Abstract
Ultrasound-targeted microbubble destruction has been utilized to deliver a drug/gene into cells in both in vitro and in vivo studies. This work was performed to investigate the feasibility of gene transfer to human retinal pigment epithelium cell line(ARPE-19) and rat retinal pigment epithelium cell line(RPE-J) by a combinatorial use of recombinant adeno-associated virus (rAAV) and ultrasound (US) or/and mi-crobubbles (MBs) and compare the difference between them. Different doses of serotype 2 rAAV encoding a enhanced green fluorescent protein (rAAV2-EGFP) gene and MBs was administered to ARPE-19 and RPE-J cells under different US conditions. Transfection efficiency and cell viability were assessed by fluorescence microscopy, flow cytometry (FCM) analysis, trypan blue staining. The results indicated that US and MBs could respectively improve rAAV2mediated gene transfer to RPE-J cells, but neither US nor MBs could do so in ARPE- 19 cells. US plus MBs could significantly enhance rAAV2-mediated gene transfer to ARPE-19 cells, however, the same effects were not seen in RPE-J cells. These findings demonstrated it is not always coincident that US, MBs and US plus MBs exert the similar effects on gene transfer in vitro RPE cells. So, it is necessary to choose appropriate RPE cell line for the study of US or/and MBs-mediated rAAV gene transfer in retinal gene therapy.
Keywords: Gene transfer, ARPE-19 cells, RPE-J cells, rAAV2, ultrasound, microbubble
INTRODUCTION
The retinal pigment epithelium (RPE) cell plays many vital functions, this single layer of polarized cells be-tween the photoreceptors and vascular choroid phago-cytizes shed photoreceptor outer segments, transports nutrients, growth factors to the rods and cones, helps remove waste products and absorbs scattered light (1). Malfunction of the RPE is involved in a wideranging of retinal diseases. Up or down regulation of RPE-specific gene expression has become one of the hot spots of therapeutic territory of retinal diseases. The viral vetor, rAAV has caused considerable interest to the development of retinal gene therapies (2,3). Studies show that rAAV can infect wide variety of cells including RPE cells, provides long-term expression of foreign genes, but does not induce inflammation or cy-totoxicity (3). However, the low transduction efficiency of rAAV limits its therapeutic effects. Enhanced trans-duction of rAAV may produce better therapeutic effects. The combination of US and MBs (US plus MBs) has been utilized to deliver a drug/gene into cells in both in vitro and in vivo studies (4-8) Although their feasibility of enhanced adenovirus-mediated gene transfer into a series of cancer and somatic cells has been examined, the research about US plus MBs enhanced rAAV-mediated gene transfer into RPE cells has hardly been reported. The human RPE cell line, ARPE-19, arose spontane-ously from a primary culture of RPE cells from a male donor. They has structural and functional properties characteristic of RPE cells in vivo (9). The rat RPE cell line, RPE-J was created from Long-Evans rats RPE primary cultures by immortalization with SV40-T. They retain many differentiated features of RPE (10). Here, we utilize this two stable, clonal, polarized RPE cell lines to in vitro study the feasibility of enhanced transduction to RPE by a combinatorial use of rAAV and US or/ and MBs and compare the difference between them.
MATERIALS AND METHODS
Adeno-associated virus
The rAAV2-EGFP were purchased from Vector Gene Technology Company Limited (Beijing, China) which express transgene under the control of cytomegalovirus promoter.
Microbubble contrast agents
SonoVue” microbubble contrast agent (Bracco, Milan, Italy) was reconstituted in saline solution supplied by the manufacturer according to the manufacturer’s protocol and resulted in a preparation that contained 2x108-5x108 MBs/ml by inversion/agitation of the unit. This microbubble contrast agent is a composition of a core of Sulfur hexafluoride gas and an envelope of Phos- pholipids. Their average diameter were about 2.5~6.0um.
Cell culture
ARPE-19 cells (CRL- 2302, ATCC, Rockville, Maryland, USA) were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS) (Gibco, Grand Island, NY, USA) at 37 °C, 5% CO2/95% air. RPE-J cells (CRL- 2240, ATCC, Rockville, Maryland, USA) were maintained in DMEM (Gibco, Grand Island, NY, USA) with 4.5g/L glucose, 2mM L-glutamine, and 0.1mM non-essential amino acids supplemented with 4% (v/v) FBS (Gibco, Grand Island, NY, USA) 33 °C, 5% CO2 /95% air.
Experimental grouping
ARPE-19 cells were put into 24-well plates every other well at the density of 2x105 per well and incubated in 500ul DMEM with 10% FBS for 24h prior to infection. Then they were co-cultured with rAAV2-EGFP at a multiplicity of infection (MOI) of 1x103 vector genomes per cell (vg/cell) (group1R alone),or in combination with MBs (group1R+MBs), US (grou- p1R +US) and US plus MBs (group1R+US+MBs). RPE-J cells were put into 24-well plates every other well at the density of 1x105 per well and incubated in 500ul DMEM with 4% FBS for 24h prior to infection. Then they were co-cultured with rAAV2-EGFP at a MOI of 1x104vg/cell (group 2R alone), or in combination with MBs (group 2R+MBs), US (group 2R+US) and US plus MBs (group 2R+US+MBs).
Ultrasound exposure protocol
A therapeutic ultrasound machine (Topteam161, Chat- tanooga, USA) was applied in this experiments. The frequency, pulse recurrent frequency (PRF) of US was set at 1MHz, 100Hz, respectively. Other parameters of US irradiation were as follow: intensity, 1,2,3 W/cm2; duration, 60,120serands; duty cycle, 10%,20%,50%, con-tinuous wave (CW) in group 1R+US and ìR+US+MBs, intensity,0.2, 0.5, 1, 2, 3W/cm2;duration, 15, 30, 60, 120,180seconds; duty cycle, 10%,20%,50%, ccntinucus wave in group 2R+US and 2R+US+MBs. The concentrations of MBs were chosen at 13.3%, 20%, 26.7%,33.3%,40% in group 1R+MBs and 1R+US+MBs, and 3.3%, 6.7%, 13.3%, 20%, 26.7% in group 2R+MBs and 2R+US+MBs. Before ultrasound exposure, the DMEM in the 24-well plates was drew out and small amount of fresh DMEM was added to the medium ensuring that the volume per well was 150μ!. The rAAV2-EGFP were diluted by DMEM, and mixed with isovolumic MBs standing for 5minutes. Thereafter, the mixed solution was added to the plates and exposed to US. When the insonation was performed, A 2-cm2 probe was placed on the bottom of the plates with a small amount of coupling medium on the surface of the probe. A home-made plastic disc with a hole at the same size of the wells in the middle was placed between the probe and the bottom of the plates to ensure the same thickness of coupling medium between the probe and the plates, as well as avoid the ultrasonic radiation for consecutive wells. Each well of the 24-well plates was supplemented medium to final volume 500μ! at 2h after transduction and was replaced with fresh DMEM with 10% FBS (ARPE- 19 cells) or 4% FBS (RPE-J cells)12h after infection.
Gene transfer efficiency assessment
After gene transfer treatment, ARPE-19 cells and RPE-J cells were incubated in DMEM with 10% FBS, 4% FBS for 48 hours, respectively. EGFP expression were ob-served and photographed using inverted fluorescence microscopy (Zeiss Axioplan2 imaging, Carl Zeiss, Germany). The ratios of EGFP-positive RPE cells were quantitatively examined by FCM (EPICS XL, Beckman Coulter Company, Miami, FL, USA) analysis.
Cell viability assessment
Cell viability were assessed by Trypan Blue exclusion test immediately after transfection. RPE cells were harvested with trypsin/EDTA, suspended in PBS. Ten μ! of cell suspension were mixed with an equal amount of 0.4% trypan blue dye (Invitrogen, USA). Blue (dead) and white (living) cells were counted microscopically in a hemocytometer (Sigma, USA)
Statistical analysis
All data were expressed as means and standard deviations (mean±SD). Analysis of variance with paired t-test, unpaired t-test, and ANOVA test was used to determine the significance of the difference in a multiple compari- son. The differences were considered significant when the P values were less than 0.05. The software packages used were SPSS, version 13 (SPSS, Chicago, USA).
RESULTS
Gene Transfer by MBs, US and US plus MBs Gene transfer by MBs, US and US plus MBs were shown in Figure 1A and 1B. MBs alone did not improve the transgene expression in the group 1R+MBs (18.42±0.75% vs.18.23±0.65%), but signifi-cantly improve the transgene expression in the group 2R+MBs (16.17±0.22 % vs. 10.53±0.43 %). According to our studies, treated by 13.3% MBs, the ratio of EGFP-positive RPE-J cells was highest than that of the group 2R alone (21.3±0.94 vs.10.53±0.43%). It was similar that US alone showed a weak trend to improve the transgene expression in the group 1R+US(21.47±0.53% vs.18.23±0.65%), but a significant trend to improve the transgene expression in the group 2R+US(17.32±1.13% vs. 10.53±0.43 %). Opti-mized US parameters, we concluded that the transfection rate of rAAV2 to RPE-J cells was maximal under the US condition of 0.5 W/cm2 intensity, 30seconds, and duty cycle 50%,which was about two times of that of the group 2R alone (22.54±0.63% vs. 10. 53±0.43%).
FIGURE 1.
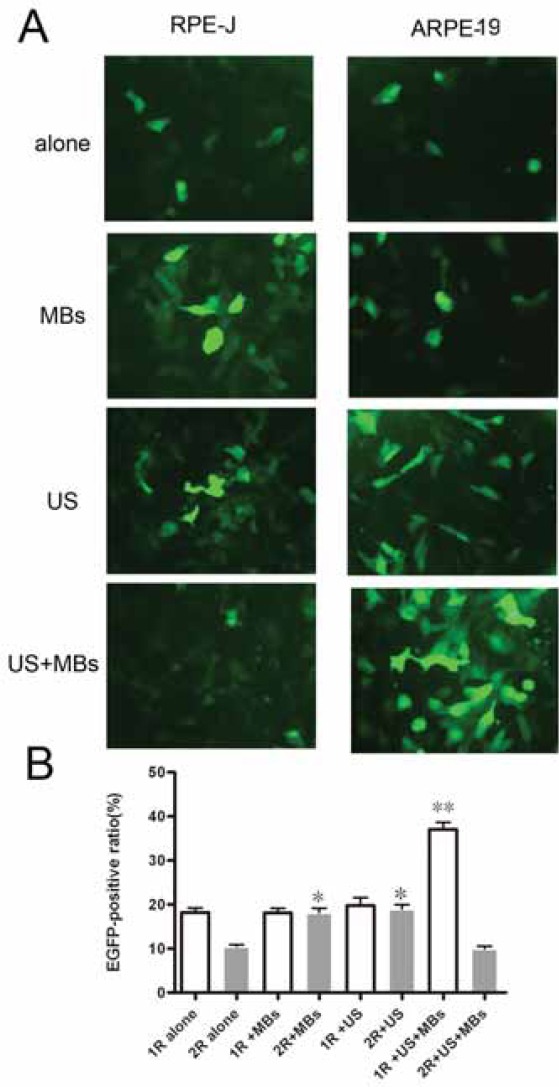
The transfectíon efficiency of r AAV in ARPE-19 and RPE-J cells under MBs, US and US plus MBs conditions. (A) EGFP expression in ARPE-19 and RPE-J cells observed 48h after infection with rAAV2-EGFP.(400xmagnification) (B) EGFP positive rate of ARPE-19 and RPE-J cells analyzed by FCM 48h after infection with rAAV2-EGFP (**p <0,01, *p<0,05). The ARPE-19 cells were infected with rAAV2-EGFP at a dose of lxlO3 vg/cell and RPE-J cells at a dose of 1x104 vg/cell in all the above experiments. All data were presented as means±SD of three independent experiments.
In group 1R+US+MBs, under any of the US plus MBs conditions described above, the ratios of EGFP-pos- itive ARPE-19 cells were significantly (1.1 to 2 times) higher than that of group1R alone (36.33±0.64%% vs.18.23±0.65%). The optimal US plus MBs conditions was 1W/cm2, 60 seconds, 50% duty cycle, 20% MBs concentration. On the contrary, in group 2R+US+MBs, the ratios of EGFP-positive RPE-J cells did not show any trend to be improved, even showed one to be descended (8.63±0.19% vs. 10.53±0.43 %). The optimal US plus MBs conditions could not been identified. Optimization of US plus MBs parameters for gene transfer to RPE cells To identify the optimal conditions to transfer genes by US plus MBs to RPE cells, the fol-lowing four parameters were examined.
MBs concentrations. Five concentrations of MBs -13.3%, 20%, 26.7%, 33.3% and 40% in the group 1R+US+MBs were examined under the US condition of 1W/cm2, 60 seconds, and duty cycle 50%. Five concentrations of MBs—3.3%, 6.7%, 13.3%, 20% and 26.7% in the group 2R+US+MBs were examined under the US condition of 0.5W/cm2,30 seconds, and duty cycle 50%. The EGFP-positive ratios were higher in cells treat-ed by US with 20% MB(37.05±0.55%) and 40% MBs (38.45±0.95%) in the group 1R+US+MBs, but they did not change, even decreased in the group 2R+US+MBs under any MBs concentrations (Figure 2A and 2B).
FIGURE 2.
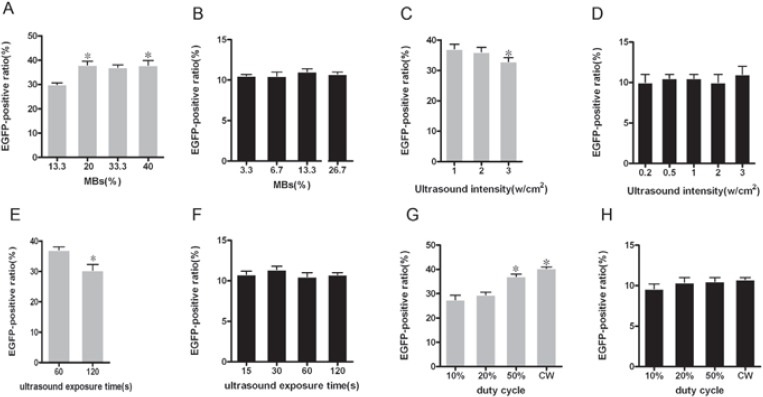
Gene transfer by US plus MBs under different conditions. EGFP-positive ratios of ARPE-19 cells at different concentrations of MBs(A), US intensities(C), US exposure times (E) and duty cycles(G) were examined by FCM analysis 48h after infection with rAAV2-EGFP (*p <0.05). Likewise, EGFP-positive ratios of RPE-J cells at different concentrations of MBs(B), US intensities(D), US exposure times (F) and duty cycles(H) were also examined by FCM analysis 48h after infection with rAAV2-EGFP (*p <0.05). All data were presented as means±SD of three independent experiments.
US Intensity. US intensities of 1, 2, and 3 W/cm2 were examined under the condition of 50% duty cycle and 60-second exposure with 20% MBs in the group 1R+US+MBs. EGFP-positive ratios of 1 W/ cm2 intensity(37.05±0.55%) were higher than that of 2,3 W/cm2 intensity (35.74±0.42%, 32.92±0.97%). US intensities of 0.2,0.5,1,2 and 3 W/cm2 were examined under the condition of 50% duty cycle and 30-second exposure with 13.3% MBs in the group 2R+US+MBs. However, any US intensity could not improve the EGFP-positive ratio (Figure 2C and 2D).
Exposure Time. US exposure times of 60,120seconds were examined under the condition of 1 W/cm2, duty cycle 50%, and 20% MBs in the group 1R+US+MBs. The EGFP-positive ratios of 60 seconds duration were significantly higher than that of 120 seconds duration (37.05±0.55% vs.28.13±0.67%). US exposure times of 15, 30, 60, 120seconds were exam-ined under the condition of 0.5 W/cm2, duty cycle 50%, and 13.3% MBs in the group 2R+US+MBs. But all US exposure times did not show a trend to improve the the EGFP-positive ratio (Figure 2E and 2F).
Duty cycle
Duty cycle of 10%, 20%, 50% and CW were examined under the condition of 2 W/cm2, 60-second exposure with 33.3% MBs in the group 1R+US+MBs and under the condition of 0.5 W/ cm2, 30-second exposure with 13.3% MBs in the group 2R+US+MBs. The EGFP-positive ratio was higher in cells treated by US with duty cycle 50% (31.6310.78%) and continuous wave (33.0610.92%) in the group 1R+US+MBs (Figure 3A and 3B). However, the EGFP-positive ratio in the group 2R+US+MBs showed no difference compared to that of the group 2R alone under any duty cycle (Figure 2G and 2H).
FIGURE 3.
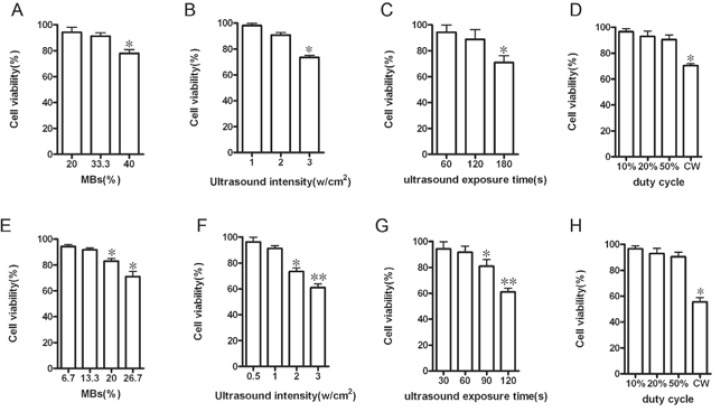
Cell viability assessed by trypan blue exclusion test. Cell viability of APRE-19 cells at different (A) MBs concentrations, (B) US intensities, (C) exposure time and (D)duty cycles, and cell viability of PRE-J cells at different (E) MBs concentrations, (F) US intensities, (G) exposure time and (H) duty cycles were assessed immediately after transfection. (’p <0,05). All data were presented as means±SD of three independent experiments.
Effect of US plus MBs on cell viability
In the group 1R+US+MBs, as long as the intensity was less than 2W/cm2, the duration was less than 120sec- onds, duty cycle was not set at CW, and the concen-tration of MBs was less than 33.3%, the cell viability of various US plus MBs would not been apparently influenced (Figure 3A, 3B, 3C and 3D, Figure 4A). The cell viability of the optimal US plus MBs conditions (1W/cm2, 60 seconds, 50% duty cycle, 20% MBs) was 96.1112.32%. In the group 2R+US+MBs, the cell viability of RPE-J cells would evidently decreased as long as the US intensity was greater than 1W/cm2; duration was greater than 60seconds, duty cycle was set at CW, and the dose of MBs was greater than 13.3% or they were applied instantaneously (Figure 3E, 3F, 3G and 3H, Figure 4D). The cell viability of the optimal MBs (13.3%), US (0.5W/cm2, 30s) conditions and their combinato-rial use was 99.7210.34%, 98.3611.22% and 95.6310.54%.
FIGURE 4.
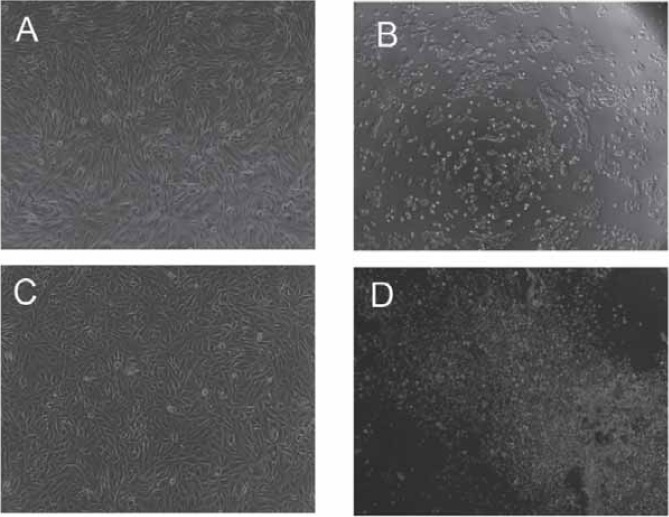
Phase-contrast photograph of ARPE-19 cells immediately after US plus MBs. No apparent cell damage was observed in (A) ARPE-19(1,2W/cm2; 60,120 seconds; 10%, 20%, 50% duty cycle; 20%, 33.3% MBs) and (C) RPE-J cells(0.5, lW/cm2; 30,60 seconds; 10%, 20%, 50% duty cycle; 6.7%,13.3%MBs). Apparent cell damage was found in (B) ARPE-19 ((3W/cm2,60 seconds, 50% duty cycle, 100%MBs) and (D) RPE-J cells (2W/cm2,90 seconds, 50% duty cycle, 26.7%MBs). (A,C,D, E: lOOxmagnification).
DISCUSSION
The AAV2 is cell type-specific and RPE cell is one of the cell types that the AAV2 preferentially transfect, which make AAV an attractive candidate for retinal gene therapy. The AAV infectious process includes multiple-step intracellular events that are approximately divided into seven stages: adhesion to the surface of the target cell (stage1), receptor-mediated endocytosis (stage 2), vesicular trafficking (stage 3), endosomal escape (stage 4), nuclear transport (stage5), viral uncoating (stage 6) and genome conversion of the single-stranded rAAV genome to double stranded DNA intermediates capable of expressing transgenes (stage 7) (11,12). US is generally used for clinical imaging, and its safety has been reliably established. Studies have demonstrated US-enhanced gene delivery to mammalian cells in vitro and in vivo in the past few years (13,14). Furthermore, it is found that cavitation is predominantly responsible for US-mediated gene transfection. The presence of MBs near the cells further increases the gene transfection efficiency by lowering the energy threshold for non-thermal cavitation needed to induce the microjets that penetrate the cellular membrane (8,15-19). In the soni-fication zone, cavitation also creates small shock waves that increase cell permeability by disruption of the membrane barrier. In case of rAAV-mediated gene delivery, US or US plus MBs were presumed to overcome most of the rate-limiting steps, from stage1 to stage 6 and therefore accelerate rAAV2-mediated gene expression.
But, the results presented here indicate that MBs, US and US plus MBs exerted different effect on rAAV-mediated gene transfer to human ARPE-19 and rat RPE-J cells. US and MBs could respectively improve the transfection efficiency of rAAV2 in RPE-J cells, but neither MBs nor US could do so in ARPE-19 cells. In contrary, US plus MBs could significantly enhance rAAV2 transfect to ARPE-19 cells, but they could not exert the same effect on RPE-J cells. In order to make clear this phenomenon, various MBs, US, and US plus MBs conditions and different doses of rAAV were examined. Similar results were gained as described above. Meanwhile, the AAV viability under the condition of US plus MBs (1W/cm2 and 3W/cm2 intensity; 50% duty cycle; 60s and 120s; 33.3%MBs) was accessed by comparing the transfection efficiency of rAAV to ARPE-19 cells treated or not by US plus MBs. No difference was found between the two conditions, which indicated the rAAV viability was normal and its infectious ability was not destroried. (data not shown). So, the difference of cell biology between ARPE-19 and RPE-J cells is mainly responsible for the different effects of MBs, US and US plus MBs on rAAV-mediated gene transfer. Compared to that of ARPE-19 cells, the polarity of Na+, K+ATPase and N-CAM in RPE-J cells differs from the in vivo localization of these proteins (10). The Na, K-ATPase is not polarized and can be detected on the apical, basal and lateral surfaces of the cells. An-other endogenous RPE-J protein, N-CAM, is localized to the lateral surface. RPE-J cells target viral proteins in a polarized fashion to the apical and basal domains. RPE cells can ingest rAAV by receptor-mediated process for binding rAAV to their surface and the non-specific phagocytic ability to phagocytize MBs. The specificity and density of rAAV receptor on the sur-face of ARPE-19 and RPE-J cells determine the intake of rAAV. The difference of their phagocytic ability for MBs is another determinative factor of rAAV intake. The diameter of SonoVue” MBs is big enough to entrap AAV vectors from the environment. When RPE cells phagocytiz MBs, they also intaked the AAV. In this study, the ARPE-19 cells were infected with rAAV2-EGFP at a dose of 1x103 vg/cell and RPE-J cells at a dose of 1x104 vg/cell in the group rAAV alone, but the transfection effiency of rAAV to ARPE-19 cells was 18.23±0.65% and 10.53±0.43 % to RPE-J cells. When they were respectively co-cultured with rAAV2-EGFP in combination with MBs at the same microbubble to RPE cell ratio, the transfection effiency of rAAV to ARPE-19 cells did not change, but the transfection ef-fiency of rAAV to RPE-J cells significantly increased. This results demonstrated the AAV infectious process in ARPE-19 cells is more efficient and there are maybe more or / and more specific rAAV receptor on the sur-face of ARPE-19, and RPE-J cells have a more powerful phagocytic ability compared to that of ARPE-19 cells. When ARPE-19 and RPE-J cells were respectively co-cultured with rAAV2-EGFP under the condition of US exposure, the bioeffects of US can result in permeability changes of the cell membrane, and hence an increased uptake of rAAV-mediated transgene. Because the AAV infectious process in ARPE-19 cells is perhaps more receptor dependence, US alone is not powerful enough to change the style of rAAV transfer into ARPE-19 cells, so the transfection effiency of rAAV showed a weak trend to be improved. However, in RPE-J cells, AAV infectious process is relatively low receptor dependence, US signif-icantly accelerate the transmembrane process of rAAV. Under the condition of US plus MBs, the bioeffects were extremely magnified. Compare to ARPE-19 cells, RPE-J cells is maybe fragile and vulnerable. According to this study, the cell viability of RPE-J cells maintain normal only at gentle conditions of US plus MBs, but it was not the case with ARPE-19 cells. Besides the modality of receptor mediated, rAAV can efficiently transfer into ARPE-19 cells via the reversible pinholes on the cell membrane. But with regard to RPE-J cells, the bioeffects of US plus MBs are too powerful to increase the intake of rAAV. One reason is that when considerable MBs collapse, RPE-J cells can not intak the rAAV by phagocytiz MBs that is maybe a vital approach for RPE-J cells to uptake rAAV. Another reason is that the microstructure change or damage of RPE-J, such as as Na+, K+ATPase(10), N-CAM(10), microvilli(10), caveolae(20), and actinbinding protein(21) under the condition of US plus MBs will result in low trans-fection efficiency of rAAV2 to RPE-J, which in fact destroy the receptor-mediated path of rAAV intake.
CONCLUSION
This study demonstrate that MBs, US and US plus MBs exert different effect on rAAV-mediated gene transfer to human ARPE-19 and rat RPE-J cells, i.e. US and MBs could respectively improve the rAAV2-mediated gene transfer to RPE-J cells, but neither US nor MBs did so in ARPE-19 cells. US plus MBs could significantly enhance rAAV2 transfect to ARPE-19 cells, but the same results was not seen in RPE-J cells. The subtle mechanisms underlying these findings remain to be clarified in future work. It is necessary to choose appropriate RPE cell line for the study of US or/and MBs-mediated AAV gene transfer in retinal gene therapy.
List ofAbbreviations
RPE - retinal pigment epithelium
rAAV - recombinant adeno-associated virus
EGFP - enhanced green fluorescent protein
US - ultrasound
MBs - microbubbles
FCM - flow cytometry
DMEM - Dulbecco’s modified Eagle’s medium
FBS - fetal bovine serum
MOI - multiplicity of infection
PRF - pulse recurrent frequency
CW - continuous wave
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China (Grant No. 30772369). Technical assistance and helpful discussion from the entire staff at the Experimental Research Center of Shanghai Jiaotong University Affili-ated First People’s Hospital is gratefully acknowledged.
REFERENCES
- 1.West K.A, Yan L, Miyagi M, et al. Proteome survey of proliferat-ing and differentiating rat RPE-J cells. Exp.Eye.Res. 2001;73:479–491. doi: 10.1006/exer.2001.1058. [DOI] [PubMed] [Google Scholar]
- 2.Alexander J.J, Umino Y, Everhart D, et al. Restoration of cone vision in a mouse model of achromatopsia. Nature Medicine. 2007;13:685–687. doi: 10.1038/nm1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pang J.J, Lauramore A, Deng W.T, et al. Comparative analysis of in vivo and in vitro AAV vector transduction in the neonatal mouse retina: Effects of serotype and site of administration. Vision Res. 2008;48:377–385. doi: 10.1016/j.visres.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Bekeredjian R, Grayburn P.A, Shohet R.V. Use of ultrasound contrast agents for gene or drug delivery in cardiovascular medicine. J.Am.Coll. Cardiol. 2005;45:329–335. doi: 10.1016/j.jacc.2004.08.067. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Miyoshi H, Nakamura M. Encapsulated ultrasound mi-crobubbles: therapeutic application in drug/gene delivery. J. Control Release. 2006;114:89–99. doi: 10.1016/j.jconrel.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Shohet R.V, Chen S, Zhou Y.T, et al. Echocardiographic destruc-tion of albumin microbubbles directs gene delivery to the myo-cardium. Circulation. 2000;101:2554–2556. doi: 10.1161/01.cir.101.22.2554. [DOI] [PubMed] [Google Scholar]
- 7.Amabile P.G, Waugh J.M, Lewis T.N, Elkins C.J, Janas W, Dake M.D. High-efficiency endovascular gene delivery via therapeutic ultrasound. J.Am.Coll. Cardiol. 2001;37:1975–1980. doi: 10.1016/s0735-1097(01)01253-0. [DOI] [PubMed] [Google Scholar]
- 8.Howard C.M, Forsberg F, Minimo C, Liu J.B, Merton D.A, Claudio P.P. Ultrasound guided site specific gene delivery system using adenoviral vectors and commercial ultrasound contrast agents. J.Cell.Physiol. 2006;209:413–421. doi: 10.1002/jcp.20736. [DOI] [PubMed] [Google Scholar]
- 9.Dunn K.C, Aotaki-Keen A.E, Putkey F.R, Hjelmeland L.M. ARPE-19, A human retinal pigment epithelial cell line with dif-ferentiated properties. Exp.Eye.Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 10.Nabi I.R, Mathews A.P, Cohen-Gould L, Gundersen D, Rodriguez-Boulan E. Immorta-lization of polarized rat retinal pigment epithelium. J.Cell.Sci. 1993;104:37–49. doi: 10.1242/jcs.104.1.37. [DOI] [PubMed] [Google Scholar]
- 11.Ding W, Zhang L, Yan Z, Engelhardt J.F. Intracellular trafficking of adeno-associated viral Vectors. Gene Therapy. 2005;12:873–880. doi: 10.1038/sj.gt.3302527. [DOI] [PubMed] [Google Scholar]
- 12.Paul C. Hendrie,DavidW.Russell.Gene Targeting with viral vec-tors. Molecular Therapy. 2005;12:9–17. doi: 10.1016/j.ymthe.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Manome Y, Nakamura M, Ohno T, Furuhata H. Ultrasound facilitates transduction of naked plasmid DNA into colon carcinoma cells in vitro and in vivo. Hum.Gene.Ther. 2000;11:1521–1528. doi: 10.1089/10430340050083252. [DOI] [PubMed] [Google Scholar]
- 14.Newman C.M, Lawrie A, Brisken A.F, Cumberland D.C. Ultra-sound gene therapy: on the road from concept to reality. Echocar-diography. 2001;18:339–347. doi: 10.1046/j.1540-8175.2001.00339.x. [DOI] [PubMed] [Google Scholar]
- 15.Taniyama Y, Tachibana K, Hiraoka K, et al. Development of safe and efficient novel nonviral gene transfer using ultrasound: en-hancement of transfection efficiency of naked plasmid DNA in skeletal muscle. Gene.Ther. 2002;9:372–380. doi: 10.1038/sj.gt.3301678. [DOI] [PubMed] [Google Scholar]
- 16.Blomley M. Which US microbubble contrast agent is best for gene therapy? Radiology. 2003;229:297–298. doi: 10.1148/radiol.2292031048. [DOI] [PubMed] [Google Scholar]
- 17.Lu Q.L, Liang H.D, Partridge T, Blomley M.J. Microbubble ul-trasound improves the efficiency of gene transduction in skeletal muscle in vivo with reduced tissue damage. Gene.Ther. 2003;10:396–405. doi: 10.1038/sj.gt.3301913. [DOI] [PubMed] [Google Scholar]
- 18.Nakashima M, Tachibana K, Iohara K, Ito M, Ishikawa M, Akamine A. Induction of reparative dentin formation by ultra-sound-mediated gene delivery of growth/ differentiation factor 11. Hum.Gene.Ther. 2003;14:591–597. doi: 10.1089/104303403764539369. [DOI] [PubMed] [Google Scholar]
- 19.Rahim A.A, Taylor S.L, Bush N.L, ter Haar G.R, Bamber J.C, Porter C.D. Spatial and acoustic pressure dependence of mi-crobubble-mediated gene delivery targeted using focused ultra-sound. J.Gene.Med. 2006;8:1347–1357. doi: 10.1002/jgm.962. [DOI] [PubMed] [Google Scholar]
- 20.Mora R.C, Bonilha V.L, Shin B.C, et al. Bipolar assembly of ca-veolae in retinal pigment epithelium. Am. J. Physiol. Cell. Physiol. 2006;290:C832–C843. doi: 10.1152/ajpcell.00405.2005. [DOI] [PubMed] [Google Scholar]
- 21.Bonilha1 V.L, Rodriguez-Boulan E. Polarity and developmental regulation of two PDZ proteins in the retinal pigment epithelium. InvestOphthalmol.Vís.Scí. 2001;42:3274–3282. [PubMed] [Google Scholar]


