We studied with the help of 13CO2 labelling and an advanced gas exchange measurement system (Tree DEMON) the strength of de novo and pool isoprenoid emissions of Scots pine during drought and rewetting. Both emission types showed a reduction, however, the de novo emissions were more strongly affected by drought and recovered slower during rewetting. The study also showed a strongly varying ratio of pool and de novo emissions for the individual isoprenoids. Furthermore an improved emission standardization algorithm was applied to correctly standardize the emission rates from de novo and pool emissions to determine the emission potential under different drought stress states.
Keywords: 13CO2, de novo BVOC emissions, drought, dynamic plant chambers, monoterpenes, Scots pine
Abstract
Monoterpene (MT) emissions of conifer tree species, emitted from de novo synthesis and storage pools, play an important role in plant ecology and physiology. During drought stress both emission sources are affected differently and with conventional measuring techniques they are difficult to separate. We investigated 13C labelled MT emission of eight 3-year-old Scots pine seedlings in a drought stress experiment using a dynamic gas exchange chamber system (Tree DEMON). Monoterpene, water vapour and CO2 gas exchange were measured for a 2-day normal watering, a 11-day treatment and a 3-day re-watering period. In each period all trees were 13C labelled once for 5 h. Results showed the expected decrease of MT, water vapour and CO2 gas exchange with decreasing soil water content. However, during re-watering water vapour and CO2 gas exchange recovered fast to pre-drought levels, whereas MT increased to a lower level compared to the initial non-stressed phase. The 13C labelling showed highly variable %13C values for different MTs, which ranged compound-specific from 0.5 to 95 % for unstressed trees. Overall, around 36 ± 5 % of the total emission rate originated from de novo synthesized MTs during the 2-day prior to stress period. During full drought, the de novo fraction was reduced to 3 %. For the re-watering phase de novo emissions recovered only partly to 20 %, while pool emissions reached pre-drought conditions. Thus, emissions of de novo synthesized MTs of Scots pine are down-regulated by soil drought rather than MT emissions from pools.
Introduction
Conifer trees, which dominate colder regions of the northern hemisphere, contribute around 10 % to the total monoterpene (MT) emissions into the atmosphere (Guenther et al. 2012), which have a significant role in atmospheric ozone and secondary organic aerosol chemistry (Yu et al. 1999; Calfapietra et al. 2013; Emanuelsson et al. 2013). Pinus sylvestris (Scots pine) is a widely distributed conifer tree species and emits a significant amount of different MTs as well as sesquiterpenes (e.g. Janson 1992; Shao et al. 2001; Komenda and Koppmann 2002; Holzke et al. 2006; Bäck et al. 2012; Yassaa et al. 2012). The emitted MTs derive de novo from directly synthesized compounds as well as from prior synthesized compounds stored in pools, e.g. resin ducts in wood or leaves or in the liquid phase of the leaves (Shao et al. 2001; Ghirardo et al. 2010). Stored MT compounds also present in other pine species (e.g. Petrakis et al. 2001; Macchioni et al. 2003; Gao et al. 2005) protect against herbivory (Manninen et al. 1998; Mumm and Hilker 2006) or aerial pathogens (Gao et al. 2005), whereas newly synthesized compounds reduce oxidative stress (Graßmann et al. 2005).
Abiotic and biotic stressors (Holopainen and Gershenzon 2010; Loreto and Schnitzler 2010) can lead to both an increase and decrease of emissions. Mechanical damage can evoke emissions through, e.g., burst of resin ducts and subsequent release of compounds (Komenda and Koppmann 2002) or induced production of compounds in order to protect open wounds against infections (Fäldt et al. 2006). In contrast, drought reduces photosynthetic capacity and thus carbon allocation, which is then lacking for MT synthesis reducing their emission (Lüpke et al. 2016).
Tree seedlings are more sensitive to extended stress periods than adult trees (Niinemets 2010), since seedling carbon pools are much smaller and could be depleted during long stress phases and are then missing for the recovery. However, they have a higher plasticity than adult trees; e.g., during drought seedlings show a more anisohydric behaviour keeping stomata open longer (Mediavilla and Escudero 2004) and they morphologically adapt faster to drought stress (Royo et al. 2001).
Increased drought intensity and frequency during the last two decades (see, e.g., Carnicer et al. 2011; Spinoni et al. 2014) has led to severe forest diebacks often observed in P. sylvestris stands (Allen et al. 2010). In order to assess drought effects and to develop potential adaptation strategies, seedling studies have frequently been performed (see, e.g., Llusià and Peñuelas 1998; Wu et al. 2015; Aaltonen et al. 2016), since they allow easier manipulations and faster replication compared to adult trees.
Although MT synthesis only uses a small percentage of the plants’ carbon pool (Kesselmeier and Staudt 1999), its emission can be used as a proxy to measure non-invasively their stress responses (Niinemets 2010). Drought stress response can be seen in recently synthesized isoprenoids (e.g. Brilli et al. 2007; Wu et al. 2015), which mostly origin from carbon allocated by photosynthesis via the methylerythritol phosphate (MEP) pathway (Ghirardo et al. 2014). However, they can also be sustained via other pathways using stored carbon such as starch or sugars (Kreuzwieser et al. 2002; Schnitzler et al. 2004). These other pathways are able to contribute a substantial amount of freshly synthesized isoprenoids to the total emissions during a drought phase and they use up stored carbon as shown by Brilli et al. (2007) for isoprene.
Sources of MT emissions (de novo synthesized vs. stored in pools) are investigated by short-term 13C isotopic labelling of the target compounds by 13CO2 enrichment in the air surrounding the plants. 13C is photosynthesized and then further synthesized to MT. Since 13CO2 is less abundant in ambient air than 12CO2 (1.098 % vs. 98.892 % of total CO2; Dawson et al. 2002) and is moreover discriminated by plant photosynthesis (Farquhar et al. 1989), emitted compounds normally incorporate a very low amount of 13C. However, if pure 13CO2 is supplied for photosynthesis, this carbon isotope is enriched in the corresponding downstream compounds. Loreto et al. (1996), Shao et al. (2001) and Harley et al. (2014) showed that 13C labelling increases the compound mass by one mass unit for each labelled C-atom. This method has been used in prior studies on, e.g., broad-leaved species such as Quercus rubra (Delwiche and Sharkey 1993), Quercus ilex (Loreto et al. 1996; Ghirardo et al. 2010; Wu et al. 2015), Betula pendula (Ghirardo et al. 2010) and Fagus sylvatica (Wu et al. 2015). Also conifer species, known for their large MT pools, were instigated with 13C labelling such as Larix decidua. (Ghirardo et al. 2010), Picea abies (Schürmann et al. 1993; Ghirardo et al. 2010), Pinus ponderosa P.Lawson & C.Lawson and Pinus nigra (Harley et al. 2014), Pinus pinea (Noe et al. 2006) and P. sylvestris (Shao et al. 2001; Ghirardo et al. 2010; Kleist et al. 2012; Wu et al. 2015). Two analytical methods have been used to measure labelled MT emissions, as compound groups by proton-transfer-reaction mass spectrometer (PTR-MS) (e.g. Ghirardo et al. 2010; Harley et al. 2014) and by pre-concentration of labelled compounds on adsorbent tubes, allowing single MT compound detection in a GC/MS (e.g. Shao et al. 2001; Lüpke et al. 2016).
Many earlier studies investigated only 13C recently fixed by photosynthesis but not alternative carbon pools for isoprenoid synthesis. Ghirardo et al. (2010) proposed an advanced method to include both sources, which was also applied on two other pine species by Harley et al. (2014) and in this study.
We investigated MT emissions of Scots pine seedlings under controlled light and temperature conditions, however, with fast changes of soil water content (SWC) in a drought experiment, which comprised normal watering, a drought phase as well as a final re-watering phase.
During these three phases, three 13CO2 labelling campaigns were performed in order to test the following key hypothesis: drought stress reduces single compound MT emissions from pools and from de novo synthesis in a similar magnitude. We focused on the four research questions: (1) Does multi-labelling cause interferences in the %13C? (2) How large are the de novo fractions of different MT compounds? (3) How strongly do trees respond to drought stress and to stress relief by re-watering? (4) How strongly does the drought stress affect the de novo emissions and at which point in time emissions originates only from pools?
Methods
Experimental setup
The combined drought and 13C labelling experiment comprised two replications, from 01 July 2015 until 16 July 2015 and from 24 July 2015 until 8 August 2015, respectively, with two treated (trt) and two control (ctr) Scots pine specimen each. Measurements were performed under similar environmentally controlled conditions in a dynamic plant chamber system. The experiments were undertaken at a seasonal point when needles were fully developed and no new needle growth was observed.
Plant and soil material.
Eight out of 25 morphologically similarly sized 4-year-old Scots pine trees (seed origin: Mittelfränkisches Hügelland of Germany, 49.497°N, 11.184°E) were selected for the experiment. All trees had been planted in November 2013 at an age of 2 years into 5-L pots containing a soil mixture of 70 % sand and 30 % humus. This soil mixture allowed a fast drought application. The plants were raised under greenhouse conditions and watered manually during wintertime and in summertime with a dripping water system (Netafim Ltd, Tel Aviv, Israel). The trees were fertilized with a 1.2 g L−1 solution (FERTY® 1, Planta Düngemittel GmbH, Regenstauf, Germany) each once per week from 15 June 2015 till 17 August 2015 in order to avoid nutrient shortage.
Dynamic plant chambers.
In each replication four whole tree canopies were installed into a system with four dynamic plant chambers for gas exchange assessment called Tree DEMON (tree drought emission monitor) (see Lüpke et al. 2016; Lüpke et al. 2017). The plant chambers (~30 L volume) were made of transparent PVDF (polyvinylidene fluoride with ~97 % photosynthetically active radiation (PAR) transmissibility) mounted on a stainless steel flange. These top chambers were connected to two duraluminium ground plates, where air in- and outlets, sensors and tree stems were installed. Each chamber was supplied with 9 Ln min−1 of mass flow controlled conditioned inlet air (VOC free, humidified and constant CO2 mixing ratio) over stainless steel tubes with multiple micro inlets [see Supporting Information for detailed conditioning procedure and Fig. S1 photo of the Tree DEMON]. This technique generated sufficient air mixing of the chamber volume with slight overpressure to avoid air leaking in and generated steady-state conditions (see also Lüpke et al. 2017 for a detailed technical evaluation).
Each plant chamber was equipped with two thermocouples type K (L-0044K-IEC, Omega Engineering Ltd, Northbank, Irlam, Manchester, UK) to measure leaf temperature as well as one air temperature/relative humidity sensor (FF-IND-10V-TE1, B+B Thermo-Technik GmbH, Donaueschingen, Germany). Before the tree canopies were installed into the plant chambers, a PTFE band was wrapped around the stem (below canopy) for protection and better sealing. Trees were installed 2 days before actual sampling start in order to adjust to the plant chambers and environmental conditions.
Climate chamber environmental settings.
The Tree DEMON was placed in a climate chamber which guaranteed stable environmental conditions comparable between both replications. The climate chamber was set to a constant temperature of 24 °C with a relative humidity of 50 %. Light levels were ramped to simulate a diurnal pattern with following steps starting at 0700 h local time (CEST): 1 h at 70 µmol PAR m2 s−1, 1 h at 125 µmol PAR m2 s−1, 1 h at 200 µmol PAR m2 s−1 and 8 h at 385 µmol PAR m2 s−1 and then reverse steps back to 0 µmol PAR m2 s−1, resulting in a 16-h day and 8-h night cycle. Photosynthetically active radiation was measured by one sensor (HOPL, Skye Instruments Ltd, Llandrindod Wells, Powys, UK) at mid-height of the plant chambers.
Watering regimes.
The drought experiment in each replication comprised the following watering regime (Table 1): the normal watering of the first 2 days encompassed 300 mL tap water manually added to each tree at 1300 h. After this phase, two of the four trees were subjected to drought from Day 3 to Day 12 by a total stop of watering, while the control trees were normally watered. At Days 13 and 14, the two treated trees were intensively re-watered in order to recover, whereas the control trees still received their normal watering. Soil water content was monitored by time domain reflectometry probes (SM300, Delta-T Devices, Cambridge, UK) installed horizontally ~5 cm above the base of the pots.
Table 1.
Irrigation and 13CO2 labelling schemes of the experiment. *Note: Tree DEMON measurements were performed until 1300 h, whereas irrigation started after 1300 h. Two replications with each two trees per treatment and control groups were performed.
| Day* (1300–1300 h) | Irrigation (mL day−1) of control trees (N = 4) | Irrigation (mL day−1) of treated trees (N = 4) | 13CO2 labelling |
|---|---|---|---|
| 1–3 | 300 (Days 1 and 2) | 300 (Days 1 and 2) | Day 2 |
| 3–13 | 300 | 0 | Day 8 |
| 13 | 300 | 1200 | |
| 14 | 300 | 600 | |
| 15–16 | 300 | 300 | Day 15 |
13C labelling.
The 13C labelling experiment was performed simultaneously at all trees on Days 2, 8 and 15 of each replication from 1300 to 1800 h by replacing the mass flow controlled added CO2 (99.995 % purity, Rießner Gase, Lichtenfels, Germany) of the supply air (~400 µmol mol−1 CO2) via a manual switch valve by 13CO2 (99.9 % 13CO2, Sigma Aldrich Chemie GmbH, Munich, Germany).
CO2 and water vapour gas exchange.
The differential gas exchange measurement of CO2 and water vapour was performed with an infrared gas analyser (IRGA) (CIRAS 2 DC, PP-Systems, Amesbury, MA, USA) at each chamber air in- and outlet sequentially every 5 min by a magnetic valve manifold. Since 13CO2 affected the sensitivity of the IRGA CO2 channel, consequently leading to biased net photosynthesis rates during the labelling experiment, CO2 exchange data were not analysed during the isotopic labelling experiment. Thus, steady-state gas exchange conditions in the course of the experiment were identified by data from the not affected H2O channel only. Transpiration and photosynthesis rates were calculated according to Caemmerer and Farquhar (1981) from the differential measurement of CO2 and water vapour at chamber in- and outlets by the IRGA.
Biomass assessment.
Biomass and leaf area of all individuals were measured after each replication. Trees were harvested and dried in an oven for 48 h at 60 °C and stem and needle dry mass were weighed separately. The specific leaf area (SLA) was determined by optical scanning of 5 g of dried needles of a tree and deriving the leaf area from the scans with ImageJ (Schneider et al. 2012). The whole canopy leaf area (Aleaf) for each tree was determined by upscaling of SLA with the total needle mass.
BVOC sampling and analysis
BVOC samples were taken with adsorbent tubes (ATs) for 50 min with a flow rate of 150 mL min−1 at 0400 and 0500 h during night and 1100 and 1200 h during day at 385 µmol PAR m2 s−1. On 13C labelling days, additional samples were taken at 1500, 1600, 1700 and 1800 h to monitor the labelling rate.
More precisely, outlet air of each chamber was sampled mass flow controlled (SMART 4S GCS, Vögtlin Instruments AG, Aesch, Switzerland) by a sampler string with each four ports consisting of an AT installed between two pneumatically controlled stainless steel valves (VXA2120M-01F-1-B, SMC Pneumatik GmbH, Gröbenzell, Germany). Each port separated the AT from the chamber outlet airflow until the start of the sampling period. ATs were made of inert stainless steel (Camsco, Houston, TX, USA) with a two-stage adsorbent bed containing 70 mg Tenax TA© and 40 mg Carbograph® 5TD both with a 60/80 mesh size. The sampled compounds on the AT were thermally desorbed within 12 h after sampling with a Perkin Elmer ATD 650 (Perkin Elmer, Waltham, MA, USA) by a dual-stage desorption with a cold trap [see Supporting Information Thermal desorption method]. Desorbed compounds were transferred over a heated glass tube to a Clarus© SQ8 GC/MS system (Perkin Elmer, Waltham, MA, USA) and separated by an Elite 5MS column (30 m length, 250 µm diameters, Perkin Elmer, USA) by a ramped temperature programme [see Supporting Information GC method].
The separated compounds were split into two detectors at the end of the column which both received the same amount of analyte within similar retention times. One detector was a flame ion detector (FID) running at 300 °C and the second a quadrupole mass spectrometer (MS) Clarus© SQ8 (Perkin Elmer, Waltham, MA, USA) with electro-ionization at 70 eV running in full scan mode (m/z 33 to 330). Compounds were identified by their mass spectra with the NIST library and additionally confirmed by a 16-component BVOC gas standard (C5–C12 with a mixing ratio ranging from 1.81 to 2.22 nmol mol−1 and expanded uncertainty ranging from 0.09 to 0.30 nmol mol−1, NPL, Teddington, Middlesex, UK [see Supporting Information—Table S1]). The gas standard was also used for calibration together with an internal standard of Δ2-carene (mixing ratio of 87 ± 8.7 nmol mol−1 expanded uncertainty, SIAD Austria GmbH, St. Pantaleon, Austria). Fifty millilitres of the internal standard were added to every AT before actual sampling in order to compensate for system fluctuations. The quantification of compounds was performed by the FID which had a detection limit ranging from 0.001 to 0.02 nmol mol−1. The target compounds had to be present in the gas standard and in the trees and had to be unbiased by co-eluting compounds.
Quantification of %13C.
For all MT target compounds, their 12C part (M12) was determined by the integrated signal area of the main fragment of the molecule obtained by the mass spectrometer, e.g., at m/z 93 for most identified MTs in this study. For the 13C part (M13) the sum of the integrated signal of the isotopologues from m/z 94 to m/z 100 was used, since m/z increased for each additional built-in 13C-atom. For some compounds other main fragments and isotopologues were used, e.g. m/z 154 for 1,8-cineole (isotopologues m/z 155 to 164) or m/z 119 for p-cymene (isotopologues m/z 120 to 128), respectively. The 13C share (%13C) was calculated after Equation 1:
| (1) |
Due to the presence of 1.1 % 13C within ambient air CO2, non-labelled MT molecules and their fragments contained natural occurring 13C. Therefore, the non-labelled %13C (average of 1100 and 1200 h sample) was subtracted from the most labelled %13C (1700 h sample) in order to obtain the pure share of the 13C labelling.
Emission rate calculation.
BVOC emission rates (nmol m−2 s−1) were calculated after Niinemets et al. (2011) by Equation 2:
| (2) |
in which χin and χout are in- and outlet concentration (nmol mol−1). In the used setup χin is zero due to filtered inlet air. Aleaf is the leaf area (m2) of the tree and Fin the molar flow rate passing in the chamber (mol s−1) set by the inlet mass flow controller. In a further step, this emission rate is corrected by additional water vapour transpired by the plants (E in Equation 2 with mol m−2 s−1) which corrects the mass balance between in- and outlet air.
De novo emission and pool emission standardization
De novo emissions rely not only on pathways using under normal conditions recently fixed carbon by photosynthesis but can also use stored carbohydrates (see, e.g., Brilli et al. 2007). The 13C labelled emission refers only to photosynthetically fixed carbon and does not incorporate de novo emissions from other carbon sources. However, some compounds, e.g. isoprene, 3-methyl-2-butanone (MBO) or 1,8-cineole, can be considered as purely de novo emitted since they have no or very small storage pools and show highly light-dependent emissions (Harley et al 2014; Wu et al. 2015). During 13C labelling these compounds show high ratios of fast labelled %13C and the remaining %12C is almost exclusively related to pathways with alternative carbon sources (e.g. starch). Since the de novo part of a mixed emission compound (pools and de novo) relies on similar pathways like pure de novo emitted compounds, a correction method used by Ghirardo et al. (2010) and Harley et al. (2014) was applied. Here, the %13C of compounds with mixed emissions was normalized by %13C of a purely de novo emitted compound, which was 1,8-cineole in this study. This compound is completely de novo emitted by Scots pine as shown by previous studies of Wu et al. (2015), Kleist et al. (2012) and Ghirardo et al. (2010).
Out of the derived de novo fraction the pool and de novo emission rates were calculated and standardized to 1000 µmol PAR m−2 s−1 by the following algorithms. For the de novo part a combined light and temperature standardization algorithm was used (Guenther 1997) as shown in Equation 3:
| (3) |
In Equation 3, the measured de novo emission rate EMde novo sample was standardized by the correction term for leaf temperature f(TL) to 30 °C and for the correction term f(Q) to light level of 1000 µmol PAR m−2 s−1. The used parameters for f(Q) and f(TL) were the same as used by Guenther (1997) [see Supporting Information Standardization algorithm]. The pool emission EMpool was corrected by a pure temperature algorithm of Guenther et al. (1995) in Equation 4 with an exponential functional containing an empirical value β of 0.09, leaf temperature TL in K and the standard temperature Tstd of 303.15 K (30 °C):
| (4) |
In a further step the standardized (30 °C and 1000 µmol PAR m−2 s−1) de novo fraction fde novo was calculated by Equation 5:
| (5) |
During normal sampling a separation of pools and de novo is not possible, thus the mixed type emission was corrected by a combined algorithm (also used by, e.g., Schuh et al. 1997; Ghirardo et al. 2010; Harley et al. 2014) in Equation 6:
| (6) |
Since fde novo highly depends on the MT synthesis capacity and should change under stress condition, a fixed constant cannot be used. In order to estimate fde novo for the non-labelled emissions, a non-linear model with a Michaelis–Menten function (see Equation 7) was fitted between the standardized emission rate of 1,8-cineole and standardized fde novo of each compound.
| (7) |
Statistical analyses
Data processing and statistics were performed with the software R (R Core Team 2017, Version 3.3). Reported values are means of each group with the respective standard error.
The paired Wilcoxon signed-rank test was used to identify how many days after 13C labelling were needed to reach %13C of the pre-labelling period in order to identify how long the labelling signature was present (residence time). Here, the 1700 h sampling (13C labelling; Day 2, N = 8) as well as the post-labelling samples at 1200 h from Day 3 to Day 7 (N = 8) were compared with the 1200 h (non-labelled, Day 2, N = 8) sample.
The gas exchange rates of the control and stress group were compared for three phases defined by SWC as well as gas exchange of CO2 and water vapour of the treatment group each lasting 3 days: (I) non-stressed phase: plants were well watered and showed a stable gas exchange; (II) fully drought stressed: SWC indicated drought conditions (SWC < 0.06 m3 m−3) and gas exchange rates were close to zero and (III) recovering phase: re-watering of the treated group. For each 3-day phase two-sample Student’s t-tests were used to compare the physiological and environmental parameters of control and treatment groups with both replications pooled together. More specifically, averaged noon measurements of gas exchange (CO2, water vapour and BVOC), TL and SWC (1100 and 1200 h) were compared (typical N = 12 per group).
Results
Morphological parameters
After harvesting, the biomass parameters measured for the control (ctr) and treatment (trt) group indicated similar sizes of the trees’ canopies. The average leaf biomass was 18.4 ± 2.0 g (ctr)/21.1 ± 2.0 g (trt) and the stem biomass 10.5 ± 2.0 g (crt)/10.6 ± 0.8 g (trt), respectively. The mean leaf area was 936.7 ± 107.2 cm2 (ctr)/1094.6 ± 78.2 cm2 (trt) and the mean height was 43.1 ± 5.2 cm/41.1 ± 1.5 cm. For both treatment groups no apparent growth of needles during the experiment was visible.
SWC and gas exchange rates (CO2, water vapour, 1,8-cineole) during the experiment
Days 3 to 5 of both replications were selected for the analysis of phase I (normal watering), since at the first 2 days of replication one neon light row failed which resulted in a reduction of PAR by 50 µmol m−2 s−1 and a decrease of gas exchange (see Fig. 1C). Although all studied trees were equally watered prior to the experiment in the greenhouse, in this phase I (and also on Days 1 and 2) the trt group already showed a significant lower mean SWC between 0.18 and 0.12 m3 m−3 (Fig. 1A, see Table 2 for detailed rates and significances), likely because watering was stopped at Day 3. However, the trt group in phase I can be still considered as non-stressed, since with decreasing SWC gas exchange rates were constant till a SWC of 0.08 m3 m−3 [see Supporting Information—Fig. S2 for SWC gas exchange relationship]. Therefore, Days 1 to 6 can be regarded as non-stressed conditions.
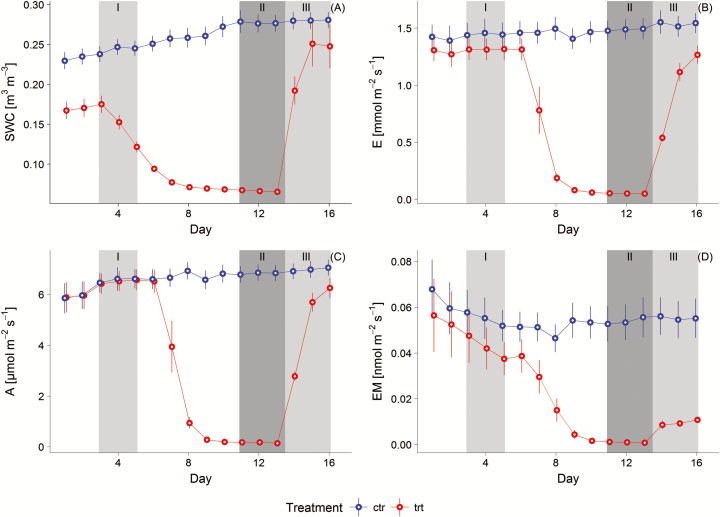
Figure 1.
Mean daytime (1100 and 1200 h) measurements of (A) SWC, (B) net photosynthesis rate A, (C) transpiration rate E and (D) 1,8-cineole BVOC emission rate EM – light/temperature corrected by the Guenther 97 algorithm (Guenther et al. 1997). Mean and standard error are given for four control (ctr) and four treated (trt) trees of the two repetitions. The three periods (I to III, see also Table 1) are split into phase I non-stressed (light grey, Days 3 to 5 noon), phase II fully drought stressed (from Day 11 afternoon to Day 13 noon) and phase III re-watering (light grey area, Days 14 to 16).
Table 2.
Mean and standard error (SE) of SWC, transpiration rate (E), photosynthesis rate (A), leaf temperature (TL) and emission rate (EM) of 1,8-cineole of both groups (trt: treatment, ctr: control) during the three phases (phase I non-stressed: Days 3 to 5, phase II fully drought stressed: Days 11 to 13 and phase III re-watering: Days 14 to 16). EM was standardized to 30 °C and 1000 µmol PAR m−2 s−1. P indicates the significance level of the t-test, while df gives the degree of freedom.
| Parameter | Phase | ctr ± SE | ctr ± SE | P | df |
|---|---|---|---|---|---|
| SWC (m3 m−3) | I | 0.24 ± 0.01 | 0.15 ± 0.01 | <0.01 | 43.4 |
| II | 0.27 ± 0.01 | 0.07 ± 0.02 | <0.01 | 26.4 | |
| III | 0.28 ± 0.01 | 0.23 ± 0.02 | <0.01 | 28.2 | |
| E (mmol m−2 s−1) | I | 1.45 ± 0.06 | 1.31 ± 0.05 | 0.10 | 43.9 |
| II | 1.49 ± 0.05 | 0.05 ± 0.00 | <0.01 | 23.1 | |
| III | 1.54 ± 0.05 | 0.99 ± 0.08 | <0.01 | 29.2 | |
| A (µmol m−2 s−1) | I | 6.56 ± 0.22 | 6.50 ± 0.22 | 0.86 | 45 |
| II | 6.82 ± 0.18 | 0.16 ± 0.01 | <0.01 | 23.1 | |
| III | 6.98 ± 0.18 | 5.00 ± 0.37 | <0.01 | 31.6 | |
| T L (°C) | I | 25.34 ± 0.08 | 25.47 ± 0.07 | 0.23 | 42.3 |
| II | 25.52 ± 0.06 | 28.14 ± 0.09 | <0.01 | 39.7 | |
| III | 25.42 ± 0.06 | 26.2 ± 0.16 | <0.01 | 27.9 | |
| EM1,8-cineole (nmol m−2 s−1) | I | 0.055 ± 0.005 | 0.042 ± 0.005 | 0.09 | 44.6 |
| II | 0.054 ± 0.004 | 0.001 ± 0.001 | <0.01 | 23.1 | |
| III | 0.055 ± 0.005 | 0.01 ± 0.001 | <0.01 | 24.6 |
In Fig. 1, an overview of the course of the experiment is given, while Table 2 shows the means of gas exchange (CO2, water vapour and 1,8-cineole emission) and SWC with results of the t-test.
In phase I, no significant differences in gas exchange between both groups were detected (see also Fig. 1B and C and Table 2 for details). In respect to BVOC emissions, only 1,8-cineole, which was completely de novo emitted (see Fig. 1D and Table 2), could be correctly standardized for light and temperature and is thus included in this comparison. Here, both groups showed similar 1,8-cineole emission rates.
After Days 6 and 7, gas exchanges of the trt group started to respond to decreasing SWC and declined to rates close to zero on Day 10 (see Fig. 1B and C). Consequently, Days 11 to 13 were considered as phase II with fully drought-stressed plants and SWC near the permanent wilting point. Phase II was characterized by extremely low gas exchange rates of the trt group (reduction of A by 98 %; E by 94 % and EM by 75 % from phase I to II) and by increased TL (+2.67 °C from phase I to II), all parameters significantly differing from the ctr group (see Table 2).
Within the re-watering phase III (Days 14 to 16) SWC of the trt group increased to 0.23 ± 0.2 m3 m−3 above phase I levels. This led to an increase of gas exchange rates (see parameters A and E in Table 2), which however did not reach phase I levels. EM increased slightly, but stayed below phase I levels. Additionally, the trt group still performed significantly worse than the ctr group.
In order to assess the emission rates of compounds with pool and de novo fractions correctly, these had to be standardized by a mixed correction algorithm (see Methods). The algorithm parameters were obtained in the simultaneous 13C labelling experiment.
13C labelling of isoprenoids and 13C residence time
In order to check how long the 13C signature after the first 13C labelling on Day 2 was still present, the %13C of all trees from Day 2 (labelling day) till Day 7 (day prior to the next labelling) were investigated. Although an effect of decreasing SWC on gas exchange was observed on Day 7 (see Fig. 1), this day was included into this test in order to check if all compounds could regain pre-labelling %13C.
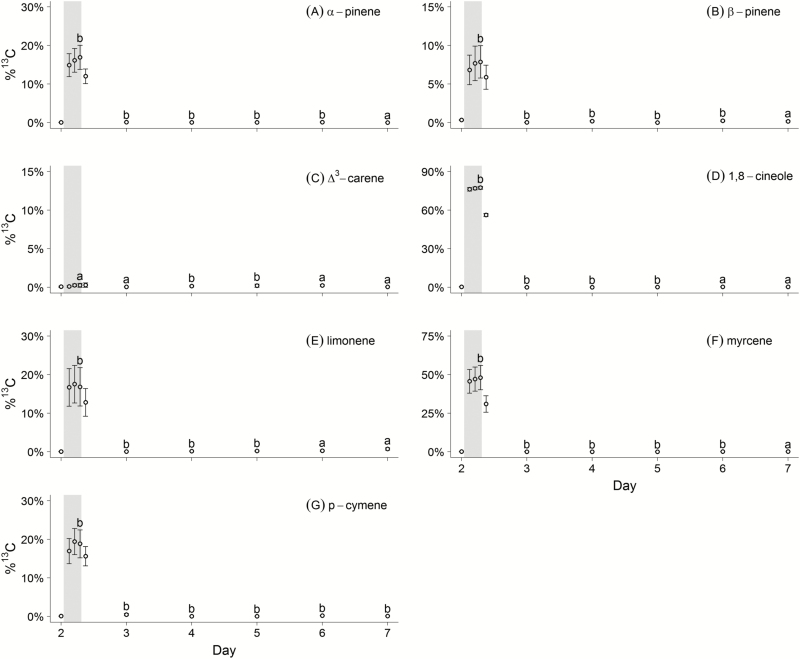
Figure 2 shows %13C of seven selected compounds before, during and after the first 13C labelling at Day 2. All %13C were highest during the 1700 h sampling, yet the increase from 1600 to 1700 h sampling was small, reaching saturation. However, each compound showed significantly different %13C before labelling (1200 h) and in the last labelling hour (1700 h, see Fig. 2A–G, except C Δ3-carene). Each single compound was assigned to two groups according to their %13C changes: (1) compounds showing small changes, such as β-pinene (%13C: 7.9 ± 2.1 %), α-pinene (%13C: 16.9 ± 3.1 %), limonene (%13C: 16.9 ± 5.0 %) and p-cymene (%13C: 18.8 ± 3.6 %), (2) compounds showing strong changes, such as and myrcene (%13C: 48.1 ± 7.9 %) and 1,8-cineole (%13C: 77.4 ± 1.1 %).
Figure 2.
Mean %13C for each compound (see Methods for calculation of each compound) for (A) α-pinene, (B) β-pinene, (C) Δ3-carene, (D) 1,8-cineole, (E) limonene, (F) myrcene and (G) p-cymene of all trees before (Day 2, 1200 h), during the 13C labelling (grey band, Day 2, 1300 h until 1800 h) and after labelling (Days 3 to 7, 1200 h). Error bars represent the standard error. Small letters show the result of a paired Wilcoxon signed-rank test by comparing Day 2 at 1200 h (non-labelled) with the 1700 h (Day 2, labelled) and the post-labelling 1200 h samples (Days 3 to 7). Letter ‘b’ indicates significant group differences for each comparison (P < 0.05), while letter ‘a’ corresponds to non-significant differences, thus pre-labelling fractions.
Within the first 18 h after labelling, %13C ratios were strongly reduced and pre-labelling %13C levels were reached between Days 6 and 7 for most compounds (see Fig. 2; paired Wilcoxon signed-rank test). Only for p-cymene, the pre-labelling ratio was not reached within the 6-day time frame. In case of Δ3-carene, two trees showed no Δ3-carene emission at all, while six ones produced Δ3-carene. For the latter ones, labelling did not show any significant increase of %13C, only a slight increase on Days 4 and 5 was observed, which decreased afterwards to pre-labelling level.
De novo and pool emission of isoprenoid at the 13C labelling days
For the three labelling days (Days 2, 8 and 15), the labelled %13C was calculated and normalized by the %13C of 1,8-cineole in order to estimate the de novo fraction fde novo sample for all compounds. For Δ3-carene, which showed a very low %13C labelling of 0.3 %, a fde novo sample of zero was used since labelled %13C was within the range of measurement uncertainty. Standardized pool and de novo emission rates (EMpool, EMde novo) for each compound were calculated from fde novo sample for all three labelling days and treatments. EMpool and EMde novo were then used to determine the standardized de novo fraction fde novo (see Table 3).
Table 3.
Mean de novo (EMde novo, nmol m−2 s−1), pool (EMpool, nmol m−2 s−1), de novo fractions (fde novo) for each labelling day and treatment (ctr control, trt drought stress). SE represents the standard error. EMde novo and EMpool are corrected to standard temperature and light conditions (30 °C and 1000 µmol PAR m−2 s−1, see Equations 3 and 4). Both fractions were derived from the fde novo sample which is calculated by normalizing labelled %13C of a specific compound by the labelled %13C 1.8-cineole. fde novo is the fraction of EMde novo in the total standardized emissions rate.
| Compound | Treatment | Day | EMde novo ± SE | EMpool ± SE | f de novo ± SE |
|---|---|---|---|---|---|
| α-Pinene | ctr | 2 | 0.016 ± 0.003 | 0.026 ± 0.005 | 0.37 ± 0.05 |
| 8 | 0.013 ± 0.001 | 0.022 ± 0.001 | 0.37 ± 0.02 | ||
| 15 | 0.013 ± 0.002 | 0.023 ± 0.002 | 0.35 ± 0.04 | ||
| trt | 2 | 0.016 ± 0.003 | 0.075 ± 0.032 | 0.23 ± 0.08 | |
| 8 | 0.006 ± 0.003 | 0.036 ± 0.015 | 0.18 ± 0.09 | ||
| 15 | 0.005 ± 0.001 | 0.044 ± 0.018 | 0.15 ± 0.06 | ||
| β-Pinene | ctr | 2 | 0.002 ± 0.001 | 0.013 ± 0.005 | 0.09 ± 0.04 |
| 8 | 0.002 ± 0 | 0.018 ± 0.005 | 0.11 ± 0.02 | ||
| 15 | 0.002 ± 0.001 | 0.027 ± 0.009 | 0.08 ± 0.03 | ||
| trt | 2 | 0.001 ± 0 | 0.024 ± 0.018 | 0.07 ± 0.04 | |
| 8 | 0 ± 0 | 0.007 ± 0.006 | 0.04 ± 0.04 | ||
| 15 | 0 ± 0 | 0.013 ± 0.011 | 0.02 ± 0.02 | ||
| Δ3-Carene | ctr | 2 | 0 ± 0 | 0.096 ± 0.013 | 0 ± 0 |
| 8 | 0 ± 0 | 0.095 ± 0.024 | 0 ± 0 | ||
| 15 | 0 ± 0 | 0.103 ± 0.03 | 0 ± 0 | ||
| trt | 2 | 0 ± 0 | 0.056 ± 0.049 | 0.01 ± 0.01 | |
| 8 | 0 ± 0 | 0.025 ± 0.022 | 0 ± 0 | ||
| 15 | 0 ± 0 | 0.064 ± 0.06 | 0 ± 0 | ||
| 1,8-Cineole | ctr | 2 | 0.070 ± 0.022 | 0 ± 0 | 1 ± 0 |
| 8 | 0.059 ± 0.010 | 0 ± 0 | 1 ± 0 | ||
| 15 | 0.060 ± 0.013 | 0 ± 0 | 1 ± 0 | ||
| trt | 2 | 0.058 ± 0.024 | 0 ± 0 | 1 ± 0 | |
| 8 | 0.009 ± 0.005 | 0 ± 0 | 1 ± 0 | ||
| 15 | 0.009 ± 0.002 | 0 ± 0 | 1 ± 0 | ||
| Limonene | ctr | 2 | 0.005 ± 0.002 | 0.006 ± 0.002 | 0.32 ± 0.12 |
| 8 | 0.004 ± 0.001 | 0.006 ± 0.001 | 0.41 ± 0.04 | ||
| 15 | 0.005 ± 0.001 | 0.008 ± 0.001 | 0.39 ± 0.05 | ||
| trt | 2 | 0.004 ± 0.002 | 0.099 ± 0.072 | 0.18 ± 0.12 | |
| 8 | 0.001 ± 0 | 0.036 ± 0.027 | 0.17 ± 0.1 | ||
| 15 | 0.001 ± 0.001 | 0.028 ± 0.017 | 0.10 ± 0.09 | ||
| Myrcene | ctr | 2 | 0.035 ± 0.008 | 0.005 ± 0 | 0.85 ± 0.04 |
| 8 | 0.031 ± 0.005 | 0.006 ± 0.002 | 0.85 ± 0.03 | ||
| 15 | 0.036 ± 0.008 | 0.008 ± 0.003 | 0.81 ± 0.06 | ||
| trt | 2 | 0.033 ± 0.012 | 0.047 ± 0.031 | 0.53 ± 0.17 | |
| 8 | 0.011 ± 0.004 | 0.012 ± 0.006 | 0.47 ± 0.13 | ||
| 15 | 0.010 ± 0.001 | 0.019 ± 0.012 | 0.51 ± 0.14 | ||
| p-Cymene | ctr | 2 | 0.005 ± 0.002 | 0.011 ± 0.002 | 0.31 ± 0.07 |
| 8 | 0.005 ± 0.001 | 0.008 ± 0.001 | 0.37 ± 0.05 | ||
| 15 | 0.005 ± 0.001 | 0.009 ± 0.001 | 0.37 ± 0.07 | ||
| trt | 2 | 0.004 ± 0.002 | 0.007 ± 0.002 | 0.27 ± 0.11 | |
| 8 | 0.001 ± 0 | 0.003 ± 0.001 | 0.16 ± 0.07 | ||
| 15 | 0.001 ± 0 | 0.005 ± 0.002 | 0.12 ± 0.05 | ||
| Total | ctr | 2 | 0.132 ± 0.037 | 0.158 ± 0.022 | 0.43 ± 0.04 |
| 8 | 0.114 ± 0.019 | 0.154 ± 0.031 | 0.43 ± 0.02 | ||
| 15 | 0.121 ± 0.026 | 0.178 ± 0.042 | 0.41 ± 0.06 | ||
| trt | 2 | 0.116 ± 0.044 | 0.308 ± 0.094 | 0.29 ± 0.07 | |
| 8 | 0.027 ± 0.012 | 0.120 ± 0.037 | 0.18 ± 0.06 | ||
| 15 | 0.026 ± 0.005 | 0.173 ± 0.055 | 0.20 ± 0.09 |
At all three labelling days, the total EMde novo of the ctr group stayed constant at 0.12 ± 0.01 nmol m−2 s−1 (see Table 2) and mostly consisted of 1,8-cineole, myrcene and α-pinene. The mean total fde novo of the ctr group was 0.42 ± 0.02, while the trt group showed a lower total fde novo of 0.29 ± 0.07 at Day 2 (total unstressed mean fde novo of 0.36 ± 0.05 of both groups pooled together), which decreased further to 0.18 ± 0.06 (Day 8) and 0.20 ± 0.09 (Day 15). The EMde novo of the trt group was 0.12 ± 0.04 nmol m−2 s−1 at Day 2. On Day 8, EMde novo was reduced to 0.03 ± 0.04 nmol m−2 s−1 and was on a similar level at Day 15 with 0.03 ± 0.05 nmol m−2 s−1. Furthermore, trt group %13C of 1,8-cineole were reduced from 94.1 ± 9.6 % (Day 2) to 71.5 ± 9.6 % (Day 8) and increased back to 84.6 ± 2.7 % (Day 15), while the ctr group showed a mean %13C of 96.0 ± 0.5 % over all labelling days.
On Day 2, the trt group had double total EMpool (see Table 3, 0.31 ± 0.09 nmol m−2 s−1) than the crt group (0.16 ± 0.02 nmol m−2 s−1), which was mostly caused by α- and β-pinene, myrcene and limonene; however, the higher EMpool of limonene was only caused by one tree. During and after the drought treatment EMpool was reduced by 61.1 % to 0.12 ± 0.04 nmol m−2 s−1 on Day 8 during the drought-stressed period and by 43.9 % to 0.17 ± 0.05 nmol m−2 s−1 (Day 15 in the re-watering period), while respective values of the crt group were 0.15 ± 0.03 nmol m−2 s−1 (Day 8) and 0.18 ± 0.04 nmol m−2 s−1 (Day 15).
Isoprenoid emission correction
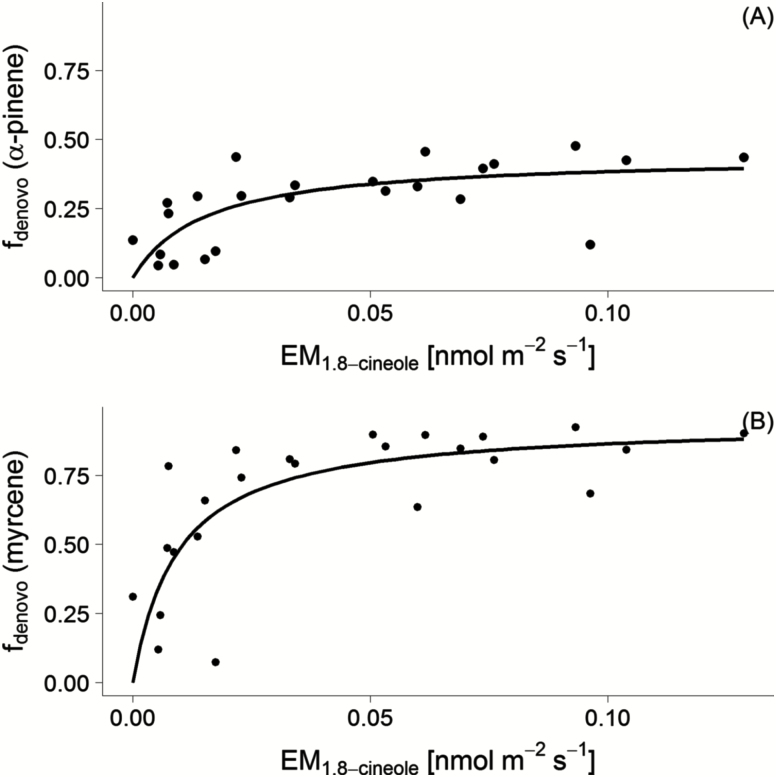
In the course of the drought experiment a decrease of the de novo fraction and emission was expected as indicated by the decline of the purely de novo emitted 1,8-cineole (see Fig. 1D) and by the decrease of fde novo for many compounds during the labelling experiment at Day 8 (see Tables 2 and 3). In order to estimate fde novo for the correction algorithm for mixed type emitted compounds (see Equation 5), a non-linear model based on the data of the labelling days was used (Equation 7). In Fig. 3, exemplary fits for α-pinene and myrcene are shown [see Supporting Information—Fig. S3 for remaining non-linear model fits].
Figure 3.
Non-linear fitting between standardized emission of 1,8-cineole and fde novo of α-pinene (A) and myrcene (B). Data points were selected from the 1700 h sample at the %13C labelling days (Days 2, 8 and 15).
The resulting functions were used to estimate fde novo for each compound by the measured 1,8-cineole emission. Based on these fde novo data, standardized emission rates could be calculated with the mixed correction algorithm. In the case of Δ3-carene emissions, only the pure temperature correction (fde novo = 0) and in case of 1,8-cineole only the light and temperature correction (fde novo = 1) were applied.
Standardized total isoprenoid emissions during the experiment
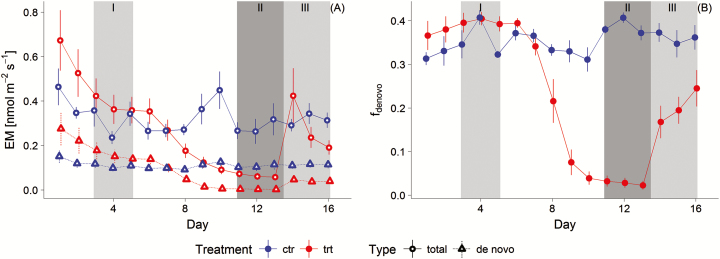
In phase I, total BVOC emission rates (EMtotal) (see Fig. 4A) did not differ significantly between the treatment groups, but a significantly higher fde novo for the trt group was revealed (see Table 4 for statistics).
Figure 4.
(A) Mean daytime (1100 and 1200 h) de novo (triangle) and total (circle) emission rates (EM) of total sum of compounds (black bar) at each day and each group (trt = treatment (N = 4), ctr = control (N = 4)) are displayed. (B) Estimated mean fde novo by the non-linear model for both groups. Error bars represent the standard error.
Table 4.
Mean and standard error (SE) of total emission rates (EMtotal), split into pool and de novo emission rates (EMpool, EMde novo) and the de novo fraction (fde novo) for both groups (trt: treatment, ctr: control) during the three phases (phase I non-stressed: Days 3 to 5, phase II fully drought stressed: Days 11 to 13 and phase III re-watering: Days 14 to 16). EM was standardized to 30 °C and 1000 µmol PAR m−2 s−1 by the mixed correction algorithm. P is showing the significance level of the t-test, while df gives the degree of freedom.
| Parameter | Phase | ctr ± SE | trt ± SE | P | df |
|---|---|---|---|---|---|
| EMtotal (nmol m−2 s−1) | I | 0.32 ± 0.01 | 0.38 ± 0.01 | 0.20 | 44.4 |
| II | 0.28 ± 0.01 | 0.06 ± 0.02 | <0.01 | 26.3 | |
| III | 0.32 ± 0.01 | 0.28 ± 0.02 | 0.45 | 31.0 | |
| EMpool (nmol m−2 s−1) | I | 0.21 ± 0.03 | 0.23 ± 0.02 | 0.58 | 43.0 |
| II | 0.18 ± 0.02 | 0.06 ± 0.01 | <0.01 | 29.1 | |
| III | 0.20 ± 0.02 | 0.24 ± 0.05 | 0.48 | 27.1 | |
| EMde novo (nmol m−2 s−1) | I | 0.11 ± 0.01 | 0.16 ± 0.2 | 0.03 | 34.5 |
| II | 0.11 ± 0.01 | 0 ± 0 | <0.01 | 23.2 | |
| III | 0.11 ± 0.01 | 0.04 ± 0 | <0.01 | 35.7 | |
| f de novo | I | 0.36 ± 0.01 | 0.40 ± 0.01 | 0.02 | 40.6 |
| II | 0.39 ± 0.01 | 0.03 ± 0.01 | <0.01 | 44.0 | |
| III | 0.36 ± 0.02 | 0.2 ± 0.2 | <0.01 | 39.9 |
Increasing water deficit in phase II decreased EMtotal and EMpool of the trt group significantly and EMde novo to almost zero compared to the ctr group emitting constant EMde novo and only marginally decreased EMpool.
In phase III, EMtotal of the trt group increased after recovering and did not differ significantly from the ctr group. In detail, however, fde novo and thus EMde novo were significantly lower than the control, while EMpool was similar. The treatment fde novo recovered only to 50 % of the phase I fraction. On Day 14, drought-stressed trees showed a burst of EMtotal of 0.42 nmol m−2 s−1 compared 0.24 and 0.19 nmol m−2 s−1 at the following Days 15 and 16, which was mainly related to increased EM of Δ3-carene, α-pinene, p-cymene, myrcene and limonene coming from the pool. The split-up of the emissions into the de novo and pool parts clearly revealed that initially high EM as well as some peaks afterwards were due to EMpool (see Fig. 4).
Discussion
Does multi-labelling cause interferences in the %13C?
Overall, the effect by stored 13C was negligible after 18 h and vanished completely after 5 to 6 days for most compounds except p-cymene and showed that multiple short interval labelling is feasible without causing interferences in the %13C by the prior 13C labelling.
In detail, the temporal behaviour in first 18 h complied with the results of Shao et al. (2001). Some of the short-term effects should however be discussed further. Shortly after the labelling stopped, the increased %13C in the target compounds might be explained by lag effects of the system as well as by labelled precursor substances, e.g. geranyl diphosphate, as shown for Scots pine by Ghirardo et al. (2010). Another possibility is that some of the compounds synthesized during 13C labelling were stored in non-specified short-term pools (Noe et al. 2006). The slow return to pre-labelling %13C levels over 5 to 6 days may be due to residual 13C fixed in primary metabolites which are then used in the alternative MT synthesis pathways (see Brilli et al. 2007). This reuse of 13C labelled carbohydrates explained the 2-day delayed increase of %13C for Δ3-carene, which is obviously a compound not related to current photosynthesis.
How large are the de novo fractions of different MT compounds?
The multiple 13C labelling showed that the fraction of the de novo emission can range between 0 and 1, depending on the specific compound. This high variability of fde novo/%13C between single compound species was also reported for other pine and conifer species by Shao et al. (2001), Kleist et al. (2012), Harley et al. (2014) and Wu et al. (2015).
The determined %13C agreed with studies of Shao et al. (2001), Ghirardo et al. (2010) and Kleist et al. (2012) in case of α-pinene and for the almost non-labelled Δ3-carene. However, the total fde novo was lower in our study (42 % for the control/29 % for the treatment) compared to 58 % reported by Ghirardo et al. (2010). This can be well explained by different shares of de novo/pool emitting plant tissue (e.g. whole tree canopy including stem vs. branch cuvettes on twigs). Further, the different plant parts can differ in their composition (Ghirardo et al. 2010), size of storage pools (Manninen et al. 2002) and their capability for synthesis of new compounds due to access to free carbon/precursor pools. Many of the lower labelled compounds, such as α-pinene and β-pinene, are known to reside in large amounts in storage pools such as needle resin ducts (see, e.g., Hiltunen and Laakso 1995; Manninen et al. 2002; Achotegui-Castells et al. 2013). Thus, the lower %13C can be explained by the high pool fraction interfering with the labelled signature.
Besides, there are also methodological differences with unknown effects on total fde novo: our study had to discard compounds with a low abundance as well as co-eluting compounds, whereas Ghirardo et al. (2010) used a PTR-MS, measuring the sum of MTs and thus can report the total de novo fraction.
The reason for certain compounds to predominantly originate from pools might be linked to permanently required functions (see Langenheim 1994; Cheng et al. 2007), whereas de novo dominated compounds are only required to combat shortly induced stress (Loreto et al. 2010; Niinemets 2010).
How strongly do trees respond to drought stress and to stress relief by re-watering?
The drought application resulted in a decline of A, E and the purely de novo emitted 1,8-cineole, but also of EMtotal. This drought-induced reduction of MT emission of Scots pine was equally observed by Wu et al. (2015) for 1,8-cineole and by Lüpke et al. (2016) for EMtotal. This response was also confirmed for other tree species (e.g. Llusià, and Peñuelas 1998; Llusià et al. 2011; Šimpraga et al. 2011; Lüpke et al. 2017). The short period of re-watering led only to a partial recovery of A and E compared to a preceding experiment on the same species (Lüpke et al. 2016) with similar environmental settings, gas exchange rates during stress and re-watering were lower, but within the same magnitude. This difference might be related to different soil material used (complete organic vs. predominantly sandy material in this study), plant age, investigated months (end of August and October vs. July) and different provenances studied (Italy, Spain and North-Eastern Germany vs. Southern Germany).
The MT 1,8-cineole which is almost exclusively synthesised de novo (~77.4 labelled %13C) (see also of Ghirardo et al. 2010; Kleist et al. 2012; Wu et al. 2015) revealed to be a good indicator compound for the drought impacts via reduced gas exchange. During the 13C labelling on Day 8, when several treated plants responded already to shortages in SWC by, e.g., a drop in photosynthesis to 1/6, 1,8-cineole showed a reduction of %13C to 71.5 % and drop in EM to 1/3. In the fully drought-stressed phase, EM of 1,8-cineole was almost zero. Brilli et al. (2007) showed a similar reduction for isoprene emission on drought-stressed poplar trees despite a much reduced photosynthesis and related the ongoing isoprene emission to using stored carbon. An effect of stomatal conductance on EM of MTs is not very likely, since partial pressures of the internal leaf gaseous space increase with stomata closure, and consequently the EM flux is sustained (Niinemets and Reichstein 2003; Harley et al. 2014).
During re-watering, EM of 1,8-cineole showed a lagged and moderated response with only reaching 25 % of the initial rates, whereas photosynthesis had already recovered to 77 % showing that re-available carbon from photosynthesis is not used immediately in 1,8-cineol synthesis. Preferably, recently allocated carbon might then be used for other processes than MT synthesis such as maintenance, growth (Wiley and Helliker 2012) or refilling of carbon pools (Brilli et al. 2007).
Quite interesting is the pronounced spike in pool emission on Day 14 after re-watering, a phenomenon which has been described by Brilli et al. (2007) for isoprene. Here, the increased EM during re-watering was related to still active alternative pathways/enzymes which based on stored and newly synthesised carbon allowing the quick production and release of terpenoids during the first re-watering phase. However, such a emission burst might result from a rapid change of the MT partial pressure until a new equilibrium between outside air and leaf gas space was reached after the initial stomata opening after re-watering (Niinemets and Reichstein 2003). Further, due to increase of the volume of water leading organs during re-watering the oleoresin pressure in woody plant parts is increased shortly (Rissanen et al. 2016) and thus increasing emissions from stored MTs.
How strongly does the drought stress affect the de novo emissions and at which point in time emissions originates only from pools?
In case of EMtotal, drought stress reduced both pool and de novo emission evident on labelling Day 8 and during full drought within phase II, with much stronger effects on the de novo part. The major reduction of the de novo emission is tightly linked to the reduction of recently synthesized carbon by photosynthesis (Brilli et al. 2007), while the still ongoing de novo emission is sustained by carbon from other sources through alternative pathways (Kreuzwieser et al. 2002; Schnitzler et al. 2004; Ghirardo et al. 2014).
The reduction of pool emissions by drought might be caused by a de novo part which could not be assessed by 13C-pulse-labelling and was thus included in the pool part and/or by a decreased xylem water potential in the woody parts affecting EM as shown by Rissanen et al. (2016) and Vanhatalo et al. (2015) for stem emissions. A similar effect was revealed in our study for the pure pool emitted Δ3-carene, which increased during the re-watering phase probably caused by refilling the xylem.
Standardization of emission rates
In order to compare MT emissions from the control and drought-impacted groups, a mixed emission correction algorithm was applied with a non-linear model to predict fde novo for each individual compound. A fixed correction on the EMtotal was rated as not suitable for Scots pine showing chemo species with different compound compositions (see, e.g., Bäck et al. 2012; Lüpke et al. 2016). In its current implementation the applied correction method is a quite coarse approach, which could be improved using more measurements especially in the low fde novo range during drought. Additionally, also other pure de novo compounds, such as isoprene or MBO as suggested by Ghirardo et al. (2010) or Harley et al. (2014), should be considered for this approach, especially in conditions where 1,8-cineole emissions are very low.
Caveats in the experiment
Some caveats should be discussed, although the effect on the results is likely to be minor. The Tree DEMON was designed for 5 L pots, likely reducing plant growth in long-term experiments (see, e.g., Passioura 2006; Poorter et al. 2012), but in case of this study it was advantageous by achieving drought conditions faster. The varying plant water uptake led to some SWC variations. However, this issue was eased by the analysis of the relation between SWC and gas exchange of CO2 revealing a constant gas exchange of Scots pine until a SWC threshold of 0.08 m3 m−3. As reported in other studies (Loreto et al. 2000; Komenda and Koppmann 2002) mechanical plant damage, which is difficult to avoid even with careful handling, enhanced pool emissions in the beginning. Small variations in temperature caused by differences in transpiration did not affect the results due to temperature correction of the emission rate. Thus, the technical limitations were solved sufficiently and should not have affected any of the results.
The largest caveat is linked to the inherent variability within the specimen of the studied provenance. Four replicates in each treatment group comprising even two chemo species may have influenced the results of single compound emissions. To account for this observation, the drought effect was discussed on the total de novo and pool emissions and the purely de novo emitted 1,8-cineole.
Future research
In this study, chemo species led to a high variability for specific single compound emissions. Since it is so far unknown if chemo species have different de novo shares, future studies should first screen a larger set of trees and split drought stress and 13C labelling experiments by chemo species.
In future studies, the tipping point between moderate stress and full drought response should be higher temporally resolved to improve modelling of such events. Further, to observe full emissions recovery a longer recovery period is needed in future studies.
A so far less and difficult to investigate issue is how adult tree and young tree emissions respond to similar applied drought, since both have different access to stored carbon and water and show a different plant growth dynamic. This would require a field site with both age types available and the possibility to investigate both age groups under similar conditions, a technical challenge and huge effort.
Conclusions
In our study, for the first time, information from 13C labelling was used to improve the standardization of emission rates of single compound emissions with varying de novo synthesis. This method could provide a methodological improvement also for other mixed type emitting tree species; however, due to the large variation of emitted compounds and their de novo fractions individual measurements are needed.
The experiment revealed that both pool and de novo emissions were affected by drought stress; however, fde novo highly varied with compound and physiological state. Since 13C labelling is expensive and complex, it was compared with the night-day difference method. This method was more prone to disturbances and should be applied only if environmental control can be regulated very precisely. Drought stress reduced MT emission from de novo synthesis more than from pools; however, the emission of single compounds with higher de novo fractions as such and with protective functions against drought was preferentially supported. A more detailed investigation on different plant parts, optimally simultaneously, during drought stress application, will allow a better understanding of the different MT pools and responses to drought.
Supporting Information
The following additional information is available in the online version of this article—
Figure S1. Photo of the Tree DEMON.
Figure S2. Relationship between soil water content and gas exchange.
Figure S3. Non-linear model fits for single compound fde novo and 1,8-cineol and fitting parameters.
Table S1. Mixing ratios of single compound of the calibration gas standard and target compound.
Sources of Funding
This study was financed by the European Research Council under the European Union Seventh Framework Program (FP7/2007-2013/ERC grant agreement No. 282250). This work was supported by the German Research Foundation (DFG) and the Technical University of Munich (TUM) in the framework of the Open Access Publishing Program.
Contributions by the Authors
M.L. planned and ran the experiment and performed the data analysis. M.L. was the main author of this manuscript. R.S., M.L. and A.M. conceived the study, participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest
None declared.
Supplementary Material
Acknowledgements
Special thanks to J. B. Cabrera for helping with the experimental work and analysis within his study project. Further thanks to M. Matiu for helping on statistical questions and to H. Seidel for helping with the setup and care of the plants within the greenhouse.
Literature Cited
- Aaltonen H, Lindén A, Heinonsalo J, Biasi C, Pumpanen J. 2017. Effects of prolonged drought stress on Scots pine seedling carbon allocation. Tree Physiology 37:418–427. [DOI] [PubMed] [Google Scholar]
- Achotegui-Castells A, Llusià J, Hódar JA, Peñuelas J. 2013. Needle terpene concentrations and emissions of two coexisting subspecies of Scots pine attacked by the pine processionary moth (Thaumetopoea pityocampa). Acta Physiologiae Plantarum 35:3047–3058. [Google Scholar]
- Allen CD, Macalady AK, Chenchouni H, Bachelet D, McDowell N, Vennetier M, Kitzberger T, Rigling A, Breshears DD, Ted Hogg EH, Gonzalez P, Fensham R, Zhang Z, Castro J, Demidova N, Lim JH, Allard G, Running SW, Semerci A, Cobb N. 2010. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. Forest Ecology Management 259:660–684. [Google Scholar]
- Bäck J, Aalto J, Henriksson M, Hakola H, He Q, Boy M. 2012. Chemodiversity of a Scots pine stand and implications for terpene air concentrations. Biogeosciences 9:689–702. [Google Scholar]
- Brilli F, Barta C, Fortunati A, Lerdau M, Loreto F, Centritto M. 2007. Response of isoprene emission and carbon metabolism to drought in white poplar (Populus alba) saplings. The New Phytologist 175:244–254. [DOI] [PubMed] [Google Scholar]
- Caemmerer S von, Farquhar GD. 1981. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376–387. [DOI] [PubMed] [Google Scholar]
- Calfapietra C, Fares S, Manes F, Morani A, Sgrigna G, Loreto F. 2013. Role of biogenic volatile organic compounds (BVOC) emitted by urban trees on ozone concentration in cities: a review. Environmental Pollution 183:71–80. [DOI] [PubMed] [Google Scholar]
- Carnicer J, Coll M, Ninyerola M, Pons X, Sanchez G, Penuelas J. 2011. Widespread crown condition decline, food web disruption, and amplified tree mortality with increased climate change-type drought. Proceedings of the National Academy of Sciences of the United States of America 108:1474–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AX, Lou YG, Mao YB, Lu S, Wang LJ, Chen XY. 2007. Plant terpenoids: biosynthesis and ecological functions. Journal of Integrative Plant Biology 49:179–186. [Google Scholar]
- Dawson TE, Mambelli S, Plamboeck AH, Templer PH, Tu KP. 2002. Stable isotopes in plant ecology. Annual Review of Ecology and Systematics 33:507–559. [Google Scholar]
- Delwiche CF, Sharkey TD. 1993. Rapid appearance of 13C in biogenic isoprene when 13CO2 is fed to intact leaves. Plant, Cell & Environment 16:587–591. [Google Scholar]
- Emanuelsson EU, Hallquist M, Kristensen K, Glasius M, Bohn B, Fuchs H, Kammer B, Kiendler-Scharr A, Nehr S, Rubach F, Tillmann R, Wahner A, Wu H-C, Mentel TF. 2013. Formation of anthropogenic secondary organic aerosol (SOA) and its influence on biogenic SOA properties. Atmospheric Chemistry and Physics 13:2837–2855. [Google Scholar]
- Fäldt J, Solheim H, Långström B, Borg-Karlson AK. 2006. Influence of fungal infection and wounding on contents and enantiomeric compositions of monoterpenes in phloem of Pinus sylvestris. Journal of Chemical Ecology 32:1779–1795. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Ehleringer JR, Hubick KT. 1989. Carbon isotope discrimination and photosynthesis. Annual Review of Plant Physiology and Plant Molecular Biology 40:503–537. [Google Scholar]
- Gao Y, Jin Y-J, Li H-D, Chen H-J. 2005. Volatile organic compounds and their roles in bacteriostasis in five conifer species. Journal of Integrative Plant Biology 47:499–507. [Google Scholar]
- Ghirardo A, Koch K, Taipale R, Zimmer I, Schnitzler JP, Rinne J. 2010. Determination of de novo and pool emissions of terpenes from four common boreal/alpine trees by 13CO2 labelling and PTR-MS analysis. Plant, Cell & Environment 33:781–792. [DOI] [PubMed] [Google Scholar]
- Ghirardo A, Wright LP, Bi Z, Rosenkranz M, Pulido P, Rodríguez-Concepción M, Niinemets Ü, Brüggemann N, Gershenzon J, Schnitzler JP. 2014. Metabolic flux analysis of plastidic isoprenoid biosynthesis in poplar leaves emitting and nonemitting isoprene. Plant Physiology 165:37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassmann J, Hippeli S, Spitzenberger R, Elstner EF. 2005. The monoterpene terpinolene from the oil of Pinus mugo L. in concert with α-tocopherol and β-carotene effectively prevents oxidation of LDL. Phytomedicine 12:416–423. [DOI] [PubMed] [Google Scholar]
- Guenther A. 1997. Seasonal and spatial variations in natural volatile organic compound emissions. Ecological Applications 7:34–45. [Google Scholar]
- Guenther A, Hewitt CN, Erickson D, Fall R, Geron C, Graedel T, Harley P, Klinger L, Lerdau M, Mckay WA, Pierce T, Scholes B, Steinbrecher R, Tallamraju R, Taylor J, Zimmerman P. 1995. A global model of natural volatile organic compound emissions. Journal of Geophysical Research 100:8873. [Google Scholar]
- Guenther AB, Jiang X, Heald CL, Sakulyanontvittaya T, Duhl T, Emmons LK, Wang X. 2012. The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN2.1): an extended and updated framework for modeling biogenic emissions. Geoscientific Model Development 5:1471–1492. [Google Scholar]
- Harley P, Eller A, Guenther A, Monson RK. 2014. Observations and models of emissions of volatile terpenoid compounds from needles of ponderosa pine trees growing in situ: control by light, temperature and stomatal conductance. Oecologia 176:35–55. [DOI] [PubMed] [Google Scholar]
- Hiltunen R, Laakso I. 1995. Gas chromatographic analysis and biogenetic relationships of monoterpene enantiomers in Scots pine and juniper needle oils. Flavour and Fragrance Journal 10:203–210. [Google Scholar]
- Holopainen JK, Gershenzon J. 2010. Multiple stress factors and the emission of plant VOCs. Trends in Plant Science 15:176–184. [DOI] [PubMed] [Google Scholar]
- Holzke C, Hoffmann T, Jaeger L, Koppmann R, Zimmer W. 2006. Diurnal and seasonal variation of monoterpene and sesquiterpene emissions from Scots pine (Pinus sylvestris L.). Atmospheric Environment 40:3174–3185. [Google Scholar]
- Janson R. 1992. Monoterpene concentrations in and above a forest of Scots pine. Journal of Atmospheric Chemistry 14:385–394. [Google Scholar]
- Kesselmeier J, Staudt M. 1999. Biogenic volatile organic compounds (VOC): an overview on emission, physiology and ecology. Journal of Atmospheric Chemistry 33:23–88. [Google Scholar]
- Kleist E, Mentel TF, Andres S, Bohne A, Folkers A, Kiendler-Scharr A, Rudich Y, Springer M, Tillmann R, Wildt J. 2012. Irreversible impacts of heat on the emissions of monoterpenes, sesquiterpenes, phenolic BVOC and green leaf volatiles from several tree species. Biogeosciences 9:5111–5123. [Google Scholar]
- Komenda M, Koppmann R. 2002. Monoterpene emissions from Scots pine (Pinus sylvestris): field studies of emission rate variabilities. Journal of Geophysical Research 107:ACH 1-1–ACH 1-13. [Google Scholar]
- Kreuzwieser J, Graus M, Wisthaler A, Hansel A, Rennenberg H, Schnitzler JP. 2002. Xylem-transported glucose as an additional carbon source for leaf isoprene formation in Quercus robur. New Phytologist 156:171–178. [DOI] [PubMed] [Google Scholar]
- Langenheim JH. 1994. Higher plant terpenoids: a phytocentric overview of their ecological roles. Journal of Chemical Ecology 20:1223–1280. [DOI] [PubMed] [Google Scholar]
- Llusià J, Peñuelas J. 1998. Changes in terpene content and emission in potted Mediterranean woody plants under severe drought. Canadian Journal of Botany 76:1366–1373. [Google Scholar]
- Llusià J, Peñuelas J, Alessio GA, Ogaya R. 2011. Species-specific, seasonal, inter-annual, and historically-accumulated changes in foliar terpene emission rates in Phillyrea latifolia and Quercus ilex submitted to rain exclusion in the Prades Mountains (Catalonia). Russian Journal of Plant Physiology 58:126–132. [Google Scholar]
- Loreto F, Ciccioli P, Cecinato A, Brancaleoni E, Frattoni M, Fabozzi C, Tricoli D. 1996. Evidence of the photosynthetic origin of monoterpenes emitted by Quercus ilex L. leaves by 13C labeling. Plant Physiology 110:1317–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loreto F, Nascetti P, Graverini A, Mannozzi M. 2000. Emission and content of monoterpenes in intact and wounded needles of the Mediterranean Pine, Pinus pinea. Functional Ecology 14:589–595. [Google Scholar]
- Loreto F, Schnitzler JP. 2010. Abiotic stresses and induced BVOCs. Trends in Plant Science 15:154–166. [DOI] [PubMed] [Google Scholar]
- Lüpke M, Leuchner M, Steinbrecher R, Menzel A. 2016. Impact of summer drought on isoprenoid emissions and carbon sink of three Scots pine provenances. Tree Physiology 36:1382–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüpke M, Steinbrecher R, Leuchner M, Menzel A. 2017. The tree drought emission monitor (Tree DEMON), an innovative system for assessing biogenic volatile organic compounds emission from plants. Plant Methods 13:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macchioni F, Cioni PL, Flamini G, Morelli I, Maccioni S, Ansaldi M. 2003. Chemical composition of essential oils from needles, branches and cones of Pinus pinea, P. halepensis, P. pinaster and P. nigra from central ltaly. Flavour and Fragrance Journal 18:139–143. [Google Scholar]
- Manninen AM, Tarhanen S, Vuorinen M, Kainulaine P. 2002. Comparing the variation of needle and wood terpenoids in Scots pine provenances. Journal of Chemical Ecology 28:211–228. [DOI] [PubMed] [Google Scholar]
- Manninen A-M, Vuorinen M, Holopainen JK. 1998. Variation in growth, chemical defense, and herbivore resistance in Scots pine provenances. Journal of Chemical Ecology 24:1315–1331. [Google Scholar]
- Mediavilla S, Escudero A. 2004. Stomatal responses to drought of mature trees and seedlings of two co-occurring Mediterranean oaks. Forest Ecology and Management 187:281–294. [Google Scholar]
- Mumm R, Hilker M. 2006. Direct and indirect chemical defence of pine against folivorous insects. Trends in Plant Science 11:351–358. [DOI] [PubMed] [Google Scholar]
- Niinemets Ü. 2010. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: past stress history, stress interactions, tolerance and acclimation. Forest Ecology and Management 260:1623–1639. [Google Scholar]
- Niinemets Ü, Kuhn U, Harley PC, Staudt M, Arneth A, Cescatti A, Ciccioli P, Copolovici L, Geron C, Guenther A, Kesselmeier J, Lerdau MT, Monson RK, Peñuelas J. 2011. Estimations of isoprenoid emission capacity from enclosure studies: measurements, data processing, quality and standardized measurement protocols. Biogeosciences 8:2209–2246. [Google Scholar]
- Niinemets Ü, Reichstein M. 2003. Controls on the emission of plant volatiles through stomata: differential sensitivity of emission rates to stomatal closure explained. Journal of Geophysical Research 108:ACH 2-1–ACH 2-17. [Google Scholar]
- Noe S, Ciccioli P, Brancaleoni E, Loreto F, Niinemets U. 2006. Emissions of monoterpenes linalool and ocimene respond differently to environmental changes due to differences in physico-chemical characteristics. Atmospheric Environment 40:4649–4662. [Google Scholar]
- Passioura JB. 2006. The perils of pot experiments: the perils of pot experiments. Functional Plant Biology 33:1075. [DOI] [PubMed] [Google Scholar]
- Petrakis PV, Tsitsimpikou C, Tzakou O, Couladis M, Vagias C, Roussis V. 2001. Needle volatiles from five Pinus species growing in Greece. Flavour and Fragrance Journal 16:249–252. [Google Scholar]
- Poorter H, Bühler J, van Dusschoten D, Climent J, Postma JA. 2012. Pot size matters: a meta-analysis of the effects of rooting volume on plant growth. Functional Plant Biology 39:839. [DOI] [PubMed] [Google Scholar]
- R Core Team 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; https://www.R-project.org/ (15 March 2017). [Google Scholar]
- Rissanen K, Hölttä T, Vanhatalo A, Aalto J, Nikinmaa E, Rita H, Bäck J. 2016. Diurnal patterns in Scots pine stem oleoresin pressure in a boreal forest. Plant, Cell & Environment 39:527–538. [DOI] [PubMed] [Google Scholar]
- Royo A, Gil L, Pardos JA. 2001. Effect of water stress conditioning on morphology, physiology and field performance of Pinus halepensis Mill. seedlings. New Forests 21:127–140. [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH image to imagej: 25 years of image analysis. Nature Methods 9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler JP, Graus M, Kreuzwieser J, Heizmann U, Rennenberg H, Wisthaler A, Hansel A. 2004. Contribution of different carbon sources to isoprene biosynthesis in poplar leaves. Plant Physiology 135:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh G, Heiden AC, Hoffmann TH, Kahl J, Rockel P, Rudolph J, Wildt J. 1997. Emissions of volatile organic compounds from sunflower and beech: dependence on temperature and light intensity. Journal of Atmospheric Chemistry 27:291–318. [Google Scholar]
- Schürmann W, Ziegler H, Kotzias D, Schänwitz R, Steinbrecher R. 1993. Emission of biosynthesized monoterpenes from needles of Norway Spruce. Naturwissenschaften 80:276–278. [Google Scholar]
- Šimpraga M, Verbeeck H, Demarcke M, Joó É, Pokorska O, Amelynck C, Schoon N, Dewulf J, van Langenhove H, Heinesch B, Aubinet M, Laffineur Q, Müller JF, Steppe K. 2011. Clear link between drought stress, photosynthesis and biogenic volatile organic compounds in Fagus sylvatica L. Atmospheric Environment 45:5254–5259. [Google Scholar]
- Shao M, Czapiewski KV, Heiden AC, Kobel K, Komenda M, Koppmann R, Wildt J. 2001. Volatile organic compound emissions from Scots pine: mechanisms and description by algorithms. Journal of Geophysical Research 106:20483–20491. [Google Scholar]
- Spinoni J, Naumann G, Carrao H, Barbosa P, Vogt J. 2014. World drought frequency, duration, and severity for 1951–2010. International Journal of Climatology 34:2792–2804. [Google Scholar]
- Vanhatalo A, Chan T, Aalto J, Korhonen JF, Kolari P, Hölttä T, Nikinmaa E, Bäck J. 2015. Tree water relations can trigger monoterpene emissions from Scots pine stems during spring recovery. Biogeosciences 12:5353–5363. [Google Scholar]
- Wiley E, Helliker B. 2012. A re-evaluation of carbon storage in trees lends greater support for carbon limitation to growth. The New Phytologist 195:285–289. [DOI] [PubMed] [Google Scholar]
- Wu C, Pullinen I, Andres S, Carriero G, Fares S, Goldbach H, Hacker L, Kasal T, Kiendler-Scharr A, Kleist E, Paoletti E, Wahner A, Wildt J, Mentel TF. 2015. Impacts of soil moisture on de novo monoterpene emissions from European beech, Holm oak, Scots pine, and Norway spruce. Biogeosciences 12:177–191. [Google Scholar]
- Yassaa N, Song W, Lelieveld J, Vanhatalo A, Bäck J, Williams J. 2012. Diel cycles of isoprenoids in the emissions of Norway spruce, four Scots pine chemotypes, and in Boreal forest ambient air during HUMPPA-COPEC-2010. Atmospheric Chemistry and Physics Discussions 12:10425–10460. [Google Scholar]
- Yu J, Cocker DR III, Griffin RJ, Flagan RC, Seinfeld JH. 1999. Gas-phase ozone oxidation of monoterpenes: gaseous and particulate products. Journal of Atmospheric Chemistry 34:207–258. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.