Abstract
Background
To determine brain areas involved in the antidepressant-related behavioral effects of the selective neuronal nitric oxide synthase inhibitor 1-(2-Trifluoro-methyl-phenyl) imidazole (TRIM) and experimental test compound 4-((3,5-dichloro-2-hydroxybenzyl)amino)-2-hydroxybenzoic acid (ZL006), an inhibitor of the PSD of 95 kDa/neuronal nitric oxide synthase interaction in the N-methyl-D-aspartic acid receptor signalling pathway, regional specific expression of the neuronal activation marker c-FOS was assessed following exposure to the forced swimming test in the Wistar Kyoto rat.
Methods
Wistar Kyoto rats were subjected to a 15-minute swim pretest (pre-forced swimming test) period on day 1. At 24, 5, and 1 hour prior to the 5-minute test, which took place 24 hours following the pre-forced swimming test, animals were treated with TRIM (50 mg/kg; i.p.), ZL006 (10 mg/kg; i.p.), or saline vehicle (1 mL/kg i.p). Behavior was recorded during both pretest and test periods.
Results
Both TRIM and ZL006 decreased immobility time in Wistar Kyoto rats in the forced swimming test. Exposure to the forced swimming test increased c-FOS immunoreactivity in the lateral septum, paraventricular nucleus of the hypothalamus, periaqueductal grey, dentate gyrus, and ventral CA1 of the hippocampus compared with non-forced swimming test-exposed controls. Forced swimming test-induced c-FOS immunoreactivity was further increased in the lateral septum, periaqueductal gray, and paraventricular nucleus of the hypothalamus following treatment with TRIM or ZL006. By contrast, forced swimming test-induced c-FOS immunoreactivity was reduced in dorsal dentate gyrus and ventral CA1 following treatment with TRIM or ZL006. Exposure to the forced swimming test resulted in an increase in NADPH diaphorase staining in the paraventricular nucleus of the hypothalamus. This forced swimming test-induced increase was attenuated following treatment with ZL006 and points to the paraventricular nucleus as a brain region where ZL006 acts to attenuate forced swimming test-induced neuronal nitric oxide synthase activity while concomitantly regulating region specific neuronal activation associated with an antidepressant-related response.
Conclusions
This study identified a pattern of enhanced and reduced forced swimming test-related c-FOS immunoreactivity indicative of a regulated network where inhibition of nitric oxide coupled to the N-methyl-D-aspartic acid receptor leads to activation of the lateral septum, periaqueductal gray, and paraventricular nucleus of the hypothalamus with concomitant inhibition of the hippocampus.
Keywords: stress, forced swimming test, nNOS, PSD-95, c-FOS
Significance Statement
Targeting PSD-95 nNOS interface on the NMDA-R signalling pathway with the small molecule inhibitor, ZL006, produces an antidepressant-related effect in the WKY rat model of depression. Moreover, ZL006 differentially influences stress-induced neuronal activation of several limbic brain regions, providing insight into an NMDA-R-NO regulated network associated with its action.
Introduction
Previous studies have demonstrated that NOS inhibitors are as efficacious as tricyclic antidepressants in preclinical antidepressant screening procedures in attenuating behavioral deficits associated with animal models of depression (Harkin et al., 2003; Ulak et al., 2008; Gigliucci et al., 2010, 2014; Silva et al., 2012; Doucet et al., 2013; Sherwin et al., 2017). The intracellular domain of the N-methyl-D-aspartic acid receptor (NMDA-R) is associated with the postsynaptic density (PSD), a membrane-bound scaffolding complex that functions in postsynaptic signaling. PSD of 95 kDa (PSD-95) associates with the NMDA-R, allowing for coupling to neuronal nitric oxide synthase (nNOS) through protein-protein PDZ interactions (Alderton et al., 2001; Guix et al., 2005; Doucet et al., 2012). We hypothesized that uncoupling nNOS from the NMDA-R through PSD-95 would produce behavioral antidepressant effects similar to NOS inhibitors (Doucet et al., 2012). Small-molecule inhibitors of the PSD-95/nNOS interaction, IC87201 (0.01–2mg/kg) and ZL006 (10mg/kg), produce antidepressant-like responses in the forced swimming test (FST) and tail suspension test (TST) in mice and support the hypothesis that targeting the PSD-95/nNOS interaction downstream of NMDA-R produces antidepressant effects and may represent a novel class of therapeutics for major depressive disorders (Doucet et al., 2012).
ZL006 is a small molecule inhibitor of the PSD-95/nNOS interface that acts by binding to the internal PDZ motif of nNOS to inhibit its interaction with PSD-95 in postsynaptic neurons. ZL006 has been shown to inhibit NMDA-R-dependent nitric oxide (NO) synthesis in cortical neurons with a high IC50 value of 82 nM. Furthermore, ZL006 has been shown to have no effect on the PDZ interactions of NR2B/PSD-95, CAPON/nNOS, and synGAP/nNOS, suggesting that the drug is specific only for the PSD-95/nNOS interaction and is capable of attenuating NMDA-R/nNOS signalling without affecting the other signalling pathways associated with NMDA-R activation (Zhou et al., 2010). Further biochemical investigations indicate that the mechanism of action of ZL006 may not be via direct binding to the extended nNOS-PDZ domain as originally proposed and that given its reported potency, ZL006 may bind to other parts of the large and complex 321-kDa homodimer nNOS protein or to other proteins affecting the nNOS/PSD-95 system (Bach et al., 2015). Others have confirmed that ZL006 directly inhibits binding of PSD95 and nNOS protein in AlphaScreen without altering the binding of PSD95 to ErbB4 (Lee et al., 2015). ZL006 has been shown to readily pass the blood brain barrier when injected systemically without having any noticeable effect upon arterial blood pressure, platelet aggregation, or cerebral blood flow (Zhou et al., 2010). In vitro and in vivo experiments have demonstrated the neuroprotective properties of ZL006 against glutamate-induced excitotoxicity (Zhou et al., 2010; Doucet et al., 2014). ZL006 blocks NMDA-R-dependent NO synthesis in cultured cortical neurons in a concentration-dependent manner and reverses neuronal atrophy of cultured cortical neurons induced by high concentrations of glutamate (Zhou et al., 2010). In an animal model of cerebral ischemia, ZL006 reduced infarct size and improved neurological scores in both mice and rats while also reducing the formation of the PSD-95/nNOS complex in the cortex (Zhou et al., 2010).
The inbred Wistar Kyoto (WKY) rat has been proposed as a model of depressive-like behavior, as it endogenously expresses some behavioral, endocrine, and neurotransmitter changes akin to those exhibited in depressed human patients (Redei et al., 2001; Rittenhouse et al., 2002; Nagasawa et al., 2012; Nam et al., 2014). The WKY rat displays a higher level of immobility in the FST relative to Wistar and Sprague-Dawley strains and is routinely used to assess the efficacy of novel antidepressant compounds (Carr et al., 2010; Malkesman et al., 2012; Akinfiresoye et al., 2013; Gormley et al., 2016). In the present study, the antidepressant-related effects of ZL006 and, for comparison purposes, the selective nNOS inhibitor, TRIM, were assessed in the FST in the WKY rat model. In addition, the patterns of neuronal activation of specific brain regions were assessed by means of c-FOS immunohistochemistry. Furthermore, it was of interest to determine if nNOS activity was affected following exposure to the FST and following administration of ZL006. Brain sections were stained for nicotinamide adenine dinucleotide (NADPH) diaphorase, a marker of NOS activity, and as such, observable staining for NADPH-diaphorase is indicative of all NOS isoforms. However, as ZL006 targets PSD-95-associated nNOS, alterations in NADPH-diaphorase staining observed may be considered to be due to inhibition of NMDA-R-associated nNOS activity.
Methods
Animals and Drug Treatments
Male WKY (Harlan Olac) rats weighing 280 to 330 g at the beginning of the study were acclimatized to the animal unit and subsequently singly housed in standard, medium-sized polypropylene cages (41x24 cm). The animals were maintained at a constant temperature (22 ± 2°C) and exposed to standard lighting conditions (12-hour-light/-dark cycle; lights on from 8:00 am to 8:00 pm). Food and water were available ad libitum except when the animals were subjected to behavioral testing. WKY rats were handled each day for 7 days prior to the commencement of the experiment to acclimatize the animals to the experimenter and reduce any stressful effects associated with handling. TRIM was dissolved by sonication in saline solution to reach a final concentration of 50 mg/mL. TRIM was administered via the i.p. cavity in an injection volume of 1 mL/kg to achieve a dose of 50 mg/kg. The dose of TRIM was selected based upon previous studies of TRIM-related antidepressant activity in rats and mice (Doucet et al., 2013; Gigliucci et al., 2014). ZL006 was prepared by dissolving in saline solution at alkaline pH, which was then adjusted to pH 7.5, to reach a concentration of 5 mg/mL. ZL006 was administered i.p. in an injection volume of 2 mL/kg to deliver a dose of 10 mg/kg. The dose of ZL006 was selected based on previous studies of ZL006-related antidepressant activity in mice (Doucet et al., 2013). Research involving animals in Trinity College Dublin is governed by Directive 2010/63/EU on the protection of animals used for scientific purposes in accordance with the requirements of the S.I No 543 of 2012 and reviewed and approved by the Animal Research Ethics Committee prior to submission to the Health Products Regulatory Authority for regulatory approval.
FST
The FST was performed in accordance with the protocol originally described by Porsolt (1977). On day 1, rats were removed from their home cage and individually placed into a clear glass tank (40 cm high and 20 cm in diameter) filled with 30 cm of water (22–23°C) and allowed to swim for 15 minutes (pre-FST). The first 5 minutes of the pre-FST session were scored for time spent immobile (seconds) with a stopwatch and used as a baseline to assess for any further increase in immobility when the animals were reexposed. The first injection of ZL006, TRIM, or saline vehicle was administered 1 hour following the pre-FST session. Animals then received a second and a third injection of ZL006, TRIM, or saline vehicle 5 and 1 hour prior to the 5-minute FST, which took place 24 hours following the pre-FST session. In the test session, rats were allowed to swim for 5 minutes, and the time spent immobile was again scored. The animals were considered immobile when floating in the water without struggling and making only those movements necessary to keep their heads above the water. The individual scoring the behavior was blind to the treatments administered to the animals.
Immunohistochemistry
At 90 minutes following the FST, animals were anesthesized with an i.p. injection of urethane (Sigma-Aldrich). Rats were intracardially perfused with phosphate buffered saline (PBS; 10 mM, pH 7.4) followed by 4% paraformaldehyde in PBS. Brains were removed and postfixed for 24 hours in 4% paraformaldehyde and stored for at least 36 hours in 30% sucrose for cryoprotection. Coronal sections (40 μm) were obtained in series of 6 on a cryostat, and brain sections were stored in a cryoprotectant solution (30% ethylene glycol, 30% sucrose in 10 mM PBS, pH 7.4). Free-floating brain sections were then processed for c-FOS immunohistochemistry as previously described (Sherwin et al., 2017). In brief, sections were blocked with 10% normal goat serum (Sigma-Aldrich). They were then washed with PBS and incubated overnight at room temperature with c-FOS anti-sera (1 in 6500 in PBS; anti-rabbit IgG, sc-52, Santa Cruz Biotechnology Inc.). Following incubation in the primary anti-serum, the sections were washed in PBS and sequentially incubated with a biotinylated goat anti-rabbit IgG (1 in 1000 in PBS, Vectastain ABC kit, Vector Laboratories). Sections were then processed by the avidin-biotin immunoperoxidase method (Vectastain ABC kit, Vector Laboratories). c-FOS immunoreactivity was visualized by the addition of the chromogen 3’,3-diaminobenzidine (with 0.02% H2O2 in PBS, Sigma-Aldrich). Sections were rinsed in PBS and then mounted on glass slides and allowed to dry overnight at room temperature. Sections were then dehydrated in ethanol, cleared in xylene, and coverslipped with mounting medium. To assess antibody specificity, incubation with the primary antibody was omitted for several sections, and no significant staining was observed in this case. To assess basal antigen immunoreactivity, a subset of animals underwent the same treatment of vehicle, TRIM (50 mg/kg), or ZL006 (10 mg/kg) but were not exposed to the FST.
NADPH Diaphorase Histology
In the ZL006 experiment, NADPH diaphorase (NADPH-d) staining was carried out to confirm nNOS activity was altered in the brain following exposure to the FST and following treatment with ZL006 in some brain regions. Experiments performed by Hope and colleagues (Hope et al., 1991) identified that NADPH-d corresponds to nNOS expression, molecular weight, and enzymatic activity. Morris and colleagues (Morris et al., 1997) determined that the intensity of NADPH-d staining reflects the level of nNOS enzyme activity at the time of tissue fixation. Moreover, the nonselective NOS inhibitor, L-NAME, and the selective nNOS inhibitor, 7-NI, have been also shown to reduce NADPH-d staining in several brain regions (Amir et al., 1997; Joung et al., 2012). Sections were incubated in the NADPH-d reaction mixture (0.5 mg/mL β-NADPH, 0.2 mg/mL nitrotetrazolium blue chloride, and 0.3% Triton-X in PBS [pH 7.4]) for approximately 110 minutes at 37°C in a water bath. A negative control consisted of incubating several sections in the reaction mixture with the exception of the β-NADPH substrate. The photosensitive nature of β-NADPH requires that the reaction be completed with minimal exposure to light. Sections were rinsed several times in PBS and then mounted on glass slides and allowed to dry overnight at room temperature. Sections were then dehydrated in ethanol, cleared in xylene, and cover slipped with mounting medium. To assess basal NOS activity, brain sections from non-FST-exposed animals were subjected to NADPH-d histology.
Cell Counting
c-FOS immunoreactivity was observed as a brown dot under the microscope representing the nucleus of cells. NADPH-d histology was observed as a dark blue/purple stain localized within the cell soma under the microscope. The number of c-FOS positive nuclei or NADPH-d positive cells was manually counted using a computerized image analysis system (Cell^d, Olympus). For each group, 2 sections from each animal were evaluated per brain region along the rostro-caudal plane. For bilateral structures, the mean value was calculated and an overall average value for each animal was obtained. Neuroanatomical sites were identified with the help of the Paxinos and Watson’s atlas (Paxinos and Watson, 1998). The anterior-posterior (AP) localization from bregma of the analyzed regions for c-FOS immunoreactivity were as follows: cingulate cortex (Cgx; AP: 2.70 mm), prelimbic cortex (PLx; AP: 2.70 mm), infralimbic cortex (ILx; AP: 2.70 mm), lateral septum (LS; AP: 0.70 mm), nucleus accumbens (NAc; AP: 0.70 mm), paraventricular nucleus of the hypothalamus (PVN; AP: -1.80 mm), basolateral amygdala (BlA; AP: -3.14 mm), medial amygdala (MeA; AP: -3.14 mm), central nucleus of the amygdala (CeA; AP: -3.14 mm), paraventricular thalamic nucleus (PVT; AP: -3.30 mm), dorsal dentate gyrus of the hippocampus (dDG; AP: -3.30 mm), ventral CA1 of the hippocampus (vCA1; AP: -4.80 mm), dorsal raphe nucleus (DRN; AP: -7.64 mm), and the dorsolateral periaqueductal gray matter (dlPAG; AP: -7.64 mm). Brain regions assessed for NADPH diaphorase staining included the PVN of the hypothalamus (AP: -1.80 mm), shell and core of the NAc (AP: 0.70 mm), the dDG and vCA1 of the hippocampus (AP: -3.30 mm and -4.80 mm), DRN (AP: -7.64 mm), and the dlPAG (AP: -7.64 mm). PVN sections were dual stained for c-FOS and NADPH diaphorase to assess neuronal activation within cells staining positively for NADPH-d.
Data Analysis
Data are expressed as group mean with SEM and were analyzed by ANOVA. The behavioral data were analyzed by means of a 2-way ANOVA followed by Newman-Keuls’ posthoc test. c-FOS immunohistochemistry nuclei counting and NADPH-d staining were analyzed by 2-way ANOVA followed by Newman-Keuls’ posthoc test. Data were considered significant when P<.05. All analysis was carried out with the GB-Stat v10 statistical package.
Results
TRIM and ZL006 Reduce Immobility Time in the FST
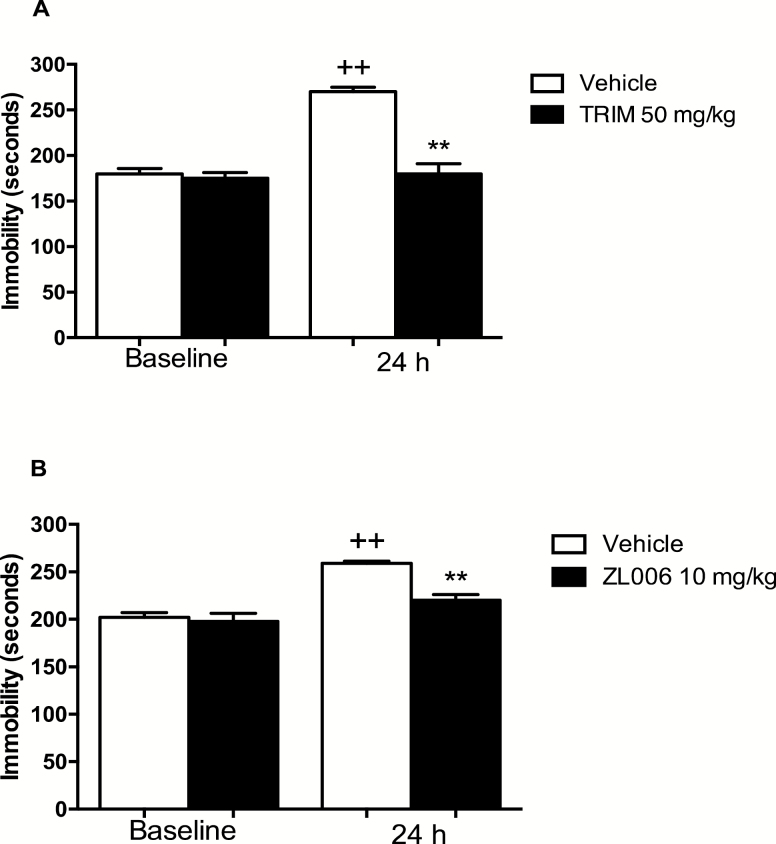
Two-way ANOVA of immobility time showed an effect of the pre-FST session [F(1,23)= 39.03; P<.001] and an effect of TRIM [F(1,23) = 39.38; P<.001] in the FST. Two-way ANOVA also revealed a significant pre-FST x drug interaction [F(1,23)= 5.62; P<.001]. Posthoc comparisons revealed that reexposure to the 5-minute FST 24 hours following the 15-minute pre-FST session facilitated an increase in immobility time compared with the first 5 minutes of the pretest session (P<.01), which was reversed following treatment with TRIM (50 mg/kg; i.p) (P<.01) (Figure 1A).
Figure 1.
Treatment with the neuronal nitric oxide synthase (nNOS) inhibitor, 1-(2-Trifluoro-methyl-phenyl) imidazole (TRIM), or the PSD of 95 kDa (PSD-95)/nNOS inhibitor, 4-((3,5-dichloro-2-hydroxybenzyl)amino)-2-hydroxybenzoic acid (ZL006) lowers immobility time of Wistar Kyoto (WKY) rats in the forced swimming test (FST). Animals were subjected to a 15-minute pre-FST session in which the first 5 minutes were scored for immobility. One hour following the pre-FST session, rats were administered vehicle (i.p.), TRIM (50 mg/kg; i.p.), or ZL006 (10 mg/kg; i.p.). A second and a third injection were administered 5 and 1 hours prior to the 5-minute FST the following day. Data expressed as mean and SEM (n=5–6 per group for TRIM and n=10 for ZL006). ++P<.01 relative to baseline vehicle group. **P<.01 relative to 24-hour vehicle group.
Two-way ANOVA of immobility time showed an effect of the pre-FST session [F(1,32)= 31.42; P<.01] and an effect of ZL006 [F(1,32)= 30.39; P<.01] in the FST. Two-way ANOVA also revealed a significant pre-FST x drug interaction [F(1,32)= 4.34; P<.001]. Posthoc comparisons revealed that reexposure to the 5-minute FST 24 hours following the 15-minute pretest provoked an increase in immobility time compared with the first 5 minutes of the pretest session (P<.01), which was attenuated following treatment with ZL006 (10 mg/kg; i.p.) (P<.01) (Figure 1B).
Effect of the nNOS Inhibitor TRIM on FST-Induced Regional c-FOS Immunoreactivity
In non-FST animals, 2-way ANOVA revealed that TRIM had no significant effect on c-FOS immunoreactivity in any brain region examined with the exception of the central nucleus of the amygdala [F(1,20)= 61.08; P<.0001; Table 1]. Posthoc comparisons revealed that TRIM increased c-FOS immunoreactivity in the central nucleus of the amygdala in naïve animals relative to naïve vehicle controls (P<.01). Two-way ANOVA revealed a significant effect of FST exposure on c-FOS immunoreactivity in the lateral septum [F(1,20)= 674.77; P<.001], PVN of the hypothalamus [F(1,20)=113.43; P<.001], the dlPAG [F(1,20)=438.42; P<.001], dorsal dentate gyrus [F(1,20)=53.19; P<.0001], and ventral CA1 [F(1,20)=73.07; P<.001] in addition to the cingulate cortex [F(1,20)=34.42; P<.001], the shell [F(1,20)=15.42; P<.001], and core [F(1,20)= 21.34; P<.001] of the nucleus accumbens, paraventricular thalamic nucleus [F(1,20)= 13.53; P<.01], basolateral nucleus of the amygdala [F(1,20)= 31.12; P<.001], central nucleus of the amygdala [F(1,20)= 63.08; P<.001], and DRN [F(1,20)= 17.89; P<.01]. Posthoc comparisons revealed that exposure to the FST significantly enhanced c-FOS immunoreactivity in these brain regions relative to non-FST vehicle counterparts (P<.01) (Figure 2A–E; Table 1).
Table 1.
Regional c-FOS Immunoreactivity in Non-FST and in FST Exposed WKY Rats Treated with Vehicle or TRIM
| Brain Region | Non-FST Vehicle | Non-FST TRIM | FST Vehicle | FST TRIM |
|---|---|---|---|---|
| Cortical | ||||
| PLx | 134.4 ± 11.35 | 145.7 ± 17.93 | 168.5 ± 15.31 | 145.5 ± 9.17 |
| ILx | 81.60 ± 8.93 | 103.2 ± 11.62 | 106.3 ± 14.40 | 109.6 ± 4.42 |
| Cgx | 59.10 ± 3.56 | 53.90 ± 14.08 | 124.3 ± 14.80++ | 121.9 ± 5.67 |
| LS | 33.60 ± 3.82 | 34.90 ± 2.94 | 95.33 ± 1.64++ | 170.6 ± 6.05** |
| Subcortical | ||||
| NAc (shell) | 46.30 ± 8.62 | 39.70 ± 2.79 | 74.92 ± 6.02+ | 58.60 ± 5.06 |
| NAc (core) | 31.20 ± 5.12 | 33.00 ± 5.57 | 66.25 ± 6.74++ | 52.90 ± 5.60 |
| PVN | 30.50 ± 3.47 | 30.40 ± 3.39 | 64.17 ± 1.52++ | 102.2 ± 9.03** |
| PVT | 41.20 ± 4.62 | 37.00 ± 3.53 | 52.00 ± 2.65++ | 50.20 ± 1.65 |
| CeA | 9.30 ± 1.37 | 30.40 ± 4.87++ | 30.83 ± 2.70++ | 63.20 ± 3.90** |
| BlA | 8.60 ± 1.43 | 10.10 ± 2.24 | 26.92 ± 3.98++ | 27.40 ± 3.76 |
| MeA | 17.40 ± 3.56 | 19.80 ± 2.13 | 25.67 ± 3.74 | 23.00 ± 2.43 |
| Hippocampus | ||||
| dDG | 11.00 ± 1.34 | 11.50 ± 1.35 | 36.58 ± 2.17++ | 16.60 ± 2.98** |
| vCA1 | 18.30 ± 2.89 | 17.30 ± 1.87 | 55.08 ± 2.22++ | 18.00 ± 1.41** |
| Brainstem | ||||
| DRN | 28.80 ± 3.63 | 27.80 ± 2.88 | 37.17 ± 2.41+ | 17.60 ± 1.20** |
| dlPAG | 20.20 ± 2.38 | 21.90 ± 0.99 | 51.42 ± 1.66++ | 73.00 ± 2.51** |
+P<.05 relative to corresponding vehicle group. ++P<.01 relative to corresponding vehicle group. **P<.01 relative to corresponding FST vehicle-treated group.
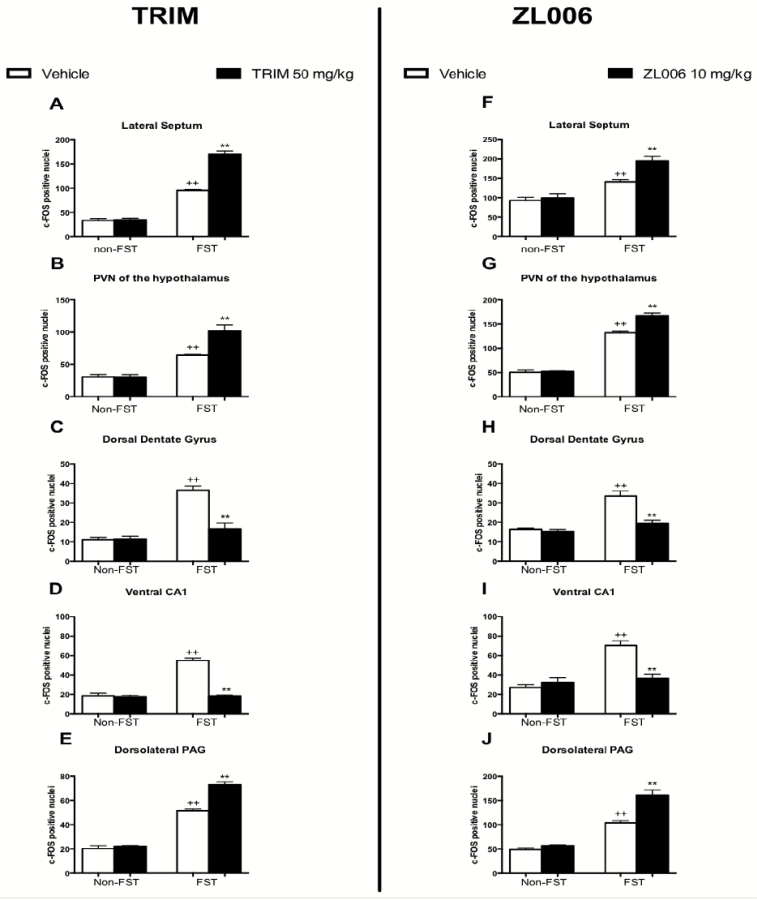
Figure 2.
Effect of the neuronal nitric oxide synthase (nNOS) inhibitor, 1-(2-Trifluoro-methyl-phenyl) imidazole (TRIM), and the PSD of 95 kDa (PSD-95)/nNOS inhibitor, 4-((3,5-dichloro-2-hydroxybenzyl)amino)-2-hydroxybenzoic acid (ZL006), on forced swimming test (FST)-induced c-FOS immunoreactivity in cortical and subcortical brain regions of the Wistar Kyoto (WKY) rat. Animals were subjected to a 15-minute pre-FST session or handled. At 1 hour following the pre-FST session, rats were administered vehicle (i.p.), TRIM (50 mg/kg; i.p.), or ZL006 (10 mg/kg; i.p.). A second and a third injection were administered 5 and 1 hours prior to the 5-minute FST the following day. Non-FST animals were handled at this time. Data expressed as mean and SEM (n=5–6 per group for TRIM and ZL006 experiments). +P<.05 relative to non-FST-exposed vehicle-treated group. ++P<.01 relative to non-FST-exposed vehicle-treated group. **P<.01 relative to corresponding vehicle-treated group.
Two-way ANOVA also revealed a significant FST x drug interaction in the lateral septum [F(1,20)= 94.70; P<.0001], the PVN of the hypothalamus [F(1,20)= 14.82; P<.001], the dlPAG [F(1,20)= 25.57; P<.0001], the dorsal dentate gyrus [F(1,20)= 23.70; P<.001], and the ventral CA1 [F(1,20)= 67.72; P<.0001] in addition to the dorsal raphe nucleus [F(1,20)= 12.02; P<.01]. Posthoc comparisons revealed that treatment with TRIM significantly enhanced FST-induced c-FOS immunoreactivity in the lateral septum (Figure 2A), the PVN of the hypothalamus (Figure 2B), the dlPAG (Figure 2E), and central nucleus of the amygdala (Table 1) relative to vehicle-treated controls (P<.01). Posthoc comparisons revealed a concomitant reduction in FST-induced c-FOS immunoreactivity in the dorsal dentate gyrus (Figure 2C), ventral CA1 (Figure 2D) of the hippocampus, and in the dorsal raphe nucleus (Table 1) (P<.01).
Effect of the Small Molecule Inhibitor ZL006 on FST-Induced Regional c-FOS Immunoreactivity
Two-way ANOVA revealed a significant effect of FST exposure on c-FOS immunoreactivity in the cingulate cortex [F(1,23)= 35.07; P<.0001], lateral septum [F(1,23)= 57.51; P<.0001], the shell [F(1,22)=26.21; P<.001], and core [F(1,22)=34.78; P<.001] of the nucleus accumbens, PVN of the hypothalamus [F(1,23)=541.56; P<.0001], paraventricular thalamic nucleus [F(1,22)= 37.83; P<.01], dorsal dentate gyrus [F(1,20)= 30.60; P<.000], ventral CA1 [F(1,23)= 28.61; P<.0001], and the dlPAG [F(1,23)=146.88; P<.0001]. Posthoc comparisons revealed that exposure to the FST significantly increased c-FOS immunoreactivity within these brain regions compared with their non-FST vehicle-treated counterparts (P<.05) (Figure 2F–J; Table 2).
Table 2.
Regional c-FOS Immunoreactivity in Non-FST and in FST Exposed WKY Rats Treated with Vehicle or ZL006
| Brain region |
Non-FST
Vehicle |
Non-FST
ZL006 |
FST Vehicle | FST ZL006 |
|---|---|---|---|---|
| Cortical | ||||
| PLx | 128.8 ± 3.67 | 123.6 ± 8.02 | 166.0 ± 13.05 | 281.8 ± 28.65** |
| ILx | 124.4 ± 5.31 | 125.7 ± 15.15 | 104.2 ± 4.78 | 172.3 ± 22.69** |
| Cgx | 69.60 ± 8.27 | 68.70 ± 8.36 | 197.8 ± 12.75++ | 119.0 ± 22.11** |
| LS | 93.25 ± 7.73 | 99.00 ± 11.03 | 140.4 ± 6.47++ | 195.5 ± 11.64** |
| Subcortical | ||||
| NAc (shell) | 47.08 ± 4.30 | 48.75 ± 4.22 | 121.5 ± 24.67++ | 124.8 ± 14.32 |
| NAc (core) | 54.67 ± 6.20 | 52.33 ± 4.09 | 90.67 ± 11.52+ | 133.9 ± 16.04** |
| PVN | 50.58 ± 4.46 | 52.17 ± 1.08 | 132.3 ± 2.88++ | 166.5 ± 6.44** |
| PVT | 42.50 ± 2.09 | 41.17 ± 3.20 | 82.50 ± 7.57++ | 73.83 ± 11.58 |
| CeA | 34.25 ± 3.82 | 33.92 ± 2.91 | 28.00 ± 2.73 | 34.17 ± 5.85 |
| Bla | 38.90 ± 4.25 | 40.25 ± 4.06 | 35.58 ± 2.05 | 38.83 ± 4.74 |
| MeA | 35.00 ± 1.29 | 37.75 ± 6.93 | 42.92 ± 3.62 | 40.83 ± 4.54 |
| Hippocampus | ||||
| dDG | 16.40 ± 0.53 | 15.13 ± 1.23 | 33.42 ± 2.65++ | 19.33 ± 1.67** |
| vCA1 | 27.08 ± 2.69 | 32.17 ± 5.03 | 70.17 ± 5.12++ | 36.42 ± 4.40** |
| Brainstem | ||||
| DRN | 67.00 ± 12.19 | 69.83 ± 11.74 | 90.50 ± 13.77 | 118.0 ± 9.71 |
| dlPAG | 48.92 ± 3.29 | 57.00 ± 2.12 | 103.8 ± 4.98++ | 160.8 ± 11.46** |
+P<.05 relative to corresponding vehicle group. ++P<.01 relative to corresponding vehicle group. **P<.01 relative to corresponding FST vehicle-treated group.
Two-way ANOVA also revealed a FST x drug interaction in the cingulate cortex [F(1,21)= 6.68; P<.01], lateral septum [F(1,23)= 6.78; P<.01], core of the nucleus accumbens [F(1,22)= 5.22; P<.01], PVN of the hypothalamus [F(1,23)= 14.95; P<.001], dorsal dentate gyrus [F(1,20)= 11.15; P<.01], ventral CA1 [F(1,23)= 19.26; P<.01], and the dlPAG [F(1,23)= 14.00; P<.01]. Posthoc comparisons revealed that treatment with ZL006 significantly enhanced FST-induced c-FOS immunoreactivity in the lateral septum (Figure 2F), PVN of the hypothalamus (Figure 2G), core of the nucleus accumbens (Table 2), and within the dlPAG (Figure 2J) relative to the vehicle-treated FST-exposed group (P<.01). Posthoc comparisons revealed that treatment with ZL006 significantly attenuated FST-induced c-FOS immunoreactivity within the cingulate cortex (Table 2), dorsal dentate gyrus (Figure 2H), and ventral CA1 of the hippocampus (Figure 2I) compared with the vehicle-treated FST-exposed group (P<.01).
Effect of the Small Molecule Inhibitor ZL006 on FST-Induced Regional NADPD-d Histology
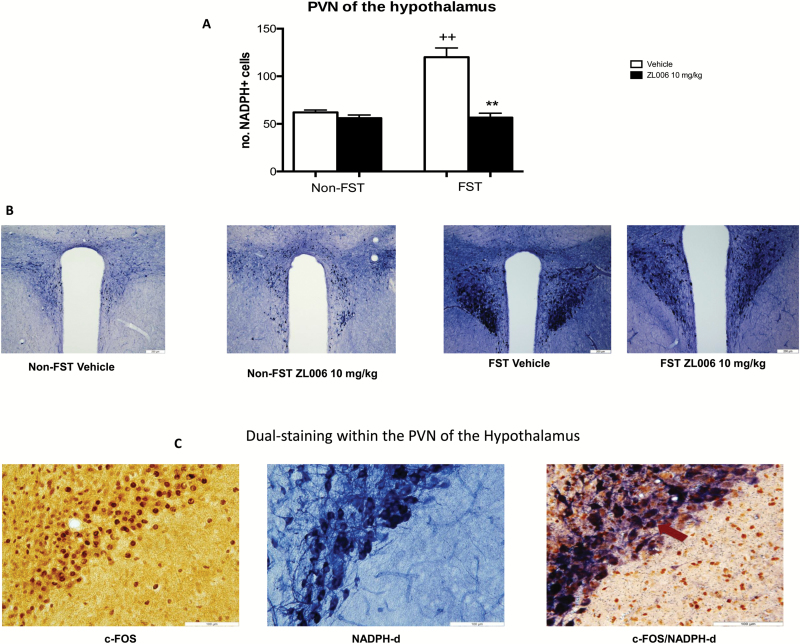
Two-way ANOVA revealed an effect of FST exposure on the number of NADPH-d-positive cells in the PVN of the hypothalamus [F(1,23)= 25.46; P<.01], shell of the nucleus accumbens [F(1,23)= 17.26; P<.01], ventral CA1 of the hippocampus [F(1,23)= 11.06; P<.05], dorsal raphe nucleus [F(1,21)= 59.33; P<.01], and dlPAG [F(1,23)= 16.14; P<.01]. Posthoc comparisons revealed that exposure to the FST increased the number of NADPH-d-positive cells in these brain regions relative to non-FST vehicle-treated counterparts (P<.05) (Figure 3A; Table 3). Two-way ANOVA revealed a significant FST x drug interaction in the PVN of the hypothalamus [F(1,23)= 24.53; P<.01] and in the shell of the nucleus accumbens [F(1,23)=17.26; P<.01]. Posthoc comparisons revealed that treatment with ZL006 reduced the number of NADPH-d-positive cells in the PVN of the hypothalamus (Figure 3A) and in the shell of the nucleus accumbens (Table 3) relative to their vehicle-treated counterparts (P<.01).
Figure 3.
Effect of 4-((3,5-dichloro-2-hydroxybenzyl)amino)-2-hydroxybenzoic acid (ZL006) administration on NADPH-d histochemistry in the paraventricular nucleus of the hypothalamus (PVN) of the hypothalamus of FST-exposed animals. Animals were subjected to a 15-minute pre-FST swim session or handled. At 1 hour following the pretest, animals were administered vehicle (i.p.) or ZL006 (10 mg/kg; i.p.). A second and a third injection were administered 5 hours and 1 hour prior to the 5-minute FST test session on the following day. Non-FST were handled at this time. Treatment with ZL006 attenuated FST-induced NADPH-d staining in the PVN of the hypothalamus (Figure 3A). Data expressed as mean and SEM (n=4–6). +P<.05 relative to non-FST vehicle-treated group. ++P<.01 relative to non-FST vehicle-treated group. **P<.01 relative to corresponding FST vehicle-treated group. Representative photomicrographs of PVN sections stained for NADPH-d from the treatment groups Vehicle Non FST; ZL006 Non FST; Vehicle FST; ZL006 FST are shown in 3B. Representative photomicrographs of dual staining for c-FOS and NADPH-d depicting colocalization within the PVN from the Vehicle FST treatment group is shown in 3C.
Table 3.
Regional NADPH Diaphorase Histochemistry in Non-FST and in FST Exposed WKY Rats Treated with Vehicle or ZL006
| Brain region | Non-FST Vehicle | Non-FST ZL006 | FST Vehicle | FST ZL006 |
|---|---|---|---|---|
| NAc (shell) | 34.25 ± 1.73 | 40.00 ± 1.88 | 47.25 ± 2.36++ | 34.08 ± 2.93** |
| NAc (core) | 45.92 ± 2.41 | 54.83 ± 2.12 | 53.08 ± 3.71 | 44.00 ± 4.15 |
| PVN | 62.08 ± 2.53 | 56.00 ± 3.25 | 120.1 ± 9.79++ | 56.54 ± 4.65** |
| dDG | 24.25 ± 2.02 | 23.83 ± 2.59 | 28.83 ± 2.43 | 26.67 ± 3.03 |
| vCA1 | 20.25 ± 2.12 | 22.08 ± 3.01 | 33.00 ± 3.948+ | 32.58 ± 4.437 |
| DRN | 41.00 ± 3.92 | 41.00 ± 2.78 | 71.00 ± 2.08++ | 73.75 ± 8.01 |
| dlPAG | 46.92 ± 3.46 | 53.00 ± 2.86 | 62.00 ± 3.57++ | 63.58 ± 2.79 |
+P<.05 relative to corresponding vehicle group. ++P<.01 relative to corresponding vehicle group. **P<.01 relative to corresponding FST vehicle-treated group.
Discussion
TRIM and ZL006 Produce an Antidepressant Response in the FST in the WKY Rat
The results of the current experiments demonstrate that treatment with TRIM and ZL006 reduce immobility time of WKY rats in the FST indicative of an antidepressant response in the test. The present study is in line with findings reported by Doucet and colleagues (Doucet et al., 2013), who demonstrated that ZL006 produces antidepressant-like effects in mice in both the FST and TST in a similar fashion to the NMDA-R antagonist, ketamine, and the nNOS inhibitor, TRIM. However, the present study demonstrates that ZL006 displays efficacy in reducing immobility behavior in an animal model of depression that is often reported to be resistant to conventional antidepressants (i.e., SSRIs) (Lahmame et al. 1997; Durand et al., 1999; Griebel et al., 1999;Tejani-Butt et al., 2003). While there is a lack of conflicting reports on the mechanism of action of ZL006 (Zhou et al., 2010; Bach et al., 2015), considerable evidence suggests it is through targeting the PSD-95/nNOS interface. The ability of ZL006 to reduce immobility time of WKY rats in the FST highlights the potential of targeting the NMDA-R/nNOS pathway for the development of novel, more efficacious antidepressants.
Regional-Specific Modulation of FST-Induced Neuronal Activation following TRIM or ZL006 Administration
Lateral Septum (LS)
Exposure to the FST enhanced c-FOS immunoreactivity within the LS, indicative of a stress-induced increase in neuronal activation within this brain region. We have previously shown that exposure to the FST increases neuronal activation within the LS of Sprague-Dawley rats (Sherwin et al., 2017). Treatment with either TRIM or ZL006 further enhanced FST-induced c-FOS immunoreactivity within the LS, which is consistent with previous work describing regional-specific modulation of neuronal activation associated with NOS inhibitors in the FST (Sherwin et al., 2017) and suggests that the region is involved in mediating antidepressant-related effects in the FST. Indeed, it was previously reported that chronic fluoxetine treatment enhances the spontaneous firing rate of septal neurons while coinciding with an antidepressant-like response in the FST (Contreras et al., 2001). TRIM or ZL006 do not share the same pharmacological mechanism of action as fluoxetine. Instead, it is likely that modulating the activity of the LS is a determinant in mediating behavioral responses in the FST.
PVN of the Hypothalamus
In the present study, exposure to the FST increased c-FOS immunoreactivity within the PVN of the hypothalamus, which was further potentiated following treatment with either TRIM or ZL006. Enhancement of neuronal activation within the PVN has previously been reported with several other antidepressants. The SSRIs fluoxetine and citalopram have both been shown to increase c-FOS immunoreactivity within this brain region in naïve animals (Jensen et al., 1999; Lino-de-Oliveira et al., 2001). Additionally, drugs with antidepressant-related properties that target the glutamatergic system such as the mGluR5 antagonist, MPEP, have been shown to elicit the same response (Inta et al., 2012). Thus, as described for the LS, modulation of the PVN may serve to influence the behavior of animals exposed to the FST.
dlPAG
Exposure to the FST enhanced c-FOS immunoreactivity within the dlPAG, indicative of stress-induced neuronal activation within this brain region. Treatment with either TRIM or ZL006 further enhanced the FST-induced c-FOS immunoreactivity. The dlPAG has been shown to mediate fight-or-flight, fearful or anxiogenic responses in rats, which is suggested to be dependent on the local activation of the NMDA-R/nNOS pathway (Tonetto et al., 2009). However, its role in mediating a depressive-like phenotype in the FST is not well documented. Inactivation of the dlPAG has previously been shown to increase the time rats spend immobile in the FST, indicating that this brain region is involved in determining passive coping behavior (Lino-de-Oliveira et al., 2002). The enhancement of FST-induced c-FOS immunoreactivity within the dlPAG following treatment with TRIM or ZL006 in the current study suggests that this region is part of a brain network regulated by NMDA-R/nNOS signaling. It was previously shown that inhibition of nNOS in the dlPAG leads to an increase in the activation of this brain region in rats (Xing et al., 2012). Stimulation of the dlPAG has also been shown to increase the latency of immobility in the FST, suggesting that activation of this region mediates antidepressant-like effects (Lino-de-Oliveira et al., 2002). Yet another study demonstrated that nNOS knockout mice display no difference in c-FOS expression in the dlPAG in response to the FST relative to wild-type counterparts (Salchner et al., 2004). Despite the paucity of information relating to a role for NOS in the activation of the dlPAG, the current study indicates that increased c-FOS immunoreactivity within this structure following treatment with TRIM or ZL006 reflects an increase in escape-orientated behavior in the FST.
Hippocampus
In the current experiments, both TRIM and ZL006 attenuated FST-induced c-FOS immunoreactivity within the dorsal DG and ventral CA1 of the hippocampus. Activation of the dorsal and ventral hippocampus in response to the FST is likely to have distinct individual functions, which gives rise to immobility time. Suppression of the FST-induced activation of the dorsal DG may relate to the learned response that gives rise to the animal adopting a passive, immobile response when reexposed to the FST, whereas activation of the ventral hippocampus contributes through regulation of the HPA axis response (Nettles et al., 2000; Bannerman et al., 2004). Given the proposed role of the ventral hippocampus in regulation of the HPA axis (Herman and Mueller 2006), reduced FST-induced c-FOS immunoreactivity in the ventral CA1 following treatment with TRIM and ZL006 may be associated with concomitant changes in neuronal activation within the hypothalamus. Further studies are, however, required to substantiate links between differential patterns of activation and regulation within circuits extending between the ventral hippocampus and PVN of the hypothalamus.
TRIM Specific Action in the CeA and DRN
Despite the aforementioned similarities between TRIM and ZL006 in the modulation of FST-induced neuronal activation, there were notable differences also. TRIM, but not ZL006, increased c-FOS immunoreactivity in naïve and FST-exposed animals in the CeA. This effect was not observed previously in rats following L-NA treatment under similar experimental conditions (Sherwin et al., 2017). Activation of the CeA has also been observed with conventional antidepressants. For instance, acute administration of citalopram and fluoxetine have been reported to increase c-FOS immunoreactivity within the CeA at baseline and in response to the FST (Miyata et al., 2005; Choi et al., 2013; Kawahara et al., 2013). The ability of antidepressants such as fluoxetine and citalopram to facilitate an increase in neuronal activation within the CeA may relate to pharmacologically induced alterations in 5-HT transmission within the structure. It is important to note, however, that a higher basal level of c-FOS immunoreactivity within the amygdala (CeA, BIA, and MeA) was observed in the experiment involving treatment with ZL006. An FST-related increase in c-FOS immunoreactivity, however, was observed in the DRN of both experiments where treatment with TRIM, but not ZL006, was found to attenuate the FST-related increase. Here a TRIM-specific modulation of neuronal activation within the DRN nucleus may be due to the close association between nNOS and 5-HT in the DRN (Chanrion et al. 2007). In addition to its interaction with PSD-95, nNOS is known to associate with the serotonin transporter. TRIM may thus influence serotonin transporter-associated variants of nNOS accounting for differences with ZL006. Activation of the DRN following exposure to stress is suggested to shift cognitive perception of stress from controllable to uncontrollable, giving rise to depressive-related behavior (Amat et al., 2005). The reduction in c-FOS immunoreactivity within the DRN following treatment with TRIM may contribute to the observed reduction in immobility. Further work is required to investigate and clarify the actions of TRIM in the DRN. A DRN-related action, however, is unlikely to account for the antidepressant-related activity of ZL006 in the test.
ZL006-Specific Modulation of FST-Induced Neuronal Activation in Cortical Areas and the Nucleus Accumbens (Core)
In the current experiments, there were instances where ZL006, but not TRIM, altered FST-induced neuronal activation, namely in the PLx, ILx, Cgx, and the core of the NAc. These specific effects of ZL006 on neuronal activation are likely due to its action, which is distinct from that of TRIM (Zhou et al., 2010; Bach et al., 2015; Lee et al., 2015). In the PLx and ILx, neither ZL006 nor FST alone influenced c-FOS immunoreactivity. However, in combination an increased response was obtained compared with either treatment alone, indicating a possible synergy. By contrast, ZL006 attenuated the FST-induced increase in c-FOS immunoreactivity in the Cgx, indicating that this region responds differently to ZL006 compared with the other cortical areas. The role of the Cgx in mediating behavioral responses in the FST or its response to antidepressants are not well investigated to date. A study by Li and colleagues (Li et al., 2012) demonstrated that lesions to the anterior Cgx have no impact on immobility time in the FST. However, another study determined that lesions to the Cgx serve to increase immobility time of rats in the FST (Bissiere et al., 2006). This would indicate that activation of the Cgx following exposure to the FST is unlikely to relate to immobility and that modulation of the Cgx following treatment with ZL006 is unlikely to have a bearing upon its antidepressant-like action in the FST.
Both shell and core of the NAc are implicated in mediating behavioral responses to antidepressants (Shirayama and Chaki 2006). Studies have shown that neuronal activation (in terms of both c-FOS and ΔFosB expression) within the core and shell can be influenced by both acute and chronic antidepressant treatments (Vialou et al., 2010a; Silva et al., 2012). Induction of ΔFosB in the NAc following chronic antidepressant treatment is linked with an increase in stress resilience (Vialou et al., 2010b). In the present investigation, treatment with ZL006 enhanced FST-induced C-FOS immunoreactivity in the core of the NAc. Silva and colleagues (Silva et al., 2012) reported reduced c-FOS immunoreactivity within the NAc following treatment with the nNOS inhibitor, 7-NI, although no such action of TRIM was observed in the current investigation. Evidence suggests that nNOS inhibitors, when injected peripherally or injected locally into the accumbens, can suppress NO production within the NAc. Moreover, when NMDA is directly applied to the NAc, it increases the production of NO, suggesting that NO production is regulated through an NMDA-R/nNOS pathway (Prast et al., 2015). This suggests that the NAc could be a target region modulated by ZL006. In support, ZL006 attenuated FST-related increase in NADPH-d staining indicative of reduced nNOS activity. However, these findings overall do not provide a mechanism likely to relate to the observed antidepressant-like actions in the FST, as TRIM reduced immobility yet failed to influence FST-induced c-FOS immunoreactivity in the NAc.
Reduced NADPH-Diaphorase Staining in the PVN of the Hypothalamus following ZL006 Administration
Stress has been shown to increase NADPH-d staining in the PVN of the hypothalamus, amygdala, hippocampus, and the DRN in rats (Krukoff and Khalili 1997; Sánchez et al., 1999; Echeverry et al., 2004; Joung et al., 2012). In the present study, exposure to the FST resulted in an increase in NADPH-d staining in the NAc, vCA1 of the hippocampus, DRN, dlPAG, and PVN of the hypothalamus consistent with previous literature (Sánchez et al., 1999).
While NADPH is a cofactor for all isoforms of NOS, dual staining studies with nNOS in the PVN of the hypothalamus have identified that stress-induced increases in NADPH-d staining occurs within nNOS-expressing cells (Echeverry et al., 2004). Thus, it is likely that the observed increase in NADPH-d staining in the current study represents increased nNOS activity. Treatment with ZL006 attenuated FST-induced NADPH-d staining indicative of a suppression of nNOS activity. In support, a previous study has shown that treatment with the selective nNOS inhibitor, 7-NI, but not the nonselective NOS inhibitor, L-NAME, attenuated stress-induced NADPH-d staining, accompanied by a decrease in the level of nitrate metabolites, in the PVN of the hypothalamus, and the lateral dorsal tegmental nucleus in rats (Joung et al., 2012). This is conisistent with the present investigation where the absence of an effect of ZL006 on NADPH-d staining in non-FST-exposed animals indictaes that stress is required to trigger an increase in NADPH-d staining that is attenuated following ZL006 administration.
The PVN of the hypothalamus was the only region in which c-FOS immunoreactivity and NADPH-d staining were both modulated following treatment with ZL006. The FST-related increase in c-FOS enhanced by ZL006 was present concomitant with an FST-related increase in NADPH-d attenuated by ZL006. Given that ZL006 has such opposing actions, it was of interest to determine that c-FOS colocalized with NADPH-d positive cells within the PVN, providing further support for the likelihood that enhanced FST-induced neuronal activation following treatment with ZL006 was associated with the local inhibition of nNOS via inhibition of the NMDA-R/PSD-95/nNOS pathway. ZL006 also attenuated FST-induced NADPH-d staining within the shell of the nucleus accumbens (Table 3). Whether this is related to the antidepressant-related properties of ZL006 is undetermined. However, as ZL006 failed to influence FST-induced c-FOS immunoreactivity in this region, it is unlikely that inhibition of nNOS within the NAc is associated with its antidepressant-related properties in the FST. A lack of effects of ZL006 on stress-induced NADPH-d staining in other brain regions may relate to the relative activity of the NMDA-NO signaling pathway in modulating nNOS activity in response to stress in these regions.
Conclusions
ZL006, a small molecule inhibitor of the PSD-95/nNOS interface, displays antidepressant activity in the FST in the WKY rat. These data build on previous work targeting the NMDA-R pathway for antidepressant-related properties in mice (Doucet et al., 2013). Furthermore, the antidepressant-like effects of ZL006 are associated with the modulation of stress-induced neuronal activation in several brain regions. The observed changes in c-FOS immunoreactivity in the current study align with previous findings of NOS inhibitor modulation of FST-induced neuronal activation (Sherwin et al., 2017) and extend previous findings by targeting NMDA-R associated nNOS. Moreover, exposure to the FST was sufficient to facilitate a stress-induced increase in NADPH-d reflective of NOS activity in the PVN. This FST-induced increase was attenuated following treatment with ZL006, consistent with previous reports of nNOS inhibitors attenuating enzymatic activity within this brain region. The observations of the current study identify the PVN of the hypothalamus as a brain region where ZL006 acts to attenuate FST-induced nNOS activity while concomitantly regulating region specific neuronal activation associated with an antidepressant related response.
Statement of Interest
None
Acknowledgments
This research was funded by the Health Research Board (HRB) of Ireland. E.S. was supported by a postgraduate research studentship from Trinity College Dublin.
References
- Akinfiresoye L, Tizabi Y (2013) Antidepressant effects of AMPA and ketamine combination: role of hippocampal BDNF, synapsin, and mTOR. Psychopharmacol 230:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderton WK, Cooper CE, Knowles RG (2001) Nitric oxide synthases: structure, function and inhibition. Biochem J 357:593–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF (2005) Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci 8:365–371. [DOI] [PubMed] [Google Scholar]
- Amir S, Rackover M, Funk D (1997) Blockers of nitric oxide synthase inhibit stress activation of c-fos expression in neurons of the hypothalamic paraventricular nucleus of the rat. Neurosci 77:623–627. [DOI] [PubMed] [Google Scholar]
- Bach A, Pedersen SW, Dorr LA, Vallon G, Ripoche I, Ducki S, Lian LY (2015) Biochemical investigations of the mechanism of action of small molecules ZL006 and IC87201 as potential inhibitors of the nNOS-PDZ/PSD-95-PDZ interactions. Sci Rep 5:12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JNP, McHugh SB, Deacon RMJ, Yee BK, Bast T, Zhang WN, Pothuizen HHJ, Feldon J (2004) Regional dissociations within the hippocampus: memory and anxiety. Neurosci Biobehav Rev 28:273–283. [DOI] [PubMed] [Google Scholar]
- Bissiere S, McAllister KH, Olpe HR, Cryan JF (2006) The rostral anterior cingulate cortex modulates depression but not anxiety-related behavior in the rat. Behav Brain Res 175:195–199. [DOI] [PubMed] [Google Scholar]
- Carr GV, Bangasser DA, Bethea T, Young M, Valentino RJ, Lucki I (2010) Antidepressant-like effects of kappa-opioid receptor antagonists in Wistar Kyoto rats. Neuropsychopharmacol 35:752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanrion B, Mannoury La Cour C, Bertaso F, Lerner-natoli M, Freissmuth M, Millan MJ, Bockaert J and Marin P (2007) Physical interaction between the serotonin transporter and neuronal nitric oxide synthase underlies reciprocal modulation of their activity. Proc Nat Acad Sci USA 104:8119–8124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Chung S, Cho JH, Cho YH, Kim JW, Kim JK, Jeong H, Kim HJ, Shin KH (2013) Changes in c-Fos expression in the forced swimming test: common and distinct modulation in rat brain by desipramine and citalopram. Korean J Physiol Pharmacol 17:321–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras CM, Rodriguez-Landa JF, Gutiérrez-Garca AG, Bernal-Morales B (2001) The lowest effective dose of fluoxetine in the forced swim test significantly affects the firing rate of lateral septal nucleus neurones in the rat. J Psychopharmacol 15:231–236. [DOI] [PubMed] [Google Scholar]
- Doucet MV, Harkin A, Dev KK (2012) The PSD-95/nNOS complex: new drugs for depression? Pharmacol Ther 133:218–229. [DOI] [PubMed] [Google Scholar]
- Doucet MV, Levine H, Dev KK, Harkin A (2013) Small-molecule inhibitors at the PSD-95/nNOS interface have antidepressant-like properties in mice. Neuropsychopharmacol 38:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet MV, O’Toole E, Connor T, Harkin A (2014) Small-molecule inhibitors at the PSD-95/nNOS interface promote outgrowth in primary cortical neurons. Neurosci 301:421–438. [DOI] [PubMed] [Google Scholar]
- Durand M, Berton O, Aguerre S, Edno L, Combourieu I, Mormède P, Chaouloff F (1999) Effects of repeated fluoxetine on anxiety-related behaviors, central serotonergic systems, and the corticotropic axis axis in SHR and WKY rats. Neuropharmacol 38:893–907. [DOI] [PubMed] [Google Scholar]
- Echeverry MB, Guimarães FS, Del Bel EA (2004) Acute and delayed restraint stress-induced changes in nitric oxide producing neurons in limbic regions. Neurosci 125:981–93. [DOI] [PubMed] [Google Scholar]
- Gigliucci V, Buckley KB, Nunan J, O’Shea K, Harkin A (2010) A role for serotonin in the antidepressant activity of NG-Nitro-L-arginine, in the rat forced swimming test. Pharmacol Biochem Behav 94:524–533. [DOI] [PubMed] [Google Scholar]
- Gigliucci V, Gormley S, Gibney S, Rouine J, Kerskens C, Connor TJ, Harkin A (2014) Characterisation of the antidepressant properties of nitric oxide synthase inhibitors in the olfactory bulbectomised rat model of depression. Eur Neuropsychopharmacol 24:1349–1361. [DOI] [PubMed] [Google Scholar]
- Gormley S, Rouine J, McIntosh A, Kerskens C, Harkin A (2016) Glial fibrillary acidic protein (GFAP) immunoreactivity correlates with cortical perfusion parameters determined by bolus tracking arterial spin labelling (bt-ASL) magnetic resonance (MR) imaging in the Wistar Kyoto rat. Physiol Behav 160: 66–79. [DOI] [PubMed] [Google Scholar]
- Griebel G, Cohen C, Perrault G, Sanger DJ (1999) Behavioral effects of acute and chronic fluoxetine in Wistar-Kyoto rats. Physiol Behav 67:315–320. [DOI] [PubMed] [Google Scholar]
- Guix FX, Uribesalgo I, Coma M, Muñoz FJ (2005) The physiology and pathophysiology of nitric oxide in the brain. Prog Neurobiol 76:126–152. [DOI] [PubMed] [Google Scholar]
- Harkin A, Connor TJ, Walsh M, St John N, Kelly JP (2003) Serotonergic mediation of the antidepressant-like effects of nitric oxide synthase inhibitors. Neuropharmacol 44: m616–623. [DOI] [PubMed] [Google Scholar]
- Herman JP, Mueller NK (2006) Role of the ventral subiculum in stress integration. Behav Brain Res 174:215–224. [DOI] [PubMed] [Google Scholar]
- Hope BT, Gregory MJ, Knigge KM, Vincent SR (1991) Neuronal NADPH diaphorase is a nitric oxide synthase. Proc Nat Acad Sci USA 88:2811–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inta D, Filipovic D, Lima-Ojeda JM, Dormann C, Pfeiffer N, Gasparini F, Gass P (2012) The mGlu5 receptor antagonist MPEP activates specific stress-related brain regions and lacks neurotoxic effects of the NMDA receptor antagonist MK-801: significance for the use as anxiolytic/antidepressant drug. Neuropharmacol 62:2034–2039. [DOI] [PubMed] [Google Scholar]
- Jensen JB, Jessop DS, Harbuz MS, Mørk A, Sánchez C, Mikkelsen JD (1999) Acute and long-term treatments with the selective serotonin reuptake inhibitor citalopram modulate the HPA axis activity at different levels in male rats. J Neuroendocrinol 11:465–471. [DOI] [PubMed] [Google Scholar]
- Joung HY, Jung EY, Kyungsoo K, Lee MS, Her S, Shim I (2012) The differential role of NOS inhibitors on stress-induced anxiety and neuroendocrine alterations in the rat. Behav Brain Res 235:176–181. [DOI] [PubMed] [Google Scholar]
- Kawahara R, Soeda F, Kawaura K, Honda S, Miki R, Noguchi T, Shirasaki T, Takahama K (2013) Effect of tipepidine with novel antidepressant-like action on c-fos-like protein expression in rat brain. Brain Res 1513:135–142. [DOI] [PubMed] [Google Scholar]
- Krukoff TL, Khalili P (1997) Stress-induced activation of nitric oxide-producing neurons in the rat brain. J Compar Neurol 377:509–519. [DOI] [PubMed] [Google Scholar]
- Lahmame A, Del Arco C, Pazos A, Yritia M, Armario A (1997) Are Wistar-Kyoto rats a genetic animal model of depression resistant to antidepressants? Eur J Pharmacol 337:115–123. [DOI] [PubMed] [Google Scholar]
- Lee WH, Xu Z, Ashpole NM, Hudmon A, Kulkarni PM, Thakur GA, Lai YY, Hohmann AG (2015) Small molecule inhibitors of PSD95-nNOS protein-protein interactions as novel analgesics. Neuropharmacol 97:464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Li M, Cao W, Xu Y, Luo Y, Zhong X, Zhang J, Dai R, Zhou X- F, Li Z, Li C (2012) Anterior cingulate cortical lesion attenuates food foraging in rats. Brain Res Bull 88:602–608. [DOI] [PubMed] [Google Scholar]
- Lino-de-Oliveira C, Sales AJ, Del Bel EA, Leite Silveira MC, Guimarāes FS (2001) Effects of acute and chronic fluoxetine treatments on restraint stress-induced Fos expression. Brain Res Bull 55:747–754. [DOI] [PubMed] [Google Scholar]
- Lino-de-Oliveira C, De Lima TCM, Carobrez AP (2002) Dorsal periaqueductal gray matter inhibits passive coping strategy elicited by forced swimming stress in rats. Neurosci Lett 335:87–90. [DOI] [PubMed] [Google Scholar]
- Malkesman O, Austin DR, Tragon T, Wang G, Rompala G, Hamidi AB, Cui Z, Young WS, Nakazawa K, Zarate CA Jr, Manji HK, Chen G (2012) Acute D-serine treatment produces antidepressant-like effects in rodents. Int J Neuropsychopharmacol 15:1135–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Hamamura T, Lee Y, Miki M, Habara T, Oka Y, Endo S, Taoka H, Kuroda S (2005) Contrasting Fos expression induced by acute reboxetine and fluoxetine in the rat forebrain: neuroanatomical substrates for the antidepressant effect. Psychopharmacol 177:289–295. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Simpson CS, Mundell S, Macheachern K, Johnston HM, Nolan AM (1997) Dynamic changes in NADPH-diaphorase staining reflect activity of nitric oxide synthase: evidence for a dopaminergic regulation of striatal nitric oxide release. Neuropharmacol 36:1589–1599. [DOI] [PubMed] [Google Scholar]
- Nagasawa M, Ogino Y, Kurata K, Otsuka T, Yoshida J, Tomonaga S, Furuse M (2012) Hypothesis with abnormal amino acid metabolism in depression and stress vulnerability in Wistar Kyoto rats. Amino Acids 43:2101–2111. [DOI] [PubMed] [Google Scholar]
- Nam H, Clinton SM, Jackson NL, Kerman IA (2014) Learned helplessness and social avoidance in the Wistar-Kyoto rat. Front Behav Neurosci 8:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettles KW, Pesold C, Goldman MB (2000) Influence of the ventral hippocampal formation on plasma vasopressin, hypothalamic-pituitary-adrenal axis, and behavioral responses to novel acoustic stress. Brain Res 858:181–190. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1998) The Rat Brain Atlas. San Diego: Academic Press, Inc. [Google Scholar]
- Porsolt R. (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266:730–732. [DOI] [PubMed] [Google Scholar]
- Prast H, Hornick A, Kraus MM, Philippu A (2015) Origin of endogenous nitric oxide released in the nucleus accumbens under real-time in vivo conditions. Life Sci 134:79–84. [DOI] [PubMed] [Google Scholar]
- Redei EE, Solberg L, Kluczynski JM, Pare WP (2001) Paradoxical hormonal and behavioral responses to hypothyroid and hyperthyroid states in the Wistar-Kyoto rat. Neuropsychopharmacol 24:632–639. [DOI] [PubMed] [Google Scholar]
- Rittenhouse PA, López-Rubalcava C, Stanwood GD, Lucki I (2002) Amplified behavioral and endocrine responses to forced swim stress in the Wistar–Kyoto rat. Psychoneuroendocrinol 27:303–318. [DOI] [PubMed] [Google Scholar]
- Salchner P, Lubec G, Engelmann M, Orlando GF, Wolf G, Sartori SB, Hoeger H, Singewald N (2004) Genetic functional inactivation of neuronal nitric oxide synthase affects stress-related Fos expression in specific brain regions. Cell Mol Life Sci 61:1498–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez F, Moreno MN, Vacas P, Carretero J, Vásquez R (1999) Swim stress enhances the NADPH-diaphorase histochemical staining in the paraventricular nucleus of the hypothalamus. Brain Res 828:159–162. [DOI] [PubMed] [Google Scholar]
- Sherwin E, Gigliucci V, Harkin A (2017) Regional specific modulation of neuronal activation associated with nitric oxide synthase inhibitors in an animal model of antidepressant activity. Behav Brain Res 316:18–28. [DOI] [PubMed] [Google Scholar]
- Shirayama Y, Chaki S (2006) Neurochemistry of the nucleus accumbens and its relevance to depression and antidepressant action in rodents. Curr Neuropharmacol 4:277–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M, Aguiar DC, Diniz CRA, Guimarās FS (2012) Neuronal NOS inhibitor and conventional antidepressant drugs attenuate stress-induced Fos expression in overlapping brain regions. Cell Mol Neurobiol 32:443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejani-Butt S, Kluczynski J, Paré WP (2003) Strain-dependent modification of behavior following antidepressant treatment. Prog Neuro-psychopharmacol Biol Psych 27:7–14. [DOI] [PubMed] [Google Scholar]
- Tonetto LL, Terzian AL, Del Bel E, Guimarāes FS, Resstel LBM (2009) Inhibition of the NMDA receptor/Nitric Oxide pathway in the dorsolateral periaqueductal gray causes anxiolytic-like effects in rats submitted to the Vogel conflict test. Beh Brain Funct 5:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulak G, Mutlu O, Akar FY, Komsuoglu FI, Tanyeri P, Erden BF (2008) Neuronal NOS inhibitor 1-(2-trifluoromethylphenyl)-imidazole augment the effects of antidepressants acting via serotonergic system in the forced swimming test in rats. Pharmacol Biochem Behav 90:563–568. [DOI] [PubMed] [Google Scholar]
- Vialou V, Robison AJ, LaPlant QC, Covington HE III, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ III, Watts EL, Wallace DL, Iñiguez SD, Ohnishi YH, Steiner MA, Warren BL, Krishnan V, Bolaños CA, Neve RL, Ghose S, Berton O, Tamminga CA, Nestler EJ (2010a) DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci 13:745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Maze I, Renthal W, LaPlant QC, Watts EL, Mouzon E, Ghose S, Tamminga CA, Nestler EJ (2010b) Serum response factor promotes resilience to chronic social stress through the induction of DeltaFosB. J Neurosci 30:14585–14592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Kong J, Lu J, Li J (2012) Angiotensin-(1–7) inhibits neuronal activity of dorsolateral periaqueductal gray via a nitric oxide pathway. Neurosci Lett 522:156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Li F, Xu H- B, Luo C- X, Wu H- Y, Zhu M- M, Lu W, Ji X, Zhou Q- G, Zhu D- A (2010) Treatment of cerebral ischemia by disrupting ischemia-induced interaction of nNOS with PSD-95. Nat Med 16:1439–1443. [DOI] [PubMed] [Google Scholar]