed] [Google Scholar]
- 162.Gibson DG, Benders GA, Andrews-Pfannkoch C, Denisova EA, Baden-Tillson H, Zaveri J, Stockwell TB, Brownley A, Thomas DW, Algire MA, Merryman C, Young L, Noskov VN, Glass JI, Venter JC, Hutchison CA, 3rd, Smith HO. Complete chemical synthesis, assembly and cloning of a Mycoplasma genitalium genome. Science. 2008;319(5867):1215–20. doi: 10.1126/science.1151721. [DOI] [PubMed] [Google Scholar]
- 163.Glorius P, Baerenwaldt A, Kellner C, Staudinger M, Dechant M, Stauch M, Beurskens FJ, Parren PW, Winkel JG, Valerius T, Humpe A, Repp R, Gramatzki M, Nimmerjahn F, Peipp M. The novel tribody [(CD20)(2)xCD16] efficiently triggers effector cell-mediated lysis of malignant B cells. Leukemia. 2013;27(1):190–201. doi: 10.1038/leu.2012.150. [DOI] [PubMed] [Google Scholar]
- 164.Kipriyanov SM, Cochlovius B, Schafer HJ, Moldenhauer G, Bahre A, Le Gall F, Knackmuss S, Little M. Synergistic antitumor effect of bispecific CD19 x CD3 and CD19 x CD16 diabodies in a preclinical model of non-Hodgkin’s lymphoma. J Immunol. 2002;169(1):137–44. doi: 10.4049/jimmunol.169.1.137. [DOI] [PubMed] [Google Scholar]
- 165.Kellner C, Bruenke J, Stieglmaier J, Schwemmlein M, Schwenkert M, Singer H, Mentz K, Peipp M, Lang P, Oduncu F, Stockmeyer B, Fey GH. A novel CD19-directed recombinant bispecific antibody derivative with enhanced immune effector functions for human leukemic cells. J Immunother. 2008;31(9):871–84. doi: 10.1097/CJI.0b013e318186c8b4. [DOI] [PubMed] [Google Scholar]
- 166.Kellner C, Bruenke J, Horner H, Schubert J, Schwenkert M, Mentz K, Barbin K, Stein C, Peipp M, Stockmeyer B, Fey GH. Heterodimeric bispecific antibody-derivatives against CD19 and CD16 induce effective antibody-dependent cellular cytotoxicity against B-lymphoid tumor cells. Cancer Lett. 2011;303(2):128–39. doi: 10.1016/j.canlet.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 167.Portner LM, Schonberg K, Hejazi M, Brunnert D, Neumann F, Galonska L, Reusch U, Little M, Haas R, Uhrberg M. T and NK cells of B cell NHL patients exert cytotoxicity against lymphoma cells following binding of bispecific tetravalent antibody CD19 x CD3 or CD19 x CD16, Cancer immunology. immunotherapy : CII. 2012;61(10):1869–75. doi: 10.1007/s00262-012-1339-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Johnson S, Burke S, Huang L, Gorlatov S, Li H, Wang W, Zhang W, Tuaillon N, Rainey J, Barat B, Yang Y, Jin L, Ciccarone V, Moore PA, Koenig S, Bonvini E. Effector cell recruitment with novel Fv-based dual-affinity re-targeting protein leads to potent tumor cytolysis and in vivo B-cell depletion. J Mol Biol. 2010;399(3):436–49. doi: 10.1016/j.jmb.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 169.Bruenke J, Barbin K, Kunert S, Lang P, Pfeiffer M, Stieglmaier K, Niethammer D, Stockmeyer B, Peipp M, Repp R, Valerius T, Fey GH. Effective lysis of lymphoma cells with a stabilised bispecific single-chain Fv antibody against CD19 and FcgammaRIII (CD16) Br J Haematol. 2005;130(2):218–28. doi: 10.1111/j.1365-2141.2005.05414.x. [DOI] [PubMed] [Google Scholar]
- 170.Schlenzka J, Moehler TM, Kipriyanov SM, Kornacker M, Benner A, Bahre A, Stassar MJ, Schafer HJ, Little M, Goldschmidt H, Cochlovius B. Combined effect of recombinant CD19 x CD16 diabody and thalidomide in a preclinical model of human B cell lymphoma. Anticancer Drugs. 2004;15(9):915–9. doi: 10.1097/00001813-200410000-00013. [DOI] [PubMed] [Google Scholar]
- 171.Schubert I, Kellner C, Stein C, Kugler M, Schwenkert M, Saul D, Stockmeyer B, Berens C, Oduncu FS, Mackensen A, Fey GH. A recombinant triplebody with specificity for CD19 and HLA-DR mediates preferential binding to antigen double-positive cells by dual-targeting. MAbs. 2012;4(1):45–56. doi: 10.4161/mabs.4.1.18498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Schubert I, Kellner C, Stein C, Kugler M, Schwenkert M, Saul D, Mentz K, Singer H, Stockmeyer B, Hillen W, Mackensen A, Fey GH. A single-chain triplebody with specificity for CD19 and CD33 mediates effective lysis of mixed lineage leukemia cells by dual targeting. MAbs. 2011;3(1):21–30. doi: 10.4161/mabs.3.1.14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Silla LM, Chen J, Zhong RK, Whiteside TL, Ball ED. Potentiation of lysis of leukaemia cells by a bispecific antibody to CD33 and CD16 (Fc gamma RIII) expressed by human natural killer (NK) cells. Br J Haematol. 1995;89(4):712–8. doi: 10.1111/j.1365-2141.1995.tb08406.x. [DOI] [PubMed] [Google Scholar]
- 174.Singer H, Kellner C, Lanig H, Aigner M, Stockmeyer B, Oduncu F, Schwemmlein M, Stein C, Mentz K, Mackensen A, Fey GH. Effective elimination of acute myeloid leukemic cells by recombinant bispecific antibody derivatives directed against CD33 and CD16. J Immunother. 2010;33(6):599–608. doi: 10.1097/CJI.0b013e3181dda225. [DOI] [PubMed] [Google Scholar]
- 175.Kugler M, Stein C, Kellner C, Mentz K, Saul D, Schwenkert M, Schubert I, Singer H, Oduncu F, Stockmeyer B, Mackensen A, Fey GH. A recombinant trispecific single-chain Fv derivative directed against CD123 and CD33 mediates effective elimination of acute myeloid leukaemia cells by dual targeting. Br J Haematol. 2010;150(5):574–86. doi: 10.1111/j.1365-2141.2010.08300.x. [DOI] [PubMed] [Google Scholar]
- 176.Bruenke J, Fischer B, Barbin K, Schreiter K, Wachter Y, Mahr K, Titgemeyer F, Niederweis M, Peipp M, Zunino SJ, Repp R, Valerius T, Fey GH. A recombinant bispecific single-chain Fv antibody against HLA class II and FcgammaRIII (CD16) triggers effective lysis of lymphoma cells. Br J Haematol. 2004;125(2):167–79. doi: 10.1111/j.1365-2141.2004.04893.x. [DOI] [PubMed] [Google Scholar]
- 177.Hombach A, Jung W, Pohl C, Renner C, Sahin U, Schmits R, Wolf J, Kapp U, Diehl V, Pfreundschuh M. A CD16/CD30 bispecific monoclonal antibody induces lysis of Hodgkin’s cells by unstimulated natural killer cells in vitro and in vivo. International journal of cancer. Journal international du cancer. 1993;55(5):830–6. doi: 10.1002/ijc.2910550523. [DOI] [PubMed] [Google Scholar]
- 178.Hartmann F, Renner C, Jung W, Deisting C, Juwana M, Eichentopf B, Kloft M, Pfreundschuh M. Treatment of refractory Hodgkin’s disease with an anti-CD16/CD30 bispecific antibody. Blood. 1997;89(6):2042–7. [PubMed] [Google Scholar]
- 179.Hartmann F, Renner C, Jung W, Pfreundschuh M. Anti-CD16/CD30 bispecific antibodies as possible treatment for refractory Hodgkin’s disease. Leukemia & lymphoma. 1998;31(3–4):385–92. doi: 10.3109/10428199809059232. [DOI] [PubMed] [Google Scholar]
- 180.Renner C, Pfreundschuh M. Treatment of heterotransplanted Hodgkin’s tumors in SCID mice by a combination of human NK or T cells and bispecific antibodies. Journal of hematotherapy. 1995;4(5):447–51. doi: 10.1089/scd.1.1995.4.447. [DOI] [PubMed] [Google Scholar]
- 181.Renner C, Hartmann F, Pfreundschuh M. Treatment of refractory Hodgkin’s disease with an anti-CD16/CD30 bispecific antibody. Cancer immunology, immunotherapy : CII. 1997;45(3–4):184–6. doi: 10.1007/s002620050428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Reiners KS, Kessler J, Sauer M, Rothe A, Hansen HP, Reusch U, Hucke C, Kohl U, Durkop H, Engert A, von Strandmann EP. Rescue of impaired NK cell activity in hodgkin lymphoma with bispecific antibodies in vitro and in patients. Mol Ther. 2013;21(4):895–903. doi: 10.1038/mt.2013.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Renner C, Hartmann F, Jung W, Deisting C, Juwana M, Pfreundschuh M. Initiation of humoral and cellular immune responses in patients with refractory Hodgkin’s disease by treatment with an anti-CD16/CD30 bispecific antibody. Cancer immunology, immunotherapy : CII. 2000;49(3):173–80. doi: 10.1007/s002620050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sahin U, Kraft-Bauer S, Ohnesorge S, Pfreundschuh M, Renner C. Interleukin-12 increases bispecific-antibody-mediated natural killer cell cytotoxicity against human tumors. Cancer immunology, immunotherapy : CII. 1996;42(1):9–14. doi: 10.1007/s002620050245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Arndt MA, Krauss J, Kipriyanov SM, Pfreundschuh M, Little M. A bispecific diabody that mediates natural killer cell cytotoxicity against xenotransplantated human Hodgkin’s tumors. Blood. 1999;94(8):2562–8. [PubMed] [Google Scholar]
- 186.da Costa L, Renner C, Hartmann F, Pfreundschuh M. Immune recruitment by bispecific antibodies for the treatment of Hodgkin disease. Cancer chemotherapy and pharmacology. 2000;(46 Suppl):S33–6. doi: 10.1007/pl00014047. [DOI] [PubMed] [Google Scholar]
- 187.Elsasser D, Stadick H, Stark S, Van de Winkel JG, Gramatzki M, Schrott KM, Valerius T, Schafhauser W. Preclinical studies combining bispecific antibodies with cytokine-stimulated effector cells for immunotherapy of renal cell carcinoma. Anticancer Res. 1999;19(2C):1525–8. [PubMed] [Google Scholar]
- 188.Ferrini S, Cambiaggi A, Sforzini S, Canevari S, Mezzanzanica D, Colnaghi MI, Moretta L. Use of anti-CD3 and anti-CD16 bispecific monoclonal antibodies for the targeting of T and NK cells against tumor cells. Cancer detection and prevention. 1993;17(2):295–300. [PubMed] [Google Scholar]
- 189.Weiner LM, Clark JI, Ring DB, Alpaugh RK. Clinical development of 2B1, a bispecific murine monoclonal antibody targeting c-erbB-2 and Fc gamma RIII. Journal of hematotherapy. 1995;4(5):453–6. doi: 10.1089/scd.1.1995.4.453. [DOI] [PubMed] [Google Scholar]
- 190.Weiner LM, Clark JI, Davey M, Li WS, Garcia de Palazzo I, Ring DB, Alpaugh RK. Phase Itrial of 2B1 a bispecific monoclonal antibody targeting c-erbB-2 and Fc gamma RIII. Cancer Res. 1995;55(20):4586–93. [PubMed] [Google Scholar]
- 191.Ferrini S, Prigione I, Miotti S, Ciccone E, Cantoni C, Chen Q, Colnaghi MI, Moretta L. Bispecific monoclonal antibodies directed to CD16 and to a tumor-associated antigen induce target-cell lysis by resting NK cells and by a subset of NK clones. International journal of cancer. Journal international du cancer. 1991;48(2):227–33. doi: 10.1002/ijc.2910480213. [DOI] [PubMed] [Google Scholar]
- 192.Gleason MK, Verneris MR, Todhunter DA, Zhang B, McCullar V, Zhou SX, Panoskaltsis-Mortari A, Weiner LM, Vallera DA, Miller JS. Bispecific and trispecific killer cell engagers directly activate human NK cells through CD16 signaling and induce cytotoxicity and cytokine production. Molecular cancer therapeutics. 2012;11(12):2674–84. doi: 10.1158/1535-7163.MCT-12-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Gleason MK, Ross JA, Warlick ED, Lund TC, Verneris MR, Wiernik A, Spellman S, Haagenson MD, Lenvik AJ, Litzow MR, Epling-Burnette PK, Blazar BR, Weiner LM, Weisdorf DJ, Vallera DA, Miller JS. CD16xCD33 bispecific killer cell engager (BiKE) activates NK cells against primary MDS and MDSC CD33+ targets. Blood. 2014;123(19):3016–26. doi: 10.1182/blood-2013-10-533398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wiernik A, Foley B, Zhang B, Verneris MR, Warlick E, Gleason MK, Ross JA, Luo X, Weisdorf DJ, Walcheck B, Vallera DA, Miller JS. Targeting Natural Killer Cells to Acute Myeloid Leukemia In Vitro with a CD16 × 33 Bispecific Killer Cell Engager and ADAM17 Inhibition. Clin Cancer Res. 2013;19(14):3844–3855. doi: 10.1158/1078-0432.CCR-13-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Vallera DA, Zhang B, Gleason MK, Oh S, Weiner LM, Kaufman DS, McCullar V, Miller JS, Verneris MR. Heterodimeric Bispecific Single-Chain Variable-Fragment Antibodies Against EpCAM and CD16 Induce Effective Antibody-Dependent Cellular Cytotoxicity Against Human Carcinoma Cells. Cancer Biother Radiopharm. 2013 doi: 10.1089/cbr.2012.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schmohl JU, Felices M, Todhunter D, Taras E, Miller JS, Vallera DA. Tetraspecific scFv construct provides NK cell mediated ADCC and self-sustaining stimuli via insertion of IL-15 as a cross-linker. Oncotarget. 2016;7(45):73830–73844. doi: 10.18632/oncotarget.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Schmohl JU, Felices M, Oh F, Lenvik AJ, Lebeau AM, Panyam J, Miller JS, Vallera DA. Engineering of Anti-CD133 Tri-Specific Molecule Capable of Inducing NK Expansion and Driving Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC) Cancer Res Treat. 2017 doi: 10.4143/crt.2016.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Vallera DA, Felices M, McElmurry R, McCullar V, Zhou X, Schmohl JU, Zhang B, Lenvik AJ, Panoskaltsis-Mortari A, Verneris MR, Tolar J, Cooley S, Weisdorf DJ, Blazar BR, Miller JS. IL15 Trispecific Killer Engagers (TriKE) Make Natural Killer Cells Specific to CD33+ Targets While Also Inducing Persistence, In Vivo Expansion, and Enhanced Function. Clin Cancer Res. 2016;22(14):3440–50. doi: 10.1158/1078-0432.CCR-15-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]