Abstract
Objectives
We sought to determine Thai National AIDS Program (NAP) outcomes and gaps, and success in reaching the WHO 90:90:90 goals.
Methods
Retrospective study of treatment outcomes, mortality and loss to follow-up (LTFU), of all individuals aged >15 years who registered to the NAP from 2000 to 2014. We focused outcomes on data from 2008 when the NAP was linked to the death registry.
Results
A total of 429,294 patients registered to the NAP up to November 2014, and 309,313 patients aged >15 years started ART. Median (IQR) age was 37 (31–43) years; 51% were male. From 2008 to 2014, long-term follow-up rates per 100 person-years were 3.2 in those who started ART vs 3.5 in those who did not (P<0.001) and mortality rates per 100 person-years were 3.5 in those who started ART vs 4.9 in those who did not (P<0.001). Mortality reduced from 16% in 2008 to 3% in 2014 for those who started ART. For patients starting treatment since 2000, 87% of those alive and with a recent viral load (VL) result had <50 copies/mL, and 6% had VL ≥1000 copies/mL. In a continuum-of-care analysis from 2008 to 2014, 68% were living and retained on ART, and 46% of diagnosed individuals were virally suppressed at <50 copies/mL.
Conclusions
In the Thai NAP, death and LTFU are major factors disrupting the care-continuum, and many patients initiate ART with low CD4 cell counts. Rolling out systems for early detection and treatment for all, regardless of CD4 cell count, are essential and under way.
Keywords: HIV, treatment outcomes, Thailand, universal health coverage
Introduction
Thailand has the highest adult HIV prevalence in Southeast Asia with approximately 445,000 adults aged >15 years living with HIV in 2014 [1]. Approximately 8000 new infections and 20,000 AIDS-related deaths occurred in 2014 [1].
Thailand was one of the first Asian countries to offer first-and second-line antiretroviral treatment (ART) regimens. A free, National Access to Antiretroviral Therapy Program (NAPHA) pilot programme was initiated in 2000, and this, together with lower drug prices from generic products contributed to a rapid increase in the number of patients receiving ART from approximately 1710 in 2001, to 48,101 in 2004 and 81,385 in 2006 [2,3]. In 2008, Thailand massively scaled up its ART programmes under the National AIDS Program (NAP) managed by the National Health Security Office (NHSO). This comprehensive treatment and care programme is available for all HIV-infected Thai patients without health coverage through their employers. The NAP-Plus programme additionally includes data on patients covered under the Social Security Scheme and some patients with coverage under the Civil Servant Medical Benefits Scheme. Treatment is initiated according to national treatment guidelines [4]. First- and second-line ART are provided, along with 6 monthly CD4 cell count and laboratory safety parameters, HIV RNA after 6 months of ART and yearly thereafter, and resistance testing if HIV RNA after ART is >1000 copies/mL.
Reporting outcomes of patients in national ART programmes is critical to demonstrate programme effectiveness and identify opportunities for improvement. Several studies have demonstrated the feasibility of implementing HAART in developing countries, with outcomes often similar to those in developed countries [5–7]. In this study we conducted a retrospective review of adult patients initiating ART from 2000 to 2014 through the Thai NAP. Our aims were to describe the continuum of patient care after diagnosis, causes of dropout, virological outcomes and mortality rates in the largest observational cohort of HIV-infected patients aged ≥15 years, in a resource-limited setting in Asia.
Methods
Antiretroviral treatment programme and settings
The history and milestones of the ART programme in Thailand from 2000 to 2007 have previously been described [3]. With rapid scale-up in 2008, 1001 hospitals from 77 provinces were recruited to work with the NAP. Each hospital has at least one HIV study co-ordinator, and healthcare professionals who receive regular training. Non-governmental organisation (NGO) staff and people living with HIV (PLWH) provide adherence counselling, non-clinic-based care or home visits if needed. ART drug supply is managed by the NHSO and Government Pharmaceutical Organization (GPO) using a vendor-managed inventory system, ensuring ARV supply to sites within 3 days of placing an order.
ART eligibility and regimens
From 2001 to 2007, HIV-infected individuals were eligible for ART with CD4 cell counts ≤200 cells/mm3, or if they had a symptomatic HIV diagnosis with a CD4 cell count of 200–250 cells/mm3. The Centers for Disease Control and Prevention (CDC) clinical stages A, B and C were used to define whether patients had asymptomatic HIV, symptomatic HIV or AIDS, respectively. In 2008, eligibility criteria for ART initiation were expanded to all HIV-infected patients with CD4 cell counts ≤250 cells/mm3, or a symptomatic HIV diagnosis with a CD4 cell count ≤350 cells/mm3. Thai national HIV treatment guidelines were updated in 2010 [8], and criteria for ART initiation in asymptomatic HIV were changed to CD4 cell counts ≤350 cells/mm3 or symptomatic HIV/AIDS at any CD4 cell count. The most recent guidelines from 2014 recommend initiating ART at any CD4 cell count [4].
Until 2010, first-line treatment regimens were zidovudine or stavudine (d4T) with lamivudine and nevirapine (fixed-dose combination of GPOvir S: stavudine /lamivudine/nevirapine; or GPOvir Z: zidovudine 250 mg /lamivudine/nevirapine). Stavudine phase-out was applied in the 2010 national guidelines, and current recommended first-line regimens are tenofovir plus lamivudine or emtricitabine plus efavirenz [4].
Second-line regimens for those failing NNRTI-based regimens are two new NRTIs (or at least one active drug) plus boosted protease inhibitors recommended by the 2014 national treatment guidelines. Lopinavir/ritonavir is the preferred option due to its lower cost; the alternative is atazanavir/ritonavir [4]. Third-line regimens are not yet fully supported by NAP.
Patient monitoring
After treatment initiation, visits are scheduled monthly for 3 months, then 3-monthly thereafter. Laboratory testing is performed according to routine procedures at primary or referral hospitals. CD4 T cell testing is carried out by flow cytometry, and HIV RNA testing is performed with Thai FDA approved commercially available assays. All laboratory sites for CD4 cell, HIV RNA and resistance testing have been validated and are prospectively monitored by the Medical Technology Council.
Programme monitoring
An electronic database was created for the NAP, and local healthcare providers complete visit-specific online forms at each patient visit. Gender, date of birth, body weight, HIV transmission risk, CDC staging, active opportunistic infections (OI) and CD4 cell counts are recorded at registration. At all subsequent visits, patient weight, OIs, comorbidities and/or ART toxicities such as diabetes mellitus, hypertension, lipodystrophy are captured, in addition to ART regimen details, concomitant medications, safety labs, and ART resistance profiles if indicated are recorded.
The National Death Registry has been electronically linked to the NHSO database by a 13 digit National Identification Number since 2008; Thai Law requires deaths to be reported within 24 hours.
In Bangkok, an HIV expert committee comprising HIV specialists from all university hospitals and NHSO officers, review 3-monthly reports summarising the frequency of HIV-RNA testing, and proportion of patients with HIV RNA <50 copies/mL or >200 copies/mL. Hospitals where the proportion of patients with undetectable HIV-RNA is <75%, or HIV-RNA testing is less than once a year are re-trained in Programme procedures.
Ethics approval
This study was approved by the Institutional Review Board (IRB) of the Institute for Development of Human Research Protection, Ministry of Public Health, Thailand. NAP data were extracted from the NHSO database and de-identified by NHSO personnel before analysis.
Statistical analysis
Statistical analysis was conducted with SAS version 9 (SAS Institute, Cary, NC, USA) and Stata version 12 (StataCorp, College Station, TX, USA). We described outcomes in the cohort of patients aged >15 years, who had registered to the NAP between January 2000 and November 2014. Descriptions were made separately by period of ART initiation 2000–2007, and 2008 –2014 after cohort linkage with the death register. Death and lost to follow-up (LTFU) in subjects who registered to the programme were described separately by calendar year and period of programme registration. LTFU was defined as not attending a clinic visit for ≥6 months and ≥12 months for patients starting ART and those who did not, respectively, and with no date of death recorded in the death registry. Subjects with follow-up information who were lost from the programme were censored at their last clinic visit. In these analyses, baseline was defined as the date of ART initiation. Baseline CD4 cell count was measured within a 1-year window before, to 1 month after the ART initiation date; when more than one result fell within the window period, the measurement closest to the ART initiation date was used. We described the proportion of subjects who had a plasma HIV-RNA assessment >6 months and ≤1 year after initiating ART, and the proportion of patients with viral load <50 copies/mL, or >1000 copies/mL at the most recent clinic visit, by calendar year of follow-up. We also described the proportion of subjects with undetectable viral load, who had repeated viral load tests according to the recommended monitoring schedule. A z-test of independent proportions was used to compare lost to follow-up and mortality proportions in patients starting ART versus those who did not. Formal comparisons of subject characteristics by period of starting ART were made using a Student's t-test or Wilcoxon test for continuous covariates, and a chi-square test for categorical covariates. Crude mortality and LTFU rates were calculated based on patient years of follow-up.
As a quality improvement exercise, we conducted additional analyses in the NAP database, 2008–2010, and 2011–2014, for patients with at least 1 year of follow-up, in line with the UNAIDS 90-90-90 target recommendations in 2014 [9]. We calculated the percentage of patients who were prescribed ART, and who had HIV viral suppression, from the denominator of patients in whom an HIV diagnosis was reported.
Results
NAP: subject disposition and baseline characteristics
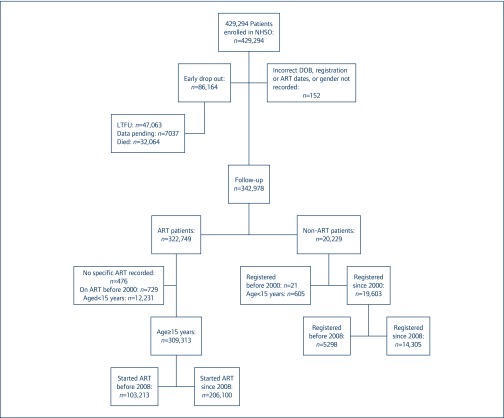
The subject disposition is shown in Figure 1. Of the 429,294 patients registered to the NAP up to November 2014, 152 (0.04%) were excluded predominantly due to errors in date fields, and 86,164 (20%) had only a registration visit recorded. Of those with only a registration visit recorded, 47,063/86,164 (53%) were lost or transferred to another ART programme, 32,064 (37%) died and 7037 (8%) had registered to the programme in 2014 and had follow-up visit data pending.
Figure 1.
The National AIDS Program (NAP) database description and analysis population. LFTU: lost to follow-up
The majority of patients (342,978/429,294, 80%) had subsequent follow-up visits. Of these, 322,749 (94%) patients started ART; 12,231 paediatric patients aged <15 years and 1205 patients on ART (476 with no ART information and 729 who started ART before 2000) were excluded. Characteristics of the 309,313 adults who started ART in or after 2000 are shown in Table 1. Approximately 67% of patients started ART in or after 2008; just over 50% were males with a median (interquartile range [IQR]) age at ART initiation of 37 (31–43) years. Although national guidelines shifted towards ART initiation at CD4 cell count <350 cells/mm3 in 2010, and any CD4 cell count in 2014 [4,8], many patients started ART with low CD4 cell counts. In 137,484/206,409 patients with a CD4 cell count recorded at ART initiation between 2008 and 2014, the median (IQR) CD4 cell count was 115 (35–244) cells/mm3, and many had symptomatic HIV disease or clinical AIDS. Ninety-three percent of subjects initiated ART with NNRTI-based ART, most commonly nevirapine-based regimens.
Table 1.
Characteristics of NAP patients starting antiretroviral therapy from 2000 to 2014, by period of initiation
| Characteristic | Years | Years | Total | P-value |
|---|---|---|---|---|
| 2000–2007
n |
2008–2014
n |
n | ||
| Participants | 103,213 | 206,100 | 309,313 | |
| Male n(%) | 52,981 (51) | 110,409 (54) | 163,390 (53) | <0.001 |
| Median (IQR) age at ARV initiation (years) | 36 (32–41) | 37 (31–43) | 37 (31–43) | <0.001 |
| Median (IQR) years of follow-up | 7.5 (7.1–8.4) | 2.5 (1–4.5) | 4.2 (1.6–7.1) | <0.001 |
| Year of starting ART, n (%) | ||||
| 2000 | 395 (0.4) | 395 (0.1) | ||
| 2001 | 715 (0.7) | 715 (0.2) | ||
| 2002 | 2046 (2) | 2046 (1) | ||
| 2003 | 4260 (4) | 4260 (1) | ||
| 2004 | 8128 (8) | 8128 (3) | ||
| 2005 | 9702 (9) | 9702 (3) | ||
| 2006 | 13,513 (13) | 13,513 (4) | ||
| 2007 | 64,454 (62) | 64,454 (21) | ||
| 2008 | 30,451 (15) | 30,451 (10) | ||
| 2009 | 27,905 (14) | 27,905 (9) | ||
| 2010 | 29,241 (14) | 29,241 (9) | ||
| 2011 | 28,342 (14) | 28,342 (9) | ||
| 2012 | 32,238 (16) | 32,238 (10) | ||
| 2013 | 34,742 (17) | 34,742 (11) | ||
| 2014 | 23,181 (11) | 23,181 (7) | ||
| Partcipants with a CD4 cell count at ART initiation n (%) | 18,399 (18) | 137,484 (67) | 155,883 (50) | |
| Median (IQR) (cells/mm3) | 192 (69–371) | 115 (35–244) | 125 (37–257) | <0.001 |
| First regimen | <0.001 | |||
| NNRTI-based (all) | 98,736 (96) | 188,495 (91) | 287,231 (93) | |
| NVP-based | 81,087 (79) | 121,891 (59) | 202,978 (66) | |
| EFV-based | 17,649 (17) | 66,604 (32) | 84,253 (27) | |
| PI-based | 3223 (3) | 14,982 (7) | 18,205 (6) | |
| Other | 1254 (1) | 2623 (1) | 3877 (1) | |
| HIV clinical stage at baseline | <0.001 | |||
| Asymptomatic HIV | 23,531 (23) | 95,878 (47) | 119,409 (39) | |
| Symptomatic HIV | 24,368 (24) | 47,742 (23) | 72,110 (23) | |
| AIDS | 43,115 (42) | 58,714 (28) | 101,829 (33) | |
| Unknown | 12,199 (12) | 3766 (2) | 15,965 (5) |
ART: antiretroviral therapy; ARV antiretroviral; EFV: efavirenz; IQR: interquartile range; NNRTI: non-nucleoside reverse transcriptase inhibitors; NVP: nevirapine; PI: protease inhibitors.
Loss to follow-up and mortality rates
Sixty-four percent of those with follow-up (220,405/342,978) registered to the programme since 2008, and 87% (206,100/220,405) started treatment after enrolment. A further 19,603 had not yet started ART but remained in follow-up; 21 who registered to the programme before 2000 and 605 aged <15 years were excluded from analysis. The majority of patients not taking ART (14,305/19,603, 73%) registered since 2008. Median (IQR) age at registration was 35 (28–42) years, median CD4 cell count was 395 (IQR 93–571) cells/mm3 and 8555(59%) were male (Table 2). Total follow-up time in these patients was 106,782 years; 37% died and 26% were lost to follow-up. The crude mortality rate was 4.9 (4.8–5.0) per 100 person-years of follow-up (PYFU), and the lost to follow-up rate was 3.5 (3.3–3.6) PYFU.
Table 2.
Characteristics of patients with follow-up visits who did not start ART, cared for under the NAP, by period of programme registration
| Characteristic | Years | Years | Total | P-value |
|---|---|---|---|---|
| 2000–2007 | 2008–2014 | |||
| n | n | n | ||
| Participants | 5298 | 14,305 | 19,603 | |
| Male, n (%) | 2316 (44) | 8555 (60) | 10,871 (55) | <0.001 |
| Median (IQR) age at registration | 33 (27–39) | 35 (28–42) | 34 (28–42) | <0.001 |
| Year of registration | ||||
| 2000 | 13 (0.2) | 13 (0.07) | ||
| 2001 | 27 (0.5) | 28 (0.1) | ||
| 2002 | 127 (2.4) | 131 (0.7) | ||
| 2003 | 628 (11.8) | 649 (3.3) | ||
| 2004 | 1030 (19.4) | 1077 (5.5) | ||
| 2005 | 869 (16.4) | 911 (4.6) | ||
| 2006 | 525 (9.9) | 565 (2.9) | ||
| 2007 | 2079 (39.2) | 2205 (11.2) | ||
| 2008 | 1942 (13.6) | 2042 (10.4) | ||
| 2009 | 1996 (14.0) | 2054 (10.5) | ||
| 2010 | 1938 (13.5) | 1993 (10.2) | ||
| 2011 | 1763 (12.3) | 1801 (9.2) | ||
| 2012 | 2111 (14.8) | 2138 (10.9) | ||
| 2013 | 2386 (16.7) | 2413 (12.3) | ||
| 2014 | 2169 (15.1) | 2188 (11.2) | ||
| Median (IQR) years of follow-up (years) | 3.4 (1.2–6.7) | 0.5 (0.1–1.9) | 1 (0.2–3.1) | <0.001 |
| Person-time years | 262,445 | 106,782 | 369,227 | |
| Participants who died, n (%) | 2482 (47) | 5235 (37) | 7717 (39) | <0.001 |
| Crude death rate per 100 PYFU (95%CI) | 0.9 (0.9–1) | 4.9 (4.8–5.0) | 2.1 (2–2.1) | |
| LTFU, n (%) | 1789 (34) | 3685 (26) | 5474 (28) | <0.001 |
| Lost rate per 100 PYFU (95%CI) | 0.68 (0.65–0.71) | 3.5 (3.3–3.6) | 1.48 (1.44–1.52) | |
| CD4 cell count at registration, n (%) | 3057 (58) | 11,750 (82) | 14,807 (76) | |
| Median (IQR) CD4 cell count at registration (cells/mm3) | 450 (255–654) | 395 (93–571) | 404 (117– 589) | <0.001 |
IQR: interquartile range; LTFU: lost to folllow-up; PYFU: person-years follow-up
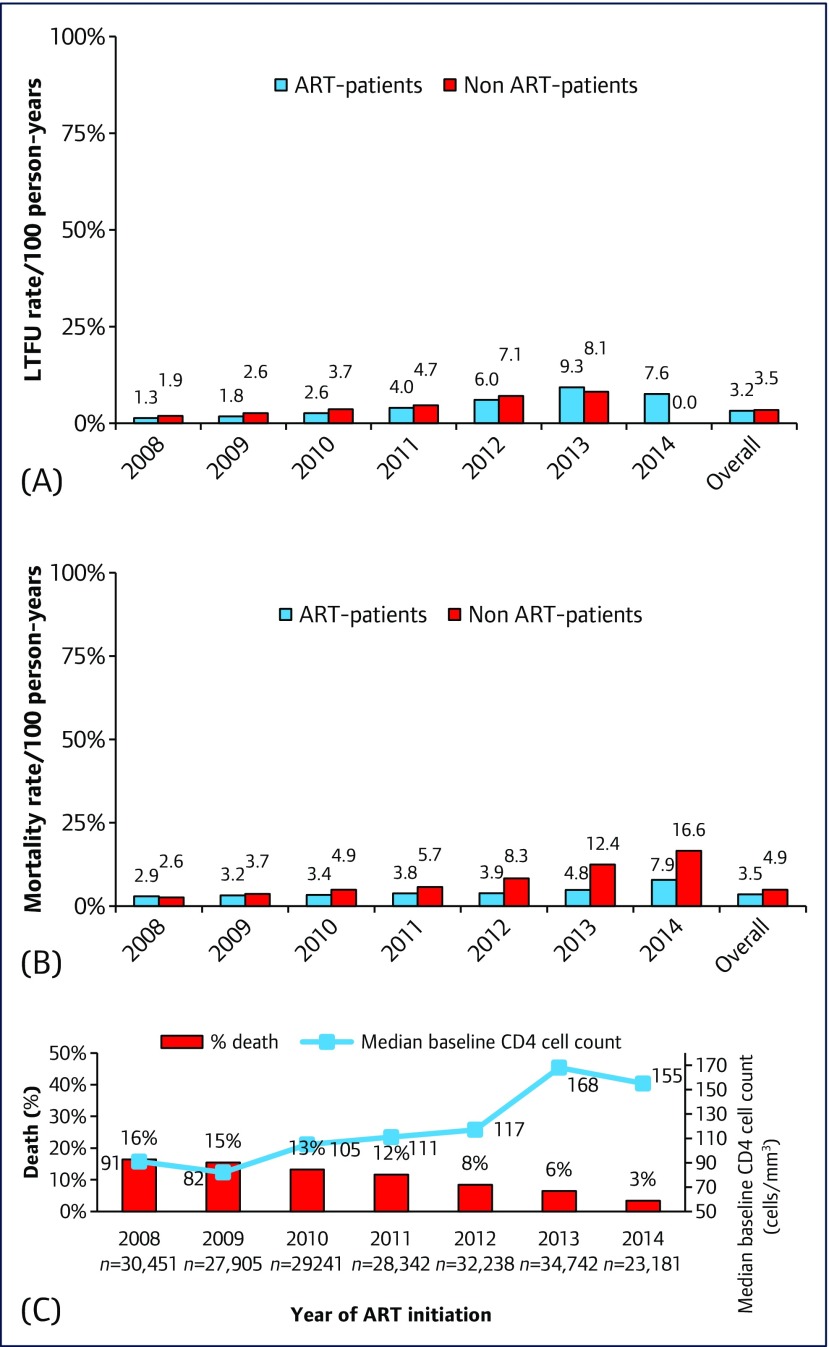
In the period after linkage with the death register, the mortality rates were significantly higher in those who registered to the programme but did not start ART, versus those who started ART in the same calendar year (Figures 2A and 2B), whereas the LTFU rate was similar. Over 1,437,767 PYFU, 34,531 patients died. The crude mortality rate in the period from 2008 to 2014 was 3.5 (95% CI 3.46–3.55), and the LTFU rate was 3.22 (95% CI 3.18–3. 27) (Table 3). The proportion of patients who died decreased with year of ART initiation and corresponded with increasing median CD4 cell counts at cART initiation (Figure 2C).
Figure 2.
(A) Loss to follow-up rates in the NAP, by year of ART-initiation (ART patients) or year of registration (non-ART patients). (B) Mortality rates in the NAP, by year of ART-initiation (ART patients) or year of registration (non ART-patients). (C) Proportion of subjects who started ART in the NPA and died, against the median patient baseline CD4 cell count, by year of ART initiation. Difference in proportions in Figures 2A and 2B is significant in each year at P<0.001
Table 3.
Treatment outcomes and ART regimens of ART-patients under the NAP at most recent visit, by period of ART initiation
| Characteristic | Years | Years | Total | P-value |
|---|---|---|---|---|
| 2000–2007 | 2008–2014 | |||
| Participants (n) | 103,213 | 206,100 | 309,313 | |
| Median (IQR) current age (years) | 44(39–49) | 40(34-–46) | 42(36–47) | <0.001 |
| Median(IQR) time on ART(years) | 7.5(7.1–8.4) | 2.5(1–4.5) | 4.2(1.6–7.1) | <0.001 |
| Person-time of follow-up(years) | 802,858 | 634,909 | 1,437,767 | |
| Participants who died, n(%) | 12,291(12) | 22,240(11) | 34,531(11) | <0.001 |
| Crude death rate per 100 PYFU(95%CI) | 1.53(1.50–1.56) | 3.5(3.46–3.55) | 2.4(2.38–2.43) | |
| Lost to follow-up, n(%) | 5693(6) | 20,465(10) | 26,158(8) | <0.001 |
| Lost to follow-up rate, per 100 PYFU(95%CI) | 0.71(0.69–0.73) | 3.22(3.18–3.27) | 1.82(1.80–1.84) | |
| Viral load tested at last visit, n (%) | 94,394(91) | 155,911(76) | 250,305(81) | |
| Patients with <50 copies/mL, n(%) | 84,493(90) | 132,686(85) | 217,179(87) | <0.001 |
| <200 copies/mL, n(%) | 88,181(93) | 141,800(91) | 229,981(92) | <0.001 |
| ≥1,000 copies/mL, n(%) | 4,598(5) | 10,644(7) | 15,242(6) | <0.001 |
| CD4 cell count tested at last visit, n(%) | 99,970(97) | 195,078(95) | 295,048(95) | |
| Median(IQR) current CD4 cell count(cells/mm3) | 496(346–669) | 368(208–531) | 411(247–582) | <0.001 |
| Current regimen | <0.001 | |||
| NNRTI-based | 89,538(87) | 178,880(87) | 268,418(87) | |
| PI-based | 12,275(12) | 25,281(12) | 37,556(12) | |
| Third-line-based(DRV/r, ETR, RAL) | 88(0.1) | 124(0.1) | 212(0.1) | |
| Others | 1312(1) | 1815(1) | 3127(1) |
ART: antiretroviral therapy; DRV/r: ritonavir boosted darunavir; ETR: etravirine; NNRTI: non-nucleoside reverse transcriptase inhibitor; PI: protease inhibitor; PYFU: Person-years follow-up; RAL: raltegravir
Virological control rates and CD4 cell count outcomes
Immunological and virological outcomes of adults who started ART are described in Table 3. After excluding patients LTFU and those who died, at the patient's most recent visit, median CD4 cell count was 411 (247–582) cells/mm3, and 132,686/155,911 (85%) patients with a viral load test who started treatment between 2008 and 2014 had a plasma viral load of <50 copies/mL, 15,242 (6%) had a viral load >1000 copies/mL, and the majority (87%) remained on NNRTI-based regimens. These proportions were similar to patients who started treatment in the period 2000–2007.
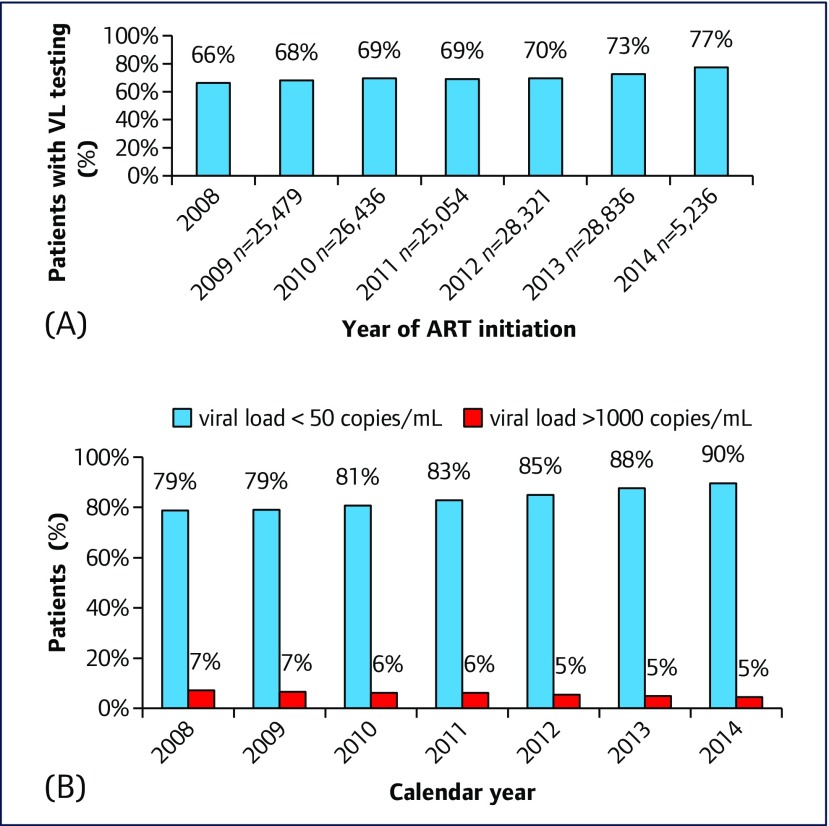
The proportion of patients starting treatment from 2008 to 2014 who had a plasma HIV-RNA test according to guidelines (i.e. >6 months and <1 year after starting ART) increased by 10%, from 66% in 2008 to 77% in 2014 (Figure 3A). In those who had repeated viral load testing, the percentage with viral load <50 copies/mL increased from 79% in 2008 to 90% in 2014. The percentage of patients with viral load >1000 copies/mL decreased from 7% to 5% in the same period (Figure 3B).
Figure 3.
(A) Proportion of patients, starting ART in NAP between 2008 and 2014, who had a plasma HIV-1 RNA (viral load, VL) test >6 months and <1 year after ART initiation. (3) Proportion of patients in the NAP program, with plasma HIV-1 RNA (VL) < 50 copies/mL and VL > 1,000 copies/mL, who had repeated viral load tests, and initiated treatment from 2008–2014
HIV care cascade
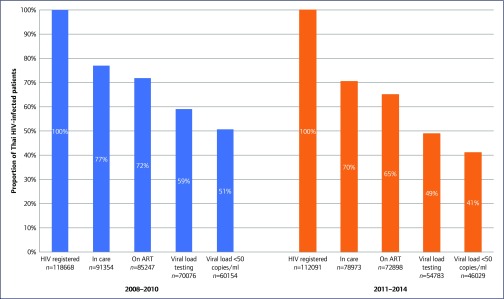
In the HIV care continuum analyses, 23% (11% died and 12% LTFU) and 30% (8% died and 22% LTFU) of patients diagnosed with HIV and registered in the NAP system were lost from the cascade before initiating ART in the periods from 2008 to 2010 and 2011 to 2014, respectively (Figure 4). An additional 13% (6% died, 2% LTFU and 5% no VL testing) and 16% (4% died, 4% LTFU, and 8% no VL testing) were lost from the cascade after starting ART in the periods 2008–2010 and 2011–2014, respectively, and an additional 8% in each period failed to achieve virological suppression. Therefore, in November 2014, of 230,759 patients who knew their HIV status, 158,145 (68%) were treated with ART, and only 106,188 (46%) were virally suppressed.
Figure 4.
HIV care continuum in the NAP from 2008 to 2010, and 2011 to 2014. Estimates for 2011–2014 are those patients with at least 1 year of follow-up
Discussion
Thailand provides HIV treatment and care through a National Universal Healthcare policy, and has recently reaffirmed its commitment to end AIDS by 2030 in line with UNAIDS strategic goals [10]. Thailand's existing healthcare infrastructure together with real-time HIV service reimbursement-based and ARV supply-based national databases linked with the death registry, gave us an opportunity to investigate outcomes and leakage from the HIV continuum of care. These data also provided the opportunity to analyse barriers to reaching the UNAIDS target of 90-90-90 by 2020, and 95-95-95 by 2030.
Leakage from the HIV care system
Of 230,759 HIV-seropositive cases reported registered to the NHSO system in the period 2008–2013 and followed-up until 2014, 74% were retained in care. However, only 68%, 54% and 46% were prescribed ART, had viral load testing and had reported HIV viral load <50 copies/mL, respectively. Leakage between registration and ART initiation was approximately 26%: the causes of leakage at this first stage were pre-ART death (10%), and LTFU or failure to link to care (16%). This translates to 68% of diagnosed people prescribed ART, 79% (124,859/158,145) of people initiated on treatment with viral load testing, and 85% (106,183/124,859) of people retained in care with virological suppression.
Of the individuals prescribed ART, 14% were not retained (5% died, 3% were lost to follow-up and 6% did not have viral load testing), and 8% were not virally suppressed. Comparison of treatment cascade information between reports is complicated by different methodologies and denominators that are applied [11]. However, large disparities in the treatment cascade had been noted in high-income countries. Of the total number of PLWH in the US, only 25.3% are reported to be virally supressed [12]. Similar comparisons in Australia and Denmark were 72% and 70%, respectively [13,14]. Estimates in low-middle income countries are also highly variable. In Latin America, the proportion of ART patients retained on ART in the period 2011–2012 was 92% in Mexico, 80% in Brazil and 53% in Colombia; only 25% of PLWH in Mexico and 14% of PLWH in Columbia had undetectable viral load; no viral load data were reported for Brazil [15].
Outcomes of the NAP programme
Based on 206,100 individuals with 634,909 PYFU since the automated linkage with the death register, we noted a dramatic decline in mortality from 16% in 2008 to only 6% in 2013 (Figure 2C). The most likely reason is because patients initiated ART with higher baseline CD4 cell counts than in earlier years. However, other possible factors include availability of better ARV regimens (from zidovudine or stavudine to tenofovir disoproxil fumarate; and from nevirapine to efavirenz), more experienced physicians and healthcare workers and a standard protocol for providing patient care. High mortality rates in patients presenting with low CD4 cell counts have been noted in many settings [6,15]. Our crude death rate between 2008 and 2014 was 3.5/100 person-years (PY) in patients taking ART. This is similar to the crude mortality rate of 3.2/100 PYFU for individuals commencing ART in Uganda in a publicly funded HIV management programme [16]. As in our study, mortality rates decreased over time among PLWH in the Botswana National HIV/AIDS Treatment programme, and patients treated with tenofovir had better outcomes than those taking stavudine or zidovudine [5].
Gaps and room for improvement in the Thailand NAP
Several areas for improvement were identified from our Thai NAP analysis. First, patients were diagnosed or commenced ART with low CD4 cell counts. Second, death was significantly higher amongst those who had not started ART, whereas LTFU rates were similar for both groups (Figures 2A and B). Third, in the most recent year with complete data capture, viral load tests were performed in only 73% of patients who were alive and taking ART for ≥6 months (Figure 3A). Therefore, effective implementation of the new Thai guidelines in commencing ART regardless of CD4 cell count is critical to avoid both loss of life and loss from the care cascade.
Strengths and challenges for Thailand to end AIDS by 2030
Thailand has a number of strengths that make improvements to the HIV continuum care system feasible, and strengthen its ability to achieve the UNAIDS target of 90-90-90. First, there is clear leadership and commitment of relevant stakeholders including the government, the Ministry of Public Health (MOPH), and NHSO. Second, strong HIV working committees comprising persons from the MOPH, NHSO, academia, NGOs and people living with HIV/AIDS, work at the country and provincial levels to solve system problems. Third, a reliable national database is in place that is linked to the National Death Registry, and this allows performance monitoring of each province to the hospital level. Fourth, high-quality implementation science and clinical research addresses knowledge gaps relating to policies and/or guidelines and is conducted within Thailand and opportunities exist for close collaboration with international organisations such as UNAIDS. Lastly, the availability of generic, low-cost antiretrovirals through the Governmental Pharmaceutical Organization (GPO) favourably impacts on budgets and programme sustainability.
Despite these strengths, ending AIDS by 2030 will be challenging for Thailand and many other countries. Important challenges include earlier diagnosis and treatment, effectively monitoring and improving care services of each site, and integrating pre-exposure prophylaxis (PrEP) for key populations with high HIV incidence into the NAP.
Informing evidence-based strategies for earlier diagnosis and treatment
A number of implementation research studies are exploring approaches and/or systems for early diagnosis and treatment, before widespread rollout. In 2012, the Thai Red Cross AIDS Research Center (TRC-ARC) in collaboration with the Department of Disease Control (DDC) of the MOPH, conducted the first test-and-treat study among 810 Thai men who have sex with men (MSM) and transgender women (TG) in Bangkok, Ubonratchathani, Lampang and Mahasarakam [17]. HIV prevalence was 16.5%, 49% had CD4 cell counts >350 cells/mm3, and immediate ART acceptance rate was 83%. Among MSM and TG who started ART, detectable HIV RNA in blood, semen and rectal secretions decreased from 100%, 61% and 67% at baseline to 3.5%, 1.2% and 1.4% at month 12, respectively [18]. Among those who were initially HIV-negative, HIV incidence was 5.5/100 PYFU despite provision of intensive risk-reduction counselling, condoms, lubricants, post-exposure prophylaxis (PrEP), and screening and treatment of asymptomatic sexually transmitted infections (STI). These data highlighted the importance of including PrEP in the 2014 Thai national guidelines on HIV treatment and prevention. In 2015, the TRCARC, DDC, the Thai MOPH-US CDC Collaboration, and USAID/PEPFAR launched parallel implementation studies to explore test-and-treat and PrEP strategies in hospital-based and community-based settings, in a study involving approximately 8000 MSM and TG in seven provinces [19,20]. The community-based model is currently implemented in six community-based organisations (CBO) in Bangkok, Chiang Mai, Chonburi and Songkhla. HIV testing, STI screening, point-of-care CD4 cell count, immediate ART referral, and PrEP services in the community-based model are provided solely by trained CBO staff, with continued quality assessment and quality improvement processes managed by the TRCARC. Preliminary data demonstrated high HIV prevalence of 16%, and 56% had CD4 cell counts above 350 cells/mm3. The majority (82%) of those who were diagnosed with HIV infection successfully commenced ART within 15 days after diagnosis. Knowledge gained from such programmes is important for scaling up treatment for all, regardless of CD4 cell count, and also for preventing infections in high-risk key populations with PrEP.
There are several limitations of our study. It is well known that outcomes appear to improve with longer periods in care, and thus some outcomes appear better in the period 2000–2007 than in the period 2008–2014, since the follow-up in the former group is longer. The actual number of PLWH in Thailand is not known. It is therefore not possible to estimate the number of PLWH who are recruited to care following a diagnosis. Although the NAP database includes most PLWH, some who are civil servants and those who are treated in private hospitals are not included and the actual numbers are not known. Nonetheless, the detailed analyses of NAP in this study of 309,313 HIV-infected individuals with a total of 1,437,767 (PYFU) provides a meaningful snapshot of outcomes, and guidance for improving the overall national HIV universal coverage programme of Thailand.
In conclusion, the Thai NAP has led to a continuing decline in mortality in the past decade, although gaps of late diagnosis and linkage to commencing ART remain. Rolling out of new guidelines for ART initiation regardless of CD4 cell count, novel programmes aiming to improve early diagnosis and treatment of PLWHA, and the integration of PrEP programmes among key populations will help Thailand improve the HIV continuum care cascade, and improve the chances of successfully ending AIDS by 2030.
Acknowledgements
Conflicts of interests
KR has received the Senior Research Scholar from Thailand Research Fund (TRF) and received honoraria or consultation fees from Merck, Roche, Jensen-Cilag, Tibotec, Mylan and the Governmental Pharmaceutical Organization, Thailand). He has also participated in a company-sponsored speaker's bureau from Abbott, Gilead, Bristol-Myers Squibb, Merck, Roche, Jensen-Cilag, GlaxoSmithKline, and Thai GPO. The remaining authors declare no conflict of interests.
Funding
No specific funding source.
Authors’ contributions
SC, SB, KR, AT, PS, SO, AA, PB, NP, SS, SM, PP designed aspects of the study. SC, BS, KR, AT, PS, SO, AA, PB, NP, SS, SM, PP were responsible for data collection or oversaw program implementation. KR, ST, SJK, AT conducted and advised on the analysis. KR, AA, ST, SJK, NP drafted the manuscript. All authors critically reviewed the manuscript and approved the manuscript for submission.
References
- 1. Thai National AIDS Committee Thailand AIDS Response Progress Report. Thailand: 2015. Available at: www.unaids.org/sites/default/files/country/documents/THA_narrative_report_2015.pdf ( accessed September 2017).
- 2. Chasombat S, Lertpiriyasuwat C, Thanprasertsuk S et al. . The national access to antiretroviral program for PHA (NAPHA) in Thailand. Southeast Asian J Trop Med Public Health 2006; 37: 704– 715. [PubMed] [Google Scholar]
- 3. Chasombat S, McConnell MS, Siangphoe U et al. . National expansion of antiretroviral treatment in Thailand, 2000–2007: program scale-up and patient outcomes. J Acquir Immune Defic Syndr 2009; 50: 506– 512. [DOI] [PubMed] [Google Scholar]
- 4. Manosuthi W, Ongwandee S, Bhakeecheep S et al. . Guidelines for antiretroviral therapy in HIV-1 infected adults and adolescents 2014, Thailand. AIDS Res Ther 2015; 12: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Farahani M, Price N, El-Halabi S et al. . Trends and determinants of survival for over 200 000 patients on antiretroviral treatment in the Botswana National Program: 2002–2013. AIDS 2015; 30: 477– 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang F, Dou Z, Ma Y et al. . Five-year outcomes of the China National Free Antiretroviral Treatment Program. Ann Intern Med 2009; 151: 241– 251. [DOI] [PubMed] [Google Scholar]
- 7. Srikantiah P, Ghidinelli M, Bachani D et al. . Scale-up of national antiretroviral therapy programs: progress and challenges in the Asia Pacific region. AIDS 2010; 24 Suppl 3: S62– 71. [DOI] [PubMed] [Google Scholar]
- 8. Sungkanuparph S, Techasathit W, Utaipiboon C et al. . Thai national guidelines for antiretroviral therapy in HIV-1 infected adults and adolescents 2010. Asian Biomedicine 2010; 4: 515– 528. [Google Scholar]
- 9. UNAIDS 90-90-90: An ambitious treatment target to help end the AIDS epidemic. 2014. Available at: www.unaids.org/en/resources/documents/2017/90-90-90 ( accessed September 2017).
- 10. UNAIDS Thailand committed to ending AIDS by 2030. 2014. Available at: https://unaidsap.wordpress.com/2014/10/27/1278/ ( accessed September 2017).
- 11. Medland NA, McMahon JH, Chow EP et al. . The HIV care cascade: a systematic review of data sources, methodology and comparability. J Int AIDS Soc 2015; 18: 20634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Skarbinski J, Rosenberg E, Paz-Bailey G et al. . Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern Med 2015; 175: 588– 596. [DOI] [PubMed] [Google Scholar]
- 13. Helleberg M, Haggblom A, Sonnerborg A, Obel N. HIV care in the Swedish-Danish HIV cohort 1995–2010, closing the gaps. PLoS One 2013; 8: e72257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kirby Institute HIV, viral hepatitis and sexually transmissible infections in Australia Annual Surveillance Report 2015. Available at: https://kirby.unsw.edu.au/report/annual-surveillance-report-hiv-viral-hepatitis-stis-2015 ( accessed September 2017).
- 15. Pineirua A, Sierra-Madero J, Cahn P et al. . The HIV care continuum in Latin America: challenges and opportunities. Lancet Infect Dis 2015; 15: 833– 839. [DOI] [PubMed] [Google Scholar]
- 16. Mills EJ, Bakanda C, Birungi J et al. . Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann Intern Med 2011; 155: 209– 216. [DOI] [PubMed] [Google Scholar]
- 17. Phanuphak N, Chamnan P, Pathipvanich P et al. . Factors associated with uptake of immediate ART regardless of CD4 count among Thai MSM and TG in the Test and Treat program. International AIDS Conference, July 2014, Melbourne, Australia. Abstract WEPE431.
- 18. Pattanachaiwit S, Pankam T, Chamnan P et al. . Prevalence of STI and HIV RNA levels in ano-genital compartments among Thai MSM and transgender women after antiretroviral therapy: implications for treatment as prevention program. 8th IAS Conference on HIV Pathogenesis, Treatment and Prevention, July 2015, Vancouver, Canada. Abstract WEPEC635.
- 19. Thai Red Cross AIDS Research Centre Study to evaluate the feasibility of community-based test and treat strategies among men who have sex with men and transgender women to increase the uptake of HIV testing and treatment services in Thailand. 2015-ongoing Available at: www.clinicaltrials.gov/ct2/show/NCT02383602 ( accessed September 2017).
- 20. Thai Red Cross Research Centre Evaluation of a facility-based test, treat, and prevent HIV program among men who have sex with men and transgender women in Thailand. 2015-ongoing Available at: www.clinicaltrials.gov/ct2/show/NCT02369887 ( accessed September 2017). [DOI] [PMC free article] [PubMed]