Abstract
The aging U.S. population and the recent rise in the prevalence of obesity are two phenomena of great importance to public health. In addition, research suggests that midlife body mass index (BMI) is a risk factor for dementia, a particularly costly disease, in later life. BMI could influence brain health by adversely impacting cerebral white matter. Recently, greater BMI has been associated with lower white matter fractional anisotropy (FA), an index of tissue microstructure, as measured by diffusion‐tensor imaging in midlife. The aim of this study was to investigate the role of abdominal obesity, the most metabolically active adipose tissue compartment, and white matter microstructure in midlife. Community dwelling participants (N = 168) between the ages of 40–62 underwent MRI scanning at 3T and a general health assessment. Inferences were made on whole brain white matter tracts using full‐tensor, high‐dimension normalization, and tract‐based spatial statistics. Higher waist circumference was associated with higher FA, indicating more directional diffusion in several white matter tracts controlling for age, sex, triglycerides, systolic blood pressure, fasting glucose, and HDL‐cholesterol. Post hoc analysis revealed that greater waist circumference was associated with lower axial diffusivity, indicating lower parallel diffusion; lower radial diffusivity, indicating lower perpendicular diffusion; and lower mean diffusivity, indicating restricted diffusion. This is the first study to report a positive relationship between obesity and FA, indicating a more complicated view of this relationship in the aging brain. Hum Brain Mapp 38:3337–3344, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: diffusion tensor imaging, aging, MRI, adiposity, waist circumference
INTRODUCTION
Dementia is a particularly costly disease at the public health level and is devastating to individuals and families. The annual cost of formal care for patients with dementia in the United States was estimated to be $109 billion in 2010 [Hurd et al., 2013]. The increasing proportion of older adults in the U.S. population is projected to increase the cost of dementia care 79% by 2040 [Hurd et al., 2013]. In addition, the prevalence of obesity in the United States has more than doubled in the past 30 years [Baskin et al., 2005]. If obesity in midlife is a risk factor for dementia in later life, as several epidemiological studies have indicated [Hassing et al., 2009; Kivipelto et al., 2005; Rosengren et al., 2005; Whitmer et al., 2005; Xu et al., 2011], the recent rise in obesity rates in midlife will likely further escalate the projected increase in late‐life dementia. However, a large epidemiological study recently identified obesity in midlife as a protective factor for later‐life dementia, seemingly contradicting previous studies [Qizilbash et al., 2015]. The controversy in the epidemiological literature underscores the need for characterizing brain differences associated with obesity in midlife to help identify potential mechanisms that may contribute to pathological aging.
Obesity has been associated with deleterious effects on the microstructure of regional white matter measured by diffusion tensor imaging (DTI) [Bettcher et al., 2013; Karlsson et al., 2013; Mueller et al., 2011; Verstynen et al., 2012]. DTI is an MRI modality that measures the directional diffusion of water. In healthy, intact white matter, water diffuses within myelin in the direction parallel to the axon. In damaged white matter, water diffuses more freely. The most commonly used DTI metric is fractional anisotrophy (FA), a scalar measure of the anisotrophy of the diffusion tensor. FA values are calculated from the diffusivities along the three axes of the diffusion tensor and can be thought of as the amount of diffusion in the principle direction relative to the amount of diffusion in the perpendicular direction. FA values range from zero to one, with a value of zero representing equal diffusivity along all three axes and a value of one representing diffusivity solely along the principle axis. Since axonal loss and demyelination result in more isotropic diffusion, it is thought that FA is an indicator of white matter health. FA is sensitive to white matter microstructural changes with both aging and dementia [Alves et al., 2012; Good et al., 2001]. Despite its proven sensitivity to a variety of white matter pathologies, FA does not fully describe the direction, shape, or magnitude of the diffusion tensor [Alexander et al., 2007]. It is possible to get the same FA values from different combinations of diffusivities along the three axes. Therefore, other diffusion metrics are needed to fully understand the diffusion tensor. Mean diffusivity (MD) averages diffusivity from the three axes and is thought to measure membrane density. For instance, tightly packed axons with a high density of membranes will have lower MD. Axial diffusivity (AD) is the diffusivity in the principle direction and is thought to inversely measure axonal injury. For example, axonal loss will result in less restricted diffusion in the parallel direction and a lower AD. Radial diffusivity (RD) is the diffusivity perpendicular to the principle direction and is thought to inversely represent myelination. Demyelination results in less restricted diffusion perpendicular to the axon and therefore greater RD. Particularly when the microstructural differences underlying FA are unknown, MD, AD, and RD more fully characterize the diffusion tensor and provide additional insight into the pathology underlying microstructural differences.
The epidemiological literature linking midlife obesity to dementia and studies linking obesity to changes in white matter microstructure cited above exclusively use body mass index (BMI) to measure obesity. BMI is a ratio of height and weight that is sensitive to both adiposity and lean mass. With increasing age, there is evidence that BMI is a less reliable measure of adiposity, particularly in the overweight range [Romero‐Corral et al., 2008]. Obesity quantified by waist circumference specifically measures abdominal obesity and is believed to be a greater risk factor for negative metabolic and cardiovascular outcomes compared with other measures of obesity [Després et al., 2001; Klein et al., 2007]. There is growing evidence that adiposity specifically in the abdominal region is independently associated with metabolic disease and the release of pro‐inflammatory cytokines that contribute to systemic inflammation [Fontana et al., 2007; Lee et al., 2013; Maury and Brichard, 2010]. Further, declines in inflammatory markers have been associated with higher FA in older adults [Bettcher et al., 2015], raising the intriguing possibility of interventions to rescue brain function by increasing white matter integrity through treating inflammation related to abdominal obesity.
The controversy in the epidemiological literature linking midlife BMI to later life dementia warrants research that utilizes waist circumference, a measure of abdominal obesity that is independently associated with inflammation, and has implications for white matter health. The aim of the current study was to investigate the independent role of abdominal obesity on white matter microstructure in midlife, possibly a critical time period for establishing aging trajectories. Dyslipidemia, hyperglycemia, and hypertension frequently cluster with obesity and were controlled for as they are risk factors for cardiovascular disease and white matter damage in their own right. Accordingly, we tested the association between FA and waist circumference controlling for age, sex, fasting glucose, triglycerides, systolic blood pressure, and HDL‐cholesterol throughout the white matter using tract‐based spatial statistics (TBSS). We hypothesized that higher waist circumference would be independently associated with lower FA based on previous research reporting negative associations between global obesity and FA [Bettcher et al., 2013; Karlsson et al., 2013; Mueller et al., 2011; Verstynen et al., 2012].
MATERIAL AND METHODS
Participants
Participants included 172 apparently healthy adults between the ages of 40‐ and 62‐years old recruited from the Austin, Texas community through flyers, newspaper advertisements, and Craigslist. Participants with histories of cardiovascular disease (e.g., coronary artery disease, angina pectoris, myocardial infarction), neurological disease (e.g., Parkinson's disease, clinically significant traumatic brain injury), major psychiatric illness (e.g., schizophrenia), substance abuse, smoking, or contraindications to MRI were excluded.
All participants underwent a medical history interview, general health assessment, and brain imaging. All participants gave written informed consent for all study procedures and the institutional review board at the University of Texas at Austin approved all procedures.
Measures
General health assessment
BMI and waist circumference were measured as indices of global and abdominal obesity. A blood sample was collected from the antecubital vein by venipuncture after an 8‐h fast. Standard enzymatic techniques were used to quantify plasma concentrations of glucose, HDL‐cholesterol, and triglycerides. Brachial blood pressure was determined after 15 min of supine rest using a semi‐automated device (VP‐1000plus, Omron Healthcare, Bannockburn, IL).
Diffusion tensor imaging
MRI was performed using a 3T Siemens Skyra system (Siemens Medical Solutions, Malvern, PA) with a 32‐channel head coil. A diffusion‐weighted, spin‐echo, echo planar imaging pulse sequence was used to acquire images in 64 directions at b = 700 s/mm. The encoding directions spanned the entire sphere. One image with b = 0 was collected for a nondiffusion weighted reference image. Contiguous 2 mm slices with voxel resolution of 2 × 2 × 2 mm were collected from anterior to posterior with the following parameters: FOV = 256 mm, TR = 9,600 ms, TE = 84 ms, GRAPPA 2. Advanced shimming was performed before diffusion weighted imaging to optimize the homogeneity of the magnetic field across the brain and to minimize EPI distortions. All images were visually inspected and four images deemed poor quality were not included in the analysis (n = 186).
Processing diffusion‐weighted images for TBSS followed recommendations of Bach et al. [2014], which include high dimensional registration to a study specific template using DTI‐TK. Preprocessing included correction for motion and eddy current distortions with affine transformations using the eddy tool in FSL [Jenkinson et al., 2012]. Non‐brain signal was removed using FSL's brain extraction tool [Smith, 2002]. Tensor fitting was performed using FSL's dtifit function.
Normalization was achieved using the full tensor, instead of tensor‐derived indices such as FA, based on the methods of Hui Zhang et al. [2007]. A study‐specific template was created using iterative rigid, affine, and diffeomorphic alignments of the full tensor in DTI‐TK (http://www.nitrc.org/projects/dtitk/). This study‐specific template was based on a subset of 122 participants. Participant data not included in the template was collected after the creation of the study template. The template subset did not differ from the larger study sample in age (t = −0.41, P = 0.69), sex (χ2 = 0.14, P = 0.71) years of education (t = −0.57, P = 0.57), HDL‐cholesterol (t = 0.28, P = 0.78), systolic blood pressure (t = −0.30, P = 0.77), triglycerides (t = 0.45, P = 0.65), fasting glucose (t = 1.65, P = 0.10), and waist circumference (t = −0.01, P = 0.99). Each participant's tensor map was then normalized to the study‐specific template using one warp that combined affine and diffeomorphic alignments with final isotropic 1 mm3 resolution.
For TBSS processing, high‐resolution participant maps in template space were averaged to create a high‐resolution population mean. FA was calculated with DTI‐TK's TVtool for each individual participant and the population mean image. The white matter skeleton was generated from the FA map of the population mean and a threshold of 0.2 was applied using the FSL TBSS pipeline [Smith et al., 2006]. Lastly, FA data for each participant was projected onto the white matter skeleton.
Statistical Analyses
To test the relationship between obesity and FA, voxelwise statistical analysis was performed on the skeletonized FA data using the tool randomize, which is implemented in FSL [Winkler et al., 2014]. Randomise uses nonparametric, permutation‐based (k = 5,000) inferences within a general linear model. Waist circumference, was entered into the model as a continuous predictor variables with fasting glucose, triglycerides, systolic blood pressure, HDL‐cholesterol, age and sex as covariates. Statistical significance was determined using threshold‐free cluster enhancement (TFCE) for 2D data, using family wise error correction, and a 5% alpha.
Since the association between FA and waist circumference was in the positive direction, contradicting previous reports and our own hypothesis, further characterization of the tensor with mean diffusivity, axial diffusivity, and radial diffusivity was performed to provide more information about the differences underlying this result. We extracted MD, AD, and RD by deprojecting significant voxels back onto each participant's image in template space. This image was binarized and then multiplied by MD, AD, and RD images calculated for each participant. The average voxel value was then extracted for each index in each participant.
RESULTS
Demographics
The current sample is representative of the community and included: 59% Caucasian, 21% Latino, 10% African American, 3% Asian, and 8% other or no response. Participant demographic variables are reported in Table 1. The sample consisted of 96 women (57%), and 72 men (43%). Thirty‐three participants (20%) reported taking blood pressure medications, 19 participants (11%) reported taking cholesterol medications, and 17 participants (10%) reported taking diabetes medications.
Table 1.
Participant demographics
| Range | M | SD | |
|---|---|---|---|
| Age (years) | 40–62 | 49.5 | 6.4 |
| Education (years) | 10–20 | 16.2 | 2.3 |
| BMI (kg/m2) | 17–53 | 29.8 | 6.7 |
| Waist circumference(cm) | 68–154 | 97.7 | 16 |
| HDL‐cholesterol (mg/dL) | 21–100 | 52.6 | 16 |
| Systolic blood pressure(mmHg) | 91–166 | 123.7 | 14 |
| Triglycerides (mg/dL) | 46–340 | 116.8 | 63 |
| Fasting Glucose (mg/dL) | 65–268 | 97.8 | 24 |
FA
Permutation models using waist circumference to predict FA along white matter tracts controlling for age, sex, fasting glucose, triglycerides, systolic blood pressure, and HDL‐cholesterol found significant positive associations between waist circumference and FA in the posterior white matter bilaterally, including the superior corona radiata, superior longitudinal fasciculus, body of the corpus callosum, cingulum, posterior limb of the internal capsule, retrolenticular part of the internal capsule, splenium of the corpus callosum, the posterior thalamic radiation, anterior limb of the internal capsule, the external capsule and thalamic white matter (Fig. 1). There were no negative associations between waist circumference and FA. To assess the potential confounding effects of metabolic and cardiovascular medications, these same models were carried out excluding participants who reported taking medications indicated for the treatment of diabetes, hyperlipidemia, or hypertension (n = 120). The same relationships persisted, although the significant positive relationship between waist circumference and FA was attenuated to only the right hemisphere. Regions in the right hemisphere that remained significant included the superior corona radiata, the superior longitudinal fasciculus, the cingulum, the posterior thalamic radiation, the splenium of the corpus callosum, the posterior limb of the internal capsule, and the external capsule. Regions in the right hemisphere that were no longer significant included the body of the corpus callosum, thalamic white matter, and the anterior limb of the internal capsule.
Figure 1.
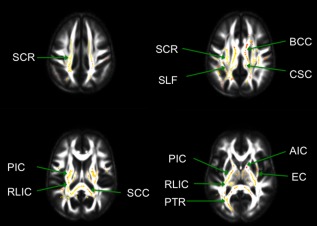
Areas of white matter tracts with significant increases in FA with increasing waist circumference are displayed in the red–yellow spectrum in radiological convention (α < 0.05, Threshold‐free cluster enhancement (TFCE), family‐wise error correction). Tracts include the superior corona radiata (SCR), superior longitudinal fasciculus (SLF), body of the corpus callosum (BCC), cingulum (CGC), posterior limb of the internal capsule (PIC), retrolenticular part of the internal capsule (RLIC), splenium of the corpus callosum(SCC), the posterior thalamic radiation (PTR), anterior limb of the internal capsule (AIC), and the external capsule (EC).
Given that positive associations were found between waist circumference and FA, contradicting our hypothesis, a secondary analysis of age was conducted to better characterize this aging sample. Consistent with prior literature, age was negatively associated with FA in anterior white matter (Fig. 2). There were no positive associations with age. In addition, we tested for an interaction between age and obesity on the effect of FA, controlling for age, sex, fasting glucose, triglycerides, systolic blood pressure, and HDL‐cholesterol. The results of this model indicated no such relationship (β = 0.06, t(159) = 0.83, P = 0.41).
Figure 2.
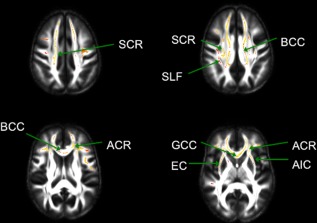
Areas of white matter tracts with significant decreases in FA with increasing age are displayed in the redyellow spectrum in radiological convention (α < 0.05, TFCE, family‐wise error correction). Tracts include the superior corona radiata (SCR), superior longitudinal fasciculus (SLF), body of the corpus callosum (BCC), anterior corona radiata (ACR), genu of the corpus callosum (GCC), external capsule (EC), and the anterior limb of the internal capsule (AIC).
MD, AD, and RD
Since positive associations were found between waist circumference and FA, contradicting our hypothesis, post hoc analysis of MD, AD, and RD were performed to better characterize the underlying differences in diffusion properties. MD, AD, and RD from each participant was extracted from voxels that were significant in the FA TBSS analysis. Three separate linear regression models were used with waist circumference as the independent variable and AD, RD, and MD as the dependent variable. Age, sex, HDL‐cholesterol, triglycerides, fasting glucose, and systolic blood pressure were included as covariates. These models revealed a negative relationship between waist circumference and AD, a negative relationship between waist circumference and RD, and a negative relationship between waist circumference and MD (Table 2). Since lower MD could be the result of a global decrease in the diffusion signal with increasing waist circumference, global MD was extracted from all participants and linear regression analyses were run with global MD as the dependent variable and waist circumference as the independent variable, controlling for age and sex. There was no evidence that waist circumference was associated with a global loss of diffusion signal (β = −0.04, t = −0.64, P = 0.53)
Table 2.
Waist circumference and DTI indices
| β | t | P | |
|---|---|---|---|
| AD | −0.39 | −4.31 | <0.001 |
| RD | −0.44 | −4.97 | <0.001 |
| MD | −0.43 | −4.85 | <0.001 |
Linear regression models with waist circumference as the independent variable and axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (MD) as the dependent variables. Age, sex, HDL‐cholesterol, triglycerides, fasting glucose, and systolic blood pressure were included as covariates.
DISCUSSION
The current study examined the relationship between abdominal obesity and white matter microstructure while controlling for other cardiovascular and metabolic factors in midlife. The strengths of the current study include the large sample that is well characterized on metabolic function. To date, it is the largest study to investigate the relationship between obesity and DTI metrics. The sample is also fairly representative of the community. In particular, the current sample included a greater proportion of Latinos (20.4%) than previous studies. The current study also utilized several advantageous methodologies, including full‐tensor, high‐dimensional normalization, a study specific template, and TBSS. These methods have been shown to improve alignment and possibly prevent spurious results from misalignment in closely adjacent tracts [Bach et al., 2014; Keihaninejad et al., 2012].
In the current study, abdominal obesity was associated with more directional diffusion in several regions of white matter, contradicting previous studies that found the inverse relationship between global obesity and FA. We, therefore, conducted post hoc analyses for significant regions in which FA was positively associated with abdominal obesity. The results suggested that greater FA was driven by lower AD, which is thought to reflect lower axon transport and damage; lower RD, which is thought to reflect greater myelination; and lower MD, which indicates restricted diffusion and membrane density. A analysis on the effect of age found decreasing FA with increasing age in anterior regions, which is consistent with prior literature [Bendlin et al., 2010]. The negative relationship between FA and age suggests that the unexpected results with waist circumference were not due to an abnormal aging sample (e.g., a strong cross‐sectional cohort effect), or a methodological problem. The contrast between the anterior age effect and the posterior obesity effect mirrors research suggesting that anterior white matter changes are associated with normal aging, while posterior changes are associated with pathological aging [Gunning‐Dixon et al., 2009; Head et al., 2004; Yoshita et al., 2006]. If higher FA observed with greater abdominal obesity in this study reflects underlying pathology, the results would be consistent with pathological aging that differentially affects posterior white matter.
Although high FA is usually associated with higher AD, lower RD, or both, our results indicate that lower AD and lower RD underlie the association of higher FA observed. In this case, linear regression revealed that with higher waist circumference, the magnitude of lower RD is greater than the magnitude of lower AD, resulting in higher FA. These results would be consistent with greater differences in myelination that are playing a dominant role in the FA calculation. In primary demyelination, axonal degradation temporally follows demyelination after the failure of repair mechanisms [Franklin and Ffrench‐Constant, 2008]. These results could reflect white matter pathology including repair mechanisms that exert reductions in RD, that precede a large effect of axonal degeneration that occurs later in life. Despite the consensus that lower FA is associated with white matter pathology, there is some evidence of higher FA with white matter pathology and repair processes. Increasing FA has been observed longitudinally in gadolinium‐enhancing lesions, indicating acute inflammatory processes, among patients with multiple sclerosis [Fox et al., 2011], and remyelination was posited to underlie these effects. Conversely, these results could also be explained by secondary, or Wallerian demyelination, during which demyelination temporally follows axonal loss [Franklin and Ffrench‐Constant, 2008]. In this situation, if higher waist circumference is associated with greater baseline myelination (lower RD), these results could represent the beginning of axonal loss (lower AD), before large decreases in myelination occur in later life.
Another possible pathological explanation of the present results could be a difference in white matter organization corresponding to higher waist circumference. Ryan et al. [2013] speculate that a loss of long white matter tracts with relative sparing of short interneurons could account for higher FA in the bilateral thalamus and left caudate among presymptomatic presenilin 1 mutation carriers, an autosomal dominant cause of Alzheimer's disease. Bender and Raz [2015] report that reductions in FA were associated with greater retest gains on an associative memory task over two years among adults 19–78 years of age. They speculate that participants with greater gains may have experienced white matter reorganization that resulted in more heterogeneous diffusion directionality. In addition, Harris et al. hypothesizes that reorganization may account for temporally dependent increases in FA following traumatic brain injury [2016]. The findings of the present study, higher FA associated with abdominal obesity, may reflect a deficit of white matter reorganization in the context of aging. In summary, there is a substantial literature that offers possible mechanisms among degenerative processes (myelin repair, loss of long white matter tracts with sparing of short interneurons, and a lack of reorganization) that could account for the association between greater abdominal adiposity and higher FA.
Another possible interpretation of these results is that the higher FA, lower RD, and lower MD indicate that abdominal obesity is associated with healthier white matter. This interpretation would be the most consistent with the interpretive consensus of FA; however, we do not know how this explanation reconciles the association with lower AD. The “obesity paradox” refers to a literature of studies that have reported protective effects of obesity, mainly in older adults and those with chronic diseases [Hainer and Aldhoon‐Hainerová, 2013]. There are several possible explanations for this paradox. A review by Hainer and Aldhoon‐Hainerová suggests that obesity, measured by BMI, is associated with better health outcomes, possibly because BMI is a better predictor of lean mass than of adiposity in older age and chronic diesease. However, abdominal obesity, measured by waist cirfumference, is consistently associated with poorer cardiovascular and metabolic outcomes. The use of waist circumference in this study and the sample of healthy middle aged adults argues against these results fitting into the current literature suggesting a protective effect of obesity. A recent epidemiological study of close to two million participants found that increasing midlife BMI was associated with lower rates of later life dementia [Qizilbash et al., 2015]. While that study remains controversial and contradicts several other smaller epidemiological studies, it challenges the dogma that adiposity is harmful to the brain and opens up the possibility of interpreting the findings observed in this study as reflecting truly healthier white matter.
It is also possible that our results reflect a more complicated picture of the relationship between the brain and obesity. The result that higher FA is associated with higher waist circumference could be explained by nonlinear effects of waist circumference on white matter across the lifespan. For instance, in a study of young adults, Warstadt et al. found positive associations between total cholesterol and FA [Warstadt et al., 2014]. They further reported that the cholesterol gene CETP (rs5882) has a positive relationship with FA in young adults and the opposite relationship in older adults. Parental family history of Alzheimer's disease, a powerful risk factor for the disease, is associated with higher FA among healthy middle aged and older adults, some of who are assumed to be in the preclinical stages of the disease [Adluru et al., 2014]. Further, Ryan et al. found that APOE ε4, a genetic risk factor for late onset Alzheimer's disease, accelerated age‐related decline of FA in a cross sectional study [2011]. Although these studies are cross sectional, they present evidence that other risk factors for neural degeneration exhibit nonlinear relationships with age and highlight the possibility that obesity has nonlinear effects on white matter microstructure with age. Although we did not find an age and obesity interaction on FA in secondary analyses, longitudinal studies and studies including a greater range of age may be better suited to test such a relationship.
The present study also found that abdominal obesity was associated with lower MD. Generally, MD is thought to indicate more restricted diffusion and is sensitive to membrane density. Lower MD with abdominal obesity would be consistent with myelination, dense axonal packing, and white matter maturation. Lower MD also raises the possibility of a decrease in the general diffusion signal as observed in Verstynen et al. [2012]. This could be the result of physiological influences of greater subcutaneous fat on the head. Mon et al. demonstrated that pig fat placed on the head changed the distribution of MR signal in T1 weighted images and MRS [Mon et al., 2013, 2016]. It is unknown if and how obesity affects the diffusion signal unrelated to the tissue microstructure. However, we found no evidence that abdominal obesity was associated with global diffusion in this study.
Although it is thought that higher inflammation and oxidative damage associated with obesity underlie changes in the white matter, the processes are unknown. It should be noted that inferences on the underlying tissue structure and pathology are speculative. It is possible that abdominal obesity captures multiple processes that exert complex and competing effects on white matter microstructure that are difficult to index. Currently most of our knowledge of how pathology affects DTI measures comes from other disease processes such as multiple sclerosis. Postmortem histopathology studies identifying white matter differences associated with obesity under the microscope are needed to further elucidate the relationship between DTI measures and brain pathology.
This study has several limitations that should be noted. As discussed above, DTI metrics are an indirect measure of white matter microstructure. The influence of crossing fibers is a limitation in all studies using DTI indices and it is possible that higher FA could result from the loss of crossing fibers. However, with the loss of crossing fibers we would also expect an increase in MD and AD, which was not observed. Therefore, we believe that these results are unlikely explained by the selective loss of crossing fibers. Another limitation that should be noted is that medication was not controlled for in this study. While medication for cardiovascular risk factors are likely to have moderating effects on brain health, it is highly unlikely that medication effects alone could explain away the results of this study since 48 out of 168 participants were on medication with great variability in drug class, dosage, and duration of treatment. In addition, all relationships persisted, albeit attenuated, if medicated participants were excluded from the analyses. A strength of this study is the greater representation of minority participants (41% of sample), who have been underrepresented in previous research, that better informs on community populations. However, it is noted that a more heterogeneous sample could possibly increase variability and muddle the observed effect. Lastly, this is a cross sectional study and inferences about changes over time should not be made. Longitudinal studies are needed to characterize how the relationship between DTI measures and abdominal obesity change over time and throughout the lifespan.
Conclusion
As measured by FA, the current study suggests that abdominal obesity is associated with more directional diffusion in the posterior white matter independent of age, sex, triglycerides, systolic blood pressure, fasting glucose, and HDL‐cholesterol. Further, the current study found that lower AD, RD, and MD underlie the relationship between waist circumference and FA. It is unclear why the results of this study seemingly contradict the results of several smaller studies investigating BMI, but this discord possibly hints at a more complicated relationship between white matter microstructure and body composition. Ultimately, the underling molecular differences and mechanisms associated with obesity are unknown and further research is needed.
REFERENCES
- Adluru N, Destiche DJ, Lu SY, Doran ST, Birdsill AC, Melah KE, Okonkwo OC, Alexander AL, Dowling NM, Johnson SC, Sager MA, Bendlin BB (2014): White matter microstructure in late middle‐age: Effects of apolipoprotein E4 and parental family history of Alzheimer's disease. Neuroimage Clin 4:730–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander AL, Lee JE, Lazar M, Field AS (2007): Diffusion tensor imaging of the brain. Neurotherapeutics 4:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves GS, O'Dwyer L, Jurcoane A, Oertel‐Knöchel V, Knöchel C, Prvulovic D, Sudo F, Alves CE, Valente L, Moreira D, Fuβer F, Karakaya T, Pantel J, Engelhardt E, Laks J (2012): Different patterns of white matter degeneration using multiple diffusion indices and volumetric data in mild cognitive impairment and Alzheimer patients. PLoS One 7:e0052859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach M, Laun FB, Leemans A, Tax CM, Biessels GJ, Stieltjes B, Maier‐Hein KH (2014): Methodological considerations on tract‐based spatial statistics (TBSS). Neuroimage 100:358–369. [DOI] [PubMed] [Google Scholar]
- Baskin ML, Ard J, Franklin F, Allison DB (2005): Prevalence of obesity in the United States. Obes Rev 6:5–7. [DOI] [PubMed] [Google Scholar]
- Bender AR, Raz N (2015): Normal‐appearing cerebral white matter in healthy adults: Mean change over 2 years and individual differences in change. Neurobiol Aging 36:1834–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendlin BB, Fitzgerald ME, Ries ML, Xu G, Kastman EK, Thiel BW, Rowley HA, Lazar M, Alexander AL, Johnson SC (2010): White matter in aging and cognition: A cross‐sectional study of microstructure in adults aged eighteen to eighty‐three. Dev Neuropsychol 35:257–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettcher BM, Walsh CM, Watson C, Miller JW, Green R, Patel N, Miller BL, Neuhaus J, Yaffe K, Kramer JH (2013): Body mass and white matter integrity: The influence of vascular and inflammatory markers. PLoS One 8:e77741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettcher BM, Yaffe K, Boudreau RM, Neuhaus J, Aizenstein H, Ding J, Kritchevsky SB, Launer LJ, Liu Y, Satterfield S, Rosano C, Health ABCs (2015): Declines in inflammation predict greater white matter microstructure in older adults. Neurobiol Aging 36:948–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Després JP, Lemieux I, Prud'homme D (2001): Treatment of obesity: Need to focus on high risk abdominally obese patients. BMJ 322:716–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S (2007): Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes 56:1010–1013. [DOI] [PubMed] [Google Scholar]
- Fox RJ, Cronin T, Lin J, Wang X, Sakaie K, Ontaneda D, Mahmoud SY, Lowe MJ, Phillips MD (2011): Measuring myelin repair and axonal loss with diffusion tensor imaging. AJNR Am J Neuroradiol 32:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench‐Constant C (2008): Remyelination in the CNS: From biology to therapy. Nat Rev Neurosci 9:839–855. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ (2001): A voxel‐based morphometric study of ageing in 465 normal adult human brains. NeuroImage 14:21–36. [DOI] [PubMed] [Google Scholar]
- Gunning‐Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS (2009): Aging of cerebral white matter: A review of MRI findings. Int J Geriatr Psychiatry 24:109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainer V, Aldhoon‐Hainerová I (2013): Obesity paradox does exist. Diabetes Care 36:S276–S281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris NG, Verley DR, Gutman BA, Sutton RL (2016): Bi‐directional changes in fractional anisotropy after experiment TBI: Disorganization and reorganization? Neuroimage 133:129–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassing LB, Dahl AK, Thorvaldsson V, Berg S, Gatz M, Pedersen NL, Johansson B (2009): Overweight in midlife and risk of dementia: A 40‐year follow‐up study. Int J Obes (Lond) 33:893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, McAvoy M, Morris JC, Snyder AZ (2004): Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: Evidence from diffusion tensor imaging. Cereb Cortex 14:410–423. [DOI] [PubMed] [Google Scholar]
- Hurd MD, Martorell P, Delavande A, Mullen KJ, Langa KM (2013): Monetary costs of dementia in the United States. N Engl J Med 368:1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012): Fsl. Neuroimage 62:782–790. [DOI] [PubMed] [Google Scholar]
- Karlsson HK, Tuulari JJ, Hirvonen J, Lepomaki V, Parkkola R, Hiltunen J, Hannukainen JC, Soinio M, Pham T, Salminen P, Nuutila P, Nummenmaa L (2013): Obesity is associated with white matter atrophy: A combined diffusion tensor imaging and voxel‐based morphometric study. Obesity (Silver Spring) 21:2530–2537. [DOI] [PubMed] [Google Scholar]
- Keihaninejad S, Ryan NS, Malone IB, Modat M, Cash D, Ridgway GR, Zhang H, Fox NC, Ourselin S (2012): The importance of group‐wise registration in tract based spatial statistics study of neurodegeneration: A simulation study in Alzheimer's disease. PLoS One 7:e45996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H, Nissinen A (2005): Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol 62:1556–1560. [DOI] [PubMed] [Google Scholar]
- Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, Kahn R, Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; American Society for Nutrition; American Diabetes Association (2007): Waist circumference and cardiometabolic risk: A consensus statement from Shaping America's Health: Association for Weight Management and Obesity Prevention; NAASO, The Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Am J Clin Nutr 85:1197–1202. [DOI] [PubMed] [Google Scholar]
- Lee MJ, Wu Y, Fried SK (2013): Adipose tissue heterogeneity: Implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med 34:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maury E, Brichard SM (2010): Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol 314:1–16. [DOI] [PubMed] [Google Scholar]
- Mon A, Abe C, Durazzo TC, Meyerhoff DJ (2013): Effects of fat on MR‐measured metabolite signal strengths: Implications for in vivo MRS studies of the human brain. NMR Biomed 26:1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mon A, Abé C, Durazzo TC, Meyerhoff DJ (2016): Fat may affect magnetic resonance signal intensity and brain tissue volumes. Obes Res Clin Pract 10:211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller K, Anwander A, Moller HE, Horstmann A, Lepsien J, Busse F, Mohammadi S, Schroeter ML, Stumvoll M, Villringer A, Pleger B (2011): Sex‐dependent influences of obesity on cerebral white matter investigated by diffusion‐tensor imaging. PLoS One 6:e18544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qizilbash N, Gregson J, Johnson ME, Pearce N, Douglas I, Wing K, Evans SJ, Pocock SJ (2015): BMI and risk of dementia in two million people over two decades: A retrospective cohort study. Lancet Diabetes Endocrinol 3:431–436. [DOI] [PubMed] [Google Scholar]
- Romero‐Corral A, Somers VK, Sierra‐Johnson J, Thomas RJ, Collazo‐Clavell ML, Korinek J, Allison TG, Batsis JA, Sert‐Kuniyoshi FH, Lopez‐Jimenez F (2008): Accuracy of body mass index in diagnosing obesity in the adult general population. Int J Obes (Lond) 32:959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren A, Skoog I, Gustafson D, Wilhelmsen L (2005): Body mass index, other cardiovascular risk factors, and hospitalization for dementia. Arch Intern Med 165:321–326. [DOI] [PubMed] [Google Scholar]
- Ryan L, Walther K, Bendlin BB, Lue LF, Walker DG, Glisky EL (2011): Age‐related differences in white matter integrity and cognitive function are related to APOE status. Neuroimage 54:1565–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan NS, Keihaninejad S, Shakespeare TJ, Lehmann M, Crutch SJ, Malone IB, Thornton JS, Mancini L, Hyare H, Yousry T, Ridgway GR, Zhang H, Modat M, Alexander DC, Rossor MN, Ourselin S, Fox NC (2013): Magnetic resonance imaging evidence for presymptomatic change in thalamus and caudate in familial Alzheimer's disease. Brain 136:1399–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM (2002): Fast robust automated brain extraction. Hum Brain Mapp 17:143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen‐Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006): Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. Neuroimage 31:1487–1505. [DOI] [PubMed] [Google Scholar]
- Verstynen TD, Weinstein AM, Schneider WW, Jakicic JM, Rofey DL, Erickson KI (2012): Increased body mass index is associated with a global and distributed decrease in white matter microstructural integrity. Psychosom Med 74:682–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warstadt NM, Dennis EL, Jahanshad N, Kohannim O, Nir TM, McMahon KL, de Zubicaray GI, Montgomery GW, Henders AK, Martin NG, Whitfield JB, Jack CR Jr, Bernstein MA, Weiner MW, Toga AW, Wright MJ, Thompson PM, Alzheimer's Disease Neuroimaging I (2014): Serum cholesterol and variant in cholesterol‐related gene CETP predict white matter microstructure. Neurobiol Aging 35:2504–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmer RA, Gunderson EP, Barrett‐Connor E, Quesenberry CP, Jr , Yaffe K (2005): Obesity in middle age and future risk of dementia: A 27 year longitudinal population based study. BMJ 330:1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014): Permutation inference for the general linear model. Neuroimage 92:381–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WL, Atti AR, Gatz M, Pedersen NL, Johansson B, Fratiglioni L (2011): Midlife overweight and obesity increase late‐life dementia risk: A population‐based twin study. Neurology 76:1568–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshita M, Fletcher E, Harvey D, Ortega M, Martinez O, Mungas DM, Reed BR, DeCarli CS (2006): Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology 67:2192–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Jahng GH, Bayne W, Mori S, Schad L, Mueller S, Du AT, Kramer JH, Yaffe K, Chui H, Jagust WJ, Miller BL, Weiner MW (2007): Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology 68:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]


