To the Editor
Secretion of cytoplasmic granules (i.e., “degranulation”) by mast cells (MCs) and basophils activated via the FcεRI plays a key role in diverse IgE-mediated immune responses including asthma, hay fever, food allergies and anaphylaxis. In both cells, secretory granule structure is thought to reflect in part electrostatic complexes between proteases (positively-charged) and proteoglycans (negatively-charged), which associate with and thereby help to store multiple bioactive molecules (e.g., histamine and certain cytokines).1, 2 Even though basophils usually represent <1% of circulating peripheral blood leukocytes, analysis of basophil activation (i.e., using basophil activation tests [BATs]) has become popular,3, 4 both because basophils may play specific roles during type 2 immune responses and allergic disorders and because blood basophils are much more readily available for analysis than are tissue-resident MCs.
Classical BATs are based on detection of proteins, e.g., the vesicular marker CD63, on the surface of activated basophils. However, CD63 is expressed by most peripheral blood leukocytes and platelets, and activated platelets can bind to various leukocytes, raising concerns that platelet binding to basophils can falsely elevate levels of “basophil” CD63.3–6 We therefore sought to develop an alternative method that ideally could be both simple to perform and be more specific and/or more sensitive than classical CD63-based BATs. We recently reported that AlexaFluor488-labeled avidin (Av.A488, a positively-charged molecule which binds to highly negatively-charged molecules) can be used to monitor degranulation of human or mouse MCs in real time, using flow cytometry in vitro or intravital imaging in vivo.7 We report herein that Av.A488 also can be used to detect activated basophils in whole blood of anonymous blood donors or subjects suffering from allergies, and can quantify the extent of such basophil activation.
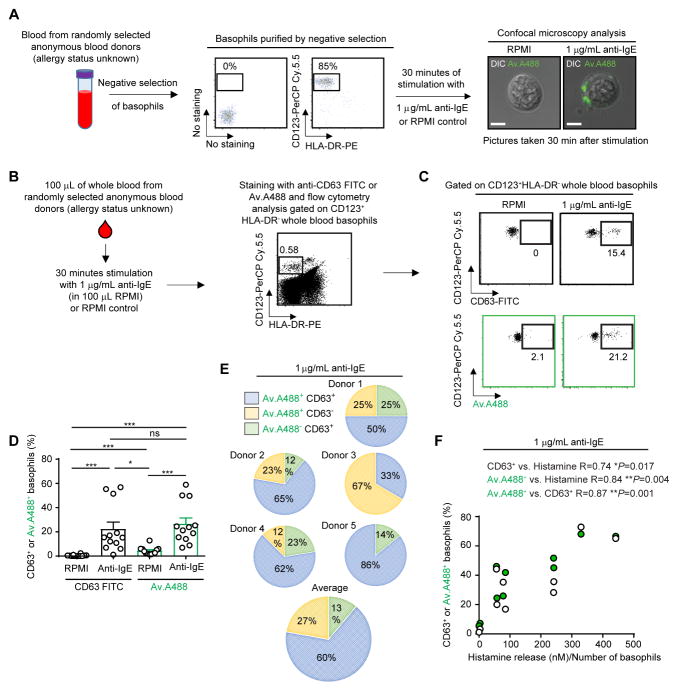
First, we purified basophils from the whole blood of anonymous blood donors and used single cell confocal imaging to determine whether Av.A488 could stain basophils stimulated with anti-IgE, which results in basophil degranulation. In accord with our previous reports on primary human and mouse MCs,7 we observed that, compared to RPMI-challenged control basophils, anti-IgE-stimulated basophils exhibited enhanced staining with Av.A488, with a pattern of staining consistent with the exteriorization and cell surface association of negatively-charged granule constituents (e.g. chondroitin sulfate- and/or heparan sulfate-containing proteoglycans) (Fig 1, A).
Figure 1. Anti-IgE challenge increases the binding of fluorochrome-labelled avidin to human blood basophils.
(A) Protocol for basophil isolation from whole blood and representative micrograph of purified basophils stimulated with RPMI or 1 μg/ml anti-IgE. Av.A488 fluorescence (green) is merged with Differential Interference Contrast (DIC). Bar=5 μm. (B) Protocol for assessing basophil activation by flow cytometry in whole blood of anonymous donors8. (C) Representative dot plot of HLA-DR−CD123+ basophils from an anonymous donor incubated with RPMI or anti-IgE and stained with anti-CD63-FITC or Av.A488. (D) Percentage of CD63+ (black) or Av.A488+ (green) basophils after RPMI or anti-IgE incubation in anonymous donors. Mean + SEM. Mann-Whitney U tests; ***P < .0005; **P < .005; *P < .05. (E) Percentage of Av.A488+CD63+, Av.A488+CD63− or Av.A488−CD63+ basophils detected simultaneously with anti-CD63-APC and Av.A488 after anti-IgE challenge in 5 anonymous donors. (F) Correlation analysis, for results from anti-IgE challenged blood specimens from 10 anonymous donors, of the percentages (%) of CD63+ or Av.A488+ basophils and the amount of histamine released (nM)/Number of basophils. Nonparametric Spearman R correlation, we considered P < .05 as statistically significant. Pairs of open or green circles are the results from the single donors analyzed.
We recently reported a simple protocol which permits BATs to be performed, either by conventional flow cytometry or by Cytometry by Time-of-Flight mass spectrometry (CyTOF), on whole blood stored at 4°C for up to 24 h before analysis.8 We used those conditions of blood storage to determine whether Av.A488 staining could be used to monitor basophil activation in whole blood from a group of 19 anonymous blood donors. We mixed 100 μL of whole blood with 1 μg/mL anti-IgE (in 100 μL of RPMI) or with 100 μL RPMI (control) alone, then gated on basophils as CD123-positive and HLA-DR-negative cells (two stable markers that, compared to FcεRI or CD203c, do not vary upon basophil activation, Fig 1, B),8 and compared detection of activated basophils by flow cytometry using either FITC-labeled anti-CD63 antibody or Av.A488 (both using the same detection channel; we defined “activated basophils” as those with a positive fluorescence signal for FITC-labeled anti-CD63 or for Av.488 as compared to the unstained and non-stimulated conditions) (Fig 1, C–D). As illustrated in Fig 1, C–D, we found that both Av.A488 and FITC-labeled anti-CD63 antibody readily detected anti-IgE-induced basophil activation in the whole blood of such subjects, whose allergy status is unknown. Importantly, no significant increase in Av.A488 staining was observed in other granulocyte populations, i.e., neutrophils and eosinophils (Fig E1, A–B), in blood incubated with anti-IgE.
Using co-staining with APC-labelled anti-CD63 antibody and Av.A488, we next assessed whether the two methods were identifying the same cell subsets within the population of anti-IgE-activated basophils in five anonymous blood donors. Notably, we identified three subsets of CD63+ or Av.A488+ basophils (i.e., CD63+Av.A488+, CD63−Av.A488+ and CD63+Av.A488−) and found that each of the 5 donors exhibited different percentages of each subset (Fig. 1, E). We also found, testing blood from 10 anonymous blood donors, that there were statistically significant positive correlations between the percentages of anti-IgE induced CD63+ or Av.A488+ basophils and between each of these percentages and the amount of histamine released in the same anti-IgE challenged specimens (Fig. 1, F). Finally, we tested whether exposure to IL-3 altered the reactivity of basophils with anti-CD63 antibody vs. Av.A488. While FITC-labeled anti-CD63 antibody did not label IL-3-stimulated basophils (a finding in accord with previous reports8), we detected a modest but significant increase (vs. RPMI stimulation) in the percentage of Av.A488+ basophils after IL-3 challenge (at 20 ng/mL) (Fig. E1, C–D); however, no histamine release above background was detected after such IL-3 challenge (data not shown).
We next investigated whether Av.A488 could be used to monitor basophil activation in whole blood from 41 of the peanut allergic patients enrolled in one of two IRB-approved clinical trials of oral immunotherapy (OIT) (32 from POISED ClinicalTrials.gov Identifier: NCT02103270 and 9 from MAP-X ClinicalTrials.gov Identifier: NCT02643862) whose demographic features are shown in Table E1 in the Online Repository. Peanut allergy in both trials was defined as having both a clinical reaction to ≤500 mg total of peanut protein in a double-blind, placebo-controlled food challenge (DBPCFC) to peanut and a positive skin prick test to peanut (≥to 5 mm).8
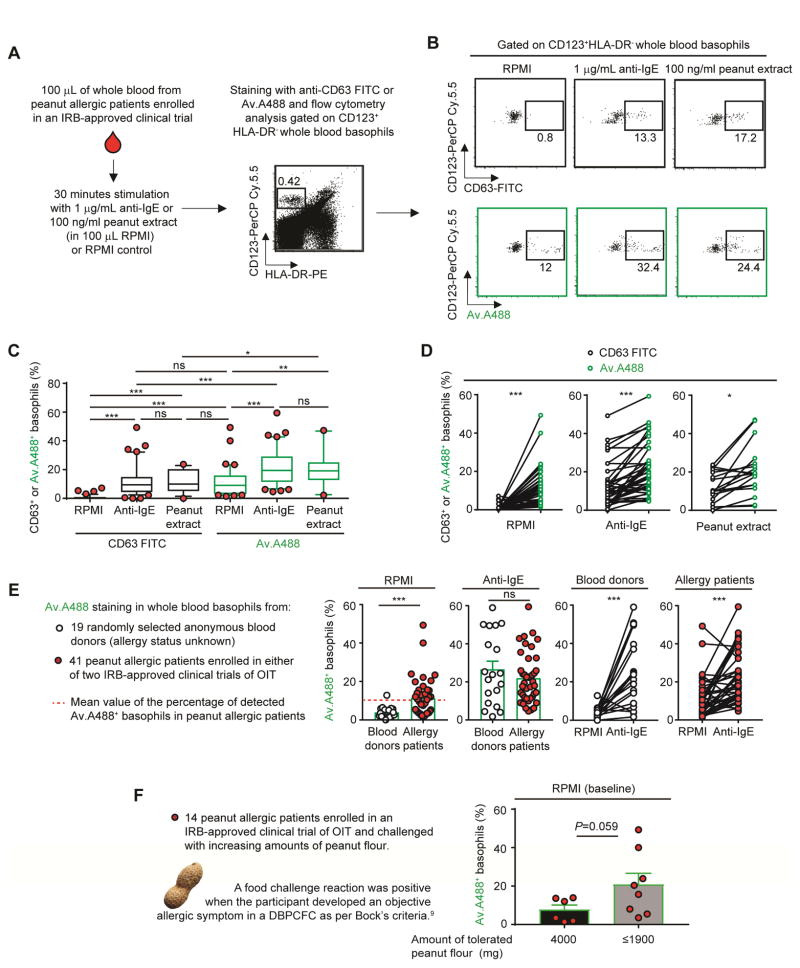
Using 100 μL of whole blood per patient, as described in Fig. 2, A–B (and in ref8 and the Online Repository), we used flow cytometry to compare the ability of FITC-labeled anti-CD63 antibody vs. Av.A488 to monitor changes in whole blood basophils after their exposure ex vivo to either RPMI (control), 1 μg/mL anti-IgE (for 41 patients) or 100 ng/mL peanut extract (for 19 of the 41 patients). Compared to CD63+ basophils, we detected a higher percentage of Av.A488+ basophils in 38 of 41 patients tested, both at baseline (i.e., after RPMI incubation) and after basophil stimulation with anti-IgE, and in 17 of the 19 patients tested after basophil stimulation with peanut extract (Fig 2, B–D). We also compared the detection of Av.A488+ basophils in the whole blood of anonymous blood donors vs. subjects suffering from peanut allergies, both at baseline (i.e., after RPMI incubation) and 30 min after anti-IgE treatment. Anti-IgE stimulation increased the percentage of Av.A488+ basophils to similar levels in healthy donors and peanut allergic patients (Fig 2, E). However, the percentage of Av.A488+ basophils at baseline was significantly higher in the blood of allergic subjects (global mean ~11.4%) vs. anonymous blood donors (global mean ~3.8%) (Fig 2, E, far left panel). In some of the same blood specimens from peanut allergic patients shown in Fig 2, E, we also analyzed basophil CD203c (a surface marker reported to be associated with basophil activation)3, 8 and found a positive correlation (P=0.01) between the percentage of detected Av.A488+ basophils and the intensity of CD203c staining at baseline (i.e., after exposure to RPMI) but this correlation did not achieve statistical significance (P=0.07) after activation by anti-IgE (Fig. E1, E). Finally, we did a pilot experiment to compare, in 14 peanut allergic patients tested either at week 104 or 117 in the POISED trial, the percentage of Av.A488+ basophils detected at baseline (i.e., after incubation with RPMI) and the ability of those subjects to tolerate peanut flour in a DBPCFC performed on the same day (after the phlebotomy). We found that subjects who passed the DBPCFC (i.e., defined by tolerance of 4000 mg of ingested peanut floor) had lower levels of Av.A488+ basophils at baseline than the patients who tolerated lower amounts of food challenge (i.e., developed a clinical reaction when challenged with ≤1900 mg of ingested peanut flour) (Fig. 2, F). Notably, 1 of the 14 patients tested failed the DBPCFC (i.e., reacted to the lowest amount of peanut flour tested) and this patient exhibited the highest level of Av.A488+ basophils at baseline among all of the 41 peanut allergic patients analyzed in this study.
Figure 2. Using fluorochrome-labelled avidin to monitor the status of basophil activation in whole blood of peanut allergic patients.
(A) Protocol for assessing basophil activation by flow cytometry in whole blood.8 (B) Representative dot plot of HLA-DR−CD123+ basophils incubated with RPMI, anti-IgE or peanut flour and stained with anti-CD63-FITC or Av.A488. (C) Percentage of CD63+ (black) or Av.A488+ (green) basophils after RPMI or anti-IgE or peanut extract challenge in 41 peanut allergic patients. Lower/higher 5% values are plotted as individual dots. Boxes extend from the 25th to 75th percentiles and whiskers represent 5th and 95th percentiles. Bars indicate medians. (D) Comparison of CD63+ (black) or Av.A488+ (green) basophils after RPMI (left) or anti-IgE (right) for each patient. Linked dots represent results from the individual patients. (E) Percentage of Av.A488+ basophils in 19 anonymous donors (open) versus 41 peanut allergic patients (black). (F) Y axis: Percentage of Av.A488+ basophils after RPMI (baseline) incubation in 14 peanut allergic patients in the POISED oral immunotherapy (OIT) trial that were challenged with increasing amounts of peanut flour; X axis: amount of tolerated peanut flour (mg) as per Bock’s criteria.9 Mean + SEM; Mann-Whitney U tests; ***P < .0005; **P < .005; *P < .05.
Taken together, these results show that fluorochrome-labelled avidin can be used to monitor basophil activation in small amounts of whole blood ex vivo and in basophils stimulated either with anti-IgE or (in peanut allergic subjects) with the patients’ offending allergen. Our data also suggest that this method might represent a more specific and sensitive alternative to CD63 detection for monitoring the activation status of blood basophils, and might even be useful in assessing the extent to which food allergic subjects are at risk for exhibiting strong clinical reactions to their offending allergen.
Some of our findings raise interesting questions that can only be answered by performing additional studies involving substantially larger groups of subjects. For example, since avidin is a constituent of egg white, the extent to which the avidin-based method can be used to detect basophil activation by egg allergen in egg allergic patients, or can be used in tests of basophil reactivity with other negatively-charged allergens, will have to be determined. It also will be important to try to understand the biological significance of the distinct basophil subsets we have observed following anti-IgE challenge (Fig. 1, E). This might reflect, at least in part, the fact that avidin molecules specifically label exteriorized basophil granule matrix (i.e., providing a direct assessment of the release of basophil granule contents), while anti-CD63 antibodies detect CD63+ vesicles which have fused with the plasma membrane during secretion (i.e., representing a more indirect and non-specific assessment of release of basophil granule contents). Therefore, it is tempting to speculate that different basophil subpopulations may coexist in variable proportions in different people (and/or perhaps in the same person under different conditions), and that some of these basophils contain a pool of CD63-negative secretory granules (most of them enriched in negatively-charged constituents [e.g., proteoglycans]) which can be externalized in response to anti-IgE challenge, whereas other basophils may exteriorize CD63 from granules/vesicles that contain low levels of Av.A488–binding content.
Finally, it will be important to explore further the possibility that the detection of high “baseline” levels of Av.A488+ basophils in food allergic patients may help to identify those subjects at risk to exhibit strong clinical reactions to the offending allergens, including patients who are enrolled in immunotherapy protocols. Perhaps the detection of Av.A488+ basophils in specimens incubated only with RPMI reflects low “basal” levels of activation even without ex vivo antigen stimulation and/or persistent effects of prior, perhaps subclinical, episodes of activation (e.g., related to the oral immunotherapy treatments or because of inadvertent exposure to small amounts of offending food allergens), and/or is due to particular intrinsic features of the basophils of some allergic subjects. It also is possible that some of the differences observed at “baseline” in the extent of Av.A488-binding vs. CD63 expression on basophils from various groups of peanut allergic subjects may reflect a longer persistence of Av.A488-biding content on the basophil surface in such subjects compared to elevated levels of CD63. These and other possible explanations of our findings will be analyzed in future studies of larger groups of subjects. However, whatever the mechanism(s) accounting for the Av.A488+ basophils observed at baseline in the blood of many of the peanut allergic subjects, such evidence of potential ongoing (or past) activation was not observed in the same specimens by assessment of CD63.
MATERIAL AND METHODS
Material and Methods are described in the “Supplemental Material” section.
Supplementary Material
Acknowledgments
We thank all members of the Valitutti and Galli laboratories for discussions, and Chen Liu for excellent technical assistance. N.G is supported by fellowships from the French ‘‘Fondation pour la Recherche Médicale FRM’’ award number SPE20130326582 and the Philippe Foundation. This work was supported by National Institutes of Health (NIH)/National Institute of Allergy and Infectious Disease (NIAID) grant 5U19AI104209 and the Department of Pathology, Stanford University.
ABBREVIATIONS USED
- BATs
Basophil activation tests
- MCs
mast cells
- Av.A488
AlexaFluor488-labeled avidin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S73–80. doi: 10.1016/j.jaci.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valent P, Bettelheim P. The human basophil. Critical reviews in oncology/hematology. 1990;10(4):327–52. doi: 10.1016/1040-8428(90)90009-h. [DOI] [PubMed] [Google Scholar]
- 3.Kleine-Tebbe J, Erdmann S, Knol EF, MacGlashan DW, Jr, Poulsen LK, Gibbs BF. Diagnostic tests based on human basophils: potentials, pitfalls and perspectives. Int Arch Allergy Immunol. 2006;141(1):79–90. doi: 10.1159/000094495. [DOI] [PubMed] [Google Scholar]
- 4.Ebo DG, Bridts CH, Hagendorens MM, Aerts NE, De Clerck LS, Stevens WJ. Basophil activation test by flow cytometry: present and future applications in allergology. Cytometry Part B, Clinical cytometry. 2008;74(4):201–10. doi: 10.1002/cyto.b.20419. [DOI] [PubMed] [Google Scholar]
- 5.Azorsa DO, Hyman JA, Hildreth JE. CD63/Pltgp40: a platelet activation antigen identical to the stage-specific, melanoma-associated antigen ME491. Blood. 1991;78(2):280–4. [PubMed] [Google Scholar]
- 6.de Bruijne-Admiraal LG, Modderman PW, Von dem Borne AE, Sonnenberg A. P-selectin mediates Ca(2+)-dependent adhesion of activated platelets to many different types of leukocytes: detection by flow cytometry. Blood. 1992;80(1):134–42. [PubMed] [Google Scholar]
- 7.Gaudenzio N, Sibilano R, Marichal T, Starkl P, Reber LL, Cenac N, et al. Different activation signals induce distinct mast cell degranulation strategies. J Clin Invest. 2016;126(10):3981–98. doi: 10.1172/JCI85538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mukai K, Gaudenzio N, Gupta S, Vivanco N, Bendall SC, Maecker HT, et al. Assessing basophil activation by using flow cytometry and mass cytometry in blood stored 24 hours before analysis. J Allergy Clin Immunol. 2016 doi: 10.1016/j.jaci.2016.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bock SA, Sampson HA, Atkins FM, Zeiger RS, Lehrer S, Sachs M, et al. Double-blind, placebo-controlled food challenge (DBPCFC) as an office procedure: a manual. J Allergy Clin Immunol. 1988;82(6):986–97. doi: 10.1016/0091-6749(88)90135-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.