Abstract
In the peripheral nervous system (PNS) in absence of tight blood barrier, neurons are at increased risk of DNA damage, yet the question of how effectively PNS neurons manage DNA damage remains largely unanswered. Genotoxins in systemic circulation include chemotherapeutic drugs that reach peripheral neurons and damage their DNA. Because neurotoxicity of platinum-based class of chemotherapeutic drugs has been implicated in PNS neuropathies, we utilized an in vitro model of Dorsal Root Ganglia (DRG) to investigate how peripheral neurons respond to cisplatin that forms intra-and interstrand crosslinks with their DNA. Our data revealed strong transcriptional upregulation of the translesion synthesis DNA polymerase kappa (Pol κ), while expression of other DNA polymerases remained unchanged. DNA Pol κ is involved in bypass synthesis of diverse DNA lesions and considered a vital player in cellular survival under injurious conditions. To assess the impact of Pol κ deficiency on cisplatin exposed DRG neurons, Pol κ levels were reduced using siRNA. Pol κ targeting siRNA diminished the cisplatin-induced nuclear Pol κ immunoreactivity in DRG neurons and decreased the extent of cisplatin-induced DNA repair synthesis, as reflected in reduced incorporation of thymidine analog into nuclear DNA. Moreover, Pol κ depletion exacerbated global transcriptional suppression induced by cisplatin in DRG neurons.
Collectively, these findings provide the first evidence for critical role of Pol κ in DNA damage response in the nervous system and call attention to implications of polymorphisms that modify Pol κ activity, on maintenance of genomic integrity and neuronal function in exogenously challenged PNS.
Keywords: DNA damage, DNA polymerase kappa (Pol κ), cisplatin, Dorsal Root Ganglion (DRG), Nucleotide Excision Repair (NER), Peripheral Nervous System (PNS), Translesion Synthesis (TLS)
INTRODUCTION
Circulating chemotherapeutic drugs readily reach neurons of the peripheral nervous system (PNS) and their neurotoxicity hinders attainment of chemotherapy goals [1]. Salient example of a chemotherapeutic agent associated with peripheral neurotoxicity is cisplatin, a drug commonly used in treatment of solid tumors [2,3]. Cisplatin belongs to the class of platinum based drugs that form bulky adducts, primarily intra-and interstrand DNA crosslinks [4]. Although bulky adducts hinder critical DNA transaction and have miscoding potential, the scope of their repair in the nervous system has not been elucidated. To gain insight into neuronal DNA damage response and cisplatin crosslinks repair in the PNS, we used an in vitro model of Dorsal Root Ganglion (DRG) neurons challenged with cisplatin. Our study reveals that in DRG neurons cisplatin and other genotoxic agents including, doxorubicin and cytarabine, strongly induce expression of the Y-family, Translesion Synthesis (TLS) DNA polymerase kappa (Pol κ), while transcription of other DNA polymerases remains unchanged.
TLS polymerases catalyze bypass synthesis of diverse DNA lesions and while considered vital players in cellular survival under injurious conditions, may also promote mutagenesis [5–9]. Although some studies showed that elevated Pol κ promotes genomic instability [5], other studies showed that Pol κ protects cells from mutagens [10,11] and that Pol κ deficiency may exacerbate mutagenesis [12,13]. Interestingly, studies also demonstrated that Pol κ participates in processing of different types of oxidative lesions, including strand breaks [14], bypass of 8-oxo-dG [15] and abasic sites [16,8], which are base excision repair (BER) substrates, as well as processing of bulky lesions that are substrates of the nucleotide excision repair (NER) pathway [17,18]. This suggests that in neurons, Pol κ might have a role in repair of diverse lesions, including cisplatin:DNA crosslinks, which are typical NER substrates [19] and in fact, cells lacking Pol κ are partially defective in interstrand crosslink removal [20,21]. Interestingly, unlike other TLS polymerases, Pol κ rarely localizes to replication forks [22], its levels are highest in the G0 phase of cell cycle and its affinity for dNTPs is higher compared to other DNA polymerases [17]. Taken together, these features are consistent with preference for non-dividing cells [23,18] supporting a role for Pol κ in replication-independent DNA repair, prerequisite in the terminally differentiated neurons. Indeed, region-specific analyses of Pol κ mRNA in the mouse brain [24] revealed constitutive expression levels second only to BER polymerase beta (Pol β), which is the major DNA polymerase in the nervous system [25,24]. Notwithstanding, to date the physiological role of Pol κ in the nervous system has not been elucidated. Here, we report marked upregulation of Pol κ expression in cultured dorsal root ganglion neurons in response to different genotoxic insults, while levels of other DNA polymerases remain unchanged under similar conditions. Moreover, we observe increasing immunoreactivity of nuclear Pol κ protein in the course of cisplatin exposure of DRG neurons and reduced post-cisplatin DNA repair synthesis when Pol κ levels are depleted. Collectively, our data suggest that Pol κ might have a central role in DNA damage response and neuronal survival in the face of endogenous and exogenous insults that challenge DNA integrity in neurons.
METHODS
Culture of mouse dorsal root ganglion neurons
Mouse-handling procedures were approved by Institutional Animal Care and Use Committee of the University of Texas Medical Branch. Dorsal root ganglion neurons were isolated from male C57BL/6 mice 3–4 months old (Harlan Laboratories, USA) as described [26–30]. Ganglia dissected from all spinal levels were placed in cold solution (130 mM NaCl, 5 mM KCl, 2 mM KH2PO4, 1.5 mM CaCl2, 6 mM MgCl2, 10 mM glucose and 10 mM Hepes, pH 7.2), incubated (1 h/37°C) with collagenase A (Roche) and trypsin followed by DNase I (Roche), dissociated by 20 slow triturations and spun (168 g/3 min). Pellets were passed through 70 μm strainer, spun and re-suspended in DMEM/F12 (Sigma) with 10% FBS, 10 ng/ml nerve growth factor (Sigma) and supplemented with penicillin/streptomycin. Cells were seeded at (3–5)×103/cm2 on precoated (10 μg/ml laminin and 100 μg/ml poly-L-ornithine) glass coverslips or plate wells. Treatments were initiated 24 h after seeding. The low sub-lethal 10 μM dose of cisplatin (Cis-Diammineplatinum (II) dichloride Sigma P4394) was selected to initiate the DNA damage response without causing significant neuronal death; cisplatin dose-linked cell death was previously determined by MTT assay and trypan blue uptake [31]. The dose agrees with earlier reports on cisplatin dosage and DRG neurons viability [32].
Delivery of siRNA
To reduce Pol κ levels in DRG neurons small interfering RNA (siRNA) was applied using the Acell system (Dharmacon Inc) designed to optimize transfection of primary cells. At 24 h after seeding siRNA was added at final concentration of 1 μM in Accell siRNA delivery medium (Dharmacon, #B-005000), and incubated for 12 h prior to the addition of 2% FBS for the duration of subsequent treatments. Transfection efficiency was assessed by imaging internalized non-targeting red fluorescent siRNA (#D-001960-01, Dharmacon) in cultures of DRG neurons and determined to be nearly 70% (supplementary Figure 1). Transfections were either with non-targeting siRNA (#D-001910-05, Dharmacon) or Pol κ targeting siRNA mixture (#A-048146-13 and #A-048146-14 Dharmacon).
Real-time quantitative PCR
Total RNA was isolated from cultured DRG neurons (4×104/3.5 cm dish) using RNeasy plus mini kit (Qiagen) and reverse transcribed with iScript RT supermix (Biorad), which contains random and oligo dT primers. Real-time PCR was done with CFX96 Real-Time System (Biorad) as we described [31,30]. 18S and B2M gene transcripts were used as internal controls. PCR reactions were assembled in duplicates with SSO FAST Evagreen supermix (Biorad). PCR program was: 95°C 2 min, 40 cycles of 95°C 5 sec, 55°C 15 sec. Data represent averages of at least 3 sets of independent biological experiments. The relative amount of target gene RNA was calculated as described [33] using the formula: −ΔΔCt=*(CT gene of interest − CT internal control) sample − (CT gene of interest − CT internal control) control]. Primer sequences are given in Supplementary Table 1.
Immunofluorescent staining
DRG neurons were seeded on pre-coated coverslips in 24-well plates and processed as we described [31,30]. Briefly, coverslips were washed 2x with PBS, fixed in 4% paraformaldehyde, rinsed, permeabilized with 0.1% Triton X-100/0.1% sodium citrate in PBS (9 min) and blocked in PBS with 3% BSA (w/v)/1% Donkey serum (v/v) for 40 min/37°C. Primary antibodies were: rabbit anti-Neurofilament 200 (1:20,000, Sigma-N4142), mouse anti Pol κ (1:12,000, Sigma-WH0051426M), mouse anti γH2AX (1:4,000, Millipore #05-636). Coverslips were washed 3x in 1% BSA in PBS, incubated 45 min in the dark with 488 and 594 Alexa-dye conjugated anti-mouse and anti-rabbit IgG (Life Technologies). Coverslips were mounted with Prolong® Gold Anti-fade with DAPI (Life Technologies) and viewed/captured with 40x objective using Olympus IX71 fitted with QIC-F-M-12-C cooled camera (QImaging, Surrey, BC) and QCapture Pro (QImaging) software. Fluorescence intensity was quantified using ImageJ (National Institutes of Health) software.
EdU incorporation
DNA repair synthesis was monitored by incorporation of EdU into newly synthesized DNA [34,35]. EdU (5-ethynyl-2′-deoxyuridine), a nucleoside analog of thymidine, was detected via the Click-iTR reaction generated fluorescence (EdU Imaging kit #C10339, Invitrogen). Of note, DNA repair synthesis, referred to as unscheduled DNA synthesis, generates very weak signal compared to strong fluorescence produced by EdU incorporation during S phase replication synthesis. To accurately quantify EdU incorporation during repair synthesis, background fluorescence measured in cells subjected to the Click-iT reaction when EdU is omitted, was subtracted from readings to set up cutoff for EdU positivity. To measure incorporation, control and cisplatin exposed DRG neurons were supplemented with 10 μM EdU for the duration of treatments. Readily measurable EdU signal was generated following 24 h cisplatin exposure, with no positive signal in non-exposed DRG neurons. After treatment, cells were fixed with 4% paraformaldehyde for 20 min, washed in PBS, permeabilized with 0.1% Triton X-100/0.1% sodium citrate (Sigma) for 9 min and rinsed with PBS. EdU detecting mix (Kit #C10339) with Alexa Fluor dye 594 was applied at 1:1000 dilution and incubated for 30 min at 25°C protected from light. Coverslips were washed with PBS and processed for immunofluorescent staining with anti Pol κ antibody as described above. EdU fluorescence was observed under Olympus IX71 microscope; images were captured with QIC-F-M-12-C cooled digital camera and nuclear fluorescence was quantified and normalized to nuclear area using the ImageJ software (NIH). Three sets of independent biological experiments were done and at least 50 DRG neurons were quantified from randomly selected fields for each experimental condition.
EU incorporation
Global RNA synthesis was assessed by imaging incorporation of EU (5-ethynyl uridine), an alkyne-modified uridine analog, into newly synthesized RNA transcripts. Incorporated EU was detected via Click-ITR reaction as previously described [34–36]. Briefly, 60 min before termination of treatments, DRGs cultures were supplemented with 0.5 mM EU. Cultures were fixed with 4% paraformaldehyde, protected from light and processed according to manufacturer with Click-iT® RNA Alexa Fluor® 594 Imaging Kit (Kit #C10330, Invitrogen,). Coverslips were then reacted with anti Pol κ antibody to concomitantly monitor Pol κ levels. Coverslips were observed with Olympus IX71 microscope; images were captured with QIC-F-M-12-C cooled digital camera and mean nuclear intensity was determined using ImageJ software (NIH). Three biological experiments were done and fluorescence of ≥ 50 DRG neurons from randomly captured fields was quantified for each experimental condition. Mean fluorescence intensity of individual nuclei was calculated and frequency histograms generated.
Statistical analysis
Data are given as mean±SEM obtained from 3–4 independent biological experiments, as indicated. One-way ANOVA was employed to compare the means among groups followed by post-test Tukey’s analysis to determine differences in means of multiple groups or as indicated. P<0.05 was considered statistically significant. MegaStat® package for Excel was used.
RESULTS
Genotoxins upregulate DNA Pol κ expression in DRG neurons
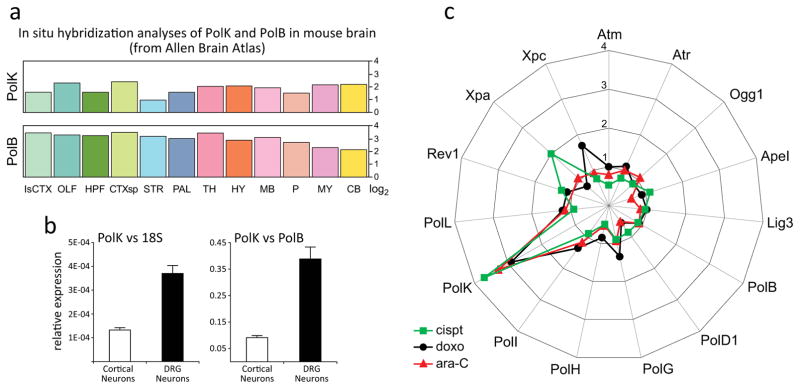
DNA Pol κ mRNA expression pattern in the mouse brain was previously analyzed by in situ hybridization (ISH) and the data are available from Allen Brain Atlas [24]. Compared to expression of other DNA polymerases, baseline level of Pol κ mRNA in the mouse brain is second only that of DNA polymerase beta (Pol β) [Fig 1a], the major DNA polymerase in the nervous system [25]. To asses Pol κ baseline mRNA levels in the central and peripheral nervous system, we carried out Real-Time (RT) qPCR analyses of RNA from cultured cortical and DRG neurons. Relative levels of Pol κ mRNA were calculated versus 18S (left) and versus Pol β (right), which is highly expressed in the nervous system [24]. Analyses revealed ~3.5-fold higher expression of Pol κ in DRG when compared to cortical neurons [Fig 1b]; relative to Pol β, Pol κ mRNA levels were ~35%- and ~10% in DRG and cortical neurons, respectively. Similar ratios of Pol κ expression were measured also when using 18S mRNA for reference [Fig 1b, left].
Fig. 1. Constitutive and genotoxin-induced expression of DNA Pol κ in DRG Neurons.
(a) Comparison of region specific PolK and PolB mRNA levels (log2) in the mouse brain (Allen Brain Atlas ISH data). (b) Real-time (RT) qPCR analyses of PolK mRNA levels relative to 18S or PolB in cortical and DRG neurons. (c) RT-qPCR analyses of genes encoding DNA repair proteins in DRG neurons exposed 24 h to 10 μM cisplatin, 0.2 μM doxorubicin or 30 μM cytarabine (ara-C). Spider graph shows mean mRNA levels of 15 DNA repair proteins. Data are pooled from 4 independent biological experiments. Fold-change is indicated on vertical axis.
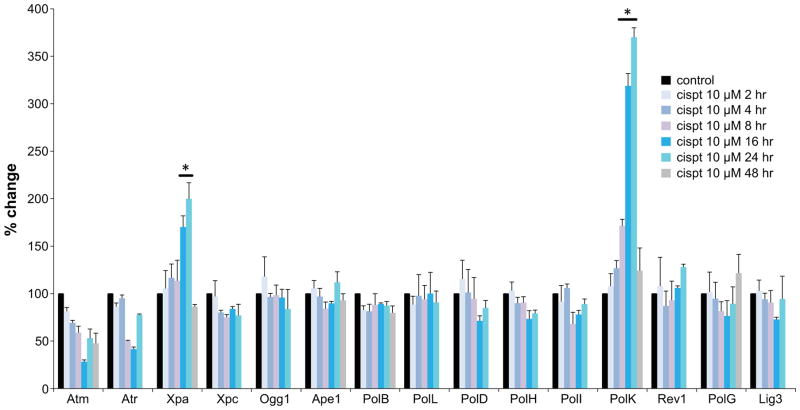
To assess Pol κ inducibility at transcriptional level, cultured DRG neurons were exposed to the DNA damaging agents cisplatin, doxorubicin and cytarabine (ara-C) that produce different types of DNA damage. Cisplatin forms intra- and interstrand crosslinks with DNA [4], doxorubicin inhibits topoisomerase ll [37] and ara-C is an antimetabolite that blocks DNA synthesis [38]. RT-qPCR analyses revealed that in DRG neurons genotoxins upregulate Pol κ transcription 3–4 fold, while the expression of other DNA polymerases remains unchanged [Fig 1c]. Because cisplatin is implicated in peripheral neurotoxicity, we examined temporal transcription patterns of DNA repair proteins in the course of cisplatin exposures [Fig 2]. RT-qPCR analyses after 2, 4, 8, 16, 24 or 48 h exposure to cisplatin (10 μM) revealed elevated expression of Pol κ by 8 h with further increases at 16 and 24 h and return to normal by 48 h. In contrast, RNA levels of the other Y-family TLS polymerases (Pol η, Pol ι and Rev1), X family Pol β and Pol λ, B family Pol δ and mitochondrial Pol γ remained unchanged. In addition to PolK, among 14 genes encoding proteins involved in DNA repair, only expression of the gene encoding Xeroderma Pigmentosum complementation group A (Xpa) protein, was also upregulated. Xpa is a zinc finger protein [39] involved in damage sensing/verification and recruitment of repair proteins to sites of damage; transcriptional regulation of Xpa protein has been reported previously [40].
Fig. 2. Temporal expression patterns of genes encoding DNA repair proteins in the course of cisplatin exposure of DRG neurons.
mRNA levels of 15 genes encoding DNA repair proteins were measured by RT-qPCR after 2, 4, 8, 16, 24 and 48 hours cisplatin exposures (48 h data given for selected genes only). Data are presented as mean ± SEM of 3 independent biological experiments; *different from untreated control P<0.05.
Cisplatin exposures induce γH2AX foci and nuclear Pol κ immunoreactivity in DRG neurons
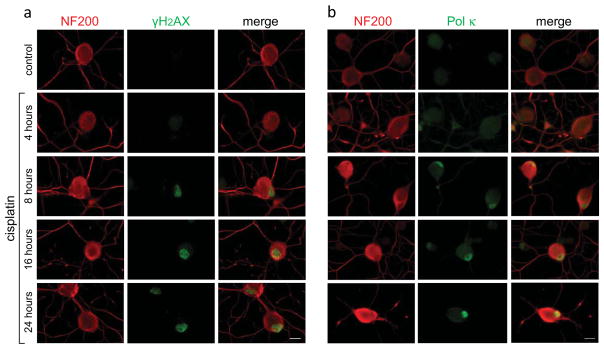
Next we asked whether exposure to sub-lethal dose of cisplatin, which upregulates Pol κ transcription, might also initiate the DNA damage response and upregulate Pol κ protein levels in DRG neurons. Continuous exposure to 10 μM cisplatin led to formation of γH2AX foci which first appeared by 4 h and temporally increased in density and intensity in the course of treatment [Fig 3a]. Nuclear γH2AX foci are considered markers of chromatin rearrangements mediated by phosphorylation of H2AX variant histone at serine 139, which facilitate accessibility of repair proteins and execution of DNA damage repair [41,42]. Induction of strong nuclear Pol κ immunoreactivity was also observed in the course of cisplatin exposure [Fig 3b], albeit temporally lagging behind formation of γH2AX foci. Baseline immunoreactivity of Pol κ, which in non-challenged DRG nuclei ranged from undetectable to weak, increased markedly in the course of 24 h cisplatin exposure, indicative of Pol κ involvement in the DNA damage response in DRG neurons.
Fig. 3. Temporal patterns of nuclear γH2AX foci formation and DNA Pol κ immunoreactivity induced by cisplatin exposure of DRG neurons.
Gradual increase of nuclear γH2AX fluorescence is observed after 4 h (a, green). Elevated immunoreactivity of Pol κ becomes apparent by 8 h with intensification over the 24-h exposure period (b, green). DRG neurons are identified by immunoreactivity of the high-molecular weight DRG specific neurofilament protein (NF200) in cell bodies and neurites (red). Merged images are shown; scale bar, 10 μm.
Cisplatin induced DNA repair synthesis is diminished by siRNA-mediated knockdown of Pol κ expression in DRG neurons
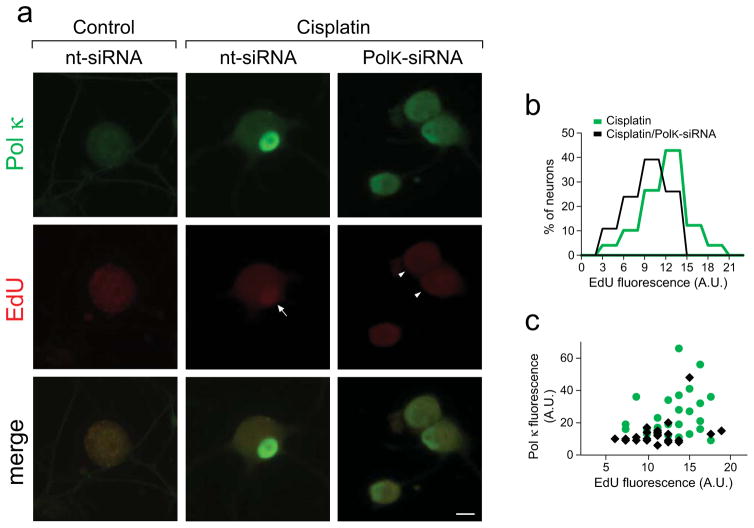
Cisplatin triggers formation of DNA crosslinks that are resolved primarily via the NER pathway. Following excision, NER process involves a critical step of gap filling DNA synthesis. Pol κ has been implicated in catalyzing this step in different models of DNA damage [17,18,43]. To assess the extent of cisplatin induced DNA repair synthesis in a system of terminally differentiated cells, DRG cultures were supplemented with EdU at the time of cisplatin addition. EdU is an alkyne-modified thymidine analog, whose incorporation into DNA is detectable in situ via fluorescence generated using Click-iT chemistry [34,35]. Incorporation of EdU during routine maintenance DNA repair synthesis in non-challenged DRG neurons did not generate fluorescence above background levels (left), whereas cisplatin exposure induced a readily measurable nuclear EdU signal that coincided with cisplatin-induced Pol κ immunoreactivity [Fig 4a, center]. To assess to what extent Pol κ deficiency might affect DNA repair synthesis in cisplatin exposed DRG neurons, Pol κ expression was reduced using small interfering RNA (PolK-siRNA). Depletion of Pol κ protein is expected within the time frame of our experimental setting since the reported Pol κ half-life is approximately 5.4 h [44]. The Acell siRNA system designed to maximize transfection efficiency of primary cells was used. At 24 h post seeding (when neurite network is established), DRG cultures were supplemented with either random/non-targeting (nt-siRNA) or Pol κ targeting (PolK-siRNA) siRNA and incubated for 12 h prior to the addition of cisplatin and for the subsequent 24 h duration of treatment. PolK-siRNA transfection led to partial reduction of cisplatin-induced Pol κ immunoreactivity in nearly 50% of DRG neurons, concordant with expectations based on the nearly 70% siRNA transfection efficiency observed for DRG neurons. Importantly, cisplatin-induced nuclear fluorescence of incorporated EdU was reduced when Pol κ was depleted by siRNA [right, arrowhead]. Following treatments mean EdU fluorescence was calculated for the individual DRG nuclei. Frequency histograms revealed reduction in cisplatin-induced EdU incorporation in DRG cultures transfected with PolK-siRNA compared to nt-siRNA [Fig 4b]. Scatter plots of fluorescent signals recorded for the individual nuclei of DRG neurons exposed to cisplatin in presence of nt-siRNA (green dots) or PolK-siRNA (black diamonds) show positive correlation between levels of Pol κ immunoreactivity and EdU fluorescence [Fig 4c].
Fig. 4. Cisplatin-induced DNA repair synthesis is diminished by siRNA mediated knockdown of Pol κ levels in DRG neurons.
(a) Representative images of cisplatin induced Pol κ immunofluorescence (green) and nuclear incorporation of EdU (red, arrow). Cisplatin-induced Pol κ immunoreactivity and EdU intensity are reduced in DRGs transfected with PolK-siRNA (right panel, arrowheads), scale bar, 10 μm. (b) Mean EdU intensities of individual nuclei are shown as frequency histograms; at least 50 nuclei were analyzed per condition and data were pooled from 3 independent biological experiments (A.U., arbitrary units). EdU signal in non-exposed control cultures did not reach the positivity cutoff (not shown). (c) Scatter plot of EdU fluorescence and Pol κ immunofluorescence after cisplatin exposure in the presence of nt-siRNA (green dots) or PolK-siRNA (black diamonds). Dots/diamonds represent values of individual nuclei. Values from one experimental set are plotted (n=20 per condition).
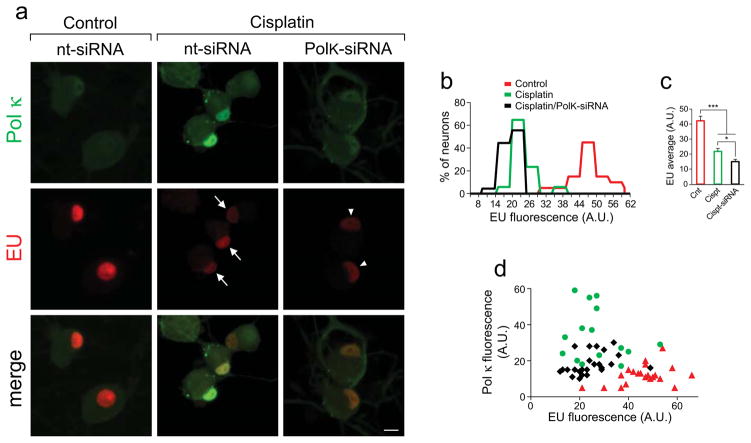
Cisplatin-induced suppression of RNA synthesis in DRG neurons is exacerbated by Pol κ depletion
Global RNA synthesis was assessed by incorporation of EU (5-ethynyl uridine), an alkyne-modified uridine analog, into newly synthesized nascent RNA. DRG cultures were incubated with 0.5 mM EU for 60 min prior to termination of treatments to measure EU incorporation into the newly synthesized RNA. Incorporated EU was visualized by Click iT chemistry [Fig. 5a, red]; detection of EU by the Click-iTR chemistry was previously described [34,35]. Strong EU fluorescence with intense nucleolar signal was observed in control cultures [Fig. 5a, left], in accordance with robust transcriptional activity of the highly metabolic DRG neurons. EU fluorescence was markedly diminished following 24 h cisplatin exposure (center, red), reflecting suppression of RNA synthesis by cisplatin. EU intensity was further reduced in DRG cultures supplemented with PolK-siRNA during cisplatin exposure (right, red). Concomitant assessment of Pol κ immunofluorescence revealed diminution by PolK-siRNA of the cisplatin-induced nuclear Pol κ immunoreactivity. EU intensity in individual nuclei was quantified using ImageJ and resultant frequency histograms revealed marked reduction of EU intensity by cisplatin, with a trend for further diminution by Pol κ knockdown [Fig 5b]. The average EU intensity in DRG nuclei was reduced by 47% by 10 μM cisplatin and by additional 14% when DRG neurons were supplemented with PolK-siRNA during cisplatin exposure [Fig 5c]. Scatter plots [Fig 5d] of individual nuclei of control neurons (red triangles) and of neurons exposed to cisplatin revealed substantial reduction of EU incorporation into newly synthesized RNA (green dots). Suppression of EU incorporation by cisplatin was further exacerbated by transfection of PolK-siRNA, which led to diminution of the cisplatin-induced nuclear Pol κ immunofluorescence (black diamonds).
Fig. 5. Cisplatin-induced suppression of RNA synthesis in DRG neurons is exacerbated by Pol κ knockdown.
(a) EU incorporation into newly synthesized RNA is detected as intense red nuclear and nucleolar fluorescence. EU signal was markedly reduced by cisplatin (arrows) with additional decrease by transfection with PolK-siRNA (right panel, arrowheads), concurrent with reduced Pol κ immunoreactivity. Bottom, merged images, scale bar, 10 μm. (b) Frequency histograms of EU fluorescence of individual nuclei (A.U., arbitrary units). (c) Bar graph shows mean ± SEM values of EU intensities of individual DRG nuclei following the different treatments. At least 50 nuclei were analyzed for each experimental condition. Data were pooled from 3 independent biological experiments; *different from PolK-siRNA, P<0.05; ***different from control, P<0.001. (d) Scatter plot of EU and Pol κ immunofluorescence of individual nuclei under control conditions (red triangles) and after cisplatin exposures with PolK-siRNA (black diamonds) or nt-siRNA (green dots); values from one experimental set are plotted (n=20 per condition).
DISCUSSION
Neuronal DNA repair pathways that process the different types of DNA damage have not been fully deciphered. This is a particular concern in the peripheral nervous system [45] where in absence of tight blood barrier, neurons are poorly shielded from circulating genotoxins, including chemotherapeutic drugs. An example of a drug that presents significant problems in the clinic is cisplatin whose neurotoxicity and associated peripheral neuropathies hinder cancer care. Here, using an in vitro system of dorsal root ganglion (DRG) neurons, we show that cisplatin exposures initiate the DNA damage response, upregulate expression of TLS DNA polymerase kappa (Pol κ) and suppress global RNA synthesis. Pol κ belongs to the Y-family of error prone TLS polymerases that are implicated in bypass of various DNA lesions [46]. While similarly to other TLS DNA polymerases Pol κ is subject for tight transcriptional and post translational controls [46,47], Pol κ is considered less error prone and its levels are not kept as low as levels of other TLS polymerases [48–50]. In fact, reports show that Pol κ catalyzes accurate bypass of multiple DNA lesions [10,11,51,52], accurate extension beyond the benzo[a]pyrene bulky adduct [53], accurate synthesis on dinucleotide microsatellite DNA [54] and on non-B DNA structures [55] as well as error free synthesis on non-damaged DNA templates suggesting that Pol κ upregulation could be advantageous without significantly increasing mutagenesis, and in some cases even promote genomic stability [56].
While Pol κ can bypass multiple types of DNA lesions, including bulky adducts, due to the structure of its active site it poorly inserts bases opposite pyrimidine dimers [57], which might be consistent with a more central role in the nervous system that is mostly sheltered from UV light. Furthermore, unlike other TLS polymerases Pol κ rarely localizes to replication forks [22] and does not absolutely depend on PCNA interactions [58]. In proliferating cells Pol κ levels are highest in the G0 phase of cell cycle and its affinity for dNTPs is higher compared to other polymerases [17]. Intriguingly, PolK−/− cells are more sensitive to cisplatin treatment in the non-S phase of cell cycle supporting a specialized role for Pol κ in the replication-independent DNA repair process [21] that is critical in terminally differentiated cells. When considered together, these features that are consistent with a preference for non-dividing cells [23,18], are suggestive of more prominent role for Pol κ in neuronal DNA repair. The fact that Pol κ is involved in handling diverse DNA lesions and indeed in DRG neurons, we detect significant induction of Pol κ in response to diverse genotoxic insults, while levels of other TLS polymerases remain unchanged, supports this notion.
Pol κ involvement in resolution of DNA lesions includes the gap-filling step of NER [17] and clearance of cisplatin crosslinks [43,59]. We found that in DRG neurons Pol κ transcription is significantly upregulated by cisplatin while expression of other TLS polymerases remains unchanged. Interestingly, mRNA levels of genes encoding DNA repair proteins involved in different repair pathways also remain unchanged under these conditions, with the exception of Xeroderma Pigmentosum complementation group A (Xpa) gene. Xpa is a zinc finger protein central in damage sensing, verification [39] and recruitment of proteins that participate in both global and transcription coupled NER processes [60]. Transcriptional control of Xpa [61,40], its binding to DNA distorting cisplatin adducts [62,63] and its involvement in resolution of cisplatin crosslinks [20] have been reported. Since Xpa has been implicated in resolution of cisplatin crosslinks and in view of our findings, it is plausible that in DRG neurons, Xpa participates in NER processes that involve Pol κ catalyzed gap filling DNA synthesis.
To gain new insight into the process of cisplatin adducts clearance in DRG neurons, the gap-filling step of NER was examined by monitoring incorporation of EdU (thymidine analog) as surrogate for gap-filling DNA repair synthesis. We found that in DRG neurons, cisplatin induces DNA repair synthesis reflected in measurable incorporation of EdU. As expected, in the non-challenged DRG neurons, EdU incorporation during routine maintenance repair synthesis is below detection limit. EdU incorporation in the course of continuous cisplatin exposure is readily detectable, plausibly because the NER process involves 30 nucleotide-long stretches of gap filling DNA synthesis [64] and high frequency of cisplatin adducts was observed following cisplatin exposures [65]. Importantly, here EdU incorporation positively correlated with cisplatin induced elevation of Pol κ immunoreactivity in DRG nuclei, whereas siRNA mediated depletion of Pol κ was associated with reduced incorporation of EdU into genomic DNA, suggesting that Pol κ is involved in gap-filling DNA repair synthesis in cisplatin exposed DRG neurons.
In view of evidence supporting the involvement of Pol κ in response to cisplatin challenge in DRG neurons, we also asked whether Pol κ deficiency might exacerbate cisplatin-induced transcriptional suppression. To this end, we measured incorporation of EU (uridine analog) into newly synthesized nascent RNA. Consistently with earlier reports [66,67,65], we found that cisplatin causes major suppression of the inherently robust global RNA synthesis in DRG neurons, plausibly reflecting formation of transcription-blocking cisplatin adducts. Importantly, we found that in DRG neurons, transcriptional suppression by cisplatin was modestly exacerbated by siRNA mediated Pol κ knockdown, suggesting that Pol κ contributes to resolution of cisplatin adducts and thereby, helps relieve the cisplatin-induced blockade of RNA synthesis in DRG neurons.
Collectively our findings indicate that in DRG neurons depletion of Pol κ slows the gap filling DNA synthesis step in the NER process, causing a delay in clearance of cisplatin adducts and thereby a delay in resumption of RNA synthesis. Hence, Pol κ might have a critical role in neuronal DNA damage repair and preservation of neuronal function, whereas Pol κ deficiency can sensitize DRG neurons to genotoxic insults. Because basal levels of Pol κ are higher in DRG compared to cortical neurons, it is plausible that in DRG neurons, which are not protected by a tight blood barrier [68,69], Pol κ affords greater capacity for coping with DNA damage [45]. Although speculative, this distinction highlights the possibility that DNA repair mechanisms necessitated by different levels of shielding may differ within the nervous system, and the less protected PNS neurons might have adapted to acquire more robust repair capacity. Together, the data provide new insights into neuronal DNA damage response and suggest that Pol κ might have a vital role in neuronal DNA damage repair.
Supplementary Material
Sequences and IDs of primers used in study.
Transfection efficiency of cultured DRG neurons was assessed in situ by imaging intracellular fluorescent siRNA (random siRNA #D-001960-01, Dharmacon). DRG neurons were seeded in 24-well plates. At 24 hours post seeding siRNA was added at final concentration of 1 μM in Accell siRNA delivery medium (Dharmacon, #B-005000). Following the addition of siRNA, cultures were incubated for 12 hours in Accell medium, prior to the addition of 2% FBS and continued incubation for the duration of treatments. (A) At 36 hours post transfection DRG neurons were visualized by immunofluorescence of the DRG neuron-specific heavy weight neurofilament protein NF-200 (green) and red fluorescence of internalized siRNA. Photomicrographs of a representative field are shown (low magnification). In the merged image orange/yellow color is indicative of internalized siRNA; scale bar = 100 μm. (B) Total number (green) and the number of siRNA positive (red) DRG neurons from 10 fields captured randomly from four culture wells, were counted. Numbers were pooled and the ratio of siRNA-positive neurons was calculated as 67±8%. (C) High magnification photomicrograph shows phase contrast/fluorescent image: intracellular siRNA (red) is seen in two of the three DRG neurons (arrow marks the siRNA negative DRG neuron); scale bar = 50 μm.
Acknowledgments
This work was supported by grants from the National Institutes of Health (ES014613) and Shriners Hospitals for Children (86700) and Surgery Department at UTMB to EWE. We thank Steve Schuenke and Eileen Figueroa for assistance with manuscript preparation.
Abbreviations
- Pol κ
DNA polymerase kappa
- BER
Base excision repair
- DRG
Dorsal root ganglion
- NER
Nucleotide excision repair
- PNS
Peripheral nervous system
- TLS
Translesion synthesis
Footnotes
Conflict of Interest/Disclosure: Authors declare no conflicts of interest.
References
- 1.Majithia N, Loprinzi CL, Smith TJ. New practical approaches to chemotherapy-induced neuropathic pain: prevention, assessment, and treatment. Oncology (Williston Park) 2016;30(11):1020–1029. [PubMed] [Google Scholar]
- 2.Dzagnidze A, Katsarava Z, Makhalova J, Liedert B, Yoon MS, Kaube H, Limmroth V, Thomale J. Repair capacity for platinum-DNA adducts determines the severity of cisplatin-induced peripheral neuropathy. J Neurosci. 2007;27(35):9451–9457. doi: 10.1523/JNEUROSCI.0523-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao WH, Zheng H, Bennett GJ. Characterization of oxaliplatin-induced chronic painful peripheral neuropathy in the rat and comparison with the neuropathy induced by paclitaxel. Neuroscience. 2012;203:194–206. doi: 10.1016/j.neuroscience.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eastman A. The formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. Pharmacol Ther. 1987;34(2):155–166. doi: 10.1016/0163-7258(87)90009-x. [DOI] [PubMed] [Google Scholar]
- 5.Bavoux C, Hoffmann JS, Cazaux C. Adaptation to DNA damage and stimulation of genetic instability: the double-edged sword mammalian DNA polymerase kappa. Biochimie. 2005;87(7):637–646. doi: 10.1016/j.biochi.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Bavoux C, Leopoldino AM, Bergoglio V, JOW, Ogi T, Bieth A, Judde JG, Pena SD, Poupon MF, Helleday T, Tagawa M, Machado C, Hoffmann JS, Cazaux C. Up-regulation of the error-prone DNA polymerase {kappa} promotes pleiotropic genetic alterations and tumorigenesis. Cancer Res. 2005;65(1):325–330. [PubMed] [Google Scholar]
- 7.Gregory MT, Park GY, Johnstone TC, Lee YS, Yang W, Lippard SJ. Structural and mechanistic studies of polymerase eta bypass of phenanthriplatin DNA damage. Proc Natl Acad Sci U S A. 2014;111(25):9133–9138. doi: 10.1073/pnas.1405739111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sale JE, Lehmann AR, Woodgate R. Y-family DNA polymerases and their role in tolerance of cellular DNA damage. Nat Rev Mol Cell Biol. 2012;13(3):141–152. doi: 10.1038/nrm3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waters LS, Minesinger BK, Wiltrout ME, D’Souza S, Woodruff RV, Walker GC. Eukaryotic translesion polymerases and their roles and regulation in DNA damage tolerance. Microbiol Mol Biol Rev. 2009;73(1):134–154. doi: 10.1128/MMBR.00034-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Yang Y, Tang TS, Zhang H, Wang Z, Friedberg E, Yang W, Guo C. Variants of mouse DNA polymerase kappa reveal a mechanism of efficient and accurate translesion synthesis past a benzo[a]pyrene dG adduct. Proc Natl Acad Sci U S A. 2014;111(5):1789–1794. doi: 10.1073/pnas.1324168111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogi T, Shinkai Y, Tanaka K, Ohmori H. Polkappa protects mammalian cells against the lethal and mutagenic effects of benzo[a]pyrene. Proc Natl Acad Sci U S A. 2002;99(24):15548–15553. doi: 10.1073/pnas.222377899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singer WD, Osimiri LC, Friedberg EC. Increased dietary cholesterol promotes enhanced mutagenesis in DNA polymerase kappa-deficient mice. DNA Repair (Amst) 2013;12(10):817–823. doi: 10.1016/j.dnarep.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 13.Stancel JN, McDaniel LD, Velasco S, Richardson J, Guo C, Friedberg EC. Polk mutant mice have a spontaneous mutator phenotype. DNA Repair (Amst) 2009;8(12):1355–1362. doi: 10.1016/j.dnarep.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, Lv L, Chen Q, Yuan F, Zhang T, Yang Y, Zhang H, Wang Y, Jia Y, Qian L, Chen B, Zhang Y, Friedberg EC, Tang TS, Guo C. Mouse DNA polymerase kappa has a functional role in the repair of DNA strand breaks. DNA Repair (Amst) 2013;12(5):377–388. doi: 10.1016/j.dnarep.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maddukuri L, Ketkar A, Eddy S, Zafar MK, Eoff RL. The Werner syndrome protein limits the error-prone 8-oxo-dG lesion bypass activity of human DNA polymerase kappa. Nucleic Acids Res. 2014;42(19):12027–12040. doi: 10.1093/nar/gku913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ling H, Boudsocq F, Woodgate R, Yang W. Snapshots of replication through an abasic lesion; structural basis for base substitutions and frameshifts. Mol Cell. 2004;13(5):751–762. doi: 10.1016/s1097-2765(04)00101-7. [DOI] [PubMed] [Google Scholar]
- 17.Ogi T, Lehmann AR. The Y-family DNA polymerase kappa (pol kappa) functions in mammalian nucleotide-excision repair. Nat Cell Biol. 2006;8(6):640–642. doi: 10.1038/ncb1417. [DOI] [PubMed] [Google Scholar]
- 18.Ogi T, Limsirichaikul S, Overmeer RM, Volker M, Takenaka K, Cloney R, Nakazawa Y, Niimi A, Miki Y, Jaspers NG, Mullenders LH, Yamashita S, Fousteri MI, Lehmann AR. Three DNA polymerases, recruited by different mechanisms, carry out NER repair synthesis in human cells. Mol Cell. 2010;37(5):714–727. doi: 10.1016/j.molcel.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Sancar A. Excision repair in mammalian cells. J Biol Chem. 1995;270(27):15915–15918. doi: 10.1074/jbc.270.27.15915. [DOI] [PubMed] [Google Scholar]
- 20.Enoiu M, Jiricny J, Scharer OD. Repair of cisplatin-induced DNA interstrand crosslinks by a replication-independent pathway involving transcription-coupled repair and translesion synthesis. Nucleic Acids Res. 2012;40(18):8953–8964. doi: 10.1093/nar/gks670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams HL, Gottesman ME, Gautier J. Replication-independent repair of DNA interstrand crosslinks. Mol Cell. 2012;47(1):140–147. doi: 10.1016/j.molcel.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogi T, Kannouche P, Lehmann AR. Localisation of human Y-family DNA polymerase kappa: relationship to PCNA foci. J Cell Sci. 2005;118(Pt 1):129–136. doi: 10.1242/jcs.01603. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann AR. New functions for Y family polymerases. Mol Cell. 2006;24(4):493–495. doi: 10.1016/j.molcel.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 24.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445(7124):168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 25.Englander EW. Brain capacity for repair of oxidatively damaged DNA and preservation of neuronal function. Mech Ageing Dev. 2008;129(7–8):475–482. doi: 10.1016/j.mad.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernyhough P. Mitochondrial dysfunction in diabetic neuropathy: a series of unfortunate metabolic events. Curr Diab Rep. 2015;15(11):89. doi: 10.1007/s11892-015-0671-9. [DOI] [PubMed] [Google Scholar]
- 27.Huang LY, Neher E. Ca(2+)-dependent exocytosis in the somata of dorsal root ganglion neurons. Neuron. 1996;17(1):135–145. doi: 10.1016/s0896-6273(00)80287-1. [DOI] [PubMed] [Google Scholar]
- 28.Lindsay RM. Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J Neurosci. 1988;8(7):2394–2405. doi: 10.1523/JNEUROSCI.08-07-02394.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc. 2007;2(1):152–160. doi: 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
- 30.Zhuo M, Gorgun MF, Englander EW. Augmentation of glycolytic metabolism by meclizine is indispensable for protection of dorsal root ganglion neurons from hypoxia-induced mitochondrial compromise. Free Radic Biol Med. 2016;99:20–31. doi: 10.1016/j.freeradbiomed.2016.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gorgun MF, Zhuo M, Englander EW. Cisplatin Toxicity in Dorsal Root Ganglion Neurons Is Relieved by Meclizine via Diminution of Mitochondrial Compromise and Improved Clearance of DNA Damage. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-0273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelley MR, Jiang Y, Guo C, Reed A, Meng H, Vasko MR. Role of the DNA base excision repair protein, APE1 in cisplatin, oxaliplatin, or carboplatin induced sensory neuropathy. PLoS One. 2014;9(9):e106485. doi: 10.1371/journal.pone.0106485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 34.Jao CY, Salic A. Exploring RNA transcription and turnover in vivo by using click chemistry. Proc Natl Acad Sci U S A. 2008;105(41):15779–15784. doi: 10.1073/pnas.0808480105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakazawa Y, Yamashita S, Lehmann AR, Ogi T. A semi-automated non-radioactive system for measuring recovery of RNA synthesis and unscheduled DNA synthesis using ethynyluracil derivatives. DNA Repair (Amst) 2010;9(5):506–516. doi: 10.1016/j.dnarep.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 36.Pallis M, Burrows F, Whittall A, Boddy N, Seedhouse C, Russell N. Efficacy of RNA polymerase II inhibitors in targeting dormant leukaemia cells. BMC Pharmacol Toxicol. 2013;14:32. doi: 10.1186/2050-6511-14-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tewey KM, Rowe TC, Yang L, Halligan BD, Liu LF. Adriamycin-induced DNA damage mediated by mammalian DNA topoisomerase II. Science. 1984;226(4673):466–468. doi: 10.1126/science.6093249. [DOI] [PubMed] [Google Scholar]
- 38.Heintz NH, Hamlin JL. In vivo effects of cytosine arabinoside on deoxyribonucleic acid replication in Chinese hamster ovary cells. 1. Resolution of differential effects on mitochondrial and nuclear deoxyribonucleic acid synthesis. Biochemistry. 1983;22(15):3552–3557. doi: 10.1021/bi00284a003. [DOI] [PubMed] [Google Scholar]
- 39.Morita EH, Ohkubo T, Kuraoka I, Shirakawa M, Tanaka K, Morikawa K. Implications of the zinc-finger motif found in the DNA-binding domain of the human XPA protein. Genes Cells. 1996;1(5):437–442. doi: 10.1046/j.1365-2443.1996.d01-252.x. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Bernauer AM, Yingling CM, Belinsky SA. HIF1alpha regulated expression of XPA contributes to cisplatin resistance in lung cancer. Carcinogenesis. 2012;33(6):1187–1192. doi: 10.1093/carcin/bgs142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Revet I, Feeney L, Bruguera S, Wilson W, Dong TK, Oh DH, Dankort D, Cleaver JE. Functional relevance of the histone gammaH2Ax in the response to DNA damaging agents. Proc Natl Acad Sci U S A. 2011;108(21):8663–8667. doi: 10.1073/pnas.1105866108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lukas J, Lukas C, Bartek J. More than just a focus: The chromatin response to DNA damage and its role in genome integrity maintenance. Nat Cell Biol. 2011;13(10):1161–1169. doi: 10.1038/ncb2344. [DOI] [PubMed] [Google Scholar]
- 43.Roy U, Scharer OD. Involvement of translesion synthesis DNA polymerases in DNA interstrand crosslink repair. DNA Repair (Amst) 2016;44:33–41. doi: 10.1016/j.dnarep.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo C, Tang TS, Bienko M, Dikic I, Friedberg EC. Requirements for the interaction of mouse Polkappa with ubiquitin and its biological significance. J Biol Chem. 2008;283(8):4658–4664. doi: 10.1074/jbc.M709275200. [DOI] [PubMed] [Google Scholar]
- 45.Englander EW. DNA damage response in peripheral nervous system: coping with cancer therapy-induced DNA lesions. DNA Repair (Amst) 2013;12(8):685–690. doi: 10.1016/j.dnarep.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goodman MF, Woodgate R. Translesion DNA polymerases. Cold Spring Harb Perspect Biol. 2013;5(10):a010363. doi: 10.1101/cshperspect.a010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McIntyre J, Woodgate R. Regulation of translesion DNA synthesis: Posttranslational modification of lysine residues in key proteins. DNA Repair (Amst) 2015;29:166–179. doi: 10.1016/j.dnarep.2015.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gerlach VL, Aravind L, Gotway G, Schultz RA, Koonin EV, Friedberg EC. Human and mouse homologs of Escherichia coli DinB (DNA polymerase IV), members of the UmuC/DinB superfamily. Proc Natl Acad Sci U S A. 1999;96(21):11922–11927. doi: 10.1073/pnas.96.21.11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohmori H, Friedberg EC, Fuchs RP, Goodman MF, Hanaoka F, Hinkle D, Kunkel TA, Lawrence CW, Livneh Z, Nohmi T, Prakash L, Prakash S, Todo T, Walker GC, Wang Z, Woodgate R. The Y-family of DNA polymerases. Mol Cell. 2001;8(1):7–8. doi: 10.1016/s1097-2765(01)00278-7. [DOI] [PubMed] [Google Scholar]
- 50.Velasco-Miguel S, Richardson JA, Gerlach VL, Lai WC, Gao T, Russell LD, Hladik CL, White CL, Friedberg EC. Constitutive and regulated expression of the mouse Dinb (Polkappa) gene encoding DNA polymerase kappa. DNA Repair (Amst) 2003;2(1):91–106. doi: 10.1016/s1568-7864(02)00189-1. [DOI] [PubMed] [Google Scholar]
- 51.Takeiri A, Wada NA, Motoyama S, Matsuzaki K, Tateishi H, Matsumoto K, Niimi N, Sassa A, Gruz P, Masumura K, Yamada M, Mishima M, Jishage K, Nohmi T. In vivo evidence that DNA polymerase kappa is responsible for error-free bypass across DNA cross-links induced by mitomycin C. DNA Repair (Amst) 2014;24:113–121. doi: 10.1016/j.dnarep.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 52.Yuan B, You C, Andersen N, Jiang Y, Moriya M, O’Connor TR, Wang Y. The roles of DNA polymerases kappa and iota in the error-free bypass of N2-carboxyalkyl-2′-deoxyguanosine lesions in mammalian cells. J Biol Chem. 2011;286(20):17503–17511. doi: 10.1074/jbc.M111.232835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jha V, Ling H. Structural basis of accurate replication beyond a bulky major benzo[a]pyrene adduct by human DNA polymerase kappa. DNA Repair (Amst) 2016 Nov 19; doi: 10.1016/j.dnarep.2016.11.001. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 54.Hile SE, Wang X, Lee MY, Eckert KA. Beyond translesion synthesis: polymerase kappa fidelity as a potential determinant of microsatellite stability. Nucleic Acids Res. 2012;40(4):1636–1647. doi: 10.1093/nar/gkr889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boyer AS, Grgurevic S, Cazaux C, Hoffmann JS. The human specialized DNA polymerases and non-B DNA: vital relationships to preserve genome integrity. J Mol Biol. 2013;425(23):4767–4781. doi: 10.1016/j.jmb.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 56.Pillaire MJ, Betous R, Hoffmann JS. Role of DNA polymerase kappa in the maintenance of genomic stability. Mol Cell Oncol. 2014;1(1):e29902. doi: 10.4161/mco.29902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lone S, Townson SA, Uljon SN, Johnson RE, Brahma A, Nair DT, Prakash S, Prakash L, Aggarwal AK. Human DNA polymerase kappa encircles DNA: implications for mismatch extension and lesion bypass. Mol Cell. 2007;25(4):601–614. doi: 10.1016/j.molcel.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 58.Okada T, Sonoda E, Yamashita YM, Koyoshi S, Tateishi S, Yamaizumi M, Takata M, Ogawa O, Takeda S. Involvement of vertebrate polkappa in Rad18-independent postreplication repair of UV damage. J Biol Chem. 2002;277(50):48690–48695. doi: 10.1074/jbc.M207957200. [DOI] [PubMed] [Google Scholar]
- 59.Shachar S, Ziv O, Avkin S, Adar S, Wittschieben J, Reissner T, Chaney S, Friedberg EC, Wang Z, Carell T, Geacintov N, Livneh Z. Two-polymerase mechanisms dictate error-free and error-prone translesion DNA synthesis in mammals. EMBO J. 2009;28(4):383–393. doi: 10.1038/emboj.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sugasawa K. Molecular mechanisms of DNA damage recognition for mammalian nucleotide excision repair. DNA Repair (Amst) 2016;44:110–117. doi: 10.1016/j.dnarep.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 61.Kang TH, Reardon JT, Sancar A. Regulation of nucleotide excision repair activity by transcriptional and post-transcriptional control of the XPA protein. Nucleic Acids Res. 2011;39(8):3176–3187. doi: 10.1093/nar/gkq1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coste F, Malinge JM, Serre L, Shepard W, Roth M, Leng M, Zelwer C. Crystal structure of a double-stranded DNA containing a cisplatin interstrand cross-link at 1.63 A resolution: hydration at the platinated site. Nucleic Acids Res. 1999;27(8):1837–1846. doi: 10.1093/nar/27.8.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vasquez KM, Christensen J, Li L, Finch RA, Glazer PM. Human XPA and RPA DNA repair proteins participate in specific recognition of triplex-induced helical distortions. Proc Natl Acad Sci U S A. 2002;99(9):5848–5853. doi: 10.1073/pnas.082193799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Staresincic L, Fagbemi AF, Enzlin JH, Gourdin AM, Wijgers N, Dunand-Sauthier I, Giglia-Mari G, Clarkson SG, Vermeulen W, Scharer OD. Coordination of dual incision and repair synthesis in human nucleotide excision repair. EMBO J. 2009;28(8):1111–1120. doi: 10.1038/emboj.2009.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yan F, Liu JJ, Ip V, Jamieson SM, McKeage MJ. Role of platinum DNA damage-induced transcriptional inhibition in chemotherapy-induced neuronal atrophy and peripheral neurotoxicity. J Neurochem. 2015;135(6):1099–1112. doi: 10.1111/jnc.13355. [DOI] [PubMed] [Google Scholar]
- 66.Jung Y, Lippard SJ. Direct cellular responses to platinum-induced DNA damage. Chem Rev. 2007;107(5):1387–1407. doi: 10.1021/cr068207j. [DOI] [PubMed] [Google Scholar]
- 67.Todd RC, Lippard SJ. Inhibition of transcription by platinum antitumor compounds. Metallomics. 2009;1(4):280–291. doi: 10.1039/b907567d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jimenez-Andrade JM, Herrera MB, Ghilardi JR, Vardanyan M, Melemedjian OK, Mantyh PW. Vascularization of the dorsal root ganglia and peripheral nerve of the mouse: implications for chemical-induced peripheral sensory neuropathies. Mol Pain. 2008;4:10. doi: 10.1186/1744-8069-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Weerasuriya A, Mizisin AP. The blood-nerve barrier: structure and functional significance. Methods Mol Biol. 2011;686:149–173. doi: 10.1007/978-1-60761-938-3_6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sequences and IDs of primers used in study.
Transfection efficiency of cultured DRG neurons was assessed in situ by imaging intracellular fluorescent siRNA (random siRNA #D-001960-01, Dharmacon). DRG neurons were seeded in 24-well plates. At 24 hours post seeding siRNA was added at final concentration of 1 μM in Accell siRNA delivery medium (Dharmacon, #B-005000). Following the addition of siRNA, cultures were incubated for 12 hours in Accell medium, prior to the addition of 2% FBS and continued incubation for the duration of treatments. (A) At 36 hours post transfection DRG neurons were visualized by immunofluorescence of the DRG neuron-specific heavy weight neurofilament protein NF-200 (green) and red fluorescence of internalized siRNA. Photomicrographs of a representative field are shown (low magnification). In the merged image orange/yellow color is indicative of internalized siRNA; scale bar = 100 μm. (B) Total number (green) and the number of siRNA positive (red) DRG neurons from 10 fields captured randomly from four culture wells, were counted. Numbers were pooled and the ratio of siRNA-positive neurons was calculated as 67±8%. (C) High magnification photomicrograph shows phase contrast/fluorescent image: intracellular siRNA (red) is seen in two of the three DRG neurons (arrow marks the siRNA negative DRG neuron); scale bar = 50 μm.