Abstract
Background
In our recent clinical trial, the addition of omalizumab to oral immunotherapy (OIT) for milk allergy improved safety but no significant clinical benefit was detected.
Objective
To investigate mechanisms by which omalizumab modulates immunity in the context of OIT, and to identify baseline biomarkers that predict subgroups of patients most likely to benefit from omalizumab.
Methods
Blood was obtained at baseline and multiple time-points during a placebo-controlled trial of OIT for milk allergy where subjects were randomized to receive omalizumab or placebo. Immunologic outcomes included measurement of basophil CD63 expression and histamine release (HR), and casein-specific CD4+ T regulatory (Treg) cell proliferation. Biomarkers were analyzed in relationship to measurements of safety and efficacy.
Results
Milk-induced basophil CD63 expression was transiently reduced in whole blood samples from both omalizumab and placebo subjects. However, IgE-dependent HR increased in washed cell preparations from omalizumab but not placebo subjects. No increase in Treg frequency was evident in either group. Subjects with lower rates of adverse reactions, regardless of arm, experienced better clinical outcomes. Pre-OIT basophil reactivity positively associated with occurrence of symptoms during OIT, while the baseline milk IgE/total IgE ratio correlated with the likelihood of achieving sustained unresponsiveness (SU). A combination of baseline basophil and serologic biomarkers defined a subset of patients where adjunctive therapy with omalizumab was associated with attainment of SU and a reduction in adverse reactions.
Conclusions
Combining omalizumab therapy with milk OIT led to distinct alterations in basophil reactivity but not T cell responses. Baseline biomarkers may identify subjects most likely to benefit from adjunctive therapy with omalizumab.
Keywords: oral immunotherapy (OIT), food allergy, milk allergy, omalizumab, desensitization, sustained unresponsiveness, basophil activation, biomarker, T regulatory cells
Introduction
Food allergy affects approximately 15 million Americans and is the most common cause of anaphylaxis outside the hospital setting.1 No FDA-approved treatment for food allergy is currently available. Recent studies suggest that oral immunotherapy (OIT), in which allergenic food is mixed into a vehicle and then ingested in gradually increasing quantities, may hold promise as a treatment for food allergy.2, 3 However, enthusiasm for this therapy has been tempered by the frequent occurrence of adverse reactions and the lack of sustained protection in most subjects once treatment is discontinued.2, 3 These limitations have sparked investigations into adjunctive therapies, including omalizumab, that could improve both safety and long-term efficacy. Omalizumab is a humanized monoclonal antibody that sequesters free IgE and prevents its binding to the high affinity IgE receptor, FcεRI. We recently completed the first double-blind placebo-controlled (DBPC) trial of omalizumab in combination with OIT in patients with severe persistent cow's milk allergy.4 Although no statistically significant benefit of omalizumab in promoting desensitization was detected compared to subjects receiving milk OIT (MOIT) alone, omalizumab-treated subjects exhibited highly significant improvements in nearly all safety parameters.4
Similar to other immunotherapy trials for food allergy, we observed significant heterogeneity in the frequency and severity of adverse reactions as well as clinical responses both within and between treatment groups.4 Identification of biomarkers that could predict which subjects are at highest risk of reactions and which are most likely to benefit from adjunctive therapies would have tremendous value in efforts to translate these therapies into clinical practice. To accomplish this goal, a greater understanding of the immunologic mechanisms underlying successful treatment is needed, as well as how adjunctive therapies, such as omalizumab, can facilitate these effects. Suppression of mast cell and basophil reactivity, increase in food-specific IgG4, decrease in food-specific IgE, and reduction in Th2 immune responses are mechanisms most often associated with successful immunotherapy to food allergens.5, 6 By preventing the interaction between circulating IgE and FcεRI, omalizumab suppresses expression of FcεRI on the surface of basophils, mast cells, and antigen-presenting cells, leading to reduced allergen-mediated activation of these cells.7-9 Some clinical studies, however, have demonstrated that basophils from a subset of patients treated with omalizumab exhibited no change or paradoxically greater responsiveness to IgE receptor cross-linking post-treatment.10-12 These subjects experienced less clinical benefit from omalizumab, particularly if they had higher baseline allergen-specific to total IgE ratios.12
In this study, we sought to dissect the effects of omalizumab on basophil and T cell allergen-induced activation in subjects undergoing MOIT, and to explore whether baseline biomarkers can predict which subjects are likely to benefit the most from adjunctive treatment with omalizumab.
Methods
Clinical study
Participants aged 7-35 years were enrolled in a randomized multi-center DBPC trial of MOIT combined with omalizumab in the treatment of challenge-confirmed IgE-mediated cow's milk allergy. The study design and clinical outcomes have been reported previously.4 Briefly, 57 subjects were randomized 1:1 to receive either omalizumab (n=28) or placebo (n=29) injections. One subject dropped out of each arm prior to starting injections. Omalizumab subjects whose dose fell outside the package insert dosing chart received 0.016 mg/kg/IgE IU; subjects who dose was greater than 750 mg were excluded. At Month 4 (M4), all subjects began MOIT dosing and were built-up to a minimum maintenance dose of 520 mg of milk protein. Subjects continued blinded omalizumab or placebo injections through M16. At M16, all subjects were unblinded and placebo subjects discontinued placebo dosing while omalizumab subjects continued active injections until M28. All randomized subjects completed a 10 gram Desensitization OFC at M28 (n=26 in omalizumab arm; n=24 in placebo arm at M28 due to 1 additional withdrawal in the omalizumab group and 4 in the placebo arm). Subjects who failed this OFC discontinued MOIT (n=2 in omalizumab and n=4 in placebo) whereas those who passed continued MOIT dosing until M30, at which point they discontinued MOIT and underwent a 10 gram Tolerance OFC at M32. Participants who passed the M32 challenge were considered to have achieved sustained unresponsiveness (SU), defined as the lack of reactivity following milk ingestion after 8 weeks of milk avoidance (n=13 in omalizumab group; n=10 in placebo). Blood was collected at baseline and at each of the time points described above, as well as M22. The clinical study was approved by the IRBs of the Icahn School of Medicine at Mount Sinai, Johns Hopkins University and Stanford University and informed consent/assent was obtained from all participants.
Basophil Assays
Whole heparinized blood or washed basophil-enriched suspensions were prepared as previously described and stimulated with milk allergen, anti-IgE, or media alone and assayed for basophil CD63 expression or histamine release (HR), respectively.13, 14 Additional details are provided in the Online Repository.
Casein-Specific T regulatory (Treg) Assay
CD4+ Treg (CD4+CD25+CD127lo/-Foxp3+) proliferation was assessed by dilution of carboxyfluorescein diacetate succinimidyl ester-labeled PBMCs as described in the Online Repository.
Immunoglobulin Measurements
Quantification of milk-, casein-, beta-lactoglobulin- specific and total IgE and IgG4 was done by ImmunoCAP assay (ThermoFisher Scientific; Michigan).
Statistical Analysis
Wilcoxon rank sum tests, analysis of covariance, and paired t-tests were used to analyze continuous variables, logistic regression and Fishers exact tests to analyze categorical data, and Spearman correlations to assess associations. We analyzed all randomized subjects, and those without OFC outcome data were considered failures.
To identify subgroups where omalizumab might be most beneficial, we first identified promising prognostic variables from graphical displays. We then applied the approach of Shuster and van Eys,15 fitting regression models which allowed the omalizumab effect to vary as a function of these prognostic factors, to construct regions with strongest evidence of omalizumab advantage using logistic regression for binary and ordinal outcomes and linear regression for continuous outcomes. Given the exploratory nature of this analysis, these resulting subgroups should be viewed as preliminary.
We describe results with two-sided p-values <0.05 as statistically significant, but results should be viewed cautiously because of the multiplicity of analyses. However, given that many of these variables are very highly correlated, concerns about multiplicity are considerably less than if these were independent variables. See Online Repository for additional details.
Results
Basophil CD63 expression
Upregulation of CD63 is frequently used as a measure of basophil responsiveness.16, 17 Basophils from omalizumab subjects showed decreased milk-induced CD63 expression relative to baseline at M4, after starting omalizumab but before initiating MOIT (p=0.0074 and 0.0135 at milk 0.1 and 10 μg/mL, respectively; Fig 1). This suppression was not evident in the placebo group at M4 (Fig 1). After adjusting for baseline, %CD63 was lower in omalizumab subjects compared to placebo at M4 (p=0.0040, 0.0510, 0.0014 for milk stimulant concentrations 0.1, 1, and 10 μg/mL, respectively). Following visit M4 when both arms initiated MOIT, %CD63 following milk stimulation decreased in both groups compared to baseline and remained significantly suppressed through M28. As previously reported, in a longitudinal analysis, CD63 expression was significantly decreased in the omalizumab arm relative to the placebo group through M28 at the three highest concentrations of milk (Fig 1).4 We also observed significant declines in CD63 expression at M28 from baseline with p<0.01 at all concentrations of milk for both arms, except for the placebo group at 10 μg/mL. 4
Fig 1.
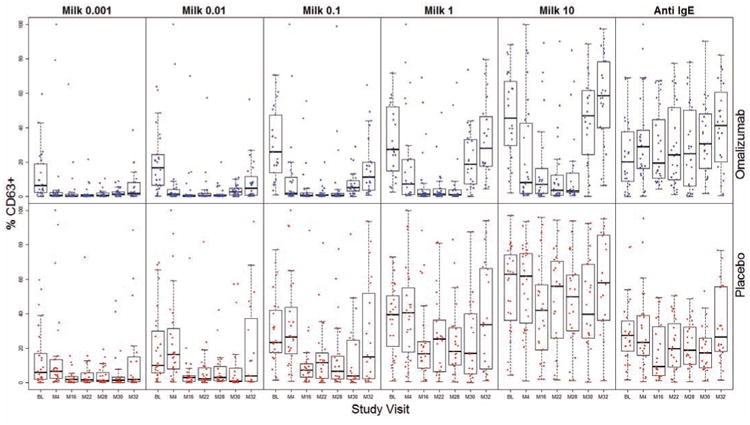
Whole blood basophil %CD63 expression in omalizumab (blue) and placebo (red) subjects following stimulation with milk (0.001, 0.01, 0.11, 10 μg/mL) or anti-lgE at baseline (BL) and visits M4, 16, 22, 28, 30 and 32. Statistically significant p-values are indicated in the text. These data were previously presented in Reference 5.
Despite continued maintenance dosing at M30, milk-induced CD63 expression increased in both groups such that the %CD63 was no longer significantly suppressed compared to baseline in either omalizumab- or placebo-treated subjects at 1 or 10 μg/mL milk (Fig 1). CD63 expression was also not lower than baseline at M32, after MOIT had been discontinued for 8 weeks (Fig 1).
Basophil CD63 expression following anti-IgE stimulation did not change over the course of the study in the placebo group (Fig 1). However, CD63 expression to anti-IgE rose steadily in the omalizumab subjects, reaching a highly significant increase from baseline at M30 (p=0.0043) and M32 (p=0.0003; Fig 1).
Basophil Histamine Release
To determine how serum factors might be influencing basophil responses during the course of the study, we prepared washed basophil-enriched suspensions from a subset of subjects, stimulated them under identical conditions as the whole blood samples, and measured HR, which correlates closely with CD63 expression.13 Although our sample size was small (n=7 omalizumab; n=10 placebo), HR to each of the 5 concentrations of milk was higher at nearly all visits compared to baseline in omalizumab-treated subjects (p=0.0240 at M16 in 0.001 μg/mL condition; p=0.0199, 0.0302, and 0.0294 at M16, M22, and M28 visits, respectively, in the 1 μg/mL condition Fig 2). This pattern was less evident in the placebo group. Furthermore, in omalizumab subjects only, HR to anti-IgE increased dramatically at M4 (p=0.0035), and remained higher compared to baseline (p<0.0005 at M16, M20, and M28) until omalizumab therapy was discontinued at M28 (Fig 2).
Fig 2.
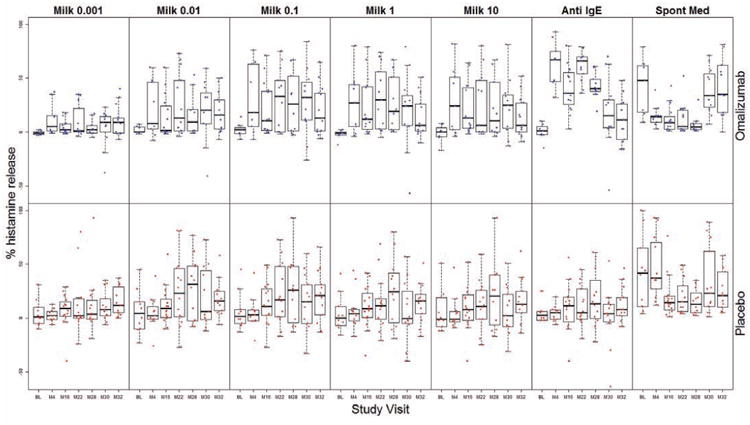
Percent histamine release from washed basophil-enriched suspensions from omalizumab (blue) and placebo (red) subjects stimulated with the 5 concentrations of milk (0.001, 0.01, 0.11, 10 μg/mL), anti-lgE, or media alone (Spont Med) at baseline (BL) and visits M4, 16, 22, 28, 30, and 32. Statistically significant p-values are indicated in the text.
Subjects receiving omalizumab exhibited a decrease in spontaneous HR at M4, although this did not reach statistical significance. This change was not evident in the placebo group (Fig 2). Subjects in both arms experienced significant decreases in spontaneous HR after the addition of MOIT (in omalizumab group, p=0.0101 at M20 and p=0.0274 at M28; in placebo group, p=0.0336 at M16 and p=0.0182 at M28; Fig 2), but levels returned to baseline by M30.
Frequency of milk-specific T regulatory cells
Neither omalizumab (n=13) nor placebo (n=11) subjects exhibited a significant increase in the percentage of casein-specific Tregs over the course of treatment (Fig E1).
Relationship Between Symptoms and Clinical Outcome
To determine the relationship between the frequency of symptoms that subjects experienced during MOIT and the degree of desensitization they achieved, we evaluated clinical responses as an ordinal variable, where SU (passed M32 OFC) is the best outcome, desensitization (passed M28 OFC but failed M32 OFC) is the intermediate outcome, and failed (failed M28 OFC) is the worst outcome. Clinical responses were highly associated with symptoms during treatment, such that the lower the rate of the symptoms, the greater the likelihood of better clinical outcome (Fig 3). This was highly statistically significant in the overall population for the three symptom categories during the entire study (p=0.0057 for moderate-severe symptoms, p=0.0038 for GI symptoms, p=0.0013 for any symptom excluding oropharyngeal symptoms). Results were also generally significant for the escalation and maintenance phases separately (Fig 3). These relationships were generally stronger in the placebo arm compared to the omalizumab arm when the groups were analyzed separately (Fig. 3).
Fig 3.
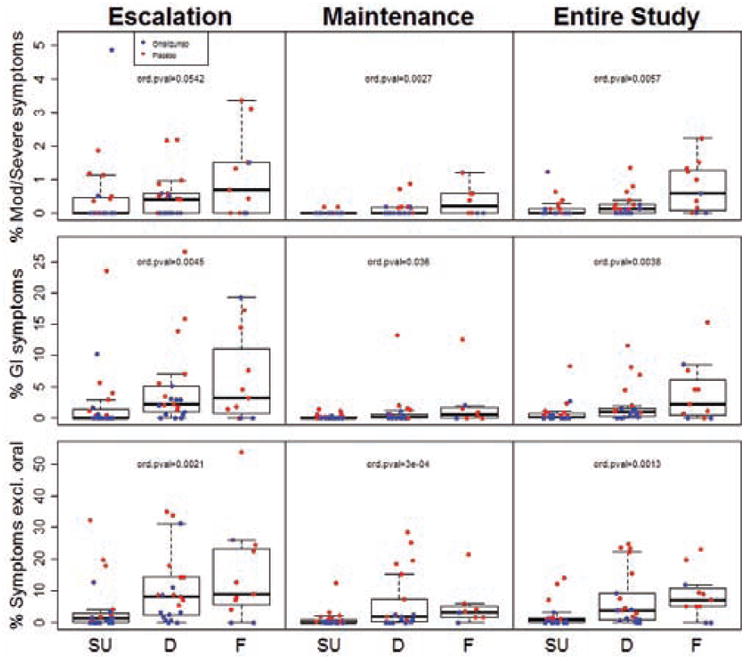
Percent of MOIT doses given during the escalation or maintenance phase of MOIT, or over the course of the entire study, that led to moderate-severe (Mod/Severe) symptoms, Gl symptoms, or any symptom excluding oropharyngeal (Symptoms excl. oral) in subjects who achieved sustained unresponsiveness (SU), were desensitized only (D), or failed the M28 desensitization OFC (F). P-values for a difference in ordinal outcomes (SU/D/F; ord. pval) are presented.
Relationship between Baseline Biomarkers and Symptoms
As illustrated in Fig 4, subjects whose basophils showed greater reactivity (%CD63 positive) to milk at baseline generally exhibited higher rates of adverse reactions, suggesting that these subjects may have the greatest potential for improved safety with omalizumab. A regression model that allowed the effect of omalizumab to vary with baseline %CD63 at 10 μg/mL milk determined a significant advantage from omalizumab starting at values of 34% for moderate-severe symptoms and 42% for GI symptoms, suggesting that 40% is a viable threshold for establishing a possible “safety subgroup” with greatest benefit from omalizumab. As shown in Fig 5, omalizumab subjects whose baseline %CD63 was above 40% experienced far fewer symptoms than the corresponding placebo subjects (p=0.0004 for moderate-severe symptoms; p<0.0001 for any symptoms other than oropharyngeal; p=0.0028 for GI symptoms). While there was some indication of an omalizumab advantage in the small cohort with baseline %CD63 below the 40% threshold, the magnitude of the treatment benefit appeared much less than that in the >40% subgroup (Fig 5). Table E1 predicts how success rates (defined as a reduction in moderate-severe symptoms, GI symptoms, and any symptom other than oropharyngeal to <0.25%, <2%, and <5%, respectively) would change in the placebo group if those subjects who fell into the “safety subgroup” had been treated with omalizumab, under the assumption that patients in this subgroup had the same success rate observed in the corresponding subgroup in the omalizumab arm.
Fig 4.
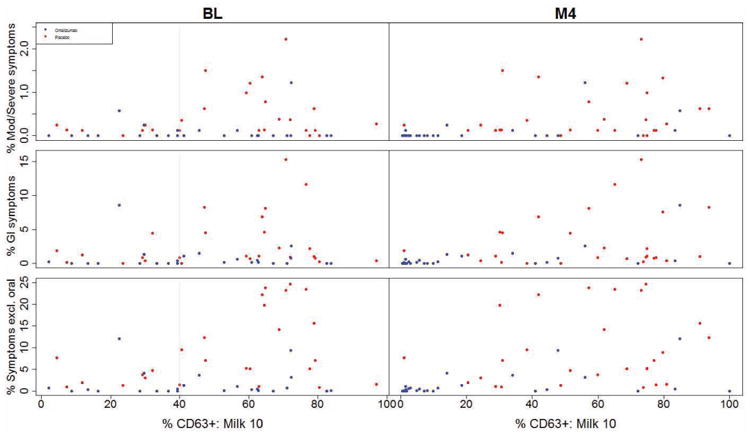
Relationship between percent of MOIT doses that led to moderate-severe (Mod/Severe) symptoms, Gl symptoms, or any symptom excluding oropharyngeal (Symptoms excl. oral) over the course of MOIT and basophil %CD63 expression at milk stimulant concentration 10 μg/mL (%CD63+: Milk 10) measured at the baseline (BL) or M4 visit. Statistically significant p-values are discussed in the text. The gray vertical line at %CD63 expression at baseline illustrates the proposed threshold for greatest treatment difference.
Fig 5.
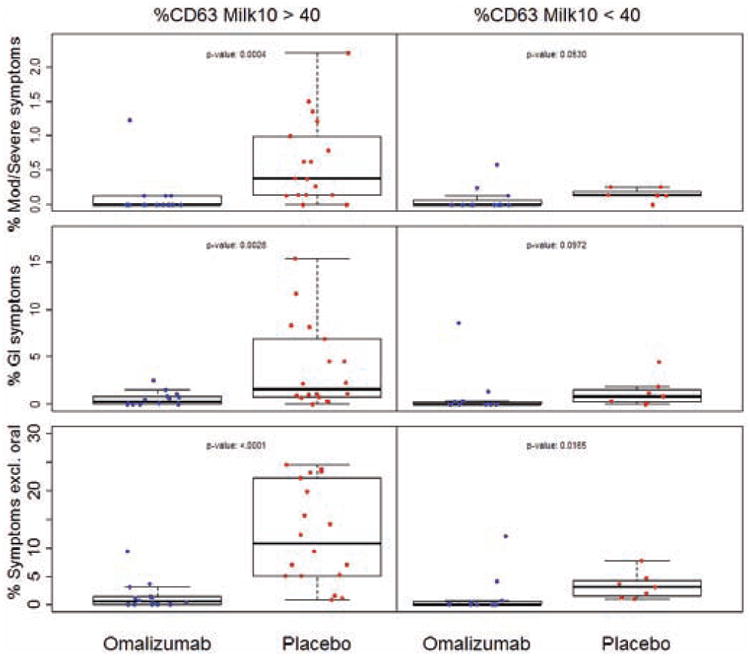
Percent of MOIT doses that led to moderate-severe (Mod/Severe) symptoms, Gl symptoms, or any symptom excluding oropharyngeal (Symptoms excl. oral) over the course of MOIT in subjects whose baseline basophil %CD63 expression at milk stimulant concentration 10μg/mL was less than or greater than 40% (%CD63 Milk 10>40 or <40, respectively). P-values are indicated in the graphs.
When all study participants were evaluated as a single group, a strong positive association was noted between the frequency of moderate-severe symptoms during MOIT and %CD63 expression using 10 μg/mL milk in the basophil assay measured at M4, before MOIT was initiated (p=0.0002; Fig 4). This same pattern was significant in the omalizumab arm only at M4 (p=0.0490), and with other categories of symptoms including GI symptoms (p=0.0004 overall and p=0.0312 in omalizumab arm at M4) and any symptoms other than oropharyngeal (p<0.0001 overall at M4; Fig 4). The observed reduction in %CD63 values from baseline to M4 in the omalizumab arm (particularly in the >40% subgroup) might be driving the large difference in the rate of symptoms during MOIT between the arms.
All other baseline measures of basophil reactivity also strongly associated with the frequency of symptoms (all 3 categories; Fig E2A-E). Age at study entry was not associated with symptom frequency (Fig E2F). Baseline milk IgE and the milk IgE/total IgE ratio were not significantly associated with moderate-severe symptoms, but overall showed moderate positive correlations with GI symptoms (Fig E2G; p=0.0138 and p=0.0309, respectively) and any symptoms other than oropharyngeal (Fig E2G; p= 0.0209 and p=0.0282, respectively). Baseline casein IgG4/casein IgE and beta-lactoglobulin IgG4/beta-lactoglobulin IgE ratios were overall not significantly associated with symptoms (Fig E2H).
Relationship between Baseline Biomarkers and Clinical Outcome
Subjects whose baseline %CD63 at 10 μg/mL milk was >40% were not more likely to achieve SU if they received omalizumab, despite the strong safety advantage in this subgroup (Fig E3). However, a logistic regression model that allowed the effect of omalizumab on achieving SU to vary with the baseline ratio of %CD63 at 10 μg/mL milk over %CD63 anti-IgE found a significant advantage in the omalizumab group starting at baseline ratios of 2.82 (Fig 6). Among omalizumab subjects whose ratio was greater than 2.82, 7 out of 9 exhibited SU in the omalizumab group compared to 0 out of 4 in the placebo group. Relationships of other baseline markers of basophil activation with clinical outcome are displayed in Fig E4A-D.
Fig 6.
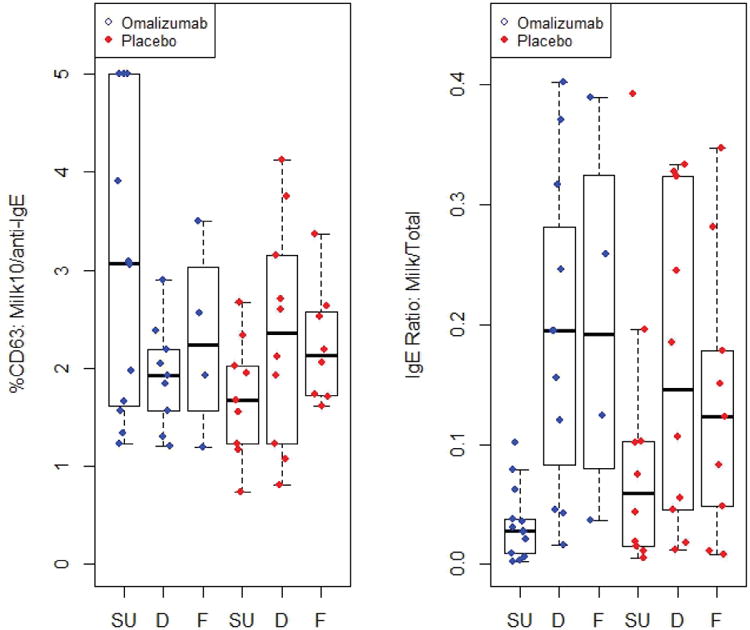
Ratio of basophil %CD63 expression at milk stimulant concentration 10 μg/mL over %CD63 anti-lgE (%CD63: Milk10/anti-lgE) or ratio of milk IgE over total IgE (IgE Ratio: Milk/Total) in placebo (red) and omalizumab (blue) subjects who achieved sustained unresponsiveness (SU), were desensitized only (D) or failed (F). Statistically significant p-values are discussed in the text.
Furthermore, the baseline milk IgE/total IgE ratio was strongly associated with the SU outcome. Subjects overall who achieved SU had significantly lower baseline milk IgE/total IgE ratios than those who failed to achieve this endpoint (Fig 6; p=0.0002). This relationship was highly significant in the omalizumab arm (Fig 6; p=0.0001) but did not reach significance in the placebo arm when analyzed separately (Fig 6). Omalizumab subjects whose ratio was low, below about 0.1, generally achieved SU which was not true for corresponding placebo subjects. Baseline milk IgE alone was also lower overall, and the casein IgG4/IgE ratio higher, in subjects who passed the M32 OFC (Fig E4E, F; p=0.0032, 0.0052, respectively) and in the omalizumab arm only (Fig E4E, F; p=0.017, 0.0083, respectively). The baseline milk IgE/total IgE ratio (Fig 6), milk IgE (Fig E4E), casein IgG4/IgE ratio (Fig E4F), and beta-lactoglobulin IgG4/IgE ratio (Fig E4G) were not significantly associated with the outcome of the M28 desensitization challenge. Age of the subject at entry into the study was not associated with the likelihood of passing the M28 or M32 OFC (Fig E4H).
Since there was some indication that the effect of omalizumab on SU might vary with both the baseline ratio of %CD63 at 10 μg/mL milk over %CD63 anti-IgE and the milk IgE/total IgE ratio, we next explored whether a combination of these biomarkers would identify a potential subgroup of patients who may most benefit from omalizumab. We fit a logistic model that allowed the effect of omalizumab on achieving SU to vary with both of these variables. This exploratory analysis found a subgroup associated with a significant omalizumab group effect, but we focused on a broader subgroup where the estimated treatment effect was positive but not necessarily significant, indicated by the shaded region in Fig 7A, where %CD63 at 10 μg/mL milk/%CD63 anti-IgE > 0.91 + 10.98× (milk IgE/total IgE), which splits the sample exactly in half (Fig 7A). Within this “efficacy subgroup”, 9/12 subjects in the omalizumab arm achieved SU versus 4/14 in the placebo arm (Fig 7B, p=0.0472). If the 14 placebo subjects who had fallen into this efficacy subgroup had been treated with omalizumab, the predicted success rate for achieving SU in the full placebo cohort would have increased from approximately 33% to 57% (Table E1), assuming that the placebo patients in this subgroup would have experienced exactly the same success rate as the corresponding subgroup in the omalizumab arm. Omalizumab subjects also experienced a significant advantage with respect to the ordered clinical response, i.e., where SU is best outcome, desensitization is intermediate, and failed is worst (p=0.0211). This same subgroup also showed a dramatic decrease in all categories of symptoms compared to placebo (Fig 7C; p=0.0025, 0.0041, <0.0001 for moderate-severe, GI, and any symptoms other than oropharyngeal, respectively).
Fig 7.
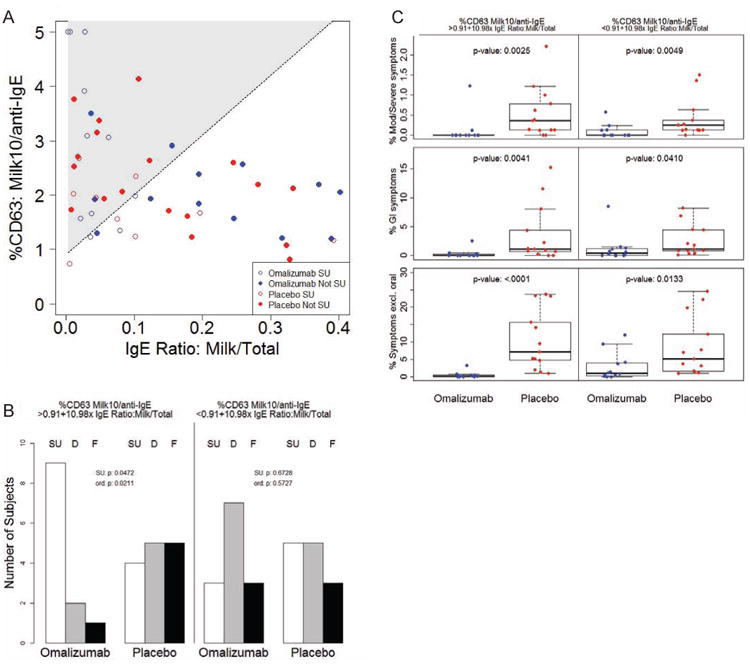
A Baseline values of the ratio of %CD63 to milk stimulant concentration 10 μg/mL over %CD63 to anti-lgE (%CD63: Milk10/anti-lgE) were evaluated in relation to the baseline milk IgE/total IgE ratio (IgE Ratio: Milk/Total) in a logistic interaction model. Shaded area indicates region of numerically positive treatment estimate in terms of likelihood of achieving SU. Subjects who achieved SU are shown in open points; those who were desensitized only or failed are depicted in closed points (Not SU). B Number of omalizumab or placebo subjects whose baseline biomarker values fell above (%CD63 Milk10/anti-lgE > 0.091+10.98× IgE Ratio: Milk/Total) or below (%CD63 Milk10/anti-lgE < 0.091+10.98× IgE Ratio: Milk/Total) the line of positive effect described in A who achieved sustained unresponsiveness (SU), were desensitized only (D) or failed (F). P-values for a difference in SU (SU. p), and for a difference in ordinal outcomes (SU/D/F; ord. p) are presented. C Percent of MOIT doses that led to moderate-severe (Mod/Severe) symptoms, GI symptoms, or any symptom excluding oropharyngeal (Symptoms excl. oral) over the course of MOIT in the two subsets of subjects described in B. P-values are indicated in the graphs.
Omalizumab subjects in the complementary subgroup (%CD63 at 10 μg/mL milk/% CD63 anti-IgE < 0.91 + 10.98× (milk IgE/total IgE)) did not have a higher rate of SU than placebo subjects (Fig 7B). This subgroup did still show significantly reduced symptoms compared to placebo (Fig 7C; p=0.0049, 0.0410, 0.0133 for moderate-severe, GI, and any symptoms other than oropharyngeal, respectively), although the extent of the reduction observed was not as great.
Relationship between Concurrent Biomarkers and Clinical Outcome
Overall, subjects who passed the M28 desensitization challenge had significantly lower %CD63 at milk concentrations 1 and 10 μg/mL and AUC, and higher casein IgG4/IgE and beta-lactoglobulin IgG4/IgE ratios, at M28 compared to those who failed the desensitization challenge (p=0.0019, 0.0007, 0.0036, 0.0304, 0.0288 respectively; Fig E5A-C, G, H). This relationship was strongest in the omalizumab arm (p=0.0067 for all 3 basophil biomarkers and p=0.0191 for the casein IgG4/IgE ratio) and did not reach significance in the placebo arm when considered separately (Fig E5A-C).
These same biomarkers measured at visit M32 were also strongly associated with the outcome of the M32 OFC. Overall, subjects who passed this OFC had a lower %CD63 at 1 and 10 μg/mL of milk and AUC, and higher casein IgG4/IgE and beta-lactoglobulin IgG4/IgE ratios, compared to those who failed (Fig E5A-C, G, H p=0.0114, 0.0369, 0.0239, 0.0018, 0.0015, respectively). No basophil biomarker measured at M28 or M32 as a ratio over %CD63 anti-IgE was significantly associated with the outcome of the M28 or M32 OFCs, respectively (Fig E5D-F).
Discussion
Several immunotherapy approaches are currently under investigation for the treatment of food allergy, with OIT providing the greatest degree of desensitization and SU. However, the clinical benefits of OIT are limited by the frequent occurrence of adverse effects, including systemic reactions and intolerable GI symptoms.2, 3 We recently reported that addition of omalizumab to a standard MOIT regimen could improve the safety of MOIT, although no benefit in enhancing desensitization or SU was detected.4 In this study, further post hoc analyses demonstrate that omalizumab exerts distinct effects on basophil activation beyond those induced by MOIT alone, but did not enhance allergen-induced Treg development. Furthermore, we identified subgroups of patients, based on their pretreatment basophil reactivity and/or allergen-specific to total IgE ratio, who may be most likely to benefit from omalizumab in terms of reducing symptoms and achieving SU.
Several trials have previously demonstrated marked suppression of allergen-induced basophil responses following monotherapy with either OIT or omalizumab.13, 18-22 We also observed decreased basophil CD63 expression at visit M4 (before initiation of MOIT) in subjects receiving omalizumab but not placebo, and in both groups after the addition of MOIT. However, combined treatment with omalizumab and MOIT led to an even greater reduction in basophil activation than that achieved with MOIT alone, particularly at the highest concentrations of milk. In contrast, no change in basophil HR was observed in washed basophil suspensions isolated from the placebo group, and HR actually increased in the omalizumab group following treatment with IgE receptor crosslinking stimuli (milk and anti-IgE). The incongruent results between these experiments suggest that serum factors, likely milk-specific IgG which increases during OIT4, may largely be responsible for the suppression in basophil reactivity observed in the whole blood samples. No change in basophil HR to milk was also reported in a previous MOIT study where washed cell preparations were used.23
The increase in HR to IgE crosslinking stimuli that we observed in washed cells from omalizumab subjects is consistent with prior studies where omalizumab was found to markedly enhance the sensitivity of basophils to degranulation.10-12 Two competing factors have been found to influence changes in basophil reactivity following omalizumab therapy: 1) decrease in allergen-specific IgE on the basophil surface; 2) increase in intrinsic sensitivity of basophils to IgE-mediated stimulation.10-12 In this study, a third variable, MOIT and the resulting increase in milk-specific IgG4, also likely influenced overall basophil responsiveness, leading to a net decrease in CD63 expression in whole blood samples, at least early in the course of treatment.
Basophils from more than 80% of food allergic children spontaneously release histamine24, and monotherapy with either omalizumab or OIT has been shown to suppress this phenomenon.13, 25, 26 We, too, observed a qualitative decrease in spontaneous HR with omalizumab (M4 compared to baseline in the treatment group) and in both groups following the start of MOIT. The decrease in both milk-induced CD63 expression and spontaneous HR are consistent with an overall suppression of IgE-mediated basophil activation with OIT.
Despite the decreases in milk-induced basophil CD63 expression early in MOIT treatment in both omalizumab and placebo groups, as previously reported, basophil reactivity returned to baseline after MOIT had been discontinued for 8 weeks (M32), and in some subjects despite continued maintenance dosing (M30).4 Immunologic suppression during OIT has been shown to be transient, and these data suggest that the addition of omalizumab is not sufficient to prevent this reversal.18 Interestingly, the degree of suppression in milk-induced CD63 expression at M28 and M32 was strongly associated with the likelihood of passing an OFC at these visits, suggesting that inhibition of basophil reactivity may be central to the underlying mechanisms responsible for desensitization to milk.
T regulatory cells (Tregs) are a class of T cells that are thought to suppress allergic immune responses and promote tolerance to food antigens. Several prior studies have investigated the role of Tregs in the development of desensitization to food allergens during OIT with conflicting results.18, 20, 27, 28 In this study, we did not find an increase in the frequency of milk-specific Tregs during MOIT, including in subjects who received omalizumab. These results are consistent with prior trials of omalizumab for asthma or milk allergy, where no changes in Treg frequency were observed.22, 29 These data suggest that the clinical benefits of omalizumab are unlikely to be mediated by Treg-dependent mechanisms, or that the increase in Tregs is transient and not captured by the time-points studied here.
OIT is often associated with a high rate of adverse reactions. Subjects who experienced the greatest frequency of reactions during MOIT also gained the least benefit clinically in terms of achieving desensitization or SU. These findings underscore the need to identify baseline biomarkers that can define this “difficult-to-treat” subset of patients who may benefit from omalizumab. Baseline allergen-specific to total IgE ratios and basophil activation have previously been shown to be important indicators of clinical responses to omalizumab.12, 30 Basophil responses have also recently been correlated with the severity and threshold of allergic reactions to peanut during OFCs, and may be predictive of children who will outgrow their food allergy.31, 32 In this study, we detected a strong positive association between nearly all measures of baseline basophil reactivity and symptoms. Subjects whose baseline milk-induced CD63 expression was below a threshold of 40% generally had infrequent symptoms (all categories) regardless of whether they received omalizumab or not, suggesting this group may not require adjunctive therapy. On the other hand, subjects whose basophils reacted above this threshold were remarkably less likely to have symptoms if they received omalizumab. This likely reflects omalizumab's ability to effectively suppress basophil activation, as indicated by the marked reduction in CD63 expression between visits M4 (after starting omalizumab) and baseline, particularly in the >40% subgroup.
A primary limitation of OIT for food allergy has been the lack of persistent clinical benefit once oral immunotherapy is discontinued. However, baseline measures of basophil activation and serologic markers identified a subset of patients where omalizumab may enhance development of SU. In this subgroup, those who received omalizumab also experienced fewer symptoms (all categories) than their placebo counterparts. Although widespread clinical use of omalizumab may not be possible given the significant costs, our data suggest there may be a subgroup of patients where this adjunctive therapy is warranted. The length of omalizumab therapy needed to confer improvements in safety and/or clinical outcomes will require comparative trials where the length of adjunctive treatment is varied.
Our study has several important limitations. First, not all mechanistic assays were performed at all time points on all subjects, which may have limited our power. Second, we sought to identify subgroups of MOIT patients who may benefit from combined therapy with omalizumab; however, the observed results are based on a small sample size which precluded an independent validation. Third, we conducted many analyses, so the p-values need to be interpreted cautiously. Nonetheless, this study demonstrates that omalizumab exerts distinct effects on basophil activation, and our analyses suggest that baseline biomarkers may predict a subset of patients where omalizumab may not only reduce the rate of adverse reactions, but also possibly promote acquisition of SU. Confirmation of these findings in a larger prospective clinical trial is needed.
Supplementary Material
Key Messages.
Combined treatment with omalizumab and OIT led to distinct alterations in IgE-dependent basophil activation that likely reflected competing effects of omalizumab on intrinsic and extrinsic basophil reactivity and the influence of serum factors induced by OIT. Addition of omalizumab did not promote an increase in casein-specific Treg frequency.
Baseline basophil CD63 expression was strongly associated with the occurrence of symptoms during OIT, and may predict a subgroup of patients who will experience the most benefit from omalizumab in reducing adverse reactions.
Baseline measures of basophil reactivity and the allergen-specific IgE over total IgE ratio may help identify subjects who are likely to benefit the most from adjunctive therapy with omalizumab during OIT in terms of both safety and likelihood of achieving sustained unresponsiveness.
Acknowledgments
We would like to thank Mohanapriya Kamalakannan, Lisa Chang, Mahlet Yishak, Molly Kidder, Madhundra Sivakumar, Akshay Bhatt, Ashish Jain, Yue Deng, Michelle Mishoe, Jessica Kinard, Peter Dawson, PhD (EMMES), Alice Henning, Shu Chen Lyu, and Melissa Paterakis for their expert assistance with this study.
Funding and Disclaimers: Omalizumab (Xolair) and omalizumab placebo for the trial were kindly provided by Genentech. The study was supported by grant AI-44236 from the National Institute of Allergy and Infectious Diseases and RR-026134 (Mount Sinai) and UL1 TR 001079 (Johns Hopkins) from the National Center for Advancing Translational Sciences, National Institutes of Health; and a supplemental grant from Food Allergy Research and Education.
P.A.F.-G. is supported by the Division of Intramural Research, NIAID, NIH. This project was funded in in part with federal funds from NCI, NIH under Contract No. HHSN26120080000IE (to W.G). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Abbreviations
- OIT
oral immunotherapy
- MOIT
milk oral immunotherapy
- DBPC
double-blind placebo-controlled
- SU
sustained unresponsiveness
- AUC
area under the curve
- OFC
oral food challenge
- HR
histamine release
- Treg
T regulatory cell
- CFDA-SE
carboxyfluorescein diacetate succinimidyl ester
- M
month
- PIPES
piperazine-N,N′ -bis(2-ethanesulfonic acid)
- PAG
PIPES/albumin/glucose
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sampson HA. Food-induced anaphylaxis. Novartis Found Symp. 2004;257:161–71. discussion 71-6, 207-10, 76-85. [PubMed] [Google Scholar]
- 2.Feuille E, Nowak-Wegrzyn A. Oral Immunotherapy for Food Allergies. Ann Nutr Metab. 2016;68(1):19–31. doi: 10.1159/000445391. [DOI] [PubMed] [Google Scholar]
- 3.Wood RA. Food allergen immunotherapy: Current status and prospects for the future. J Allergy Clin Immunol. 2016;137:973–82. doi: 10.1016/j.jaci.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Wood RA, Kim JS, Lindblad R, Nadeau K, Henning AK, Dawson P, et al. A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow's milk allergy. J Allergy Clin Immunol. 2016;137:1103–10 e11. doi: 10.1016/j.jaci.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinthrajah RS, Hernandez JD, Boyd SD, Galli SJ, Nadeau KC. Molecular and cellular mechanisms of food allergy and food tolerance. J Allergy Clin Immunol. 2016;137:984–97. doi: 10.1016/j.jaci.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berin MC, Shreffler WG. Mechanisms Underlying Induction of Tolerance to Foods. Immunol Allergy Clin North Am. 2016;36:87–102. doi: 10.1016/j.iac.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 7.MacGlashan DW, Jr, Bochner BS, Adelman DC, Jardieu PM, Togias A, McKenzie-White J, et al. Down-regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol. 1997;158:1438–45. [PubMed] [Google Scholar]
- 8.Prussin C, Griffith DT, Boesel KM, Lin H, Foster B, Casale TB. Omalizumab treatment downregulates dendritic cell FcepsilonRI expression. J Allergy Clin Immunol. 2003;112:1147–54. doi: 10.1016/j.jaci.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Holgate S, Casale T, Wenzel S, Bousquet J, Deniz Y, Reisner C. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J Allergy Clin Immunol. 2005;115:459–65. doi: 10.1016/j.jaci.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 10.Macglashan DW, Jr, Saini SS. Omalizumab increases the intrinsic sensitivity of human basophils to IgE-mediated stimulation. J Allergy Clin Immunol. 2013;132:906–11. e1–4. doi: 10.1016/j.jaci.2013.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zaidi AK, Saini SS, Macglashan DW., Jr Regulation of Syk kinase and FcRbeta expression in human basophils during treatment with omalizumab. J Allergy Clin Immunol. 2010;125:902–8 e7. doi: 10.1016/j.jaci.2009.12.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacGlashan DW, Jr, Savage JH, Wood RA, Saini SS. Suppression of the basophil response to allergen during treatment with omalizumab is dependent on 2 competing factors. J Allergy Clin Immunol. 2012;130:1130–5 e5. doi: 10.1016/j.jaci.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorelik M, Narisety SD, Guerrerio AL, Chichester KL, Keet CA, Bieneman AP, et al. Suppression of the immunologic response to peanut during immunotherapy is often transient. J Allergy Clin Immunol. 2015;135:1283–92. doi: 10.1016/j.jaci.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ford LS, Bloom KA, Nowak-Wegrzyn AH, Shreffler WG, Masilamani M, Sampson HA. Basophil reactivity, wheal size, and immunoglobulin levels distinguish degrees of cow's milk tolerance. J Allergy Clin Immunol. 2013;131:180–6. e1–3. doi: 10.1016/j.jaci.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shuster J, van Eys J. Interaction between prognostic factors and treatment. Control Clin Trials. 1983;4:209–14. doi: 10.1016/0197-2456(83)90004-1. [DOI] [PubMed] [Google Scholar]
- 16.Macglashan D., Jr Expression of CD203c and CD63 in human basophils: relationship to differential regulation of piecemeal and anaphylactic degranulation processes. Clin Exp Allergy. 2010;40:1365–77. doi: 10.1111/j.1365-2222.2010.03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knol EF, Mul FP, Jansen H, Calafat J, Roos D. Monitoring human basophil activation via CD63 monoclonal antibody 435. J Allergy Clin Immunol. 1991;88:328–38. doi: 10.1016/0091-6749(91)90094-5. [DOI] [PubMed] [Google Scholar]
- 18.Syed A, Garcia MA, Lyu SC, Bucayu R, Kohli A, Ishida S, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3) J Allergy Clin Immunol. 2014;133:500–10. doi: 10.1016/j.jaci.2013.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW, et al. Oral immunotherapy for treatment of egg allergy in children. N Engl J Med. 2012;367:233–43. doi: 10.1056/NEJMoa1200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124:292–300. e1–97. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savage JH, Courneya JP, Sterba PM, Macglashan DW, Saini SS, Wood RA. Kinetics of mast cell, basophil, and oral food challenge responses in omalizumab-treated adults with peanut allergy. J Allergy Clin Immunol. 2012;130:1123–9 e2. doi: 10.1016/j.jaci.2012.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bedoret D, Singh AK, Shaw V, Hoyte EG, Hamilton R, DeKruyff RH, et al. Changes in antigen-specific T-cell number and function during oral desensitization in cow's milk allergy enabled with omalizumab. Mucosal Immunol. 2012;5:267–76. doi: 10.1038/mi.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, Schroeder JT, Hamilton RG, Boden S, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. The Journal of allergy and clinical immunology. 2012;129:448–55. 55 e1–5. doi: 10.1016/j.jaci.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.May CD. High spontaneous release of histamine in vitro from leukocytes of persons hypersensitive to food. J Allergy Clin Immunol. 1976;58:432–7. doi: 10.1016/0091-6749(76)90124-x. [DOI] [PubMed] [Google Scholar]
- 25.Schroeder JT, Bieneman AP, Chichester KL, Keet CA, Hamilton RG, MacGlashan DW, Jr, et al. Spontaneous basophil responses in food-allergic children are transferable by plasma and are IgE-dependent. J Allergy Clin Immunol. 2013;132:1428–31. doi: 10.1016/j.jaci.2013.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, Schroeder JT, Hamilton RG, Boden S, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2012;129:448–55. 55 e1–5. doi: 10.1016/j.jaci.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vickery BP, Scurlock AM, Kulis M, Steele PH, Kamilaris J, Berglund JP, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. 2014;133:468–75. doi: 10.1016/j.jaci.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim EH, Bird JA, Kulis M, Laubach S, Pons L, Shreffler W, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. The Journal of allergy and clinical immunology. 2011;127:640–6 e1. doi: 10.1016/j.jaci.2010.12.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gruchalla RS, Sampson HA, Liu AH, Shreffler W, Wallace PK, Togias A, et al. Effects of omalizumab on T lymphocyte function in inner-city children with asthma. Pediatr Allergy Immunol. 2016;27:328–31. doi: 10.1111/pai.12508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eckman JA, Sterba PM, Kelly D, Alexander V, Liu MC, Bochner BS, et al. Effects of omalizumab on basophil and mast cell responses using an intranasal cat allergen challenge. J Allergy Clin Immunol. 2010;125:889–95 e7. doi: 10.1016/j.jaci.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santos AF, Du Toit G, Douiri A, Radulovic S, Stephens A, Turcanu V, et al. Distinct parameters of the basophil activation test reflect the severity and threshold of allergic reactions to peanut. J Allergy Clin Immunol. 2015;135:179–86. doi: 10.1016/j.jaci.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubio A, Vivinus-Nebot M, Bourrier T, Saggio B, Albertini M, Bernard A. Benefit of the basophil activation test in deciding when to reintroduce cow's milk in allergic children. Allergy. 2011;66:92–100. doi: 10.1111/j.1398-9995.2010.02432.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


