Abstract
Brucellosis is an important public health problem in Bosnia and Herzegovina. The diagnosis of bru-cellosis in the country without any experiences with this kind of infection may be very difficult. The aim of this study was to evaluate diagnostic methods: Rose Bengal test, blood cultures and ELISA IgM and IgG in the patients with brucellosis. The study included 91 brucellosis patients in the period 2004 to 2007. All the patients were treated at the Clinic for Infectious Diseases, University of Sarajevo Clinics Centre. Blood cultures were positive in 28/91 (30, 8%) patients. This method often needs a long period of incubation and specimens need to be obtained early. These limitations make serology the most useful tool for the laboratory diagnosis of Brucella infection. Rose Bengal is a rapid plate agglutination test, very sensitive irrespective of the stage of the disease. In our study, Rose Bengal test was positive in all patients 91/91 (100, 0%). Brucella IgM antibodies with ELISA were positive in 59/91 (64, 8%). Brucella IgG antibodies with ELISA were positive in 51/91 (56%). In order to determine the diagnostic value of the different tests, we compared the sensitivity among test-methods: Rose Bengal test-100.0%, blood culture-30.8%, ELISA IgM-64.8% and ELISA IgG-56.1%. Sensitivity of test methods was different in the different stages of illness. It is necessary to use combination of different tests such are blood culture, Rose Bengal test and ELISA in order to ensure the diagnosis. Rose Bengal test is excellent for the screening. Blood culture is a method of choice for the diagnosis acute infection. ELISA is a very good method for the diagnostic chronic disease and relapse.
Keywords: Brucella spp, brucellosis, laboratory diagnosis
INTRODUCTION
Brucellosis is worldwide zoonosis with a high degree of morbidity in humans. The disease remains endemie in many countries, particularly around the Mediter-ranean basin and in the Middle East, India, Mexico, and Central and South America, and thereby repre-sents an important public health problem. Recording to WHO date about 500.000 cases of this disease were registered in the world every year (1,2,3). In Bosnia and Herzegovina before the recent war (1992-1995) only one outbreak was reported on Manjača Mountain, in a military training camp during 1985/86. Brucellosis has become the increasing public health problem in Bosnia and Herzegovina since 2000 (4). Brucellosis is caused by bacteria from genus Brucella. It is Gram-negative facultative intracellular cocco-bacillus size of 0, 5-0, 7 x 0, 5-1, 5 μσι. Although the six species can be differentiated by conventional phenotypic tests, these species display a high degree of DNA homology in DNA-DNA hybridization assays (>90% identity). There- fore, it has been proposed that the Brucella genus should comprise only one species - Brucella melitensis and that the other species should be considered as biovars (5,6,7). Brucellosis is primarily an animal disease, and in them it passes as an asymptomatic chronic infection. Infections of humans are followed by outspread of brucel-losis in animals. It is usually a professional disease of cattle-breeders, farmers, butchers and members of their family, veterinarians and laboratory workers because of transmission ways (direct contact, aerosol inhalation, food). In humans, brucellosis behaves as a systemic infection with a very heterogeneous clinical spectrum. The disease usually presents as fever with no apparent focus, although there are focal forms in 20-40% of cases. The diagnosis of brucellosis in the country without any experiences with this kind of infection may be very dif- ficult. Early diagnostic of brucellosis and inclusion of adequate antibiotic therapy have a crucial importance for patients, especially for the protection development complications and appearance relapses of disease.
As the clinical picture in human’s brucellosis is fairly non-specific, a definitive diagnosis requires isolation of causative organism, or the demonstration of high levels of specific antibodies, or seroconversion (8). The blood culture is method of choice, but specimens should be taken in the early stage of the disease. The incubation of the blood culture takes a long time, be-tween five to ten days. The isolation of the bacterium is proceeding of the biological hazard for the laboratory staff. Brucellosis is one of the most common laboratory acquired infections (9). The polymerase chain reaction (PCR) based laboratory tests have been proposed in the last few years (5,6,7). PCR is a very expensive test and it can not be considered as a routine diagnostic method yet. These limitations make serology the most useful tool for the laboratory diagnosis of the Brucella infection. The antibody detection is not always sufficient to indicate the existence of active infection especially in the endemic areas in which equivocal serologic profiles among affect-ed individuals are frequent (10,11,12,13). Several reports dealing with the significance of various laboratory tests in the diagnosis of brucellosis have been published (14,15,16,17). The present study deals with usefulness and significance of blood culture and serology tests in the diagnosis of the human brucellosis. The main purpose of this work was to evaluate diagnostics methods: Rose Bengal test, blood culture and ELISA IgM and IgG in order to design diagnostic protocol applicable in hospitals.
MATERIALS AND METHODS
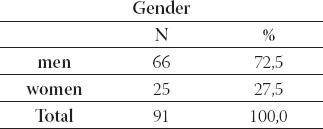
The study included 91 brucellosis patients in period 2004 to 2007. All patients were treated at the Clinic for Infec-tious Diseases, University of Sarajevo Clinics Centre. The average age of patients was 35, 9 years of life-range from 0,6 to 70 (Table 1.). Most of the cases were between 41 and 50 years (Table 2.). Gender structure of patients was: 66 (72,5%) males and 25 (27,5%) females (Table 3). All the laboratories testing for brucellosis was performed at the Institute of microbiology, immunol-ogy and parasitology, University of Sarajevo Clinics Centre. The disease was diagnosed by positive blood-culture results and/or by positive relevant serological test results (ELISA, Rose-Bengal latex agglutination).
TABLE 1.
The average age of patients
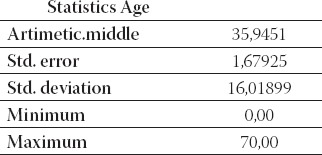
TABLE 2.
Age structure of patients
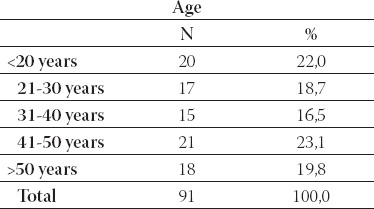
TABLE 3.
Gender structure of patients
Blood culture
The blood culture was performed by inoculation 8-10 cm3 of freshly collected blood into each of Plus aerobic/F BACTEC bottle and incubation for up to seven days in the “BACTEC 9120” semi-automated systems (Becton-Dickinson Diagnostic Instruments Systems, Maryland, USA). Bottles were examined for the presence of growth on a 10-minute cycle by the mea-surement of CO2-induced fluorescence emitted by the sensor at the bottom of the culture. Bottles giving a positive growth index were Gram stained and subcultured to blood agar plates. Brucella isolates were identified by the conventional biochemical testing (catalase, oxidase and urease activity; glucose fermentation and production of H2S). Brucella spp. suspected isolates were con-firmed by slide agglutination using type-specific antisera (Murex Diagnostics Dartford, United Kingdom). The Rose-Bengal latex agglutination (RB test) was performed according to standard procedures. Un-diluted serum samples (30μL) were mixed with an equal volume of Rose Bengal Slide Screening Test antigen (bio Merioux, Marcy L Etoile/France) on a white agglutination card. Results were rated negative when agglutination was absent and 1+ to 4+ positive according to the strength of the agglutination. Brucella IgM and IgG enzyme-linked immunosorbent assays (ELISAs) were obtained from Genzyme Virotech GmbH/Germany. The test was performed and evaluated according to the kit procedure. The test result was read automatically by BEP 2000-Behring ELISA processor. For the evaluation of the results, standard statistical methods were used. Statistical analysis was performed by using a Chi-square test. Statistically significance was defined for p<0, 05. In our study we observed ethical principles outlined in World Medical Association Declaration of Helsinki.
RESULTS
Of the 91 brucellosis patients, the blood cultures were positive in 30, 8% (28/91) (Table 4).
TABLE 4.
Results of examination blood cultures

TABLE 5.
Results of Rose Bengal test

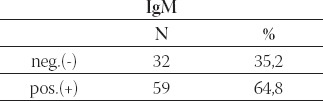
Brucella IgM antibodies with ELISA were positive in 59/91 (64, 8%) and negative in 32/91 (35, 2%) patients (Table 6.).
TABLE 6.
The value of Immunoglobulin M (IgM)
Brucella IgG antibodies with ELISA were positive in 51/91 (56%) and negative in 40/91 (44%) patients (Table 7).
TABLE 7.
The value of Immunoglobulin G (IgG)

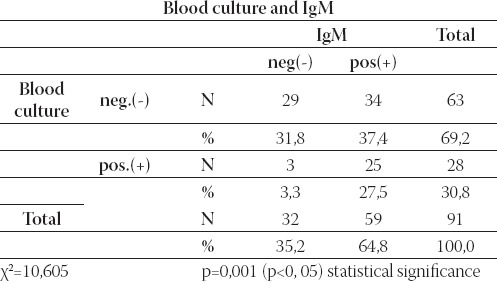
On Table 8. there was relationship between blood culture and IgM. Results of this methods corresponded at 54/91(59, 3%) patients: in 29/91(31, 8%) patients both methods were negative, and in 25/91(27, 5%) both methods were positive. χ2 (Chi square test) demonstrates statistical significance co-incidence among blood culture and IgM (p=0,001).
TABLE 8.
Review of relationship between blood culture and IgM
The relationship between IgG and IgM is shown in Table 9.
TABLE 9.
Review of relationship between IgG and IgM
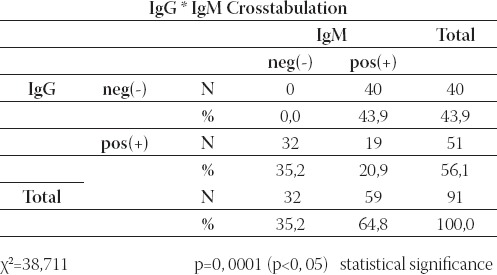
Results show that the values of IgG and IgM corresponded at 19/91(20, 9%) patients. χ2 (Chi square test) demonstrates statistical significance distinction inside number of patients with positive and negative value IgM in relation of value of IgG (p=0, 0001).
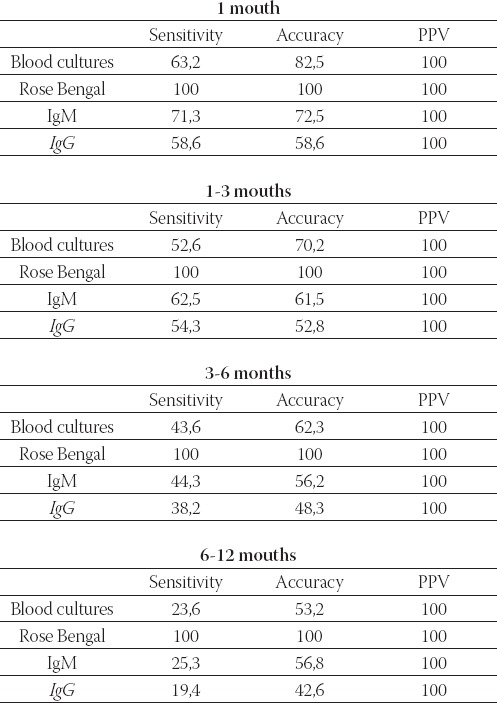
The sensitivity of the test-methods was: Rose Bengal test- 100, 0%, blood cultures-30, 8%, ELISA IgM-64, 8% and ELISA IgG-56,1%. Positive predictive values were 100, 0% for all methods (Table 10.). Sensitivity of test meth-ods was different in different stages of illness (Table 11.).
TABLE 10.
Sensitivity of test methods
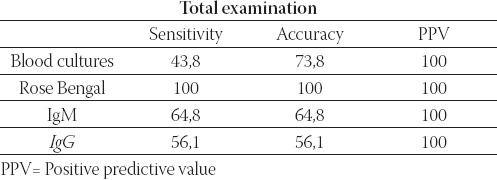
TABLE 11.
Sensitivity of test methods in different stages of illness
DISCUSSION
Brucellosis represents a prevalent disease in humans and animals in Bosnia and Herzegovina in the last ten years. The clinical features and presentation of human brucel-losis overlap with different infectious and autoimmune or neoplastic processes. Since the symptoms of brucello-sis are non-specific, the clinical diagnosis of the disease is difficult. Therefore, its accurate diagnosis necessitates the use of specific tests, mainly culture and serologic tests (8). In this study we evaluated the diagnosis of bru-cellosis by culture, Rose Bengal test, and ELISA IgM and IgG. The study included 91 bru-cellosis patients in period 2004 to 2007. Blood cultures were positive in 28/91 (30, 8%) patients. In total 21/28 (75%) patients blood cultures were positive in the first mouth of infection. This fact suggested that it is method of choice in the acute phase of the dis- ease. The examination of blood cultures gave possibility to identify species in genus Brucella and to perform an-tibiotic susceptibility testing. Although blood culture is the gold standard in the diagnosis of brucellosis, it may sometimes be abolished by the factors inherent to the microbe itself despite the strong laboratory measures undertaken. The isolation of the bacteria are hazardous and brucellosis is one of the most common laboratory acquired infection (9). Despite the important advances made in the diagnosis of human brucellosis following the general introduction of the new semi-automated methods for blood culture processing, the diagnosis of this disease is still based mostly on the demonstration of specific antibodies by means of different serological techniques. This is mainly because the greatest incidence of brucellosis is found in under-developed countries with poor technical resources and so they have not adequate equipment. Brucellosis tends to oc-cur in rural communities. Blood culture demonstrated the high sensitivity in the primary infected patients, but expressed limitations as laboratory test in a rural area in which brucellosis is endemic (10,11,12,13). A large number of different tests have been used for the serological diagnosis of brucellosis, thus demonstrating the lack of an ideal technique. Rose Bengal is a rapid plate agglutination test that uses a suspension of Brucella abortus in an acid buffer. It has a high degree of sensitivity for diagnosis infection with Brucella spp, irrespective of the stage of the disease. This high sensitivity, together with the fact that the technique is simple and rapid (4 min.), makes the Rose Bengal test ideal for the screening patients for human brucellosis. In our study, Rose Bengal test was positive in all patients 91/91 (100, 0%). Use of the Rose Bengal test as the sole diagnostic tool to establish treatment of brucellosis in endemic areas is not a reliable practice with individuals who are exposed repeatedly to the disease, or who have a recent history of the disease (14,15,16). Rose Bengal test continue to be the mainstay of the laboratory diagnosis, due to their simplicity, low cost, and ability (>90% sensitivity) in diag-nosis acute brucellosis. However, this test suffers from high false-negative rates in complicated and chronic cases. ELISA which can reveal the classes and subclasses of immunoglobulins in sensitive is more successful as-say because of the limitations of the Rose Bengal test. In our study, Brucella IgM antibodies with ELISA were positive in 59/91 (64, 8%). Brucella IgG antibodies with ELISA were positive in 51/91 (56%). Early in infection, antibodies of the IgM class predominate. The study reports that the IgM antibody may be detected during the first week following the entry of the organism. The peak level is reached four weeks later. IgG an-tibodies may be detected after 10 days. The peak level is reached after two months. The IgG antibody has delayed appearance, although it is found together with IgM four weeks after the initial antigenic stimulus; the IgM antibody level always exceeds the IgG antibody level in the acute stage of the disease. In our study, in few patients in acute phase of brucellosis with bacter- emia, only IgM antibodies were detected. Several studies have shown that ELISA is the test of choice for the diagnosis of complicated and chronic cases, especially when other tests are negative (17,18,19). Gazapo at al, stated that ELISA IgM and IgG positivity are helpful for epidemiological evaluations, whereas some false positive results can be obtained in agglutination tests due to the cross reactivity between Brucella spp. and Salmonella spp, Vibrio cholera and Yersinia bacteria (20). In many studies performed with ELISA, it was determined that IgG positivity and the increasing of the antibody titers were considerably valuable in relapsed cases and in patients with chronic infections (21,22). The sensitivity of test-methods is: Rose Bengal test-100, 0%, blood culture-30, 8%, ELISA IgM-64, 8% and ELISA IgG-56, 1%. The positive predictive values were 100, 0% for all the methods. The received diagnostic values for sensitivity were lower than values in the literature. The assumption was that this was caused by usage inadequate method for each stage of illness. This assumption was confirmed by evaluation of the sensitivity of appli-cable methods for different stages of illness. The sensitivity of test methods was different in different stages of ill-ness and only combination of blood culture, Rose Bengal test and ELISA ensured the diagnosis. Rose Bengal test is excellent for the screening. The blood culture gave excellent sensitivity results in the patients with primary infections. ELISA performed equally well in the diagnosis patients at different stages of illness including patients with acute, subacute, or chronic disease and with relapse. A combination of those different Brucellosis tests would be considered appropriate for the practical diagnostic measurement, taking into account various factors including the effectiveness for the complementary purpose of the laboratory diagnosis, the different laboratory infrastructure and eco-nomic coverage capacity for laboratory reagents etc.
CONCLUSION
Based on our results, the following conclusions can be drawn:
Huma n brucellosis is an actual public health problem in Bosnia and Herzegovina.
Rose Bengal test is excellent for screening.
Blood culture is method of choice for acute stages of illness.
ELISA (IgM, IgG) is method of choice for the diagnosis chronic disease and relapses
Sensitivity of test methods was different: Rose Bengal test-100, 0%, blood culture-30, 8%, ELISA IgM-64, 8% and ELISA IgG-56, 1%.
Sensitivity of the test methods was different in different stages of illness and only combination blood culture, Rose Bengal test and ELISA ensured the diagnosis
Establishment Reference Laboratory for Brucellosis in Bosnia and Herzegovina would be necessary in order to support the strengthening of laboratory capacity and activities of Brucellosis diagnosis and surveillance and also for national brucellosis control strategies
REFERENCES
- 1.Young E.J. An Overview of human brucellosis. Clin. Infect. Dis. 1995;21:283–290. doi: 10.1093/clinids/21.2.283. [DOI] [PubMed] [Google Scholar]
- 2.Dautović-Krkić S, Mehanić S, Ferhatović M, Čavaljuga S. Bru-cellosis-epidemiological and clinical aspects. Bosn. J. Basic Med. Sci. 2006;6(2):11–15. doi: 10.17305/bjbms.2006.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smits H, Kadri M. Brucellosis in India: a deceptive infectious disease. Indian J Med Res. 2005;122:375–384. [PubMed] [Google Scholar]
- 4.Cloeckaert A, Verger J.M, Grayon M, Grepinet O. Polymor-phism at the dnaK locus of Brucella species and identification of a Brucella melitensis species-specific marker. J. Med. Microbiol. 1996;45:200–205. doi: 10.1099/00222615-45-3-200. [DOI] [PubMed] [Google Scholar]
- 5.Cloeckaert A, Verger J.M, Grayon M, Grepinet O. Restriction site polymorphism of the genes encoding the major 25 kDa and 36 kDa outer membrane proteins of Brucella. Microbiology. 1995;141:2111–21. doi: 10.1099/13500872-141-9-2111. [DOI] [PubMed] [Google Scholar]
- 6.Araj G.F. Human brucellosis: a classical infectious disease with persistent diagnostic challenges. Clin. Lab. Sci. 1999;12:207–212. [PubMed] [Google Scholar]
- 7.Hukić M, Ljubović-Dedeić A, Šiširak M, Knežević Z. Brucellosis as laboratory infection. 20-ti simpozijum iz infektivnih bolesti s međunarodnim sudjelovanjem Sarajevo. Bosnia and Herzegovina. 2006:40. [Google Scholar]
- 8.Ruiz J, Lorente I, Perez J, et al. Diagnosis of brucellosis by using blood cultures. J Clin. Microbiol. 1997;35:2417–2418. doi: 10.1128/jcm.35.9.2417-2418.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esel D, Doganay M, Alp E, Sumerkan B. Prospective evaluation of blood cultures in a Turkish university hospital: epidemiology, microbiology and patient outcome. Clin. Microbiol. Infect. 2003;9:1038–1044. doi: 10.1046/j.1469-0691.2003.00714.x. [DOI] [PubMed] [Google Scholar]
- 10.Serra J, Vinas M. Laboratory diagnosis ofbrucellosis in a rural en-demic area in northeastern Spain. Int. Microbiol. 2004;7:53–58. [PubMed] [Google Scholar]
- 11.Ruiz-Mesa J.D, Sanches-Gonzalez J, Reguera J.M, Martin L, Lopez-Palmero S, Colmenero J.D. Rose Bengal test: diagnos-tic yield and use for the rapid diagnosis of human brucellosis in emergency departments in endemic areas. Clin. Microbiol. Infect. 2005;11:221–225. doi: 10.1111/j.1469-0691.2004.01063.x. [DOI] [PubMed] [Google Scholar]
- 12.Young E.J. Serologic diagnosis of human brucellosis: Analysis of 214 cases by agglutination tests and review of the liteature. Rev. Infect. Dis. 1991;13:359–372. doi: 10.1093/clinids/13.3.359. [DOI] [PubMed] [Google Scholar]
- 13.Araj G.F, Lulu A.R, Mustafa M.Y, Khateeb M.I. Evaluation of ELISA in the diagnosis of acute and chronic brucellosis in human beings. J. Hyg. Cambridge. 1986;97:457–469. doi: 10.1017/s0022172400063634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gad El-Rab M.O, Kambal A.M. Evaluation of Brucella enzyme immunoassay test (ELISA) in comparison with bacteriological culture and agglutination. J. Infect. Dis. 1998;36(2):197–201. doi: 10.1016/s0163-4453(98)80013-3. [DOI] [PubMed] [Google Scholar]
- 15.Gazapo E, Lahoz G.J, Subiza J, et al. Changes in IgM and IgG an-tobody concentrations in brucellosis over time: Importance for diagnosis and folow up. J Infect. Dis. 1989;159:219–225. doi: 10.1093/infdis/159.2.219. [DOI] [PubMed] [Google Scholar]
- 16.Ariza J, Pellicer T, Pallares R, Foz A, Gudiol F. Specific antibody profile in human brucellosis. Clin. Infect. Dis. 1992;14:131–140. doi: 10.1093/clinids/14.1.131. [DOI] [PubMed] [Google Scholar]
- 17.Kostoula A, Bobogianni H, Virioni G, et al. Detection of Brucella IgG, IgM and IgA antibodies with ELISA method in patients with Brucellosis. Clin Microbiol. Infect. 2001;7(suppl 1):108. [Google Scholar]


