Abstract
Although the invasive azooxanthellate corals Tubastraea coccinea and T. tagusensis are spreading quickly and outcompeting native species in the Atlantic Ocean, there is little information regarding the genetic structure and path of introduction for these species. Here we present the first data on genetic diversity and clonal structure from these two species using a new set of microsatellite markers. High proportions of clones were observed, indicating that asexual reproduction has a major role in the local population dynamics and, therefore, represents one of the main reasons for the invasion success. Although no significant population structure was found, results suggest the occurrence of multiple invasions for T. coccinea and also that both species are being transported along the coast by vectors such as oil platforms and monobouys, spreading these invasive species. In addition to the description of novel microsatellite markers, this study sheds new light into the invasive process of Tubastraea.
Keywords: Sun-coral, Clone structure, Microsatellites, Population genetics, T. coccinea, T. tagusensis
Introduction
The marine environment is continuously subjected to multiple stressors, many of which are associated with human activities (e.g., over-exploitation of resources, pollution, climate change and invasive species) (Halpern et al., 2014; Gallardo et al., 2016). Among these stressors, invasive species are considered to be a major threat to biodiversity (Molnar et al., 2008) with the potential to quickly trigger changes in native communities and the ecosystem services and functions, which can have wide-ranging negative impacts. There are numerous examples of marine invasions which impact humans or native biota, such as in the Mediterranean Sea with the invasion of the ctenophore Mnemiopsis leidyi, which caused the collapse of the fishing industry (Shiganova, 1998), the algae Womersleyella setacea, that negatively affected sponge reproduction (Caralt & Cebrian, 2013) and the lionfish Pterois spp., responsible for a reduction in the native fish recruitment in the Atlantic (Albins & Hixon, 2008).
Scleractinian corals are known to play a key role in the marine environment by building structurally complex and highly diverse ecosystems (Reaka-Kudla, 1997). As ecosystem engineers that are under threat globally (Hoegh-Guldberg, 1999; Pandolfi et al., 2003), scleractinian corals are rarely seen as an environmental risk. However, three scleractinian species from the genus Tubastraea were introduced and are spreading rapidly throughout the Western Atlantic Ocean (De Paula & Creed, 2004; Fenner, 2001; Fenner & Banks, 2004; Sammarco, Atchison & Boland, 2004; Sammarco, Porter & Cairns, 2010; Capel, 2012; Sampaio et al., 2012; Costa et al., 2014; Silva et al., 2014), threatening native and endemic species (Mantellato et al., 2011; Santos, Ribeiro & Creed, 2013; Creed, 2006) and fouling man-made structures and vessels.
Tubastraea is an azooxanthellate dendrophyllid genus from the Pacific and Indian Oceans that was first reported in the Caribbean in 1943 (Vaughan & Wells, 1943). Since then, three species have been identified in the Western Atlantic Ocean: (1) T. coccinea, now reported along 9,000 km of coastline of the Western Atlantic Ocean from Florida (26°47′N, 80°02′W) (Fenner & Banks, 2004) to Southern Brazil (27°17′S, 48°22′W) (Capel, 2012); (2) T. tagusensis, along the Brazilian coast (De Paula & Creed, 2004); and (3) T. micranthus in the Gulf of Mexico (Sammarco, Porter & Cairns, 2010). All three are considered opportunistic species most likely associated with transport on ships and/or oil platforms in the Caribbean, Gulf of Mexico and Brazilian coast (Cairns, 2000; Castro & Pires, 2001; Sammarco, Porter & Cairns, 2010).
Once established, invasive species can alter the structure of local communities, displacing and outcompeting native species (Vitousek, 1990; Mooney & Cleland, 2001; Lages, Fleury & Menegola, 2011; Cure et al., 2012; Santos, Ribeiro & Creed, 2013; Miranda, Cruz & Barros, 2016). In contrast to the native range, where Tubastraea is largely restricted to shaded or marginal habitats, studies on oil rigs in the Gulf of Mexico have shown that both T. coccinea and T. micranthus are excellent competitors and can overgrow other species (Hennessey & Sammarco, 2014; Sammarco et al., 2015). Similarly, in Brazil, T. coccinea and T. tagusensis can cover up to 100% of the available surface in some areas (Mantellato et al., 2011), killing native and endemic coral species upon direct contact (Creed, 2006; Santos, Ribeiro & Creed, 2013; Mantellato & Creed, 2014; Miranda, Cruz & Barros, 2016).
Fast growth rate, rapid range expansion, early reproductive age, propagule pressure and a wide variety of reproductive and survival strategies are biological characteristics usually associated with invasion success (Sax & Brown, 2000; Sakai et al., 2001; Lockwood, Cassey & Blackburn, 2005; Sax et al., 2007). Tubastraea species possess all of these characteristics (Cairns, 1991; Ayre & Resing, 1986; Glynn et al., 2008; Harrison, 2011; Capel et al., 2014; De Paula, Pires & Creed, 2014), which are enhanced by the fact that within the invaded areas they generally lack natural predators and dominant competitors. In addition, a large number of infested vectors (e.g., oil platforms and monobuoys) have been recorded transporting Tubastraea spp. along the Brazilian coast, leading to rapid range expansion throughout the Southwestern Atlantic Ocean (Creed et al., 2016).
Asexual reproduction improves coral ability to reach high abundance (Ayre & Miller, 2004) and may be an important trait of many invasive species, mainly in the first stage of invasion (Taylor & Hastings, 2005). When associated with early reproductive age and high propagule pressure it can rapidly increase abundance. Asexual production of brooded planulae has been reported in several anthozoans, including actinarians (Ottaway & Kirby, 1975; Black & Johnson, 1979), octocorals (Brazeau & Lasker, 1989) and scleractinians (Stoddart, 1983; Ayre & Resing, 1986). Although T. coccinea and T. diaphana appear to reproduce mainly by asexually produced larvae (Ayre & Resing, 1986), there is no information for their congeners, and the proportion of sexual versus asexual reproduction remains unknown within the genus. Furthermore, Ayre & Resing (1986) were able to score only two allozyme loci to infer asexual production of brooded larvae of Tubastraea spp. and the use of a larger number of more polymorphic loci, such as microsatellites, is desirable to corroborate their findings.
Although Tubastraea species are spreading rapidly and changing local benthic communities throughout the tropical Western Atlantic, information about their genetic diversity and reproductive strategies are still scarce. The study of reproductive strategies of invasive species is fundamental to understanding the invasion process, preventing new invasions, development of effective management strategies, and resolving the ecological and evolutionary processes involved in their invasion success (Sakai et al., 2001; Sax et al., 2007). However, to date there was no molecular marker developed to perform such studies with Tubastraea. Here, we report 12 novel microsatellite loci specifically developed for T. coccinea and cross-amplified in T. tagusensis and investigate the clonal structure and genetic diversity of populations of these alien invasive corals in the Southwestern Atlantic Ocean.
Materials and Methods
Sampling and DNA extraction
Microsatellite development was performed using samples of T. coccinea collected from Búzios Island (23°47′S, 45°08′W, 6 m in depth) and also from a monobuoy (IMODCO 4) at the São Sebastião channel (23°48′S, 45°24′W, 5 m of depth), Brazil. Additional samples of T. coccinea and T. tagusensis, collected from Todos-os-Santos Bay (TSB), northeastern Brazil (12°49′S, 38°46′W), and Ilha Grande Bay (IGB) (23°06′S, 44°15′ W), southeastern Brazil (∼24 colonies/species/locality), were used to test the markers and evaluate their genetic diversity (Fig. 1). Samples were preserved in 96% ethanol or CHAOS buffer (Fukami et al., 2004) prior to extraction. Total DNA was extracted using the Qiagen DNeasy tissue and blood kit following the manufacturer’s instructions or using the Phenol:Chloroform method described by Fukami et al. (2004).
Figure 1. Distributional range and sample localities on Southwestern Atlantic.
Map showing the distributional range of Tubastraea spp. on Southwestern Atlantic with the northern (NL) and southern (SL) limits of the distribution and sampled localities: Todos-os-Santos Bay (TSB) and Ilha Grande Bay (IGB) are showed by dark-gray stars; light-gray star represent Búzios Island and São Sebastião channel where initial collections to isolate microsatellite loci were performed. Map layout from http://d-maps.com/carte.php?num_car=1521&lang=en.
Microsatellite development and primer testing
Two genomic libraries were constructed at the National Laboratory for Scientific Computing (LNCC, Petrópolis, Brazil) using the 454 Genome Sequencer FLX platform (Fernandez-Silva et al., 2013). Reads were trimmed for adapters and quality using the FASTX-Toolkit. The software Newbler 2.3 (Roche, Basel, Switzerland) was used to perform the de novo assembly. The programs MSATCOMMANDER 0.8 (Faircloth, 2008) and SSRfinder were used to search for di-, tri-, tetra-, penta-, and hexa-nucleotide repetitions. Thirty-nine pairs of primers flanking the microsatellite regions were designed using Primer3 (http://bioinfo.ut.ee/primer3-0.4.0/) and primer characteristics were checked using OligoAnalyzer 3.1 (https://www.idtdna.com/calc/analyzer/). Forward primers were designed with a M13 tail at their 5′ end (TGT AAA ACG ACG GCC AGT) for dye labeled (6-FAM, VIC, NED, or PET) primers annealing to the replicated strand during PCR reactions (Schuelke, 2000).
A total of 47 specimens of T. coccinea and 48 T. tagusensis were amplified by Polymerase Chain Reactions (PCRs). PCRs were performed in 10 µl reactions including 0.2 µM of forward primer with M13 tail, 0.4 µM of labeled primer (M13 with VIC, NED, PET, or 6-FAM fluorescent dyes), 0.8 µM of reverse primer, 1U GoTaq (Promega, Fitchburg, WI, USA), 1× PCR Buffer (Promega), 0.20 mM dNTPs (Invitrogen, Carlsbad, CA, USA), between 1.5 and 2.5 mM MgCl2 (Table 1), 10 µg BSA (Invitrogen), and 5–10 ng of DNA. Cycling conditions were: 95°C for 3 min followed by 5 cycles at 95°C, 30 s; 52–62°C (Table 1), 30 s; 72°C, 45 s; and 30 cycles at 92°C, 30 s; 52–62°C, 30 s; 72°C, 55 s; with a final extension at 72°C for 30 min (Toonen, 1997). Amplification was verified in 2% agarose gel. PCR products were pooled with GS600-LIZ size standard (Applied Biosystems, Waltham, MA, USA) and genotyped in the ABI 3500 genetic Analyzer (Applied Biosystems). Genotypes were determined using the program Geneious 7.1.9.
Table 1. Description of Tubastraea coccinea and Tubastraea tagusensis microsatellite loci with their respective GeneBank Accession number.
Forward primers include an M13 sequence (5′-TGTAAAACGACGGCCAGT-3′).
| Locus/ Accession number | Primer sequence | Repeat motif | Species | TA (°C)/ [ ] MgCl2 (mM) | Range (bp) | TSB (N = 23-Tc/24-Tt) | IBG (N = 24-Tc/24-Tt) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Na | Ho | He | FIS | Na | Ho | He | FIS | ||||||
| Tco1/ KY198738 | F:TGTAAAACGACGGCCAGTACTTCGGTGATCGGACGAG-PET | (GTT)6 | T. coccinea | 56/2 | 567–600 | a | |||||||
| R: AGCACGGGTACTTGCTTTG | T. tagusensis | 56/2 | 2 | 0.12 | 0.18 | 0.00 | 1 | 0.00 | 0.00 | NA | |||
| Tco4/ KY198739 | F: TGTAAAACGACGGCCAGTGTGGAGAGTGAATAAGCTTGGG-NED | (TCA)4 | T. coccinea | 60/2 | 253–259 | 2 | 1.00 | 0.50 | −1.00 | 2 | 1.00 | 0.50 | −1.00 |
| R: GCCTGATGGTTTCTTGAGGTC | T. tagusensis | 58/2 | 2 | 0.40 | 0.32 | −0.14 | 2 | 0.33 | 0.28 | 0.00 | |||
| Tco5/ KY198740 | F: TGTAAAACGACGGCCAGTTCAGGAGCCGATTAATACCTG-6FAM | (GAAA)5 | T. coccinea | 54/2 | 368–432 | 5 | 0.50 | 0.76 | 0.39 | 3 | 0.20 | 0.34 | 0.50 |
| R: TGTGCAGTGAATGTGCTCAAG | T. tagusensis | 54/2.5 | 2 | 0.60 | 0.42 | −0,33 | 2 | 0.67 | 0.44 | −0.33 | |||
| Tco8/ KY198741 | F: TGTAAAACGACGGCCAGTGGTGCAGTGTAAATTGGTTCG-PET | (GGA)6 | T. coccinea | 54 /2 | 343–349 | 2 | 1.00 | 0.50 | −1.00 | 2 | 1.00 | 0.50 | −1.00 |
| R: GACAAGTGGAAAGCGGACG | T. tagusensis | 52/2 | 2 | 1.00 | 0.50 | −1.00 | 2 | 1.00 | 0.50 | −1.00 | |||
| Tco9/ KY198742 | F: TGTAAAACGACGGCCAGTTTGACCACGTACTGCCAAG-VIC | (TA)10 | T. coccinea | 60/2 | 347–357 | a | |||||||
| R: TCTGTTCAGAGAGCTCCGC | T. tagusensis | 60/2 | 2 | 0.20 | 0.18 | 0.00 | 1 | 0.00 | 0.00 | NA | |||
| Tco29/ KY198743 | F: TGTAAAACGACGGCCAGTGTGCCCTAGGTCCATGGTTT-VIC | (ATA)20 | T. coccinea | 62/1.5 | 211–222 | 3 | 0.70 | 0.51 | −0.31 | 3 | 1.00 | 0.57 | −0.71 |
| R: CCGGCTTCTATATAGGCTTCC | T. tagusensis | 58/2 | 3 | 0.20 | 0.46 | 0.64 | 1 | 0.00 | 0.00 | NA | |||
| Tco30/ KY198744 | F: TGTAAAACGACGGCCAGTGGGAATTCGGATGCAATTAT-6FAM | (ACAT)6 | T. coccinea | 60/1.5 | 252–264 | 3 | 1.00 | 0.61 | −0.63 | 3 | 1.00 | 0.58 | −-0.67 |
| R: CTCTGTGGAATGAGCTGCAA | T. tagusensis | 60/2.25 | 2 | 1.00 | 0.50 | −1.00 | 2 | 1.00 | 0.50 | −1.00 | |||
| Tco32a/ KY198745 | F: TGTAAAACGACGGCCAGTGCGTGGTCTGGTCTTTTCAT-6FAM | (ATA)13 | T. tagusensis | 58/2 | 240–246 | 2 | 1.00 | 0.50 | −1.00 | 2 | 1.00 | 0.50 | −1.00 |
| R: ACCCACTTTGAGGTGTTTGG | |||||||||||||
| Tco32b/ KY198745 | a | T. tagusensis | 270–276 | 2 | 1.00 | 0.50 | −1.00 | 3 | 1.00 | 0.61 | −0,50 | ||
| Tco34/ KY198746 | F: TGTAAAACGACGGCCAGTGCGCCTACTACCACACGAAT-PET | (TTA)19 | T. coccinea | 58/2 | 189–217 | 2 | 0.38 | 0.31 | −0.20 | 2 | 0.17 | 0.15 | 0.00 |
| R: TCCTTTCTACAGCGCACCTT | T. tagusensis | 58/2 | 3 | 0.80 | 0.58 | −0.28 | 3 | 1.00 | 0.61 | −0.50 | |||
| Tco36/ KY198747 | F: TGTAAAACGACGGCCAGTGCAATGACAACAGCCAGAAC-VIC | (ATA)15 | T. coccinea | 58/1.5 | 238–250 | b | b | ||||||
| R: TTTCGTCTGCCACATTCTTG | |||||||||||||
| Tco37/ KY198748 | F: TGTAAAACGACGGCCAGTAAACATTCGATTCCCACTCG-NED | (CTA)24 | T. coccinea | 62/1.5 | 242–263 | 4 | 1.00 | 0.74 | −0.32 | 2 | 1.00 | 0.50 | −1.00 |
| R: ACCCGGCCACTAATATTTCC | T. tagusensis | 62/1.5 | 3 | 1.00 | 0.62 | −0.50 | 3 | 1.00 | 0.61 | −0.50 | |||
| Tco38/ KY198749 | F: TGTAAAACGACGGCCAGTTTTGAGTTTGAGTTTATTGACTCCTT-NED | (TACA)6 | T. coccinea | 58/1.5 | 227–235 | b | b | ||||||
| R: GGAGTAAGCTTAGAGGGGTGCT | |||||||||||||
Notes.
- TA
- primer’s annealing temperature
- [ ]
- MgCl2 concentration of magnesium chloride
- N
- number of individuals genotyped
- Na
- number of alleles
- He
- expected heterozygosity
- Ho
- observed heterozygosity
- FIS
- inbreeding coefficient (negative values indicate an excess of heterozygotes)
Loci with evidence of linkage disequilibrium.
Loci with evidence of null alleles.
Statistical analyses
Clonal structure of each species was assessed using the ‘GenClone’ on R 3.2.3 package (R Core Team, 2015). Samples with the same alleles at all loci (ramets) were assigned to the same multilocus genotype (MLG, or genets) and considered to be a product of asexual reproduction. To check if individuals with the same MLG were truly clones, the probability of finding identical MLGs, resulting from distinct sexual reproductive events (Psex), was calculated following Arnaud-Haond et al. (2007). When Psex < 0.001, samples are considered ramets belonging to the same genet. In order to avoid the overestimation of genotype numbers due to scoring errors or somatic mutations (Douhovnikoff & Dodd, 2003), a second analysis calculating the genetic distance among all pairs of genets was performed. Based on the genetic distances, MLGs that differed at only one allele were assigned to the same multi-locus Lineage (MLL) (Arnaud-Haond et al., 2007). For the genetic diversity and population structure analyses, only unique MLLs were considered.
To assess the clonal structure of each population, two indexes were calculated as proposed by Arnaud-Haond et al. (2007): (1) clonal richness, to evaluate the proportions of clones in each population (R = G − 1∕N − 1), where G represents distinct multilocus lineages (MLL) and N is the total number of individuals sampled. The index ranges from zero (when all individuals are clones) to one (when all samples analyzed correspond to a different MLL); and (2) the genotypic evenness, to evaluate the equitability in the distribution of the MLL, calculated by the Simpson’s complement evenness index (V = (D − Dmin)∕(Dmax − Dmin)), where D represents the observed diversity, Dmax the value assumed if all genets have the same number of ramets, and Dmin the diversity value when all but one genet has one individual (Hurlbert, 1971). This index ranges from zero (when one genet dominates the population) to one (when genets each have the same number of ramets).
Quality control of loci followed Selkoe & Toonen (2006). To assess each population’s genetic diversity, the number of alleles (Na), observed (Ho) and expected heterozygosities (He) were calculated using the ‘diveRsity’ in R 3.2.3 package (R Core Team, 2015). Significant deviations from Hardy–Weinberg equilibrium (HWE) and linkage equilibrium were tested with the FSTAT program (Goudet, 1995). The occurrence of null alleles was investigated using the Micro-Checker program (Van Oosterhout et al., 2004). To measure population structure two indexes were calculated using the programs Genetix (Belkhir et al., 2004) and GenoDive (Meirmans & Van Tienderen, 2004). (1) Wright’s fixation index FST, ranging from zero, when different populations have identical alleles frequencies, to one, when each population has different fixed alleles (Wright, 1965). However, when applied to highly polymorphic markers, such as microsatellites, this index never reaches one and can underestimate genetic differentiation (Hedrick, 1999; Meirmans & Hedrick, 2011; Bird et al., 2011). The second measure, (2) Meirmans and Hedrick’s differentiation index , is a standardized measure rescaled from zero to one based on the maximum value of which simplifies interpretation of the degree of genetic differentiation among populations when using highly polymorphic microsatellite markers (Meirmans & Hedrick, 2011; Bird et al., 2011).
A Bayesian analysis was performed to estimate the number of genetic clusters in the dataset using STRUCTURE v. 2.3.4 software (Pritchard, Stephens & Donnelly, 2000) with the admixture ancestry model and correlated allele frequency. The analysis was performed with an initial burn-in of 500,000 cycles followed by 500,000 additional cycles and the number of clusters (K) tested varied from one to three with 15 iterations for each K-value. A higher range in the number of clusters (K ranging from one to five) was also tested to verify possible substructure within the populations. The most likely K-value was estimated by estimating the “log probability of data” for each value of K (mean LnP(K)) (Pritchard, Stephens & Donnelly, 2000) using STRUCTURE HARVESTER (Earl & Von Holdt, 2012). The ΔK criterion, frequently used in population genetic studies, is applied for datasets with more than two populations and as one of the hypotheses here is that the two localities are one panmitic population, this criterion was not used in the present work (Evanno, Regnaut & Goudet, 2005).
Results
Characterization of microsatellite markers
The two 454 runs resulted in a total of 329,832 reads with an average size of ±708.5 bp. A total of 1,077 regions with 2–6 bp microsatellite repeats with at least four units were found. Among these regions, 39 were selected for primer design, based on the size and position of the repeat within the sequence, and the primer characteristics (e.g., lacking primer-dimer formation). Within these, 11 and 10 were successfully amplified and genotyped for Tubastraea coccinea and T. tagusensis respectively (Accession numbers: KY198738 –KY198749). While two loci failed to amplify for T. tagusensis (Tco36 and Tco38), this species also exhibited two loci at a single locus with no evidence of linkage disequilibrium between them (Tco32a and Tco32b), so both were included in these analyses.
Evidence for null alleles for T. coccinea TSB population was observed in the same two loci (Tco36 and Tco38) that failed to amplify for T. tagusensis. Since both loci had only homozygote genotypes at the two analyzed localities, these loci were removed from the genetic diversity analyses. The loci Tco1 and Tco9 showed evidence of linkage disequilibrium with other loci and were also removed from the remaining analyses. The number of alleles per locus ranged from one to five in T. coccinea and one to four in T. tagusensis. Between localities, Ho ranged from 0.38 to 1 (TSB) and 0.17 to 1 (IGB) for T. coccinea and from 0.2 to 1 (TSB) and 0 to 1 (IGB) for T. tagusensis. He ranged from 0.31 to 0.76 (TSB) and 0.15 to 0.58 (IGB) for T. coccinea and from 0.18 to 0.62 (TSB) and 0 to 0.61 (IGB) for T. tagusensis (Table 1). In general, the observed heterozygosity was higher than expected for most loci in both populations of both species, with up to 100% of individuals being heterozygous at some loci (Table 1), although no significant deviation from HWE was observed.
Clonality
Psex values observed were highly significant (<0.001) for all but two and seven individuals of T. coccinea and T. tagusensis respectively. Thus, these data do not support the hypothesis of several individuals with the same MLG having originated by chance from distinct sexual reproduction events. A high proportion of clones were observed at both localities for both species (Table 2). For T. coccinea, at TSB of the 23 colonies sampled 13 MLLs were found, while at IGB only six MLLs out of the 24 colonies sampled were found. T. tagusensis had five (at TSB) and three (at IGB) unique MLLs among the 24 sampled colonies at each locality (Table 2). Missing values were considered as different alleles by the program, and although only specimens with missing information at no more than one locus were kept, it is important to note that the final number of MLL might be overestimated slightly.
Table 2. Genetic diversity of Tubastraea coccinea and T. tagusensis in two localities on the Southwestern Atlantic Ocean, Todos os Santos Bay (TSB) and Ilha Grande Bay (IGB), Brazil.
| Specie | Location | N | MLG | MLL | R | V | A | AR | Ap | Ho | He | FIS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T. coccinea | TSB | 23 | 13 | 13 | 0.55 | 0.845 | 21 | 2.74 | 4 | 0.80 | 0.56 | −0.380 |
| IGB | 24 | 6 | 6 | 0.21 | 1.13e–16 | 17 | 2.18 | 0 | 0.77 | 0.45 | −0.651 | |
| T. tagusensis | TSB | 24 | 7 | 5 | 0.17 | 0.54 | 25 | 1.98 | 4 | 0.67 | 0.43 | −0.468 |
| IGB | 24 | 6 | 3 | 0.09 | 1.04e–16 | 22 | 1.87 | 1 | 0.64 | 0.37 | −0.615 |
Notes.
- N
- Number of individuals sampled
- MLG
- multilocus genotype
- MLL
- multilocus lineages
- R
- clonal richness
- V
- genotypic evenness
- β
- pareteo distribution
- A
- alleles number
- AR
- allele richness
- Ap
- number of private alleles
- Ho
- observed heterozigosities
- He
- expected heterozigosities
- FIS
- inbreeding coefficient
Clonal richness observed for T. coccinea indicates that IGB is mostly composed of clones (R = 0.22), with only six MLLs out of 24 individuals, while TSB has nearly half of the individuals comprised of clones (13 MLL in 23 individuals sampled; R = 0.55) (Table 2). In addition to the low MLL diversity at IGB, 19 individuals had the same predominant MLL, which was observed by the evenness indexes (V = 1.13−16). Conversely, the TSB population of T. coccinea had more equally distributed MLLs, with the most common one being shared among only 4 individuals (V = 0.85). For T. tagusensis, both populations were composed mainly of clones, with very low clonal richness (IGB: R = 0.09; TSB: R = 0.17). Similarly to what was observed for T. coccinea, MLLs were more equally distributed at TSB, with 14 individuals belonging to the same MLL (V = 0.54), while in IGB the most common one was shared among 22 individuals (V = − 1.04p−16).
Genetic diversity and population structure
Only unique MLLs were used to assess genetic diversity and population structure in each species. For both species, TSB had higher number of alleles, allelic richness and number of private alleles compared to IGB, with T. coccinea presenting the more accentuated differences (Table 2). There were no significant deficits of heterozygosity; both observed (Ho) and expected (He) heterozygosity were similar when comparing between localities for both T. coccinea (TSB: 0.80 and 0.56; IGB: 0.77 and 0.45) and T. tagusensis (TSB: 0.67 and 0.43; IGB: 0.64 and 0.37). The inbreeding coefficient (FIS), although not significant, was negative for both localities and in both species, indicating an excess of heterozygotes (Table 2).
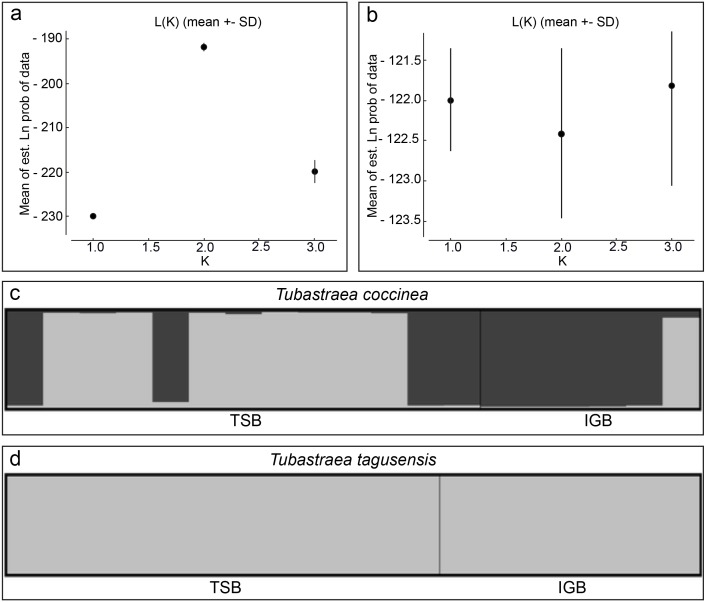
FST and values were 0.06 (p = 0.08) and 0.13 (p = 0.07) for T. coccinea and indistinguishable from zero (p = 0.69 and p = 0.69) for T. tagusensis. The lack of significant population structure among the sampled localities indicates similar allele frequencies for both species across these sites. Although Bayesian analysis recovered two genetic clusters for T. coccinea for both ranges of K tested, these groups are not a function of population structure between localities (Fig. 2), but instead, reflect the presence of population structure within each locality. Furthermore, there is no evidence of interbreeding between the two clusters, and the FST values between these sites is likely a result of the strikingly different proportion of these two groups in each site. In contrast, no clustering was observed between or within localities for T. tagusensis, with the most likely K value being one for both ranges of K tested (Fig. 2).
Figure 2. Bayesian clustering analyses for Tubastraea coccinea and T. tagusensis.
(A) and (B) shows the most likely K-value estimated by the mean of estimated “log probability of data” for each value of K for T. coccinea (K = 2) and T. tagusensis (K = 1), respectively; (C) and (D) shows the genetic clusters, where each individual is represented by a vertical bar with different colors indicating the relative proportion of each genetic cluster. TSB, Todos os Santos Bay; IGB, Ilha Grande Bay.
Discussion
The novel microsatellite markers reported herein will enable further studies regarding the genetic diversity and population structure of Tubastraea spp. corals in the Atlantic and native ranges of these invasive populations. Using these microsatellites, this study shows that both invasive coral species (T. coccinea and T. tagusensis) have high proportions of clones at both localities on the Brazilian coast with identical multilocus lineages (MLLs) found in sites separated by more than 1,500 km. The results indicate that asexual reproduction dominates in the invasive range of Tubastraea spp. in the Southwestern Atlantic and despite the large distance between localities, no significant population structure could be found. In contrast, there are clear signs of population structure across this same region in an endemic spawning coral species (Mussismilia hispida, Azevedo, 2015).
Our results support previous work reporting reproduction via asexual larvae in T. coccinea (Ayre & Resing, 1986). Likewise, the high proportion of clones found at both sampled localities for T. tagusensis indicates likely reproduction by asexual larvae for this species also, a reproductive mode previously recorded for only three scleractinian species: Pocillopora damicornis (Stoddart, 1983), Tubastraea diaphana and T. coccinea (Ayre & Resing, 1986). Indeed, a study on the reproductive strategies of T. coccinea and T. tagusensis in the Southwestern Atlantic observed a small number of spermaries and the presence of embryos and planula at different times of the year, concluding that asexual reproduction could be important for both species (De Paula, Pires & Creed, 2014). For most corals, clonality is a result of mechanical fragmentation due to physical disturbances (Foster et al., 2013; Nakajima et al., 2015). T. coccinea and T. tagusensis, however, are not prone to fragmentation, so the high number of clones observed for both species in this study seems more likely to result from asexually produced larvae. Nevertheless, it is desirable to confirm the production of asexual larvae for both T. coccinea and T. tagusensis by performing paternity studies in the future.
For invasive species, asexual reproduction can be crucial in the first stage of invasion, when sexual partners are scarce or absent, because it significantly enhances the chances of survival for the colonists (Taylor & Hastings, 2005). Successful invasions originating from a few clonal genotypes have been previously recorded for plants (Ren, Zhang & Zhand, 2005; Liu et al., 2006) and other cnidarians (Reitzel et al., 2008). Asexual reproduction is dominant in the invasive range and it may have contributed to the invasive success of Tubastraea in the Southwestern Atlantic, where the rocky shores provide a suitable habitat and release from enemies (Enemy Release Hypothesis, Keane & Crawley, 2002). At IGB both studied coral species have high percentage of clones and an extremely low genotypic evenness, indicating that most colonies are clones belonging to the same genet. Sampling more areas surrounding each collection site is needed to thoroughly examine clonal diversity for these regions, but particularly in TSB where samples were more widely spaced, this observation supports the role of asexual reproduction in increasing local abundance. Gregarious settlement has been previously observed for both T. coccinea (Glynn et al., 2008; De Paula, Pires & Creed, 2014), and T. tagusensis (De Paula, Pires & Creed, 2014), although these studies did not determine if the aggregated larvae were sexually or asexually derived. It is noteworthy that T. coccinea has higher numbers of MLLs, clonal richness and genotypic evenness at TSB than at IGB, suggesting increased occurrence of sexual reproduction or a greater number of successful colonists at the former site. Rates of sexual and asexual reproduction can be highly variable among geographic regions in other corals (Baums, Miller & Hellberg, 2006; Noreen, Harrison & Van Oppen, 2009; Combosch & Vollmer, 2011; Gorospe & Karl, 2013), but it remains unknown what governs the difference in the proportion of sexual and asexual reproduction at different localities. Several factors can influence both genotypic and genetic diversity in invasive species, including the number of invasions, the genetic diversity of the source population(s) and a variety of biological factors, such as the main reproductive strategy adopted by the species (Dlugosch & Parker, 2008). Although sexual reproduction might also occur in Tubastraea, the results obtained for T. coccinea might be an effect of the occurrence of recent multiple introductions from different native populations (Roman & Darling, 2007). Another hypothesis would be the presence of cryptic species, which has been found in other scleractinian corals (Pinzón & Weil, 2011; Warner, Van Oppen & Willis, 2015; Nakajima et al., 2017). Morphological analyses combined with molecular data including native populations are necessary to corroborate this hypothesis.
A decrease in genetic diversity as a result of a small founding population has been previously recorded for several invasive populations (Roman & Darling, 2007; Geller et al., 2008; Johnson & Woollacott, 2015; Wrange et al., 2016; but see Gaither et al., 2010; Gaither, Toonen & Bowen, 2012 for counter-examples). Here, we report excess of heterozygosity for both populations of both species and the presence of up to 100% heterozygous individuals at some loci (Table 1). High levels of heterozygosity can result from an isolate-breaking effect, when multiple introductions mix previously separated native populations (Holland, 2000; Hamilton, 2010). However, in this case, there is no evidence of mixing between the two genetic clusters (Fig. 2), indicating that they are not interbreeding. Thus, it seems more likely that TSB and IGB were colonized by different native populations followed by recent transport between localities without sufficient time for them to interbreed, although the possibility of cryptic species that are incapable of interbreeding should also be considered. If the first scenario of introduction by different native populations proves true, the high heterozygosity could be either a result of a founder effect in which the new area was, by chance, colonized by a higher number of heterozygote genotypes, or due to a higher fitness of the heterozygote genotypes, either of which could be propagated by asexual reproduction (De Meeus & Balloux, 2005). Alternatively, Gaither, Toonen & Bowen (2012) showed that introduced fishes in Hawai’i with a known history actually had higher and more even genetic diversity than was observed in the native range, and such an effect could also explain the observed pattern here. In contrast to what is observed with T. coccinea, we recover only a single genetic cluster for T. tagusensis between both populations. This single cluster could result from either invasion of both localities from the same source population, or a secondary invasion along the Brazilian coast from the original locality being spread to another. Unlike T. coccinea, which is now considered cosmopolitan (Cairns, 2000), T. tagusensis has a restricted distribution (Cairns, 1991) and may have naturally low genetic diversity. The distinction between these species is reminiscent of the pattern reported by Gaither, Bowen & Toonen (2013) in which population structure of species in their native range predicts the diversity and rate of spread in the invasive range.
Considering that (i) both T. coccinea and T. tagusensis brood larvae competent for only ∼18 days (in aquaria) that typically display gregarious settlement (Glynn et al., 2008; De Paula, Pires & Creed, 2014) and (ii) the absence of Tubastraea in extensive areas between the two localities, it is highly unlikely that they are connected through larval dispersal. On the other hand, oil platforms are known to be moved between these regions (Sampaio et al., 2012), and are considered the main vector for the introduction of Tubastraea into the southwestern Atlantic (Castro & Pires, 2001; Creed et al., 2016). Thus, our data showing a lack of structure between localities, and the occurrence of shared MLLs for each species among these distant sites, indicate that anthropogenic vectors, such as oil platforms, monobuoys, or other vessels have played an important role in dispersing these alien invasive species, and possibly assisting other species to spread along the coast (Almeida et al., 2015; Creed et al., 2016).
Conclusions
Invasive Tubastraea spp. are spreading quickly throughout the Atlantic, in some areas covering up to 100% of the available surface (Mantellato et al., 2011) and outcompeting native and endemic species (Mantellato et al., 2011; Santos, Ribeiro & Creed, 2013; Creed, 2006). Despite this documented impact and concern, little is known about the genetic diversity and reproductive strategies of Tubastraea species globally. This study provides the first survey of genetic diversity and likely reproductive strategies along the southwestern Atlantic coast, demonstrating that asexual reproduction has an important role in the population dynamics of both T. coccinea and T. tagusensis and is probably a relevant feature leading to their invasive success. Results also indicate that there were likely at least two different populations of T. coccinea introduced into the southwestern Atlantic. A molecular systematic examination of the genus is highly recommended in order to check for the occurrence of cryptic species. Future studies should focus on the identification of potential source populations and the global phylogeograpy of Tubastraea with the goal of tracking and limiting future invasions, as well as the establishment of effective management and prevention strategies.
Supplemental Information
Raw data used in the present work, including allele sizes for each genotyped individual.
Acknowledgments
We are grateful to Antonio Solé-Cava for helping editing the first set of Next-Generation Sequencing data and to Marcelo Mantellato and Projeto Coral-Sol for samples. We are also thankful to Diane Bailleul for all the support on the clonal analyses. This is Scientific Contribution No. 30 of the Projeto Coral-Sol.
Funding Statement
This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Joel C. Creed, Ciências do Mar 1137/2010); Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (Katia Cristina Cruz Capel, Joel C. Creed and Carla Zilberberg, FAPERJ-E-26/010.003031/2014 PensaRio; Joel C. Creed E26/201.286/2014); Conselho Nacional de Desenvolvimento Científico e Tecnológico (Joel C. Creed, CNPq-305330/2010-1) and Fundação de Amparo à Pesquisa do Estado de São Paulo (Marcelo V. Kitahara, FAPESP 2014/01332-0); and Award NSF-OA#14-16889 (National Science Foundation). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Robert J. Toonen is an Academic Editor for PeerJ.
Author Contributions
Katia Cristina Cruz Capel conceived and designed the experiments, performed the experiments, analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Robert J. Toonen wrote the paper, reviewed drafts of the paper.
Caio T.C.C. Rachid analyzed the data, reviewed drafts of the paper.
Joel C. Creed contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
Marcelo V. Kitahara contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
Zac Forsman wrote the paper, reviewed drafts of the paper.
Carla Zilberberg conceived and designed the experiments, contributed reagents/materials/analysis tools, wrote the paper, reviewed drafts of the paper.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
The samples used in the study were collected under the permit No 003/2014 from Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis – Ministério do Meio Ambiente, Brazil.
DNA Deposition
Data Availability
The following information was supplied regarding data availability:
The raw data has been uploaded as a Supplemental File.
References
- Albins & Hixon (2008).Albins MA, Hixon MA. Invasive Indo-Pacific lionfish Pterois volitans reduce recruitment of Atlantic coral-reef fishes. Marine Ecology Progress Series. 2008;367:233–238. doi: 10.3354/meps07620. [DOI] [Google Scholar]
- Almeida et al. (2015).Almeida ACS, Souza FBC, Gordon DP, Vieira LM. The non-indigenous bryozoan Triphyllozoon (Cheilostomata: Phidoloporidae) in the Atlantic: morphology and dispersion on the Brazilian coast. Zoologia. 2015;32:476–484. doi: 10.1590/s1984-46702015000600007. [DOI] [Google Scholar]
- Arnaud-Haond et al. (2007).Arnaud-Haond S, Duarte M, Alberto F, Serrão A. Standardizing methods to address clonality in population studies. Molecular Ecology. 2007;16:5115–5139. doi: 10.1111/j.1365-294X.2007.03535.x. [DOI] [PubMed] [Google Scholar]
- Ayre & Miller (2004).Ayre DJ, Miller KJ. Where do clonal coral larvae go? Adult genotypic diversity conflicts with reproductive effort in the brooding coral Pocillopora damicornis. Marine Ecology Progress Series. 2004;277:95–105. doi: 10.3354/meps277095. [DOI] [Google Scholar]
- Ayre & Resing (1986).Ayre DJ, Resing JM. Sexual and asexual production of planulae in reef corals. Marine Biology. 1986;90:187–190. doi: 10.1007/BF00569126. [DOI] [Google Scholar]
- Azevedo (2015).Azevedo LP. Master’s dissertation. 2015. Conectividade genética do coral endémico Mussismilia hispida (Scleractinia: Mussidae) ao longo da costa brasileira. [Google Scholar]
- Baums, Miller & Hellberg (2006).Baums IB, Miller MW, Hellberg ME. Geographic variation in clonal structure in a reef-building Caribbean coral, Acropora palmata. Ecologial Monographs. 2006;76:503–519. doi: 10.1890/0012-9615(2006)076[0503:GVICSI]2.0.CO;2. [DOI] [Google Scholar]
- Belkhir et al. (2004).Belkhir K, Borsa P, Chikhi L, Raufaste N, Bonhomme F. CNRS UMR 500, Université de Montpelier II; Montpellier: 2004. [Google Scholar]
- Bird et al. (2011).Bird CE, Karl S, Smouse PE, Toonen RJ. Detecting and measuring genetic differentiation. In: Koenemann S, Held C, Schubart C, editors. Crustacean issues: phylogeography and population genetics in Crustacea. CRC Press; Boca Raton: 2011. pp. 31–55. [Google Scholar]
- Black & Johnson (1979).Black R, Johnson MS. Asexual viviparity and population genetics of Actinia tenebrosa. Marine Biology. 1979;53:27–31. doi: 10.1007/BF00386526. [DOI] [Google Scholar]
- Brazeau & Lasker (1989).Brazeau DA, Lasker HR. The reproductive cycle and spawning in a Caribbean gorgonian. Biological Bulletin. 1989;176:1–7. doi: 10.2307/1541882. [DOI] [Google Scholar]
- Cairns (1991).Cairns SD. A revision of the ahermatypic scleractinia of the Galapagos and Cocos Islands. Smithsonian Contributions to Zoology. 1991;504:1–32. doi: 10.5479/si.00810282.504. [DOI] [Google Scholar]
- Cairns (2000).Cairns SD. A revision of the shallow-water azooxanthellate Scleractinia of the western Atlantic. Studies on the Natural History of the Caribbean Islands. 2000;75(1):1–192. [Google Scholar]
- Capel (2012).Capel KCC. Master’s dissertation. 2012. Scleractinia (Cnidaria: Anthozoa) da Reserva Biológica Marinha do Arvoredo (SC), com ênfase na estrutura espaço-temporal da formação mais meridional de corais recifais no Oceano Atlântico. [Google Scholar]
- Capel et al. (2014).Capel KC, Migotto AE, Zilberberg C, Kitahara MV. Another tool towards invasion? Polyp bail-out in Tubastraea coccinea. Coral Reefs. 2014;33:1165. doi: 10.1007/s00338-014-1200-z. [DOI] [Google Scholar]
- Caralt & Cebrian (2013).Caralt S, Cebrian E. Impact of an invasive alga (Womersleyella setacea) on sponge assemblages: compromising the viability of future populations. Biological Invasions. 2013;15:1591–1600. doi: 10.1007/s10530-012-0394-7. [DOI] [Google Scholar]
- Castro & Pires (2001).Castro CB, Pires DO. Brazilian coral reefs: what we already know and what is still missing. Bulletin of Marine Science. 2001;69:357–371. [Google Scholar]
- Combosch & Vollmer (2011).Combosch DJ, Vollmer SV. Population genetics of an ecosystem-defining reef coral Pocillopora damicornis in the Tropical Eastern Pacific. PLOS ONE. 2011;6:e21200. doi: 10.1371/journal.pone.0021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa et al. (2014).Costa TJF, Pinheiro HT, Teixeira JB, Mazzei EF, Bueno L, Hora MSC, Joyeux JC, Carvalho-Filho A, Amado-Filho G, Sampaio CLS, Rocha LA. Expansion of an invasive coral species over Abrolhos Bank, Southwestern Atlantic. Marine Pollution Bulletin. 2014;85:252–253. doi: 10.1016/j.marpolbul.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Creed (2006).Creed JC. Two invasive alien azooxanthellate corals, Tubastraea coccinea and Tubastraea tagusensis, dominate the native zooxanthellate Mussismilia hispida in Brazil. Coral Reefs. 2006;25:350. doi: 10.1007/s00338-006-0105-x. [DOI] [Google Scholar]
- Creed et al. (2016).Creed JC, Fenner D, Sammarco P, Carins S, Capel K, Junqueira AOR, Cruz I, Miranda RJ, Carlos-Junior L, Mantelatto MC, Oigman-Pszczol S. The invasion of the azooxanthellate coral Tubastraea (Scleractinia: Dendrophylliidae) throughout the world: history, pathways and vectors. Biological Invasions. 2016;19:283–305. doi: 10.1007/s10530-016-1279-y. [DOI] [Google Scholar]
- Cure et al. (2012).Cure K, Benkwitt CE, Kindinger TL, Pickering EA, Pusack TJ, McIlwain JL, Hixon MA. Comparative behavior of red lionfish Pterois volitans on native Pacific versus invaded Atlantic coral reefs. Marine Ecology Progress Series. 2012;467:181–192. doi: 10.3354/meps09942. [DOI] [Google Scholar]
- De Meeus & Balloux (2005).De Meeus T, Balloux F. F-statistics of clonal diploids structured in numerous demes. Molecular Ecology. 2005;14:2695–2702. doi: 10.1111/j.1365-294X.2005.02643.x. [DOI] [PubMed] [Google Scholar]
- De Paula & Creed (2004).De Paula AF, Creed JC. Two species of the coral Tubastraea (Cnidaria, Scleractinia) in Brazil: a case of accidental introduction. Bulletin of Marine Science. 2004;74:175–183. [Google Scholar]
- De Paula, Pires & Creed (2014).De Paula AF, Pires DO, Creed JC. Reproductive strategies of two invasive Sun corals (Tubastraea spp.) in the southwestern Atlantic. Journal of the Marine Biological Association of the United Kingdom. 2014;94:481–492. doi: 10.1017/S0025315413001446. [DOI] [Google Scholar]
- Dlugosch & Parker (2008).Dlugosch KM, Parker M. Founding events in species invasions: genetic variation, adaptive evolution, and the role of multiple introductions. Molecular Ecology. 2008;17:431–449. doi: 10.1111/j.1365-294X.2007.03538.x. [DOI] [PubMed] [Google Scholar]
- Douhovnikoff & Dodd (2003).Douhovnikoff V, Dodd RS. Intra-clonal variation and a similarity threshold for identification of clones: application to Salix exigua using AFLP molecular markers. Theoretical and Applied Genetics. 2003;106:1307–1315. doi: 10.1007/s00122-003-1200-9. [DOI] [PubMed] [Google Scholar]
- Earl & Von Holdt (2012).Earl DA, Von Holdt BM. STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conservation Genetics Resources. 2012;4:359–361. doi: 10.1007/s12686-011-9548-7. [DOI] [Google Scholar]
- Evanno, Regnaut & Goudet (2005).Evanno G, Regnaut S, Goudet J. Detecting the number of clusters of individuals using the software Structure: a simulation study. Molecular Ecology. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- Faircloth (2008).Faircloth BC. Msatcommander: detection of microsatellite repeat arrays and automated, locus-specific primer design. Molecular Ecology Resources. 2008;8:92–94. doi: 10.1111/j.1471-8286.2007.01884.x. [DOI] [PubMed] [Google Scholar]
- Fenner (2001).Fenner D. Biogeography of three Caribbean corals (Scleractinia) and the invasion of Tubastraea coccinea into the Gulf of Mexico. Bulletin of Marine Science. 2001;69:1175–1189. [Google Scholar]
- Fenner & Banks (2004).Fenner D, Banks K. Orange Cup Coral Tubastraea coccinea invades Florida and the Flower Garden Banks, Northwestern Gulf of Mexico. Coral Reefs. 2004;23:505–507. doi: 10.1007/s00338-004-0422-x. [DOI] [Google Scholar]
- Fernandez-Silva et al. (2013).Fernandez-Silva I, Whitney J, Wainwright B, Andrews KR, Ylitalo-Ward H, Bowen BW, Toonen RJ, Goetze E, Karl SA. Microsatellites for next-generation ecologists: a post-sequencing bioinformatics pipeline. PLOS ONE. 2013;8:e55990. doi: 10.1371/journal.pone.0055990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster et al. (2013).Foster NL, Baums IB, Sanchez JA, Paris CB, Chollett I, Agudelo CL, Vermeij MJA, Mumby PJ. Hurricane-driven patterns of clonality in an ecosystem engineer: the Caribbean coral Montastraea annularis. PLOS ONE. 2013;8:e53283. doi: 10.1371/journal.pone.0053283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami et al. (2004).Fukami H, Budd AF, Paulay G, Sole-Cava A, Chen CLA, Iwao K, Knowlton N. Conventional taxonomy obscures deep divergence between Pacific and Atlantic corals. Nature. 2004;427:832–835. doi: 10.1038/nature02339. [DOI] [PubMed] [Google Scholar]
- Gaither, Bowen & Toonen (2013).Gaither MR, Bowen BW, Toonen RJ. Population structure in the native range predicts the spread of introduced marine species. Proceedings of the Royal Society B: Biological Sciences. 2013;280:20130409. doi: 10.1098/rspb.2013.0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaither et al. (2010).Gaither MR, Bowen BW, Toonen RJ, Planes S, Messmer V, Earle J, Ross Robertson D. Genetic consequences of introducing allopatric lineages of Bluestriped Snapper (Lutjanus kasmira) to Hawaii. Molecular Ecology. 2010;19:1107–1121. doi: 10.1111/j.1365-294X.2010.04535.x. [DOI] [PubMed] [Google Scholar]
- Gaither, Toonen & Bowen (2012).Gaither MR, Toonen RJ, Bowen BW. Coming out of the starting blocks: extended lag time rearranges genetic diversity in introduced marine fishes of Hawaii. Proceedings of the Royal Society B: Biological Sciences. 2012;279:3948–3957. doi: 10.1098/rspb.2012.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallardo et al. (2016).Gallardo B, Clavero M, Sánchez MI, Vilà M. Global ecological impacts of invasive species in aquatic ecosystems. Global Change Biology. 2016;22:151–163. doi: 10.1111/gcb.13004. [DOI] [PubMed] [Google Scholar]
- Geller et al. (2008).Geller J, Sotka EE, Kado R, Palumbi SR, Schwindt E. Sources of invasions of a northeastern Pacific acorn barnacle, Balanus glandula, in Japan and Argentina. Marine Ecology Progress Series. 2008;358:211–218. doi: 10.3354/meps07466. [DOI] [Google Scholar]
- Glynn et al. (2008).Glynn PW, Colley SB, Mate JL, Cortes J, Guzman HM, Bailey RL, Feingold JS, Enochs IC. Reproductive ecology of the azooxanthellate coral Tubastraea coccinea in the equatorial eastern pacific: Part V. Dendrophylliidae. Marine Biology. 2008;153:529–544. doi: 10.1007/s00227-007-0827-5. [DOI] [Google Scholar]
- Gorospe & Karl (2013).Gorospe KD, Karl SA. Genetic relatedness does not retain spatial pattern across multiple spatial scales: dispersal and colonization in the coral, Pocillopora damicornis. Molecular Ecology. 2013;22:3721–3736. doi: 10.1111/mec.12335. [DOI] [PubMed] [Google Scholar]
- Goudet (1995).Goudet J. FSTAT (version 1.2), a computer program to calculate F-statistics. Journal of Heredity. 1995;86:485–486. doi: 10.1093/oxfordjournals.jhered.a111627. [DOI] [Google Scholar]
- Halpern et al. (2014).Halpern BS, Frazier M, Potapenko J, Casey KS, Koenig K, Longo C, Lowndes JS, Rochwood RC, Selig ER, Selkoe KA, Walbridge S. Spatial and temporal changes in cumulative human impacts on the world’s ocean. Nature Communications. 2014;6:7615. doi: 10.1038/ncomms8615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton (2010).Hamilton M. Population genetics. John Wiley & Sons Inc; New York: 2010. [Google Scholar]
- Harrison (2011).Harrison PL. Sexual reproduction of Scleractinian Corals. In: Dubinsky Z, Stambler N, editors. Coral reefs: an ecosystem in transition, Part 3. Springer; Dordrecht: 2011. pp. 59–85. [Google Scholar]
- Hedrick (1999).Hedrick PW. Highly variable loci and their interpretation in evolution and conservation. Evolution. 1999;53:313–318. doi: 10.2307/2640768. [DOI] [PubMed] [Google Scholar]
- Hennessey & Sammarco (2014).Hennessey SM, Sammarco PW. Competition for space in two invasive Indo-Pacific corals—Tubastraea micranthus and Tubastraea coccinea: Laboratory experimentation. Journal of Experimental Marine Biology and Ecology. 2014;459:144–150. doi: 10.1016/j.jembe.2014.05.021. [DOI] [Google Scholar]
- Hoegh-Guldberg (1999).Hoegh-Guldberg O. Climate change, coral bleaching and the future of the world’s coral reefs. Marine and Freshwater Research. 1999;50:839–866. doi: 10.1071/MF99078. [DOI] [Google Scholar]
- Holland (2000).Holland BS. Genetics of marine bioinvasions. Hydrobiologia. 2000;420:63–71. doi: 10.1007/978-94-017-2184-4_7. [DOI] [Google Scholar]
- Hurlbert (1971).Hurlbert SH. The nonconcept of species diversity: a critique and alternative parameters. Ecology. 1971;52:577–586. doi: 10.2307/1934145. [DOI] [PubMed] [Google Scholar]
- Johnson & Woollacott (2015).Johnson CH, Woollacott RM. Analyses with newly developed microsatellite markers elucidate the spread dynamics of Tricellaria inopinata d’Hondt and Occhipinti-Ambrogi, 1985–a recently established bryozoan along the New England seashore. Aquatic Invasions. 2015;10:135–145. doi: 10.3391/ai.2015.10.2.02. [DOI] [Google Scholar]
- KeaneCrawley (2002).Keane RM, Crawley MJ. Exotic plant invasions and the enemy release hypothesis. Trends in Ecology & Evolution. 2002;17:164–170. doi: 10.1016/S0169-5347(02)02499-0. [DOI] [Google Scholar]
- Lages, Fleury & Menegola (2011).Lages BG, Fleury BG, Menegola C, Creed JC. Change in tropical rocky shore communities due to an alien coral invasion. Marine Ecology Progress Series. 2011;438:85–96. doi: 10.3354/meps09290. [DOI] [Google Scholar]
- Liu et al. (2006).Liu J, Dong M, Miao SL, Li ZY, Song MH, Wang RQ. Invasive alien plants in China: role of clonality and geographical origin. Biological Invasions. 2006;8:1461–1470. doi: 10.1007/s10530-005-5838-x. [DOI] [Google Scholar]
- Lockwood, Cassey & Blackburn (2005).Lockwood JL, Cassey P, Blackburn T. The role of propagule pressure in explaining species invasions. Trends in Ecology and Evolution. 2005;20:223–228. doi: 10.1016/j.tree.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Mantellato & Creed (2014).Mantellato MC, Creed JC. Non-indigenous sun corals invade mussel beds in Brazil. Marine Biodiversity. 2014;45:605–606. doi: 10.1007/s12526-014-0282-8. [DOI] [Google Scholar]
- Mantellato et al. (2011).Mantellato MC, Mourão GG, Migotto A, Lindner A. Range expansion of the invasive corals Tubastraea coccinea and Tubastraea tagusensis in the Southwest Atlantic. Coral Reefs. 2011;30:397. doi: 10.1007/s00338-011-0720-z. [DOI] [Google Scholar]
- Meirmans & Hedrick (2011).Meirmans PG, Hedrick PW. Assessing population structure: FST and related measures. Molecular Ecology Resources. 2011;11:5–18. doi: 10.1111/j.1755-0998.2010.02927.x. [DOI] [PubMed] [Google Scholar]
- Meirmans & Van Tienderen (2004).Meirmans PG, Van Tienderen PH. GENOTYPE and GENODIVE: two programs for the analysis of genetic diversity of asexual organisms. Molecular Ecology Notes. 2004;4:792–794. doi: 10.1111/j.1471-8286.2004.00770.x. [DOI] [Google Scholar]
- Miranda, Cruz & Barros (2016).Miranda RJ, Cruz ICS, Barros F. Effects of the alien coral Tubastraea tagusensis on native coral assemblages in a southwestern Atlantic coral reef. Marine Biodiversity. 2016;163 doi: 10.1007/s00227-016-2819-9. Article 45. [DOI] [Google Scholar]
- Molnar et al. (2008).Molnar JL, Gamboa RL, Revenga C, Spalding MD. Assessing the global threat of invasive species to marine biodiversity. Frontiers in Ecology and the Environment. 2008;6:485–492. doi: 10.1890/070064. [DOI] [Google Scholar]
- Mooney & Cleland (2001).Mooney HA, Cleland EE. The evolutionary impact of invasive species. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5446–5451. doi: 10.1073/pnas.091093398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima et al. (2017).Nakajima Y, Nishikawa A, Iguchi A, Nagata T, Uyeno D, Sakai K, Mitarai S. Elucidating the multiple genetic lineages and population genetic structure of the brooding coral Seriatopora (Scleractinia: Pocilloporidae) in the Ryukyu Archipelago. Coral Reefs. 2017;36:415–426. doi: 10.1007/s00338-017-1557-x. [DOI] [Google Scholar]
- Nakajima et al. (2015).Nakajima Y, Shinzato C, Satoh N, Mitarai S. Novel polymorphic microsatellite markers reveal genetic differentiation between two sympatric types of Galaxea fascicularis. PLOS ONE. 2015;10:e0130176. doi: 10.1371/journal.pone.0130176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreen, Harrison & Van Oppen (2009).Noreen AME, Harrison PL, Van Oppen JH. Genetic diversity and connectivity in a brooding reef coral at the limit of its distribution. Proceedings of the Royal Society B. 2009;276:3927–3935. doi: 10.1098/rspb.2009.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaway & Kirby (1975).Ottaway JR, Kirby GC. Genetic relationships between brooding and brooded Actinia tenebrosa. Nature. 1975;255:221–223. doi: 10.1038/255221a0. [DOI] [PubMed] [Google Scholar]
- Pandolfi et al. (2003).Pandolfi JM, Bradbury RH, Sala R, Hughes TP, Bjorndal KA, Cooke RG, McArdle D, McClenachan L, Newman MJH, Paredes G, Warner RR, Jackson JBC. Global trajectories of the long-term decline of coral reef ecosystems. Science. 2003;301:955–958. doi: 10.1126/science.1085706. [DOI] [PubMed] [Google Scholar]
- Pinzón & Weil (2011).Pinzón JH, Weil E. Cryptic species within the Atlantic-Caribbean genus meandrina (scleractinia): a multidisciplinary approach and description of the new species Meandrina jacksoni. Bulletin of Marine Science. 2011;87:823–853. doi: 10.5343/bms.2010.1085. [DOI] [Google Scholar]
- Pritchard, Stephens & Donnelly (2000).Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2015).R Core Team . R Foundation for Statistical Computing; Vienna: 2015. [Google Scholar]
- Reaka-Kudla (1997).Reaka-Kudla ML. Global biodiversity of coral reefs: a comparison with rainforests. In: Reaka-Kudla ML, Wilson DE, Wilson EO, editors. Biodiversity II: understanding and protecting our biological resources. Joseph Henry Press; Washington, D.C.: 1997. [Google Scholar]
- Reitzel et al. (2008).Reitzel AM, Darling JA, Sullivan JC, Finnerty JR. Global population genetic structure of the starlet anemone Nematostella vectensis: multiple introductions and implications for conservation policy. Biological Invasions. 2008;10:1197–1213. doi: 10.1007/s10530-007-9196-8. [DOI] [Google Scholar]
- Ren, Zhang & Zhand (2005).Ren M-X, Zhang Q-G, Zhand D-Y. Random amplified polymorphic DNA markers reveal low genetic variation and a single dominant genotype in Eichhornia crassipes populations throughout China. Weed Research. 2005;45:236–244. doi: 10.1111/j.1365-3180.2005.00445.x. [DOI] [Google Scholar]
- Roman & Darling (2007).Roman J, Darling JA. Paradox lost: genetic diversity and the success of aquatic invasions. Trends Ecology Evolution. 2007;22:454–464. doi: 10.1016/j.tree.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Sakai et al. (2001).Sakai AK, Allendorf FW, Holt JS, Lodge DML, Molofsky J, With KA, Baughman S, Cabin RJ, Cohen JE, Ellstrand NC, McCauley DE, O’Neil P, Parker IM, Thompson JN, Weller SG. The population biology of invasive species. Annual Review of Ecology, Evolution, and Systematics. 2001;32:305–332. doi: 10.1146/annurev.ecolsys.32.081501.114037. [DOI] [Google Scholar]
- Sammarco, Atchison & Boland (2004).Sammarco PW, Atchison AD, Boland GS. Expansion of coral communities within the Northern Gulf of Mexico via offshore oil and gas platforms. Marine Ecology Progress Series. 2004;280:129–143. doi: 10.3354/meps280129. [DOI] [Google Scholar]
- Sammarco, Porter & Cairns (2010).Sammarco PW, Porter SA, Cairns SD. A new coral species introduced into the Atlantic Ocean–Tubastraea micranthus (Ehrenberg 1834) (Cnidaria, Anthozoa, Scleractinia): an invasive threat? Aquatic Invasions. 2010;5:131–140. doi: 10.3391/ai.2010.5.2.02. [DOI] [Google Scholar]
- Sammarco et al. (2015).Sammarco PW, Porter SA, Genazzio M, Sinclair J. Success in competition for space in two invasive coral species in the western Atlantic–Tubastraea micranthus and T. coccinea. PLOS ONE. 2015;10:e0144581. doi: 10.1371/journal.pone.0144581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio et al. (2012).Sampaio CLS, Miranda RJ, Maia-Nogueira R, Nunes JACC. New occurrences of the nonindigenous orange cup corals Tubastraea coccinea and T. tagusensis (Scleractinia: Dendrophylliidae) in Southwestern Atlantic. Check List. 2012;8:528–530. doi: 10.15560/8.3.528. [DOI] [Google Scholar]
- Santos, Ribeiro & Creed (2013).Santos LAH, Ribeiro FV, Creed JC. Antagonism between invasive pest corals Tubastraea spp. and the native reef-builder Mussismilia hispid in the southwest Atlantic. Journal of Experimental Marine Biology and Ecology. 2013;449:69–76. doi: 10.1016/j.jembe.2013.08.017. [DOI] [Google Scholar]
- Sax & Brown (2000).Sax DF, Brown JH. The paradox of invasion. Global Ecology & Biogeography. 2000;9:363–371. doi: 10.1046/j.1365-2699.2000.00217.x. [DOI] [Google Scholar]
- Sax et al. (2007).Sax DF, Stachowick JJ, Brown JH, Bruno JF, Dawson MN, Gaines SD, Grosberg RK, Hastings A, Holt RD, Mayfield MM, O’Connor MI, Rice WR. Ecological and evolutionary insights from species invasions. Trends in Ecology and Evolution. 2007;22:465–471. doi: 10.1016/j.tree.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Schuelke (2000).Schuelke M. An economic method for the fluorescent labeling of PCR fragments. Nature Biotechnology. 2000;18:223–234. doi: 10.1038/72708. [DOI] [PubMed] [Google Scholar]
- Selkoe & Toonen (2006).Selkoe KA, Toonen RJ. Microsatellites for ecologists: a practical guide to using and evaluating microsatellite markers. Ecology Letters. 2006;9:615–629. doi: 10.1111/j.1461-0248.2006.00889.x. [DOI] [PubMed] [Google Scholar]
- Shiganova (1998).Shiganova TA. Invasion of the Black Sea by the ctenophore Mnemiopsis leidyi and recent changes in pelagic community structure. Fisheries Oceanography. 1998;7:305–310. doi: 10.1046/j.1365-2419.1998.00080.x. [DOI] [Google Scholar]
- Silva et al. (2014).Silva AG, De Paula AF, Fleury BG, Creed JC. Eleven years of range expansion of two invasive corals (Tubastraea coccinea and Tubastraea tagusensis) through the southwest Atlantic (Brazil) Estuarine, Coastal and Shelf Science. 2014;141:9–16. doi: 10.1016/j.ecss.2014.01.013. [DOI] [Google Scholar]
- Stoddart (1983).Stoddart JA. Asexual production of planulae in the coral Pocillopora damicornis. Marine Biology. 1983;76:279–284. doi: 10.1007/BF00393029. [DOI] [Google Scholar]
- Taylor & Hastings (2005).Taylor CM, Hastings A. Allee effects in biological invasions. Ecology Letters. 2005;8:895–908. doi: 10.1111/j.1461-0248.2005.00787.x. [DOI] [Google Scholar]
- Toonen (1997).Toonen RJ. Microsatellites for ecologists: non-radioactive isolation and amplification protocols for microsatellite markers. 1997. http://biogeek.ucdavis.edu/Mstats/ http://biogeek.ucdavis.edu/Mstats/
- Van Oosterhout et al. (2004).Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P. Micro-checker: software for identifying and correcting genotyping errors in microsatellite data. Molecular Ecology Notes. 2004;4:535–538. doi: 10.1111/j.1471-8286.2004.00684.x. [DOI] [Google Scholar]
- Vaughan & Wells (1943).Vaughan TW, Wells JW. Revision of the suborders, families and genera of the Scleractinia. Geological Society of America Special Papers. 1943;44:1–363. [Google Scholar]
- Vitousek (1990).Vitousek PM. Biological invasions and ecosystem processes: towards an integration of population biology and ecosystem studies. Oikos. 1990;57:7–13. doi: 10.2307/3565731. [DOI] [Google Scholar]
- Warner, Van Oppen & Willis (2015).Warner PA, Van Oppen MJH, Willis BL. Unexpected cryptic species diversity in the widespread coral Seriatopora hystrix masks spatial-genetic patterns of connectivity. Molecular Ecology. 2015;24:2993–3008. doi: 10.1111/mec.13225. [DOI] [PubMed] [Google Scholar]
- Wrange et al. (2016).Wrange A-L, Charrier G, Thonig A, Rosenblad MA, Blomberg A, Havenhand JN, Jonsson PR, André C. The story of a hitchhiker: population genetic patterns in the invasive barnacle Balanus (Amphibalanus) improvisus Darwin 1854. PLOS ONE. 2016;11:e0147082. doi: 10.1371/journal.pone.0147082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright (1965).Wright S. The interpretation of population structure by F-statistics with special regard to systems of mating. Evolution. 1965;19:395–420. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Raw data used in the present work, including allele sizes for each genotyped individual.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data has been uploaded as a Supplemental File.