Abstract
Gentamicin is commonly used for the treatment of severe gram negative bacterial infections but inevi-tably cause renal failure during prolonged use. The aim of our study was to emphasize protective effects of pentoxifylline on glomerular basement membrane (GBM) alterations induced by gentamicin in rats. Experiments were done on 40 male Wistar rats divided in three experimental groups. GM-group was treated daily with gentamicin in dose of 100 mg/kg during 8 days. PTX-group was treated daily with pentoxifylline in dose of 45 mg/kg and the same dose of gentamicin as in GM-group during 8 days. The control group received 1 ml/day saline intraperitoneally. Morphometric parameter measured during the analysis was glomerular basement membrane thickness. In GM-group of animals glomeruli were en-larged and GMB was diffusely and unequally thickened with neutrophil cells infiltration. In proximal tu-bules epithelial cells, vacuolization of cytoplasm with coagulation-type necrosis were observed. In PTX-group of animals glomeruli were somewhat enlarged and GBM was thickened only in some segments. Coagulation-type necrosis was not found. Blood urea and serum creatinine concentration in GM-group were significantly elevated in comparison with PTX-group while potassium level was decreased. Our results suggest that PTX has protective effects on GBM and proximal tubules in GM-treated rats.
Keywords: gentamicin, pentoxifylline, glomerular basement membrane, morphometry, rats
INTRODUCTION
Gentamicin (GM) is an aminoglycoside antibiotic commonly used as an agent for the treatment of severe Gram-negative infections (1). Although nephrotoxicity as a major side effect of aminoglycosides accounting for 10-15% of all acute renal failure cases (2), the exact mechanisms of this adverse action still remain unclear. GM has been shown to enhance the generation of reactive oxygen species (ROS) (3,4) which are putative causative agents of cell death in different pathological conditions including various models of renal diseases (5, 6, 7, 8). Accordingly, the administration of several compounds with antioxidant activity has been suc-cessfully used to prevent or ameliorate GM-induced nephrotoxicity (9, 10, 11, 12). The usage of gentamicin in clinical practice would be undoubtedly increased if potent protective agent for its undesirable kidney side effects could be found. Thus, a potential therapeutic ap-proach to protect or reverse renal gentamicin damage would have very important clinical consequences (13). Pentoxifylline (PTX) is a hemorheological agent used in the treatment of peripheral vascular diseases. Recently, PTX has gained considerable interest as a ROS scaven- ger. Several in vitro studies have confirmed the potential antioxidant effects of this drug (14,15,16). Although its mechanism of action is not well understood, pentoxifyl-line decreases blood viscosity by altering erythrocyte deformability (17), reducing platelet aggregability and fi-brinogen levels (18), and increasing neutrophil mobility (19).Also, pentoxifylline is a potent stimulator of pros-tacyclin in vascular beds (18) and like other xanthines such as theophylline, is an adenosine receptor antagonist (20).Therefore, PTX is a potent anti-inflammatory agent capable of ameliorating kidney inflammation by acting on various targets including the synthesis of pro-inflammatory cytokines and chemokines, as well as the growth and activation of inflammatory mononuclear cells. The aim of our experimental investigation was to determine the potential protective effect of pen-toxifylline on glomerular basement membrane ul-trastructural changes caused by gentamicin in rats.
MATERIAL AND METHODS
All studies were performed on adult male Wistar rats, weighing 250 - 300 g. Animals were housed in a central facility under controlled conditions (12 h light/ dark cycle and room temperature of 20°C ± 2°C) and with free access to food and water. All experimental procedures were conducted in accordance with the principles for the care and use of laboratory animals in research. The investigation conforms to the regulations of the European Union and USA Guide for the Care and Use of Laboratory Animals pub-lished by the National Institute of Health (National Academy of Science Press, Washington, DC, 1996).
Experimental protocol
The total number of 40 animals was divided in 3 groups, one of which was used as a sham control. The experimental group of animals or GM-group (15 rats) received gentamicin (Galenika AD, Belgrade, Serbia) intraperitoneally in a daily dose of 100 mg/kg.
PTX-group animals (15 rats) were treated daily with pentoxifylline (Jugoremedija A.D. Niš, Serbia) intraperi-toneally in dose of 45 mg/kg and the same dose of gen-tamicin as in GM-group. The control group of animals or C-group (10 rats) received 1 ml/day saline intraperitone- ally. Both experimental and control group were treated over the period of 8 consecutive days. Following the last application, nine days after the beginning of the experiment, all animals were anaesthetized using 80mg/kg ket-amine (Ketamidor 10%, Richter Pharma AG, Wels, Austria) and then sacrificed. Immediately after vivisection 2ml blood from aorta was taken for biochemical analysis.
Histological analysis
The kidneys were sectioned and fixed in 10% paraformaldehyde (in 0,1 mol/dm3 phosphate buffer saline), dehydrated in graded alcohols and processed for paraffin wax embedding. Then, kidney slices were cut on 5 μm thick sections using HistoRange microtome (model: LKB 2218, LKB-Produkter AB, Bromma, Sweden) and stained with hematoxylin-eosin (HE), PAS (Periodic Acid Schifi) and Jones meth-enamine silver according to conventional staining protocols as described by Bancroft and Stevens (21).
Morphometric analysis
Histological slides were analyzed using light microscope (Olympus BX50, Tokyo, Japan) and Micro Image 3.0 (Olympus, Tokyo, Japan) image analysis and processing software were used for the morphometric analysis. Spatial calibration, by object micrometer (1:100), as well as optical density calibration was performed before each analysis. Morphometric parameter mea-sured during the analysis was glomerular basement membrane thickness ^m). The glomerular basement membrane thickness was estimated as a mean distance after manual tracing of two lines along both sides of the basement membrane. In each animal at least 20 glomeruli were measured, excluding columns of Bertin.
Biochemical analysis
As previously mentioned, after finishing the experiment, blood samples were taken from aorta and analyzed for markers of renal impairment. Plasma creatinine, blood urea, sodium and potassium con-centrations were measured using an automatic bio-chemical analyzer (A25 Biosystems, Barcelona, Spain).
Statistical analysis
Statistical analysis included estimation of mean values and standard deviation (SD) for parameters obtained during the morphometric and laboratory analysis. The statistical significance for the differences between the experimental and control group of animals’ parameters was tested by Student’s t - test using NCSS statistical software (NCSS Kaysville, Utah). In all cases, statistical significance was inferred for p<0,05.
RESULTS
1. Histological analysis
The kidney sections taken from the experimental GM-group of animals showed areas of proximal tubule epithelial cells necrosis and apoptosis, vacuolization of cytoplasm and epithelial desquamation (Figure 1). Glomeruli were enlarged, glomerular basement membrane was irregularly thickened, with neu-trophil cell infiltration. In the cytoplasm of viable proximal tubule epithelial cells numerous black dots (“myeloid bodies”) were found in the sections stained with silver methenamine (Figure 2). In PTX-group of animals glomeruli were somewhat enlarged, and the glomerular basement membrane was thickened only in some segments of the glomer- uli. Coagulation-type necrosis was not found. In some epithelial cells of proximal tubules dark inclusions and cytoplasm vacuolization were observed (Figure 3).
FIGURE 1.
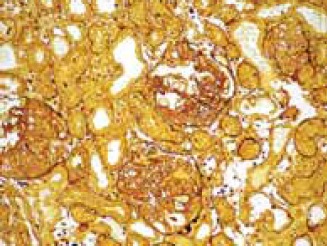
Necrosis and apoptosis, vacuolization of cytoplasm and epithelial desquamation of proximal tubule epithelial cells in GM-group of animals. Silver-methenamine staining method (magnification × 40).
FIGURE 2.
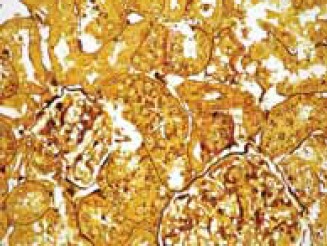
Enlarged glomeruli, glomerular basement membrane irregularly thickened and proximal tubules epithelial cells with numerous black dots (“myeloid bodies”) in GM-group of animals. Silver-methenamine staining method (magnification × 40).
FIGURE 3.
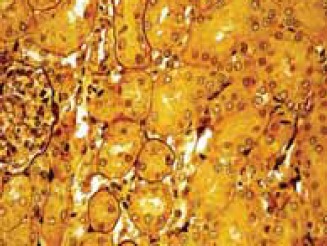
Somewhat enlarged glomeruli, glomerular basement membrane thickened only in some segments and dark inclusions in certain epithelial ceHs of proximal tubules in PTX-group of animals. Silver-methenamine staining method (magnification × 40).
2. Morphometric analysis
The morphometric analysis of glomerular basement membrane thickness between animals from the experimental GM, PTX and control groups are presented in the (Table 1).
TABLE 1.
Glomerular membrane thickness (μπι) in experimental and control group of rats.
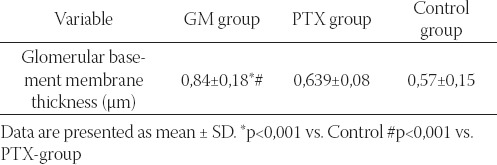
Morphometric analysis of glomerular basement membrane thickness showed statistically sig-nificant differences between the control and the experimental GM and PTX groups (Table 1). The mean glomerular membrane thickness was significantly greater in gentamicin treat-ed group of animals compared to PTX and control group of rats (p< 0,001) (Table 1). Glomerular basement membrane thickness of PTX-group of animals was greater than in control group of rats, but without statistical significance (Table 1).
3. Biochemical analysis
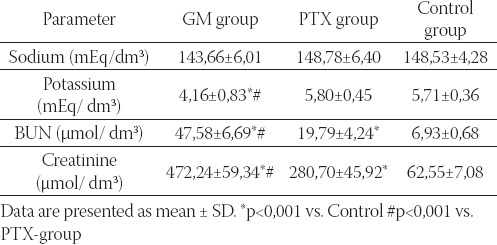
Using multivariate t-test, statistically significant differenc-es were found for values of BUN and creatinine (p<0,001). The mean values of BUN and creatinine serum concentrations found in GM and PTX groups of rats were statistically significantly elevated in com-parison to the control group of rats (p<0,001). The mean values of serum potassium concentrations in GM-group were statistically significantly reduced in comparison with PTX and control groups (p<0,001). The values of BUN and serum creatinine in GM-group were significantly elevated in comparison with PTX-group (p<0,001).
TABLE 2.
Serum concentration of sodium, potassium, BUN, and crea-tinine in experimental and control groups of rats.
DISCUSSION
Because of its strong bactericidal effect, gentamicin is widely used antibiotic in the treatment of infections caused by gram-negative microorganisms. However, the ample data also speak of its nephrotoxic effect demonstrated in a numerous experimental studies in which gentamicin acute renal insufficiency was induced (22,23). Oxidative stress is probably the most common pathogenic factor of cell damage in the com-plex pathogenesis of gentamicin nephrotoxicity. The data obtained from experiments suggest that both vascular (glomerular) and tubular targets are involved in drug-induced nephrotoxicity (24). In our study, gentamicin given in a supratherapeutic dose (100mg/ kg) induced acute renal insufficiency (ARI) in rats. Pentoxifylline penetrates the kidney tissue, where it reduces the production of free oxygen radicals due to its anti-oxidant effect. When it is applied alone or to-gether with the vitamin E, pentoxifylline shows satis-factory effects on renal damage (25,26). The increased production of TNF-alpha which induces apoptosis (27) and other cytotoxins which are important factors of cellular proliferation and ECM synthesis of mesangial cells of glomeruli and interstitial fibroblasts can be pre-vented with pentoxifylline (28). These data suggest that pentoxifylline has an ameliorative effect on the course of experimental ARI, which is in accordance with the results of other authors (29,30). The mechanism of amelioration probably involves the stimulation of renal vasodilator prostaglandins as well as the prevention of vascular congestion (31). On the other hand, it has been shown that PTX affects calcium homeostasis and inhibits calcium entry in the human erythrocyte (32). It has been reported that calcium channel blockers are ef-fective in the prevention of GM-induced nephrotoxicity (by inhibiting calcium accumulation in the kidney) (33). The most important effect of PTX in gentamicin nephrotoxicity is the increased blood flow through blood vessels which leads to significant improvement of the renal microcirculation. It also reduces the tubular dysfunction and glomerular filtration, which leads to the reduction of BUN and creatinine in plasma. The histopathological changes observed in GM-group of animals consisted of enlargement of glomeruli and glomerular basement membrane alterations with un-equal thickness in some of its segments (34). The neu-trophilic leucocytes were present in some glomeruli capillaries. The changes in the proximal tubules were dominant and manifested in the form of segmented necrosis of the coagulation type, cytoplasm vacuolization of tubular epithelial cells with preserved nuclei and multitude of dark inclusions (“myeloid body”). The structural changes in the distal tubules were not found. These changes mostly coincide with the changes already described by other authors (35,36). The presence of neutrophils in the glomerular capillaries confirms that the renal microcirculation and glomerular hemodynam-ics were impaired by the administration of gentamicin. If the changes in the kidneys and glomeruli are primarily due to changed microcirculation and hemodynamics in the capillaries, the removal of those would abolish gen-tamicin effects in renal nephrotoxicity. The PTX-group of rats showed lesser morphological and functional kidney damage in comparison to the rats treated with gentamicin only. Glomeruli were insignificantly en-larged and the basement membrane of glomerular cap-illaries was thickened only in some segments of glomeruli. There were no signs of necrosis in the proximal tubules, but only vacuolization and the presence of dark inclusions were noted in the cytoplasm of some cells, which points out the protective effect of pentoxifylline. The glomerular basement membrane (GBM) is progres-sively formed by two membranes: the lamina rara interna (LRI; formed from the endothelial cells) and the lamina rara externa (LRE; formed from the pedicels of the epithelial cells), which fuse and generate a common lamina densa (LD) in the central part. In the rat neonate, all these stages can be observed, the first differentiated (older) nephrons being in the juxta - medullary area. The urine is formed in mature glomeruli by filtration through the GBM, which stops the large molecules, such as proteins. This permselectivity of the GBM is considered to be mainly due to an electrostatic shield made of highly negatively charged heparan sulfate proteoglycans con-stituting the anionic sites situated in the LRI and LRE. Quantifying the GBM layers and anionic sites and dosing components of the extracellular matrix, Smaoui et al. (37) found that the developing GBM was abnormal in the treated neonates, and that morphological and functional alterations of the GBM were still present in the juxtamedullary glomeruli of the adult animals, i.e., 12month-old rats, prenatally exposed to gentamicin. In the early stages of GBM maturation, the fibrillar material ap-peared more important in the LRE and LRI before they joined to form the LD; in later stages, the LD itself looked larger and denser. Morphometric analysis showed that the GBM layers were different from those of controls in treated neonates: the LRI and LRE were thinner while the LD was enlarged. The counting of anionic sites showed an increase of their number per square microm-eter of the LRE or LRI and thus an increased density; glycosaminoglycans were more sulfated and quantita-tively more abundant than in controls (37). Our results revealed that glomerular basement membrane thick-ness was significantly larger in gentamicin treated rats than in the control group, as previously described (34). Morphometric analysis revealed that GMB was highly significantly thickened in GM-group of rats, compared to control and PTX groups. The thickness of GBM was higher in PTX-group compared to control group of rats, but this difference was not statistically significant. In the GM-group of animals, the biochemical analysis showed the most significant increase in serum urea and creatinine as a sign of the functional alterations of kidney. This is rather customary having in mind that the above laboratory parameters and electrolytes are secreted predominantly by glomerular filtration. The values of serum creatinine and BUN in the PTX-group of rats were enhanced in relation to the control group but reduced in relation to the GM-group.
CONCLUSION
Our results suggest that PTX has ameliorative effects on GBM and proximal tubules in GM-treated rats.
List of Abbreviations
GM - Gentamicin
PTX - Pentoxifylline
BUN - Blood urea nitrogen
ARI - Acute renal insufficiency
GBM - Glomerular basement membrane
LRI - Lamina rara interna
LRE - Lamina rara externa
LD - Lamina densa
Acknowledgment
This work was supported by the Ministry of Science Republic of Serbia grant 145004.
REFERENCES
- 1.Ali B.H. Gentamicin Nephrotoxicity in humans and animals: some recent research. Gen. Pharmac. 1995;26(7):1477–1487. doi: 10.1016/0306-3623(95)00049-6. [DOI] [PubMed] [Google Scholar]
- 2.Shifow A.A, Kumar K.V, Naidu M.U.R, Tarnakar ICS. Melatonin, a pineal hormone with antioxidant properties, protects against gentamicin-induced nephrotoxicity in rats. Nephron. 2000;85:167–174. doi: 10.1159/000045650. [DOI] [PubMed] [Google Scholar]
- 3.Cuzzocrea S, Mazzon E, Dugo L, Serraino I, Di Paola R, Britti D, De Sarro A, Pierpaoli S, Caputi A, Masini E, Salvemini D. A role for superoxide in gentamicin-mediated nephropathy in rats. Eur. J. Pharmacol. 2002;450:67–76. doi: 10.1016/s0014-2999(02)01749-1. [DOI] [PubMed] [Google Scholar]
- 4.Yanagida C, Ito K, Komiya I, Horie T. Protective effect of fosfo-mycin on gentamicin-induced lipid peroxidation of rat renal tis-sue. Chem. Biol. Interact. 2004;148:139–147. doi: 10.1016/j.cbi.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Shah S.V. Effect of enzymatically generated reactive oxygen me-tabolites on the cyclic nucleotide content in isolated rat glom-eruli. J. Clin. Invest. 1984;74:393–401. doi: 10.1172/JCI111434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCord J.M, Roy R.S, Schaffer S.W. Free radicals and myo-cardial ischemia. The role of xanthine oxidase. Adv. Myocardiol. 1985;5:183–189. [PubMed] [Google Scholar]
- 7.Grune T, Sommerburg O, Petras T, Siems W.G. Postanoxic formation of aldehydic lipid peroxidation products in human renal tubular cells. Free. Radic. Biol. Med. 1995;18:21–27. doi: 10.1016/0891-5849(94)e0093-x. [DOI] [PubMed] [Google Scholar]
- 8.Baliga R, Ueda N, Walker P.D, Shah S.V. Oxidant mechanisms in toxic acute renal failure. Drug. Metab. Rev. 1999;31:971–997. doi: 10.1081/dmr-100101947. [DOI] [PubMed] [Google Scholar]
- 9.Kopple J.D, Ding H, Letoha A, Ivanyi B, Qing D.P.-Y, Dux L, Wang H.-Y, Sonkodi S. L-carnitine ameliorates gentamicin-in-duced renal injury in rats. Nephrol. Dial. Transplant. 2002;17(12):2122–2131. doi: 10.1093/ndt/17.12.2122. [DOI] [PubMed] [Google Scholar]
- 10.Pedraza-Chaverri J, Gonzalez-Orozcoa A.E, Maldonado P.D, Barrera D, Medina-Campos ON, Hernandez-Pando R. Diallyl disulfide ameliorates gentamicin induced oxidative stress and nephropaty in rats. Eur. J. Pharmacol. 2003;473:71–78. doi: 10.1016/s0014-2999(03)01948-4. [DOI] [PubMed] [Google Scholar]
- 11.Parlakpinar H, Tasdemir S, Polat A, Bay-Karbulut A, Vardi N, Ucar M, Yanilmaz M, Kavakli A, Acet A. Protective effect of chelerythrine on gentamicin-induced nephrotoxicity. Cell. Biochem. Funct. 2006;24(1):41–48. doi: 10.1002/cbf.1182. [DOI] [PubMed] [Google Scholar]
- 12.Mazzon E, Britti D, De Sarro A, Caputi A.P, Cuzzocrea S. Effect of N-acetylcysteine on gentamicin-mediated nephropathy in rats. Eur. J. Pharmacol. 2001;424:75–83. doi: 10.1016/s0014-2999(01)01130-x. [DOI] [PubMed] [Google Scholar]
- 13.Pedraza-Chaverri J, Maldonado P.D, Medina-Campos O, Oliveares-Corichi I.M, Grandos-Silvestre M.R, Hernadez-Pando R, Ibarra-Rubio M.E. Garlic ameliorates gentamicin nephrotox-icity: relation to antioxidant enzymes. J. Free Radic. Biol. Med. 2000;29:602–611. doi: 10.1016/s0891-5849(00)00354-3. [DOI] [PubMed] [Google Scholar]
- 14.Horvath B, Marton Z, Halmosi R, Alexy T, Szapary L, Vekasi J, Biro Z, Habon T, Kesmarky G, Toth K. In vitro antioxidant properties of pentoxifylline, piracetam, and vinpocetine. Clin. Neuropharmacol. 2002;25(1):37–42. doi: 10.1097/00002826-200201000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Bhat V.B, Madyastha K.M. Antioxidant and radicals scavenging properties of 8-oxo derivatives of xanthine drugs pentoxifylline and lisofylline. Biochem. Biophys. Res. Commun. 2001;288(5):1212–1217. doi: 10.1006/bbrc.2001.5922. [DOI] [PubMed] [Google Scholar]
- 16.Freitas J.P, Filipe P, Guerra-Rodrigo F. Potential antioxidative effects of pentoxifylline. CRSeances Soc. Biol. Fil. 1995;189(3):401–405. [PubMed] [Google Scholar]
- 17.Bilto Y.Y, Player M, Stuart J. Rheological action of oxpentifylline and structurally-related xanthine derivatives on human erythro-cytes. Clin. Hemorheol. 1988;8:213–22l. [Google Scholar]
- 18.Weithmann K. U: Reduced platelet aggregation by pentoxifylline stimulated prostacyclin release. VASA. 1981;10:249–252. [PubMed] [Google Scholar]
- 19.Crocket K.Y, Lackie I.M, Rogers A.A. Effect of pentoxifylline on neutrophil behavior: stimulation of movement without adhesion changes. Biomed. Pharmacother. 1988;42:117–120. [PubMed] [Google Scholar]
- 20.Churchill P.C, Bidani A.K. Hypothesis: adenosine mediates he-modynamic changes in renal failure. Med. Hypothesis. 1982;8:275–285. doi: 10.1016/0306-9877(82)90124-4. [DOI] [PubMed] [Google Scholar]
- 21.Bancroft J.D, Stevens A. Theory and Practice of Histological Techniques. Nottingham: Churchill Livingstone; 1996. [Google Scholar]
- 22.Ademuyiwa O, Ngaha E.O, Ubah F.O. Vitamin E and selenium in gentamicin nephrotoxicity. Hum. Exp. Toxicol. 1990;9:281–288. doi: 10.1177/096032719000900504. [DOI] [PubMed] [Google Scholar]
- 23.Kavutcu A, Canbolat O, Ozturk S, Olcay E, Ulutepe S, Ekinci C, Gokhun I.H, Durak I. Reduced enzymatic antioxidant defense mechanism in kidney tissues from gentamicin treated guinea pigs: Effects of vitamin E and C. Nephron. 1996;72:269–274. doi: 10.1159/000188853. [DOI] [PubMed] [Google Scholar]
- 24.Rodriguez-Barbero A, L’Azou B, Cambar J, Lopez-Novoa J.M. Potential use of isolated glomeruli and cultured mesangial cells as in vitro models to assess nephrotoxicity. Cell. Biol. Toxicol. 2000;16:145–153. doi: 10.1023/a:1007683320660. [DOI] [PubMed] [Google Scholar]
- 25.Brunner L.J, Vadiei K, Iyer L.V, Luke D.R. Prevention of cy-closporine-induced nephrotoxicity with pentoxifylline. Ren. Fail. 1989;11(2-3):97, 104. doi: 10.3109/08860228909066950. [DOI] [PubMed] [Google Scholar]
- 26.Akpolat T, Akpolat I, Ozturk H, Sarikaya S, Coşar A.M, Bedir A, Kandemir B. Effect of vitamin E and pentoxifylline on glycer-ol-induced acute renal failure. Nephron. 2000;84(3):243–247. doi: 10.1159/000045584. [DOI] [PubMed] [Google Scholar]
- 27.Kim Y.K, Choi T.R, Kwon C.H, Kim J.H, Woo J.S, Jung J.S. Ben-eficial effect of pentoxifylline on cisplatin-induced acute renal failure in rabbits. Ren. Fail. 2003;25(6):909–922. doi: 10.1081/jdi-120026026. [DOI] [PubMed] [Google Scholar]
- 28.Strutz F, Ziesberg M, Hemmerlein B. Basic fibroblast growth factor (FGF-2) expression in increased in human renal fibrogenesis and may mediate autocrine fibroblast proliferation. Kidney Int. 2000;57:1521–1538. doi: 10.1046/j.1523-1755.2000.00997.x. [DOI] [PubMed] [Google Scholar]
- 29.Kaputlu I, Sadan G, Karayalcin B, Boz A. Beneficial effects of pentoxifylline on cyclosporine-induced nephrotoxicity. Clin. Exp. Pharmacol. Physiol. 1997;24(5):365–369. doi: 10.1111/j.1440-1681.1997.tb01203.x. [DOI] [PubMed] [Google Scholar]
- 30.Savic V, Vlahovic P, Djordjevic V, Mitic-Zlatkovic M, Avramovic V, Stefanovic V. Nephroprotective effects of pentoxifylline in experimental myoglobinuric acute renal failure. Pathol. Biol. 2002;50(10):599–607. doi: 10.1016/s0369-8114(02)00323-1. [DOI] [PubMed] [Google Scholar]
- 31.Vadiei K, Brunner L.J, Luke D.R. Effects of pentoxifylline in experimental acute renal failure. Kidney Int. 1989;36(3):466–470. doi: 10.1038/ki.1989.218. [DOI] [PubMed] [Google Scholar]
- 32.Ellory J.C, Culliford S.J, Honvitz E.R, Mojiminiyi F.B.O, Stuart J. Oxpentifylline-induced inhibition of calcium entry into human erythrocytes. Clin. Hemorheol. 1994;14:545–555. [Google Scholar]
- 33.Stojiljković N, Veljković S, Mihailović D, Stoiljković M, Radovanović D, Ranđelović P. The effect of calcium channel blocker verapamil on gentamicin nephrotoxicity in rats. Bosn. J. Basic Med. Sci. 2008;8(2):170–176. doi: 10.17305/bjbms.2008.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stojiljkovic N, Mihailovic D, Veljkovic S, Stoiljkovic M, Jovanovic I. Glomerular basement membrane alterations induced by gentamicin administration in rats. Exp. Toxicol. Pathol. 2008;60:69–75. doi: 10.1016/j.etp.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Gilbert N. Mandell G.L, Bennett J.E, Dolin R, editors. Aminoglycosides. In: Principles and Practice of Infec-tious Disease. (5th ed) :307–336. [Google Scholar]
- 36.Mingeot-Leclercq M.P, Tulkens P.M, editors. Antimicrob. Agents. Chemother 1999. Vol. 43. New York: Churchill Livingstone; 2000. Aminoglycosides: nephrotoxicity; pp. 1003–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smaoui H, Mallie J.P, Schaeverbeke M, Robert A, Schaeverbeke J. Gentamicin administered during gestation alters glom-erular basement membrane development. Antimicrob. Agents Chemother. 1993;37:1510–1517. doi: 10.1128/aac.37.7.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]


