Abstract
Drug addiction has often been described as a “hijacking” of the brain circuits involved in learning and memory. Glutamate is the principal excitatory neurotransmitter in the brain, and its contribution to synaptic plasticity and learning processes is well established in animal models. Likewise, over the past 20 years the addiction field has ascribed a critical role for glutamatergic transmission in the development of addiction. Chronic drug use produces enduring neuroadaptations in corticostriatal projections that are believed to contribute to a maladaptive deficit in inhibitory control over behavior. Much of this research focuses on the role played by ionotropic glutamate receptors directly involved in long-term potentiation and depression or metabotropic receptors indirectly modulating synaptic plasticity. Importantly, the balance between glutamate release and clearance tightly regulates the patterned activation of these glutamate receptors, emphasizing an important role for glutamate transporters in maintaining extracellular glutamate levels. Five excitatory amino acid transporters participate in active glutamate reuptake. Recent evidence suggests that these glutamate transporters can be modulated by chronic drug use at a variety of levels. In this review, we synopsize the evidence and mechanisms associated with drug-induced dysregulation of glutamate transport. We then summarize the preclinical and clinical data suggesting that glutamate transporters offer an effective target for the treatment of drug addiction. In particular, we focus on the role that altered glutamate transporters have in causing drug cues and contexts to develop an intrusive quality that guides maladaptive drug seeking behaviors.
Keywords: glutamate transporters, addiction, relapse, cellular redox, n-acetylcysteine
Introduction
Substance abuse disorders represent a major public health problem costing the United States over $740 billion annually in healthcare-related and indirect costs (NIDA, 2017). Addiction is marked by an enduring propensity to relapse. Repeated drug use produces persistent neuroadaptions in key brain circuits that promote this susceptibility to return to drug use following abstinence. Chronic drug use prominently disrupts glutamate homeostasis in nucleus accumbens (NAc). Differential effects on basal extracellular glutamate levels are observed in NAc depending on drug class (e.g., increased by alcohol, decreased by cocaine, unchanged by heroin), but reinstatement initiated by drug-associated cues triggers a large increase in extracellular glutamate across drugs (Scofield et al., 2016); thus, all drugs tested disrupt glutamate homeostasis, leading to increased synaptic glutamate spillover during a drug-seeking event. Likewise, in human drug addicts there is an increase in blood-oxygen-level dependent contrast imaging response to drug-related cues in prefrontal cortex (PFC) associated with later relapse (Goldstein and Volkow, 2011). Moreover, pharmacological interventions that restore glutamate homeostasis have demonstrated efficacy in reducing drug seeking (Reissner and Kalivas, 2010; Scofield et al., 2016).
Glutamate is a nonessential amino acid neurotransmitter responsible for most excitatory synaptic transmission in the central nervous system. Arguably, glutamate is the most important neurotransmitter for normal brain function as the cellular substrate of learning and memory (Citri and Malenka, 2007). Most evoked and spontaneous glutamate release is vesicular, occurring directly at the synapse and mediated by voltage-gated calcium channels (Ermolyuk et al., 2013). Synaptic glutamate release rapidly increases extracellular glutamate approaching millimolar concentrations within the synaptic cleft (Moussawi et al., 2011a). These elevated levels typically decline within milliseconds due to diffusion and reuptake. Glutamate transporters control signal transmission by removing glutamate from the synaptic cleft, thereby limiting its time in the extracellular space and access to the extrasynaptic compartment. Thus, under physiological conditions, glutamate transmission is temporally and spatially restricted, acting on glutamate receptors within or on the annulus of the synaptic cleft.
This review emphasizes the role of excitatory amino acid transporters (EAATs), which are ultimately responsible for controlling extracellular glutamate levels, in regulating the motivation to seek drugs. Systematic literature searches were performed using PubMed with search criteria consisting of “EAATX,” “EAATX and localization/regulation/trafficking,” and “EAATX + drug of abuse,” (e.g, EAAT1 and alcohol). Preclinical drug studies include noncontingent and contingent drug administration protocols, although for EAAT2 we mainly limit our discussion to self-administration models due to the surfeit of reports on this transporter subtype (Reissner and Kalivas, 2010; Roberts-Wolfe and Kalivas, 2015). We discuss the normal distribution and expression of glutamate transporters and how drugs of abuse affect them inclusive of in vitro and in vivo studies. Finally, we address the potential of EAATs as therapeutic targets for addiction and other psychiatric diseases.
EAATS
Glutamate transporters control glutamate homeostasis in the central nervous system and their presence, or lack thereof, creates micro-domains of varying extracellular glutamate concentrations. There are 5 sodium-dependent glutamate transporters or EAATs comprising the solute carrier 1 family (SLC1): EAAT1/GLAST (SLC1A3), EAAT2/GLT-1 (SLC1A2), EAAT3/EAAC1 (SLC1A1), EAAT4 (SLC1A6), and EAAT5 (SLC1A7). The gene products are designated by the SLC1XX nomenclature. The human proteins use a homogeneous classification system of EAAT1-5, but 3 EAATs initially cloned from rat brain and rabbit intestine were given nonstandard names still used in animal model literature: Glutamate Aspartate Transporter 1 (GLAST) for EAAT1, Glutamate transporter 1 (GLT-1) for EAAT2, and Excitatory Amino Acid Carrier 1 (EAAC1) for EAAT3 (Jensen et al., 2015; Martinez-Lozada et al., 2016). The EAATs take up glutamate against its concentration gradient driven by cotransport with 3 Na+ and 1 H+ alongside the export of 1 K+ (Figure 1) (Grewer et al., 2008). Disrupted glutamate transporter function is linked to a variety of excitotoxicity-related diseases, including Alzheimer’s, Parkinson’s, amyotrophic lateral sclerosis, epilepsy, multiple sclerosis, and stroke, and more recently psychiatric diseases like schizophrenia (Nakagawa and Kaneko, 2013).
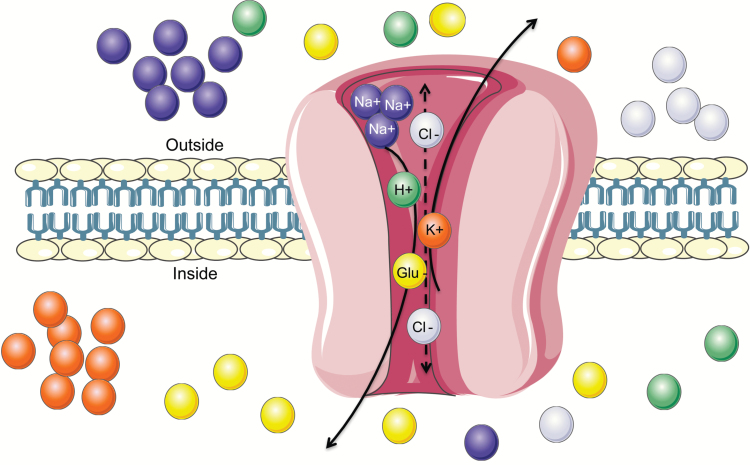
Figure 1.
Diagram of a glutamate transporter. The transport of glutamate is coupled with cotransport of 3 sodium (Na+), 1 hydrogen (H+), and 1 potassium (K+) ion along their concentration gradient. The stoichiometry of coupling has been determined for excitatory amino acid transporter (EAAT)1–4; however, the order of ion binding is not completely resolved. EAATs 1–3 compared with EAAT4 transport glutamate with considerably different kinetics and voltage dependence despite a similar uptake mechanism. Additionally, EAATs perform an uncoupled flux of chloride (Cl-) cations. This latter function is most predominant in EAAT4 and EAAT5, and nearly absent in EAAT2.
EAAT1/GLAST
Localization/Distribution
Immunocytochemistry detects moderate to strong expression of GLAST in cerebellum, hippocampus, striatum, thalamus, brainstem, and spinal cord, and low to moderate expression in neocortex, amygdala, and hypothalamus (Lehre et al., 1995; Schmitt et al., 1997) (Figure 2). In human postmortem cortical tissue, EAAT1 protein is detected in astrocytic processes bordering glutamatergic synapses and unexpectedly in the soma, axons, and dendritic spines of neurons (Roberts et al., 2014). Neuronal EAAT1 expression was suggested to be due to a truncated EAAT1/GLAST splice variant that is expressed in unhealthy neurons during hypoxia (Sullivan et al., 2007; Roberts et al., 2014). In rat hippocampal cultures GLAST and GLT1 are expressed in neurons up to 7 days in vitro, but disappear with astrocyte maturation (Plachez et al., 2004). Adding astrocyte-conditioned medium to neuronal cultures suppresses neuronal GLAST expression, demonstrating the importance of glia-neuron communication. Reciprocally, astrocytes grown without neurons show reduced GLAST and GLT1 levels (Gegelashvili et al., 1997).
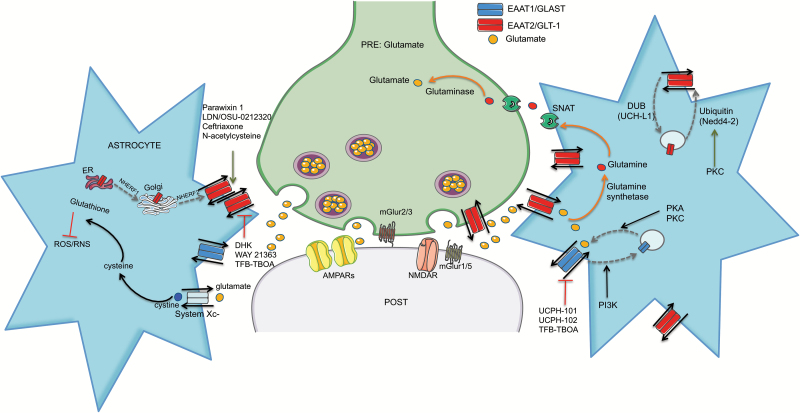
Figure 2.
Glial glutamate transporters. Excitatory amino acid transporter EAAT1/GLAST (blue) is located exclusively on astrocytes and other glial cells. EAAT2/GLT-1 (red) is likewise predominately restricted to astrocytes. Orange arrows depict the glutamate-glutamine cycle associated with glutamate uptake: glutamine synthetase converts up taken glutamate to glutamine, glutamine is transported back to the glutamatergic neuron through sodium coupled amino acid transporters (SNATs), and glutamine is converted back to glutamate by glutaminase. The gray dashed arrows depict regulation of EAAT trafficking. Endosomal trafficking of EAAT1 depends on sodium-hydrogen exchanger regulatory factor 1 and 2 (NHERF1 and 2). Rapid cell surface expression of EAATs is also prominently modulated by kinase activity. Protein Kinase A (PKA) and Protein Kinase C (PKC) inhibitors decrease EAAT1 surface expression, while Phosphoinositide 3-Kinase (PI3K) inhibitors promote surface expression. EAAT2 trafficking is regulated both constitutively and inducibly by ubiquitination/deubiquitination cycles. PKC-dependent activation of Neural precursor cell-expressed developmentally downregulated gene 4-2 (Nedd4-2) ubiquitin ligase targets EAAT2 for proteasomal degradation. Specific pharmacological activators (green arrows) and inhibitors (red bar-line) of each transporter are shown. UCPH-101 and UCPH-102 are the first selective EAAT1 inhibitors. TFB-TBOA ((3S)-3-[[3-[[4-(Trifluoromethyl)benzoyl]amino]phenyl]methoxy]-L-aspartic acid) is more selective for EAAT1 and EAAT2 but has low efficacy at EAAT3 as well. Dihydrokainate (DHK) blocks EAAT2 function. WAY-213613 is a competitive inhibitor with higher selectivity and potency for EAAT2 over EAAT1 and EAAT3. Parawixin 1, purified from the venom of spider Parawixia bistriata, selectively increases EAAT2 activity. Pyradizine analogs, including LDN/OSU-0212320 increases EAAT2 function through transactivation. Ceftriaxone and other beta-lactam antibiotics increase EAAT2 expression and function. The antioxidant pro-drug N-acetylcysteine (NAC) increases EAAT2 function but may interact with the glutamate transport system at multiple levels.
Regulation of Expression/Trafficking
There is little sequence homology between rat and human GLAST/EAAT1 promoters, although they share many predicted transcription factor binding sites (e.g., nuclear factor kappa B [NF-κB], YY1, cocaine- and amphetamine-regulated transcript [CART], specificity protein 1 [SP1]) (Unger et al., 2012). Yin Yang 1 (YY1) is an important negative regulator of EAAT1 expression, while epidermal growth factor (EGF)-dependent transactivation of EAAT1 depends on NF-κB binding (Karki et al., 2015). Reporter assays directly compared the transcriptional regulation of human and rat EAAT1 with known activators of the rat gene (dibutyryl-cyclic adenosine monophosphate [dbCAMP], pituitary adenylate cyclase-activating peptide [PACAP], epidermal growth factor [EGF], and transforming growth factor alpha [TGFa]), revealing an important species-specific regulatory role for the 3’-untranslated region. The 3’-untranslated region represses constitutive transcription in humans but is an enhancer in rodents (Unger et al., 2012). Despite this interesting difference and sequence dissimilarity, the limited studies largely confirm conserved regulatory mechanisms controlling EAAT1 expression across species.
EAAT1 function is regulated at the protein level via posttranslational modifications and trafficking. Glutamate itself can rapidly increase GLAST activity by increasing surface expression (Duan et al., 1999). Calcium/calmodulin (CAMKII)-dependent EAAT1 phosphorylation regulates constitutive transporter activity (Chawla et al., 2017), and consensus sequences for Protein Kinase A (PKA), Protein Kinase C (PKC), and Phosphoinositide 3-Kinase (PI3K) suggest that phosphorylation may also rapidly modulate EAAT1 activity. PKA or PKC inhibitors reduce cell surface EAAT1 expression, while a PI3K inhibitor increases surface protein in primary cortical cultures (Guillet et al., 2005).
EAAT1 and Drugs of Abuse
GLAST has been most well studied relating to alcohol addiction. We showed that repeated ethanol exposure (7 days, i.p. 1 g/kg) elevates basal extracellular glutamate levels and reduces glutamate uptake without changing GLT-1 or GLAST protein in NAc 24 hours after the last injection (Melendez et al., 2005) (Table 1). Alcoholism is considered a hyperglutamatergic disease, but postmortem brains of human alcoholics (Flatscher-Bader and Wilce, 2008) and chronic intermittent ethanol-exposed rats (Rimondini, 2002) show increased EAAT1/GLAST in frontal cortex. These increased GLAST levels may represent compensatory increases in protein to offset elevated extracellular glutamate. In contrast, a recent study using a free-choice ethanol-drinking model in male alcohol-preferring rats (P rats) reported no change in GLAST expression in PFC or NAc (Hakami et al., 2016). Chronic ethanol consumption in female P rats increases extracellular glutamate, decreases uptake, and reduces EAAT1 protein in ventral tegmental area (VTA) and NAc shell, with the protein deficit enduring for 2 weeks following ethanol deprivation (Ding et al., 2013). These results are consistent with another previously reported sex-specific ethanol withdrawal effect on glutamate transporter levels (Alele and Devaud, 2005). Other variability likely reflects differences in ethanol administration protocols, drug exposure periods, withdrawal periods, and brain region-dependent effects. Moreover, these data indicate that ethanol can alter GLAST function, likely through increased membrane trafficking, without affecting transcription or translation. In line with this interpretation, ethanol exposure dose-dependently modifies GLAST distribution, promoting a shift from the cytoplasm to the surface in rat astrocyte cultures (Sery et al., 2015). A GLAST knockout mouse model was used to directly examine the GLAST contribution to alcohol addiction and reinforcement with the logical hypothesis that GLAST deletion would promote alcohol intake and reward. Surprisingly, GLAST-/- show lower alcohol consumption and no ethanol preference in the conditioned place preference (CPP) paradigm (Karlsson et al., 2012); however, interpretation of these results is unclear, because endocannabinoid signaling is altered in these knockouts. Thus, more targeted genetic or pharmacologic manipulations are required to fully evaluate this question.
Table 1.
Addictive Drug-Induced Glutamate Transporter Adaptations
| Brain Region | Effect on Expression and | Species | Treatment Protocol | |
|---|---|---|---|---|
| Ethanol | ||||
|
EAAT1/
GLAST |
NAc core | ⇔ Melendez et al., 2005 | Rats | 1 g/kg i.p. x 7 days |
| PFC | ⇑ mRNA Flatscher-Bader and Wilce, 2008 | humans, postmortem | Alcoholics | |
| PFC | ⇑ mRNA Rimondini et al., 2002 | Rats | Chronic intermittent ethanol vapor | |
| PFC/NAc | ⇔ Hakami et al., 2016 | P rats | Two-bottle choice | |
| VTA/NAc shell | ⇓ Ding et al., 2013 | *Female P rats |
Two-bottle choice | |
| Cannabinoids | ||||
| Hippocampus | ⇓ Castaldo et al., 2010 | Rat | Perinatal THC | |
| Cerebellum | ⇓ Suarez et al., 2004 | Rat | Perinatal THC | |
|
EAAT2/
GLT-1 |
Cocaine | |||
| NAc core | ⇓ Knackstedt et al., 2010 | Rat | Short access SA and extinction | |
| NAc core | ⇓ Trantham-Davidson et al., 2012 | Rat | Short access SA and extinction | |
| NAc core | ⇓ Reissner et al., 2015 | Rat | Short access SA and extinction | |
| NAc core/shell | ⇓/⇔Fischer-Smith et al., 2012 | Rat | Short access SA and acute withdrawal | |
| NAc core/shell | ⇓⇓/⇓ Fischer-Smith et al., 2012 | Rat | Short access SA and long withdrawal | |
| NAc core/shell | ⇓⇓⇓/⇓⇓ Fischer-Smith et al., 2012 | Rat | Long access SA and acute withdrawal | |
| NAc core/shell | ⇓⇓⇓⇓/⇓⇓ Fischer-Smith et al., 2012 | Rat | Long access SA and long withdrawal | |
| NAc core | mRNA ⇓ Kim et al., 2016b | Rat | Extended access SA and extended withdrawal | |
| Amphetamine/Methamphetamine | ||||
| DStr | ⇑ Shirai et al., 1996 | Rat | Methamphetamine sensitization | |
| NAc core/shell | ⇔ Szumlinski et al., 2016 | Mice | Methamphetamine sensitization | |
| midbrain, NAc, Str, PFC | ⇔ Sidiripoulou et al, 2001 | Rat | Amphetamine sensitization | |
| Str and hippocampus | ⇓ Althobaiti et al., 2016 | Rat | Methamphetamine high 10 mg/kg x 4, every 2 h | |
| Ethanol | ||||
| NAc core | ⇔ Melendez et al., 2005; | Rat | 1 g/kg i.p. x 7 days | |
| NAc core/shell | ⇓ Sari et al., 2013 | P rats | Chronic drinking | |
| PFC and Str | ⇓ Abulseoud et al., 2014 | P Rats/ Wistars |
Chronic drinking followed by oral gavage | |
| VTA/NAc shell | ⇔ Ding et al., 2013 | *Female P rats | Two-bottle choice | |
| NAc core | ⇔ Griffin et al., 2015 | Mice | Chronic intermittent alcohol | |
| *white blood cells | ⇑ Ozsoy et al., 2016 | Human | Alcoholics at d 1 and d 28 withdrawal | |
| Nicotine | ||||
| NAc core | ⇓ Knackstedt et al., 2009 | Rat | SA and extinction | |
| NAc core | ⇓ Gipson et al., 2013 | Rat | SA and extinction | |
| Opiates | ||||
| NAc core | ⇓ Shen et al., 2014 | Rat | Heroin SA and extinction | |
| Cannabinoids | ||||
| Hippocampus | ⇓ Castaldo et al., 2010 | Rat | Perinatal THC or WIN 55,212-2 | |
|
EAAT3/
EAAC1 |
Amphetamine/Methamphetamine | |||
| Str | ⇓ Kerdsan et al., 2012 | Rat | Meth: acute (8 mg/kg, ip 1x) or chronic (4 mg/kg, ip 14 days) | |
| PFC | ⇓ Kerdsan et al., 2012 | Rat | Meth chronic (4 mg/kg, ip 14 days) | |
| Hippocampus | ⇓ Kerdsan et al., 2012 | Rat | Meth chronic (4 mg/kg, ip 14 days) | |
| PFC | ⇓/⇔ Lominac et al., 2016 | Mice | Chronic meth (10 mg/kg x 10 days) + 21-d withdrawal | |
| Midbrain, NAc, DStr, PFC | no change Sidiripoulou et al., 2001 | Rat | Amphetamine sensitization | |
| NAc | ⇓ Szumlinski et al., 2016 | High meth drinking mice | naïve | |
| NAc | ⇓ Szumlinski et al., 2016 | Mice | Chronic meth (10 mg/kg x 10 days) + 21 day withdrawal | |
| NAc | ⇔ Szumlinski et al., 2016 | Mice | Meth CPP | |
| Opiates | ||||
| PFC, NAc, VTA/HP | ⇑ surface/⇔Wan et al., 2016 | Mice | Morphine CPP | |
| PFC | ⇓ Wu et al., 2013 | Mice | Morphine-induced reinstatement of CPP | |
| Cannabinoids | ||||
| Cerebellum | ⇓ Suarez et al., 2004 | Rat | Perinatal THC | |
Represents protein level changes except where indicated. Downward arrows indicate a decrease, upward arrows indicate an increase, and sideways arrows indicate no change.
Data pertaining to other drugs is lacking. Nicotine increases GLAST expression through a α7 nicotinic acetylcholine receptor and fibroblast growth factor-2-dependent pathway in rat cortical cultures (Morioka et al., 2014; Morioka et al., 2015). Perinatal delta-9-tetrahydrocannabinol (THC) exposure decreases GLT-1 and GLAST protein and reduces glutamate uptake in hippocampus (Castaldo et al., 2010) and produces an enduring deficit in GLAST levels in cerebellum (Suarez et al., 2004). Repeated intermittent 3,4-methylenedioxymethamphetamine (MDMA) exposure increases GLAST expression in cortex (Kindlundh-Hogberg et al., 2008). Finally, other studies suggest that GLAST contributes to morphine tolerance and antinociceptive effects in spinal cord (Mao et al., 2002; Tai et al., 2007; Eidson et al., 2017).
EAAT2/GLT-1
Localization/Distribution
GLT-1 is one of the most abundant proteins ubiquitously expressed throughout the brain (Takahashi et al., 2015) and is responsible for ~90% of glutamate uptake. EAAT2 is the human homologue of GLT-1, and in postmortem cortical tissue EAAT2 is detected robustly in astrocytic processes and much less frequently in neuronal profiles, including within the postsynaptic density at asymmetric synapses (Roberts et al., 2014). Most GLT-1 immunoreactivity colocalizes with presynaptic marker synaptophysin (within 0.2 μm2) and is thus considered the synaptic pool, but ~40% of GLT-1 is located distally from the synapse, constituting an extrasynaptic pool (Figure 2) (Minelli et al., 2001). There are at least 3 different GLT-1 isoforms that vary at their C terminus: GLT1a, GLT1b, and GLT1c. These isoforms differ in relative abundance (for example: 90%, 6%, and 1%, respectively, in hippocampus) (Holmseth et al., 2009). There are no data to suggest that these variants alter transport function per se, but they likely contribute to differential protein expression, localization, and/or trafficking (Holmseth et al., 2009). For example, GLT1a but not GLT1b is detected in neurons, and GLT-1b contains a PDZ domain absent in GLT1a (Holmseth et al., 2009; Sogaard et al., 2013).
Regulation of Expression/Trafficking
The rat and human promoters of EAAT2/GLT-1 have many conserved sequences, including binding sites for transcription factors NF-κB, cAMP response element binding protein (CREB), and SP-1 (Sitcheran et al., 2005; Grewer et al., 2014). These sequences and their interacting transcription factors control both constitutive and/or inducible GLT-1 expression. NF-κB, for example, is involved in both basal and inducible regulation of GLT-1 expression. NF-κB mediates GLT-1 induction via cAMP, TGFa, and EGF, and is also required for TNFa-dependent GLT-1 repression in human H4 glioma cells (Sitcheran et al., 2005). Comparisons of the human and rat promoters in reporter assays reveal remarkable similarity but not complete overlap between gene regulation of GLT-1 and EAAT2. For instance, TNFa-dependent inhibition occurs through distinct mechanisms, but EGF, TGFa, and PACAP-dependent transactivation is similar between species (Allritz et al., 2010).
The 3’ untranslated region of GLT-1 has predicted binding sites for microRNAs miR-128, miR182, and miR200b, representing another potential means of transcriptional regulation, but these interactions are untested (Lauriat and McInnes, 2007). Surprisingly, miR-124a, the most abundant miR in vertebrate brain, increases GLT-1 protein without altering gene expression denoting translational rather than transcriptional regulation (Morel et al., 2013). This effect is mediated via novel exosomal transfer of neuronal miR-124a into astrocytes. Other translational activators of EAAT2 include corticosterone and retinol through an interaction with the 5’ untranslated region (Tian et al., 2007). Conversely, excessive glutamate or oxidative stress significantly inhibits EAAT2 translation (Tian et al., 2007).
EAAT2 expression is additionally regulated at the epigenetic level. There is an CpG island before GLT-1 exon 1 that displays both enrichment of repressive trimethyl-histone H3 alongside decreased acetyl-histone H4 in cerebellum compared with cortex (Perisic et al., 2012). Interestingly, EAAT2 expression in cortex but not the hypermethylated form in cerebellum is inducible by dexamethasone and HDAC inhibitors (Perisic et al., 2010, 2012). This distinction in region-specific methylation might contribute to the parallel difference in protein distribution. Likewise, lower methylation of the EAAT2 promoter is observed in astrocyte-neuron co-cultures compared with astrocytes cultured alone in accordance with higher EAAT2 expression in co-cultures (Yang et al., 2010).
Posttranslational modifications of GLT-1 regulate transporter expression, function, and trafficking. A fraction of EAAT2 is constitutively sumoylated in vivo with this form preferentially retained intracellularly (Foran et al., 2014). GLT-1 palmitoylation at cysteine 38 is required for basal glutamate uptake but does not impact expression or trafficking (Huang et al., 2010). Finally, PKC-dependent phosphorylation of Nedd4-2 ubiquitin ligase promotes the inducible internalization and degradation of GLT-1 (García-Tardón et al., 2012), while constitutive GLT-1 recycling utilizes clathrin-dependent internalization but also involves a ubiquitination cycle (Martinez-Villarreal et al., 2012).
EAAT2 and Drugs of Abuse
CocaineIn a standard short-access cocaine self-administration and exti nction model, GLT-1 is downregulated in NAc core (Knackstedt et al., 2010). GLT-1 protein in NAc core is sensitive to both increasing cocaine intake (extended 6–8 h/d self-administration compared with limited access) and increasing abstinent withdrawal period (1 vs 45 days) (Fischer-Smith et al., 2012). In NAc shell, GLT-1 expression is reduced by limited access with long withdrawal and by extended access with short or long withdrawal. Overall, NAc shell does not display the same progressive decrease in protein levels observed in core and the magnitude of change is lower (Fischer-Smith et al., 2012). Indeed, GLT-1 expression in NAc core but not shell displays a significant negative correlation with cue-induced cocaine seeking. Interestingly, while GLT-1 protein levels are altered even following short-access and limited withdrawal in NAc core, GLT-1 mRNA is only decreased following prolonged withdrawal from long access self-administration (R. Kim et al., 2016b). This decrease in GLT-1 expression is associated with hypermethylation, suggesting differential engagement of transcriptional and translational mechanisms depending on addiction state.
Amphetamine/Methamphetamine
One study shows methamphetamine sensitization increasing GLT-1 in striatum (Shirai et al., 1996), but a recent study finds no change in GLT-1 in NAc core or shell following another sensitization protocol (Szumlinski et al., 2016). Likewise, no change in GLT-1 in midbrain, NAc, dorsal striatum, or PFC is reported following amphetamine sensitization (Sidiropoulou et al., 2001). Repeated high doses of methamphetamine (10 mg/kg x 4, every 2 hours) decrease GLT-1 in striatum and hippocampus (Althobaiti et al., 2016). The disparity between these results likely reflects differences in dosing and treatment regimens. Indeed, we know that treatment protocol is important for methamphetamine-dependent glutamate dynamics, because methamphetamine self-administration with extinction training reduces basal glutamate levels in dmPFC and NAc (Parsegian and See, 2014), but extracellular glutamate is increased in NAc following withdrawal without extinction (Lominac et al., 2012). Unfortunately, the effect of amphetamine/methamphetamine self-administration on GLT-1 protein is unknown.
Ethanol
The effects of ethanol administration on GLT-1 levels and function are not fully consistent. Sari and colleagues (Sari et al., 2013) report reduced GLT-1 in NAc core and shell in male P rats following chronic drinking. In an ethanol withdrawal study that incorporated 2-bottle choice drinking followed by forced oral gavage in male P rats and outbred Wistar rats, a similar decrease in GLT-1 was observed in PFC and striatum (Abulseoud et al., 2014). Interestingly, in female P rats, EAAT1 rather than EAAT2 levels (discussed above) are reduced in the NAc shell and VTA (Ding et al., 2013). No differences in EAAT2 expression are observed in NAc following noncontingent sensitizing ethanol injections in rats (Melendez et al., 2005) or chronic intermittent ethanol vapor in mice (Griffin et al., 2015). Overall, the reduction in GLT-1 protein following ethanol consumption/exposure is most reliably reproduced in studies utilizing alcohol preferring P rats compared with outbred rats or mice. Therefore, it would be interesting to determine whether basal differences in GLT-1 expression or function exist between P rats and other outbred strains or if they emerge due to significant differences in ethanol consumption. Furthermore, it is also probable that exposure type (daily vs intermittent; choice vs noncontingent) and withdrawal period influence GLT-1 expression and function, and generally more variable results are obtained in noncontingent ethanol exposure models.
There is also clinical evidence for alcohol-dependent EAAT2 regulation. In inpatient alcoholics, EAAT2 and EAAT3 mRNA is increased in white blood cells during early (day 1) and late (day 28) withdrawal compared with healthy controls (Ozsoy et al., 2016). Proton magnetic resonance spectroscopy shows elevated glutamate levels in NAc in alcohol-dependent patients during early withdrawal compared with controls, and craving positively correlates with combined glutamate and glutamine levels in NAc and anterior cingulate cortex (Bauer et al., 2013).
Nicotine, Opiates, and Cannabinoids
Nicotine self-administration in rats decreases GLT-1 protein expression in NAc (Knackstedt et al., 2009; Gipson et al., 2013). Heroin self-administration produces functional deficits in glutamate uptake along with decreased GLT-1 surface expression in NAc (Shen et al., 2014). In a noncontingent mouse model of opiate reward, GLT-1 overexpression in NAc shell reduces morphine CPP (Fujio et al., 2005). A single study links perinatal exposure to THC or WIN 55,212-2 to decreases in GLT-1 protein and glutamate uptake in hippocampus (Castaldo et al., 2010).
EAAT3
Localization/Distribution
In the brain, EAAT3/EAAC1 expression is primarily neuronal (Velaz-Faircloth et al., 1996). EAAT3 is relatively ubiquitous, but higher densities are observed in cortex, hippocampus, basal ganglia, and cerebellum, and it is also found widely in peripheral tissues (Velaz-Faircloth et al., 1996). In hippocampus and cerebellum, synapses are less likely to be bounded by astrocytic processes, perhaps magnifying the importance of neuronal EAATs. EAAT3/EAAC1 is present in all neuronal subcompartments both pre- and postsynaptically (Figure 3) (Nieoullon et al., 2006). The majority of EAAT3/EAAC1 protein resides intracellularly (Nieoullon et al., 2006). As such, it is theorized that EAAT3/EAAC1 is important for diverse functions not necessarily related to glutamate uptake and recycling. For example, glutamate taken up by EAAT3 in gamma amino-butyric acid (GABA) neurons provides a precursor for further GABA production (Bjorn-Yoshimoto and Underhill, 2016). EAAT3 also displays high affinity for L-cysteine and thus serves as the primary source of intracellular precursor for production of the important antioxidant glutathione (GSH) (Watts et al., 2014).
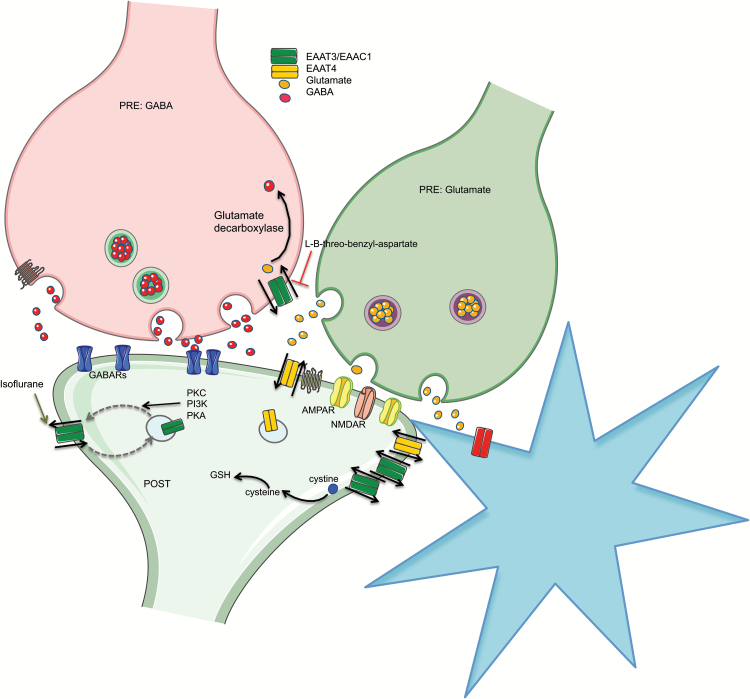
Figure 3.
Neuronal glutamate transporters. Neuronal excitatory amino acid transporter EAAT3/EAAC1 (green) is located both and pre- and postsynaptically throughout the brain. EAAT4 (yellow) is likewise a neuronal EAAT found mainly in the cerebellum but with some forebrain and midbrain expression. EAAT3 expressed presynaptically on GABAergic neurons participates in supplying glutamate precursor for GABA synthesis (orange arrow). EAAT3 also more effectively transports L-cysteine compared with the other EAATs, thus directly supplying precursor for glutathione (GSH) synthesis. Rapid regulation of cell surface expression of EAAT3 is important, because the majority of the protein normally resides intracellularly. Protein Kinase A (PKA), Protein Kinase C (PKC), and Phosphoinositide 3-Kinase (PI3K) inhibitors decrease EAAT3 surface expression, demonstrating positive regulation by these kinases. Likewise, isoflurane (and other anesthetics) promotes insertion of EAAT3 in the plasma membrane also through purported kinase-dependent mechanisms (green arrow). The elimination of the ether oxygen in the nonselective EAAT inhibitor threo-beta-hydroxyaspartate (TBOA) to yield L-B-threo-benzyl-aspartate (L-B-BA) produces an inhibitor with 10-fold preference for EAAT3 over EAAT1 and EAAT2 (red bar-line).
Regulation of Expression
The EAAT3 promoter contains a unique consensus binding site, not shared by EAAT1 or EAAT2, for transcription factor regulatory factor x-1, which positively regulates EAAT3 expression (Ma et al., 2006). Nuclear factor erythroid 2-related factor 2 interacts with a conserved ARE-related sequence in the EAAT3 promoter to induce EAAT3 expression (Escartin et al., 2011). This pathway is activated by oxidative stress to increase EAAT3 expression and augment GSH levels (Escartin et al., 2011). EAAT3 gene expression is also increased by nuclear factor retinoic acid receptor beta (Bianchi et al., 2009).
Three alternative isoforms are reported for human EAAT3 protein. Two isoforms result from skipping of exon 2 or 11 and have significant implications for predicted protein structure (Bjorn-Yoshimoto and Underhill, 2016). The third isoform, identified in humans and mice, is a result of a secondary internal promoter producing an N-terminal truncated protein (Porton et al., 2013). In humans, these alternative isoforms display differential expression in obsessive-compulsive disorder (OCD) patients, and certain variants are associated with disease transmission (Arnold et al., 2006; Porton et al., 2013). This is noteworthy given that OCD, like addiction, is a psychiatric disease related to obsessive thoughts and impulse control deficits.
Given the primarily intracellular EAAT3 localization, rapid regulation of trafficking by posttranslational modifications may be more important than transcriptional mechanisms for controlling function. EAAT3/EAAC1 is constitutively internalized via clathrin and dynamin-dependent mechanisms and recycled back to the plasma membrane via Rab11-dependent trafficking (González et al., 2007). Kinases like PKCα and PI3K participate in activity-dependent EAAT3 trafficking (Bjorn-Yoshimoto and Underhill, 2016).
EAAT3 and Drugs of Abuse
There is accumulating evidence for some EAAT3 involvement in amphetamine/methamphetamine addiction, but EAAT3 levels have never been measured following self-administration. In striatum, acute (8 mg/kg i.p., 1 day) and repeated (4 mg/kg i.p., 14 days) methamphetamine injections decrease EAAT3 levels, but in frontal cortex this effect is only significant with repeated injections (Kerdsan et al., 2012). Conversely, repeated methamphetamine increases EAAT3 levels in hippocampus, perhaps as compensatory mechanism to counter excitotoxicity in this brain region (Kerdsan et al., 2012). Chronic methamphetamine treatment (10 days, 2 mg/kg i.p.) followed by 21 days of withdrawal reduces EAAT3 levels in NAc (Szumlinski et al., 2016) but not PFC (Lominac et al., 2016). Repeated amphetamine injections fail to alter EAAT3 expression across a number of brain regions (Sidiropoulou et al., 2001). In mice bred for high vs low methamphetamine preference, high-drinking mice have lower EAAT3 levels in NAc core along with elevated extracellular glutamate basally and upon methamphetamine challenge (Szumlinski et al., 2016). The EAAT3 phenotype fails to correlate with methamphetamine place preference in outbred C57BL/6J mice, but the hyperglutamatergic profile observed in NAc of drug-naive methamphetamine high-drinking mice is phenocopied by outbred mice withdrawn from a sensitizing methamphetamine regimen, suggesting that this molecular profile represents both a cause and consequence of methamphetamine addiction (Szumlinski et al., 2016).
There is evidence that EAAT3 trafficking and not just expression can be altered by amphetamines, perhaps contributing to inconsistent results observed with total protein. In midbrain dopamine cells, acute amphetamine treatment decreases EAAT3-specific glutamate uptake via Rho-A-dependent EAAT3 endocytosis, leading to potentiated glutamatergic synaptic transmission (Underhill et al., 2014). These researchers went on to demonstrate that acute methamphetamine similarly augments glutamatergic signaling (Li et al., 2016). These studies implicate immediate changes in EAAT3 function for acute amphetamine effects that could have implications for long-term adaptations downstream contributing to the transition to addiction.
Morphine place conditioning increases membrane EAAT3 levels in mPFC, NAc, and VTA 24 hours after the final injection in mice, although expression is unchanged in hippocampus (Wan et al., 2016). Morphine-induced CPP reinstatement in rats decreases EAAT3 in PFC measured 30 minutes after the priming injection (Wu et al., 2013). Interestingly, constitutive EAAT3 knockout does not affect acquisition, expression, or reinstatement of morphine preference, but instead morphine CPP extinguishes faster in EAAT3-/- mice, perhaps suggesting that increased EAAT3 following conditioning is a compensatory mechanism to limit reward-related behavior. Cell culture studies validate a dynamic regulation of EAAT3 expression by morphine with dose- and time-dependent effects upon initial exposure, recovery with withdrawal, and increased sensitivity upon reexposure (Wu et al., 2013), which may also go towards explaining opposing changes reported in rat vs mouse CPP studies above.
There is limited data linking EAAT3 to other drugs. Acute cocaine (10 mg/kg i.p.) administered at P15 transiently reduces D-aspartate uptake for 60 minutes associated with increased serine phosphorylation of EAAT1, EAAT2, and EAAT3 in PFC (Sathler et al., 2016). Perinatal THC exposure produces an enduring deficit in EAAT3 levels in cerebellar Purkinje neurons (Suarez et al., 2004).
Translational Studies on EAAT Pharmacotherapies for Addiction
Preclinical Models
The preclinical research linking EAAT dysfunction to drug addiction has primarily focused on identifying compounds that regulate EAAT2/GLT-1 given that GLT-1 mediates most glutamate uptake, but few drugs specifically target one EAAT over the others. Function of other EAATs may be affected or alternative mechanisms may be engaged by these manipulations. This caveat should be kept in mind when considering studies summarized below, as even the “specific” GLT-1 inhibitor dihydrokainate (DHK) can have off-target effects.
The antioxidant cystine pro-drug N-acetylcysteine (NAC) is the best-studied GLT-1 modulating drug. The over-the-counter dietary supplement is commonly indicated for acetaminophen overdose. NAC can suppress NF-κB signaling and prevent downstream activation of proinflammatory cytokines (Oka et al., 2000). NF-κB interacts with the GLT-1 promoter at multiple sites and has been implicated in transcriptional repression and activation (Sitcheran et al., 2005). Furthermore, NAC provides cystine for GSH synthesis and can interact directly with reactive oxygen species (Badisa et al., 2013). Unfortunately, as an oral medication NAC has low bioavailability, thereby requiring large doses. NAC amide (AD4, NAC-amide) is purported to display better bioavailability properties (Jastrzębska et al., 2016).
In rodent self-administration models, NAC inhibits cue- and cocaine-induced reinstatement of cocaine seeking and restores GLT-1 expression (Baker et al., 2003a, 2003b; Madayag et al., 2007; Knackstedt et al., 2010; Amen et al., 2011), and this effect is long lasting following daily NAC administration prior to extinction sessions (Moussawi et al., 2011b; Reichel et al., 2011). A single study using NAC-amide similarly shows reduced cue-induced and cocaine-primed reinstatement (Jastrzębska et al., 2016). A vivo-morpholino anti-sense strategy was used to show that NAC’s ability to decrease cue- and cocaine-primed reinstatement depends on increased GLT-1 (Reissner et al., 2015). Daily NAC also reduces extinction responding and cue-induced reinstatement for heroin and nicotine seeking (Zhou and Kalivas, 2008; Ramirez-Nino et al., 2013). Surprisingly, NAC has no effect on cue-induced reinstatement of ethanol seeking (Weiland et al., 2015), but blocks behavioral sensitization to ethanol and associated brain changes in ΔFosB in PFC and NAc (Morais-Silva et al., 2016).
Compared with the overall positive NAC treatment effects on abstinent drug seeking above, the effects on drug reward during ongoing intake are equivocal. NAC does not alter the acute rewarding effects of cocaine during short access (1–2 h/d) self-administration or the acute locomotor stimulating effects (Madayag et al., 2007). However, chronic NAC treatment prevents locomotor sensitization to cocaine (Madayag et al., 2007). One unique study used a second-order schedule to assess effects of NAC on cocaine seeking vs taking within the same experiment, and NAC dose-dependently reduces drug seeking but not taking at acquisition and maintenance stages (Murray et al., 2012). Yet, in the long access self-administration model that produces escalated drug intake, NAC blunts this escalation (Madayag et al., 2007); however, if escalated cocaine intake is preestablished, NAC treatment is ineffective at reducing escalation progression or modifying motivation to seek a drug under a progressive ratio schedule (Ducret et al., 2016). NAC increases sensitivity to punishment in a “voluntary abstinence” model in long access rats only, and in NAC-treated rats, cocaine intake never returns to control levels following punished suppression (Ducret et al., 2016). Taken together, these studies point to a state-dependent NAC treatment effect on measures of cocaine addiction and bring to mind the progressive adaptations in GLT-1 protein and gene expression observed in NAc core with increasing cocaine exposure and withdrawal length (R. Kim et al., 2016b). This state dependence is further supported by the fact that NAC does not increase GLT-1 levels in controls (Knackstedt et al., 2010). Additionally, NAC may be more effective as a relapse prevention therapy for cocaine addiction delivered during abstinence rather than active use, an idea supported by the clinical research. NAC may offer more promise as a suppressant of active drug intake for other substances. Both acute and chronic NAC reduce nicotine self-administration (Ramirez-Nino et al., 2013). Chronic NAC reduces ethanol intake during maintenance drinking while having no effect during acquisition (Quintanilla et al., 2016).
Beta-lactam antibiotics stimulate GLT1 expression and function (Rothstein et al., 2005). These include ceftriaxone, cefazolin, amoxicillin, ampicillin, and cefoperazone, of which ceftriaxone has been best studied. As indicated by their nomenclature, these antibiotics contain a beta-lactam ring conferring their glutamate-regulating activity. Like NAC, the purported mechanism for ceftriaxone to increase GLT-1 expression involves NF-κB-dependent transactivation (Seok-Geun Lee et al., 2008). Repeated dosing is required for optimal effects on behavior and changes in GLT-1 (Rao et al., 2015a).
Ceftriaxone inhibits cue- and cocaine-induced reinstatement of cocaine seeking and restores GLT-1 expression (Sari et al., 2009; Knackstedt et al., 2010; Sondheimer and Knackstedt, 2011). Similarly, ceftriaxone inhibits cue-induced reinstatement of heroin seeking and reverses deficits in GLT-1 expression and glutamate uptake (Shen et al., 2014). Intra-NAc DHK and DL-threo-benzyloxyaspartic acid (DL-TBOA) microinfusions block the effects of ceftriaxone on cocaine seeking (Fischer et al., 2013), and an anti-sense morpholino strategy confirmed GLT-1 dependence of ceftriaxone’s effects on heroin seeking (Shen et al., 2014). Ceftriaxone reduces drug-induced reinstatement of methamphetamine and nicotine CPP (Abulseoud, 2012) (Alajaji et al., 2013). Likewise, ceftriaxone reduces physical, somatic, and affective nicotine withdrawal signs in ICR mice (Alajaji et al., 2013) and nicotine intake in female P rats (Sari et al., 2016). Subthreshold doses of MK-801, an NMDAR antagonist, plus ceftriaxone reduce the acquisition of morphine CPP and block extinction and reinstatement of preference (Fan et al., 2012). Therapeutic doses of ceftriaxone alone given during extinction block reinstatement of morphine CPP and normalize plasticity in NAc shell medium spiny neurons (Hearing et al., 2016). Repeated ceftriaxone treatment reduces alcohol drinking in male and female P rats coincident with an increase in GLT-1 levels in PFC and NAc (Sari et al., 2011, 2016). Other beta-lactams, ampicillin, cefazolin, cefoperazone, and amoxicillin, decrease ethanol intake and increase GLT-1 expression in PFC and NAc (Rao et al., 2015b; Hakami et al., 2016). Further, ceftriaxone alleviates alcohol withdrawal syndrome symptoms (Abulseoud et al., 2014) and reduces relapse-like drinking behavior modeled by restoration of drinking following a 2-week withdrawal (Qrunfleh et al., 2013; Rao and Sari, 2014). Finally, cefazolin and ceftriaxone reduce cue-induced reinstatement to ethanol (Weiland et al., 2015).
Several other agents with glutamate uptake-regulating capabilities have been investigated to a lesser extent. Clavulanic acid, a structural beta-lactam analog with some beta-lactamase inhibitory activity, has greater oral availability and brain penetrability compared with ceftriaxone and negligible antibiotic activity (J. Kim et al., 2016a). Chronic treatment with clavulanic acid increases GLT-1 expression in NAc and reduces motivation for cocaine under a progressive ratio schedule (J. Kim et al., 2016a). Clavulanic acid also reduces morphine CPP and locomotor sensitization (Schroeder et al., 2014). Riluzole, a neuroprotective drug approved for treating amyotrophic lateral sclerosis, increases GLT-1 transcription through a supposed heat shock factor 1-dependent mechanism (Liu et al., 2011) and augments GLT-1 protein levels and function (Carbone et al., 2012). Riluzole reduces expression of locomotor sensitization to methamphetamine (Itzhak and Martin, 2000) and blocks morphine and amphetamine CPP, although on its own it produces a preference (Tzschentke and Schmidt, 1998). Acute and chronic riluzole treatment reduces ethanol self-administration in mice and withdrawal-induced convulsions (Besheer et al., 2009). MS-153 [(R)-(-)-5-methyl-l-nicotinoyl-2-pyrazoline] is a synthetic pyrazoline analog that enhances glutamate uptake (Shimada, 1999). MS-153 blocks the development of amphetamine and morphine CPP (Nakagawa, 2005). It also reduces alcohol intake and increases GLT-1 in NAc, hippocampus, and amygdala in association with increased NF-κB65 and decreased IκB-α in P rats (Alhaddad et al., 2014; Aal-Aaboda et al., 2015). GPI-1046 is a neuroimmunophilin that reduces ethanol consumption and increases GLT-1 levels in PFC and NAc and enhances GSH synthesis in striatum (Tanaka et al., 2002; Sari and Sreemantula, 2012). Propentofylline is a methylxanthine derivative that reduces astroglial activation (Sweitzer and De Leo, 2011) and increases GLT-1 levels in NAc of cocaine withdrawn rats. Accordingly, 5 daily injections of propentyphylline reduce reinstated cocaine seeking (Reissner et al., 2014).
PRECLINICAL MODELS
Clinical trials using NAC to treat drug addiction have been systematically reviewed recently (Deepmala et al., 2015; Nocito Echevarria et al., 2017). There are 5 NAC clinical trials for cocaine dependence. In the largest double-blind placebo-controlled study, NAC does not differ from placebo in self-reported cocaine use or drug-negative urines; however, in a subset of patients that were abstinent at study initiation, their days to relapse increased (LaRowe et al., 2013). In a smaller trial, NAC decreased self-reported use and money spent on cocaine (Mardikian et al., 2007). Proof of principle laboratory trials show decreases in self-reported craving and attentional bias in response to cocaine cues or drug infusion (LaRowe et al., 2007; Amen et al., 2011). These results are in line with NAC acting more as a relapse prevention medication rather than cocaine cessation treatment, which is supported by rodent studies where NAC is largely ineffective at modifying cocaine intake under short access self-administration conditions but reliably reduces reinstatement when given during extinction (Baker et al., 2003a; Madayag et al., 2007; Reissner et al., 2015). In 2 trials conducted for methamphetamine dependence, results are equivocal. Methamphetamine-dependent individuals receiving NAC + naltrexone received no benefit over placebo (Grant et al., 2010); however, a more recent double-blind crossover trial shows NAC reduced craving (Mousavi et al., 2015).
There are 3 clinical trials for NAC with cannabis dependence and 6 for nicotine use or dependence. A small open-label trial shows decreased self-reported cannabis use and craving in adolescents (Gray et al., 2010), although there was no difference between NAC treatment and control in the follow-up study (Roten et al., 2013). However, in a larger, double-blind, placebo-controlled study conducted in adolescent and young adult cannabis-dependent patients, NAC increased drug-negative urines (Gray et al., 2012). A large, multi-site, clinical study through the National Institute on Drug Abuse Clinical Trials Network titled Achieving Cannabis Cessation – Evaluating NAC treatment was established to replicate positive findings in a larger sample (McClure et al., 2014). The results of nicotine clinical trials with NAC vary. This may be due in part to complications associated with high comorbidity between smoking with other drug use and psychiatric disorders. For example, in a study on substance use in bipolar patients, nicotine use was not reduced by NAC (Bernardo et al., 2009), while in a study of pathological gamblers, NAC temporarily reduced nicotine dependence measures (Grant et al., 2014). In a double-blind, placebo-controlled, pilot study of heavy smokers, NAC did not reduce craving or withdrawal symptoms, but the first cigarette smoked after brief abstinence reportedly felt less rewarding (Schmaal et al., 2011). Dependent smokers treated with chronic NAC self-reported reduced daily cigarettes smoked, although actual carbon monoxide levels, craving, and withdrawal systems were unaffected (Knackstedt et al., 2009). Recently, an open-label pilot trial of NAC plus varenicline reported a modest reduction in daily cigarettes smoked (McClure et al., 2015), and a 12-week, double-blind, placebo-controlled pilot of NAC in patients with therapy-resistant tobacco use disorder showed a reduction in daily cigarette use, exhaled carbon monoxide, and depression scores (Prado et al., 2015). Ongoing clinical trials will clarify effects of NAC on substance use and craving.
The clinical data is lacking as it concerns other glutamate uptake modulators in addiction. A single, multi-arm, modified blinded, placebo-controlled efficacy screening trial of cocaine-dependent individuals found riluzole ineffective at reducing drug-negative urines or decreasing craving beyond placebo (Ciraulo et al., 2005). There is currently one double-blind placebo-controlled study recruiting cocaine-dependent participants to assess safety and drug interactions between clavulanic acid and cocaine (NCT02563769).
PERSPECTIVES
Is GLT1 the Whole Story?
Recent research from Underhill and colleagues (Underhill et al., 2014) has ignited interest in neuronal transporter EAAT3 in relation to amphetamine/methamphetamine addiction, but this transporter might be similarly affected by other psychostimulants. AMPH reduces EAAT3 function in midbrain dopamine neurons, resulting in potentiated glutamatergic transmission (Underhill et al., 2014; Li et al., 2016). Acute administration of cocaine similarly induces long-term potentiation in VTA dopamine cells (Ungless et al., 2001), and it is hypothesized that this early synaptic potentiation contributes to the transition to compulsive drug use (Lüscher, 2013). Thus, it would be interesting to determine if EAAT3 dysfunction contributes to this effect for cocaine and other drugs. Moreover, outside of addiction, it has been shown that EAAT3 plays a critical role in learning and memory. Fear conditioning rapidly increases surface EAAT3 expression in hippocampus, and EAAT3-/- mice display deficits in context and cue-related learning and memory (Wang et al., 2014). These data could have implications for cue and context reward associations during drug self-administration and reinstatement if drugs of abuse modify EAAT3. Interestingly, learning and memory deficits in EAAT3-/- are rescued by NAC treatment likely due to restoration of redox status (see below) (Cao et al., 2012).
We have not discussed EAAT4 or EAAT5. EAAT5 is restricted to retina and thus irrelevant to this discussion. However, EAAT4 is a neuronal glutamate transporter expressed abundantly in cerebellar Purkinje cells, but also at lower levels throughout the forebrain and midbrain with the highest levels in substantia nigra pars compacta, VTA, habenula-interpedunclar system, supraoptic nucleus, lateral posterior thalamic nucleus, and subiculum (Massie et al., 2008). EAAT4 can act as a glutamate-gated chloride channel adding to the ways this transporter might regulate synaptic transmission (Furuta et al., 1997). Little is known about the potential role of EAAT4 as a substrate for drug addiction, but given that EAAT4 protein is detected in midbrain and forebrain regions, the study of drug effects on this transporter should be expanded. This is further supported by evidence of antipsychotic treatments impacting EAAT4 expression (Huerta, 2006; Segnitz, 2009), and the demonstration that learned helpless rats display decreased levels of EAAT4 in the hippocampus and cerebral cortex given the connection between substance abuse and other affective disorders (Zink et al., 2010).
How Much Does Regulation of Redox State Contribute to NAC Treatment Efficacy?
Alcohol, psychostimulants, and opiates affect cellular redox status and can lead to the formation of oxidative or nitrosative stress (ROS and RNS). Redox-sensitive posttranslational modifications on cysteine residues, such as S-glutathionylation and S-nitrosylation, can influence the function of many addiction-related signaling proteins, and there is direct evidence for the involvement of these processes in cocaine addiction. GSH is one of the most important cellular antioxidants, and it is modified to GSH disulfide under conditions of oxidative stress. The GSH:GSH disulfide ratio is a common measure of intracellular redox status. Acute cocaine increases redox potential and S-glutathionylation of proteins in NAc (Uys et al., 2011). Currently, studies are underway to determine which proteins are modified as a result of chronic drug use. In addition to its ability to increase GLT-1, NAC is a precursor for GSH. NAC treatment reverses GSH depletion in rat astroglial cells following cocaine-induced oxidative stress (Badisa et al., 2013).
Nitric oxide is another important molecule regulated by redox state. Cocaine increases nitric oxide levels in dorsal striatum (D. K. Lee et al., 2011). Cue-induced reinstatement of cocaine seeking increases s-nitrosylation and enzymatic activity of extracellular matrix enzymes matrix metalloproteinases (MMPs) in NAc core (Smith et al., 2017). This activation of matrix metalloproteinases during cue-induced reinstatement generalizes across abused drugs and is required for cue-reinstated drug seeking (Smith et al., 2014). In vitro studies indicate that NAC can block activation of pro-MMP-9 and inhibit enzymatic function of activated protein (Pei et al., 2006), thus another parallel mechanism through which NAC could regulate drug seeking.
Targeting Glutamate Transport in Other Psychiatric Diseases
An emerging hypothesis states that addiction is one disease exemplary of a class of disorders that carry intrusive thinking as a symptomatic endophenotype, including OCD, major depression, and schizophrenia (Kalivas and Kalivas, 2016). Each of these diseases has a suggested etiology involving dysregulated glutamatergic transmission with varying degrees of evidence demonstrating EAAT involvement. As mentioned before, the EAAT3 genetic locus is associated with OCD risk (Arnold et al., 2006; Porton et al., 2013), and glutamate-modulating drugs including NAC, riluzole, and minocycline have garnered interest as alternative therapies for resistant OCD with several clinical trials at various stages of completion (Pittenger, 2015). In human postmortem studies, deficits in EAAT protein and RNA are reported in frontal cortex and striatum of depressed patients (McCullumsmith and Meador-Woodruff, 2002; Miguel-Hidalgo et al., 2010). Likewise, a chronic unpredictable stress rat model of depression markedly downregulates EAAT2 and EAAT3 in the hippocampus (Zhu et al., 2017). Also, acute stress in rats causes an enduring reduction of GLT-1 in the NAc (Garcia-Keller et al., 2016), and a recent double-blind clinical trial in veterans comorbid for posttraumatic stress disorder and substance use disorder revealed that NAC reduced both drug craving and symptoms of posttraumatic stress disorder (Back et al., 2016). Sub-anesthetic ketamine, an off-label treatment for refractory depression increasingly used in the clinic, rescued this deficit and the associated behavior (Zhu et al., 2017). Similar results were reported with decreased GLT-1 levels in hippocampus of depressed rats ameliorated by the traditional antidepressant fluoxetine, suggesting that normalization of these proteins may be important for antidepressant efficacy (Chen et al., 2014). Accordingly, double-blind studies in depressed patients show that NAC reduces symptoms of depression (Fernandes et al., 2016; however, see Berk et al., 2014). For schizophrenia, the glutamate hypotheses originated from observations that NMDAR antagonists like phencyclidine and ketamine produce schizophrenia-like symptoms. Examination of human postmortem brains reveals consistent alterations at the level of morphology of glutamatergic neurons and synapses, but alterations of mRNA and proteins related to glutamate transmission, including EAATs, are varied (Hu et al., 2015). Nonetheless, therapies targeting glutamate transmission at multiple levels are being investigated as adjunctive and stand-alone schizophrenia treatments, and NAC in combination with antipsychotic medication significantly improves symptoms of schizophrenia over control (Berk et al., 2008). Altogether, these examples in no way constitute a comprehensive analysis of this literature, as that is beyond the scope of this review, but these data are highlighted to illustrate remarkable mechanistic overlap between addiction and other often co-morbid mental diseases. Thus, EAAT-modulating drugs may have translational relevance across compulsive psychiatric disorders.
Conclusions
Disruptions in glutamate homeostasis following chronic drug use are a well-conserved neuropathology contributing to the lasting vulnerability to relapse characteristic of addiction, and we posit that contributions of glutamate transporters to this phenomenon warrant additional investigation. This requires developing more specific pharmacological tools and additional examination of mechanisms underlying existing drugs. We introduce an emergent role for cellular redox status in response to chronic drugs. We propose that this dual and interactive role between glutamate transporters and redox status be kept in mind as addiction pharmacotherapies are developed and tested. Finally, based on positive clinical trials with NAC that restores GLT-1 levels in animal models of addiction, we suggest that glutamate transport may offer a therapeutic target extending beyond addiction to other psychiatric disorders characterized by intrusive thoughts.
Statement of Interest
None.
Acknowledgments
This work was supported by the National Institute on Drug Abuse at the National Institutes of Health (DA003906, DA012513, DA015369 to P.W.K. and DA041462 to S.S.) and the Burroughs Wellcome Fund (1012607).
References
- Aal-Aaboda M, Alhaddad H, Osowik F, Nauli SM, Sari Y (2015) Effects of (R)-(-)-5-methyl-1-nicotinoyl-2-pyrazoline on glutamate transporter 1 and cysteine/glutamate exchanger as well as ethanol drinking behavior in male, alcohol-preferring rats. J Neurosci Res 93:930–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abulseoud OA, Miller JD, Wu J, Choi DS, Holschneider DP (2012) Ceftriaxone upregulates the glutamate transporter in medial prefrontal cortex and blocks reinstatement of methamphetamine seeking in a condition place preference paradigm. Brain Res 1456:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abulseoud OA, Camsari UM, Ruby CL, Kasasbeh A, Choi S, Choi DS (2014) Attenuation of ethanol withdrawal by ceftriaxone-induced upregulation of glutamate transporter EAAT2. Neuropsychopharmacology 39:1674–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alajaji M, Bowers MS, Knackstedt L, Damaj MI (2013) Effects of the beta-lactam antibiotic ceftriaxone on nicotine withdrawal and nicotine-induced reinstatement of preference in mice. Psychopharmacology (Berl) 228:419–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alele PE, Devaud LL (2005) Differential adaptations in GABAergic and glutamatergic systems during ethanol withdrawal in male and female rats. Alcohol Clin Exp Res 29:1027–1034. [DOI] [PubMed] [Google Scholar]
- Alhaddad H, Kim NT, Aal-Aaboda M, Althobaiti YS, Leighton J, Boddu SH, Wei Y, Sari Y (2014) Effects of MS-153 on chronic ethanol consumption and GLT1 modulation of glutamate levels in male alcohol-preferring rats. Front Behav Neurosci 8:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allritz C, Bette S, Figiel M, Engele J (2010) Comparative structural and functional analysis of the GLT-1/EAAT-2 promoter from man and rat. J Neurosci Res 88:1234–1241. [DOI] [PubMed] [Google Scholar]
- Althobaiti YS, Alshehri FS, Almalki AH, Sari Y (2016) Effects of ceftriaxone on glial glutamate transporters in Wistar rats administered sequential ethanol and methamphetamine. Front Neurosci 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amen SL, Piacentine LB, Ahmad ME, Li SJ, Mantsch JR, Risinger RC, Baker DA (2011) Repeated N-acetyl cysteine reduces cocaine seeking in rodents and craving in cocaine-dependent humans. Neuropsychopharmacology 36:871–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold PD, Sicard T, Burroughs E, Richter MA, Kennedy JL (2006) Glutamate transporter gene SLC1A1 associated with obsessive-compulsive disorder. Arch Gen Psychiatry 63:769–776. [DOI] [PubMed] [Google Scholar]
- Back SE, McCauley JL, Korte KJ, Gros DF, Leavitt V, Gray KM, Hamner MB, DeSantis SM, Malcolm R, Brady KT, Kalivas PW (2016) A double-blind randomized controlled pilot trial of n-acetylcysteine in veterans with PTSD and substance use disorders. J Clin Psychiatry 77:e1439–e1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badisa RB, Goodman CB, Fitch-Pye CA (2013) Attenuating effect of N-acetyl-L-cysteine against acute cocaine toxicity in rat C6 astroglial cells. Int J Mol Med 32:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Toda S, Kalivas PW (2003a) N-acetyl cysteine-induced blockade of cocaine-induced reinstatement. Ann N Y Acad Sci 1003:349–351. [DOI] [PubMed] [Google Scholar]
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW (2003b) Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci 6:743–749. [DOI] [PubMed] [Google Scholar]
- Bauer J, Pedersen A, Scherbaum N, Bening J, Patschke J, Kugel H, Heindel W, Arolt V, Ohrmann P (2013) Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacology 38:1401–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk M, Copolov D, Dean O, Lu K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Judd F, Katz F, Katz P, Ording-Jespersen S, Little J, Conus P, Cuenod M, Do KQ, Bush AI (2008) N-acetyl cysteine as a glutathione precursor for schizophrenia--a double-blind, randomized, placebo-controlled trial. Biol Psychiatry 64: 361–368. [DOI] [PubMed] [Google Scholar]
- Berk M, Dean OM, Cotton SM, Jeavons S, Tanious M, Kohlmann K, Hewitt K, Moss K, Allwang C, Schapkaitz I, Robbins J, Cobb H, Ng F, Dodd S, Bush AI, Malhi GS (2014) The efficacy of adjunctive N-acetylcysteine in major depressive disorder: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry 75:628–636. [DOI] [PubMed] [Google Scholar]
- Bernardo M, Dodd S, Gama CS, Copolov DL, Dean O, Kohlmann K, Jeavons S, Schapkaitz I, Anderson-Hunt M, Bush AI, Berk M (2009) Effects of N-acetylcysteine on substance use in bipolar disorder: a randomised placebo-controlled clinical trial. Acta Neuropsychiatr 21:285–291. [DOI] [PubMed] [Google Scholar]
- Besheer J, Lepoutre V, Hodge CW (2009) Preclinical evaluation of riluzole: assessments of ethanol self-administration and ethanol withdrawal symptoms. Alcohol Clin Exp Res 33:1460–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MG, Gazzola GC, Cagnin S, Kagechika H, Bussolati O (2009) The ATRA-dependent overexpression of the glutamate transporter EAAC1 requires RARbeta induction. Biochim Biophys Acta 1788:1861–1868. [DOI] [PubMed] [Google Scholar]
- Bjorn-Yoshimoto WE, Underhill SM (2016) The importance of the excitatory amino acid transporter 3 (EAAT3). Neurochem Int 98:4–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Li L, Zuo Z (2012) N-acetylcysteine reverses existing cognitive impairment and increased oxidative stress in glutamate transporter type 3 deficient mice. Neuroscience 220:85–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone M, Duty S, Rattray M (2012) Riluzole elevates GLT-1 activity and levels in striatal astrocytes. Neurochem Int 60:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaldo P, Magi S, Cataldi M, Arcangeli S, Lariccia V, Nasti AA, Ferraro L, Tomasini MC, Antonelli T, Cassano T, Cuomo V, Amoroso S (2010) Altered regulation of glutamate release and decreased functional activity and expression of GLT1 and GLAST glutamate transporters in the hippocampus of adolescent rats perinatally exposed to Delta(9)-THC. Pharmacol Res 61:334–341. [DOI] [PubMed] [Google Scholar]
- Chawla AR, Johnson DE, Zybura AS, Leeds BP, Nelson RM, Hudmon A (2017) Constitutive regulation of the glutamate/aspartate transporter EAAT1 by Calcium-Calmodulin-Dependent Protein Kinase II. J Neurochem 140:421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JX, Yao LH, Xu BB, Qian K, Wang HL, Liu ZC, Wang XP, Wang GH (2014) Glutamate transporter 1-mediated antidepressant-like effect in a rat model of chronic unpredictable stress. J Huazhong Univ Sci Technol Med Sci 34:838–844. [DOI] [PubMed] [Google Scholar]
- Ciraulo DA, Sarid-Segal O, Knapp CM, Ciraulo AM, LoCastro J, Bloch DA, Montgomery MA, Leiderman DB, Elkashef A (2005) Efficacy screening trials of paroxetine, pentoxifylline, riluzole, pramipexole and venlafaxine in cocaine dependence. Addiction 100:12–22. [DOI] [PubMed] [Google Scholar]
- Citri A, Malenka RC (2007) Synaptic plasticity: multiple forms, functions, and mechanisms. Neuropsychopharmacology 33:18–41. [DOI] [PubMed] [Google Scholar]
- Deepmala , Slattery J, Kumar N, Delhey L, Berk M, Dean O, Spielholz C, Frye R (2015) Clinical trials of N-acetylcysteine in psychiatry and neurology: a systematic review. Neurosci Biobehav Rev 55:294–321. [DOI] [PubMed] [Google Scholar]
- Ding Z-M, Rodd ZA, Engleman EA, Bailey JA, Lahiri DK, McBride WJ (2013) Alcohol drinking and deprivation alter basal extracellular glutamate concentrations and clearance in the mesolimbic system of alcohol preferring (P) rats. Addict Biol 18:297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan S, Anderson CM, Stein BA, Swanson RA (1999) Glutamate induces rapid upregulation of astrocyte glutamate transport and cell-surface expression of GLAST. J Neurosci 19: 10193–10200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducret E, Puaud M, Lacoste J, Belin-Rauscent A, Fouyssac M, Dugast E, Murray JE, Everitt BJ, Houeto JL, Belin D (2016) N-acetylcysteine facilitates self-imposed abstinence after escalation of cocaine intake. Biol Psychiatry 80:226–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidson LN, Inoue K, Young LJ, Tansey MG, Murphy AZ (2017) Toll-like receptor 4 mediates morphine-induced neuroinflammation and tolerance via soluble tumor necrosis factor signaling. Neuropsychopharmacology 42:661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermolyuk YS, Alder FG, Surges R, Pavlov IY, Timofeeva Y, Kullmann DM, Volynski KE (2013) Differential triggering of spontaneous glutamate release by P/Q-, N- and R-type Ca2+ channels. Nat Neurosci 16:1754–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escartin C, Won SJ, Malgorn C, Auregan G, Berman AE, Chen PC, Deglon N, Johnson JA, Suh SW, Swanson RA (2011) Nuclear factor erythroid 2-related factor 2 facilitates neuronal glutathione synthesis by upregulating neuronal excitatory amino acid transporter 3 expression. J Neurosci 31: 7392–7401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Niu H, Rizak JD, Li L, Wang G, Xu L, Ren H, Lei H, Yu H (2012) Combined action of MK-801 and ceftriaxone impairs the acquisition and reinstatement of morphine-induced conditioned place preference, and delays morphine extinction in rats. Neurosci Bull 28:567–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes BS, Dean OM, Dodd S, Malhi GS, Berk M (2016) N-acetylcysteine in depressive symptoms and functionality: a systematic review and meta-analysis. J Clin Psychiatry 77:e457–466. [DOI] [PubMed] [Google Scholar]
- Fischer KD, Houston AC, Rebec GV (2013) Role of the major glutamate transporter GLT1 in nucleus accumbens core versus shell in cue-induced cocaine-seeking behavior. J Neurosci 33:9319–9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith KD, Houston AC, Rebec GV (2012) Differential effects of cocaine access and withdrawal on glutamate type 1 transporter expression in rat nucleus accumbens core and shell. Neuroscience 210:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatscher-Bader T, Wilce PA (2008) Impact of alcohol abuse on protein expression of midkine and excitatory amino acid transporter 1 in the human prefrontal cortex. Alcohol Clin Exp Res 32:1849–1858. [DOI] [PubMed] [Google Scholar]
- Foran E, Rosenblum L, Bogush A, Pasinelli P, Trotti D (2014) Sumoylation of the astroglial glutamate transporter EAAT2 governs its intracellular compartmentalization. Glia 62: 1241–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujio M, Nakagawa T, Sekiya Y, Ozawa T, Suzuki Y, Minami M, Satoh M, Kaneko S (2005) Gene transfer of GLT-1, a glutamate transporter, into the nucleus accumbens shell attenuates methamphetamine- and morphine-induced conditioned place preference in rats. Eur J Neurosci 22:2744–2754. [DOI] [PubMed] [Google Scholar]
- Furuta A, Martin LJ, Lin CL, Dykes-Hoberg M, Rothstein JD (1997) Cellular and synaptic localization of the neuronal glutamate transporters excitatory amino acid transporter 3 and 4. Neuroscience 81:1031–1042. [DOI] [PubMed] [Google Scholar]
- Garcia-Keller C, Kupchik YM, Gipson CD, Brown RM, Spencer S, Bollati F, Esparza MA, Roberts-Wolfe DJ, Heinsbroek JA, Bobadilla AC, Cancela LM, Kalivas PW (2016) Glutamatergic mechanisms of comorbidity between acute stress and cocaine self-administration. Mol Psychiatry 21:1063–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Tardón N, González-González IM, Martínez-Villarreal J, Fernández-Sánchez E, Giménez C, Zafra F (2012) Protein Kinase C (PKC)-promoted endocytosis of glutamate transporter GLT-1 requires ubiquitin ligase Nedd4-2-dependent ubiquitination but not phosphorylation. J Biol Chem 287:19177–19187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegelashvili G, Danbolt NC, Schousboe A (1997) Neuronal soluble factors differentially regulate the expression of the GLT1 and GLAST glutamate transporters in cultured astroglia. J Neurochem 69:2612–2615. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith AC, Stankeviciute N, Hensley-Simon ME, Kalivas PW (2013) Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci U S A 110:9124–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND (2011) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12:652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González MI, Susarla BTS, Fournier KM, Sheldon AL, Robinson MB (2007) Constitutive endocytosis and recycling of the neuronal glutamate transporter, excitatory amino acid carrier 1. J Neurochem 103:1917–1931. [DOI] [PubMed] [Google Scholar]
- Grant JE, Odlaug BL, Kim SW (2010) A double-blind, placebo-controlled study of N-acetyl cysteine plus naltrexone for methamphetamine dependence. Eur Neuropsychopharmacol 20:823–828. [DOI] [PubMed] [Google Scholar]
- Grant JE, Odlaug BL, Chamberlain SR, Potenza MN, Schreiber LR, Donahue CB, Kim SW (2014) A randomized, placebo-controlled trial of N-acetylcysteine plus imaginal desensitization for nicotine-dependent pathological gamblers. J Clin Psychiatry 75:39–45. [DOI] [PubMed] [Google Scholar]
- Gray KM, Watson NL, Carpenter MJ, LaRowe SD (2010) N-acetylcysteine (NAC) in young marijuana users: an open-label pilot study. Am J Addict 19:187–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Carpenter MJ, Baker NL, DeSantis SM, Kryway E, Hartwell KJ, McRae-Clark AL, Brady KT (2012) A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry 169:805–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewer C, Gameiro A, Zhang Z, Tao Z, Braams S, Rauen T (2008) Glutamate forward and reverse transport: from molecular mechanism to transporter-mediated release after ischemia. IUBMB life 60:609–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewer C, Gameiro A, Rauen T (2014) SLC1 glutamate transporters. Pflugers Arch 466:3–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, Ramachandra VS, Knackstedt LA, Becker HC (2015) Repeated cycles of chronic intermittent ethanol exposure increases basal glutamate in the nucleus accumbens of mice without affecting glutamate transport. Front Pharmacol 6:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillet BA, Velly LJ, Canolle B, Masmejean FM, Nieoullon AL, Pisano P (2005) Differential regulation by protein kinases of activity and cell surface expression of glutamate transporters in neuron-enriched cultures. Neurochem Int 46:337–346. [DOI] [PubMed] [Google Scholar]
- Hakami AY, Hammad AM, Sari Y (2016) Effects of amoxicillin and augmentin on cystine-glutamate exchanger and glutamate transporter 1 isoforms as well as ethanol intake in alcohol-preferring rats. Front Neurosci 10:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearing MC, Jedynak J, Ebner SR, Ingebretson A, Asp AJ, Fischer RA, Schmidt C, Larson EB, Thomas MJ (2016) Reversal of morphine-induced cell-type-specific synaptic plasticity in the nucleus accumbens shell blocks reinstatement. Proc Natl Acad Sci U S A 113:757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmseth S, Scott HA, Real K, Lehre KP, Leergaard TB, Bjaalie JG, Danbolt NC (2009) The concentrations and distributions of three C-terminal variants of the GLT1 (EAAT2; slc1a2) glutamate transporter protein in rat brain tissue suggest differential regulation. Neuroscience 162:1055–1071. [DOI] [PubMed] [Google Scholar]
- Hu W, MacDonald ML, Elswick DE, Sweet RA (2015) The glutamate hypothesis of schizophrenia: evidence from human brain tissue studies. Ann N Y Acad Sci 1338:38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K, Kang MH, Askew C, Kang R, Sanders SS, Wan J, Davis NG, Hayden MR (2010) Palmitoylation and function of glial glutamate transporter-1 is reduced in the YAC128 mouse model of Huntington disease. Neurobiol Dis 40: 207–215. [DOI] [PubMed] [Google Scholar]
- Huerta I, McCullumsmith RE, Haroutunian V, Giménez-Amaya JM, Meador-Woodruff JH (2006) Expression of excitatory amino acid transporter interacting protein transcripts in the thalamus in schizophrenia. 59:394–402. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL (2000) Effect of riluzole and gabapentin on cocaine- and methamphetamine-induced behavioral sensitization in mice. Psychopharmacology (Berl) 151:226–233. [DOI] [PubMed] [Google Scholar]
- Jastrzębska J, Frankowska M, Filip M, Atlas D (2016) N-acetylcysteine amide (AD4) reduces cocaine-induced reinstatement. Psychopharmacology (Berl) 233:3437–3448. [DOI] [PubMed] [Google Scholar]
- Jensen AA, Fahlke C, Bjorn-Yoshimoto WE, Bunch L (2015) Excitatory amino acid transporters: recent insights into molecular mechanisms, novel modes of modulation and new therapeutic possibilities. Curr Opin Pharmacol 20:116–123. [DOI] [PubMed] [Google Scholar]
- Kalivas BC, Kalivas PW (2016) Corticostriatal circuitry in regulating diseases characterized by intrusive thinking. Dialogues Clin Neurosci 18:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki P, Kim C, Smith K, Son D-S, Aschner M, Lee E (2015) Transcriptional regulation of the astrocytic excitatory amino acid transporter 1 (EAAT1) via NF-κB and Yin Yang 1 (YY1). J Biol Chem 290:23725–23737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson RM, Adermark L, Molander A, Perreau-Lenz S, Singley E, Solomon M, Holmes A, Tanaka K, Lovinger DM, Spanagel R, Heilig M (2012) Reduced alcohol intake and reward associated with impaired endocannabinoid signaling in mice with a deletion of the glutamate transporter GLAST. Neuropharmacology 63:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerdsan W, Thanoi S, Nudmamud-Thanoi S (2012) Changes in the neuronal glutamate transporter EAAT3 in rat brain after exposure to methamphetamine. Basic Clin Pharmacol Toxicol 111:275–278. [DOI] [PubMed] [Google Scholar]
- Kim J, John J, Langford D, Walker E, Ward S, Rawls SM (2016a) Clavulanic acid enhances glutamate transporter subtype I (GLT-1) expression and decreases reinforcing efficacy of cocaine in mice. Amino Acids 48:689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim R, Williams E, Sepulveda-Orenga M, Healey K, Reissner K (2016b) A dual mechanism for regulation of GLT-1 expression by cocaine. In: American College of Neuropsychopharmacology. Hollywood, FL: Nature. [Google Scholar]
- Kindlundh-Hogberg AM, Blomqvist A, Malki R, Schioth HB (2008) Extensive neuroadaptive changes in cortical gene-transcript expressions of the glutamate system in response to repeated intermittent MDMA administration in adolescent rats. BMC Neurosci 9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, LaRowe S, Mardikian P, Malcolm R, Upadhyaya H, Hedden S, Markou A, Kalivas PW (2009) The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry 65:841–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW (2010) Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry 67:81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRowe SD, Myrick H, Hedden S, Mardikian P, Saladin M, McRae A, Brady K, Kalivas PW, Malcolm R (2007) Is cocaine desire reduced by N-acetylcysteine? Am J Psychiatry 164:1115–1117. [DOI] [PubMed] [Google Scholar]
- LaRowe SD, Kalivas PW, Nicholas JS, Randall PK, Mardikian PN, Malcolm RJ (2013) A double-blind placebo-controlled trial of N-acetylcysteine in the treatment of cocaine dependence. Am J Addict 22:443–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauriat TL, McInnes LA (2007) EAAT2 regulation and splicing: relevance to psychiatric and neurological disorders. Mol Psychiatry 12:1065–1078. [DOI] [PubMed] [Google Scholar]
- Lee DK, Ahn SM, Shim YB, Koh WC, Shim I, Choe ES (2011) Interactions of dopamine D1 and N-methyl-D-aspartate receptors are required for acute cocaine-evoked nitric oxide efflux in the dorsal striatum. Exp Neurobiol 20:116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-G, Su Z-Z, Emdad L, Gupta P, Sarkar D, Borjabad A, Volsky DJ, Fisher PB (2008) Mechanism of ceftriaxone induction of excitatory amino acid transporter-2 expression and glutamate uptake in primary human astrocytes. J Biol Chem 283:13116–13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC (1995) Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci 15:1835–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Underhill SM, Reed C, Phillips TJ, Amara SG, Ingram SL (2017) Amphetamine and methamphetamine increase NMDAR-GluN2B synaptic currents in midbrain dopamine neurons. Neuropsychopharmacology 42:1539–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AYC, Mathur R, Mei N, Langhammer CG, Babiarz B, Firestein BL (2011) Neuroprotective drug riluzole amplifies the heat shock factor 1 (HSF1)- and glutamate transporter 1 (GLT1)-dependent cytoprotective mechanisms for neuronal survival. J Biol Chem 286:2785–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominac KD, Sacramento AD, Szumlinski KK, Kippin TE (2012) Distinct neurochemical adaptations within the nucleus accumbens produced by a history of self-administered vs non-contingently administered intravenous methamphetamine. Neuropsychopharmacology 37:707–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lominac KD, Quadir SG, Barrett HM, McKenna CL, Schwartz LM, Ruiz PN, Wroten MG, Campbell RR, Miller BW, Holloway JJ, Travis KO, Rajasekar G, Maliniak D, Thompson AB, Urman LE, Kippin TE, Phillips TJ, Szumlinski KK (2016) Prefrontal glutamate correlates of methamphetamine sensitization and preference. Eur J Neurosci 43:689–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher C. (2013) Cocaine-evoked synaptic plasticity of excitatory transmission in the ventral tegmental area. Cold Spring Harb Perspect Med 3:a012013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma K, Zheng S, Zuo Z (2006) The transcription factor regulatory factor X1 increases the expression of neuronal glutamate transporter type 3. J Biol Chem 281:21250–21255. [DOI] [PubMed] [Google Scholar]
- Madayag A, Lobner D, Kau KS, Mantsch JR, Abdulhameed O, Hearing M, Grier MD, Baker DA (2007) Repeated N-acetylcysteine administration alters plasticity-dependent effects of cocaine. J Neurosci 27:13968–13976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Sung B, Ji RR, Lim G (2002) Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci 22:8312–8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardikian PN, LaRowe SD, Hedden S, Kalivas PW, Malcolm RJ (2007) An open-label trial of N-acetylcysteine for the treatment of cocaine dependence: a pilot study. Prog Neuropsychopharmacol Biol Psychiatry 31:389–394. [DOI] [PubMed] [Google Scholar]
- Martinez-Lozada Z, Guillem AM, Robinson MB (2016) Transcriptional regulation of glutamate transporters: from extracellular signals to transcription factors. Adv Pharmacol 76:103–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Villarreal J, Garcia Tardon N, Ibanez I, Gimenez C, Zafra F (2012) Cell surface turnover of the glutamate transporter GLT-1 is mediated by ubiquitination/deubiquitination. Glia 60:1356–1365. [DOI] [PubMed] [Google Scholar]
- Massie A, Cnops L, Smolders I, McCullumsmith R, Kooijman R, Kwak S, Arckens L, Michotte Y (2008) High-affinity Na+/K+-dependent glutamate transporter EAAT4 is expressed throughout the rat fore- and midbrain. J Comp Neurol 511:155–172. [DOI] [PubMed] [Google Scholar]
- McClure EA, Baker NL, Gipson CD, Carpenter MJ, Roper AP, Froeliger BE, Kalivas PW, Gray KM (2015) An open-label pilot trial of N-acetylcysteine and varenicline in adult cigarette smokers. Am J Drug Alcohol Abuse 41:52–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure EA, Sonne SC, Winhusen T, Carroll KM, Ghitza UE, McRae-Clark AL, Matthews AG, Sharma G, Van Veldhuisen P, Vandrey RG, Levin FR, Weiss RD, Lindblad R, Allen C, Mooney LJ, Haynes L, Brigham GS, Sparenborg S, Hasson AL, Gray KM (2014) Achieving cannabis cessation -- evaluating N-acetylcysteine treatment (ACCENT): design and implementation of a multi-site, randomized controlled study in the National Institute on Drug Abuse Clinical Trials Network. Contemp Clin Trials 39:211–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullumsmith RE, Meador-Woodruff JH (2002) Striatal excitatory amino acid transporter transcript expression in schizophrenia, bipolar disorder, and major depressive disorder. Neuropsychopharmacology 26:368–375. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW (2005) Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcohol Clin Exp Res 29:326–333. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ, Waltzer R, Whittom AA, Austin MC, Rajkowska G, Stockmeier CA (2010) Glial and glutamatergic markers in depression, alcoholism, and their comorbidity. J Affect Disord 127:230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minelli A, Barbaresi P, Reimer RJ, Edwards RH, Conti F (2001) The glial glutamate transporter GLT-1 is localized both in the vicinity of and at distance from axon terminals in the rat cerebral cortex. Neuroscience 108:51–59. [DOI] [PubMed] [Google Scholar]
- Morais-Silva G, Alves GC, Marin MT (2016) N-acetylcysteine treatment blocks the development of ethanol-induced behavioural sensitization and related DeltaFosB alterations. Neuropharmacology 110:135–142. [DOI] [PubMed] [Google Scholar]
- Morel L, Regan M, Higashimori H, Ng SK, Esau C, Vidensky S, Rothstein J, Yang Y (2013) Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J Biol Chem 288:7105–7116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka N, Tokuhara M, Nakamura Y, Idenoshita Y, Harano S, Zhang FF, Hisaoka-Nakashima K, Nakata Y (2014) Primary cultures of rat cortical microglia treated with nicotine increases in the expression of excitatory amino acid transporter 1 (GLAST) via the activation of the alpha7 nicotinic acetylcholine receptor. Neuroscience 258:374–384. [DOI] [PubMed] [Google Scholar]
- Morioka N, Harano S, Tokuhara M, Idenoshita Y, Zhang FF, Hisaoka-Nakashima K, Nakata Y (2015) Stimulation of alpha7 nicotinic acetylcholine receptor regulates glutamate transporter GLAST via basic fibroblast growth factor production in cultured cortical microglia. Brain Res 1625:111–120. [DOI] [PubMed] [Google Scholar]
- Mousavi SG, Sharbafchi MR, Salehi M, Peykanpour M, Karimian Sichani N, Maracy M (2015) The efficacy of N-acetylcysteine in the treatment of methamphetamine dependence: a double-blind controlled, crossover study. Arch Iran Med 18:28–33. [PubMed] [Google Scholar]
- Moussawi K, Riegel A, Nair S, Kalivas PW (2011a) Extracellular glutamate: functional compartments operate in different concentration ranges. Front Syst Neurosci 5:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Zhou W, Shen H, Reichel CM, See RE, Carr DB, Kalivas PW (2011b) Reversing cocaine-induced synaptic potentiation provides enduring protection from relapse. Proc Natl Acad Sci U S A 108:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Everitt BJ, Belin D (2012) N-Acetylcysteine reduces early- and late-stage cocaine seeking without affecting cocaine taking in rats. Addict Biol 17:437–440. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Kaneko S (2013) SLC1 glutamate transporters and diseases: psychiatric diseases and pathological pain. Curr Mol Pharmacol 6:66–73. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Fujio M, Ozawa T, Minami M, Satoh M (2005) Effect of MS-153, a glutamate transporter activator, on the conditioned rewarding effects of morphine, methamphetamine and cocaine in mice. Behav Brain Res 156:233–239. [DOI] [PubMed] [Google Scholar]
- NIDA (2014) Trends & Statistics. Retrieved from https://www.drugabuse.gov/related-topics/trends-statistics. Accessed June 30, 2017.
- Nieoullon A, Canolle B, Masmejean F, Guillet B, Pisano P, Lortet S (2006) The neuronal excitatory amino acid transporter EAAC1/EAAT3: does it represent a major actor at the brain excitatory synapse? J Neurochem 98:1007–1018. [DOI] [PubMed] [Google Scholar]
- Nocito Echevarria MA, Andrade Reis T, Ruffo Capatti G, Siciliano Soares V, da Silveira DX, Fidalgo TM (2017) N-acetylcysteine for treating cocaine addiction: a systematic review. Psychiatry Res 251:197–203. [DOI] [PubMed] [Google Scholar]
- Oka S, Kamata H, Kamata K, Yagisawa H, Hirata H (2000) N-acetylcysteine suppresses TNF-induced NF-kappaB activation through inhibition of IkappaB kinases. FEBS Lett 472:196–202. [DOI] [PubMed] [Google Scholar]
- Ozsoy S, Asdemir A, Karaaslan O, Akalin H, Ozkul Y, Esel E (2016) Expression of glutamate transporters in alcohol withdrawal. Pharmacopsychiatry 49:14–17. [DOI] [PubMed] [Google Scholar]
- Parsegian A, See RE (2014) Dysregulation of dopamine and glutamate release in the prefrontal cortex and nucleus accumbens following methamphetamine self-administration and during reinstatement in rats. Neuropsychopharmacology 39:811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei P, Horan MP, Hille R, Hemann CF, Schwendeman SP, Mallery SR (2006) Reduced nonprotein thiols inhibit activation and function of MMP-9: implications for chemoprevention. Free Radic Biol Med 41:1315–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perisic T, Zimmermann N, Kirmeier T, Asmus M, Tuorto F, Uhr M, Holsboer F, Rein T, Zschocke J (2010) Valproate and amitriptyline exert common and divergent influences on global and gene promoter-specific chromatin modifications in rat primary astrocytes. Neuropsychopharmacology 35:792–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perisic T, Holsboer F, Rein T, Zschocke J (2012) The CpG island shore of the GLT-1 gene acts as a methylation-sensitive enhancer. Glia 60:1345–1355. [DOI] [PubMed] [Google Scholar]
- Pittenger C. (2015) Glutamatergic agents for OCD and related disorders. Curr Treat Options Psychiatry 2:271–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plachez C, Martin A, Guiramand J, Recasens M (2004) Astrocytes repress the neuronal expression of GLAST and GLT glutamate transporters in cultured hippocampal neurons from embryonic rats. Neurochem Int 45:1113–1123. [DOI] [PubMed] [Google Scholar]
- Porton B, Greenberg BD, Askland K, Serra LM, Gesmonde J, Rudnick G, Rasmussen SA, Kao HT (2013) Isoforms of the neuronal glutamate transporter gene, SLC1A1/EAAC1, negatively modulate glutamate uptake: relevance to obsessive-compulsive disorder. Transl Psychiatry 3:e259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado E, Maes M, Piccoli LG, Baracat M, Barbosa DS, Franco O, Dodd S, Berk M, Vargas Nunes SO (2015) N-acetylcysteine for therapy-resistant tobacco use disorder: a pilot study. Redox Rep 20:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qrunfleh AM, Alazizi A, Sari Y (2013) Ceftriaxone, a beta-lactam antibiotic, attenuates relapse-like ethanol-drinking behavior in alcohol-preferring rats. J Psychopharmacol 27:541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla ME, Rivera-Meza M, Berrios-Carcamo P, Salinas-Luypaert C, Herrera-Marschitz M, Israel Y (2016) Beyond the “first hit”: marked inhibition by n-acetyl cysteine of chronic ethanol intake but not of early ethanol intake. Parallel effects on ethanol-induced saccharin motivation. Alcohol Clin Exp Res 40:1044–1051. [DOI] [PubMed] [Google Scholar]
- Ramirez-Nino AM, D’Souza MS, Markou A (2013) N-acetylcysteine decreased nicotine self-administration and cue-induced reinstatement of nicotine seeking in rats: comparison with the effects of N-acetylcysteine on food responding and food seeking. Psychopharmacology (Berl) 225:473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PS, Sari Y (2014) Effectiveness of ceftriaxone treatment in preventing relapse-like drinking behavior following long-term ethanol dependence in P rats. J Addict Res Ther 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PS, Saternos H, Goodwani S, Sari Y (2015a) Effects of ceftriaxone on GLT1 isoforms, xCT and associated signaling pathways in P rats exposed to ethanol. Psychopharmacology (Berl) 232:2333–2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PS, Goodwani S, Bell RL, Wei Y, Boddu SH, Sari Y (2015b) Effects of ampicillin, cefazolin and cefoperazone treatments on GLT-1 expressions in the mesocorticolimbic system and ethanol intake in alcohol-preferring rats. Neuroscience 295:164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Moussawi K, Do PH, Kalivas PW, See RE (2011) Chronic N-acetylcysteine during abstinence or extinction after cocaine self-administration produces enduring reductions in drug seeking. J Pharmacol Exp Ther 337:487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Kalivas PW (2010) Using glutamate homeostasis as a target for treating addictive disorders. Behav Pharmacol 21:514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Brown RM, Spencer S, Tran PK, Thomas CA, Kalivas PW (2014) Chronic administration of the methylxanthine propentofylline impairs reinstatement to cocaine by a GLT-1-dependent mechanism. Neuropsychopharmacology 39:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Gipson CD, Tran PK, Knackstedt LA, Scofield MD, Kalivas PW (2015) Glutamate transporter GLT-1 mediates N-acetylcysteine inhibition of cocaine reinstatement. Addict Biol 20:316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M (2002) Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J 16:27–35. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Roche JK, McCullumsmith RE (2014) Localization of excitatory amino acid transporters EAAT1 and EAAT2 in human postmortem cortex: a light and electron microscopic study. Neuroscience 277:522–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts-Wolfe DJ, Kalivas PW (2015) Glutamate transporter GLT-1 as a Therapeutic target for substance use disorders. CNS Neurol Disord Drug Targets 14:745–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roten AT, Baker NL, Gray KM (2013) Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav 38:1788–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB (2005) Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature 433:73–77. [DOI] [PubMed] [Google Scholar]
- Sari Y, Sakai M, Weedman JM, Rebec GV, Bell RL (2011) Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol 46:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Smith KD, Ali PK, Rebec GV (2009) Upregulation of GLT1 attenuates cue-induced reinstatement of cocaine-seeking behavior in rats. J Neurosci 29:9239–9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sreemantula SN (2012) Neuroimmunophilin GPI-1046 reduces ethanol consumption in part through activation of GLT1 in alcohol-preferring rats. Neuroscience 227:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Sreemantula SN, Lee MR, Choi DS (2013) Ceftriaxone treatment affects the levels of GLT1 and ENT1 as well as ethanol intake in alcohol-preferring rats. J Mol Neurosci 51:779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari Y, Toalston JE, Rao PS, Bell RL (2016) Effects of ceftriaxone on ethanol, nicotine or sucrose intake by alcohol-preferring (P) rats and its association with GLT-1 expression. Neuroscience 326:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathler MF, Stutz B, Martins RS, Dos Santos Pereira M, Pecinalli NR, Santos LE, Taveira-da-Silva R, Lowe J, de Freitas IG, de Melo Reis RA, Manhaes AC, Kubrusly RC (2016) Single exposure to cocaine impairs aspartate uptake in the pre-frontal cortex via dopamine D1-receptor dependent mechanisms. Neuroscience 329:326–336. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Berk L, Hulstijn KP, Cousijn J, Wiers RW, van den Brink W (2011) Efficacy of N-acetylcysteine in the treatment of nicotine dependence: a double-blind placebo-controlled pilot study. Eur Addict Res 17:211–216. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Asan E, Püschel B, Kugler P (1997) Cellular and regional distribution of the glutamate transporter GLAST in the CNS of Rats: nonradioactive in situhybridization and comparative immunocytochemistry. J Neurosci 17:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JA, Tolman NG, McKenna FF, Watkins KL, Passeri SM, Hsu AH, Shinn BR, Rawls SM (2014) Clavulanic acid reduces rewarding, hyperthermic and locomotor-sensitizing effects of morphine in rats: a new indication for an old drug? Drug Alcohol Depend 142:41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, Roberts-Wolfe D, Kalivas PW (2016) The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharmacol Rev 68:816–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segnitz N, Schmitt A, Gebicke-Härter PJ, Zink M (2009) Differential expression of glutamate transporter genes after chronic oral treatment with aripiprazole in rats. Neurochem Int 55:619–628. [DOI] [PubMed] [Google Scholar]
- Sery O, Sultana N, Kashem MA, Pow DV, Balcar VJ (2015) GLAST but not least--distribution, function, genetics and epigenetics of L-glutamate transport in brain--focus on GLAST/EAAT1. Neurochem Res 40:2461–2472. [DOI] [PubMed] [Google Scholar]
- Shen HW, Scofield MD, Boger H, Hensley M, Kalivas PW (2014) Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. J Neurosci 34:5649–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada F, Shiga Y, Morikawa M, Kawazura H, Morikawa O, Matsuoka T, Nishizaki T, Saito N (1999) The neuroprotective agent MS-153 stimulates glutamate uptake. Eur J Pharmacol 386:263–270. [DOI] [PubMed] [Google Scholar]
- Shirai Y, Shirakawa O, Nishino N, Saito N, Nakai H (1996) Increased striatal glutamate transporter by repeated intermittent administration of methamphetamine. Psychiatry Clin Neurosci 50:161–164. [DOI] [PubMed] [Google Scholar]
- Sidiropoulou K, Chao S, Lu W, Wolf ME (2001) Amphetamine administration does not alter protein levels of the GLT-1 and EAAC1 glutamate transporter subtypes in rat midbrain, nucleus accumbens, striatum, or prefrontal cortex. Molecular Brain Research 90:187–192. [DOI] [PubMed] [Google Scholar]
- Sitcheran R, Gupta P, Fisher PB, Baldwin AS (2005) Positive and negative regulation of EAAT2 by NF-κB: a role for N-myc in TNFα-controlled repression. EMBO J 24:510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Kupchik YM, Scofield MD, Gipson CD, Wiggins A, Thomas CA, Kalivas PW (2014) Synaptic plasticity mediating cocaine relapse requires matrix metalloproteinases. Nat Neurosci 17:1655–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AC, Scofield MD, Heinsbroek JA, Gipson CD, Neuhofer D, Roberts-Wolfe DJ, Spencer S, Garcia-Keller C, Stankeviciute NM, Smith RJ, Allen NP, Lorang MR, Griffin WC 3rd, Boger HA, Kalivas PW (2017) Accumbens nNOS interneurons regulate cocaine relapse. J Neurosci 37:742–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogaard R, Borre L, Braunstein TH, Madsen KL, MacAulay N (2013) Functional modulation of the glutamate transporter variant GLT1b by the PDZ domain protein PICK1. J Biol Chem 288:20195–20207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondheimer I, Knackstedt LA (2011) Ceftriaxone prevents the induction of cocaine sensitization and produces enduring attenuation of cue- and cocaine-primed reinstatement of cocaine-seeking. Behav Brain Res 225:252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez I, Bodega G, Rubio M, Fernandez-Ruiz JJ, Ramos JA, Fernandez B (2004) Prenatal cannabinoid exposure down-regulates glutamate transporter expressions (GLAST and EAAC1) in the rat cerebellum. Dev Neurosci 26:45–53. [DOI] [PubMed] [Google Scholar]
- Sullivan SM, Macnab LT, Bjorkman ST, Colditz PB, Pow DV (2007) GLAST1b, the exon-9 skipping form of the glutamate-aspartate transporter EAAT1 is a sensitive marker of neuronal dysfunction in the hypoxic brain. Neuroscience 149:434–445. [DOI] [PubMed] [Google Scholar]
- Sweitzer S, De Leo J (2011) Propentofylline: glial modulation, neuroprotection, and alleviation of chronic pain. Handb Exp Pharmacol:235–250. [DOI] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Campbell RR, Cohen M, Fultz EK, Brown CN, Miller BW, Quadir SG, Martin D, Thompson AB, von Jonquieres G, Klugmann M, Phillips TJ, Kippin TE (2016) Methamphetamine addiction vulnerability: the glutamate, the bad, and the ugly. Biol Psychiatry 81:959–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai YH, Wang YH, Tsai RY, Wang JJ, Tao PL, Liu TM, Wang YC, Wong CS (2007) Amitriptyline preserves morphine’s antinociceptive effect by regulating the glutamate transporter GLAST and GLT-1 trafficking and excitatory amino acids concentration in morphine-tolerant rats. Pain 129:343–354. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Foster JB, Lin CL (2015) Glutamate transporter EAAT2: regulation, function, and potential as a therapeutic target for neurological and psychiatric disease. Cell Mol Life Sci 72:3489–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Yoshioka M, Miyazaki I, Fujita N, Ogawa N (2002) GPI1046 prevents dopaminergic dysfunction by activating glutathione system in the mouse striatum. Neurosci Lett 321:45–48. [DOI] [PubMed] [Google Scholar]
- Tian G, Lai L, Guo H, Lin Y, Butchbach MER, Chang Y, Lin C-lG (2007) Translational control of glial glutamate transporter EAAT2 expression. J Biol Chem 282:1727–1737. [DOI] [PubMed] [Google Scholar]
- Trantham-Davidson H, LaLumiere RT, Reissner KJ, Kalivas PW, Knackstedt LA (2012) Ceftriaxone normalizes nucleus accumbens synaptic transmission, glutamate transport, and export following cocaine self-administration and extinction training. J Neurosci 32:12406–12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ (1998) Blockade of morphine- and amphetamine-induced conditioned place preference in the rat by riluzole. Neurosci Lett 242:114–116. [DOI] [PubMed] [Google Scholar]
- Underhill SM, Wheeler DS, Li M, Watts SD, Ingram SL, Amara SG (2014) Amphetamine modulates excitatory neurotransmission through endocytosis of the glutamate transporter EAAT3 in dopamine neurons. Neuron 83:404–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger T, Lakowa N, Bette S, Engele J (2012) Transcriptional regulation of the GLAST/EAAT-1 gene in rat and man. Cell Mol Neurobiol 32:539–547. [DOI] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A (2001) Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411:583–587. [DOI] [PubMed] [Google Scholar]
- Uys JD, Knackstedt L, Hurt P, Tew KD, Manevich Y, Hutchens S, Townsend DM, Kalivas PW (2011) Cocaine-induced adaptations in cellular redox balance contributes to enduring behavioral plasticity. Neuropsychopharmacology 36:2551–2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velaz-Faircloth M, McGraw TS, alandro MS, Fremeau RT Jr., Kilberg MS, Anderson KJ (1996) Characterization and distribution of the neuronal glutamate transporter EAAC1 in rat brain. Am J Physiol 270:C67–75. [DOI] [PubMed] [Google Scholar]
- Wan L, Bi J, Li J, Zuo Z (2016) Glutamate transporter type 3 participates in maintaining morphine-induced conditioned place preference. Neuroscience 344:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Park SH, Zhao H, Peng S, Zuo Z (2014) A critical role of glutamate transporter type 3 in the learning and memory of mice. Neurobiol Learn Mem 114:70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts SD, Torres-Salazar D, Divito CB, Amara SG (2014) Cysteine transport through excitatory amino acid transporter 3 (EAAT3). PLoS One 9:e109245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiland A, Garcia S, Knackstedt LA (2015) Ceftriaxone and cefazolin attenuate the cue-primed reinstatement of alcohol-seeking. Front Pharmacol 6:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Xia S, Lin J, Cao D, Chen W, Liu L, Fu Y, Liang J, Cao M (2013) Effects of the altered activity of delta-opioid receptor on the expression of glutamate transporter type 3 induced by chronic exposure to morphine. J Neurol Sci 335:174–181. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gozen O, Vidensky S, Robinson MB, Rothstein JD (2010) Epigenetic regulation of neuron-dependent induction of astroglial synaptic protein GLT1. Glia 58:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Kalivas PW (2008) N-acetylcysteine reduces extinction responding and induces enduring reductions in cue- and heroin-induced drug-seeking. Biol Psychiatry 63:338–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Ye G, Wang Z, Luo J, Hao X (2017) Sub-anesthetic doses of ketamine exert antidepressant-like effects and upregulate the expression of glutamate transporters in the hippocampus of rats. Neuroscience letters 639:132–137. [DOI] [PubMed] [Google Scholar]
- Zink M, Vollmayr B, Gebicke-Haerter PJ, Henn FA (2010) Reduced expression of glutamate transporters vGluT1, EAAT2 and EAAT4 in learned helpless rats, an animal model of depression. Neuropharmacology 58:465–473. [DOI] [PubMed] [Google Scholar]