Abstract
Background
HIV-infected individuals have an increased risk of avascular bone necrosis (AVN). Antiretroviral therapy (ART) and particularly protease inhibitors (PI) have been implicated as a risk factor. We aimed to study the associations of ART with the occurrence of AVN among Swiss HIV Cohort Study participants (SHCS).
Methods
We used incidence density sampling to perform a case control study within the Swiss HIV Cohort Study (SHCS) comparing prospectively collected AVN cases and controls by conditional logistic regression analysis. To evaluate the effect of ART, multivariable models were adjusted for HIV transmission risk group, age, alcohol consumption, use of corticosteroids, CD4 nadir, maximum viral load, and pancreatitis.
Results
We compared 74 AVN cases and 145 controls. Associations with AVN were shown for heterosexual HIV acquisition (odds ratio [OR], 3.4; 95% confidence interval [CI], 1.1–10), alcohol consumption (OR, 2.7; 95% CI, 1.3–5.7), and hyperlipidemia (OR, 3.6; 95% CI, 1.4–9.6). After adding ART substances to the multivariable base model, there was evidence of an association for treatment with tenofovir (TDF) >1 year (OR, 4.4; 95% CI, 1.4–14) with AVN. Neither exposure to specific frequently prescribed ART combinations or ART drug classes nor cumulative ART exposure showed any associations with AVN.
Conclusions
In the HIV-infected population, a combination of risk factors such as heterosexual HIV acquisition, moderate to severe alcohol intake, and hyperlipidemia seem to contribute to AVN. ART does not seem to be a relevant risk factor for AVN. The association of prolonged TDF exposure with AVN needs to be confirmed.
Keywords: antiretroviral therapy, avascular bone necrosis, HIV, tenofovir
With the introduction of modern antiretroviral therapy (ART), HIV infection has become a chronic condition, with the HIV-infected population facing potential long-term drug toxicities and increasing co- and multimorbidity, along with the natural aging process [1, 2]. Furthermore, certain medical conditions are considerably more prevalent in HIV-infected persons compared with the general population [3, 4], including avascular bone necrosis (AVN) [5, 6]. The pathogenesis of AVN seems to be related to ischemia of bone, with established risk factors including corticosteroid use, alcohol consumption, smoking, hyperlipidemia, hemodialysis, sickle cell disease, pancreatitis, collagen vascular disease, irradiation therapy, and hypercoagulability [5–9].
Among HIV-positive persons, AVN incidence is up to 100 times higher than in the general population, and, rarely, AVN occurs at multiple sites [7, 8, 10]. It remains unclear to what extent this is related to increased physician awareness of AVN [5, 6] or to prolonged survival of HIV-infected persons [11]. Along with other established risk factors, ART and especially protease inhibitors (PIs) have been implicated as risk factors for AVN, although by unknown mechanisms. Due to the rarity of AVN, no conclusive link has emerged between HIV, ART, and AVN [6, 8–10, 12]. We aimed to study the association of different ART classes (PIs, non-nucleoside reverse transcriptase inhibitors [NNRTI], nucleoside reverse transcriptase inhibitor [NRTI]) and individual ART agents with the occurrence of AVN among Swiss HIV Cohort Study participants (SHCS). Extending our own series of 26 AVN cases published in 2004 [6], we now offer an updated analysis, with additional prospectively recorded AVN cases including detailed information on longitudinal alcohol intake and illicit drug exposure available for all SHCS participants.
MATERIALS AND METHODS
Data Source
The Swiss HIV Cohort Study (SHCS) is an observational study representative of >70% of adult HIV-infected persons in Switzerland. At 6-monthly follow-up visits, demographic, clinical, laboratory, and ART information has been prospectively recorded since 1988 [13]. In addition, the SHCS collects information on comorbidities with central adjudication within the Data collection on Adverse events of Anti-HIV Drugs (D:A:D) Study framework [14]. Detailed information on bone-related events (AVN, osteoporosis, fractures with/without adequate trauma and DEXA scan) has been collected since 2008. The study was approved by local ethics committees, and written informed consent was obtained from all participants.
Definitions
There is no operational case definition for AVN in the medical literature. In our study, 67.6% AVN cases were diagnosed with magnetic resonance imaging (MRI) and the remaining cases with scintigraphy or x-ray. Body mass index (BMI; kg/m2) was categorized into <18.5 (underweight), 18.5–24.9 (normal), 25–29.9 (overweight), and ≥30 (obese) [15]. Hyperlipidemia was defined as serum cholesterol level >5 mmol/L and/or a triglyceride level >2 mmol/L. Alcohol use was stratified according to the World Health Organization definitions of severe (female >40 g/d, male >60 g/d), moderate (female 20–40 g/d, male 40–60 g/d), and light (female <20 g/d, male <40 g/d). We distinguished 2 smoking categories (nonsmokers and ever/current smokers), and among the latter the number of pack-years was recorded. For cases and controls, information on the use of corticosteroids (yes/no, use of corticosteroids ≥3 months, dosage of corticosteroids) [16, 17], testosterone (yes/no), and anabolic steroids (yes/no) was obtained from medical records.
Information on HIV infection included presumed mode of HIV transmission; era of HIV diagnosis; duration of HIV infection at diagnosis of AVN; CD4 cell count and log10 HIV RNA at diagnosis of AVN; nadir CD4 cell count; log10 HIV RNA maximum value and exposure to drug classes including protease inhibitors; non-nucleoside reverse transcriptase inhibitor (NNRTI); nucleoside reverse transcriptase inhibitor (NRTI)– and integrase inhibitor (INI)–based ART combinations; individual drugs including didanosine (DDI), stavudine (d4T), zidovudine (AZT), abacavir (ABC), lamivudine (3TC), emtricitabine (FTC), tenofovir (TDF), saquinavir (SQV), ritonavir (RTV), nelfinavir (NFV), lopinavir (LPV), indinavir (IDV), darunavir (DRV), atazanavir (ATV), nevirapine (NVP), efavirenz (EFV), and raltegravir (RGV); and most commonly prescribed drug regimens (3TC/AZT/LPV/RTV, FTC/TDF/LPV/RTV, 3TC/ABC/LPV/RTV, FTC/TDF/ATV/RTV, FTC/TDF/DRV/RTV, FTC/TDF/EFV) in years.
Study Participants
Twenty-five out of the 26 patients from our 2004 publication on AVN [6] and 55 additional prospective cases of AVN were identified within the SHCS. Since our first analysis, AVN cases have accumulated 10 additional years of ART exposure with a large number of individual drugs. We performed incidence density sampling to identify controls. In detail: For each case with confirmed AVN, we randomly selected SHCS participants without any signs of AVN and matched these controls for gender, SHCS registration date (within –6/+12 months of the registration of corresponding cases) and last follow-up date (last follow-up date after the date of AVN diagnosis of the corresponding case). Medical charts of both cases and controls were reviewed by the treating physician using a standardized questionnaire to validate the AVN diagnoses and complete risk factors, which were not part of the standard SHCS questionnaires. These additional risk factors included use of megestrol acetate, testosterone, corticosteroids, hypercoagulability, pulmonary embolism, deep vein thrombosis, pancreatitis, sickle cell anemia, radiation, collagenosis, and hemodialysis. After excluding unvalidated cases and their respective controls, we analyzed 74 well-documented cases of AVN and 145 controls (Supplementary Figure 1).
Statistical Analysis
Characteristics were measured at the date of AVN diagnosis in cases and at the SHCS visit closest to this date in controls. Differences between cases and controls were analyzed by the chi-square test for categorical variables and by the Kruskal-Wallis test for continuous variables. We assessed factors associated with AVN by the use of uni- and multivariable conditional logistic regression analysis. Factors with a P < .2 were included in the multivariable models. Final models were adjusted for HIV transmission risk group, age, alcohol consumption, use of corticosteroids, CD4 nadir, maximum viral load, pancreatitis, hyperlipidemia, smoking >20 pack-years, and use of testosterone. We used Stata/SE (version 14.2, StataCorp, College Station, Texas) for analyses.
RESULTS
We analyzed 219 SHCS participants, 74 with AVN and 145 controls. Of 74 AVN cases, 4 (5%) were diagnosed before 1997, 20 (27%) between 1997 and 2002, and 50 (68%) between 2002 and 2014. AVN was located in the femoral head in 59 (80%) participants, in the shoulder in 3 (4%), and in another bone in 11 (15%). One participant concomitantly had AVN in the shoulder and in the femoral head.
Baseline Characteristics
Characteristics of participants are shown in Table 1. Compared with controls, participants with AVN were more likely to be intravenous drug users (IDUs) or to have acquired HIV heterosexually. In addition, they were more likely to have used corticosteroids for more than 3 months, to have hyperlipidemia, to drink alcohol, or to have experienced pancreatitis in the past; 54% of the cases and 42% of the controls were smokers with >20 pack-years. The median durations of HIV infection among cases and controls were 12.6 years (interquartile range [IQR], 5.4–19 years) and 13 years (IQR, 7.0–18 years), respectively. There was a trend for lower nadir CD4 cell counts among cases compared with controls. Median exposure time to NRTI-, PI-, or NNRTI-based ART regimens was similar in both groups. Compared with controls, previous use of 3TC and TDF was more common among AVN cases (P < .05) (Table 2).
Table 1.
Characteristics of Participants With Avascular Bone Necrosis and Controls
| Baseline Characteristics | Total (n = 219) |
Cases With AVN (n = 74) |
Controls (n = 145) |
P Value |
|---|---|---|---|---|
| Sex | ||||
| Male, n (%) | 162 (74) | 55 (74) | 107 (74) | naa |
| Registration date in SHCS, median year (IQR) | 1996 (1991–1999) | 1996 (1991–1999) | 1996 (1991–1999) | naa |
| Follow-up until diagnosis of AVN, median (IQR), y | 8.3 (4.0–15) | 8.5 (3.8–15) | 8.1 (4.2–15) | naa |
| Race | ||||
| White, n (%) | 200 (91) | 66 (89) | 134 (92) | .44 |
| HIV transmission risk group | .003 | |||
| Men who have sex with men, n (%) | 94 (43) | 22 (30) | 72 (50) | |
| Heterosexual, n (%) | 61 (28) | 27 (36) | 34 (23) | |
| Injection drug use, n (%) | 64 (29) | 25 (34) | 39 (27) | |
| Age at diagnosis of AVN, median (IQR), y | 45 (38–53) | 42 (37–52) | 46 (38–53) | .29 |
| Year of diagnosis | .66 | |||
| <1997, n (%) | 12 (5.5) | 4.0 (5.4) | 8.0 (5.5) | |
| 1997–2002, n (%) | 55 (25) | 20 (27) | 35 (24) | |
| >2002, n (%) | 152 (69) | 50 (68) | 102 (70) | |
| Smoking | .08 | |||
| <20 pack-years, n (%) | 118 (54) | 34 (46) | 84 (58) | |
| ≥20 pack-years, n (%) | 101 (46) | 40 (54) | 61 (42) | |
| BMI at diagnosis of AVN, median (IQR), kg/m2 | 24 (23–25) | 24 (23–25) | 24 (23–25) | .72 |
| Alcohol consumption | .001 | |||
| None/light, n (%) | 150 (68) | 40 (54) | 110 (76) | |
| Moderate/severe, n (%) | 69 (32) | 34 (46) | 35 (24) | |
| Comorbidities | ||||
| Diabetes mellitus, n (%) | 19 (8.7) | 5.0 (6.8) | 14 (9.7) | .445 |
| Hypertension, n (%) | 64 (29) | 20 (27) | 44 (30) | .62 |
| Hyperlipidemia, n (%) | 164 (75) | 64 (86) | 100 (69) | .002 |
| Pancreatitis, n (%) | 9.0 (4.1) | 6.0 (8.0) | 3.0 (2.0) | .05 |
| Pulmonary embolism/deep venous thrombosis, n (%) | 18 (8.2) | 8.0 (11) | 10 (6.9) | .308 |
| Radiation therapy, n (%) | 9.0 (4.1) | 2.0 (2.7) | 7.0 (4.8) | .49 |
| Haemodialyis, n (%) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | na |
| Collagenosis, n (%) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | na |
| Sickle cell anemia, n (%) | 0.0 (0.0) | 0.0 (0.0) | 1 .0 (1.0) | na |
| Co-infections | ||||
| CMV-seropositive, n (%) | 169 (79) | 56 (76) | 113 (80) | .441 |
| Toxoplasmosis-seropositive, n (%) | 91 (43) | 30 (41) | 61 (44) | .69 |
| HBs-Ag positive, n (%) | 16 (7.6) | 7.0 (9.7) | 9.0 (6.5) | .289 |
| HCV seropositive, n (%) | 73 (33) | 28 (38) | 45 (31) | .361 |
| Comedications | ||||
| Corticosteroid use, n (%) | 60 (27) | 24 (32) | 36 (25) | .23 |
| Corticosteroid use >3 mo, n (%) | 17 (7.8) | 11 (15) | 6.0 (4.1) | .009 |
| Testosterone/anabolics use, n (%) | 12 (5.5) | 2.0 (2.7) | 10 (6.9) | .24 |
| Megestrol acetate use, n (%) | 0.0 (0.0) | 0.0 (0.0) | 0.0 (0.0) | na |
| AIDS at diagnosis of AVN, n (%) | 71 (32) | 30 (41) | 41 (28) | .093 |
| CD4+ T-cell count | ||||
| CD4 count at diagnosis of AVN, median (IQR), cells/uL | 431 (295–587) | 398 (259–547) | 460 (329–610) | .33 |
| CD4 nadir, median (IQR), cells/uL | 141 (57–250) | 117 (38–200) | 160 (70–265) | .20 |
| CD4 nadir <200 cells/uL, n (%) | 144 (66) | 55 (74) | 89 (61) | .05 |
| HIV-RNA | ||||
| HIV RNA at diagnosis of AVN below detection limit, n (%) | 140 (66) | 45 (63) | 95 (67) | .49 |
| RNA first value, median (IQR), log10 RNA | 4.5 (3.7–5.1) | 4.5 (3.5–5.2) | 4.5 (3.8–5.0) | .52 |
| RNA maximum value, median (IQR), log10 RNA | 5.0 (4.3–5.4) | 5.1 (4.5–5.7) | 5.0 (4.3–5.2) | .17 |
| RNA AUC, median (IQR), log10 copy-years | 40 (24–60) | 43 (25–62) | 39 (24–58) | .89 |
| HIV treatment | ||||
| Duration of HIV infection at diagnosis of AVN, median (IQR), y | 13 (6.2–19) | 13 (5.6–19) | 13 (7.2–19) | .07 |
| Duration of untreated HIV infection at diagnosis of AVN, median (IQR), y | 6.4 (1.5–10) | 6.1 (1.1–9.7) | 6.8 (1.9–11) | .34 |
Alcohol use: severe (female >40 g/d, male >60 g/d), moderate (female 20–40 g/d, male 40–60 g/d), light (female <20 g/d, male <40 g/d). Hyperlipidemia: cholesterol >5 mmol/L and/or triglyceride >2 mmol/L.
Abbreviations: 3TC, lamivudin; ABC, abacavir; ART, antiretroviral treatment; ATV, atazanavir; AVN, avascular necrosis; AZT, zidovudine; d4T, stavudin; DDI, didanosin; DRV, darunavir; EFV, efavirenz; ETC, emtricitabine; FI, fusion inhibitor; HIV, human immunodeficiency virus; IDV, indinavir; IQR, interquartile range; LPV, lopinavir; NFV, nelfinavir; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitor; RGV, raltegravir; RNA, ribonucleic acid; RTV, ritonavir; SQV, saquinavir; TDF, tenofovir.
aWe did not analyze factors that were matched on.
Table 2.
Exposure to Antiretroviral Therapy
| ART Substance, Regimen, or Drug Class | Total | Cases With AVN | Controls | P Valuea | P Valueb |
|---|---|---|---|---|---|
| n = 219 | n = 74 | n = 145 | |||
| ETC | .75 | .82 | |||
| Never, n (%) | 157 (72) | 53 (72) | 104 (72) | ||
| <1 y, n (%) | 15 (6.9) | 4.0 (5.4) | 11 (7.6) | ||
| ≥1 y, n (%) | 47 (21) | 17 (23) | 30 (21) | ||
| DDI | .70 | .93 | |||
| Never, n (%) | 117 (53) | 40 (54) | 77 (53) | ||
| <1 y, n (%) | 35 (16) | 10 (14) | 25 (17) | ||
| ≥1 y, n (%) | 67 (31) | 24 (32) | 43 (30) | ||
| DDC | .36 | .30 | |||
| Never, n (%) | 186 (85) | 66 (89) | 120 (83) | ||
| ≥1 y, n (%) | 15 (6.8) | 3.0 (4.1) | 12 (8.3) | ||
| >1 y, n (%) | 18 (8.2) | 5.0 (6.8) | 13 (9) | ||
| D4T | .57 | .71 | |||
| Never, n (%) | 109 (50) | 39 (53) | 70 (48) | ||
| <1 y, n (%) | 27 (12) | 7.0 (9.5) | 20 (14) | ||
| ≥1 y, n (%) | 83 (38) | 28 (38) | 55 (38) | ||
| AZT | .53 | .11 | |||
| Never, n (%) | 54 (25) | 16 (22) | 38 (26) | ||
| <1 y, n (%) | 34 (16) | 14 (19) | 20 (14) | ||
| ≥1 y, n (%) | 131 (60) | 44 (59) | 87 (60) | ||
| ABC | .59 | .036 | |||
| Never, n (%) | 150 (68) | 54 (73) | 96 (66) | ||
| <1 y, n (%) | 14 (6.4) | 4.0 (5.4) | 10 (6.9) | ||
| ≥1 y, n (%) | 55 (25) | 16 (22) | 39 (27) | ||
| 3TC | .011 | .044 | |||
| Never, n (%) | 49 (22) | 11 (15) | 38 (26) | ||
| <1 y, n (%) | 17 (7.8) | 10 (14) | 7.0 (4.8) | ||
| ≥1 y, n (%) | 153 (70) | 53 (72) | 100 (69) | ||
| TDF | .032 | .34 | |||
| Never, n (%) | 123 (56) | 36 (49) | 87 (60) | ||
| <1 y, n (%) | 17 (7.8) | 6.0 (8.1) | 11 (7.6) | ||
| ≥1 y, n (%) | 79 (36) | 32 (43) | 47 (32) | ||
| SQV | .77 | .93 | |||
| Never, n (%) | 169 (77) | 56 (76) | 113 (80) | ||
| <1 y, n (%) | 19 (8.7) | 8.0 (11) | 11 (7.6) | ||
| ≥1 y, n (%) | 31 (14) | 10 (14) | 21 (15) | ||
| RTV | .58 | .70 | |||
| Never, n (%) | 186 (85) | 61 (82) | 125 (86) | ||
| <1 y, n (%) | 20 (9.1) | 7.0 (9.5) | 13 (9.0) | ||
| ≥1 y, n (%) | 13 (5.9) | 6.0 (8.1) | 7.0 (4.8) | ||
| NFV | .15 | .09 | |||
| Never, n (%) | 134 (61) | 49 (66) | 85 (59) | ||
| <1 y, n (%) | 24 (11) | 10 (14) | 14 (9.7) | ||
| ≥1 y, n (%) | 61 (28) | 15 (20) | 46 (32) | ||
| LPV | .11 | .34 | |||
| Never, n (%) | 161 (74) | 50 (68) | 111 (77) | ||
| <1 y, n (%) | 16 (7.3) | 9.0 (12) | 7.0 (4.8) | ||
| ≥1 y, n (%) | 42 (19) | 15 (20) | 27 (19) | ||
| IDV | .39 | .95 | |||
| Never, n (%) | 135 (62) | 42 (57) | 93 (64) | ||
| <1 y, n (%) | 28 (13) | 12 (16) | 16 (11) | ||
| ≥1 y, n (%) | 56 (26) | 20 (27) | 36 (25) | ||
| DRV | .98 | .87 | |||
| Never, n (%) | 205 (94) | 69 (93) | 136 (94) | ||
| <1 y, n (%) | 3.0 (1.4) | 1.0 (1.4) | 2.0 (1.4) | ||
| ≥1 y, n (%) | 11 (5.0) | 4.0 (5.4) | 7.0 (4.8) | ||
| ATV | .15 | .25 | |||
| Never, n (%) | 161 (74) | 50 (68) | 111 (77) | ||
| <1 y, n (%) | 13 (5.9) | 4 (5.4) | 9 (6.2) | ||
| ≥1 y, n (%) | 45 (21) | 20 (27) | 25 (17) | ||
| NVP | .13 | .17 | |||
| Never, n (%) | 184 (84) | 67 (91) | 117 (81) | ||
| <1 y, n (%) | 15 (6.8) | 2.0 (2.7) | 13 (9.0) | ||
| ≥1 y, n (%) | 20 (9.1) | 5.0 (6.8) | 15 (10) | ||
| EFV | .72 | .24 | |||
| Never, n (%) | 124 (57) | 40 (54) | 84 (58) | ||
| <1 y, n (%) | 30 (14) | 10 (14) | 20 (14) | ||
| ≥1 y, n (%) | 65 (30) | 24 (32) | 41 (28) | ||
| RGV | .90 | .37 | |||
| Never, n (%) | 209 (95) | 70 (95) | 139 (96) | ||
| <1 y, n (%) | 5.0 (2.3) | 2.0 (2.7) | 3.0 (2.1) | ||
| ≥1 y, n (%) | 5.0 (2.3) | 2.0 (2.7) | 3.0 (2.1) | ||
| 3TC/AZT/LPV/RTV | .24 | .12 | |||
| Never, n (%) | 199 (91) | 64 (87) | 135 (93) | ||
| <1 y, n (%) | 11 (5.0) | 5.0 (6.8) | 6.0 (4.1) | ||
| ≥1 y, n (%) | 9.0 (4.1) | 5.0 (6.8) | 4.0 (2.8) | ||
| ETC/TDF/LPV/RTV | .07 | .32 | |||
| Never, n (%) | 210 (96) | 71 (96) | 139 (96) | ||
| <1 y, n (%) | 4.0 (1.8) | 0.0 (0.0) | 4.0 (2.8) | ||
| ≥1 y, n (%) | 5.0 (2.3) | 3.0 (4.1) | 2.0 (1.4) | ||
| 3TC/ABC/LPV/RTV | .54 | .37 | |||
| Never, n (%) | 213 (97) | 73 (99) | 140 (97) | ||
| <1 y, n (%) | 1.0 (0.5) | 0.0 (0) | 1.0 (0.69) | ||
| ≥1 y, n (%) | 5.0 (2.5) | 1.0 (1.4) | 4.0 (2.8) | ||
| ETC/TDF/ATV/RTV | .75 | .26 | |||
| Never, n (%) | 194 (89) | 64 (86) | 130 (90) | ||
| <1 y, n (%) | 8.0 (3.7) | 3.0 (4.1) | 5.0 (3.4) | ||
| ≥1 y, n (%) | 17 (7.8) | 7.0 (9.5) | 10 (6.9) | ||
| ETC/TDF/DRV/RTV | .30 | .45 | |||
| Never, n (%) | 213 (97) | 73 (99) | 140 (97) | ||
| <1 y, n (%) | 3.0 (1.4) | 1.0 (1.4) | 2.0 (1.4) | ||
| ≥1 y, n (%) | 3.0 (1.4) | 0.0 (0.0) | 3.0 (2.1) | ||
| ETC/TDF/EFV | .92 | .37 | |||
| Never, n (%) | 202 (92 | 69 (93) | 133 (92) | ||
| <1 y, n (%) | 4.0 (1.8 | 1.0 (1.4) | 3.0 (2.1) | ||
| ≥1 y, n (%) | 13 (5.9) | 4.0 (5.4) | 9.0 (6.2) | ||
| 3TC/ABC/EFV | .79 | .77 | |||
| Never, n (%) | 207 (95 | 69 (93.) | 138 (95) | ||
| <1 y, n (%) | 3.0 (1.4 | 1.0 (1.4) | 2.0 (1.4) | ||
| ≥1 y, n (%) | 9.0 (4.1) | 4.0 (5.4) | 5.0 (3.4) | ||
| NRTI | .74 | .16 | |||
| Never, n (%) | 19 (8.7) | 6.0 (8.1) | 13 (9.0) | ||
| <1 y, n (%) | 13 (5.9) | 4.0 (5.4) | 9.0 (6.2) | ||
| ≥1 y, n (%) | 187 (83) | 64 (86) | 123 (85) | ||
| NNRTI | .43 | .049 | |||
| Never, n (%) | 102 (47) | 32 (43) | 70 (48) | ||
| <1 y, n (%) | 25 (11) | 11 (15) | 14 (9.7) | ||
| ≥1 y, n (%) | 92 (42) | 31 (42) | 61 (42) | ||
| PI | .65 | .63 | |||
| Never, n (%) | 45 (21) | 13 (18) | 32 (22) | ||
| <1 y, n (%) | 17 (7.8) | 7.0 (9.5) | 10 (6.9) | ||
| ≥1 y, n (%) | 157 (72) | 54 (73) | 103 (71) | ||
| ART | .74 | .217 | |||
| Never, n (%) | 19 (8.7) | 6.0 (8.1) | 13 (9.0) | ||
| <1 y, n (%) | 13 (5.9) | 4.0 (5.4) | 9.0 (6.2) | ||
| ≥1 y, n (%) | 187 (85) | 64 (86) | 123 (85) |
Abbreviations: 3TC, lamivudin; ABC, abacavir; ART, antiretroviral treatment; ATV, atazanavir; AVN, avascular necrosis; AZT, zidovudine; d4T, stavudin; DDI, didanosin; DRV, darunavir; EFV, efavirenz; ETC, emtricitabine; FI, fusion inhibitor; HIV, human immunodeficiency virus; IDV, indinavir; IQR, interquartile range; LPV, lopinavir; NFV, nelfinavir; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; NVP, nevirapine; PI, protease inhibitor; RGV, raltegravir; RNA, ribonucleic acid; RTV, ritonavir; SQV, saquinavir; TDF, tenofovir.
aGlobal P value across the 3 categories never, <1 year, ≥1 year (from conditional logistic regression analysis).
b P value ever versus never (from conditional logistic regression analysis).
Overall Associations With Avascular Necrosis
There was no association of AVN with advancing age and BMI. An univariable association with AVN was recorded for heterosexual or IDU acquisition of HIV, moderate/severe alcohol consumption, pancreatitis, hyperlipidemia, and corticosteroid use ≥3 months. There was a trend of an association with CD4 nadir <200 cellsµ/L (OR, 1.9; 95% CI, 0.99–3.7), maximum HIV-1 viral load (OR, 3.6; 95% CI, 0.89–15), and >20 pack-years of smoking (OR, 1.6; 95% CI, 0.94–2.7).
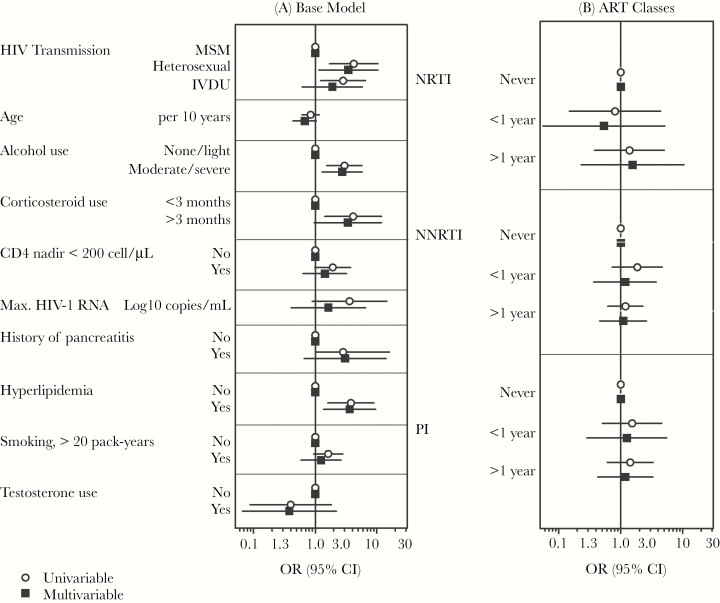
In adjusted models (Table 3; Figure 1), associations of AVN were documented with heterosexual HIV acquisition (OR, 3.4; 95% CI, 1.1–10), alcohol consumption (OR, 2.7; 95% CI, 1.3–5.7), hyperlipidemia (OR, 3.6; 95% CI, 1.4–9.6), and corticosteroid use <3 months (OR, 3.4; 95% CI, 0.97–12, trend). Other associations with AVN were no longer significant after mutual adjustment (CD4 nadir < 200 cells/µL: OR, 1.4; 95% CI, 0.63–3.2; maximum HIV RNA: OR, 1.6; 95% CI, 0.41–6.6; and pancreatitis: OR, 3.0; 95% CI, 0.66–14).
Table 3.
Uni- and Multivariable Conditional Logistic Regression of Avascular Bone Necrosis in 74 Cases and 145 Concurrent Controls
| Factor | Univariable Model OR (95% CI) |
P Value | Multivariable Modelsa OR (95% CI) |
P Value |
|---|---|---|---|---|
| Transmission risk group MSM | 1 | |||
| Heterosexual | 4.2 (1.7–10) | .002 | 3.5 (1.1–10) | .027 |
| Intravenous drug use | 2.8 (1.2–6.5) | .014 | 1.9 (0.61–5.8) | .268 |
| Age per 10 y | 0.84 (0.61–1.2) | .29 | 0.67 (0.43–1.0) | .073 |
| Alcohol consumptionb | 3.0 (1.6–5.8) | .001 | 2.7 (1.3–5.7) | .008 |
| Corticosteroid usec | 4.1 (1.4–12) | .009 | 3.4 (0.97–12) | .057 |
| CD4 nadir <200 cells/µL | 1.9 (0.99–3.7) | .054 | 1.4 (0.63–3.2) | .392 |
| Log10 maximal HIV-1 viral load | 3.6 (0.89–15) | .071 | 1.6 (0.41–6.6) | .491 |
| Pancreatitis | 4.0 (1.0–16) | .05 | 3.0 (0.66–14) | .153 |
| Hyperlipidemiad | 3.8 (1.6–9.0) | .002 | 3.6 (1.4–9.6) | .010 |
| Smoking, >20 pack-years | 1.6 (0.94–2.8) | .081 | 1.2 (0.59–2.63) | .565 |
| Testosterone use | 0.40 (0.09–1.8) | .237 | 0.38 (0.07–2.2) | .279 |
Abbreviations: CI, confidence interval; OR, odds ratio.
aMultivariable models were adjusted for transmission risk group, age, alcohol consumption, use of corticosteroids, CD4 nadir, maximum viral load, pancreatitis, hyperlipidemia, smoking, and testosterone use.
bAlcohol use: moderate (female 20–40 g/d, male 40–60 g/d) and severe use (female >40 g/d, male >60 g/).
cCorticosteroid intake for more than 3 months.
dHyperlipidemia: cholesterol >5 mmol/L and/or triglyceride >2 mmol/L.
Figure 1.
Uni- and multivariable associations with avascular bone necrosis derived from logistic regression analyses. Results for drug classes were derived from separate multivariable models adjusted for all variables of the base model. Hyperlipidemia: cholesterol level >5 mmol/L and/or a triglyceride level >2 mmol/L. Alcohol use: severe (female >40 g/d, male >60 g/d), moderate (female 20–40 g/d, male 40–60 g/d), and light (female <20 g/d, male <40 g/d). Abbreviations: CI, confidence interval; NNRTI, non-nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; OR, odds ratio; PI, protease inhibitor.
Associations of ART and Avascular Necrosis
Univariable associations with AVN were present for exposure to 3TC <1 year (OR, 6.8; 95% CI, 1.6–28) and TDF >1 year (OR, 3.5; 95% CI, 1.3–10) (Table 3). There was a trend toward an association with exposure to ATV >1 year (OR, 2.2; 95% CI, 0.98–4.8). Neither exposure to the most frequently prescribed ART combinations (3TC/AZT/LPV, ETC/TDF/LPV, 3TC/ABC/LPV, ETC/TDF/ATV, ETC/TDF/DRV, ETC/TDF/EFV, 3TC/ABC/EFV) nor exposure to individual drug classes (PIs, NRTIs, NNRTIs, FIs) or cumulative duration of ART exposure showed a univariable association with AVN.
By adding ART agents to the multivariable base model (Figure 1), there was an association for treatment with TDF >1 year (OR, 4.4; 95% CI, 1.4–14) and ever vs never use of TDF (OR, 3.5; 95% CI, 1.2–10). There was no evidence of an association for cumulative use (per year), current use of TDF, or use of regimens containing TDF. Further, there were trends toward an association with exposure to 3TC <1 year (OR, 5.1; 95% CI, 0.92–28), cumulative 3TC use (OR, 0.88; 95% CI, 0.76–1.0), and cumulative ABC use (OR, 0.86; 95% CI, 0.72–1.0). No further significant associations were seen for use of any ART, other ART substances, or ART drug classes.
DISCUSSION
In this case control study with 74 cases and 145 controls, nested in a prospective cohort study, we found an association of AVN with heterosexual HIV acquisition, moderate/severe alcohol consumption, hyperlipidemia, and a trend for corticosteroid use. These risk factors are also established for osteoporosis and osteoporotic fractures [18, 19], which hints toward a potential similarity of risk factors for AVN and osteoporosis in HIV-seropositive persons. We did not find evidence for an association of AVN with any ART exposure except for a TDF signal.
Heterosexual HIV acquisition was associated with AVN in our study. This finding is possibly mediated by advanced immunosuppression, considering the fact that heterosexual participants of our study were more likely to be late presenters compared with other transmission groups (data not shown) [6]. This hypothesis was supported by a univariable association of AVN with a CD4 nadir >200 cells/µL and corticosteroid use >3 months. Prolonged corticosteroid treatment is more often used in late presenters (eg, as adjunctive treatment to opportunistic infections) or subsequent to ART start (eg, management of immune reconstitution inflammatory syndrome) [5, 6, 10, 12, 20, 21]. In contrast to other studies, we did not observe an association with the use of testosterone or megestrol acetate. Alcohol use has previously been reported as a risk factor for AVN among HIV-positive persons [6, 10, 20, 21]. The longitudinal design of the SHCS including our quantitative assessment of alcohol consumption at 6-monthly intervals in the SHCS since 2008 [13] allowed us to extend these findings. We confirmed that, compared with light alcohol consumption, participants with moderate/heavy alcohol use were more likely to experience AVN. We found an association of AVN with hyperlipidemia in our study population. Altered lipid metabolism may be present in the setting of HIV, hence promoting bone ischemia and osteonecrosis [21]. Indeed, HIV-positive persons tend to drink more alcohol [22], to smoke more [23], and to have higher lipid levels [24] compared with the general population. Thus, HIV-positive persons might be prone to AVN because of an unfavorable lifestyle profile [5, 8, 21]. Of note, a history of pancreatitis, which can be a late sequel of both chronic alcohol intake and hyperlipidemia [21], was also associated with AVN in the univariate model.
Recently, investigators from EuroSIDA published a study on fractures (n = 496) and osteonecrosis (n = 73, 12 of whom were also included in our study), but when compared with our study, it identified quite a different set of risk factors for AVN: white race, lower nadir CD4, prior fracture/osteonecrosis, and prior AIDS [25]. We found some signal for CD4-nadir in the univariable model but not with AIDS. We have therefore decided not to include the latter in the final multivariable models, also to avoid overfitting because both parameters were strongly correlated (P < .001). Other differences are probably explained by the limited availability of well-known risk factors such as use of alcohol and corticosteroids in the EuroSIDA study [25]. Further, different methodological approaches with a case-control design in our study and a time-to-event analysis with GEE models, including multiple end points and an arbitrary baseline in January 2004 in the EuroSIDA study, may also have contributed to the observed differences. EuroSIDA found associations for AVN with the ever vs never use of DDI, IDV, SQV, LPV/r (all P < .05), and TDF (P = .051) when analyzed individually but not when mutually adjusted for other drugs. In our study, only the 2 TDF exposures ever vs never (P = .024) and never vs <1 year vs ≥1 year of use (P = .022) were associated with an increase of AVN. There was no evidence of an association for cumulative use (per year), current use of TDF, or use of regimens containing TDF. Of note, the power to detect signals of more recently introduced drugs were limited because one-third of AVN diagnoses were made before 2002 (Table 1), when TDF was approved for clinical use. The current clinical situation is quite different than the pre-ART and early-ART era. Hence, the significance of the isolated finding for TDF, the association of AVN with prior fractures [25] and osteoporosis [26], remains to be elucidated in future studies. It might be speculated that once bone damage has occurred (ie, diminished bone mineral density of any origin), the bone will be more prone to ischemia and hence occurrence of AVN [26]. In addition, a subnormal regenerative capacity of osteoblasts has been postulated as a common feature of osteoporosis and AVN [26]. Of note, in the subset of patients with available DEXA scans in our study (n = 55), cases were more likely to have a lower bone mineral density compared with controls (data not shown). Therefore, emphasis should be placed on maintaining bone health with optimization of vitamin D and calcium intake in HIV-infected persons, in particular whenever prolonged corticosteroid treatment is initiated.
Prolonged use of ART, particularly exposure to protease inhibitors, has also been implicated as a potential AVN risk factor, possibly via PI-aggravated dyslipidemia (especially hypertriglyceridemia) and impaired circulation [18, 19]. A recently published meta-analysis [18, 19] on 68 AVN cases and 126 controls showed an increased odds ratio for AVN among PI-exposed HIV-infected patients. In contrast to this meta-analysis, we did not find an association with any PI except for a weak ATV (ATV > 1 year: OR, 2.4; 95% CI, 0.86–6.4) and NFV signal (NVF ever vs never use, 2.4: 95% CI, 0.86–6.4).
Strengths of our study include a detailed chart review for both cases and controls with ascertainment of AVN diagnoses, validation and completion of risk factors collected in the SHCS (demographics; alcohol, nicotine, and drug use; lipids; CD4; HIV-1 RNA; ART), and identifying additional risk factors (eg, corticosteroid, testosterone, and megesterolacetate use; radiation therapy; collagenosis; hypercoagulability; pancreatitis; sickle cell anemia; radiation; and hemodialysis). Due to the completeness of the SHCS database, we were also able to control for ART exposure itself, exposure to specific ART substances and ART groups. Our study has also some limitations. We could not analyze time trends in the occurrence of AVN because of the incidence density matching. Simultanously, incidence density matching is a strength of the current study as we had controls with contemporary ART. As our study has a case-control design, recorded data are subject to observation bias [27]. The association of AVN with HET remains partially unexplained. Bivariable analyses with all available potential confounders did not reveal relevant reductions in the associations of HET with AVN. Therefore, we cannot exclude residual confounding. We performed numerous analyses looking at all individual drugs (n = 18) and the 7 most frequently prescribed combination regimens without Bonferroni corrections. Thus, we cannot exclude false positive findings due to multiple testing. The biological plausibility of the TDF signal is not clear yet and remains to be elucidated. DEXA scans and measurements of vitamin D levels were not systematically done in all participants.
In conclusion, we confirm previously recorded AVN risk factors such as heterosexual HIV acquisition, moderate/severe alcohol consumption [10, 21], hyperlipidemia, and corticosteroid use >3 months (trend) [5, 8, 10, 12, 21]. Our observation that TDF contributes to AVN is potentially worrisome and needs to be confirmed in other patient populations.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Acknowledgments
We thank patients participating in the Swiss HIV Cohort Study, all study physicians, study nurses, and data managers at the clinics and in the data center providing the data.
Financial support. This study was financed within the framework of the Swiss HIV Cohort Study, supported by the Swiss National Science Foundation (grant number 148522), by SHCS project number 771, and by the SHCS Research Foundation. The data were gathered by the 5 Swiss university hospitals, 2 Cantonal hospitals, 15 affiliated hospitals, and 36 private physicians (listed in http://www.shcs.ch/180-health-care-providers). The authors have no other related disclosures.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Members of the Swiss HIV Cohort Study. Aubert V, Battegay M, Bernasconi E, Böni J, Braun DL, Bucher HC, Calmy A, Cavassini M, Ciuffi A, Dollenmaier G, Egger M, Elzi L, Fehr J, Fellay J, Furrer H (Chairman of the Clinical and Laboratory Committee), Fux CA, Günthard HF (President of the SHCS), Haerry D (deputy of “Positive Council”), Hasse B, Hirsch HH, Hoffmann M, Hösli I, Kahlert C, Kaiser L, Keiser O, Klimkait T, Kouyos RD, Kovari H, Ledergerber B, Martinetti G, Martinez de Tejada B, Marzolini C, Metzner KJ, Müller N, Nicca D, Pantaleo G, Paioni P, Rauch A (Chairman of the Scientific Board), Rudin C (Chairman of the Mother & Child Substudy), Scherrer AU (Head of Data Centre), Schmid P, Speck R, Stöckle M, Tarr P, Trkola A, Vernazza P, Wandeler G, Weber R, Yerly S.
Contributor Information
the Swiss HIV Cohort Study:
V Aubert, M Battegay, E Bernasconi, J Böni, DL Braun, HC Bucher, A Calmy, M Cavassini, A Ciuffi, G Dollenmaier, M Egger, L Elzi, J Fehr, J Fellay, H Furrer, CA Fux, HF Günthard, D Haerry, B Hasse, HH Hirsch, M Hoffmann, I Hösli, C Kahlert, L Kaiser, O Keiser, T Klimkait, RD Kouyos, H Kovari, B Ledergerber, G Martinetti, B Martinez de Tejada, C Marzolini, KJ Metzner, N Müller, D Nicca, G Pantaleo, P Paioni, A Rauch, C Rudin, AU Scherrer, P Schmid, R Speck, M Stöckle, P Tarr, A Trkola, P Vernazza, G Wandeler, R Weber, and S Yerly
References
- 1. Hasse B, Ledergerber B, Furrer H et al. ; Swiss HIV Cohort Study Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis 2011; 53:1130–9. [DOI] [PubMed] [Google Scholar]
- 2. Guaraldi G, Orlando G, Zona S et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis 2011; 53:1120–6. [DOI] [PubMed] [Google Scholar]
- 3. Schouten J, Wit FW, Stolte IG et al. ; AGEhIV Cohort Study Group Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis 2014; 59:1787–97. [DOI] [PubMed] [Google Scholar]
- 4. Hasse B, Tarr PE, Marques-Vidal P et al. Strong impact of smoking on multimorbidity and cardiovascular risk among human immunodeficiency virus-infected individuals in comparison with the general population. Open Forum Infect Dis 2015; 2:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Glesby MJ, Hoover DR, Vaamonde CM. Osteonecrosis in patients infected with human immunodeficiency virus: a case-control study. J Infect Dis 2001; 184:519–23. [DOI] [PubMed] [Google Scholar]
- 6. Hasse B, Ledergerber B, Egger M et al. ; Swiss HIV Cohort Study Antiretroviral treatment and osteonecrosis in patients of the Swiss HIV Cohort Study: a nested case-control study. AIDS Res Hum Retroviruses 2004; 20:909–15. [DOI] [PubMed] [Google Scholar]
- 7. Reddy R, Daftary MN, Delapenha R et al. Avascular necrosis and protease inhibitors. J Natl Med Assoc 2005; 97:1543–6. [PMC free article] [PubMed] [Google Scholar]
- 8. Mehta P, Nelson M, Brand A, Boag F. Avascular necrosis in HIV. Rheumatol Int 2013; 33:235–8. [DOI] [PubMed] [Google Scholar]
- 9. Plate AM, Boyle BA. Review of avascular necrosis and HIV. AIDS Read 2000; 10:570–3. [PubMed] [Google Scholar]
- 10. Lawson-Ayayi S, Bonnet F, Bernardin E et al. Avascular necrosis in HIV-infected patients: a case-control study from the Aquitaine Cohort, 1997–2002, France. Clin Infect Dis 2005; 40:1188–93. [DOI] [PubMed] [Google Scholar]
- 11. Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 2008; 372:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Whitlock GG, Herbert S, Copas A et al. Avascular necrosis in HIV patients: a case-control study. Int J STD AIDS 2013; 24:799–803. [DOI] [PubMed] [Google Scholar]
- 13. Schoeni-Affolter F, Ledergerber B, Rickenbach M et al. ; Swiss HIV Cohort Study Cohort profile: the Swiss HIV Cohort study. Int J Epidemiol 2010; 39:1179–89. [DOI] [PubMed] [Google Scholar]
- 14. Friis-Møller N, Sabin CA, Weber R et al. ; Data Collection on Adverse Events of Anti-HIV Drugs (DAD) Study Group Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med 2003; 349:1993–2003.14627784 [Google Scholar]
- 15. WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363:157–63. [DOI] [PubMed] [Google Scholar]
- 16. Leslie WD, Lix LM, Langsetmo L et al. Construction of a FRAX model for the assessment of fracture probability in Canada and implications for treatment. Osteoporos Int 2011; 22:817–27. [DOI] [PubMed] [Google Scholar]
- 17. Kanis JA, Johnell O, De Laet C et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone 2004; 35:375–82. [DOI] [PubMed] [Google Scholar]
- 18. Yin MT, Brown TT. HIV and bone complications: understudied populations and new management strategies. Curr HIV/AIDS Rep 2016; 13:349–58. [DOI] [PubMed] [Google Scholar]
- 19. Finnerty F, Walker-Bone K, Tariq S. Osteoporosis in postmenopausal women living with HIV. Maturitas 2017; 95:50–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller KD, Masur H, Jones EC et al. High prevalence of osteonecrosis of the femoral head in HIV-infected adults. Ann Intern Med 2002; 137:17–25. [DOI] [PubMed] [Google Scholar]
- 21. Scribner AN, Troia-Cancio PV, Cox BA et al. Osteonecrosis in HIV: a case-control study. J Acquir Immune Defic Syndr 2000; 25:19–25. [DOI] [PubMed] [Google Scholar]
- 22. Conen A, Fehr J, Glass TR et al. ; Swiss HIV Cohort Study Self-reported alcohol consumption and its association with adherence and outcome of antiretroviral therapy in the Swiss HIV Cohort Study. Antivir Ther 2009; 14:349–57. [PubMed] [Google Scholar]
- 23. Huber M, Ledergerber B, Sauter R et al. ; Swiss HIV Cohort Study Group Outcome of smoking cessation counselling of HIV-positive persons by HIV care physicians. HIV Med 2012; 13:387–97. [DOI] [PubMed] [Google Scholar]
- 24. Carr A, Samaras K, Thorisdottir A et al. Diagnosis, prediction, and natural course of HIV-1 protease-inhibitor-associated lipodystrophy, hyperlipidaemia, and diabetes mellitus: a cohort study. Lancet 1999; 353:2093–9. [DOI] [PubMed] [Google Scholar]
- 25. Borges ÁH, Hoy J, Florence E et al. ; EuroSIDA Antiretrovirals, fractures, and osteonecrosis in a large International HIV Cohort. Clin Infect Dis 2017; 64:1413–21. [DOI] [PubMed] [Google Scholar]
- 26. Fessel WJ, Chau Q, Leong D. Association of osteonecrosis and osteoporosis in HIV-1-infected patients. AIDS 2011; 25:1877–80. [DOI] [PubMed] [Google Scholar]
- 27. Chokotho L, Harrison WJ, Lubega N, Mkandawire NC. Avascular necrosis of the femoral head in HIV positive patients-an assessment of risk factors and early response to surgical treatment. Malawi Med J 2013; 25:28–32. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.