Abstract
Psychiatric research has been hampered by an explanatory gap between psychiatric symptoms and their neural underpinnings, which has resulted in poor treatment outcomes. This situation has prompted us to shift from symptom-based diagnosis to data-driven diagnosis, aiming to redefine psychiatric disorders as disorders of neural circuitry. Promising candidates for data-driven diagnosis include resting-state functional connectivity MRI (rs-fcMRI)-based biomarkers. Although biomarkers have been developed with the aim of diagnosing patients and predicting the efficacy of therapy, the focus has shifted to the identification of biomarkers that represent therapeutic targets, which would allow for more personalized treatment approaches. This type of biomarker (i.e., “theranostic biomarker”) is expected to elucidate the disease mechanism of psychiatric conditions and to offer an individualized neural circuit-based therapeutic target based on the neural cause of a condition. To this end, researchers have developed rs-fcMRI-based biomarkers and investigated a causal relationship between potential biomarkers and disease-specific behavior using functional MRI (fMRI)-based neurofeedback on functional connectivity. In this review, we introduce a recent approach for creating a theranostic biomarker, which consists mainly of 2 parts: (1) developing an rs-fcMRI-based biomarker that can predict diagnosis and/or symptoms with high accuracy, and (2) the introduction of a proof-of-concept study investigating the relationship between normalizing the biomarker and symptom changes using fMRI-based neurofeedback. In parallel with the introduction of recent studies, we review rs-fcMRI-based biomarker and fMRI-based neurofeedback, focusing on the technological improvements and limitations associated with clinical use.
Keywords: psychiatric disorder, resting-state functional connectivity, neurofeedback, theranostic biomarker
Introduction
Although great advancements in psychiatric research have been made in recent years, an explanatory gap between phenomenological entities and neurobiological underpinnings remains (Montague et al., 2012). This gap has prevented precise diagnosis and dramatic improvements in treatment outcomes in the field of clinical psychiatry (Insel and Cuthbert, 2015). Our lack of understanding of the disease mechanisms is reflected by the fact that the 2 world-wide standard psychiatric diagnosis systems—the Diagnostic and Statistical Manual of Mental Disorders (DSM) (American Psychiatric Association, 2013) and International Classification of Diseases (ICD) (World Health Organization, 1990)—adopt symptom-based approaches, in which underlying biological substrates are not taken into consideration (Insel and Cuthbert, 2009), except in the case of dementia and sleep disorder for which DSM-5 acknowledges biological measures such as genetic and neuroimaging testing as informative regarding diagnostic confirmation. Consequently, these symptom-based diagnostic systems may artificially draw distinctions among conditions that actually share common biological etiologies and therefore may fail to provide effective biology-based treatments directed toward specific pathogenic processes associated with these conditions (Owen, 2014). Therefore, the recent initiative of research domain criteria has proposed an important paradigm shift from the conventional symptom-based categories to data-driven dimensional approaches based on observable behaviors and neurobiological measures (Insel and Cuthbert, 2009), with the aim of eliminating the gap between disease-related behaviors and neurobiological substrates. In this review, we first explain that brain functional measures provided by fMRI, particularly the resting-state functional connectivity (rs-fc) MRI, play crucial roles for the development of “biomarkers” that provide dimensions along which various psychiatric disorders could be defined. Then, we illustrate the potential and power of the fMRI- and biomarker-based neurofeedback methods in the treatments of disease-related behaviors. By illustrating the development of rs-fcMRI-based biomarkers and fMRI-based neurofeedback, we claim that these 2 lines of new research converge in filling the abovementioned gap.
Towards Development of Theranostic Biomarker for Psychiatric Disorder
Recent psychiatric neuroimaging research has begun to bridge the explanatory gap by redefining psychiatric disorders as disorders of neural circuitry (Insel and Cuthbert, 2015). Indeed, rs-fcMRI represents a promising platform for identifying affected neural circuitry. Traditionally, alterations in neural circuitry have been studied by examining brain activation and/or functional connectivity (FC) during specific task conditions using a limited number of participants. However, more recent whole-brain rs-fcMRI studies have applied state-of-the-art machine-learning algorithms to “big data” to identify brain features that predict the diagnostic status and/or severity of psychiatric disease (Clementz et al., 2016; Yahata et al., 2016, 2017; Arbabshirani et al., 2017). Since brain features are identified in a data-driven manner, this approach is free of potential biases that may derive from explicit hypotheses regarding affected brain regions, FCs, or functions. Furthermore, relative to task-based fMRI, rs-fcMRI is more suited to the clinical investigation of patients and young children who have difficulty in performing tasks (Poldrack and Farah, 2015). In addition, previous studies have shown that resting brain signals generate highly structured spatiotemporal patterns that correspond well to those observed when performing tasks (Smith et al., 2009; Laird et al., 2011). These signals predict brain activation evoked by several kinds of tasks at the level of individual participant (Tavor et al., 2016). These studies indicate that rs-fcMRI data may include abundant information regarding individual characteristics and that these data can therefore be used as a substitute for task-based fMRI data. Together with the relative simplicity and low variability in data acquisition setups, rs-fcMRI serves as the platform by which large amounts of clinical data can be analyzed to develop appropriate machine-learning algorithms.
Many studies along this line of psychiatric research share the goal of identifying biological measures of altered neural circuitry that represent “biomarkers” for psychiatric disorders (Perlis, 2011; Abi-Dargham and Horga, 2016). Indeed, to date, a number of structural and functional MRI studies have claimed to have identified such “biomarkers” for various psychiatric disorders (Fan et al., 2008; Sun et al., 2009; Kim et al., 2010; Sui et al., 2015; Ivleva et al., 2016; Kambeitz et al., 2016; Drysdale et al., 2017; Li et al., 2017). However, the significance of these identified biomarkers varies greatly depending on study aims and designs. Here, we propose that so-called “biomarkers” should be categorized into the following 4 types: (1) biomarker “candidates” that correlate with diagnosis in a sample pool, (2) those that generalize over the studied samples and therefore predict diagnosis of a disease of interest in a general population, (3) those that predict the effect of a therapy (i.e., surrogate endpoint), and (4) those that correspond to the disease mechanism and may therefore be regarded as therapeutic targets. Biomarker types 1 and 2 are similar in that the measure simply represents a correlation with the disease status, though they are critically different regarding whether the scope of the biomarker is limited to the sample dataset (1) or has the capacity to generalize over the disease of interest in general, beyond the sample (2). In this sense, only biomarker types 2 to 4 qualify as true “biomarkers” (Abi-Dargham and Horga, 2016). While biomarker types 2 and 3 can be used as an auxiliary test in clinical practice and are expected to provide important information regarding the diagnosis and treatment strategy, the clinical importance of these 2 types of biomarkers does not necessarily indicate that these measures account for the disease mechanism. For instance, low-density lipoprotein (LDL) cholesterol has been used as a surrogate marker for a clinically meaningful endpoint for the heart disease. However, lowering LDL cholesterol does not necessarily lead to the prevention of heart disease at the individual level, because the density of LDL cholesterol may not be associated with the underlying cause of heart disease (Albert, 2011). In contrast, when the biomarker corresponds to elements of the disease mechanism as in type 4, its significance is 2-fold: as a measure of diagnostic status and/or the severity of symptoms, and as a therapeutic target. Therefore, we refer to this type of biomarker as a “theranostic biomarker” (Yahata et al., 2017). The development of such theranostic biomarkers will result in breakthroughs not only in basic biological research but also in clinical psychiatry practice, providing patients with individually tailored therapeutic targets and allowing for the elimination of unnecessary treatments and adverse effects (Ahn, 2016).
Hereafter, we introduce several recent studies that have suggested that some rs-fcMRI-based biomarkers satisfy prerequisites for type 2, 3, and even 4 biomarkers for major psychiatric disorders. That is, these biomarkers may explain elements of disease mechanisms and identify a therapeutic target for a range of neuromodulation interventions, including neuropharmacology, repetitive transcranial magnetic stimulation, and neurofeedback. To strictly verify that the rs-fcMRI-based biomarker represents the disease mechanism, the following 3 levels of evidence are necessary: (1) The rs-fcMRI-based biomarker predicts diagnostic status and/or the severity of symptoms with high accuracy for the general population of a disease of interest; (2) normalization of the biomarker via neuromodulation interventions leads to the alleviation of symptoms; and (3) alterations of neural circuits that are represented by the biomarker are caused by a whole range of known risk factors for the disease of interest, including genes, molecules, cells, circuits, cognition, behavior, and the physical and social environments. However, it is very difficult to provide the third level of evidence due to the limited datasets obtained in human studies. Consequently, we discuss the first 2 levels of evidence to examine whether rs-fcMRI-based biomarkers can act as theranostic biomarkers for psychiatric disorders. Concretely, we first introduce the development of the rs-fcMRI-based biomarkers for autism spectrum disorder (ASD), major depressive disorder (MDD), schizophrenia (SCZ), and obsessive compulsive disorder (OCD)—which utilized state-of-the-art machine-learning algorithms that achieved high classification accuracy and generalized well for independent validation cohorts. Secondly, we introduce the preliminary results of recent proof-of-concept studies that have examined whether the normalization of rs-fcMRI-based biomarker can be achieved via fMRI-based neurofeedback on FC and whether such normalization leads to the improvement of symptoms in depression. In addition, we also refer to technical difficulties in the development of rs-fcMRI-based biomarkers and fMRI-based neurofeedback and discuss recent advances in overcoming these challenges.
Rs-Fcmri-Based Biomarker for Psychiatric Disorder
The Importance of rs-fcMRI-Based Biomarkers for Psychiatric Disorders
The brain generates highly structured spatiotemporal patterns even in the absence of explicit task execution (i.e., under resting-state conditions) (Smith et al., 2009; Laird et al., 2011). This finding suggests that rich information may be decoded by applying machine-learning algorithms to rs-fcMRI data in the individual brain. Indeed, a series of studies has successfully used such algorithms to predict various characteristics in healthy individuals, including age (Dosenbach et al., 2010), intelligence (Smith et al., 2015), working memory (Yamashita et al., 2015), and sustained attention (Rosenberg et al., 2016). Based on these successful applications, a growing number of studies have sought to develop rs-fcMRI-based biomarkers for various psychiatric disorders (Arbabshirani et al., 2017), such as ASD (Anderson et al., 2011), MDD (Drysdale et al., 2017), SCZ (Kaufmann et al., 2015), and ADHD (Deshpande et al., 2015).
The Generalization Ability of rs-fcMRI-Based Biomarkers
A number of rs-fcMRI-based biomarker studies have claimed high accuracy in discrimination between individuals with a disease of interest and healthy controls (HCs) for most major psychiatric disorders. However, to date, no such biomarkers have been identified for use in routine clinical practice. Aside from issues related to economic and practical feasibility in clinical settings, one major issue with previously developed biomarkers is that accuracy in discrimination of the biomarker is validated only for a single sample cohort that is shared with the training of the biomarker. Therefore, the generalizability of the biomarker is usually untested beyond the sample dataset, and highly accurate discrimination is likely to fail when that biomarker is applied to an independent cohort. More specifically, if the developed biomarker is fitted to noise structures that are specific to the training dataset (e.g., demographic distributions and measurement conditions such as the type of MRI scan protocol), the prediction is inflated for the training data but catastrophic to the independent validation dataset, which does not contain the same noise structure (Whelan and Garavan, 2014; Huys et al., 2016; Yahata et al., 2017).
To develop clinically meaningful rs-fcMRI-based biomarkers, it is necessary to prove the generalizability of the biomarker using independent datasets as validation cohorts. For this step to be successful, the development of optimal machine-learning algorithms that alleviate overfitting to the noise structures of the training data is critical. Such overfitting often occurs when a large number of parameters are included relative to the number of participants, and when the model does not sufficiently remove the effect of nuisance variables that are included in training dataset (Whelan and Garavan, 2014; Yahata et al., 2017). Therefore, for the model to be reliable, the number of parameters in the model should be reduced based on the number of participants, and the brain features that reflect disease-related factors (e.g., diagnostic status and symptom severity) should be extracted after removing the data-specific noise structure. In the following section, we review the development of rs-fcMRI-based biomarkers that satisfy the aforementioned conditions for ASD (Yahata et al., 2016), MDD (Ichikawa et al., 2017), SCZ (Yoshihara et al., 2017), and OCD (Takagi et al., 2017). In illustrating these cases, we show that the overfitting problem was successfully alleviated by the development of novel machine-learning algorithms, which resulted in the identification of a small number of altered FCs capable of discriminating between individuals with a specific medical condition of interest and HCs or typically developed controls (TDs). The resultant biomarker has achieved high accuracy for a discovery cohort (i.e., training data) together with good generalizability for independent validation cohorts (i.e., test data).
The rs-fcMRI-Based Biomarker for ASD
Although it is generally believed that abnormal FCs may underlie ASD (Menon, 2011), whether such abnormalities involve underconnectivity, overconnectivity, or distance-dependent alterations (Just et al., 2012; Supekar et al, 2013; Long et al., 2016) remains unknown. Several research groups have attempted to solve this problem by developing rs-fcMRI-based biomarkers. However, none of these biomarkers has been validated in an independent cohort (Anderson et al., 2011). One study that attempted to validate the generalizability of the biomarker observed poor performance below chance in an independent cohort (Yoshihara et al., 2011). Among these unsatisfactory attempts to develop an rs-fcMRI biomarker for ASD, Yahata et al. (2016), aimed to achieve a desired level of generalizability by controlling the 2 causes of overfitting: the number of parameters in the model and the interference of nuisance variables. Specifically, they developed a unique combination of machine-learning algorithms of L1 regularized sparse canonical correlation analysis (L1-SCCA) followed by sparse logistic regression (SLR; Yamashita et al., 2008). Briefly, in this algorithm, L1-SCCA was applied to extract FC features associated with diagnostic labels (e.g., ASD or TD), while removing FC features associated with nuisance variables (e.g., age, sex, medication, scan protocol). Then, sparse estimation performed by L1-SCCA and SLR reduced the number of explanatory variables (i.e., FCs) in the biomarker. Therefore, the combination of L1-SCCA and SLR is highly suited for controlling the aforementioned 2 causes of over-fitting inherent to machine-learning studies using multicenter rs-fcMRI data.
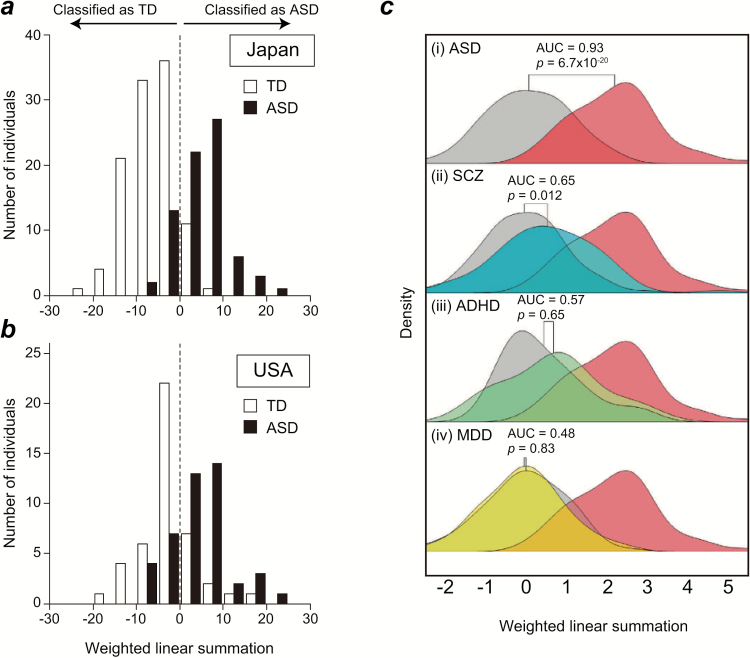
Yahata et al. (2016) applied this novel machine-learning algorithm to rs-fcMRI data from 74 high-functioning adults with ASD and 107 TD adults obtained from 3 different sites in Japan. FC data in each individual were analyzed as a correlation matrix representing the Pearson correlation values for 9730 pairs of time-series data extracted from 140 regions in the sulci-based anatomical atlas (extended Brainvisa Sulci Atlas; Perrot et al., 2011). Using the correlation matrices of 181 individuals as inputs, the machine-learning algorithm of L1-SCCA and SLR generated a classifier consisted of only 16 FCs (0.2% of all FCs) that distinguished between ASDs and TD with a high accuracy of 85% and an area under the curve (AUC) of 0.93 (Figure 1a).
Figure 1.
Distribution of weighted linear summations (WLS) calculated by functional connections. (a) The white and black bars denote the number of typically developing (TD) and autism spectrum disorder (ASD) individuals in the Japanese dataset, respectively. A horizontal axis denotes WLS score. If the WLS score is positive, an individual is classified as having ASD, while a negative WLS score indicates TD. (b) A histogram shows the distribution of WLS scores for the US ABIDE dataset. (c) The density distribution of WLS when applying the ASD classifier to various psychiatric conditions, such as ASD, schizophrenia (SCZ), ADHD, and major depressive disorder (MDD). In each panel, TD/HC distribution is gray and ASD distribution is red. The distribution of other psychiatric conditions (i.e., SCZ, ADHD, and MDD) is colored with blue, green, and yellow, respectively. Area under the curve (AUC) values are based on the classification between each psychiatric condition and TD/HC. P values are obtained by the Benjamini-Hochberg-corrected Kolmogorov-Smirnov test. The TD distribution of WLS at each panel is adjusted to have the same median and SD for the visualization purpose. Adapted, with permission, from Figures 1 and 5 in Yahata et al. A small number of abnormal connections predicts adult autism spectrum disorder. Nature Communications, DOI: 10.1038/ncomms11254 (2016).
Because the biomarker for ASD was developed using Japanese datasets only, it must be validated using independent cohort datasets, which, in this study, were collected in countries with different cultural and ethnic backgrounds than those in Japan. Therefore, the US ABIDE dataset was selected as an independent cohort (Di Martino et al., 2014), which consisted of 44 high-functioning adults with ASD and 44 demographically matched TD controls. Indeed, the biomarker developed in Japan generalized well and exhibited a high classification accuracy of 75% (AUC=0.76) (Figure 1b). To our knowledge, this is the first study to demonstrate high generalizability for an independent cohort across cultures and ethnicities. The generalizability of the biomarker was further confirmed in a second independent cohort collected in Japan (accuracy=70%, AUC=0.77). Lastly, the selected FCs used in the developed biomarker predicted with high accuracy not only diagnostic status but also the severity of communication problems, based on communication domain scores of the Autism Diagnostic Observation Schedule (Lord et al., 2000) (r = 0.44, P < .001). These results indicate that, with the proper use of machine-learning algorithms for controlling over-fitting, we are able to develop a reliable biomarker from the rs-fcMRI data that predicts ASD with high accuracy.
Further, we applied this ASD biomarker to other psychiatric disorders (SCZ, ADHD, and MDD) to investigate whether the selected FCs could discriminate patients with these psychiatric disorders from HCs. That is, we aimed to determine whether the biomarker is specific to ASD diagnosis. Our results indicated that this biomarker could not significantly differentiate individuals with ADHD or MDD from their respective controls (ADHD: AUC = 0.57, P =.65, MDD: AUC = 0.48, P = .83), although moderate differentiation of those with SCZ was observed (AUC = 0.65, P = .012) (Figure 1c). This modest generalizability of the constructed ASD biomarker only to SCZ indicates that the weighted summation of the extracted FCs for the biomarker may reflect the extent of “ASD-ness,” or more precisely liability of ASD, as individuals throughout any population—including those with other psychiatric conditions—may possess ASD-like traits (Yahata et al., 2016). This speculation is biologically plausible considering the evidence that ASD is closer to SCZ than ADHD and MDD in terms of genetic, behavioral, and neuroimaging findings (King and Lord, 2011; Cross-Disorder Group of the Psychiatric Genomics Consortium, 2013).
The Generalizable rs-fcMRI-Based Biomarkers for Depression, Schizophrenia, and Obsessive Compulsive Disorder
The classifier construction algorithm developed in Yahata et al. (2016) was applied to patients with melancholic depression, SCZ, and OCD. Ichikawa et al. (2017) restricted the training samples to individuals with melancholic depression (n = 66) and demographically matched HCs with Beck Depression Inventory-II scores ≤ 10 (Beck et al., 1996) (n = 66) to avoid issues in the heterogeneity of depression (CONVERGE consortium, 2015). The resulting classifier identified 12 diagnosis-specific FCs (of 9316 connections) and achieved high discriminability (AUC = 0.77, accuracy = 70%) in the training dataset and high generalizability (AUC = 0.62, accuracy=65%) to the independent validation cohort (i.e., test data), which included individuals with melancholic depression (n = 11) and HCs (n = 40). (These HCs were matched based on Beck Depression Inventory-II score to those in the training dataset.)
A biomarker for SCZ (AUC = 0.83, accuracy = 76%) using a Japanese training dataset consisting of patients in the chronic stage (duration of illness = 12.8 ± 7.8 years), scanned at 2 imaging sites in Japan, was also developed (Yujiro Yoshihara, personal communication, March 30, 2017). The SCZ biomarker also worked well for 2 independent validation cohorts of patients in the chronic stage (United States site 1: duration of illness = 14.2 ± 11.5 years, AUC = 0.75, accuracy = 70%, EU site: duration of illness = 5.9 ± 5.8 years, AUC = 0.66, accuracy = 61%), but not for a test dataset of patients experiencing their first episode (United States site 2: duration of illness = 16.8 ± 9.6 months, AUC = 0.42, accuracy = 45%). These findings suggest that each stage of SCZ is associated with specific pathological processes manifested as differentially altered FCs, in accordance with the findings of previous studies (Anticevic et al., 2015; Li et al., 2016).
For the discrimination of OCD, Takagi et al. (2017) adopted the methodology for developing rs-fcMRI-based biomarkers described by Yahata et al. (2016) in conjunction with principal component analysis for feature selection of FCs, partly because a small training dataset from a single imaging site was available. All principal components were analyzed using the aforementioned algorithm (L1-SCCA and SLR). The biomarker for the training dataset (nOCD = 52, nHC = 56) exhibited high accuracy (AUC: 0.81, accuracy: 73%), while that for the test dataset (nOCD = 18, nHC = 10) exhibited high generalizability (AUC: 0.70).
Neurofeedback
fMRI-Based Neurofeedback
Neurofeedback is an auxiliary technique for self-regulating the neural activity that underpins specific behaviors or symptoms by providing participants with real-time feedback that represents the current activity state of the neural activity of interest (Sitaram et al., 2016; Thibault et al., 2016). In contrast to other neuromodulation methods that rely on externally applied factors (e.g., electromagnetic field and pharmacological agents), neurofeedback is a method of internally (either volitionally or conditionally) regulating neural activity. As such, this method provides a means to aid participants to learn to induce brain activity toward a desired pattern of neural activity relying only on participants’ own endogenous factors. Among several neuroimaging modalities including electroencephalography (EEG) and functional near-infrared spectroscopy, fMRI-based neurofeedback has attracted considerable attention for its potential as a novel method of therapeutic treatment in clinical neuroscience (Fovet et al., 2015; Morimoto and Kawato, 2015). To date, several studies have applied fMRI-based neurofeedback methods to psychiatric patients by training them to upregulate or downregulate the level of activation in single or multiple regions-of-interest (ROI) (Sitaram et al., 2016). This fMRI-based neurofeedback method was shown to significantly alleviate symptoms in several conditions, including depression (Linden et al., 2012), subclinical OCD (Scheinost et al., 2013), ADHD (Zilverstand et al., 2017), and schizophrenia (Sitaram et al., 2014). In particular, strong evidence for therapeutic effect of this type of fMRI-based neurofeedback on MDD has been demonstrated utilizing a double-blind, placebo-controlled, randomized clinical paradigm (Young et al., 2017). This study demonstrated that patients with MDD learned to upregulate the amygdala activity, which resulted in larger decrease in depressive symptoms when they were assigned to the real neurofeedback condition, compared with a control condition where they were required to increase the intra-parietal activity. Besides the use of the randomized controlled trial design, this study has 2 additional characteristics that may deserve particular attention: (1) participants were unmedicated during a current episode, and (2) participants were asked to recall positive memory during the neurofeedback training for both experimental and control conditions; therefore, the cognitive strategy was controlled between conditions. These characteristics of the carefully designed study strengthened the conclusion that fMRI-based neurofeedback is effective for the treatment of MDD. Furthermore, recent simultaneous EEG and fMRI neurofeedback studies have suggested that amygdala activity change induced by the fMRI-based neurofeedback may be achieved by a more accessible EEG neurofeedback (Keynan et al., 2016; Zotev et al., 2016). These studies demonstrated that amygdala blood oxygen level-dependent signal could be successfully predicted by EEG. In one of these studies, based on the prediction model, they performed EEG neurofeedback training to decrease amygdala activity and showed that the neurofeedback helped participants downregulate the target blood oxygen level-dependent signal and improve implicit emotion regulation.
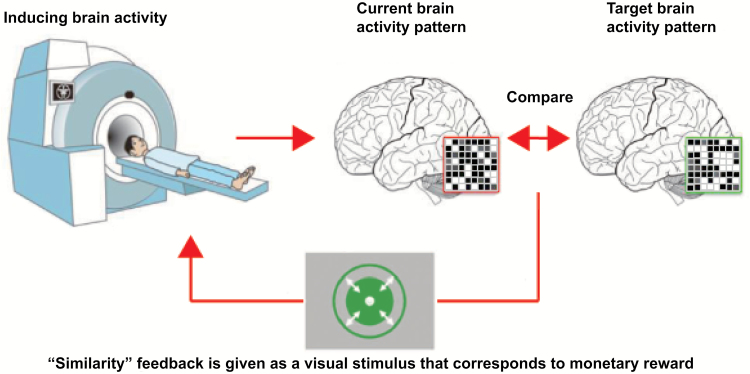
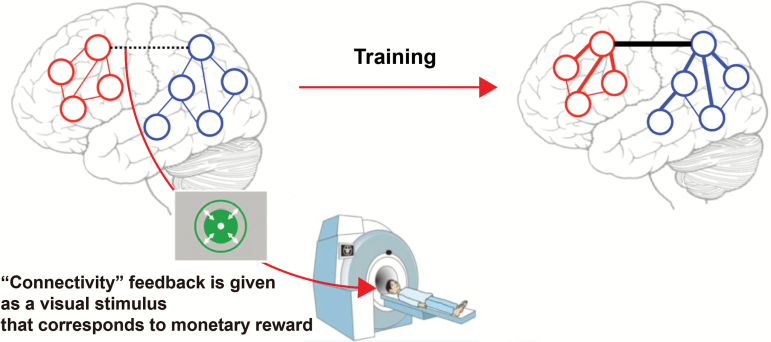
In addition to the conventional fMRI-based neurofeedback that aims to manipulate the level of activation in predefined ROIs, recent advances in fMRI data acquisition and analysis have enabled us to evaluate fMRI signals with greater spatial and temporal precision in real-time. This technological advance has yielded 2 novel lines of fMRI-based neurofeedback: decoded neurofeedback (DecNef) and functional-connectivity-based neurofeedback (FCNef). DecNef is a novel method of controlling distributed activity patterns of multiple voxels within a circumscribed ROI (Shibata et al., 2011) (Figure 2), thereby greatly increasing spatial precision over the previous method of regulation of localized activity in coarsely defined ROIs. In contrast, FCNef allows for the modulation of spatiotemporal activation patterns across multiple ROIs (Koush et al., 2013, 2015) as well as intrinsic functional networks (Megumi et al., 2015) (Figure 3). In the following sections, we review recent successful applications of DecNef and FCNef, which, in our view, have potential as novel interventions for the treatment of psychiatric disorders via powerful regulation of neural activity (Yanagisawa et al., 2016; Sakai, 2015).
Figure 2.
The procedure of decoded neurofeedback (DecNef). During training, participants were instructed to self-regulate brain activity to maximize the feedback score. This was represented by, for example, the size of a green disc, which corresponded to the participant’s success in inducing a current brain activity pattern as similar as possible to the target brain activity pattern.
Figure 3.
The procedure of functional connectivity (FC)-based neurofeedback (FCNef). During training, participants were instructed to self-regulate brain activity to maximize the feedback score. This was represented by, for example, the size of a green disc, which reflected the degree of success in achieving target FC.
DecNef
DecNef is a novel neurofeedback method in which participants learn to induce specific activation patterns of multiple voxels in a given brain region. This neurofeedback method is based on recently developed fMRI decoding techniques (Kamitani and Tong, 2005), which allow researchers to infer the mental experiences and states of participants via analysis of multi-voxel patterns of activation. DecNef is thus based on the assumption that, by determining a particular multi-voxel pattern as a target pattern that corresponds to a specific mental experience or state, experimenters can calculate the similarity between the target and the current multi-voxel pattern online and return it to participants as a feedback score. DecNef studies are usually composed of 3 types of experiments: (1) pre- and postbehavioral tests, (2) fMRI decoder construction, and (3) DecNef training (Shibata et al., 2011, 2016; Amano et al., 2016; Koizmi et al., 2016; Cortese et al., 2016, 2017). The pre- and postbehavioral tests examine whether the DecNef method can alter the target behavior in the desired direction (e.g., whether DecNef can enhance visual perceptual learning). In the fMRI decoder construction stage, multi-voxel patterns of activation corresponding to the target mental experience or state (e.g., visual perception of gratings with orientation of 45 degrees) are identified via a stimulus-driven and task-based fMRI experiment. In the DecNef training stage, participants learn to induce the decoded multi-voxel pattern of activation matched with the target mental experience or state through neurofeedback. In the following paragraph, we mainly review 3 DecNef studies, in which researchers aimed to alter 3 types of behavior: (1) visual perceptual learning, (2) meta-cognition, and (3) fear response.
Shibata et al. (2011) demonstrated that the DecNef method may aid participants in inducing target spatiotemporal patterns of activation in the primary visual cortex corresponding to a specific orientation of Gabor patches, without the presentation of a matched stimulus. Furthermore, the authors observed that this method resulted in visual perceptual learning specific to the target orientation. These results and characteristics indicate that DecNef has the potential to induce sufficient neuroplasticity for perceptual learning in the adult early visual cortex with high selectivity.
Based on the findings of this seminal study, DecNef has now been extended to the associative learning (Amano et al., 2016), face preference (Shibata et al., 2016), meta-cognition (Cortese et al., 2016), and fear extinction paradigms (Koizumi et al., 2016). In the following paragraphs, we focus on the latter 2 paradigms, which are thought to be related to the etiology and/or pathogenesis of psychiatric disorders. Cortese et al. (2016) selected confidence ratings as the target behavior during a 2-choice dot-motion discrimination task in a cross-over DecNef study. (That is, the same participant performed two types of DecNef in a random order, separated by a 1-week interval: one aimed at increasing confidence ratings and one aimed at decreasing confidence ratings.) The authors reported that DecNef could be used to modulate activity in fronto-parietal regions to produce bidirectional alterations in confidence ratings without affecting task accuracy. Such a result would indicate that confidence is well decoded in higher cognitive areas, and that DecNef can be used to bidirectionally alter this meta-cognition-related behavior.
Koizumi et al. (2016) investigated whether the DecNef paradigm could be applied to fear extinction. In this experiment, prebehavioral testing included fear-conditioning, in which fear responses were induced by pairing 2 kinds of colored circles (target fear-conditioned stimulus CS+ and control CS+) with electric shocks. Participants then underwent 3 days of DecNef training in which rewards were paired with the multi-voxel patterns of activity in V1/V2 matched to the target CS+. After the training, fear responses as assessed by skin conductance response were selectively reduced for the target CS+ but not for control CS+. We emphasize that this counter-conditioning occurred even though participants were not explicitly exposed to any fear-related stimuli, but rather implicitly exposed to the neural activity patterns matched with the target CS+.
The results of the latter 2 types of experiments—which may reflect alterations in meta-cognitive processes and the Pavlovian conditioned fear response—suggest that DecNef may be useful as an adjunctive therapy for psychiatric disorders, as meta-cognition and fear are closely related to behavioral changes associated with mental illness (David et al., 2012). In particular, counter-conditioning DecNef may benefit patients with fear-related disorders such as phobias and posttraumatic stress disorder, as explicit exposure to traumatic situations (e.g., prolonged exposure therapy) may be too difficult for some patients (Schnurr et al., 2007).
FCNef
The scope of fMRI-based neurofeedback now extends beyond controlling activation levels or patterns within ROIs to include regulation of FC between brain regions. Kim et al. (2015) demonstrated that the combination of ROI- and FC-based neurofeedback for heavy smokers aided participants in inducing increased ROI activation and FC, which was accompanied by reduced cravings for nicotine. Another FCNef study used dynamic causal modeling to enhance the flow of information from the dorsomedial prefrontal cortex to the amygdala—the putative neural circuit associated with the cognitive control of emotions—successfully reducing state anxiety (Koush et al., 2015).
These results indicate that specific regulation of FCs can indeed be achieved using FCNef, in turn leading to desired changes in behavior and function. Although such findings suggest that FCNef may represent a novel treatment method for psychiatric disorders, it remains unclear for how long FCNef-induced connectivity changes are retained. This issue was addressed by another recent study in which FC between the lateral parietal and primary motor areas, which were negatively correlated prior to training, was enhanced via a 4-day, FC-based neurofeedback training protocol (Megumi et al., 2015). This increase in FC during the training period resulted in positive alteration of the rs-fcMRI between the default-mode and motor/visuo-spatial networks, which include the 2 ROIs, respectively. Intriguingly, this effect lasted for more than 2 months after the training. These results indicate that FCNef may be capable of inducing robust and long-lasting plasticity in target FCs, which is clinically significant for the treatment of psychiatric disorders. Yamashita et al. (2017) further demonstrated that FCNef induced bidirectional changes in behavior by changing the sign of a neurofeedback signal. Our hypothesis is as follows: If an rs-fcMRI-based biomarker capable of discriminating between individuals with a psychiatric condition and HCs with high accuracy can be developed, successful normalization of the individual’s own FC pattern using FCNef would lead to a reduction in psychiatric symptoms.
Unique Characteristics of DecNef and FCNef
DecNef and the latter 2 FCNef (Megumi et al., 2015; Yamashita et al., 2017) studies have the following 3 unique characteristics: (1) implicitness, (2) monetary reward, and (3) spatially limited influence. First, no verbal instruction regarding any explicit strategy was given to participants, and no participant became aware of how feedback was increased or the mechanisms underlying the neurofeedback experiment. Second, monetary reward was given to participants in proportion to the success of voxel pattern or FC induction. Third, induced information by DecNef and FCNef was largely constrained in the brain region. As for (1), no participant adopted a rational cognitive strategy that was fitted to the respective experimental designs, as revealed in postexperiment interviews (Shibata et al., 2011, 2016; Megumi et al., 2015; Amano et al., 2016; Koizumi et al., 2016; Cortese et al., 2016, 2017). When an efficient cognitive strategy is unavailable, desired brain activation can be reinforced by providing contingent feedback and/or rewards, rather than by the cognitive strategy itself. Therefore, reinforcement learning—or more specifically, neural operant conditioning—may well explain the training mechanism of DecNef and FCNef. However, to develop a clinically useful neurofeedback paradigm, it is necessary to compare the effect of various instruction, feedback, and reward conditions in future studies.
Neurofeedback and Pharmacology
When applying these fMRI-based neurofeedback methods to individuals with psychiatric disorders, it is necessary to consider the relationship between neurofeedback and pharmacology, as many patients with such conditions are prescribed psychotropic agents. Neurofeedback learning apparently depends on induction of changes in synaptic efficiency where neurochemical environments, including neurotransmitters and receptors, play crucial roles (Sitaram et al., 2016). Although what types of learning systems constitute neurofeedback has not been exactly identified, a type of associative learning, operant conditioning, is thought to be one of the major components of neurofeedback learning. Previous studies have shown that NMDA receptors, dopamine, and serotonin affect synaptic plasticity during associative learning (Gruart et al., 2015; Khani and Rainer, 2016). To test the roles of these neurochemicals in neurofeedback learning, a previous study manipulated several pharmacological agents and examined how neurochemicals and receptors mediate neurofeedback learning in rodents (Ishikawa et al., 2014). In this study, the authors first successfully induced hippocampal neuronal activity through a neural operant conditioning method using electrical stimulation of lateral hypothalamus as a contingent reward. Then, the authors further demonstrated that the administration of an NMDA receptor antagonist and dopamine D1 receptor antagonist abolished the neural operant conditioning. In addition, depression model mice conditioned by forced swimming failed to induce target neural activity. However, neural operant conditioning was successfully induced in the same mice following treatment with fluoxetine, a selective serotonin reuptake inhibitor. These results suggest that successful application of fMRI-based neurofeedback in humans also depends on specific neurochemical environments determined by molecules including dopamine, glutamate, and serotonin. Because these environments of neurotransmitters and neuromodulators are often significantly altered in psychiatric diseases because of either disease itself or medication, further animal research is indispensable for identifying molecular conditions where fMRI-based neurofeedback is clinically applicable. Fruitful interaction with pharmacology is critical for the development of fMRI-based neurofeedback as a realistic option for clinical application and for maximizing the effect of neurofeedback depending on the pharmaceutical conditions of patients.
Neurofeedback Therapy Based on Neuroimaging Biomarkers
Based on the promising results of the aforementioned studies regarding rs-fcMRI-based biomarkers and FCNef, several proof-of-concept studies have examined the potential efficacy of FCNef in the treatment of patients with MDD and ASD (Hashimoto 2013; Kawato 2013; Yahata et al., 2016 and 2017). The most recent study consists of rs-fcMRI-based biomarker construction (see The rs-fcMRI-Based Biomarker for ASD) and normalization (i.e., FCs consisted of the biomarker) using an FCNef protocol. The protocol for normalization of target FCs was determined in large part based on a previous study (Megumi et al., 2015). Briefly, the FCNef training was held over 4 successive days. In each trial during the training, participants were instructed to manipulate brain activity to increase as much as possible the size of a green disc in the display, which represented the degree of target FC normalization. The following paragraphs discuss the preliminary findings of recent proof-of-concept experiments in individuals with MDD and ASD. These studies were approved by the ethical committee of Kyoto University and Showa University, respectively. All volunteers gave written informed consent prior to the study, in accordance with the Declaration of Helsinki.
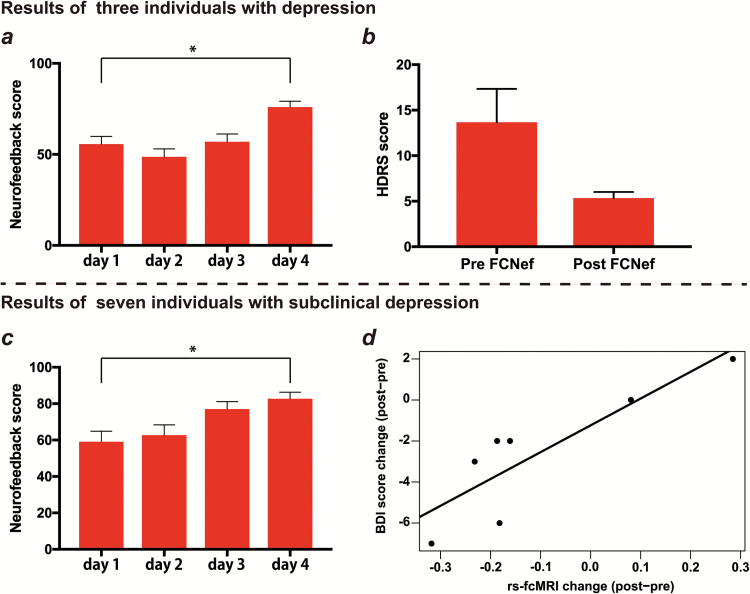
In the study of MDD, we selected FC between the left dorsolateral prefrontal cortex and left precuneus/posterior cingulate cortex as a target for FCNef, based on the following steps. First, we constructed 2 types of rs-fcMRI-based biomarker, one for predicting diagnosis (i.e., depression or healthy) (Ichikawa et al., 2017) and the other for predicting the severity of depressive symptoms (i.e., the score of BDI) (Yamashita et al., 2015). The target FC was defined as that included in both types of biomarkers. The identified FC was consistent with the findings of previous studies that have demonstrated an imbalance in anticorrelation between the default mode and fronto-parietal networks as a neural correlate for MDD (Kaiser et al., 2015; Northoff, 2016; Rayner et al., 2016). During neurofeedback training, participants aimed to decrease the correlation of the target FC. In the most recent study, FCNef has been conducted for 3 individuals with MDD and 7 individuals with subclinical depression. Participants with average BDI-II scores >10 at 2 different time points prior to training were categorized into the subclinical depression group. Figure 4a shows the neurofeedback scores of all 3 patients with MDD on each day of training. Scores exhibited an upward trend across the 4 days of training, and this was confirmed using a multiple regression model that included 2 explanatory variables (i.e., each training day and subject) and one response variable (i.e., neurofeedback scores), showing significant positive effect of the training day (95% CI 1.9–9.1 of the coefficient). Post-hoc t-tests revealed that scores for all 3 participants were significantly higher on the last day than on the first (t = 4.01, P < .001). These results consistently demonstrated that participants learned to induce negative correlation for the target FC throughout the training. Furthermore, all 3 patients exhibited decreased scores on the Hamilton Depression Rating Scale (Hamilton, 1980), which represents the severity of depressive symptoms, after the training (Figure 4b). Similar to those of the depression group, neurofeedback scores also tended to increase over the training period among the 7 individuals with subclinical depression (Figure 4c). One-way ANOVA revealed a significant main effect of training day (P = .011), while posthoc paired t tests revealed that neurofeedback scores were significantly higher on the last day of training than on the first (P = .0046). A tendency for reduced BDI scores following training was also observed (P = .07). Furthermore, 5 of the 7 participants also reduced the target rs-fcMRI in the normal direction, and the change in rs-fcMRI between pre- and post-FCNef was significantly correlated with that of BDI score (r = 0.87, P = .011) (Figure 4d). These results indicated the possibility that the target FC between the left dorsolateral prefrontal cortex and left precuneus/posterior cingulate cortex may be a theranostic biomarker for depression, as more than one-half of the participants decreased the target rs-fc in accordance with BDI score through our FCNef training. Taken together, these findings suggest that our therapeutic package for depression can be used to detect potential theranostic biomarkers and ameliorate depressive symptoms using circuit-specific fMRI-based neurofeedback.
Figure 4.
Results from 3 individuals with depression and 7 subclinical participants. (a) and (b) show the results of participants with depression. (c) and (d) show the results of participants with subclinical depression. (a) Neurofeedback scores across the 4 training days. Red bar denotes the mean of neurofeedback scores for all trials. Error bar denotes SEM. Asterisk shows the statistical significance (P < .001). (b) Hamilton Depression Rating Scale scores at pre- and postfunctional connectivity-based neurofeedback (FCNef). Red bar denotes the mean of Hamilton Depression Rating Scale scores and error bar shows SEM. (c) Neurofeedback scores in the same format as a. Asterisk shows the statistical significance (P < .01). (d) Scatter plot of the change in the Beck Depression Inventory (BDI) score vs the change of the target resting-state functional connectivity (FC) MRI (rs-fcMRI) between post- and preneurofeedback. Each dot represents individual data. The line denotes the linear regression of the change of BDI score from the change of the target rs-fcMRI.
The integration of the FCNef technique with disease-specific rs-fcMRI-based biomarkers may also aid in the development of a novel therapeutic treatment for ASD. Using the highly accurate rs-fcMRI-based biomarker for ASD (Yahata et al., 2016), we conducted a proof-of-concept study of this approach in which a small number of adults with high-functioning ASD underwent 4 to 5 successive days of FC-neurofeedback training (Hashimoto 2013; Yahata et al., 2016, 2017). In contrast to MDD, multiple FCs included in the ASD biomarker were selected as targets of intervention. Although the results are still preliminary and several aspects of the protocol must be refined, we observed steady improvement in feedback scores throughout the training in some participants. This observation indicates that some individuals with ASD are indeed capable of learning to change their altered FC patterns in the direction toward the typically developed pattern, even in adulthood. Furthermore, we observed cases in which the neurofeedback training had a long-lasting impact on the FC pattern during the resting state. The outputs of the rs-fcMRI-based biomarker closely approached the neurotypical level not only during the training sessions but also more than 3 weeks after the training, whereas, prior to training, the biomarker outputs had been invariably ASD-like. A typical case is shown in Figure 5. We acknowledge that even more robust changes in rs-fcMRI may be required to induce behavioral changes that may significantly improve patient quality of life. Furthermore, several difficulties may exist that are specific to ASD, such as the altered sensitivity to reward (Dichter et al., 2012; Kohls et al., 2013). However, recent preliminary results suggest that real-time FCNef guided by the disease-specific rs-fcMRI-based biomarker may provide a foundation for the development of a novel neuro-circuitry-based therapy, particularly for conditions in which the effects of standard interventions are very limited, such as ASD.
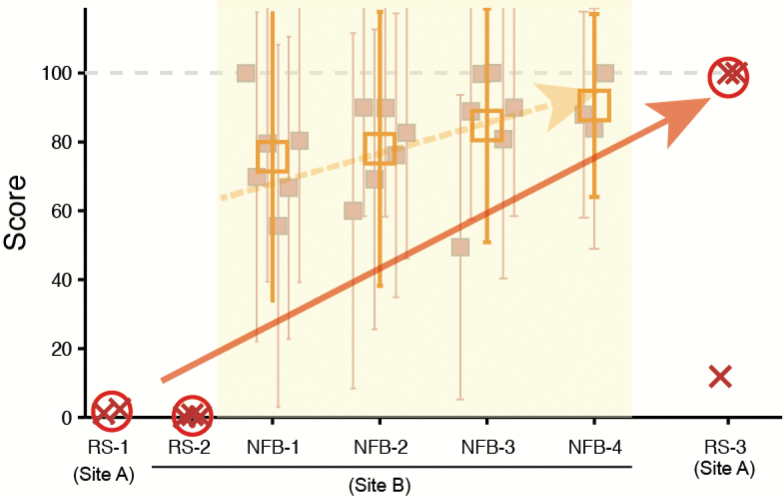
Figure 5.
Neurofeedback-induced change of functional connectivity (FC) toward the neurotypical pattern in a case of adult high-functioning autism spectrum disorder (ASD). The graph shows the feedback scores during the training sessions (blank squares and error bars) and the outputs of the ASD biomarker (Yahata et al., 2016) using the resting-state FC data collected before (i.e., RS-1 and RS-2) and after (i.e., RS-3) the neurofeedback training (x signs). The open circle denotes the mean output of the ASD biomarker across the three rs-fcMRI sessions conducted in a single day. Although the linear weighted summation of FCs in the ASD biomarker ranged between 0 (neurotypical pattern) and 1 (typical ASD pattern), the value was fed into a mathematical transformation involving a sigmoid function, such that the output of the ASD biomarker ranged between 0 (typical ASD) to 100 (neurotypical). In each training day, there were 6 runs (filled squares and error bars; except for three runs in the final day), each of which had 10 trials. Note that, whereas the outputs of the biomarker had remained close to 0 before the training, the resting state FCs exhibited the neurotypical pattern at least twice out of 3 scans in the posttraining, which was acquired 3 weeks after the training.
Conclusion
In this review, we discussed recent progress in computational psychiatric studies and the findings of our research program focusing on rs-fcMRI-based biomarkers and fMRI-based neurofeedback (DecNef project 2017). While not utilized in clinical psychiatry at present, these approaches have the potential to change the conventional method of symptom-based diagnosis to a data-driven method, allowing for more precise treatment with psychotropic agents and circuit-specific therapies such as neurofeedback and repetitive transcranial magnetic stimulation. Furthermore, the combination of rs-fcMRI-based biomarkers and FCNef may allow for the simultaneous diagnosis and treatment of psychiatric disorders, thus establishing a theranostic biomarker, which has yet to be achieved in clinical psychiatry.
Funding
This research is conducted as the “Application of DecNef for Development of Diagnostic and Cure System for Mental Disorders and Construction of Clinical Application Bases” of the Strategic Research Program for Brain Sciences from Japan Agency for Medical Research and Development AMED. T.Y. was also partially supported by the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (16K10236) and grants from SENSHIN Medical Research Foundation.
Statement of Interest
M.K., N.Y., R.H., and N.K. are inventors of a patent owned by Advanced Telecommunications Research (ATR) Institute International related to the present work [PCT/JP2014/061543(WO2014178322)]. M.K., N.Y., R.H., and N.K. are inventors of a patent owned by ATR Institute International related to the present work [PCT/JP2014/061544 (WO2014178323)]. M.K. and N.Y. are inventors of a patent application submitted by ATR Institute International related to the present work [JP2015-228970]. H.T. received research grants from Takeda. H.T. has received honoraria for lectures by Otsuka, Meiji Seika Pharma, MSD, Dainippon-Sumitomo, and GlaxoSmithKline. For the past 3 years, Y.O. declares the following potential conflicts of interest, although they are all unrelated to the current study. Y.O. has received honoraria for lectures by Otsuka, Dainippon Sumitomo, Astellas, Pfizer, Eli Lilly, Janssen, Meiji Seika Pharma, Mochida, Yoshitomi Yakuhin, Eizai, and GlaxoSmithKline.
Acknowledgments
We thank T. Kochiyama for the assistance with MRI data analysis. We thank A. Yamashita and S. Hayasaka for the assistance in planning neurofeedback paradigm.
References
- Abi-Dargham A, Horga G (2016) The search for imaging biomarkers in psychiatric disorders. Nat Med 22:1248–1255. [DOI] [PubMed] [Google Scholar]
- Ahn BC. (2016) Personalized medicine based on theranostic radioiodine molecular imaging for differentiated thyroid cancer. Biomed Res Int 2016:1680464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert MA. (2011) Biomarkers and heart disease. J Clin Sleep Med 7:S9–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano K, Shibata K, Kawato M, Sasaki Y, Watanabe T (2016) Learning to associate orientation with color in early visual areas by associative decoded fMRI neurofeedback. Curr Biol 26:1861–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders. 5th ed Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Anderson JS, Nielsen JA, Froehlich AL, DuBray MB, Druzgal TJ, Cariello AN, Cooperrider JR, Zielinski BA, Ravichandran C, Fletcher PT, Alexander AL, Bigler ED, Lange N, Lainhart JE (2011) Functional connectivity magnetic resonance imaging classification of autism. Brain 134:3742–3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Corlett PR, Cole MW, Savic A, Gancsos M, Tang Y, Repovs G, Murray JD, Driesen NR, Morgan PT, Xu K, Wang F, Krystal JH (2015) N-methyl-D-aspartate receptor antagonist effects on prefrontal cortical connectivity better model early than chronic schizophrenia. Biol Psychiatry 77:569–580. [DOI] [PubMed] [Google Scholar]
- Arbabshirani MR, Plis S, Sui J, Calhoun VD (2017) Single subject prediction of brain disorders in neuroimaging: promises and pitfalls. NeuroImage 145:137–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W (1996) Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal Pers Assess 67:588–597. [DOI] [PubMed] [Google Scholar]
- Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, Keshavan MS, Tamminga CA (2016) Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry 173:373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONVERGE consortium (2015) Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature 523:588–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese A, Amano K, Koizumi A, Kawato M, Lau H (2016) Multivoxel neurofeedback selectively modulates confidence without changing perceptual performance. Nat Comm 7:13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese A, Amano K, Koizumi A, Lau H, Kawato M (2017) Decoded fMRI neurofeedback can induce bidirectional confidence changes within single participants. NeuroImage 149:323–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium (2013) Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 381:1371–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David AS, Bedford N, Wiffen B, Gilleen J (2012) Failures of metacognition and lack of insight in neuropsychiatric disorders. Philos Trans R Soc Lond B Biol Sci 367:1379–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decoded Neurofeedback Project within Strategic Research Program for Brain Sciences (SRPBS) (2017) BMI Technology Application of DecNef for development of diagnostic and care system for mental disorders and construction of clinical application bases. Available from http://www.cns.atr.jp/decnefpro/. [Google Scholar]
- Deshpande G, Wang P, Rangaprakash D, Wilamowski B (2015) Fully connected cascade artificial neural network architecture for attention deficit hyperactivity disorder classification from functional magnetic resonance imaging data. IEEE Trans Cybern 45:2668–2679. [DOI] [PubMed] [Google Scholar]
- Di Martino A, et al. (2014) The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry 19:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, Bodfish JW (2012) Reward circuitry function in autism spectrum disorders. Soc Cogn Affect Neurosci 7:160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Nardos B, Cohen AL, Fair DA, Power JD, Church JA, Nelson SM, Wig GS, Vogel AC, Lessov-Schlaggar CN, Barnes KA, Dubis JW, Feczko E, Coalson RS, Pruett JR Jr, Barch DM, Petersen SE, Schlaggar BL (2010) Prediction of individual brain maturity using fMRI. Science 329:1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale AT, et al. (2017) Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med 23:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Resnick SM, Wu X, Davatzikos C (2008) Structural and functional biomarkers of prodromal Alzheimer’s disease: a high-dimensional pattern classification study. NeuroImage 41:277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetz EE. (1969) Operant conditioning of cortical unit activity. Science 163:955–958. [DOI] [PubMed] [Google Scholar]
- Fovet T, Jardri R, Linden D (2015) Current issues in the use of fMRI-based neurofeedback to relieve psychiatric symptoms. Curr Pharmaceu Des 21:3384–3394. [DOI] [PubMed] [Google Scholar]
- Gruart A, Leal-Campanario R, Lopez-Ramos JC, Delgado-Garcia JM (2015) Functional basis of associative learning and its relationships with long-term potentiation evoked in the involved neural circuits: Lessons from studies in behaving mammals. Neurobiol Learn Mem 124:3–18. [DOI] [PubMed] [Google Scholar]
- Hamilton M. (1980) Rating depressive patients. J Clin Psychiatry 41:21–24. [PubMed] [Google Scholar]
- Hashimoto R. (2013) Non-invasive neuroimaging and neurofeedback. The 36th Meeting of Japan Neuroscience Society; 20–23 June; Kyoto, Japan. [Google Scholar]
- Huys QJ, Maia TV, Frank MJ (2016) Computational psychiatry as a bridge from neuroscience to clinical applications. Nat Neurosci 19:404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa N, Lisi G, Yahata N, Okada G, Takamura M, Yamada M, Suhara T, Hashimoto R, Yamada T, Yoshihara Y, Takahashi H, Kasai K, Kato N, Yamawaki S, Kawato M, Morimoto J, Okamoto Y (2017) Identifying melancholic depression biomarker using whole-brain functional connectivity. arXiv:1704.01039 [q-bio.NC].
- Insel TR, Cuthbert BN (2009) Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol Psychiatry 66:988–989. [DOI] [PubMed] [Google Scholar]
- Insel TR, Cuthbert BN (2015) Medicine. Brain disorders? Precisely. Science 348:499–500. [DOI] [PubMed] [Google Scholar]
- Ishikawa D, Matsumoto N, Sakaguchi T, Matsuki N, Ikegaya Y (2014) Operant conditioning of synaptic and spiking activity patterns in single hippocampal neurons. J Neurosci 34:5044–5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivleva EI, Clementz BA, Dutcher AM, Arnold SJ, Jeon-Slaughter H, Aslan S, Witte B, Poudyal G, Lu H, Meda SA, Pearlson GD, Sweeney JA, Keshavan MS, Tamminga CA (2016) Brain structure biomarkers in the psychosis biotypes: findings from the bipolar-schizophrenia network for intermediate phenotypes. Biol Psychiatry 82:26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just MA, Keller TA, Malave VL, Kana RK, Varma S (2012) Autism as a neural systems disorder: a theory of frontal-posterior underconnectivity. Neurosci Biobehav Rev 36:1292–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA (2015) Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry 72:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambeitz J, Cabral C, Sacchet MD, Gotlib IH, Zahn R, Serpa MH, Walter M, Falkai P, Koutsouleris N (2016) Detecting neuroimaging biomarkers for depression: a meta-analysis of multivariate pattern recognition studies. Biol Psychiatry. doi: 10.1016/j.biopsych.2016.10.028. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Kamitani Y, Tong F (2005) Decoding the visual and subjective contents of the human brain. Nat Neurosci 8:679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawato M. (2013) Computational neuroscience approach to biomarkers and treatments for psychiatric diseases. The 11th World Congress of Biological Psychiatry; 20–23 June; Kyoto, Japan. [Google Scholar]
- Keynan JN, Meir-Hasson Y, Gilam G, Cohen A, Jackont G, Kinreich S, Ikar L, Or-Borichev A, Etkin A, Gyurak A, Klovatch I, Intrator N, Hendler T (2016) Limbic activity modulation guided by functional magnetic resonance imaging-inspired electroencephalography improves implicit emotion regulation. Biol Psychiatry 80:490–496. [DOI] [PubMed] [Google Scholar]
- Khani A, Rainer G (2016) Neural and neurochemical basis of reinforcement-guided decision making. J Neurophysiol 116:724–741. [DOI] [PubMed] [Google Scholar]
- Kim DI, Sui J, Rachakonda S, White T, Manoach DS, Clark VP, Ho BC, Schulz SC, Calhoun VD (2010) Identification of imaging biomarkers in schizophrenia: a coefficient-constrained independent component analysis of the mind multi-site schizophrenia study. Neuroinformatics 8:213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Yoo SS, Tegethoff M, Meinlschmidt G, Lee JH (2015) The inclusion of functional connectivity information into fMRI-based neurofeedback improves its efficacy in the reduction of cigarette cravings. J Cog Neurosci 27:1552–1572. [DOI] [PubMed] [Google Scholar]
- King BH, Lord C (2011) Is schizophrenia on the autism spectrum? Brain Res 1380:34–41. [DOI] [PubMed] [Google Scholar]
- Kohls G, Schulte-Ruther M, Nehrkorn B, Muller K, Fink GR, Kamp-Becker I, Herpertz-Dahlmann B, Schultz RT, Konrad K (2013) Reward system dysfunction in autism spectrum disorders. Soc Cogn Affect Neurosci 8:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi A, Amano K, Cortese A, Shibata K, Yoshida W, Seymour B, Kawato M, Lau H (2016) Fear reduction without fear through reinforcement of neural activity that bypasses conscious exposure. Nat Human Behav 1:0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koush Y, Rosa MJ, Robineau F, Heinen K, S WR, Weiskopf N, Vuilleumier P, Van De Ville D, Scharnowski F (2013) Connectivity-based neurofeedback: dynamic causal modeling for real-time fMRI. NeuroImage 81:422–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koush Y, Meskaldji DE, Pichon S, Rey G, Rieger SW, Linden DE, Van De Ville D, Vuilleumier P, Scharnowski F (2015) Learning control over emotion networks through connectivity-based neurofeedback. Cereb Cortex. 2015 Dec 17. pii: bhv311. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Laird AR, Fox PM, Eickhoff SB, Turner JA, Ray KL, McKay DR, Glahn DC, Beckmann CF, Smith SM, Fox PT (2011) Behavioral interpretations of intrinsic connectivity networks. J Cog Neurosci 23:4022–4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Karnath HO, Xu X (2017) Candidate biomarkers in children with autism spectrum disorder: a review of MRI studies. Neurosci Bull 33:219–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, et al. (2016) Brain-wide analysis of functional connectivity in first-episode and chronic stages of schizophrenia. Schizophr Bull 43:436–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden DE, Habes I, Johnston SJ, Linden S, Tatineni R, Subramanian L, Sorger B, Healy D, Goebel R (2012) Real-time self-regulation of emotion networks in patients with depression. PLoS One 7:e38115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Z, Duan X, Mantini D, Chen H (2016) Alteration of functional connectivity in autism spectrum disorder: effect of age and anatomical distance. Sci Rep 6:26527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, DiLavore PC, Pickles A, Rutter M (2000) The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30:205–223. [PubMed] [Google Scholar]
- Megumi F, Yamashita A, Kawato M, Imamizu H (2015) Functional MRI neurofeedback training on connectivity between two regions induces long-lasting changes in intrinsic functional network. Front Hum Neurosci 9:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V. (2011) Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 15:483–506. [DOI] [PubMed] [Google Scholar]
- Montague PR, Dolan RJ, Friston KJ, Dayan P (2012) Computational psychiatry. Trends Cogn Sci 16:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto J, Kawato M (2015) Creating the brain and interacting with the brain: an integrated approach to understanding the brain. J R Soc Interface 12:20141250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G (2016) Spatiotemporal psychopathology I: no rest for the brain’s resting state activity in depression? Spatiotemporal psychopathology of depressive symptoms. J Affect Disord 190:854–866. [DOI] [PubMed] [Google Scholar]
- Owen MJ. (2014) New approaches to psychiatric diagnostic classification. Neuron 84:564–571. [DOI] [PubMed] [Google Scholar]
- Perlis RH. (2011) Translating biomarkers to clinical practice. Mol Psychiatry 16:1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot M, Riviere D, Mangin JF (2011) Cortical sulci recognition and spatial normalization. Med Image Anal 15:529–550. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Farah MJ (2015) Progress and challenges in probing the human brain. Nature 526:371–379. [DOI] [PubMed] [Google Scholar]
- Rayner G, Jackson G, Wilson S (2016) Cognition-related brain networks underpin the symptoms of unipolar depression: evidence from a systematic review. Neurosci Biobehav Rev 61:53–65. [DOI] [PubMed] [Google Scholar]
- Rosenberg MD, Finn ES, Scheinost D, Papademetris X, Shen X, Constable RT, Chun MM (2016) A neuromarker of sustained attention from whole-brain functional connectivity. Nat Neurosci 19:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai Y. (2015) Neural substrate of OCD and possible clinical application of Decoded Neurofeedback to its treatment. The 38th Annual Meeting of the Japan Neuroscience Society; 28–31 July; Kobe, Japan. [Google Scholar]
- Scheinost D, Stoica T, Saksa J, Papademetris X, Constable RT, Pittenger C, Hampson M (2013) Orbitofrontal cortex neurofeedback produces lasting changes in contamination anxiety and resting-state connectivity. Transl Psychiatry 3:e250. doi: 10.1038/tp.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnurr PP, Friedman MJ, Engel CC, Foa EB, Shea MT, Chow BK, Resick PA, Thurston V, Orsillo SM, Haug R, Turner C, Bernardy N (2007) Cognitive behavioral therapy for posttraumatic stress disorder in women: a randomized controlled trial. JAMA 297:820–830. [DOI] [PubMed] [Google Scholar]
- Shibata K, Watanabe T, Sasaki Y, Kawato M (2011) Perceptual learning incepted by decoded fMRI neurofeedback without stimulus presentation. Science 334:1413–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata K, Watanabe T, Kawato M, Sasaki Y (2016) Differential activation patterns in the same brain region led to opposite emotional states. PLoS Biol 14(9):e1002546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaram R, Caria A, Veit R, Gaber T, Ruiz S, Birbaumer N (2014) Volitional control of the anterior insula in criminal psychopaths using real-time fMRI neurofeedback: a pilot study. Front Behav Neurosci 8:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaram R, Ros T, Stoeckel L, Haller S, Scharnowski F, Lewis-Peacock J, Weiskopf N, Blefari ML, Rana M, Oblak E, Birbaumer N, Sulzer J (2016) Closed-loop brain training: the science of neurofeedback. Nat Rev Neurosci 18:86–100. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF (2009) Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci U S A 106:13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE, Vidaurre D, Winkler AM, Behrens TE, Glasser MF, Ugurbil K, Barch DM, Van Essen DC, Miller KL (2015) A positive-negative mode of population covariation links brain connectivity, demographics and behavior. Nat Neurosci 18:1565–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui J, Pearlson GD, Du Y, Yu Q, Jones TR, Chen J, Jiang T, Bustillo J, Calhoun VD (2015) In search of multimodal neuroimaging biomarkers of cognitive deficits in schizophrenia. Biol Psychiatry 78:794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D, van Erp TG, Thompson PM, Bearden CE, Daley M, Kushan L, Hardt ME, Nuechterlein KH, Toga AW, Cannon TD (2009) Elucidating a magnetic resonance imaging-based neuroanatomic biomarker for psychosis: classification analysis using probabilistic brain atlas and machine learning algorithms. Biol Psychiatry 66:1055–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supekar K, Uddin LQ, Khouzam A, Phillips J, Gaillard WD, Kenworthy LE, Yerys BE, Vaidya CJ, Menon V (2013) Brain hyperconnectivity in children with autism and its links to social deficits. Cell Rep 5:738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, Sakai Y, Lisi G, Yahata N, Abe Y, Nishida S, Nakamae T, Morimoto J, Kawato M, Narumoto J, Tanaka SC (2017) A neural marker of obsessive-compulsive disorder from whole-brain functional connectivity. Sci Rep [In press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavor I, Parker Jones O, Mars RB, Smith SM, Behrens TE, Jbabdi S (2016) Task-free MRI predicts individual differences in brain activity during task performance. Science 352:216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault RT, Lifshitz M, Raz A (2016) The self-regulating brain and neurofeedback: experimental science and clinical promise. Cortex 74:247–261. [DOI] [PubMed] [Google Scholar]
- Whelan R, Garavan H (2014) When optimism hurts: inflated predictions in psychiatric neuroimaging. Biol Psychiatry 75:746–748. [DOI] [PubMed] [Google Scholar]
- World Health Organization (1990) International statistical classification of diseases and related health problems. 10th revision Geneva: WHO. [Google Scholar]
- Yahata N, Morimoto J, Hashimoto R, Lisi G, Shibata K, Kawakubo Y, Kuwabara H, Kuroda M, Yamada T, Megumi F, Imamizu H, Nanez JE Sr, Takahashi H, Okamoto Y, Kasai K, Kato N, Sasaki Y, Watanabe T, Kawato M (2016) A small number of abnormal brain connections predicts adult autism spectrum disorder. Nat Comm 7:11254. doi: 10.1038/ncomms11254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahata N, Kasai K, Kawato M (2017) Computational neuroscience approach to biomarkers and treatments for mental disorders. Psychiatry Clin Neurosci. 71:215–237. [DOI] [PubMed] [Google Scholar]
- Yamashita A, Hayasaka S, Lisi G, Ichikawa N, Takamura M, Okada G, Morimoto J, Yahata N, Okamoto Y, Kawato M, Imamizu H (2015) Common functional connectivity between depression and depressed mood. The 37th annual meeting of the Japanese society of biological psychiatry; 24–26 September; Tokyo, Japan. [Google Scholar]
- Yamashita A, Hayasaka S, Kawato M, Imamizu H (2017) Connectivity neurofeedback training can differentially change functional connectivity and cognitive performance. Cereb Cortex doi: 10.1093/cercor/bhx177. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Yamashita M, Kawato M, Imamizu H (2015) Predicting learning plateau of working memory from whole-brain intrinsic network connectivity patterns. Sci Rep 5:7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita O, Sato MA, Yoshioka T, Tong F, Kamitani Y (2008) Sparse estimation automatically selects voxels relevant for the decoding of fMRI activity patterns. NeuroImage 42:1414–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa T, Fukuma R, Seymour B, Hosomi K, Kishima H, Shimizu T, Yokoi H, Hirata M, Yoshimine T, Kamitani Y, Saitoh Y (2016) Induced sensorimotor brain plasticity controls pain in phantom limb patients. Nat Comm 7:13209. doi: 10.1038/ncomms13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KD, Siegle GJ, Zotev V, Phillips R, Misaki M, Yuan H, Drevets WC, Bodurka J (2017) Randomized clinical trial of real-time fmri amygdala neurofeedback for major depressive disorder: effects on symptoms and autobiographical memory recall. Am J Psychiatry: appiajp201716060637. doi: 10.1176/appi.ajp.2017.16060637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara Y, Sugihara G, Deoni S, Ishizuka A, Yogo H, Matsumoto K, Tsuchiya K, Tsujii M, Nakamura K, Murphy DG, Mori N, Takei N (2011) Discrimination of Autism Adults from controls using data on structural MRI in a Japanese sample. The 17th annual meeting of the organization on human brain mapping; 26–30 June; Quebec City, Canada. [Google Scholar]
- Zilverstand A, Sorger B, Slaats-Willemse D, Kan CC, Goebel R, Buitelaar JK (2017) fMRI neurofeedback training for increasing anterior cingulate cortex activation in adult attention deficit hyperactivity disorder: an exploratory randomized, single-blinded study. PLoS One 12:e0170795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zotev V, Yuan H, Misaki M, Phillips R, Young KD, Feldner MT, Bodurka J (2016) Correlation between amygdala BOLD activity and frontal EEG asymmetry during real-time fMRI neurofeedback training in patients with depression. NeuroImage Clin 11:224–238. [DOI] [PMC free article] [PubMed] [Google Scholar]