Abstract
Background
Cariprazine, a D3-preferring dopamine D2/D3 receptor partial agonist, is a new antipsychotic drug recently approved in the United States for the treatment of schizophrenia and bipolar mania. We recently demonstrated that cariprazine also has significant antianhedonic-like effects in rats subjected to chronic stress; however, the exact mechanism of action for cariprazine’s antidepressant-like properties is not known. Thus, in this study we examined whether the effects of cariprazine are mediated by dopamine D3 receptors.
Methods
Wild-type and D3-knockout mice were exposed to chronic unpredictable stress for up to 26 days, treated daily with vehicle, imipramine (20 mg/kg), aripiprazole (1 and 5 mg/kg), or cariprazine (0.03, 0.1, 0.2, and 0.4 mg/kg), and tested in behavioral assays measuring anhedonia and anxiety-like behaviors.
Results
Results showed that cariprazine significantly attenuated chronic unpredictable stress-induced anhedonic-like behavior in wild-type mice, demonstrating potent antidepressant-like effects comparable with aripiprazole and the tricyclic antidepressant imipramine. This antianhedonic-like effect of cariprazine was not observed in D3-knockout mice, suggesting that the cariprazine antidepressant-like activity is mediated by dopamine D3 receptors. Moreover, cariprazine significantly reduced drinking latency in the novelty-induced hypophagia test in wild-type mice, further confirming its antianhedonic-like effect and showing that it also has anxiolytic-like activity.
Conclusions
In combination with previous studies, these results suggest that cariprazine has a unique pharmacological profile and distinct dopamine D3 receptor-dependent mechanism of action that may be beneficial in the treatment of schizophrenia, bipolar disorder, and major depressive disorder.
Keywords: cariprazine, antidepressant, anxiolytic, dopamine D3 receptor, anhedonia
Significance Statement
In a mouse model of depression, cariprazine produces antidepressant-like activity through action on dopamine D3 receptors. Indeed, cariprazine reversed anhedonia-like and anxiety-like deficits in this chronic stress model. These results suggest that cariprazine has a unique and distinct dopamine D3 receptor-dependent mechanism of action that may be beneficial in the treatment of schizophrenia, bipolar disorder, and major depressive disorder.
Introduction
Major depressive disorder is a common, chronic, recurring illness associated with significant functional and social impairment (Duric and Duman, 2013). Effective treatment of depression is challenging, with most patients failing to achieve remission after initial antidepressant treatment. As a result, antipsychotics are increasingly used as adjunct therapy to enhance antidepressant efficacy, especially since augmentation with atypical agents improves pharmacological and side effect profiles (Nelson and Papakostas, 2009). Although central nervous system mechanisms underlying antidepressant actions of atypical antipsychotics are still unclear, it has been proposed that a compound that exhibits high affinity and occupancy of both D2 and D3 dopamine receptors may be effective as a treatment for depressive disorders as well as the negative symptoms of schizophrenia. A dopamine D3 receptor strategy for treatment of depression or schizophrenia is based on the brain distribution and the putative role of D3 receptors (Gross and Drescher, 2012); these receptors have the highest density in rat and human ventral striatum (Sokoloff et al., 1990; Gurevich and Joyce, 1999), one of the core areas implicated in the pathology of schizophrenia as well as depression and anxiety. The potential involvement of dopamine D3 receptors in the effects of antidepressants was suggested by several preclinical studies demonstrating that administration of antidepressant drugs enhances D3 receptor gene expression and/or binding in distinct brain regions (Maj et al., 1998; Lammers et al., 2000).
Cariprazine, a novel, orally active antipsychotic agent that is a potent dopamine D2/D3 receptor partial agonist with preferential binding to D3 receptors, was recently approved by the U.S. Food and Drug Administration for the treatment of both schizophrenia and acute manic or mixed episodes associated with bipolar I disorder (Ágai-Csongor et al., 2012; Veselinovic et al., 2013; Findlay et al., 2016). Among atypical antipsychotics, cariprazine displays the highest D3 receptor binding affinity and D3 vs. D2 receptor selectivity (by approximately 6- to 8-fold) (Kiss et al., 2010; McCormick et al., 2010; Ellenbroek and Cesura, 2015). These features could be responsible for cariprazine showing a more balanced dopamine D2 and D3 receptor brain occupancy in vivo in both rodents (Gyertyán et al., 2011; Kiss et al., 2012) and patients (Girgis et al., 2016) compared with other antipsychotics, which displayed preferential occupancy for D2 vs. D3 receptors (Graff-Guerrero et al., 2009; McCormick et al., 2010; Mizrahi et al., 2011). Furthermore, cariprazine was also found to be unique among antipsychotics in its ability to increase dopamine D3 receptor levels in D3 receptor-rich brain regions following chronic treatment (Choi et al., 2014). The nucleus accumbens shell is one of the brain regions of particular interest where cariprazine produced this effect, and an increase in D3 receptor expression in this region has been proposed to be a common neurobiological mechanism of antidepressant treatments (Lammers et al., 2000).
Cariprazine has demonstrated antidepressant-like activity in the chronic mild stress model in rats and was shown to reduce anhedonia-like behavior, a hallmark symptom of depression and negative symptoms of schizophrenia (Papp et al., 2014); however, the mechanism of action underlying cariprazine’s antidepressant-like effects has not been elucidated. A previous study of cariprazine in dopamine D3 receptor-knockout (D3-KO) mice showed that cariprazine has D3 receptor-dependent positive/protective effects on cognitive function, suggesting that the efficacy of cariprazine in some symptom domains is at least partly mediated through its D3-receptor activity (Zimnisky et al., 2013). Therefore, in the current study, we investigated the antidepressant- and anxiolytic-like effects of cariprazine in a mouse model of chronic unpredictable stress (CUS) and determined whether these effects are mediated by D3 receptors using mutant D3-KO mice. Moreover, we explored whether there were any differences in the antidepressant- and anxiolytic-like effects of cariprazine compared with aripiprazole, another atypical antipsychotic and dopamine receptor partial agonist, which, unlike cariprazine, has a higher affinity for dopamine D2 vs. D3 receptors.
Materials and Methods
Animals
Adult male C56BL/6 mice (Jackson Laboratory) were used for all studies, except for the experiment using mutant D3-KO and littermate wild-type (WT) mice obtained from Dr. Marc Caron (Duke University, Durham, NC) (Beaulieu et al., 2007). Mice were housed in groups of 1 to 5 per cage under a 12-h-light/-dark cycle at constant temperature (25°C) and humidity with ad libitum access to food and water (except when indicated). Prior to any treatments or experiments, animals were allowed at least 1 week of habituation to the housing conditions. All mice were age and weight matched (~25–30 g) at the time of the first stressor. The maintenance of mouse colonies and all animal treatments and procedures were in strict accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Yale Animal Care and Use Committee.
Chronic Unpredictable Stress
Mice were exposed to a variable sequence of unpredictable stressors as described in our recent studies (Koo and Duman, 2008; Duric et al., 2010; Koo et al., 2010). Overall, mice were subjected to 10 different stressors, 2/d for up to 26 consecutive days. Stressors included: restraint stress (1 hour), cold (1 hour), forced swim (10 minutes), light/dark cycle disturbance, strobe light, odor, no/wet bedding, and cage tilt. Control mice were handled daily during vehicle (e.g., Tween 80 [1%]) administration and weighing. In conjunction with CUS exposure, imipramine (20 mg/kg; q.d.), aripiprazole (1 and 5 mg/kg; b.i.d.), or cariprazine (0.03, 0.1, 0.2, and 0.4 mg/kg; b.i.d.) were administered i.p. for 26 days (n = 6–8 mice/group). Twice-daily (i.e., b.i.d.) dosing regimen was used for cariprazine due to its relatively short plasma t1/2 of approximately 3.2 hours. This was based on our previous pharmacokinetic studies in male mice (26–32 g) administered (i.p.) 1 mg/kg of cariprazine (data on file, G. Richter Plc). Moreover, vehicle was administrated i.p. to the control vehicle group (b.i.d.) and the imipramine group once per day to standardize the total number of injections per animal. Behavioral testing started after 18 days of CUS with continued exposure to stress and drug treatments. Each test was performed a minimum of 12 hours after the last stressor and last injection.
Behavioral Testing
Sucrose and Water Consumption Tests
The effects of CUS and drug administration on anhedonic-like behavior were assessed by measuring sucrose consumption (Pothion et al., 2004) as previously described in our laboratory (Duric et al., 2010). Mice were single-housed and habituated to palatable 1% sucrose solution (Sigma) for 48 hours (free access) prior to testing. Following overnight fluid deprivation, animals were exposed to sucrose solution for 1 hour, and the total volume consumed was measured by weight and recorded. Results are expressed in grams of sucrose consumed during a 1-hour test period. For the water consumption test, the same procedure was repeated with tap water. Both tests were conducted after at least 18 days of consecutive CUS exposure.
Novelty-Induced Hypophagia Test
Mice were habituated with diluted (1:3 milk/water) sweetened condensed milk (Carnation, Nestle USA) for 1 hour on 3 consecutive days. At first, mice were tested in the home cage under normal lighting by replacing the water bottle with the milk (1 mL) presented in a small petri dish placed in the middle of the cage, and the latency to drink was recorded with a maximum cutoff set at 5 minutes. For novel cage testing, latency to drink was recorded after mice were placed in different clean cages of the same dimensions (no bedding) under dim lighting (~50 lux) with white paper under cages to enhance aversion. Animals with latency to drink exceeding 10 minutes in the home cage were excluded from the novelty-induced hypophagia analysis.
Locomotor Activity Test
Locomotor activity was measured during the CUS treatment paradigm to control for changes in ambulatory activity. Mice were placed in novel home cage-like arenas, and their behavior was video-recorded over the next 30 minutes. Total locomotor activity was determined as distance traveled (in meters) using the video-tracking software ANY-maze (Stoelting Co).
Statistical Analysis
One-way ANOVA analysis (StatView 5.0; SAS Institute) was used to determine whether CUS produced significant effects on sucrose consumption or drinking latency in the novelty-induced hypophagia test. Since imipramine was used as a positive control and the low dose of cariprazine was not tested in D3-KO mice, the experimental design was asymmetric. We excluded animal groups not represented in both genotypes for the 2-way ANOVA analysis testing for significant interactions between genotype and treatment for sucrose consumption, water consumption, locomotor activity, and drinking latency. All mouse groups were included in the posthoc 1-way ANOVA and pairwise comparison using Fisher’s probable least-squares difference (PLSD).
Results
Cariprazine Produces Antidepressant- and Anxiolytic-Like Effects in CUS Mice
In the first phase of the study, we investigated the dose range of cariprazine required to reverse CUS-induced anhedonia-like and anxiety-like deficits in mice. Mice were exposed to a variable sequence of unpredictable stressors (2/d) for a total of 26 days (Figure 1a). Throughout the stress paradigm, mice were administered vehicle or cariprazine, or imipramine that served as positive control; there was also a separate group not exposed to the CUS procedure that received vehicle.
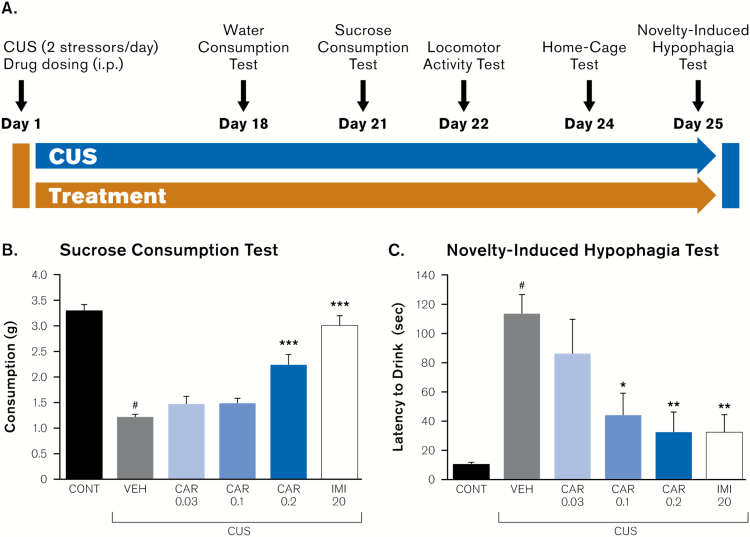
Figure 1.
Influence of chronic unpredictable stress (CUS) and drug treatment on anhedonia- and anxiety-like behavioral responses. (A) Mice were exposed to the CUS paradigm or control conditions for 26 days and were administered vehicle, cariprazine, or imipramine. Sucrose consumption, water consumption, novelty-induced hypophagia, and locomotor activity were determined. Behavioral results for (B) sucrose consumption test (day 21 of CUS) and (C) novelty-induced hypophagia test (day 25 of CUS) are shown, expressed as mean ± SEM (n = 6–7). All doses are in mg/kg. #P < .0001 compared to nonstressed control group; *P<.05 compared with CUS + vehicle group; **P<.001 compared to CUS + vehicle group; ***P<.0001 compared with CUS + vehicle group (1-way ANOVA and Fisher’s PLSD posthoc analysis). CAR, cariprazine; CONT, control; IMI, imipramine; VEH, vehicle.
One-way ANOVA analysis revealed that 21 days of CUS exposure resulted in significant changes in sucrose consumption (day 21; F5,35=30.28, P <.0001) (Figure 1b). Vehicle-treated mice subjected to CUS exhibited a significant decrease in sucrose consumption, indicating CUS-induced anhedonia-like deficits (P <.0001) (Figure 1b). In addition, treatments with cariprazine 0.2 mg/kg (but not 0.03 or 0.1 mg/kg) significantly attenuated the CUS-induced decrease of sucrose consumption (P <.0001 vs. CUS + vehicle; Figure 1b), demonstrating cariprazine’s antidepressant-like activity. Likewise, chronic administration of imipramine (20 mg/kg) also robustly increased sucrose consumption in CUS animals (P <.0001 vs. CUS + vehicle; Figure 1b), which further validated the assay sensitivity to classical antidepressant treatment (Monleon et al., 1995; Elsayed et al., 2012; Papp et al., 2014). Analysis of water consumption (day 18; F5,35=0.39, P =.85; Table 1) and locomotor activity (day 22; F5,35=1.15, P =.35; Table 1) showed no significant difference between groups, indicating that effects of CUS on sucrose consumption were not due to experimental bias related to changes in drinking behavior or overall ambulatory activity, respectively.
Table 1.
Influence of CUS and Drug Treatment on Water Consumption and Locomotor Activity in Wild-Type Mice
| Treatment Group | Water ConsumptionMean (g) ± SEM | Locomotor ActivityMean (m) ± SEM |
|---|---|---|
| Control (vehicle) | 1.87 ± 0.11 | 35.1 ± 1.6 |
| CUS + vehicle | 1.95 ± 0.09 | 35.8 ± 4.7 |
| CUS + CAR (0.03 mg/kg; b.i.d.) | 2.01 ± 0.09 | 30.1 ± 1.6 |
| CUS + CAR (0.1 mg/kg; b.i.d.) | 1.96 ± 0.10 | 30.4 ± 2.0 |
| CUS + CAR (0.2 mg/kg; b.i.d.) | 2.03 ± 0.10 | 29.1 ± 1.4 |
| CUS + IMI (20 mg/kg) | 2.03 ± 0.10 | 30.6 ± 3.4 |
Abbreviations: b.i.d., twice a day; CAR, cariprazine; CUS, chronic unpredictable stress; IMI, imipramine.
There were no overall significant effects of CUS or drug treatment using ANOVA.
The potential effects of cariprazine on CUS-induced anxiety-like behaviors were also addressed in the same cohort of mice using the novelty-induced hypophagia test. ANOVA analysis indicated no significant effect of CUS or drug treatment on latency to drink sweetened milk in home cage conditions (day 24; F5,33=0.72, P =.61; data not shown). Conversely, ANOVA analysis showed significant effects of CUS or drug treatment when the same test was conducted in the novel arena (i.e., novelty-induced hypophagia test; day 25; F5,35=6.41, P =.0003). Specifically, the posthoc analysis revealed that latency to drink was significantly increased in mice exposed to CUS, demonstrating an increase in anxiety-like behavior (P <.0001 vs. control; Fig 1c). Further posthoc analysis showed that cariprazine treatment robustly attenuated novelty-induced hypophagia, suggesting anxiolytic-like effects, as significant reductions in drinking latency were observed in CUS mice treated with both cariprazine 0.1 mg/kg (P =.003 vs. CUS + vehicle; Figure 1c) and 0.2 mg/kg doses (P =.0007 vs. CUS + vehicle; Figure 1c). Furthermore, the anxiolytic action of cariprazine was comparable with the anxiolytic response to imipramine (20 mg/kg), since we found significantly reduced drinking latency in CUS mice treated with imipramine (P =.0006 vs. CUS + vehicle) (Figure 1c).
Antidepressant Actions of Cariprazine Require Dopamine D3 Receptors
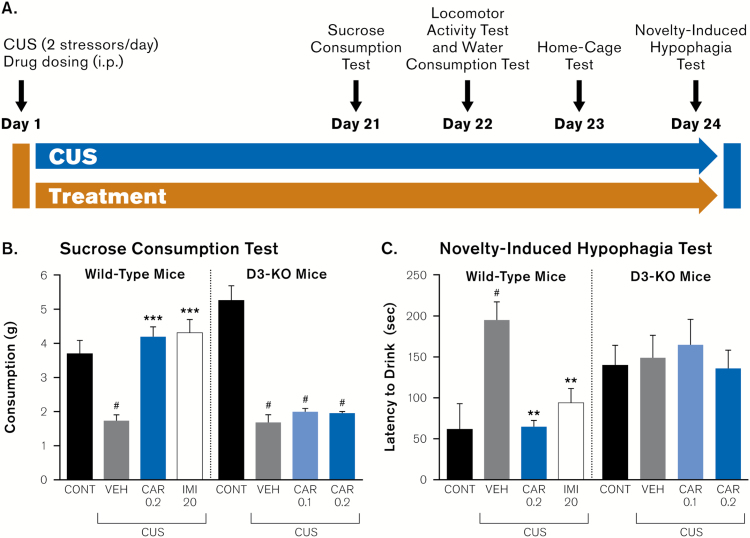
To determine whether antidepressant-like properties of cariprazine are mediated through dopamine D3 receptors, in the second phase of the study we examined if deletion of D3 receptors influences the behavioral response to chronic stress (Figure 2a). Notably, previous reports have shown that D3-KO mice do not exhibit baseline anxiety- or depressive-like behaviors (Chourbaji et al., 2008; Leggio et al., 2008). CUS-induced decreases in sucrose consumption were observed in both D3-KO and wild-type littermate mice, establishing the validity of the CUS model in D3-KO mice and the lack of exacerbation of the anhedonia-like effects of CUS in D3-KO mice (day 21; F7,52=20.50, P <.0001; Figure 2b). Furthermore, 2-way ANOVA analysis showed a significant interaction between genotype and cariprazine treatment (F2,42=18.74, P <.0001). Consistent with the initial experiment, cariprazine 0.2 mg/kg attenuated CUS-induced decreases in sucrose consumption (P <.0001 vs. CUS + vehicle; Figure 2b) in wild-type mice; the magnitude of this effect was similar to that observed with imipramine (P <.0001 vs. CUS + vehicle; Figure 2b). However, in D3-KO mice, cariprazine did not reverse CUS-induced decreases in sucrose consumption at any dose (P =.56 vs. CUS + vehicle; Figure 2b), suggesting that the antidepressant-like activity of cariprazine required D3 receptor activation. Finally, there were no significant effects of CUS, cariprazine treatment, or genotype on water consumption (day 22; F7,52=1.90, P =.09; Table 2) or locomotor activity (day 22; F7,52=1.19, P =.32; Table 2), indicating no difference in overall liquid consumption and ambulatory behavior between D3-KO and wild-type mice.
Figure 2.
Influence of dopamine D3 receptor deletion on cariprazine actions in behavioral models of depression. (A) Experimental paradigm for behavioral testing and CUS exposure of D3 knockout mice (D3-KO) and wild-type (WT) littermates (n = 6–8). Both genotypes were tested in the (B) sucrose consumption test (day 21 of chronic unpredictable stress [CUS]) and (C) novelty-induced hypophagia test (day 24 of CUS). All doses are in mg/kg. Results are expressed as mean ± SEM; #P<.0001 compared to non-stressed control group; **P<.01 compared to CUS + vehicle group; ***P<.0001 compared to CUS + vehicle group (1-way ANOVA and Fisher’s PLSD posthoc analysis). CAR, cariprazine; CONT, control; IMI, imipramine; VEH, vehicle.
Table 2.
Influence of CUS and Drug Treatment on Water Consumption and Locomotor Activity in the D3 Knockout (D3-KO) Mice and Wild-Type Littermates
| Genotype | Treatment Group | Water ConsumptionMean (g) ± SEM | Locomotor ActivityMean (m) ± SEM |
|---|---|---|---|
| Wild-type mice | Control (vehicle) | 1.70 ± 0.07 | 34.3 ± 3.0 |
| CUS + vehicle | 1.61 ± 0.05 | 39.7 ± 1.8 | |
| CUS + CAR (0.2 mg/kg; b.i.d.) | 1.64 ± 0.07 | 36.7 ± 2.7 | |
| CUS + IMI (20 mg/kg) | 1.45 ± 0.08 | 40.4 ± 2.2 | |
| D3-KO mice | Control (vehicle) | 3.15 ± 1.03 | 36.7 ± 2.8 |
| CUS + vehicle | 1.76 ± 0.07 | 42.6 ± 2.4 | |
| CUS + CAR (0.1 mg/kg; b.i.d.) | 1.70 ± 0.14 | 41.1 ± 5.4 | |
| CUS + CAR (0.2 mg/kg; b.i.d.) | 1.73 ± 0.13 | 43.1 ± 3.0 |
Abbreviations: b.i.d., twice a day; CAR, cariprazine; CUS, chronic unpredictable stress; D3-KO, dopamine D3 receptor knockout mice; IMI, imipramine.
There were no overall significant effects of genotype, drug treatment, or CUS using ANOVA.
As in the previous experiment, the effect of D3 receptor deletion on anxiety-like behaviors was evaluated in the novelty-induced hypophagia test. In home cage conditions, 1-way ANOVA showed no significant effect of CUS on drinking latency (day 23; F7,52=2.10, P =.06; data not shown), while 2-way ANOVA further revealed no main effect of cariprazine 0.2 mg/kg treatment (F2,42=1.270, P =.29) or genotype (F1,42=1.058, P =.31) but a significant genotype x treatment interaction (F2,42=5.015, P <.01). In the novelty-induced hypophagia test, posthoc analysis confirmed that CUS induced a significant increase in latency to drink (day 24; F7,52=3.60, P =.003) (Figure 2c). In addition, 2-way ANOVA analysis revealed a significant effect of cariprazine treatment (F2,42=4.48, P =.017) and a significant interaction between the drug treatment and genotype (F2,42=3.27, P =.048). In wild-type animals, negative effects of CUS on drinking latency were significantly inhibited by both cariprazine 0.2 mg/kg (P =.005 vs. CUS + vehicle; Figure 2c) and imipramine (P =.0008 vs. CUS + vehicle; Figure 2c) treatments. However, there were no significant effects of CUS or drug treatment in the D3-KO mice when tested in novel cage conditions. D3-KO mice not subjected to CUS exhibited elevated rearing and exploratory behavior. This “distracted” behavior explains the significantly higher latency to drink in D3-KO when compared to WT controls (P =.03). Lastly, CUS-exposed D3-KO animals showed similar latency to drink as D3-KO control mice (Figure 2c). As a result, we confirmed the anxiolytic properties of cariprazine in the novelty suppressed feeding test but could not conclusively determine the involvement of dopamine D3 receptors in this effect since the D3-KO mice exhibited this particular behavior and experimental confound.
Comparison of Antidepressant and Anxiolytic Actions of Cariprazine and Aripiprazole
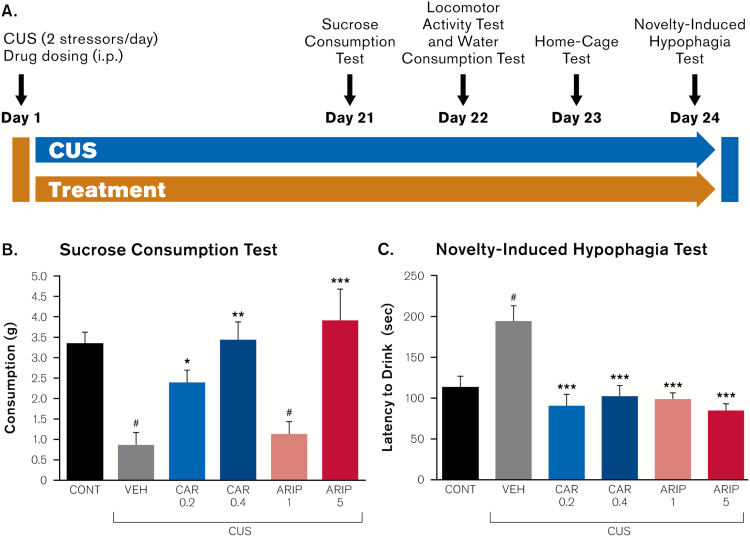
We compared the antidepressant and anxiolytic actions of cariprazine to aripiprazole in mice exposed to the CUS paradigm or control conditions (Figure 3a). In wild-type mice exposed to CUS, both cariprazine and aripiprazole demonstrated robust antidepressant-like (day 21; F5,42=8.41, P <.0001; Figure 3b) and anxiolytic-like (day 21; F5,41=9.68, P <.0001; Figure 3c) effects in sucrose consumption and novelty-induced hypophagia tests, respectively. Moreover, cariprazine significantly attenuated CUS-induced anhedonia-like behavior at both 0.2 mg/kg (P =.018 vs. CUS + vehicle) and 0.4 mg/kg (P =.0002 vs. CUS + vehicle), while aripiprazole was effective only at the higher 5 mg/kg dose (P <.0001 vs. CUS + vehicle) (Figure 3b). Mice treated with 5 mg/kg aripiprazole also exhibited a significant increase in water consumption (day 22; F5,42=6.419; P <.001; posthoc Fisher’s PLSD P =.0085 vs. control, P =.0009 vs. CUS + vehicle; Table 3), suggesting that changes in sucrose consumption observed following 5 mg/kg aripiprazole administration may, in part, be attributed to an overall increase in drinking behavior. Furthermore, no effects of cariprazine or aripiprazole were observed on the locomotor activity (Table 3).
Figure 3.
Antidepressant and anxiolytic pharmacological profile of cariprazine is compared with aripiprazole in the chronic unpredictable stress (CUS) model. (A) Mice were exposed to the CUS paradigm or control conditions for 26 days and were administered either vehicle, cariprazine or aripiprazole. The effects of drug treatment on (B) anhedonia- and (C) anxiety-like behavioral responses are shown. All doses are in mg/kg. Results are expressed as mean ± SEM; #P<.0001 compared to non-stressed control group; *P<.05 compared with CUS + vehicle group; **P<.001 compared with CUS + vehicle group; ***P<.0001 compared with CUS + vehicle group (1-way ANOVA and Fisher’s PLSD posthoc analysis). ARIP, aripiprazole; CAR, cariprazine; CONT, control; VEH, vehicle.
Table 3.
Effects of Cariprazine or Aripiprazole Treatments on Water Consumption and Locomotor Activity in Wild-Type CUS Mice
| Treatment Group | Water ConsumptionMean (g) ± SEM | Locomotor ActivityMean (m) ± SEM |
|---|---|---|
| Control (vehicle) | 1.45 ± 0.23 | 17.7 ± 1.1 |
| CUS + vehicle | 1.23 ± 0.21 | 18.5 ± 0.8 |
| CUS + CAR (0.2 mg/kg; b.i.d.) | 0.84 ± 0.15 | 16.7 ± 1.9 |
| CUS + CAR (0.4 mg/kg; b.i.d.) | 0.94 ± 0.15 | 18.0 ± 1.1 |
| CUS + ARIP (1 mg/kg; b.i.d.) | 1.41 ± 0.26 | 18.0 ± 0.7 |
| CUS + ARIP (5 mg/kg) | 2.20 ± 0.12a | 21.0 ± 1.1 |
Abbreviations: ARIP, aripiprazole; b.i.d., twice a day; CAR, cariprazine; CUS, chronic unpredictable stress.
ANOVA analysis showed statistically significant differences in the water consumption test (F5,42 = 6.42, P < .001).
aPosthoc analysis revealed significant difference when compared to control (vehicle) or CUS + vehicle groups.
Discussion
Cariprazine is a newly developed antipsychotic medication that was approved for the treatment of schizophrenia and manic or mixed episodes associated with bipolar I disorder based on results from a number of phase 2/3 clinical trials (Durgam et al., 2014, 2015a, 2015b; Calabrese et al., 2015; Kane et al., 2015; Sachs et al., 2015). Cariprazine is a partial agonist at both dopamine D2 and D3 receptors as well as serotonin 5-HT1A receptors and in this regard is relatively similar to the currently available atypical antipsychotics, aripiprazole, and brexpiprazole. However, the multifunctional pharmacological properties of cariprazine at dopamine receptors, specifically its superior affinity and selectivity for D3 receptors, differentiate it from most atypical antipsychotic agents (including compounds with partial agonist as well as those with full antagonist properties at D2/D3 receptors) (Ellenbroek and Cesura, 2015; Stahl, 2016).
Cariprazine is unique in its ability to bind D3 receptors with higher affinity than even dopamine itself, which essentially results in blockade of D3 receptors (Freedman et al., 1994; Sautel et al., 1995; Kiss et al., 2010). In contrast, most other atypical antipsychotics have relatively lower affinities for the D3 receptor and in the presence of normal brain levels of dopamine are unable to block these receptors (Stahl, 2016). Furthermore, since D3 receptors are thought to be involved in the regulation of mood, cognition, and motivation (Gross and Drescher, 2012), compounds like cariprazine that exhibit high affinity and occupancy of these receptors could also potentially be useful in the treatment of depression and the negative symptoms of schizophrenia. This has been further supported by our previous studies showing that cariprazine has potent antidepressant-like, antipsychotic-like, and procognitive effects in rodent models (Gyertyán et al., 2011; Zimnisky et al., 2013; Papp et al., 2014; Neill et al., 2016; Watson et al., 2016). Moreover, in recent clinical studies in patients suffering from schizophrenia with predominant negative symptoms, cariprazine provided significantly greater improvement than risperidone for both negative symptoms and functionality (Debelle et al., 2015). These results further support the differentiating features of cariprazine.
The antidepressant-like efficacy of cariprazine was demonstrated in a chronic mild stress model in rats by reducing anhedonic-like behavior (Papp et al., 2014); however neural mechanisms underlying these effects are still unclear. In the current study, cariprazine significantly attenuated CUS-induced sucrose consumption in a novel environment in wild-type mice, demonstrating antidepressant-like activity that was comparable with the tricyclic antidepressant imipramine. Interestingly, the same antianhedonic-like actions of cariprazine were not observed in D3-KO mice, indicating that dopamine D3 receptors are required to mediate the antidepressant-like effects of cariprazine. Furthermore, previous studies in D3-KO mice have suggested that blockade of D3 receptors in the brain may produce anxiolytic effects (Steiner et al., 1997). In this study, we demonstrated that cariprazine is an effective anxiolytic in wild-type mice by significantly reducing latency to drink in the novelty-induced hypophagia test. However, D3-KO mice not exposed to CUS showed elevated latency to drink in the novelty-induced hypophagia test, possibly due to distracted behavior displayed by D3-KO control mice. Similar increases in rearing activity have been previously observed in D3-KO mice (Yarkov et al., 2010). We found no effect of CUS exposure or drug treatment in D3-KO mice, probably because of the elevated baseline exhibited in the D3-KO animals. Although this experimental confound precluded the determination of clear involvement of D3 receptors in the cariprazine effect in this test, we confirmed in 3 independent cohorts that cariprazine exerts anxiolytic properties and reverses the effect of chronic stress exposure.
The neuropharmacological profile of cariprazine is generally considered to be similar to aripiprazole, another atypical antipsychotic that is commonly used for the treatment of schizophrenia and bipolar disorder, except that cariprazine has a greater affinity and selectivity for D3 vs. D2 receptors (Tadori et al., 2011; Ellenbroek and Cesura, 2015; Findlay et al., 2016; Stahl, 2016). Aripiprazole also has been shown to be effective as an adjunct treatment for major depressive disorder (Bourin et al., 2009; Lenze et al., 2015; Zhou et al., 2015). Our results show that administration of a high dose of aripiprazole produced an antidepressant-like response, but also induced increases in total fluid consumption, which could be due to polydipsia and/or polyuria, potential side effects of some antipsychotic agents (Bersani et al., 2007; Meulendijks et al., 2010; Gandhi et al., 2016). In this regard, cariprazine has a more specific antidepressant-like effect that was independent of changes in overall liquid consumption. We found that both drugs have similar anxiolytic-like effects in the novelty-induced hypophagia test. The antidepressant- and anxiolytic-like effects of aripiprazole were not explored in D3-KO mice because of the nonspecificity of its anhedonia-like actions and the difficulty in interpreting anxiolytic-like activity of drugs in D3-KO animals.
Overall, in combination with previous studies, these data indicate that cariprazine has a unique pharmacological profile and, with its distinct dopamine D3 receptor-preferring mechanism of action, may have potential efficacy in the treatment of depressive disorders and negative symptoms of schizophrenia.
Funding
This study was supported by Forest Research Institute, Inc., an Allergan affiliate, and Gedeon Richter Plc. Editorial support was provided by Paul Ferguson of Prescott Medical Communications Group, Chicago, IL, a contractor of Allergan.
Statement of Interest
R.S. Duman has received honoraria from Bristol Myers Squibb, Lilly, Lundbeck, Johnson and Johnson, Naurex, and Forest Laboratories, Inc.; research contracts from Lundbeck, Sunovion, Johnson and Johnson, Allergan, Naurex, Taisho, Navitor, and Forest Research Institute; and consulting fees from Taisho, Naurex, and Johnson and Johnson. M. Banasr has received research contracts from Servier (IRIS) and BioHaven Inc and is listed as an inventor on provisional patent No. 62/310,409. V. Duric, T. Franklin, and A. Lepack have nothing to disclose. N. Adham is an employee of Allergan. B. Kiss is an employee of Gedeon Richter Plc. I. Gyertyán was an employee of Gedeon Richter Plc. at the time of the study and is an inventor in patents US 7,737,142 B2 / EP 1 663 996 B1, which cover cariprazine.
Acknowledgments
The dopamine D3 receptor knockout mice were a gift from Dr. Marc Caron (Duke University).
References
- Ágai-Csongor E, Domány G, Nógrádi K, Galambos J, Vágó I, Keserű GM, Greiner I, Laszlovszky I, Gere A, Schmidt E, Kiss B, Vastag M, Tihanyi K, Sághy K, Laszy J, Gyertyán I, Zájer-Balázs M, Gémesi L, Kapás M and Szombathelyi Z (2012) Discovery of cariprazine (RGH-188): a novel antipsychotic acting on dopamine D3/D2 receptors. Bioorg Med Chem Lett 22:3437–3440. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Tirotta E, Sotnikova TD, Masri B, Salahpour A, Gainetdinov RR, Borrelli E, Caron MG (2007) Regulation of Akt signaling by D2 and D3 dopamine receptors in vivo. J Neurosci 27:881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersani G, Pesaresi L, Orlandi V, Gherardelli S, Pancheri P (2007) Atypical antipsychotics and polydipsia: a cause or a treatment? Hum Psychopharmacol 22:103–107. [DOI] [PubMed] [Google Scholar]
- Bourin M, Chenu F, Prica C, Hascoet M (2009) Augmentation effect of combination therapy of aripiprazole and antidepressants on forced swimming test in mice. Psychopharmacology (Berl) 206:97–107. [DOI] [PubMed] [Google Scholar]
- Calabrese JR, Keck PE Jr, Starace A, Lu K, Ruth A, Laszlovszky I, Nemeth G and Durgam S (2015) Efficacy and safety of low- and high-dose cariprazine in acute and mixed mania associated with bipolar I disorder: a double-blind, placebo-controlled study. J Clin Psychiatry 76:284–292. [DOI] [PubMed] [Google Scholar]
- Choi YK, Adham N, Kiss B, Gyertyán I, Tarazi FI (2014) Long-term effects of cariprazine exposure on dopamine receptor subtypes. CNS Spectr 19:268–277. [DOI] [PubMed] [Google Scholar]
- Chourbaji S, Brandwein C, Vogt MA, Dormann C, Mueller R, Drescher KU, Gross G, Gass P (2008) Dopamine receptor 3 (D3) knockout mice show regular emotional behaviour. Pharmacol Res 58:302–307. [DOI] [PubMed] [Google Scholar]
- Debelle M, Németh G, Szalai E, Szatmári B, Harsányi J, Barabássy A, Laszlovszky I (2015) Cariprazine as monotherapy for the treatment of schizophrenia patients with predominant negative symptoms: a double-blind, active controlled trial. Eur Neuropsychopharamcol 25:S510. [DOI] [PubMed] [Google Scholar]
- Durgam S, Cutler AJ, Lu K, Migliore R, Ruth A, Laszlovszky I, Nemeth G, Meltzer HY (2015a) Cariprazine in acute exacerbation of schizophrenia: a fixed-dose, phase 3, randomized, double-blind, placebo- and active-controlled trial. J Clin Psychiatry 76:e1574–1582. [DOI] [PubMed] [Google Scholar]
- Durgam S, Starace A, Li D, Migliore R, Ruth A, Németh G, Laszlovszky I (2014) An evaluation of the safety and efficacy of cariprazine in patients with acute exacerbation of schizophrenia: a phase II, randomized clinical trial. Schizophr Res 152:450–457. [DOI] [PubMed] [Google Scholar]
- Durgam S, Starace A, Li D, Migliore R, Ruth A, Németh G, Laszlovszky I (2015b) The efficacy and tolerability of cariprazine in acute mania associated with bipolar I disorder: a phase II trial. Bipolar Disord 17:63–75. [DOI] [PubMed] [Google Scholar]
- Duric V, Banasr M, Licznerski P, Schmidt HD, Stockmeier CA, Simen AA, Newton SS, Duman RS (2010) A negative regulator of MAP kinase causes depressive behavior. Nat Med 16:1328–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duric V, Duman RS (2013) Depression and treatment response: dynamic interplay of signaling pathways and altered neural processes. Cell Mol Life Sci 70:39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbroek BA, Cesura M (2015) Antipsychotics and the dopamine serotonin connection. Top Med Chem 13:1–50. [Google Scholar]
- Elsayed M, Banasr M, Duric V, Fournier NM, Licznerski P, Duman RS (2012) Antidepressant effects of fibroblast growth factor-2 in behavioral and cellular models of depression. Biol Psychiatry 72:258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay LJ, El-Mallakh PL, El-Mallakh RS (2016) Cariprazine for the treatment of bipolar disorder. Perspect Psychiatr Care doi: 10.1111/ppc.12150. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Freedman SB, Patel S, Marwood R, Emms F, Seabrook GR, Knowles MR, McAllister G (1994) Expression and pharmacological characterization of the human D3 dopamine receptor. J Pharmacol Exp Ther 268:417–426. [PubMed] [Google Scholar]
- Gandhi S, McArthur E, Reiss JP, Mamdani MM, Hackam DG, Weir MA, Garg AX (2016) Atypical antipsychotic medications and hyponatremia in older adults: a population-based cohort study. Can J Kidney Health Dis 3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis RR, Slifstein M, D’Souza D, Lee Y, Periclou A, Ghahramani P, Laszlovszky I, Durgam S, Adham N, Nabulsi N, Huang Y, Carson RE, Kiss B, Kapas M, Abi-Dargham A, Rakhit A (2016) Preferential binding to dopamine D3 over D2 receptors by cariprazine in patients with schizophrenia using PET with the D3/D2 receptor ligand [11C]-(+)-PHNO. Psychopharmacology (Berl) 233:3503–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Guerrero A, Mizrahi R, Agid O, Marcon H, Barsoum P, Rusjan P, Wilson AA, Zipursky R, Kapur S (2009) The dopamine D2 receptors in high-affinity state and D3 receptors in schizophrenia: a clinical [11C]-(+)-PHNO PET study. Neuropsychopharmacology 34:1078–1086. [DOI] [PubMed] [Google Scholar]
- Gross G, Drescher K (2012) The role of dopamine D(3) receptors in antipsychotic activity and cognitive functions. Handb Exp Pharmacol 167–210. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, Joyce JN (1999) Distribution of dopamine D3 receptor expressing neurons in the human forebrain: comparison with D2 receptor expressing neurons. Neuropsychopharmacology 20:60–80. [DOI] [PubMed] [Google Scholar]
- Gyertyán I, Kiss B, Sághy K, Laszy J, Szabó G, Szabados T, Gémesi LI, Pásztor G, Zájer-Balázs M, Kapás M, Csongor EA, Domány G, Tihanyi K, Szombathelyi Z (2011) Cariprazine (RGH-188), a potent D3/D2 dopamine receptor partial agonist, binds to dopamine D3 receptors in vivo and shows antipsychotic-like and procognitive effects in rodents. Neurochem Int 59:925–935. [DOI] [PubMed] [Google Scholar]
- Kane JM, Zukin S, Wang Y, Lu K, Ruth A, Nagy K, Laszlovszky I, Durgam S (2015) Efficacy and safety of cariprazine in acute exacerbation of schizophrenia: results from an international, phase III clinical trial. J Clin Psychopharmacology 35:367–373. [DOI] [PubMed] [Google Scholar]
- Kiss B, Horti F, Bobok A (2012) Cariprazine, a D3/D2 dopamine receptor partial agonist antipsychotic, displays greater D3 receptor occupancy in vivo compared with other antipsychotics. Schizophrenia Res 136:S190. [Google Scholar]
- Kiss B, Horváth A, Némethy Z, Schmidt E, Laszlovszky I, Bugovics G, Fazekas K, Hornok K, Orosz S, Gyertyán I, Ágai-Csongor E, Domány G, Tihanyi K, Adham N, Szombathelyi Z (2010) Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther 333:328–340. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS (2008) IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci U S A 105:751–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS (2010) Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc Natl Acad Sci U S A 107:2669–2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers CH, Diaz J, Schwartz JC, Sokoloff P (2000) Selective increase of dopamine D3 receptor gene expression as a common effect of chronic antidepressant treatments. Mol Psychiatry 5:378–388. [DOI] [PubMed] [Google Scholar]
- Leggio GM, Micale V, Drago F (2008) Increased sensitivity to antidepressants of D3 dopamine receptor-deficient mice in the forced swim test (FST). Eur Neuropsychopharmacol 18:271–277. [DOI] [PubMed] [Google Scholar]
- Lenze EJ, Mulsant BH, Blumberger DM, Karp JF, Newcomer JW, Anderson SJ, Dew MA, Butters MA, Stack JA, Begley AE, Reynolds CF, . 3rd (2015) Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet 386:2404–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj J, Dziedzicka-Wasylewska M, Rogoz R, Rogoz Z (1998) Effect of antidepressant drugs administered repeatedly on the dopamine D3 receptors in the rat brain. Eur J Pharmacol 351:31–37. [DOI] [PubMed] [Google Scholar]
- McCormick PN, Kapur S, Graff-Guerrero A, Raymond R, Nobrega JN, Wilson AA (2010) The antipsychotics olanzapine, risperidone, clozapine, and haloperidol are D2-selective ex vivo but not in vitro. Neuropsychopharmacology 35:1826–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulendijks D, Mannesse CK, Jansen PA, van Marum RJ, Egberts TC (2010) Antipsychotic-induced hyponatraemia: a systematic review of the published evidence. Drug Saf 33:101–114. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, Agid O, Borlido C, Suridjan I, Rusjan P, Houle S, Remington G, Wilson AA, Kapur S (2011) Effects of antipsychotics on D3 receptors: a clinical PET study in first episode antipsychotic naive patients with schizophrenia using [11C]-(+)-PHNO. Schizophr Res 131:63–68. [DOI] [PubMed] [Google Scholar]
- Monleon S, D’Aquila P, Parra A, Simon VM, Brain PF, Willner P (1995) Attenuation of sucrose consumption in mice by chronic mild stress and its restoration by imipramine. Psychopharmacology (Berl) 117:453–457. [DOI] [PubMed] [Google Scholar]
- Neill JC, Grayson B, Kiss B, Gyertyán I, Ferguson P, Adham N (2016) Effects of cariprazine, a novel antipsychotic, on cognitive deficit and negative symptoms in a rodent model of schizophrenia symptomatology. Eur Neuropsychopharmacol 26:3–14. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Papakostas GI (2009) Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo-controlled randomized trials. Am J Psychiatry 166:980–991. [DOI] [PubMed] [Google Scholar]
- Papp M, Gruca P, Lason-Tyburkiewicz M, Adham N, Kiss B, Gyertyán I (2014) Attenuation of anhedonia by cariprazine in the chronic mild stress model of depression. Behav Pharmacol 25:567–574. [DOI] [PubMed] [Google Scholar]
- Pothion S, Bizot JC, Trovero F, Belzung C (2004) Strain differences in sucrose preference and in the consequences of unpredictable chronic mild stress. Behav Brain Res 155:135–146. [DOI] [PubMed] [Google Scholar]
- Sachs GS, Greenberg WM, Starace A, Lu K, Ruth A, Laszlovszky I, Németh G, Durgam S (2015) Cariprazine in the treatment of acute mania in bipolar I disorder: a double-blind, placebo-controlled, Phase III trial. J Affect Disord 174:296–302. [DOI] [PubMed] [Google Scholar]
- Sautel F, Griffon N, Levesque D, Pilon C, Schwartz JC, Sokoloff P (1995) A functional test identifies dopamine agonists selective for D3 versus D2 receptors. Neuroreport 6:329–332. [DOI] [PubMed] [Google Scholar]
- Sokoloff P, Giros B, Martres MP, Bouthenet ML, Schwartz JC (1990) Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature 347:146–151. [DOI] [PubMed] [Google Scholar]
- Stahl SM. (2016) Mechanism of action of cariprazine. CNS Spectr 21:123–127. [DOI] [PubMed] [Google Scholar]
- Steiner H, Fuchs S, Accili D (1997) D3 dopamine receptor-deficient mouse: evidence for reduced anxiety. Physiol Behav 63:137–141. [DOI] [PubMed] [Google Scholar]
- Tadori Y, Forbes RA, McQuade RD, Kikuchi T (2011) In vitro pharmacology of aripiprazole, its metabolite and experimental dopamine partial agonists at human dopamine D2 and D3 receptors. Eur J Pharmacol 668:355–365. [DOI] [PubMed] [Google Scholar]
- Veselinovic T, Paulzen M, Grunder G (2013) Cariprazine, a new, orally active dopamine D2/3 receptor partial agonist for the treatment of schizophrenia, bipolar mania and depression. Expert Rev Neurother 13:1141–1159. [DOI] [PubMed] [Google Scholar]
- Watson DJ, King MV, Gyertyan I, Kiss B, Adham N, Fone KC (2016) The dopamine D(3)-preferring D(2)/D(3) dopamine receptor partial agonist, cariprazine, reverses behavioural changes in a rat neurodevelopmental model for schizophrenia. Eur Neuropsychopharmacol 26:208–224. [DOI] [PubMed] [Google Scholar]
- Yarkov AV, Der TC, Joyce JN (2010) Locomotor activity induced by MK-801 is enhanced in dopamine D3 receptor knockout mice but suppression by dopamine D3/D2 antagonists does not occur through the dopamine D3 receptor. Eur J Pharmacol 627:167–172. [DOI] [PubMed] [Google Scholar]
- Zhou X, Ravindran AV, Qin B, Del Giovane C, Li Q, Bauer M, Liu Y, Fang Y, da Silva T, Zhang Y, Fang L, Wang X, Xie P (2015) Comparative efficacy, acceptability, and tolerability of augmentation agents in treatment-resistant depression: systematic review and network meta-analysis. J Clin Psychiatry 76:e487–498. [DOI] [PubMed] [Google Scholar]
- Zimnisky R, Chang G, Gyertyán I, Kiss B, Adham N, Schmauss C (2013) Cariprazine, a dopamine D(3)-receptor-preferring partial agonist, blocks phencyclidine-induced impairments of working memory, attention set-shifting, and recognition memory in the mouse. Psychopharmacology (Berl) 226:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]