Abstract
Alzheimer’s disease (AD) is a multifactorial disease but its aetiology and pathophisiology are still not fully understood. Epidemiologic studies examining the association between lipids and dementia have reported conflicting results. High total cholesterol has been associated with both an increased, and decreased, risk of AD and/or vascular dementia (VAD), whereas other studies found no association. The aim of this study was to investigate the serum lipids concentration in patients with probable AD, as well as possible correlation between serum lipids concentrations and cognitive impairment.
Our cross-sectional study included 30 patients with probable AD and 30 age and sex matched control subjects. The probable AD was clinically diagnosed by NINCDS-ADRDA criteria. Serum total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C) and triglyceride (TG) levels were determined at the initial assessment using standard enzymatic colorimetric techniques. Low-den- sity lipoprotein cholesterol (LDL-C) and very low density lipoprotein cholesterol (VLDL-C) levels were calculated. Subjects with probable AD had significantly lower serum TG (p<0,01), TC (p<0,05), LDL-C (p<0,05) and VLDL-C (p<0,01) compared to the control group. We did not observe signifi-cant difference in HDL-C level between patients with probable AD and control subjects. Negative, although not significant correlation between TG, TC and VLDL-C and MMSE in patients with AD was observed. In the control group of subjects there was a negative correlation between TC and MMSE but it was not statistically significant (r = -0,28). Further studies are required to explore the possibility for serum lipids to serve as diagnostic and therapeutic markers of AD.
Keywords: Alzheimer’s disease, lipids, cognitive impairment, Mini-Mental State Examination
INTRODUCTION
Dementia is a clinicopathological state whose name literally means “loss of the ability to think.” Alzheimer’s disease (AD) and vascular dementia (VAD) are the most common forms of dementias. Alzheimers disease is a multifactorial disease but its aetiology and pathophisi-ology are still not fully understood. Studies so far have shown that pathomechanisms responsible for AD are excessive production of nitric oxide (NO), hypoperfu-sion of brain vasculature and increased oxidative stress. Oxidative modification of lipids in serum and cerebro-spinal fluid have important role in AD pathogenesis (1). Efforts have been made to discover biological markers for different types of dementia during the last few decades. Such markers may play a key role in early diagnosis and management of these disorders. Serum lipids are measured routinely in every-day practice and, therefore may constitute tempting potential biomarkers for many diseases (2). Epidemiologic studies examining the association be-tween serum cholesterol and dementia have reported conflicting results. Among longitudinal studies, high total cholesterol (TC) level has been associated with both an increased and decreased risk of AD and/or VAD, whereas other studies found no association (3). In a Swedish study, low cholesterol level was associated with dementia even 9 or more years before the diagnosis (3). In another study, although high levels of cholesterol at midlife represented a risk factor for AD, there was no detectable difference in cholesterol levels in late life (4). At least two autopsy studies supported that midlife, but not late life lipids are somehow related to Al- zheimer’s disease-type pathology (5,6). In a previ-ous report from the Honolulu-Asia Aging Study, low midlife TC level was associated with a lower num-ber of neuritic, amyloid plaques and neurofibril-lary tangles (the characteristic pathologic changes of AD), while late-life TC and low density lipopro-tein cholesterol (LDL-C) levels were not associated with any of the AD neuropathologic markers (5). In cross-sectional studies, lower HDL-C levels have been associated with lower Mini-Mental State Examination (MMSE) scores (7) and with higher risks for dementia (8,7,9) and AD (10). However, most longitudinal studies have failed to detect similar associations: HDL-C levels have not been associated with AD (11-15, 16), VAD (11,13,15), or other forms of dementia (15).
The aim of the study
The aim of this study was to investigate serum lip-ids concentration in patients with probable AD, as well as possible correlation between serum lipids concentration and cognitive impairment tested by MMSE in patients with probable AD.
MATERIALS AND METHODS
Patients
The study was designed as a cross-sectional study which included patients with probable AD and control subjects. Group of patients with probable AD (AD group) enrolled in the present study comprised of 30 patients, 24 females and 6 males, with clinically diag-nosed probable AD. Control group (CG) included 30 community dwelling age-matched apparently healthy, asymptomatic persons without dementia (22 females and 8 males). All subjects were aged 65 and over. Patients in AD group were patients currently institution-alized at specialized unit for patients with dementia with-in Health-Care Hospice for person with disabilities and other persons in Sarajevo, Bosnia and Herzegovina. The probable AD was clinically diagnosed by standardized clinical examination conducted by a senior staff neurol-ogist and psychiatrist by NINCDS-ADRDA criteria (17). Subjects underwent history and clinical examination. Anamnesis was taken from patient’s caregivers by a one-to-one interview with special emphasis on previous symptomatic cerebrovascular diseases. Clinical examination included physical examination, risk factors assessment for probable Alzheimer disease and Cranial Computed tomography (CT). Physical examination included clinical neurological examination, blood pressure and Body Mass Index (BMI) mea- surement. BMI for each subject was calculated (weight in kilograms divided by height in meters squared). Height was measured with stadiometer and weight was measured with Toledo self-zeroing electronic digital scale (Mettler-Toledo, Inc., Worthington, OH.). Trained persons measured blood pressure using a mer-cury sphygmomanometer (MD10XX, MEDI, Shanghai, China) on the right arm after at least a 5-min rest. For both groups of subjects, exclusion criteria were positive history of cardiovascular or thyroid disease, chronic inflammatory disease (asthma and rheumatoid arthritis), hepatic or renal insufficiency, cancer and VAD. Exclusion criteria for AD patients was AD associated with cerebrovascular lesions or probable VAD (so called “mixed dementia”) as-sessed with Hachinski ischemic score (HIS). Approval for the study was obtained by the local ethics committee. All procedures on human subjects were performed in the accordance with Helsinki Declaration of 1975. Informed con-sent was obtained from subjects and caregivers upon careful explanation of the study procedure.
Clinical diagnosis of Alzheimer’s disease
The probable AD was clinically diagnosed by standardized clinical examination conducted by a senior staff neurologist and psychiatrist by NINCDS-ADRDA criteria and MMSE score. NINCDS-ADRDA criteria for clinical diagnosis of probable AD (i.e., insidious onset of cognitive decline with progressive deterioration and the exclusion of all other causes of dementia by history, physical examination, and laboratory tests) was based on National Institute of Neurological and Cognitive Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association criteria (17).
Global cognitive function was tested with the 30-point MMSE score (18). This screening test was originally created for a clinical setting (18) and is used exten-sively in epidemiologic studies (19). The test was ad-ministered by specially trained research assistants. The MMSE includes questions on orientation to time and place, registration, attention and calculation, recall, language and visual construction. If fewer than four individual items (out of 20) were not answered by the subject, these were rated as errors (20). If a sub-ject did not answer four or more individual items, the total MMSE score was considered missing. A score of less than 23 points on the MMSE indicated cognitive impairment (18). All patients had MMSE score < 12. All subjects in CG had a MMSE score of 26-30.
Hachinski ischemic score
Hachinski ischemic score is a test which helps dif-ferentiate patients with VAD from individuals with AD. The HIS assesses the presence of thirteen clinical features and attributes a total score of 18. The score above 7 suggests a diagnosis of multi-infarct dementia (MID) and if 4 or below, it suggests AD. This score has been validated in patients with pathologically confirmed MID and AD and allows for correct identification of MID and AD cases in 84% and 76% of cases, respectively (21). In our study all patients with probable AD had HIS 4 or bellow.
Cranial Computed tomography
Cranial CT was performed in 27 patients with probable AD, while remaining 3 were uncooperative and refused a CT scan. All CT scans were performed without con-trast enhancement and with 8-mm continuous slices on spiral CT (SOMATOM Emotion Duo, Siemens, Erlan- gen, Germany) on Institute of Radiology of University of Sarajevo Clinics Centre. The scans were examined by a neuroradiology specialist. Analysis of CT scans showed that all patients with probable AD had cerebral atrophy and that 4 of these patients had also cerebellum atrophy.
Blood analysis
Blood samples for analysis were obtained from patients and subjects in fasting conditions from antecubital vein into siliconized tubes (BD Vacutainer Systems, PL6 7BP, Plymouth, UK.). Plasma TC, HDL-C and triglyceride (TG) levels were determined at the initial assess-ment using standard enzymatic colorimetric techniques, on automated apparatus (Dimension RxL Max, Dade Behring, Germany) at the Institute for Chemistry and Biochemistry University of Sarajevo Clinics Center. Low-density lipoprotein cholesterol (LDL-C) levels were calculated using the Friedewald et al. formula (22). Very low density lipoprotein cholesterol (VLDL-C) lev-els were calculated by the formula: VLDL-C = TG/2,2.
Referral value using this method for serum total cholesterol level is < 5.2 mmol/L, triglycerides <1,7 mmol/L, HDL-C 1.04 -1,55 mmol/L, LDL-C 1,4 - 4,5 mmol/L and VLDL-C 0,13 - 0,90 mmol/L. Hyperlipidaemia was defined as fasting serum trig-lycerides level >1.7 mmol/L and cholesterol level >6.5 mmol/L representing threshold for people older than 60 years without additional risk factors, or cur-rent treatment with lipid lowering therapy (23).
Statistical analysis
Statistical analyses were performed with Microsoft Office Excel 2003 and SPSS, version 12.0. Data are presented as mean ± SEM. Data distribution was determined using the Kolmogorov-Smirnov test. Since data were normally distributed, statistical difference was tested with Student t-tests. Additionally, Pearson correlations were used as measures of association for the continu-ous variables. Statistical significance was set at p<0,05.
RESULTS
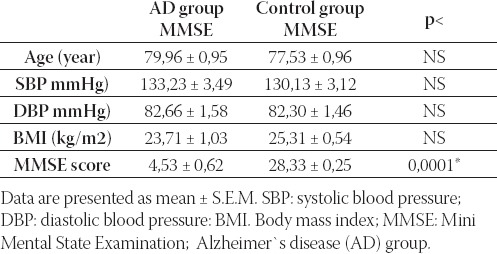
The baseline characteristics of the two groups en-rolled in the study are reported in Table 1. No dif-ference emerged in age, systolic, diastolic blood pressure and BMI between AD and control group. Subjects in AD group had significantly lower MMSE score compared with the control group (p<0,0001).
TABLE 1.
Baseline characteristics of patients with probable Alzheimer’s disease and control subjects
As presented in Table 2., negative, although not significant correlation between TG, TC and VLDL-C and MMSE in patients with AD was observed. In the control group of subjects there was a negative correlation between TC and MMSE but it was not statistically significant (r = 0,28). Subjects with probable AD had statistically signifi-cantly lower serum TG (p<0,01), TC (p<0,05), LDL-C (p<0,05) and VLDL-C (p<0,01) compared to control group (p<0,05). We did not observe statistically sig-nificant difference in HDL-C level between patients with probable AD and control subjects (Figure 1).
TABLE 2.
Pearson correlation analysis between serum lipids and MMSE score in patients with probable AD and in control subjects.
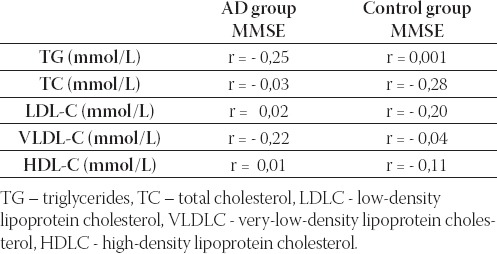
FIGURE 1.
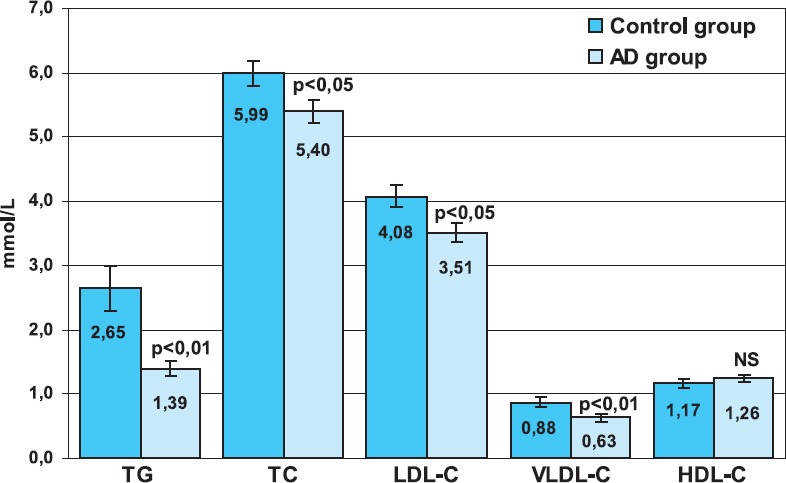
Mean serum lipids concentration in the control (C) and Alzheimer’s disease (AD) group. Bars show means and error bars show S.E.M.; TG - triglycerides, TC - total cholesterol, LDL-C - low-density lipoprotein cholesterol, VLDL-C - very-low-density lipoprotein cholesterol, HDL-C - high-density lipoprotein cholesterol, NS - not significant.
DISCUSSION
In our cross-sectional study we found significantly lower serum lipids except of HDL-C in patients with probable AD compared to control subjects. However, measure of adiposity tested by BMI was not different in AD group compared to controls which suggests that decrease in serum lipids in patients might not be due to poor nutritional status. Several studies assessed the serum lipids in patients with AD, VAD and control subjects but the re-sults are conflicting. A recent study, with approximately 5 years of follow-up, suggested that high cholesterol in late life was associated with a lower risk of AD (13). By contrast, two longitudinal studies (24,25) reported that high TC measured in mid-life was associated with an increased risk of dementia as long as 30 years later. Notkola et al. (24) found that high cholesterol was associated with a higher risk of dementia 30 years later, but before the onset of dementia cholesterol levels be-gan to decline and as a result, low cholesterol was associated with dementia cross-sectionally. The authors noted that a decrease in cholesterol immediately before the dementia diagnosis was also predictive of demen- tia. Romas et al. (12) found that decreased TC level had significant inverse association with incident AD compared with nondemented elderly, independent of APOE genotype. In their study, no other lipoprotein fragment was associated with either prevalent or incident AD. Experimental studies suggest that high cholesterol accelerates the production of β-amyloid, the putative patho-logic species in AD, by shifting amyloid precursor protein metabolism from alpha to beta cleavage products (26-28). However, cholesterol is an essential molecule for many physiologic processes and may have several bene-ficial effects as well. Cholesterol is a precursor of steroid hormones (estrogens, androgens, vitamin D), provides structural integrity and modulates fluidity of cell mem- branes, and is essential for basic synaptic integrity and neurotransmission. All these processes are compromised with aging and have been shown to be dysfunctional in patients with AD. In addition, in vitro studies have suggested that cholesterol acts as an antioxidant and therefore has a protective role in dementia pathogen- esis, possibly through intercepting pro-oxidants to create oxysterols, which are less toxic than free radicals (3). However, it has been noted by Jacobs et al. (29) else-where that low (not high) cholesterol is associated with mortality among the elderly, and the authors suggested it is possible that cholesterol might play an important role in protecting against dementia. Therefore, we believe that an alternative explanations focusing on potentially neuroprotective properties of cholesterol should be considered. It was speculated that high cholesterol may be protective through increasing gamma-glutamyltransferase. This enzyme plays a role in amino acid uptake and transport and could reduce the neurotoxic effects of amino acids (30). Hypertriglyceridemia has been confirmed as a cardiovascular risk factor. On the contrary, Dziedzic et al. (30) found that lower TG level is associated with more severe stroke but were unable to offer an explanation and potential biological mechanism responsible for inverse association between TG level and stroke severity. Dimopoulos et al. (2) found lower TG plasma levels in patients with dementia compared to the control group, but the difference was not significant. Several studies confirmed that the level of TG in the blood is not associated with memory and cognitive abilities (7,31). We did not find the data on the association between the lower triglycerides levels and risk for AD devel-opment in current literature. Unfortunately, we can not give an explanation for significantly decreased triglycerides levels in patients with possible AD.
CONCLUSION
Given that the determination of lipids in the blood is a routine, non invasive and cheap method we believe that monitoring of lipids in older individuals with dementia may be an additional tool in evaluation of patients with probable AD. Although further studies are needed serum lipids levels might serve as a potential biomarkers of AD severity.
List of Abbreviations
AD - Alzheimer s disease
VAD - Vascular dementia
MID - Multi-infarct dementia
TC - Total cholesterol
TG - Triglyceride
LDL-C - Low-density lipoprotein cholesterol
HDL-C - High-density lipoprotein cholesterol
VLDL-C - Very low density lipoprotein cholesterol
NINCDS-ADRDA criteria - National Institute of Neurological and Cognitive Disorders and Stroke/Alzheimer’s Disease and Related Disorders Association criteria
MMSE - Mini Mental State Examination
NO - Nitric oxide
CT - Computed tomography
REFERENCES
- 1.Zaciragic A, Lepara O, Valjevac A, et al. Elevated serum C-re-active protein concentration in Bosnian patients with probable Alzheimer’s disease. J. Alzheimers. Dis. 2007;12:151–156. doi: 10.3233/jad-2007-12204. [DOI] [PubMed] [Google Scholar]
- 2.Dimopoulos N, Piperi C, Salonicioti A, et al. Characterization of the lipid profile in dementia and depression in the elderly. J. Geriatr. Psychiatry Neurol. 2007;20:138–144. doi: 10.1177/0891988707301867. [DOI] [PubMed] [Google Scholar]
- 3.Mielke M.M, Zandi P.P, Sjögren M, et al. High total cholesterol levels in late life associated with a reduced risk of dementia. Neu-rology. 2005;64:1689–1695. doi: 10.1212/01.WNL.0000161870.78572.A5. [DOI] [PubMed] [Google Scholar]
- 4.Kivipelto M, Helkala E.L, Laakso M.P, et al. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann. Intern. Med. 2002;137:149–155. doi: 10.7326/0003-4819-137-3-200208060-00006. [DOI] [PubMed] [Google Scholar]
- 5.Launer L.J, White L.R, Petrovitch H, Ross G.W, Curb J.D. Cholesterol and neuropathologic markers of AD: a population-based autopsy study. Neurology. 2001;57:1447–1152. doi: 10.1212/wnl.57.8.1447. [DOI] [PubMed] [Google Scholar]
- 6.Pappolla M.A, Bryant-Thomas T.K, Herbert D, Pacheco J, Fabra Garcia M, et al. Mild hypercholesterolemia is an early risk factor for the development of Alzheimer amyloid pathology. Neurology. 2003;61:199–205. doi: 10.1212/01.wnl.0000070182.02537.84. [DOI] [PubMed] [Google Scholar]
- 7.van Exel E, de Craen A.J, Gussekloo J, et al. Association between high-density lipoprotein and cognitive impairment in the oldest old. Ann. Neurol. 2002;51:716–721. doi: 10.1002/ana.10220. [DOI] [PubMed] [Google Scholar]
- 8.Wolf H, Hensel A, Arendt T, Kivipelto M, Winblad B, Gertz H.J. Serum lipids and hippocampal volume: the link to Alzheim-er’s disease? Ann Neurol. 2004;56(5):745–748. doi: 10.1002/ana.20289. [DOI] [PubMed] [Google Scholar]
- 9.Bonarek M, Barberger-Gateau P, Letenneur L, Deschamps V, Iron A, Dubroca B, Dartigues J.F. Relationships between cholesterol, apolipoprotein E polymorphism and dementia: a cross-sectional analysis from the PAQUID study. Neuroepidemiology. 2000;19:141–148. doi: 10.1159/000026249. [DOI] [PubMed] [Google Scholar]
- 10.Merched A, Xia Y, Visvikis S, Serot J.M, Siest G. Decreased high-density lipoprotein cholesterol and serum apolipoprotein AI concentrations are highly correlated with the severity of Al-zheimer’s disease. Neurobiol. Aging. 2000;21:27–30. doi: 10.1016/s0197-4580(99)00103-7. [DOI] [PubMed] [Google Scholar]
- 11.Moroney J.T, Tang M.X, Berglund L, Small S, Merchant C, et al. Low-density lipoprotein cholesterol and the risk of dementia with stroke. JAMA. 1999;282(3):254–260. doi: 10.1001/jama.282.3.254. [DOI] [PubMed] [Google Scholar]
- 12.Romas S.N, Tang M.X, Berglund L, Mayeux R. APOE genotype, plasma lipids, lipoproteins, and AD in community elderly. Neu-rology. 1999;53:517–521. doi: 10.1212/wnl.53.3.517. [DOI] [PubMed] [Google Scholar]
- 13.Reitz C, Tang MX, Luchsinger J, Mayeux R. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch. Neurol. 2004;61:705–714. doi: 10.1001/archneur.61.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuusisto J, Koivisto K, Mykkänen L, Helkala E.L, Vanhanen M, et al. Association between features of the insulin resistance syn-drome and Alzheimer’s disease independently of apolipoprotein E4 phenotype: cross sectional population based study. BMJ. 1997;315:1045–1049. doi: 10.1136/bmj.315.7115.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li G, Shofer J.B, Kukull W.A, Peskind E.R, Tsuang D.W, et al. Serum cholesterol and risk of Alzheimer disease: a community-based cohort study. Neurology. 2005;65:1045–1050. doi: 10.1212/01.wnl.0000178989.87072.11. [DOI] [PubMed] [Google Scholar]
- 16.Tan Z.S, Seshadri S, Beiser A, Wilson P.W, Kiel D.P, et al. Plasma total cholesterol level as a risk factor for Alzheimer disease: the Framingham Study. Arch. Intern. Med. 2003;163:1053–1057. doi: 10.1001/archinte.163.9.1053. [DOI] [PubMed] [Google Scholar]
- 17.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan E.M. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheim-er’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 18.Folstein M.F, Folstein S.E, McHugh P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 19.Launer L.J. Overview of incidence studies of dementia conducted in Europe. Neuroepidemiology. 1992;11(Suppl 1):2–13. doi: 10.1159/000110954. [DOI] [PubMed] [Google Scholar]
- 20.Fillenbaum G.G, George L.K, Blazer D.G. Scoring nonre-sponse on the Mini-Mental State Examination. Psychol Med. 1988;18(4):1021–1025. doi: 10.1017/s0033291700009946. [DOI] [PubMed] [Google Scholar]
- 21.Moroney J.T, Bagiella E, Desmond D.W, Hachinski V.C, Mölsä P.K, et al. Meta-analysis of the Hachinski Ischemic Score in pathologically verified dementias. Neurology. 1997;49:10961105. doi: 10.1212/wnl.49.4.1096. [DOI] [PubMed] [Google Scholar]
- 22.Friedewald W.T, Levy R.I, Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 23.Masse I, Bordet R, Deplanque D, Al Khedr A, Richard F, et al. Lipid lowering agents are associated with a slower cognitive decline in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2005;76:1624–1629. doi: 10.1136/jnnp.2005.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Notkola I.L, Sulkava R, Pekkanen J, Erkinjuntti T, Ehnholm C, et al. Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer’s disease. Neuroepidemiology. 1998;17:14–20. doi: 10.1159/000026149. [DOI] [PubMed] [Google Scholar]
- 25.Kivipelto M, Helkala E.L, Laakso M.P, Hänninen T, Hallikainen M, et al. Midlife vascular risk factors and Alzheimer’s disease in later life: longitudinal, population based study. BMJ. 2001;322:1447–1451. doi: 10.1136/bmj.322.7300.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sparks D.L, Scheff S.W, Hunsaker J.C, 3rd, Liu H, Landers T, Gross D.R. Induction of Alzheimer-like beta-amyloid immuno-reactivity in the brains of rabbits with dietary cholesterol. Exp. Neurol. 1994;126:88–94. doi: 10.1006/exnr.1994.1044. [DOI] [PubMed] [Google Scholar]
- 27.Simons M, Keller P, De Strooper B, Beyreuther K, Dotti C.G, Simons K. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc. Natl. Acad. Sci. U S A. 1998;95(26):6460–6464. doi: 10.1073/pnas.95.11.6460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fassbender K, Simons M, Bergmann C, Stroick M, Lutjohann D, et al. Simvastatin strongly reduces levels of Alzheimer’s dis-ease beta-amyloid peptides Abeta 42 and Abeta 40 in vitro and in vivo. Proc Natl Acad Sci U S A. 2001;98:5856–5861. doi: 10.1073/pnas.081620098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacobs D, Blackburn H, Higgins M, Reed D, Iso H, et al. Report of the Conference on Low Blood Cholesterol: Mortality Associations. Circulation. 1992;86:1046–1060. doi: 10.1161/01.cir.86.3.1046. [DOI] [PubMed] [Google Scholar]
- 30.Dziedzic T, Slowik A, Gryz E.A, Szczudlik A. Lower serum trig-lyceride level is associated with increased stroke severity. Stroke. 2004;35:e151–152. doi: 10.1161/01.STR.0000128705.63891.67. [DOI] [PubMed] [Google Scholar]
- 31.Henderson V.W, Guthrie J.R, Dennerstein L. Serum lipids and memory in a population based cohort of middle age women. J. Neurol. Neurosurg. Psychiatry. 2003;74:1530–1535. doi: 10.1136/jnnp.74.11.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]


