Abstract
Background
High prices of direct acting antivirals (DAAs) for hepatitis C virus (HCV) can lead to restrictions on access to treatment in high- and middle-income countries. An increasing number of people in these countries are treating their HCV infection with generic drugs produced in India, China, Bangladesh or Egypt. This analysis assessed the efficacy of generic imported DAAs.
Methods
Patients sourced generic versions of sofosbuvir (SOF), ledipasvir (LDV) and daclatasvir (DCV) from suppliers in India, Bangladesh, China and Egypt via three buyers’ clubs. The choice of DAAs and the length of treatment were determined on baseline RNA levels, HCV genotype and stage of fibrosis. Patient HCV RNA levels were evaluated pre-treatment, during treatment, at end of treatment (EOT) and then for sustained virological response (SVR) at 4, 12, and 24 weeks, normally by a treating clinician.
Results
Overall 616 patients submitted results: 199 from an Australian buyers’ club, 205 from a South-east Asian buyers’ clubs, and 212 from an Eastern European buyers’ club. Of the 616 patients treated, 276 received SOF/LDV (35 with ribavirin [RBV]) and 340 received SOF/DCV (61 with RBV). At baseline, 61% were male, 52% had HCV genotype 1 and 11% had liver cirrhosis. The mean age was 44.3 years and the mean baseline HCV RNA was 6.9 log10 IU/mL. A rapid virological response (RVR) was observed in 314/375 (84%) of the patients treated. Based on currently available data, the percentage of patients with HCV RNA below the lower limit of quantification (LLoQ) was 99% (234/237) at EOT, 99% (299/303) at SVR4 and 99% (247/250) at SVR12.
Conclusions
In this analysis, treatment with imported generic DAAs achieved high rates of HCV RNA undetectability at the end of treatment, and SVR12 in 99% of patients evaluated to date. Mass treatment with generic DAAs is a feasible and economical alternative route of accessing curative DAAs, where the high prices for branded alternatives prevent access to treatment.
Keywords: hepatitis C, direct acting antivirals, sofosbuvir, ledipasvir, daclatasvir
Introduction
The World Health Organization (WHO) estimates there are 71 million people chronically infected with hepatitis C virus (HCV) worldwide, with this number rising despite the existence of an effective cure. Up to 20% of those chronically infected will develop cirrhosis and 2–4% hepatocellular carcinoma (HCC), causing more than 400,000 deaths per year [1]. The recent WHO sector strategy for viral hepatitis aims to reduce this death toll by 65% by 2030, a scenario only feasible through increased and targeted treatment efforts [2].
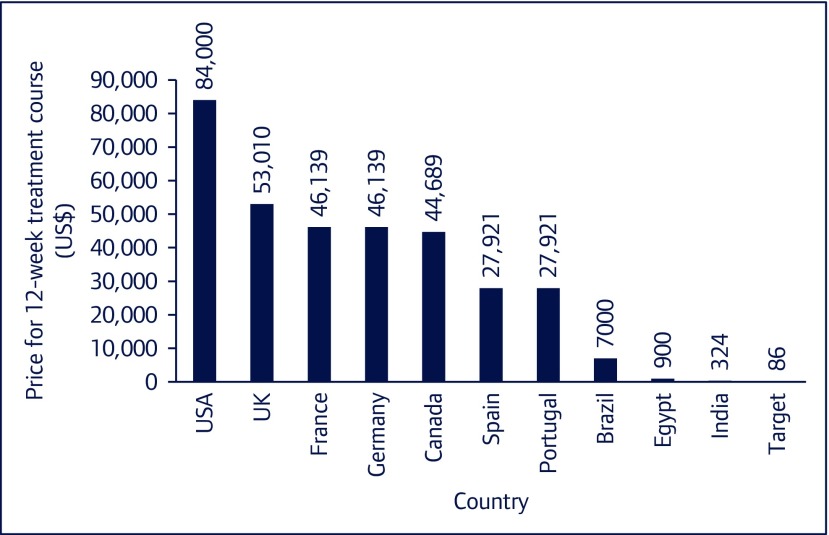
Combinations of direct acting antivirals (DAAs), new oral, well-tolerated medications, can achieve over 90% sustained virological response (SVR) rates, which is considered a cure of HCV infection [3]. The importance of two of these combinations led to their inclusion in the 2016 WHO Essential Medicines List: sofosbuvir/ledipasvir (SOF/LDV), currently recommended for HCV genotypes 1, 4, 5 and 6, and sofosbuvir/daclatasvir (SOF/DCV), recommended for HCV genotypes 1, 3 and 4 [4]. However, since their FDA approval, only 1.5 million HCV-infected patients were treated with DAAs worldwide by the end of 2016 [1]. This low treatment uptake can be attributed to a number of reasons, including the high medication cost. The price for a 12-week course of sofosbuvir in the US is currently US$84,000, despite calculations that show a 12-week course can be profitably produced for just US$86 (Figure 1) [5,6]. As part of the efforts to expand access, voluntary licences have been issued in some lower-income countries. These agreements explicitly prohibit supply to most middle- and all high-income countries, where prices of DAAs remain high [7].
Figure 1.
Lowest prices of sofosbuvir in selected countries
As a result, patients all around the world are resorting to importing generic versions of DAAs produced in India, China, Bangladesh or Egypt for prices in the range of US$400–1300 per 12-week treatment course [8]. Buyers’ clubs have been set up to act as a third party and aid patients in accessing generic medication via the internet. The import regulations vary by country; under UK and Australian law it is legal for patients to import a 3-month supply of medication for personal use [9,10]. In many other countries it is legal for people to buy medications abroad, and then to hand-carry these medications into their home country, for their personal use.
The objective of this observational, retrospective study was to assess the efficacy of generic DAAs imported by patients from countries of manufacture via three internet buyers’ clubs.
The relevance of this study is two-fold. Primarily, generic imports for personal use would provide access to DAAs in the many countries where treatment access remains limited. Secondly, empirical assessment of treatment outcomes with imported generics that are not licensed domestically, can provide an important case study for the feasibility of using buyers’ clubs for other diseases for which patented treatments are inaccessible due to high prices.
Methods
To identify the buyers’ clubs in operation worldwide, we contacted a number of community organisations who identified two Australian buyers’ clubs (FixHepC and Hep C Treatment Without Borders), the South-east Asian buyers’ clubs, and an Eastern European buyers’ club. The results from FixHepC have been reported elsewhere [11]. The three other buyers’ clubs asked individuals who were using imported generic DAAs to fill out a standardised data collection form along with a consent form. These patients were typically treated with assistance from a clinician in their country. In some cases, patients were advised on which combination of DAAs to take from an online consultation with the co-ordinators of their buyers’ club.
The information collected was:
-
•
Patient demographics such as age, sex and country of residence;
-
•
Baseline disease characteristics, such as HCV genotype, severity of liver disease, treatments and doses;
-
•
Planned dates of starting and stopping treatment;
-
•
Manufacturer and marketer of each generic medication used; and
-
•
HCV RNA levels pre-treatment, during treatment (RVR), at the end of treatment (EOT), and 4, 12 and 24 weeks after EOT (SVR4, SVR12 and SVR24, respectively).
These surveys were conducted between March 2016 and March 2017. The methods used to assess the severity of liver disease were not recorded in the questionnaire (i.e. Fibroscan versus liver biopsy). We also did not record whether patients were co-infected with hepatitis B or HIV.
The patient information was compiled into a single database. All patients included in the analysis gave informed consent for their treatment and response data to be shared. The questionnaires were sent out, compiled and analysed as part of a research project conducted at Imperial College London, UK.
The buyers’ clubs had different measures for ‘undetectable’ HCV RNA. HCV RNA <25 IU/mL at 12 and 24 weeks post treatment was taken as the definition of SVR12 and SVR24: a cure of HCV. The data were summarised by type of treatment received: sofosbuvir/ledipasvir (SOF/LDV) or sofosbuvir/daclatasvir (SOF/DCV).
Participants were only included if they were taking generic versions of both drugs in the combination regimen. Patients with HCV RNA data available from their course of DAA treatment were included in the analysis. In some cases, patients had to fund their HCV RNA testing locally, and had very limited results available at follow-up.
Treatment efficacy was calculated by dividing the number of people achieving SVR by the total number of people taking each treatment regimen; 95% confidence intervals (CI) were calculated using the Exact method.
Results
Overall, 616 patients were included in the final analysis; 199 from the Australian buyers’ club, 205 from the South-east Asian buyers’ clubs and 212 from the Eastern Europe buyers’ club.
Baseline data are shown in Table 1. Patients were from 38 different countries. Data were available on gender for 606 patients: 228/606 (38%) were female while 378/606 (62%) were male. No data were available for 10 of the patients. The mean age was 44.3 years (14–75 years) and the mean baseline plasma HCV RNA level was 6.9 log10 IU/mL; 52% had genotype 1; 5% genotype 2; 37% genotype 3; 2% genotype 4 and 5% genotype 6. METAVIR fibrosis scores were 19% F0, 23% F1, 33% F2, 11% F3, and 11% F4. The most common duration of treatment was 12 weeks.
Table 1.
Baseline characteristics
| Characteristic | Overall
n (%) |
SOF/LDV
n (%) |
SOF/DCV
n (%) |
|---|---|---|---|
| Number of participants* | 616 | 276(45) | 340(55) |
| Male | 378(61) | 172(62) | 206(61) |
| Female | 228(37) | 97(35) | 131(29) |
| Mean age(years) | 44.3 | — | — |
| HCV genotype | |||
| Genotype 1 | 318(52) | 235(85) | 83(24) |
| Genotype 2 | 31(5) | 1(0) | 30(9) |
| Genotype 3 | 227(37) | 6(2) | 221(65) |
| Genotype 4 | 11(2) | 8(3) | 3(1) |
| Genotype 5 | 1(0) | 1(0) | 0(0) |
| Genotype 6 | 28(5) | 25(9) | 3(1) |
| Fibrosis(METAVIR score) | |||
| F0 | 114(19) | 49(18) | 65(19) |
| F1 | 141(23) | 62(22) | 79(23) |
| F2 | 206(33) | 91(33) | 115(34) |
| F3 | 69(11) | 29(11) | 40(12) |
| F4(Cirrhosis) | 68(11) | 30(11) | 38(11) |
| Ribavirin | |||
| No RBV | 520(84) | 241(39) | 279(45) |
| RBV | 96(16) | 35(6) | 61(10) |
| Treatment duration | |||
| Median(weeks) | 12.1 | 12.0 | 12.1 |
| <12 | 179(29) | 88(32) | 91(27) |
| 12–23(12–13) | 378(61) | 165(60) | 213(63) |
| ≥24 | 59(10) | 23(8) | 36(11) |
| HCV RNA at baseline | |||
| Mean(log10 IU/mL) | 6.9 | ||
| Mean(IU/mL) | 7,898,550 |
No data on gender were available for 10 patients
DCV: daclatasvir; HCV: hepatitis C virus; LDV: ledipasvir; SOF: sofosbuvir
Of the 616 patients treated, 276 (45%) received SOF/LDV (13% with RBV), and 340 (55%) received SOF/DCV (61 with RBV). The generic medication was marketed by 24 different companies and organisations (Table 2). The majority (34%) were marketed by Cipla Ltd, while 30% (173/572) of patients were taking DAAs sourced from Hetero, 5.8% (33/572) from Natco and 4.2% (24/572) from Mylan.
Table 2.
Marketers of generic DAAs
| Marketer | Number of patients | ||
|---|---|---|---|
| SOF | DCV | LDV | |
| Cipla | 192 | 89 | 105 |
| Hetero | 182 | 103 | 70 |
| Natco | 45 | 17 | 16 |
| Mylan | 41 | 9 | 15 |
| West Linfield Pharmacy (Compounded API) | 12 | 3 | 11 |
| Sun Pharma | 18 | 9 | 8 |
| Incepta | 8 | 0 | 8 |
| Mesochem | 10 | 15 | 4 |
| Other | 3 | 9 | 4 |
| Zydus | 22 | 25 | 3 |
| Pharmed Healthcare | 3 | 0 | 3 |
| UTH | 3 | 0 | 3 |
| Chinese API | 3 | 31 | 1 |
| Beacon | 1 | 1 | 1 |
| Dr. Reddy's | 1 | 1 | 1 |
| Marcyrl | 6 | 5 | 0 |
| Strides Arcolab | 12 | 2 | 0 |
| Emcure | 5 | 0 | 0 |
| Aark Pharmaceuticals | 0 | 0 | 0 |
| Augispov | 1 | 0 | 0 |
| Beximco | 1 | 0 | 0 |
| Pharco Corporation | 1 | 0 | 0 |
| No data available | 46 | 21 | 23 |
DCV: daclatasvir; LDV: ledipasvir; SOF: sofosbuvir
In terms of follow-up results, as of 17 June 2017, 375 (61%) had data available for RVR; 237 (38%) for EOT; 303 (49%) for SVR4; 250 (41%) for SVR12 and 97 (16%) for SVR24.
An RVR was observed in 82% (146/177) of the patients treated with SOF/LDV and 85% (168/198) of the patients treated with SOF/DCV. The percentage of patients with HCV RNA <LLoQ was 99% (234/237) at end of treatment (EOT), 99% (299/303) SVR4 and 99% (247/250) at SVR12 (Table 3).
Table 3.
HCV RNA undetectable at follow-up by treatment regimen
| Viral status | Overall
n/total n (%) |
SOF/LDV
n/total n (%) |
SOF/DCV
n/total n (%) |
|---|---|---|---|
| RVR | 314/375(84) | 146/177(82) | 168/198(85) |
| EOT | 234/237(99) | 108/110(98) | 126/127(99) |
| SVR 4 | 299/303(99) | 131/134(98) | 168/169(99) |
| SVR 12 | 247/250(99) | 104/104(100) | 143/146(98) |
| SVR 24 | 96/97(99) | 30/30(100) | 66/67(99) |
DCV: daclatasvir; LDV: ledipasvir; SOF: sofosbuvir
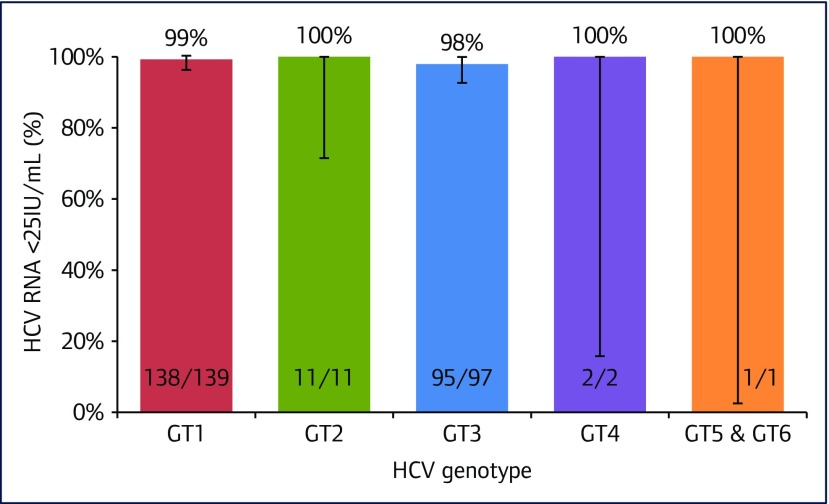
The SVR12 results by genotype are displayed in Figure 2. Of those with genotype 1 HCV 98% (95% CI 95–100) had HCV RNA <25 IU/mL at SVR4 and 99% (95% CI 96–100) at SVR12. In genotype 3, the SVR4 rate was 99% (95% CI 95–100) and SVR12 was 98% (95% CI 93–100). Sample sizes for all other genotypes were small, but all patients exhibited SVR4 and SVR12 rates of >99%.
Figure 2.
Percentage of patients with HCV RNA < LLoQ at SVR12
Discussion
This observational study demonstrates that internet-procured generic DAAs have viral suppression rates in the same range as originator drugs in Phase 3 trials [12]. Overall, 84% (314/375) of patients had an RVR, 99% (234/237) reached undetectable HCV RNA at EOT, 99% reached SVR4 (299/303) and 99% (247/250) SVR12. In genotype 1 HCV patients the SVR12 rate was 99% (138/139) and in genotype 3 SVR12 was 98% (95/97). However, these were the results of on-treatment analyses. Only 21% of the patients had follow-up data available to evaluate SVR. The survey did not include questions on discontinuing DAA treatment, adverse events, or loss to follow-up.
These generic treatments could be purchased through buyers’ clubs for a small percentage of the price for branded medications. Patients paid US$ 500–1500 per treatment course, depending on the type and duration of treatment required. These prices contrast with retail prices of sofosbuvir of up to US$ 84,000 per 12-week treatment course in the USA. However, it should be noted that most providers of HCV treatment in the USA are offered significant discounts from this $84,000 price – the size of these discounts is normally kept confidential and so is hard to evaluate.
Our analysis is limited due to its observational nature, and by its lack of a control group. As treatment and investigations were guided by clinical intent only, pharmacokinetic measures, which are commonly used to assess generic bio-equivalence, were not obtained. Furthermore, safety data were not collected in this analysis and the reasons as to the loss of follow-up were not recorded. A more detailed analysis of the FixHepC buyers’ club, presented in 2017 showed an overall SVR rate of 90%, with less than 1% of patients lost to follow-up for HCV RNA at the SVR time points [13].
The introduction of DAAs has been fairly recent and the function of global buyers’ clubs is only beginning to emerge. This analysis was limited by its small sample size and is, therefore, unrepresentative of the less common HCV genotypes. Hence, this study can be considered a pilot study for the efficacy of generics, and will be updated as buyers’ clubs grow and more data are available.
The high efficacy of generic DAAs observed in this analysis was not unexpected. Several companies currently manufacturing generic sofosbuvir are licenced by Gilead, which provides them with a full technology transfer of the manufacturing process [14]. Furthermore, the manufacturing processes of many of the companies have been certified by national regulatory bodies. Such companies include Mylan, Natco and also Cipla Ltd. Many companies also provide Western markets with other medications [14]. The Global Fund Expert Review Panel has listed four generic versions of sofosbuvir to be used in its treatment programme, one from Mylan and the other from Hetero Labs Ltd [15]. There are concerns about the potential for counterfeit medications, however, the results from these buyers’ clubs have shown no evidence that this is true in the markets included. Furthermore, the risk of counterfeit generic DAAs should not be any greater than that of more expensive patented medications, which have a greater financial incentive to counterfeit [7].
HCV drugs have now been included in the WHO prequalification programme (WPP) [16,17]. The WPP results in the quick procurement of cheaper quality-assured generic medications, which optimises treatment outcomes and resource utilisation [18] to scale up HCV treatment. The granting of the prequalification will aid buyers’ clubs by assuring the integrity of the supply chain and providing reassurance to HCV patients who source their treatment from generic manufacturers.
While it is important to investigate the quality of imported generics, questions remain as to how internet-procured medication can be practically used within health systems. This includes regulating and monitoring the online generic market while still respecting intellectual property rights.
Part of the success of the Australian, eastern European and South-east Asian buyers’ clubs is the result of the careful monitoring of patients throughout treatment, in addition to the available advice and the tailoring of treatment to each individual's needs. The majority of online suppliers require a written doctor's prescription for the purchase of DAA medications, although there are some websites selling generic HCV DAAs for importation, without the need for a prescription. However, in the UK, many doctors are uncertain about the efficacy of generic antivirals and are unwilling to prescribe and monitor patients using generic medications, fearing they may lose their licences [19]. The authors recommend that doctors should be better informed about the legalities of recommendation, prescribing and monitoring of patients on imported generic medication.
Patients are receiving conflicting advice regarding the safety of medications procured online. The Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine (ASHM) acknowledge generic DAAs as a viable option for patients [20], whereas the UK Medicines Healthcare and Regulatory Agency emphasise their concerns over counterfeit medication [19]. The Swiss Experts in Viral Hepatitis (SEVhep) have recognised that a large number of patients have bought HCV medication online and have written guidelines on how patients can safely access generic DAAs. In addition to this, a Swiss health insurance company, Concordia, included hepatitis C in its health insurance plan that reimburses up to 75% of the cost of drugs imported from the FixHepC buyers’ club [21].
Furthermore, the legality of importing medication for personal use differs internationally [22]. It is difficult for buyers’ clubs to police these laws, and making them more explicit will enable internet bought medication to be transparent in its services and transactions.
In conclusion, treatment with legally imported generic DAAs, purchased through online buyer's clubs, achieved high rates of undetectable HCV RNA at the end of treatment, and SVR in 99% patients evaluated to date. The efficacy observed is similar to Phase 3 trials of the branded medications. Mass treatment with the current generic DAAs is a feasible and economical alternative for accessing curative DAAs, while the high prices for branded DAAs limit access to treatment. The results in this analysis of selected buyers' clubs cannot be taken to prove that all medications for hepatitis C procured online will be of a similar, high quality. Each new supplier or buyers' club needs to be evaluated carefully to ensure the quality of the medications procured.
References
- 1. World Health Organization Global hepatitis report, 2017. Available at: www.who.int/hepatitis/publications/global-hepatitis-report2017/en/ ( accessed September 2017).
- 2. World Health Organization Combating hepatitis B and C to reach elimination by 2030. Available at: www.who.int/hepatitis/publications/hep-elimination-by-2030-brief/en/ ( accessed September 2017).
- 3. Infectious Diseases Society of America (IDSA), American Association for the Study of Liver Diseases (AASLD) HCV guidance: recommendations for testing, managing and treating hepatitis C. Available at: www.hcvguidelines.org/ ( accessed September 2017).
- 4. World Health Organization 2016. Guidelines for the screening, care and treatment of persons with chronic hepatitis C infection: updated version. Available at: www.who.int/hepatitis/publications/hepatitis-c-guidelines-2016/en/ ( accessed September). [PubMed]
- 5. University of Washington Hepatitis C Online Web Sofosbuvir (Solvaldi). Available at: www.hepatitisc.uw.edu/page/treatment/drugs/sofosbuvir-drug (accesed Sep 2017).
- 6. Hill A, Simmons B, Gotham D, Fortunak J. Rapid reductions in prices for generic sofosbuvir and daclatasvir to treat hepatitis C. J Virus Erad 2016; 2: 28– 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ghinea N, Lipworth W, Day R et al. . Importation of generic hepatitis C therapies: bridging the gap between price and access in high-income countries. Lancet 2017; 389: 1268– 1272. [DOI] [PubMed] [Google Scholar]
- 8. Alldaychemist Hepcinat 400mg. Available at: www.alldaychemist.com/hepcinat-400-mg.html ( accessed September 2017).
- 9. UK Government Travelling with controlled drugs. Available at: www.gov.uk/travelling-controlled-drugs ( accessed September 2017).
- 10. Moon S, Jambert E, Childs M, Schoen-Angerer T.. A win-win solution? A critical analysis of tiered pricing to improve access to medicines in developing countries. Global Health 2011; 7: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. ClinicalTrials.gov Reviewing DAA efficacy managing patient treatment in online neighbourhoods (REDEMPTION). Available at: https://clinicaltrials.gov/ct2/show/NCT02657694 ( accessed September 2017).
- 12. Rockstroh J. 2014. Summary from AASLD 2014 for hepatitis C, Boston, 7–11 November 2014. Available at: www.natap.org/2014/AASLD/AASLD_93.htm ( accessed September 2017).
- 13. Hill A, Khwairakpam G, Wang J et al. . 2017. Ninety-six % SVR rates using imported generic DAAs for patients with hepatitis C. Conference on Retroviruses and Opportunistic Infections February 2017. Seattle, WA, USA. Abstract P569.
- 14. Gilead 2014. Gilead announces generic licensing agreements to increase access to hepatitis C treatments in developing countries. Available at: www.gilead.com/news/press-releases/2014/9/gilead-announces-generic-licensing-agreements-to-increase-access-to-hepatitis-c-treatments-in-developing-countries ( accessed September 2017).
- 15. Fund G. 2017. List of antihepatitis pharmaceutical products Available at: www.theglobalfund.org/media/5876/psm_productshepatitis_list_en.pdf ( accessed September 2017).
- 16. Gilead 2014. Application for inclusion of sofosbuvir (Sovaldi) tablets on the WHO model list of essential medcines. Available at: www.who.int/selection_medicines/committees/expert/20/applications/sofosbuvir/en/ ( accessed September 2017).
- 17. World Health Organization 2015. WHO essential medecines list application: daclatasvir. Available at: www.who.int/selection_medicines/committees/expert/20/applications/daclatasvir/en/ ( accessed September 2017).
- 18. Rago L. WHO prequalification of medecines programmw (WHO- PQP): ensuring quality medecines. Available at: https://extranet.who.int/prequal/sites/default/files/documents/Lembit-Rago.pdf ( accessed September 2017).
- 19. Wilson C. Making a killing. New Scientist 2017; 234: 22– 23. [Google Scholar]
- 20. Jensen DM, Sebhatu P, Reau NS.. Generic medications for hepatitis C. Liver Int 2016; 36: 925– 928. [DOI] [PubMed] [Google Scholar]
- 21. Chandrasekhar A. 2017. Swiss bend rules to provide patients with afforable treatment. Available at: www.swissinfo.ch/eng/society/hepatitis-c_swiss-bend-rules-to-provide-patients-with-affordable-treatment-/43212072#.WTWEEB4tU7U.twitter ( accessed September 2017).
- 22. Hepatitis SA. 2016. Importing medecines: guidelines for clinicians. Available at: https://hepatitissa.asn.au/news/221-importing-medicines-guidelines-for-clinicians ( accessed September 2017).