Abstract
Introduction
HIV pre-exposure prophylaxis (PrEP) is not available on the National Health Service (NHS) in England. People are buying generic versions of tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC) on the internet, which is legal under UK import laws.
Methods
HIV-negative individuals attending our clinic who reported purchasing generic PrEP online were provided with risk-reduction advice and were evaluated for HIV, hepatitis B and C, renal function and sexually transmitted infections (STIs)on their first visit. They were offered regular follow-up visits every 3 months and given risk-reduction advice. Plasma therapeutic drug monitoring (TDM) for tenofovir and FTC was also offered.
Results
641 individuals accessed the service during 2016–2017. Median time on generic PrEP was 202 days. All were MSM, 81% were white, 75% used PrEP daily and 14% on an event-driven basis, and 67% were on generic TDF/FTC manufactured by Cipla Ltd. There were no serious adverse events. Thirty-nine percent of individuals (191/494) reported using recreational drugs in the 12 months before starting PrEP, and 29% (127/443) reported this while taking PrEP. During follow-up, 26% (142/552) of individuals were diagnosed with an STI at one or more follow-up visits. In 336 person-years of follow-up, there were no cases of HIV infection (0%, 95% CI 0%–1.1%). There were no new cases of hepatitis B and two new cases of hepatitis C.
Discussion
There were no new cases of HIV in 641 individuals using generic PrEP. At the same centre, new HIV diagnoses fell from 69 per month in October 2015 to 15 per month in June 2017. We believe that our support for individuals taking generic PrEP has contributed to this fall. There was a 10% increase in STI diagnoses during PrEP compared to baseline. Strategies to reduce STIs remain crucial.
Keywords: HIV infection, pre-exposure prophylaxis, generic, antiretroviral drugs
Introduction
The incidence of HIV infections remained stable in the UK from 2005 to 2015, despite large increases in antiretroviral treatment [1]. Pre-exposure prophylaxis (PrEP) with tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC) has been shown to significantly reduce the risk of HIV acquisition in several large-scale clinical trials [2–4].
The World Health Organization (WHO) updated its guidelines on the use of antiretrovirals for HIV treatment and prevention to recommend HIV PrEP as an additional prevention strategy [5]. As a result, several countries have included PrEP in their national guidelines. The US, as the first country to introduce PrEP in July 2015, currently has an estimated 100,00 people taking PrEP [6]. Australia recruited 6500 patients over three clinical trials, with the number of people on PrEP increasing rapidly [7–9]. More recently, Belgium made the decision to provide PrEP to high-risk populations via a reimbursement scheme [10] and Scotland has become the first country in the UK to routinely offer PrEP to eligible patients, which they estimate to be 1900 people [11].
However, the high cost of the branded TDF/FTC (Truvada) has prevented many health services from funding PrEP. The National Health Service (NHS) in England has refused to provide PrEP, but plans to start a trial to provide PrEP to at least 10,000 people in England in late 2017. However, to keep within the £10 million allocated budget, TDF/FTC will need to cost substantially less than the current NHS list price for Truvada of £4260/year [12–14].
At-risk individuals in the UK have resorted to purchasing generic versions of PrEP on the internet, using websites such as www.iwantprepnow.co.uk [15]. This is possible because voluntary licensing rules have enabled manufacturing companies to produce preparations of TDF/FTC that are bioequivalent to the branded product. The iwantprepnow website gives guidance to patients on purchasing generic PrEP safely through online pharmacies. It informs patients that under UK import laws, it is legal to import 3 months of PrEP for personal use [16]. This website only lists generic PrEP from Cipla, Mylan or the Government Pharmaceutical Organization in Thailand, which have been officially approved by the United States Food and Drug Administration (US FDA). For each patient attending the clinic for therapeutic drug monitoring (TDM), the name of the medication, manufacturer, batch number and expiry date are recorded, a blood sample is taken and levels of tenofovir and emtricitabine (FTC) are measured. Online pharmacies are only listed on www.iwantprepnow.co.uk after the sales process has been verified as safe and reliable by users or iwantprepnow staff and once adequate drug levels have been found. Currently, the five pharmacies recommended by iwantprepnow are Dynamix International, Pulse Clinic, United Pharmacies UK, All Day Chemist, and In House Pharmacy. The current price for a 1-month supply of PrEP on this site is £39–£78 [15] compared to the commercial price of over £400/month for non-discounted branded Truvada from Gilead Sciences [12].
In February 2016, a service was established at 56 Dean Street (DS) to offer plasma tenofovir and FTC TDM for people buying generic PrEP online, alongside regular tests for sexually transmitted infections (STIs) and renal function monitoring. A previous publication [17] concentrated on pharmacokinetics, and addressed initial concerns over the authenticity of the medicines purchased. Drug concentrations of generics purchased online were consistent with data from previously published Phase I pharmacokinetic clinical trials. Furthermore, no fake preparations were identified [17].
This article reports other clinical outcomes recorded in the drug monitoring service including: number of new HIV infections, diagnosis of other STIs, renal adverse events and use of recreational drugs.
Methods
All HIV-negative individuals attending DS (from February 2016 to March 2017) and who reported purchasing PrEP on the internet were offered TDM in addition to tests for HIV, hepatitis B (HBV), hepatitis C (HCV), renal function and other STIs at first visit. Those continuing on generic PrEP were offered regular follow-up visits every 3 months and were encouraged to return via text message reminders. Patients were also given risk-reduction advice.
Patient characteristics, use of recreational drugs (known in the UK as ‘chems’), treatment regimen, days on PrEP, and name of drug and manufacturer were recorded at initial appointment. Renal function, HIV, HBV and HCV testing were offered at baseline and every 3–6 months. Time on PrEP and follow-up time for HIV diagnosis were measured from the most recent HIV test. Follow-up time for STI diagnosis was measured from the date of starting PrEP. For each patient attending the clinic for therapeutic drug monitoring (TDM), the name of the medication, manufacturer, batch number and expiry date are recorded, a blood sample is taken and levels of tenofovir and emtricitabine (FTC) are measured.
Monitoring of STIs was consistent with national guidelines: HIV, Chlamydia trachomatis (CT), gonorrhoea (GC) and syphilis were routinely tested for every 3 months; HCV testing was offered to those deemed at risk; immunity to HBV was checked at baseline and those not immune were offered vaccination. Tests for other STIs were performed according to clinical indication [18].
Renal function was evaluated from estimated glomerular filtration rate (eGFR), creatinine level and/or proteinuria on urinalysis for individuals with data available at both baseline and following PrEP initiation. Comparison of renal function prior to and following the use of PrEP was performed using non-parametric Wilcoxon signed-rank test for related samples. Renal function deterioration was defined as a >10% decline in eGFR from baseline and/or an increase in proteinuria.
The methods for pharmacokinetic analysis have been described previously [17]. Briefly, drug concentrations in plasma samples taken from all patients on generic TDF/FTC were measured by ultra-performance liquid chromatography, coupled with UV detection with a linear range of 25–10,000 ng/mL.
This analysis was approved by Chelsea and Westminster Hospital NHS Foundation Trust. The database used to collect patient information was registered by the HIV/Genito-urinary Medicine Directorate and all data were recorded anonymously.
Results
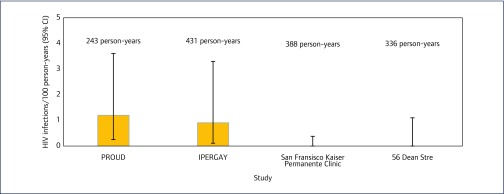
From February 2016 to March 2017, data were available for 641 patients accessing the monitoring service. Of 641 patients, 87 (14%) attended an initial appointment only and 554 (86%) attended both an initial appointment and at least one follow-up visit. We observed 336 person-years of PrEP use; the median follow-up time on generic PrEP was 7.2 months. Baseline characteristics are displayed in Table 1, compared to three large-scale studies of PrEP in men who have sex with men (MSM). PROUD [2] was a clinical trial in the UK of 544 MSM taking PrEP daily. IPERGAY [4], carried out in France and Canada, enrolled 400 men to take PrEP on demand. The San Francisco Kaiser Permanente study (KP) [19] looked at PrEP use in a real-life clinical setting, with a cohort of 657 PrEP initiators.
Table 1.
Baseline characteristics for studies of HIV oral PrEP in MSM
| Characteristics | Clinical trial | Clinical practice | ||
|---|---|---|---|---|
| IPERGAY [4] (n=199) | PROUD [2] (n=273) | 56 Dean Street (n=641) | San Francisco Kaiser Permanente [19] (n=972) | |
| MSM (percentage) | 100% | 100% | 100% | 98% |
| Median age (years) (IQR) | 34.9 (29–43) | 35 (30–43) | 37 (32–45) | 37 (18–68) |
| White race (%) | 94% | 81% | 81% | 65% |
| Once-daily PrEP (%) | 0% | 100% | 75% | 100% |
| Chem use (%) | 43% | 43% | 30% | – |
| Baseline STIs (%) | 25% | 63% | 16% | 28% |
Of the 641 men initiating PrEP at DS, the median age was 37, 81% of the population were white, and of those who disclosed their method of PrEP use, 75% (481) took PrEP daily compared to 14% (90) who used event-driven PrEP. The PrEP dosing regimen was not stated in 11%. Reported recreational drug use in the 12 months preceding PrEP was 30% (191/641) at DS, compared to 43% in both PROUD and IPERGAY. The baseline STI rate was lower at DS than in the other trials, 16% (91/573) compared to 25%, 63% and 28% in PROUD, IPERGAY and KP, respectively. However, baseline STIs at DS only included diagnoses in the 3 months pre-PrEP, compared to 12 months in the other studies. The most common STIs diagnosed, as in the other three studies, were Chlamydia trachomatis (42%) and gonorrhoea (40%).
At baseline, of the patients disclosing how PrEP was sourced, 202 (62%) used United Pharmacies, 55 (17%) All Day Chemist, 12 (4%) All Day Pharmacy, and 3/324 (0.9%) UK Pharmacy. Tenvir-EM, marketed by Cipla Ltd, was the most common preparation (97%). However, during follow-up, many patients switched from Tenvir-EM to Ricovir-EM, marketed by Mylan, from an online pharmacy called Dynamix [20].
In 336 person-years of follow-up, there were no cases of HIV infection (0%, 95% CI 0%–1.1%). This is comparable to the other three studies in Figure 1. There were no new cases of HBV infection. Of the 492 people tested for HCV, there were two new diagnoses during follow-up.
Figure 1.
Incidence rate of HIV infections per 100 person-years, 56 Dean Street compared to other studies
STI results at baseline and follow-up are compared in Table 2. In 199 person-years of STI follow-up, 26% (142/552) of patients were diagnosed with an STI at one or more follow-up visits, compared to 16% (91/573) at baseline.
Table 2.
Individuals diagnosed with an STI at baseline (3 months pre-PrEP) and during follow-up
| Sexually transmitted infectio | Baseline | Follow-up |
|---|---|---|
| Individuals diagnosed (n, %) | ||
| Any | 91/573 (16%) | 142/552 (25%) |
| Episodes diagnosed (n, %) | ||
|---|---|---|
| Chlamydia trachomatis (CT) | 45/107(42%) | 166/373(45%) |
| Gonorrhoea(GT) | 43/107(40%) | 158/373(42%) |
| Syphilis | 12/107(11%) | 31/373(8%) |
| Shigella | 2/107(2%) | 0(0%) |
| Herpes simplex virus(HSV) | 4/107(4%) | 7/373(2%) |
| Lymphogranuloma venereum (LGV) | 1/107(1%) | 11/373(3%) |
The incidence of STIs decreased during PrEP in the PROUD and IPERGAY trials by 5% and 6%, respectively, whereas the 9% increase seen at DS was lower than the Kaiser Permanente study, which saw an increase of 22% [2,4,19].
Renal function testing at both baseline and follow-up was conducted in 282 individuals. Baseline eGFR (>60 mL/min/1.73 m2) and/or urinalysis was normal in 99% (537/545) of individuals. Two (0.7%) patients had reductions in eGFR while on PrEP; one was taking protein and creatine supplements, which are known to underestimate true renal function, and had no proteinuria. PrEP was not discontinued in this patient. The second patient did not respond to efforts to contact him. Three (1%) patients had trace proteinuria at baseline but otherwise normal renal function results while on PrEP; two patients (0.7%) had normal urinalysis results at baseline but showed trace proteinuria during follow-up on PrEP.
Table 3 displays the results for recreational drug use at DS. Of patients reporting this, 39% (191/494) reported using recreational drugs in the 12 months before starting PrEP, and 29% (127/443) whilst taking PrEP. The most popular drugs used were gammahydroxybutyrate (GHB) or gammabutyrolactone (GBL) (23%), mephedrone (21%) and methamphetamine (16%). Injecting drug use (IDU) was reported in 61 (12%) individuals at baseline and 42 (13%) during follow-up. Nineteen individuals (15%) were referred for recreational drug support.
Table 3.
Recreational drug use at baseline (1 year pre-PrEP) and during follow-up
| Recreational drugs used | Baseline | Follow-up |
|---|---|---|
| Individuals who used (n, %) | ||
| Any | 191/494 (39%) | 127/443 (29%) |
| Drugs used (episodes, n, %) | ||
|---|---|---|
| Cocaine | 48/530(9%) | 27/315(9%) |
| Methamphetamine | 75/530(14%) | 51/315(16%) |
| MDMA/ecstasy | 48/530(9%) | 27/315(9%) |
| Gammahydroxybutyrate(GHB) or gammahydroxybutyrolactone (GBL) | 107/530(20%) | 71/315(23%) |
| Methadone | 38/530(7%) | 19/315(6%) |
| Mephedrone | 102/530(19%) | 67/315(21%) |
| Amyl nitrite(poppers) | 40/530(8%) | 19/315(6%) |
| Amphetamine sulphate | 35/530(7%) | 16/315(5%) |
| Sildenafil(Viagra) | 37/530(7%) | 18/315(6%) |
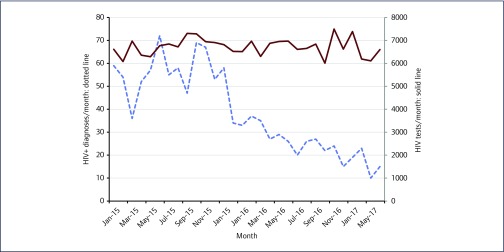
As shown in Figure 2, DS has seen a reduction in new HIV-positive diagnoses between October 2015 (69 HIV-positive diagnoses), and June 2017 (15 HIV-positive diagnoses). Over this time, the number of HIV tests carried out remained constant.
Figure 2.
HIV tests and positive diagnoses at 56 Dean Street, London, 2015–2017
Discussion
At DS, 641 MSM purchased generic TDF/FTC online over 13 months, to be used as PrEP for HIV. In 336 person-years of follow-up, there were no new cases of HIV and no serious adverse events. The recreational drug use reported by those taking generic PrEP decreased during PrEP use. However, there was a 10% increase in individuals diagnosed with an STI during PrEP follow-up, compared to those diagnosed in the 3 months before starting PrEP.
These results are consistent with clinical trial results with patented TDF/FTC by Gilead; very few HIV diagnoses were seen in PROUD and IPERGAY studies in 243 and 431 person-years of follow-up, respectively, and including after the trials ended [21,22]. Furthermore, the KP study also saw an increase in STIs during PrEP use, supporting the evidence that STI risk reduction remains crucial.
Although the data reported in this paper are from the original cohort of online PrEP users attending our service, DS now monitors over 1000 individuals on generic PrEP. In parallel with the introduction of the PrEP monitoring service, DS has seen a decrease in the number of HIV diagnoses overall, from 69 positive diagnoses in October 2015, to 15 in June 2017. PrEP is believed to have contributed to this reduction; however, there are a number of other interventions over this time period that have influenced HIV rates in our service. These include: the rapid STI results and treatment within hours provided at Dean Street Express, early treatment initiation in individuals diagnosed with HIV and provision of HIV post-exposure prophylaxis (PEP) [23].
DS is not the only clinic to have observed a reduction in HIV diagnoses since PrEP use became increasingly popular. Public Health England (PHE) report a decrease in HIV diagnoses in four other London clinics in the last quarter of 2016. However, this is not the case for all UK clinics; PHE have found a large diversity in HIV diagnoses UK-wide [24].
It is worth noting that the median age of PrEP users in our service was 37 years, while the median age at diagnosis of MSM in England in 2015 was 33, reflecting the increasing proportion of new diagnoses in under-35-year-olds in recent years [25]. Despite the fact that generic PrEP is significantly cheaper than Truvada, it is likely that younger individuals would be less able to afford it than older MSM.
Limitations
There were several limitations to this analysis. As this was a service evaluation rather than a research study, we could not develop rigorous baseline data, nor impose a requirement for mandatory follow-up. This made it difficult to observe an accurate link between baseline and follow-up with regard to STI acquisition, recreational drug use or changes in renal function. The increase in attendances at follow-up, as a result of measures put in place to encourage patients to attend, may have contributed to the increase in STIs seen in this analysis. Even so, the analysis was still limited by lack of follow-up data for many patients. Some patients may have attended other clinics, having only attended DS for PrEP consultation and TDM. Others may have only attended with symptoms, resulting in under-documenting the presence of asymptomatic disease. Further investigation into this relationship is needed; however, it is crucial that individuals on PrEP are appropriately followed up to ensure that they remain adherent to treatment and that incident STIs, including HIV infection, are not missed and to identify and address cases of renal dysfunction.
Previously, recreational drug use at DS had not been reported systematically. However, recently DS have found an improvement in patient reporting as a result of a ‘no blame, no judgement’ approach. In this service, the percentage referred for recreational drug use support was low (15%); however, walk-in support clinics are available and widely advertised within the service.
Renal function monitoring was performed inconsistently despite the availability of a clinic guideline, with individuals being monitored with urinalysis or eGFR/creatinine or a combination of both at different time points during follow-up. However, the PROUD, IPERGAY and other PrEP studies suggest a good TDF/FTC safety profile with few serious attributable adverse events [2,4,26].
Screening for HCV is currently performed according to risk, so we were unable to observe a link between HCV and PrEP. The sexual health services at the Royal Free Hospital and the Mortimer Market Centre in London have seen a general increase in new diagnoses of HCV [27]. Further work is needed to ascertain whether the same increase in new HCV diagnoses is occurring at DS; however, anecdotal data currently suggest that this is not the case.
Generic TDF/FTC has been registered for the treatment of HIV in 154 countries [28]; as such, the high clinical efficacy of PrEP seen in our cohort was expected. Furthermore, generic TDF/FTC has been added to the list of WHO prequalified medicines for the treatment of HIV [29], not just for its prevention. Although counterfeit medications remain a concern [30], no fake preparations were found in any of the sources of PrEP at DS. Although these results suggest that the pharmacies listed on the www.iwantprepnow.co.uk website are safe, this may not apply for other suppliers.
To fully ensure the safe procurement, monitoring and regulation of generic PrEP along with the other HIV prevention tools, PrEP should be available through the NHS. In Australia, patients can access PrEP free of charge, but can only obtain continued supplies if they have the appropriate follow-up tests, ensuring adequate monitoring of adverse events and STIs.
There is thought to be a large demand for PrEP in the UK; DS alone had interest from over 300 patients in one day following the announcement of a commercial PrEP trial in 2016. Australia, a country with one-fifth of the population of the UK, has recruited over 6500 patients to PrEP trials across the country [7–9].
There were approximately 3000 new HIV infections in MSM in the UK in 2015 [31]. The incidence of HIV infection in MSM was estimated to be 1.3% in 2012 [23]. Using this estimate, 77 men would need to access PrEP for 1 year to avert one HIV infection, assuming the effectiveness seen in the PROUD and IPERGAY studies. Therefore, if PrEP was the only contributor to HIV prevention, 230,769 people would need to be taking PrEP for 1 year to prevent all HIV infections in MSM in the UK. To provide this many people with access to PrEP in the UK, a considerable reduction in the cost of antiretroviral drugs is necessary [32]. While the NHS finalises the funding for this, personally imported generic medication that can be purchased for only £40 a month is an affordable option to many patients.
Given the success of PrEP in MSM, other programmes to provide PrEP to black and minority ethnic (BME) groups, commercial sex workers or other people at high risk of HIV infection should be considered. However, work still needs to be done to identify appropriate implementation models for these groups.
Summary
The price of branded PrEP from Gilead is prohibitively high for most at-risk individuals. Online generic PrEP provides an opportunity for patients to access HIV prevention more affordably. A safe network of online suppliers and appropriate monitoring will enable individuals at risk of HIV infection to access an effective means of prevention as an interim solution until PrEP is available on the NHS to all who need it. Similar methods of access to generics could be established in other countries where branded PrEP is not available.
References
- 1. Chau C, Kirwan P, Brown A et al. . HIV diagnoses, late diagnoses and numbers accessing treatment and care. 2016 report. Available at: www.gov.uk/government/uploads/system/uploads/attachment_data/file/602945/HIV_diagnoses_late_diagnoses_and_numbers_accessing_treatment_and_care.pdf ( accessed September 2017).
- 2. McCormack S, Dunn D, Desai M et al. . Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet 2016; 387: 53– 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grant RM, Lama JR, Anderson PL et al. . Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010; 363: 2587– 2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Molina J-M, Capitant C, Spire B et al. . On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med 2015; 373: 2237– 2246. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization (WHO) Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV, September 2015. Available at: www.who.int/hiv/pub/guidelines/earlyrelease-arv/en ( accessed September 2017). [PubMed]
- 6. Mera Giler R, Magnusen D, Trevor H et al. . Changes in Truvada for HIV pre-exposure prophylaxis utilization in the USA: 2012–2016. 9th International AIDS Society Conference on HIV Science. July 2017. Paris, France. Abstract WEPEC0919.
- 7. PrEPX South Australia. Alfred Health. Available at: www.alfredhealth.org.au/research/research-areas/infectious-diseases-research/prepx-south-australia ( accessed September 2017).
- 8. EPIC-NSW – Expanded PrEP Implementation in Communities in NSW. Available at: https://epic-nswstudy.org.au/ ( accessed September 2017).
- 9. QPrepd project – #ComePrEPd. Available at: www.comeprepd.info/qprepd-project/ ( accessed September 2017).
- 10. Belgium – PrEPWatch. Available at: www.prepwatch.org/belgium/ ( accessed September 2017).
- 11. BBC News NHS Scotland to fund 'game-changer' Prep HIV drug. Available at: www.bbc.co.uk/news/uk-scotland-39552641 ( accessed September 2017).
- 12. Hill AM, Pozniak AL.. How can we achieve universal access to low-cost treatment for HIV? J Virus Erad 2016; 2: 193– 197. [PMC free article] [PubMed] [Google Scholar]
- 13. Medicines and Healthcare products Regulatory Agency. Available at: www.gov.uk/government/organisations/medicines-and-healthcare-products-regulatory-agency ( accessed September 2017)
- 14. NHS England NHS England announces major extension of national HIV prevention programme with Public Health England and funding for ten new specialised treatments. Available at: www.england.nhs.uk/2016/12/hiv-prevention-pregramme/ ( accessed September 2017)
- 15. iwantprepnow Buy PrEP now. Where to buy genuine generic PrEP online now. Available at: www.iwantprepnow.co.uk/buy-prep-now ( accessed September 2017).
- 16. HS England August update on the commissioning and provision of pre-exposure prophylaxis (PREP) for HIV prevention. Available at: www.england.nhs.uk/2016/08/august-update-on-the-commissioning-and-provision-of-pre-exposure-prophylaxis-prep-for-hiv-prevention/ ( accessed September 2017).
- 17. Wang X, Nwokolo N, Korologou-Linden R et al. . InterPrEP: internet-based pre-exposure prophylaxis with generic tenofovir disoproxil fumarate/emtrictabine in London – analysis of pharmacokinetics, safety and outcomes. HIV Med 2017. [DOI] [PubMed] [Google Scholar]
- 18. McCormack S, Fidler S, Waters L et al. . BHIVA BASHH Position Statement on PrEP in UK. Available at: www.bhiva.org/documents/Publications/Practical-PrEP-guidance.pdf ( accessed September 2017).
- 19. Volk JE, Marcus JL, Phengrasamy T et al. ( 2015). No new HIV infections with increasing use of HIV preexposure prophylaxis in a clinical practice setting. Clin Infect Dis 2015; 61: 1601– 1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dynamix International UK product page. Available at: www.purchase-prep.com/product-category/u-k/ ( accessed September 2017).
- 21. White E, Dunn D, Gilson R et al. . Long term follow up of PROUD: evidence for high continued HIV exposure and durable effectiveness of PrEP. 9thIAS Conference on HIV Science. Paris, France. July 2017. Abstract TUAC0101.
- 22. Molina J-M, Charreau I, Spire B et al. . Efficacy, safety, and effect on sexual behaviour of on-demand pre-exposure prophylaxis for HIV in men who have sex with men: an observational cohort study. Lancet HIV 2017; 4: e402– e410. [DOI] [PubMed] [Google Scholar]
- 23. World Health Organization Priority interventions HIV/AIDS prevention, treatment and care in the health sector. 2009. Available at: www.who.int/hiv/pub/priority_interventions_web.pdf ( accessed September 2017).
- 24. Delpech V, Desai M.. Towards elimination of HIV amongst gay and bisexual men in the United Kingdom. 2017. Available at: www.bhiva.org/documents/Conferences/2017Liverpool/Presentations/170405/ValerieDelpech-MonicaDesai.pdf ( accessed September 2017).
- 25. Public Health England HIV in the UK 2016 Report. Available at: www.gov.uk/government/uploads/system/uploads/attachment_data/file/602942/HIV_in_the_UK_report.pdf ( accessed September 2017).
- 26. Gandhi M, Glidden DV, Liu AY et al. . Higher cumulative TFV/FTC Levels in PrEP associated with decline in renal function. Conference on Retroviruses and Opportunistic Infections. Boston, MA, USA. February 2016. Abstract 866.
- 27. Giraudon I, Ruf M, Maguire H et al. . Increase in diagnosed newly acquired hepatitis C in HIV-positive men who have sex with men across London and Brighton, 2002–2006: is this an outbreak? Sex Transm Infect 2008; 84: 111– 115. [DOI] [PubMed] [Google Scholar]
- 28. Moore K. The Medicines Patent Pool (MPP) broadens collaboration with Gilead Sciences: signs licence for Phase III medicine tenofovir alafenamide (TAF). Melbourne, 2014. Available at: www.medicinespatentpool.org/the-medicines-patent-pool-mpp-broadens-collaboration-with-gilead-sciences-signs-licence-for-phase-iii-medicine-tenofovir-alafenamide-taf/ ( accessed September 2017
- 29. World Health Organization ( 2017). WHO Public Assessment Reports (WHOPARs). 2017. Available at: https://extranet.who.int/prequal/key-resources/prequalification-reports/whopars?field_whopar_therapeutic_area=20 ( accessed September 2017).
- 30. Ghinea N, Lipworth W, Day R et al. . Importation of generic hepatitis C therapies: bridging the gap between price and access in high-income countries. Lancet 2017; 389: 1268– 1272. [DOI] [PubMed] [Google Scholar]
- 31. National AIDS Trust UK HIV statistics. Available at: www.nat.org.uk/we-inform/HIV-statistics/UK-statistics ( accessed September 2017).
- 32. Ong KJ, Desai S, Desai M et al. . Cost and cost-effectiveness of an HIV pre-exposure prophylaxis (PrEP) programme for high-risk men who have sex with men in England: results of a static decision analytical model. Lancet 2015; 386: S16. [Google Scholar]