Abstract
Environmental exposures have been recognized as critical in the initiation and exacerbation of asthma, one of the most common chronic childhood diseases. The National Institute of Allergy and Infectious Diseases (NIAID), National Institute of Environmental Health Sciences (NIEHS), National Heart, Lung, and Blood Institute (NHLBI), and Merck Childhood Asthma Network (MCAN) sponsored a joint workshop to discuss the current state of the science with respect to the indoor environment and its effects on the development and morbidity of childhood asthma. The workshop included U.S. and international experts with backgrounds in allergy/allergens, immunology, asthma, environmental health, environmental exposures and pollutants, epidemiology, public health, and bioinformatics. Workshop participants provided new insights into the biologic properties of indoor exposures, indoor exposure assessment and exposure reduction techniques. This informed a primary focus of the workshop-- to critically review trials and research relevant to the prevention or control of asthma through environmental intervention. The participants identified important limitations and gaps in the scientific methodologies and knowledge, and proposed and prioritized areas for future research. The group reviewed socioeconomic and structural challenges to changing environmental exposure and offered recommendations for creative study design to overcome these challenges in trials to improve asthma management. The recommendations of this workshop can serve as guidance for future research in the study of the indoor environment and on environmental interventions as they pertain to the prevention and management of asthma and airway allergies.
Keywords: asthma, allergy, child health, indoor allergens, pollutants, environmental intervention, clinical trials
Introduction
Many trials aiming to improve asthma outcomes by altering the indoor environment have been conducted over the past two decades in response to observational studies suggesting that indoor environmental exposures influenced childhood asthma incidence and morbidity. The NIH Institutes NIAID, NIEHS and NHLBI, in collaboration with the MCAN, sponsored a joint workshop to discuss the current state of the science with respect to the indoor environment and its effects on the development and morbidity of childhood asthma. The workshop included US and international experts from a variety of relevant disciplines and addressed the unmet need to critically review environmental intervention asthma trials aiming at reducing asthma incidence and improving asthma control. In addition, workshop participants discussed indoor exposure assessment methodologies, and the biologic properties of allergens and indoor pollutants as they relate to the risk of asthma and asthma morbidity, as well as to possible protective effects that some of those exposures may have. This report, authored by all participants, presents the deliberations of the workshop with specific recommendations for current research needs in the field. The workshop was held in 2014, but all authors contributed current updates in both recommendations and key publications. The authors hope that the report will stimulate the next generation of scientific projects and clinical trials related to the role of the environment in childhood asthma and respiratory allergy.
New insights into indoor exposure assessment
The indoor environment contains numerous exposures with the potential to influence asthma development and morbidity. Exposures include biologics (allergens, bacteria, or fungi), pollutant gases and particulate matter from indoor (e.g. gas stoves and cigarette smoke) and outdoor sources. Infiltrating ambient particulate matter contains a heterogeneous mix of inorganic, organic, and biologic components. (1, 2) Indoor particle sampling can include collection of house dust (vacuumed or swiped from surfaces) or air samples (collected actively or by passive settling). Experience with nasal samplers and other personal monitoring devices for assessment of bioaerosol inhalation exposure is limited. (3–5)
The gold standard for measurement of exposure to individual allergens in dust or air samples has been enzyme-linked immunosorbent assays (ELISA), which have been improved with reduction of assay time and use of amplification to increase sensitivity. In the past decade, for standardized measurement of multiple allergens in epidemiologic studies, ELISA has largely been replaced by fluorescent multiplex array technology, with measurements shown to be reproducible within and between laboratories. (6–8) New laboratory approaches, advances in field sampling equipment and real-time data monitoring, including rapid tests for allergens (9–11) may contribute insight into the spectrum of indoor exposures (Table 1). (6, 7) Technologies for allergen measurement including qPCR, mass spectrometry and allergen biosensors are in development, including those supported by the NIH PRISMS (Pediatric Research using Integrated Sensor Monitoring Systems) program.(12) Mass spectrometry has been used as a high sensitivity method for detection of grass pollen allergens, and is also being evaluated for food allergen detection.(13–15) A first generation of allergen biosensors can measure Der p 1, Der p 2, Asp f 1 and Ara h 1, and advances in personal air sampling methodology have led to new insight into critical allergen exposure locations.(16–19)
Table 1.
Recent and Emerging Technologies for Indoor Exposure Assessment of Biologic Environmental Exposures
| Description/Comments | |
|---|---|
| Allergens | |
| Fluorescent Multiplex Array(6–8) | Bead-based fluorescent suspension array allows for simultaneous detection of up to 11 allergens. Also being developed for food allergens. |
| Biosensors(16–19) | Variety of sensor technologies (AAO film, gold nanoparticle, magnetic beads, DNA-stem loop probe). High sensitivity; could be smart phone enabled for personal exposure measures. |
| Mass Spectrometry(13–15) | Fragmentation of analyte and quantification of mass to charge units. Methods developed for grass pollen, food allergens. High sensitivity, but high throughput capacity is limited; measurements are expensive |
| Bacteria | |
| 16S rDNA microarrays(33) | Requires higher quantities (~500ng) of 16S rDNA compared to sequencing. Broad range of taxa identifiable, but some rare micro-organisms may be missed. |
| 16S rDNA sequencing(25–28, 33–35) | 16S rDNA is amplified and sequenced. Sequencing technologies: Roche 454 pyrosequencing, Illumina HiSeq, MiSeq, Ion Torrent, PacBio. Reference databases for comparison: Greengenes, Ribosomal Database Project (RDP). |
| Fungi | |
| 18S/28S/ITS rDNA sequencing(29, 30, 36) | rDNA from 18S, 28S, or ITS regions is amplified and sequenced. Sequencing technologies: Roche 454 pyrosequencing, Illumina HiSeq, MiSeq, Ion Torrent, PacBio. Reference databases (SILVA, FMP) limited but increasing. |
| All Biologics | |
| Whole genome shotgun sequencing(34) | All DNA from an environmental sample is extracted and sequenced. More expensive than rDNA methods, often less depth taxonomically for lower abundance microbes. Offers potential for functional metagenomics (i.e. abundance of microbial metabolic pathway genes). |
For the characterization of indoor microbial communities in dust and air, prior to the availability of culture independent technology enabling metagenomics, environmental microbial taxa were measured either by culture, qPCR of select taxa, or quantification of presence or activity of bioactive indoor pathogen-associated molecular patterns (PAMPs). Gram negative bacteria endotoxin bioactivity has been quantified using both kinetic Limulus amebocyte lysate (LAL) and recombinant Factor C (rFC) assays.(20–22) Endotoxin and the gram positive PAMP biomarker, peptidoglycan (N-acetyl muramic acid) have been also measured by gas chromatography/mass spectrometry (GC/MS). These methods are now complemented by culture independent metagenomic characterization of communities of microbes originating from a multitude of sources (e. g., humans, pets, mice, cockroaches, dust mites, water, soil, plants, building materials).(23, 24) Amplification and sequencing of select regions (16S rDNA for bacteria; 18S or ITS for fungi) of ribosomal DNA (rDNA), the gene that encodes for ribosomal RNA, yields information on the taxonomic composition of the environmental microbiome.(23–32) Alternatively, rDNA microarrays can be used to characterize bacterial taxonomic abundance. Microarrays are less agnostic than rDNA sequencing, and may require larger quantities of 16S rDNA. (33) Whole genome shotgun sequencing of all DNA extracted from an environmental sample also yields information on taxonomic composition of bacteria, fungi and viruses, although depth of coverage may be less than for rDNA amplification and sequencing. It also provides characterization of potential function through metagenomics estimation of the proportion of genes detected for given microbial metabolic pathways.(34) All of these metagenomic techniques generate relative abundance data for taxonomic composition or representation of functional pathways, but do not measure total bacterial or fungal microbial load, a task that requires qPCR. Also, they do not adequately address the actual function of household bacteria, and the relevance to that function to metabolic products (including breakdown of household chemicals) that could influence human health, or to colonization of the human microbiome.
Research Priorities
-
Analytical/Technological Improvements
Personal monitoring devices for allergen, pollutant, and microbial exposures, including capacity for continuous monitoring, real-time data capture and spatial mapping
Development of techniques for uncontaminated and unbiased collection, extraction and processing of environmental microbiome samples in air and dust
Expansion of methods to measure environmental microbial functional potential and viability
Assessment of the metabolism of household chemicals by environmental microbes
-
Development of methods for:
Quantification of multiple combined as well as individual indoor environmental allergens, microbes, pollutants, and household chemicals (“the exposome”)
Assessment of their relevance to human compartmental (e.g., upper airway, lower airway, gut) exposures during critical life stages
Insights into biologic properties of indoor exposures and associations with respiratory allergy and asthma outcomes
Molecular studies of allergens, adjuvants and other environmental stimuli
Allergy is classically manifested by an IgE antibody response to something that is normally considered harmless, typically a protein. The role of allergens in cross-linking pre-formed IgE on mast cells followed by the recruitment of Th2 cells, basophils and eosinophils and resulting in immediate and late allergic responses, is well understood. Given that not all proteins are allergenic, other biologic properties of allergens that are less understood may be responsible for their allergenic potential. Recent studies focus on the importance of allergen proteases (37) in disrupting airway epithelial barrier integrity and function and allowing for more effective antigen uptake by innate immune cells.(38–40) Also, non-antigenic stimulation of pattern recognition receptors on epithelial cells can produce alarmins like TSLP, IL-33, IL-25 leading to Type 2 immune response polarization. (41, 42)
Human and in-vitro laboratory studies have suggested a variety of adverse or protective airway responses to inhaled allergens modulated by co-exposure to natural adjuvants, (e.g. bacterial components) that depend on dose, timing of exposure and host characteristics. In some mouse models, endotoxin was found to be the primary adjuvant in common house dust for promoting Th17 responses and neutrophilic inflammation characteristic of steroid-resistant asthma, but this microbial product was dispensable for priming the Th2 responses that are associated with allergic asthma.(43) In contrast, bacterial flagellin stimulated strong Th2 responses to ovalbumin, and was an important adjuvant component in some samples of house dust. (44) Thus, in mouse models, microbial ligands found in house dust can act in a dose-dependent manner to direct discrete types of immune responses to inhaled allergens.(45, 46) Human studies support this general notion that concomitant exposure to allergens and microbes can shape the type of immune response to the allergen that develops. (23, 24, 43, 44)
Allergen and microbial exposures may interact with each other and with pollutants, leading to harmful or, in certain cases, beneficial immunologic and clinical effects.(47, 48) Tobacco smoke and other inhalant toxins appear to alter epithelial cell gene expression throughout the respiratory tract and are likely to be important co-factors in immune response to allergens and perpetuation of asthma.(38) Metabolites of microbes and other organisms can also act as adjuvants. For example, chitin, a polysaccharide in allergens, fungi and insects, has been shown to be an adjuvant for Th2 responses.(45, 46, 49)
The effects of allergens, adjuvants and other environmental stimuli on the human airway epithelium can be studied in vitro with the use of primary cell cultures. Nasal brushing yielding upper airway respiratory epithelial cells from the inferior turbinate offers targeted opportunities for epigenetic and gene expression characterization of airway responses potentially relevant to asthma and allergic rhinitis.(50) While it is a minimally invasive procedure, nasal brushing is perceived with variable levels of comfort/discomfort by children and adults.(51) A recent study suggest that gene expression responses to tobacco smoke in the nasal epithelium correlate well with that in lower airway epithelial cells. (52, 53)
Population level studies of allergen exposure
While the prospective relation of home allergen levels to allergy development has been well-studied in specific birth cohorts, including those with clinical trial designs (Table 3), the National Survey of Lead and Allergens in Housing and National Health and Nutrition Examination Survey 2005–2006 were the first US population-level studies of cross-sectional associations between allergen exposures, allergic conditions and sensitization. (54, 55) These surveys indicated that almost half of the US population was sensitized to aeroallergens and that exposure to multiple allergens in homes was common. While many prospective as well as cross-sectional studies show adverse associations of allergens or their sources with allergic sensitization, wheeze or asthma, protective associations have also been found with exposures to animal allergens or their mammalian sources,(56–60) and in one multi-city disadvantaged urban U.S. cohort study, to multiple allergens including cockroaches and dust mite.(24) Collectively, these findings underscore the need to understand time windows of susceptibility to allergic sensitization and the complex dose-response relationships between allergen exposure, other heritable or environmental co-exposures (e.g., stress, pollutants) and sensitization.
Table 3.
Randomized controlled trials in primary prevention of asthma using HDM allergen avoidance
| Studies | Year/s recruited | N | Assessments (Years) | Major findings |
|---|---|---|---|---|
| Isle of Wight (110–112) | 1990 | 120 | 1, 2, 4, 8, 18 | Reduced asthma and atopy at all ages 1–18 |
| MAAPPS*(109, 115, 116) | 1995–1997 | 291 | 1, 3, 5, 8, 16 | Reduced severe wheezing (infancy) Improved lung function (age 3 years) Increased mite sensitization (age 3 years) |
| CaPPS(113, 114, 149) | 1995 | 545 | 1, 2, 7, 15 | Reduced asthma (up to age 7 and at age 15 in females only) |
| PIAMA(118–120) | 1996/97 | 810 | 1, 2, 3, 4, 5, 6, 7, 8 | Reduced asthma at age 2. No effect at other ages. |
| CAPS(121) | 1997–1999 | 616 | 18 mo., 3, 5 | No difference in asthma, wheeze or atopy. Eczema higher in intervention group. |
Published outcomes of the intervention in MAAPPS available for ages one and three years only
Research Priorities
Studies on biochemical characteristics, such as protease or lipid binding activity, of a wide variety of allergens, to elucidate their contribution to allergy.
Studies of individual and combined influences of natural adjuvants, microbial substances, and inhaled irritants and toxicants on immune and airway responses relevant to allergy and asthma, using in vitro, in vivo, and human studies that take into account dose, timing, vulnerability and susceptibility.
Studies of airway respiratory epithelial cell responses to environmental stimuli with further development of more consistently comfortable upper airway sampling methods yielding outcomes relevant to the lower airways and asthma.(50)
Exposure reduction techniques: Fundamental concepts/methods and new insights for environmental interventions
Air pollutants found indoors, that can trigger asthma symptoms, originate from outdoor (e.g., traffic) and indoor sources (e.g., secondhand smoke (SHS), gas stove emissions). Elimination of SHS through smoking cessation and home smoking bans should always be considered a first-line indoor environmental intervention for children with asthma. Technologic improvements have been made in the efficacy of High Efficiency Particulate Air (HEPA) particle filtration designed to remove targeted indoor air pollutants such as fine particulate matter (PM2.5).(61) Other than replacement of gas stoves with electric stoves, fewer methods are currently available for indoor NO2 reduction of indoor or outdoor origin.(62, 63) In homes with smokers, recent home and school-based intervention trials in children report significant reductions in particulate matter with HEPA filter use, (64–66) but without reduction in indoor gases, without consistent reductions in markers of cigarette smoke, and with mixed success in improving child asthma symptoms. The efficacy in reducing indoor pollutants is dependent on room dimensions and building structure and conditions. While air cleaners have been also been used as adjunct interventions in multipronged environmental intervention trials (67) successful in reducing asthma symptoms, their independent contributions to health is uncertain, and the physical settings in which they may reduce exposure sufficiently to contribute to asthma control are also not well defined.
Indoor fungi originate through penetration from outdoors as well as from indoor sources, especially in damp and water damaged buildings.(68–70) They have a multitude of forms, properties and components. Fungi and their irritant or toxicant components can have adverse airway irritant as well as allergenic properties, and asthma symptoms can occur in individuals not sensitized, as well as in those sensitized to fungi.(71, 72) The mechanisms for effects of individual fungal components and interactive effects with other indoor exposures on airway and immune responses are not well-understood. Paradoxically, some observational birth cohort studies suggest that specific microbial communities or early life diversity of microbial agents, including fungi, may protect against allergy development,(73) but this is not a justification for discouraging fungal remediation in water damaged homes or poorly maintained moldy homes.
In symptomatic patients with asthma, fungal prevention and remediation strategies and their success in reducing exposures or improving health in damp or water damaged buildings can vary by housing stock, climatic region, and resident behaviors. New building materials, ventilation systems, and home furnishings, particularly those harboring humidity, may introduce new challenges requiring novel strategies to minimize fungal growth. While a review of studies to reduce mold in buildings and assess health outcomes recommended “better research, preferably with a randomized controlled study design and with more validated outcome measures,” (74–76) imaginative study designs are needed to fit extreme situations investigators and communities are at times confronted with. In disasters with clear-cut mold damage the health risks can be obvious, but building remediation solutions may be challenging. The post-Hurricane Katrina HEAL study reported improvement of asthma symptoms with implementation of a hybrid intervention with asthma counselors and environmental remediation, but in the midst of post-disaster changes, investigators could not disentangle the extent to which the active study environmental interventions were responsible for the observed fungal reduction or symptom improvement.(77)
A variety of multi-pronged community-based strategies have been used to decrease indoor allergen exposures (78, 79) with varying success in reducing exposure and in improving asthma control. This inconsistency may be due to variable levels of intensity of the intervention, provided resources, participant education, social resources or adherence. More confounders include other changes in environmental exposures, differences in tailoring the interventions to individual sensitivities of the participants, baseline levels of allergens, and effect modification of the intervention health effect by the presence of co-exposures like stress or ETS (i.e., SHS).
While many studies have sampled and tested efficacy of interventions in individual indoor homes, effects of the structure and building components of housing, including multi-unit structures, on exposure are less well studied. In Northeastern and mid-Atlantic cities, asthma prevalence is often high in multifamily low-income housing sites where multiple and interrelated housing-related exposures are present. A few studies have evaluated indoor environmental and respiratory health before and after alterations in single or multifamily homes that undergo ‘green’ construction, renovation or weatherization under construction guidelines aimed to conserve energy while maintaining adequate ventilation, using “environmentally friendly” materials. Such studies take advantage of costly interventions already taking place but have the potential limitations of uncontrolled observational study designs.(80) In one Boston-based study, asthmatic children living in green homes experienced substantially lower risk of asthma symptoms, hospital visits, and asthma-related school absences than children living in conventional public housing.(80) A study of green housing in the South Bronx (81) showed improvements in asthma symptoms and urgent care visits for asthma and a Chicago-based study showed self-reported asthma symptom improvements.(82) However, given the variable application of “green” construction approaches, the potential risks of responding to financial pressures by reduction of air exchange or inadequate maintenance even in new buildings, and study design limitations, uncertainty remains about which aspects of new construction may improve asthma.
Table 2 offers a summary of selected published studies on exposure reduction and on associations between exposure reduction with asthma control.
Table 2.
Select studies on building/home-based exposure reduction and asthma outcomes in children (2000–2017).
| Reference | Population | Study design | Exposure focus | Intervention | Exposure outcome | Asthma outcome | Comments |
|---|---|---|---|---|---|---|---|
| Carter et. al.(83) | 104 enrolled 6–16 y/o inner-city children with asthma (Atlanta, GA, US) | RCT Single blinded |
Dust mite and cockroach allergen |
Intervention 1 (n=35): allergen impermeable covers + effective roach bait, instructions to wash bedding once/wk. in hot water, and education re dust mite and cockroach cleaning measures Intervention 2 (placebo) (n=34): allergen permeable covers, instructions to wash bedding once/wk. in cold water Control 2 (n=35) Routine medical care; no home visits [85 completed study; 30/25/30] Significant allergen reduction defined as 70% decrease |
No difference between intervention vs placebo in percent attaining 70% decrease in allergen reduction; cockroach allergen reduction measures ineffective |
|
|
| Morgan et al. (2004) (84) | 937 5–11 y/o inner-city children with asthma sensitized to ≥ 1/11 indoor allergen (7 US cities; ICAS) | RCT | Indoor allergens. ETS |
Intervention (n=469): Multifaceted: 1 yr. of education + allergen impermeable covers + HEPA vacuum cleaner + bedroom HEPA air cleaner + remediation with IPM tailored to each child’s sensitization/exposure profile Control (n=468): Evaluation every 6 months |
Reduction in dust mite and cockroach allergen levels |
|
|
| Phipatanakul et al.(2004) (85) | 18 mouse infested homes of mouse sensitized inner city asthmatic children (Boston, US) | RCT | Mouse allergen |
Intervention (n=12): Professionally delivered IPM Control (n=6): No IPM |
Reduction (~75%) in settled dust mouse allergen levels in intervention vs. control homes |
|
|
| Krieger et al. (2005) (86) | 274 4–12y/o children with asthma from low income families (Seattle-King County WA, US) | RCT | Multiple asthma “triggers” |
Intervention (n=138): Multifaceted: 5–8 home visits by community health worker over 1 year including home assessment, education, support for behavior change and resources to reduce exposures Control(n=136): 1 visit, limited resources |
N/A |
|
|
| Eggleston et al.(2005)(87) | 100 6–12 y/o children with asthma from low income families (Baltimore MD, US) | RCT | PM10 & PM2.5; indoor allergens (focus on cockroach, mouse) |
Intervention( n=50): Multifaceted:1 yr. of education + allergen impermeable covers + bedroom HEPA air cleaner + remediation with IPM for mice and for cockroach (if infestation signs or if child sensitized) Control (n=50): treated at end of 1-yr trial |
Reductions of ~39% in PM10 & PM2.5, and ~50% in cockroach allergen |
|
|
| Chew et al. (2006) (88) | 3 uninhabited water-damaged homes after a major hurricane (New Orleans, LA; US) | Pre-post treatment comparison | Mold (spore counts, cultures, PCR analysis, glucan), endotoxin, and PM | Intervention: Removal of drywall, carpet, insulation, and all water-damaged furnishings | Reductions in mold and endotoxin pre-post, but high levels during clean-up | N/A |
|
| Kercsmar et al. (2006) (74) | 62 2–17 y/o children with asthma in homes with mold (Cuyahoga County, IL, US) | RCT | Mold scores; allergen levels |
Intervention (n=29) and Control (n=33): asthma action plan, education, individualized problem solving Intervention group only: + Household repairs and modifications |
|
|
|
| Sever et al. (2007)(89) | 60 cockroach infested homes (NC, US) | 3-arm RCT | Cockroach/Bla g 1 |
Intervention 1 (n=20): 12-mo professional entomologist pest control Intervention 2 (n=20):12-mo contract-based services performed by pest control companies Control (n=20) |
Compared to control:
|
N/A |
|
| Pongracic et al. (2008) (90) | 312 5–11 y/o inner-city children with asthma and sensitization to a rodent (subset of ICAS; 7 US cities) | RCT | Rodent allergen/Mus m 1 |
Intervention (N=150): ICAS (Inner-City Asthma Study) rodent module: 1 yr. of education + allergen impermeable covers + HEPA vacuum cleaner + bedroom HEPA air cleaner +filling rodent access points and setting traps throughout home Control (N=155): 97% received at least 1 other module |
80% bedrooms had detectable mouse allergen.
|
|
|
| Howden-Chapman et al. (2008) (91) | 409 households of 6–12 y/o children with asthma (5 New Zealand communities) | RCT | Nitrogen dioxide |
Intervention (N=200): Installation of a non-polluting more effective heater (heat pump, wood pellet burner, or flued (vented) gas) before winter Control (N=209): Given replacement heater at end of 1 yr. trial |
Reduction in NO2 levels in living rooms and bedrooms |
|
|
| Bryant-Stephens et al. (2009)(92) | 264 2–16 y/o children with asthma (Philadelphia) PA, US) | Randomized 6-mo Crossover | Dust, pests, pets, ETS | Immediate (n=144) or delayed (n=120) intervention: 6-mo (5 visit) family education+ supplies for trigger reduction (allergen impermeable covers, roach bait, mice traps, cleaning airs, storage bins, replacement for curtains/carpet) given by lay health educators |
|
|
|
| Breysse et al. (2011) (93) | 49 adults, 29 children from 31 units in a low income 3-building, 60-unit apartment complex (MN, US) | Cross-sectional health survey of pre-immediately post renovation health, followed by survey 12–18 mo. post renovation | Green specifications targeting ventilation, moisture, mold, pests, radon | Intervention: Renovation according to Enterprise Green Communities green specifications, using “healthy Housing” features. New mechanical ventilation installed(94) |
|
Immediately post renovation:
|
|
| Butz et al. (2011) (64) | 126 children with asthma, residing with a smoker (Baltimore MD, US) | RCT | Indoor PM and ETS exposure |
Intervention 1 (n=41):6-mo air cleaner Intervention 2 (n=41):Air cleaner + health coach Control (n=44): Delayed air cleaner |
|
|
|
| Lanphear et al.(2011) (65) | 215 6–12 y/o children with asthma exposed to ≥5 cigarettes/day (Cincinnati OH, US) | RCT Double-blinded |
Particle counts: >0.3μm >0.5μm |
Intervention (n=110):2 active HEPA air cleaners Control (n=115): 2 inactive HEPA air cleaners |
|
|
|
| Mitchell et al. (2012) (95) | 182 4–12 y/o children with moderate to severe asthma living in post Hurricane Katrina flooded areas (New Orleans LA, US) | Observational, pre-post intervention study | Indoor allergens, moisture and mold | Intervention: Individually tailored multi-faceted environmental intervention plus asthma counselor (timing of introduction of counselor varied) |
|
|
|
| Hoppe et al. (2012) (96) | Families living in 73 flood/water damaged homes (Cedar Rapids IA, US) | Cross-sectional assessment of homes and health at two levels of remediation (in-progress (n=24) or complete (n=49)) | Extensive, (e.g., Mold, bacteria, endotoxin, PM, allergens) | Intervention: Removal of drywall, carpet, insulation, and all water-damaged furnishings |
|
|
|
| Turyk et al. (2013) (97) | 218 <18 y/o children with asthma from 138 families (Chicago IL, US) | Observational, pre-post intervention study | Intervention: Asthma management education, plus individually tailored low-cost asthma home trigger remediation (e.g., allergen impermeable covers, home walkthrough covering reduction in asthma triggers, provision of environmental remediation tools), and referrals to social or medical agencies when appropriate |
|
|
|
|
| Breysse et al. (2014)(98) | 102 low income households in rental properties with 1 or more children with not well-controlled asthma(King Country WA, US) | Observational, pre-post intervention study with historical comparison group |
Intervention (n=34): Weatherization plus community health worker(CHW) education Historical comparison group (n=68): CHW education without weatherization |
|
|
|
|
| Colton et al. (2014) (99) | 31 low income households in rental housing | Observational comparison of exposures and health in green vs conventional housing, including in those who move between housing types. |
Intervention (n=18): Move from conventional to new buildings designed to green standards. Smoke-free policies and IPM practices employed. Control 1(n=6): Move from conventional to conventional housing Control 2(n=7): Live in conventional housing [61 visits including pre and post for 24 who moved] |
Green vs conventional housing:
|
|
|
|
| Colton et al. (2015)(80) | 235 households in 3 Boston public housing 188 residents (80%) with 2 visits | Observational comparison of conditions and health in green vs conventional housing. Visits included home inspection and questionnaire. | Visits to Green Units (n = 201) and conventional Public Housing Units (n = 222) |
Fewer reports and observations of mold, pests, inadequate ventilation, and secondhand smoke in green compared to conventional housing. |
|
|
|
| DiMango et al. (2016)(100) | 110 adults and 137 children with asthma sensitized and exposed to at least 1 indoor allergen | RCT | Key allergens in vacuumed settled dust (cat, dog, dust mite, cockroach and mouse) | Following optimization of asthma treatment and control, randomization to: Intervention (n=125): Multifaceted: 40-wk education + allergen impermeable covers + HEPA vacuum cleaner + bedroom HEPA air cleaner Control (n=122): education not related to allergen avoidance |
|
|
|
| Matsui et al (2017)(101) | 350 children and adolescents with asthma sensitized and exposed to mouse allergen (Baltimore MD; Boston MA, US) | RCT | Mouse allergen |
IPM+Education Group (n=181): application of rodenticide, sealing holes that could serve as entry points for mice, trap placement, targeted cleaning, allergen-proof mattress/pillow encasements, portable air purifiers. If infestation persisted or recurred, additional treatments were delivered. Education (n=180): Written material and demonstration of the materials needed to set traps and seal holes. |
|
|
|
| Rabito et al (2017)(102) | 102 5–7 y/o children with moderate to severe asthma in cockroach-infested homes(New Orleans, LA; US) | RCT | Cockroach allergen |
Intervention (n=53): 12-mo with trapping & bait placement at baseline, 1, 3, 6, 9, and 12 mo in areas with evidence of cockroach. Control (n=49): 12-mo with trapping but no bait placement at baseline, 1, 3, 6, 9, and 12 mo after baseline. |
|
|
|
| Murray et al (2017)(103) | 286 3–17 y/o mite-sensitized children with emergency hospital attendance for asthma exacerbation (North-West England) | RCT [Age group (3–10 y; 11–17 y) stratified] | Dust mite allergen |
Intervention (n=146): 12-mo with mite-impermeable bed encasings. Control (n=138): 12-mo with no encasings. |
|
|
|
RCT=Randomized controlled trial; ETS=environmental tobacco smoke; IPM=integrated pest management; PM=particulate mass; ICS=inhaled corticosteroids; QOL=quality of life; ICAS=Inner City Asthma Study. References (2000–2016) selected by Workshop participants as representative and illustrative of asthma management intervention studies in children.
Research Priorities
Well-designed (and, if feasible, blinded and controlled) trials to test the conditions under which free-standing air filtration systems, structural interventions, and other emerging building-level interventions reduce indoor pollutants, allergens, and other contaminants at home or in schools. This is a precondition to assessing whether exposure reduction improves respiratory health.
Development of effective mold reduction strategies tailored to specific individual risk factors (e.g., poorly controlled asthma) and building, geographic, and climatic factors.
Tailoring of multipronged strategies for indoor exposure reduction to the specific physical and social situations of urban families and their housing situations. Effective strategies may require changes in physical infrastructure, as well as in building management practices and occupant behavior.
Assessment, with engagement of building management and construction engineers, of effects of new building approaches (including ‘green’ building) and building characteristics (e.g., humidity, structural integrity) on indoor exposures and health.
Assessment of effects of housing policy interventions, such as housing mobility programs, on indoor exposures.
For highly mobile populations or for populations with little control over the structure of their homes, testing of low cost interventions easily transferable from home to home or interventions that can be applied to any home without the need for structural changes.
Development of novel technologies for particle or gas filtration (including NO2 reduction) in home and school environments.
With community engagement, development of interventions that can be applied to low-income populations with limited resources, especially those with high mobility.
All environmental interventions should include cost-benefit estimations.
Indoor environmental interventions for primary prevention of asthma
Primary prevention of asthma is an enviable goal that, if achieved, could reduce the prevalence of the disease. Of a large number of potentially modifiable risk factors for asthma development identified in the literature, (104) allergen exposure is one that has attracted considerable attention.(105, 106) Observational epidemiologic studies have identified early life allergen exposures as risk factors for subsequent allergic sensitization, and early allergic sensitization is a major risk factor for asthma.(107) However, the concept of allergen avoidance for primary prevention of asthma has been challenged by investigators who argue that this approach is limited by a) the ubiquitous nature of aeroallergens in some ecologic and cultural settings; b) the dominance of genetic factors in influencing the course of asthma; c) the importance of early priming by other factors (microbes or microbial components, in utero smoking, vitamin D, etc.) or, most recently d) the benefits from early allergen exposure as manifested by studies in food allergy and e) the protective effect against wheezing of high aeroallergen exposure in the first years of life. Evidence for potential benefits of early exposure to allergens or their sources for allergic sensitization, wheeze or asthma have been reported by observational birth cohort studies including the Massachusetts-based Epidemiology of Home Allergens and Asthma Study (EHAAS), the Wisconsin Childhood Origins of Asthma Study (COAST), the Detroit Childhood Allergy Study (CAS) and the Urban Environment and Childhood Asthma (URECA) Study. (24, 56, 58, 108) Most of these observational studies report protective associations with early-life mammalian exposures, especially dogs and associated allergens or microbes. The URECA data indicated that early-life multiple exposures including cockroach and mouse are protective, (24) whereas EHAAS found these two exposures to be risk factors. Multiple differences in cofactors and exposure levels may be responsible for the contrasts in these observational studies.
Dust mite allergen avoidance and prevention studies
Long-term follow up in primary allergen prevention trials focused on house dust mite reduction vary in terms of their success in asthma prevention. (109) The first such study was the Isle of Wight primary prevention study which recruited 120 children and used a multifaceted approach to reduce both common food allergens and house dust mite (HDM) exposure during infancy, with follow-up extending to 18 years. This study has shown a consistent reduction of asthma, but not atopy, in the allergen avoidance group (Figure 1).(110–112) A multifaceted approach for infants with a family history of allergic disease was also tested in the Canadian Asthma Primary Prevention Study (Table 3). The intervention, which began during pregnancy, yielded mixed results with a significant reduction in asthma, but not atopy, at 1, 2 and 7 years. At 15 years of age, the reduction in asthma risk was seen only in females.(113, 114) The Manchester Asthma and Allergy Primary Prevention Study (MAAPPS) tested the effect of stringent indoor allergen avoidance measures in a relatively large (n=291) randomized controlled trial. (115) By age 3 years HDM sensitization was more common in the intervention group and there was no difference between the groups in physician-diagnosed asthma.(116, 117) Finally, in the Prevention and Incidence of Asthma and Mite Allergy (PIAMA) study, 810 allergic mothers were enrolled during pregnancy and randomized to impermeable mattress- and pillow-covers or placebo covers. Apart from a reduction in asthma prevalence at age 2 years, no preventive effect on asthma or allergic sensitization up to 8 years was observed.(118–121)
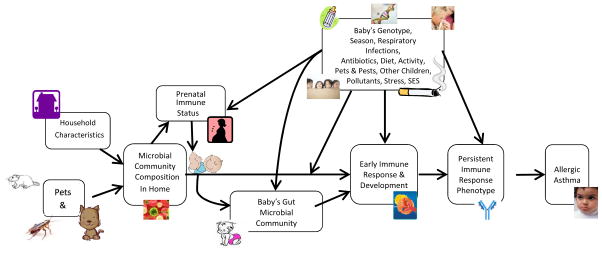
Figure 1.
A schematic model describing presumed relationships between the microbiome and allergic asthma. Adapted with permission from (153)
There are a number of explanations for the inconsistent findings across studies of HDM allergen avoidance. It might be that only a multifaceted intervention is effective.(122) Another potential explanation is that the baseline mite allergen levels in the PIAMA study were so low that further reduction could not have significant clinical effect.(115, 123) It is also possible, but less likely, that genetic variations in Isle of Wight and Canadian children made them more receptive to allergen avoidance (124) or that genes or environmental co-factors in the home, or outside of the home modify either the magnitude or even the direction of the response. Overall, interpretation of these findings is difficult because the relationships between the levels of allergen exposure and their biologic effects are not clear.
Other potentially modifiable environmental factors for asthma prevention
An explanation for protective associations with pets might be that the ecology of the home microbiome is affected by the presence of a pet, which in turn may influence the gastrointestinal microbiome of the infant.(125–127) Whether the microbial ecology of a child’s home is affected by outdoor microbes brought in by the pet, or by the pet’s own microbiome is unknown (Figure 1). The mechanisms through which this protection may occur are unknown, but the role of the microbiome and its biochemical products as modulators of innate immune system responses that may suppress allergy is an area of intense focus.(24, 128) One recent animal model validated the Detroit birth cohort observation that pet dust could be protective against allergic responses.(129)
Asthma disproportionately affects certain ethnic groups, and patterns of allergen and microbial exposure vary according to socioeconomic status, area of residence, and race or ethnicity.(130, 131) For example, non-Hispanic Blacks and Hispanics in the US Northeast are more likely to be exposed to mouse and cockroach allergens (but less likely to be exposed to HDM, dog, and cat allergens) than non-Hispanic whites.(132) In addition, stressful experiences, such as home or community violence, may contribute to the high prevalence of asthma in these communities.(133–135) Such experiences can disturb stress regulation and thus adversely influence immune function and increase susceptibility to asthma.(136) Primary prevention studies in asthma should strive to account for relevant social, cultural and demographic factors, as well as for the role of diet, stress and other lifestyle factors. (137)
Other potentially modifiable factors such as micronutrients, antioxidants and others, which are not considered classic environmental pollutants, allergens, or bioaerosols, are beyond the scope of this article. However, such factors are being actively investigated in the context of asthma prevention. (138–146)
Research Priorities
Additional observational and animal model validation studies to assess the role of dose, route, timing and pattern of single or multiple exposures, as well as genetic inheritance, in determining the relation of exposure to allergy or asthma development. This will optimize design of asthma prevention trials focused on allergen, pollutant, and microbial exposures.
Sufficiently powered observational study of multiple early-life environmental influences on asthma and allergy development in diverse communities in the United States. The recent collaboration of U.S. birth cohorts through the NIH-sponsored effort “Environmental Influences on Child Health Outcomes” (ECHO) offers a unique opportunity to achieve this goal; ECHO will facilitate characterization of children manifesting a variety of asthma phenotypes or endotypes that may be differentially influenced by indoor environmental exposures.(147, 148)
Studies to identify early patterns of human microbiome and its metabolic output in the gastrointestinal tract, the airways and the skin that are associated with the development of allergic diseases, and how they are influenced by the indoor environment, including environmental microbes, their metabolic products, and their functional components.
Randomized multifaceted environmental interventions for asthma prevention designed to account for each element of the intervention and for social, cultural and other demographic factors.
Randomized controlled trials (RCTs) that include primary prevention of asthma, through stress reduction measures tailored to ethnic and cultural diversity, and assessment of interactive effects of stress reduction with environmental interventions on asthma development.
-
For each of major potentially modifiable factors,
Identify the subpopulations that would benefit from the intervention, and subpopulations that might be at adverse risk, or not benefit.
Define, develop, refine and test interventions that would be of benefit to most children (e.g., smoking cessation).
Indoor Environmental Interventions for Asthma Management
Although indoor environmental interventions aimed at reducing asthma morbidity have been more successful than those aimed at primary prevention of asthma, questions remain about their role in asthma management. Table 2 provides an overview of the most recent environmental intervention trials and highlights their findings and limitations in influencing exposure reduction and asthma control. Effective environmental interventions are typically multi-faceted, tailored to the specific exposures and sensitivities of the target subject, and intensive.(84, 150) Publication bias leads to less publication of unsuccessful intervention trials, but the few that have been published suggest that single-allergen interventions and low-intensity efforts are ineffective. One such negative publication (151) exemplifies the challenge of translating an efficacious intervention from a tightly controlled clinical trial setting to a broader population: when the provision of allergen-proof mattress/pillow encasements to adults with asthma was tested in primary care, no effect was found with this untailored intervention. Although the study population was adults, the notion that health benefits observed in tightly controlled RCTs may not easily translate to more “real-world” settings is applicable to environmental interventions in children, too. In addition, families face a number of barriers to remediating environmental exposures, including costs, preferences, home ownership status, life-long behavioral practices, and education. For example, low-income urban populations are highly mobile and have limited resources with which to address environmental concerns. Also, residents often do not control the structure of their buildings since they rent, rather than those who own their homes.
The Inner-City Asthma Study (ICAS) may be the most successful environmental intervention study conducted to date; the intervention was targeted at specific allergen reduction in asthmatic children who were both sensitized and exposed to those allergens, but the intervention was also multi-facetted including integrated pest management targeted to specific allergen sensitivities, provision of HEPA vacuum cleaners, free standing bedroom HEPA filter air cleaners, and allergen-impermeable mattress and pillow covers. Primary trial results reported in 2004 found that the environmental intervention group experienced significant and clinically meaningful reductions in a range of asthma outcomes compared to controls.(84) Benefits were seen up to 12 months after the environmental intervention and cost-effectiveness analysis derived a cost of $750 to $1000 per year per family to implement, a cost they estimated was equivalent to cost of mid-range inhaled corticosteroid and albuterol for a child with moderately severe asthma. This translated to almost $28 per gained symptom-free day.(152) Because a multi-faceted, patient-tailored, intervention was tested in ICAS, and direct measures of environmental tobacco smoke exposure reduction were not made, it is not possible to determine the relative contribution of individual components of the environmental intervention and exposure reduction to the successful outcome. Notably, both arms of the ICAS (environmental intervention and physician feedback) were successful without other interaction with the health care systems, but optimally environmental control trials should be designed in the context of optimal access to health care, access to medications, and appropriate clinical asthma management.
Research Priorities
Further define the role of environmental interventions in asthma management by conducting randomized, multifaceted clinical trials designed to account for each element of the intervention and for social, cultural and other demographic factors.
Determine the most feasible, and cost- and clinically-effective approach to environmental interventions by conducting head-to-head comparisons of various forms of environmental intervention.
Determine which environmental intervention components can be effectively implemented and the best approaches to implementation. Studies are needed that will test how to effectively implement optimal environmental control schemes into healthcare, public health policy, housing policy, and clinical practice.
Specific, detailed research questions for each priority area in environmental interventions for asthma management described above are listed in Table 4. Addressing these research priorities will have clear implications for how healthcare providers, public health agencies, healthcare systems, communities, and insurers implement and support environmental intervention as an integral component of asthma management.
Table 4.
Research Questions for Priority Areas for Environmental Interventions and Asthma Management in Children
| Priority: Further define the role of environmental interventions (EI) in asthma management |
| • Are the findings from positive trials replicable? Scalable? |
| • Are there behavioral interventions that can improve adherence to EI behaviors? |
| • How, and in which settings, should EI be implemented? Are EIs effective as a public health intervention delivered at the community or school, rather than health care, level? |
| • What populations benefit most from EIs? Should EIs be targeted primarily/only to high morbidity populations, such as low-income and minority children with uncontrolled disease? Are EIs effective in populations that have not been studied (e.g., adults, suburban, rural)? |
| • Is further tailoring of the intervention, considering the whole environment (e.g., social stressors) as well as environments not typically studied (e.g., outdoor allergens) more effective than focusing on home and indoor allergens? |
| • Do EIs improve asthma outcomes by lowering indoor allergens, by their “bystander” effect on other factors related to asthma (e.g. medication adherence, second hand smoke exposure) or both? |
| • Do EIs have beneficial effects above and beyond those obtained by the use of controller medications? |
| • Do EIs reduce controller medication requirements/needs? |
| • Does EI mitigate the costs and side effects of controller medications? |
| Priority: Determine the most feasible, and cost- and clinically-effective approach to EI |
| • Which component(s) of EI are clinically effective and cost-effective, in order to maximize clinical effectiveness and limit costs? |
| • What is the minimal EI that retains efficacy and what components are required to retain efficacy (e.g., minimum frequency of visits, location of visits, activities performed at visits, duration of intervention?) |
| • Are there specific populations for whom the cost-benefit balance is favorable? |
| • How would coverage of EI by a healthcare system affect asthma morbidity and costs among its pediatric patients with asthma? |
| • In consideration of cost-effectiveness, which environmental intervention measures are most appropriate for specific populations and what is the optimal duration of a specific intervention? |
| • What are the benefits of one size fits all EIs, how to they compare to tailored EIs, and how do the cost-benefits compare? |
| Priority: Determine which EI components can be effectively implemented and the best approaches to implementation (Implementation Science) |
| • What are the systems obstacles to implementing EIs and how can they best be overcome? |
| • How should the population that will receive EIs be defined and identified in a non-research setting? |
| • How should staff be identified and trained (Are community health workers enough? Are more advanced credentials needed? Is professional integrated pest management necessary? How does training used in a clinical trial setting translate to the healthcare or community setting?) |
| • How can the intervention be supported financially? |
| • How should tools developed for clinical trials be replicated/developed/adapted for use beyond the clinical trial EI? |
| • When do adaptations no longer make the EI evidence-based, and what study designs are sufficient to evaluate continued efficacy? |
Conclusions
With a focus on indoor allergens, microbes and pollutants, workshop participants assessed current methods, and prioritized new method development for measurement of indoor environmental exposures potentially relevant to asthma development and asthma management. We assessed new insights into the biologic properties of many of these exposures, and prioritized needs for future elucidation of these properties. We reviewed the state-of-knowledge of the efficacy of targeted and multi-pronged environmental interventions in changing environmental exposures, and the social and structural challenges in influencing environment interventions, with recommendations for future directions. Finally, we reviewed the efficacy of primary prevention trials to reduce asthma development by altering the indoor biologic or physical environment, and the efficacy of trials to improve asthma management and asthma control by improving the indoor home or school environment. For each covered topic, the workshop offered recommendations on research priorities to inform the next generation of asthma prevention or asthma management trials that include environmental components. There was uncertainty as to whether efforts at primary intervention should include a trial of changes in the indoor environment. It is anticipated that the newly funded, U.S.-wide NIH-initiative Environmental Influences on Child Health Outcomes (ECHO) as well as complementary mechanistic studies with functional validation of observational findings might further inform future directions. Ultimately, new trials and translation of trial findings into public policy will need to take into account the family, social, economic and neighborhood context of participants and, for children with established asthma, their access to adequate health care, including appropriate asthma medications.
Acknowledgments
This workshop was supported by funds from the Division of Allergy, Immunology, and Transplantation, National Institute of Allergy and Infectious Diseases, the Division of Lung Diseases, National Heart, Lung, and Blood Institute, the Division of Intramural Research, National Institute of Environmental Health Sciences, and the Merck Childhood Asthma Network (MCAN), Inc. The authors would like to give special thanks to Dr. Floyd Malveaux for his MCAN leadership and support of this workshop. The findings and conclusions in this manuscript are those of the authors and do not necessarily represent the official position of any of the above-referenced NIH Institutes or the CDC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Diane R. Gold, Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School; Department of Environmental Health, Harvard T.H. Chan School of Public Health; Boston, MA.
Gary Adamkiewicz, Department of Environmental Health, Harvard T.H. Chan School of Public Health, Boston, MA.
Syed Hasan Arshad, The David Hide Asthma and Allergy Research Centre, Isle of Wight, and Clinical and Experimental Sciences, Faculty of Medicine, University of Southampton, Southampton, United Kingdom.
Juan C. Celedón, Division of Pulmonary Medicine, Allergy and Immunology, Children’s Hospital of Pittsburgh of the University of Pittsburgh Medical Center, University of Pittsburgh, Pittsburgh, Pennsylvania, U.S.A.
Martin D. Chapman, Indoor Biotechnologies, 700 Harris Street, Charlottesville, VA 22903.
Ginger L. Chew, Centers for Disease Control and Prevention (CDC), National Center for Environmental Health, Division of Environmental Hazards and Health Effects | Air Pollution and Respiratory Health Branch, Atlanta, GA.
Donald N. Cook, Immunity, Inflammation and Disease Laboratory, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC 27709.
Adnan Custovic, Section of Paediatrics and MRC and Asthma UK Centre in Allergic Mechanisms of Asthma, Imperial College London, London.
Ulrike Gehring, Institute for Risk Assessment Sciences, Utrecht University, Yalelaan 2, 3584CM, Utrecht, The Netherlands.
James E. Gern, Departments of Pediatrics and Medicine, University of Wisconsin School of Medicine and Public Health, K4/918 CSC, 600 Highland Avenue, Madison WI 53792-9988.
Christine C. Johnson, Department of Public Health Sciences, Henry Ford Hospital & Health System, Detroit Michigan USA.
Suzanne Kennedy, Department of Pediatrics, NC Children’s Hospital, University of North Carolina, Chapel Hill, NC.
Petros Koutrakis, Department of Environmental Health, Harvard T.H. Chan School of Public Health; Boston, MA.
Brian Leaderer, Yale School of Public Health, Yale School of Medicine, Yale School of Forestry and Environmental Studies, Center for Perinatal, Pediatric and Environmental Epidemiology (CPPEE), New Haven, CT
Herman Mitchell, Rho Federal Systems Division, Rho, Inc., Chapel Hill, NC.
Augusto A. Litonjua, Channing Division of Network Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, MA, USA.
Geoffrey A. Mueller, Genome Integrity and Structural Biology Laboratory, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC
George T. O’Connor, Pulmonary Center, Boston University School of Medicine, Boston, MA, USA.
Dennis Ownby, Division of Allergy-Immunology and Rheumatology, Department of Pediatrics, Augusta University, Augusta, GA.
Wanda Phipatanakul, Harvard Medical School, Boston Children’s Hospital, Asthma, Allergy, and Immunology, Boston, MA.
Victoria Persky, Division of Epidemiology and Biostatistics, School of Public Health, University of Illinois at Chicago, Chicago, IL.
Matthew S. Perzanowski, Department of Environmental Health Sciences, Mailman School of Public Health, Columbia University, 722 West 168th St, 11th Floor, New York, NY 10032
Clare D. Ramsey, Departments of Medicine and Community Health Sciences, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, Manitoba, Canada.
Päivi M. Salo, Division of Intramural Research, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park
Julie M. Schwaninger, Division of Allergy, Immunology, and Transplantation, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, MD, USA.
Joanne E. Sordillo, Channing Division of Network Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Avrum Spira, Division of Computational Biomedicine, Department of Medicine, Boston University School of Medicine, Boston, Massachusetts, USA.
Shakira F. Suglia, Department of Epidemiology, Rollins School of Public Health, Emory University, Atlanta, GA
Alkis Togias, Division of Allergy, Immunology, and Transplantation, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, MD, USA
Darryl C. Zeldin, Division of Intramural Research, National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC
Elizabeth C. Matsui, Division of Pediatric Allergy/Immunology, Johns Hopkins University, Baltimore, MD.
References
- 1.Habre R, Moshier E, Castro W, Nath A, Grunin A, Rohr A, et al. The effects of PM2.5 and its components from indoor and outdoor sources on cough and wheeze symptoms in asthmatic children. J Expo Sci Environ Epidemiol. 2014;24(4):380–7. doi: 10.1038/jes.2014.21. [DOI] [PubMed] [Google Scholar]
- 2.Habre R, Coull B, Moshier E, Godbold J, Grunin A, Nath A, et al. Sources of indoor air pollution in New York City residences of asthmatic children. J Expo Sci Environ Epidemiol. 2014;24(3):269–78. doi: 10.1038/jes.2013.74. [DOI] [PubMed] [Google Scholar]
- 3.Gore RB, Hadi EA, Craven M, Smillie FI, O’Meara TJ, Tovey ER, et al. Personal exposure to house dust mite allergen in bed: nasal air sampling and reservoir allergen levels. Clin Exp Allergy. 2002;32(6):856–9. doi: 10.1046/j.1365-2222.2002.01403.x. [DOI] [PubMed] [Google Scholar]
- 4.Renstrom A, Karlsson AS, Tovey E. Nasal air sampling used for the assessment of occupational allergen exposure and the efficacy of respiratory protection. Clin Exp Allergy. 2002;32(12):1769–75. doi: 10.1046/j.1365-2222.2002.01545.x. [DOI] [PubMed] [Google Scholar]
- 5.Graham JA, Pavlicek PK, Sercombe JK, Xavier ML, Tovey ER. The nasal air sampler: a device for sampling inhaled aeroallergens. Ann Allergy Asthma Immunol. 2000;84(6):599–604. doi: 10.1016/s1081-1206(10)62410-6. [DOI] [PubMed] [Google Scholar]
- 6.Earle CD, King EM, Tsay A, Pittman K, Saric B, Vailes L, et al. High-throughput fluorescent multiplex array for indoor allergen exposure assessment. J Allergy Clin Immunol. 2007;119(2):428–33. doi: 10.1016/j.jaci.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Filep S, Tsay A, Vailes L, Gadermaier G, Ferreira F, Matsui E, et al. A multi-allergen standard for the calibration of immunoassays: CREATE principles applied to eight purified allergens. Allergy. 2012;67(2):235–41. doi: 10.1111/j.1398-9995.2011.02750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.King EM, Filep S, Smith B, Platts-Mills T, Hamilton RG, Schmechel D, et al. A multi-center ring trial of allergen analysis using fluorescent multiplex array technology. J Immunol Methods. 2013;387(1–2):89–95. doi: 10.1016/j.jim.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsay A, Williams L, Mitchell EB, Chapman MD Multi-Centre Study G. A rapid test for detection of mite allergens in homes. Clin Exp Allergy. 2002;32(11):1596–601. doi: 10.1046/j.1365-2222.2002.01533.x. [DOI] [PubMed] [Google Scholar]
- 10.Winn AK, Salo PM, Klein C, Sever ML, Harris SF, Johndrow D, et al. Efficacy of an in-home test kit in reducing dust mite allergen levels: results of a randomized controlled pilot study. J Asthma. 2016;53(2):133–8. doi: 10.3109/02770903.2015.1072721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tovey ER, Willenborg CM, Crisafulli DA, Rimmer J, Marks GB. Most personal exposure to house dust mite aeroallergen occurs during the day. PLoS One. 2013;8(7):e69900. doi: 10.1371/journal.pone.0069900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institute of Biomedical Imaging and Bioengineering. Pediatric Research Using Integrated Sensor Monitoring Systems. [cited 2017 24 Feb]. Available from: https://www.nibib.nih.gov/research-funding/prisms.
- 13.Chapman MD, Briza P. Molecular approaches to allergen standardization. Curr Allergy Asthma Rep. 2012;12(5):478–84. doi: 10.1007/s11882-012-0282-3. [DOI] [PubMed] [Google Scholar]
- 14.Fenaille F, Nony E, Chabre H, Lautrette A, Couret MN, Batard T, et al. Mass spectrometric investigation of molecular variability of grass pollen group 1 allergens. J Proteome Res. 2009;8(8):4014–27. doi: 10.1021/pr900359p. [DOI] [PubMed] [Google Scholar]
- 15.Monaci L, Pilolli R, De Angelis E, Godula M, Visconti A. Multi-allergen detection in food by micro high-performance liquid chromatography coupled to a dual cell linear ion trap mass spectrometry. J Chromatogr A. 2014;1358:136–44. doi: 10.1016/j.chroma.2014.06.092. [DOI] [PubMed] [Google Scholar]
- 16.Kurita R, Yanagisawa H, Niwa O. Indoor allergen assessment quantified by a thin-layer electrochemical cell and magnetic beads. Biosens Bioelectron. 2013;48:43–8. doi: 10.1016/j.bios.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Sun X, Guan L, Shan X, Zhang Y, Li Z. Electrochemical detection of peanut allergen Ara h 1 using a sensitive DNA biosensor based on stem-loop probe. J Agric Food Chem. 2012;60(44):10979–84. doi: 10.1021/jf3027233. [DOI] [PubMed] [Google Scholar]
- 18.Kim D, Wang SX. A Magneto-Nanosensor Immunoassay for Sensitive Detection of Aspergillus Fumigatus Allergen Asp f 1. IEEE Trans Magn. 2012;48(11):3266–8. doi: 10.1109/TMAG.2012.2195163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai JJ, Bau IJ, Chen HT, Lin YT, Wang GJ. A novel nanostructured biosensor for the detection of the dust mite antigen Der p2. Int J Nanomedicine. 2011;6:1201–8. doi: 10.2147/IJN.S20757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alwis KU, Milton DK. Recombinant factor C assay for measuring endotoxin in house dust: comparison with LAL, and (1 --> 3)-beta-D-glucans. Am J Ind Med. 2006;49(4):296–300. doi: 10.1002/ajim.20264. [DOI] [PubMed] [Google Scholar]
- 21.Milton DK, Feldman HA, Neuberg DS, Bruckner RJ, Greaves IA. Environmental endotoxin measurement: the Kinetic Limulus Assay with Resistant-parallel-line Estimation. Environ Res. 1992;57(2):212–30. doi: 10.1016/s0013-9351(05)80081-7. [DOI] [PubMed] [Google Scholar]
- 22.Park JH, Szponar B, Larsson L, Gold DR, Milton DK. Characterization of lipopolysaccharides present in settled house dust. Appl Environ Microbiol. 2004;70(1):262–7. doi: 10.1128/AEM.70.1.262-267.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujimura KE, Demoor T, Rauch M, Faruqi AA, Jang S, Johnson CC, et al. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proc Natl Acad Sci U S A. 2014;111(2):805–10. doi: 10.1073/pnas.1310750111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lynch SV, Wood RA, Boushey H, Bacharier LB, Bloomberg GR, Kattan M, et al. Effects of early-life exposure to allergens and bacteria on recurrent wheeze and atopy in urban children. J Allergy Clin Immunol. 2014;134(3):593–601. e12. doi: 10.1016/j.jaci.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kembel SW, Meadow JF, O’Connor TK, Mhuireach G, Northcutt D, Kline J, et al. Architectural design drives the biogeography of indoor bacterial communities. PLoS One. 2014;9(1):e87093. doi: 10.1371/journal.pone.0087093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konya T, Koster B, Maughan H, Escobar M, Azad MB, Guttman DS, et al. Associations between bacterial communities of house dust and infant gut. Environ Res. 2014;131:25–30. doi: 10.1016/j.envres.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Song SJ, Lauber C, Costello EK, Lozupone CA, Humphrey G, Berg-Lyons D, et al. Cohabiting family members share microbiota with one another and with their dogs. Elife. 2013;2:e00458. doi: 10.7554/eLife.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taubel M, Rintala H, Pitkaranta M, Paulin L, Laitinen S, Pekkanen J, et al. The occupant as a source of house dust bacteria. J Allergy Clin Immunol. 2009;124(4):834–40. e47. doi: 10.1016/j.jaci.2009.07.045. [DOI] [PubMed] [Google Scholar]
- 29.Amend AS, Seifert KA, Samson R, Bruns TD. Indoor fungal composition is geographically patterned and more diverse in temperate zones than in the tropics. Proc Natl Acad Sci U S A. 2010;107(31):13748–53. doi: 10.1073/pnas.1000454107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adams RI, Miletto M, Taylor JW, Bruns TD. The diversity and distribution of fungi on residential surfaces. PLoS One. 2013;8(11):e78866. doi: 10.1371/journal.pone.0078866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lax S, Smith DP, Hampton-Marcell J, Owens SM, Handley KM, Scott NM, et al. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science. 2014;345(6200):1048–52. doi: 10.1126/science.1254529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meadow JF, Altrichter AE, Kembel SW, Moriyama M, O’Connor TK, Womack AM, et al. Bacterial communities on classroom surfaces vary with human contact. Microbiome. 2014;2(1):7. doi: 10.1186/2049-2618-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujimura KE, Rauch M, Matsui E, Iwai S, Calatroni A, Lynn H, et al. Development of a standardized approach for environmental microbiota investigations related to asthma development in children. J Microbiol Methods. 2012;91(2):231–9. doi: 10.1016/j.mimet.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tringe SG, Zhang T, Liu X, Yu Y, Lee WH, Yap J, et al. The airborne metagenome in an indoor urban environment. PLoS One. 2008;3(4):e1862. doi: 10.1371/journal.pone.0001862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schloss PD, Jenior ML, Koumpouras CC, Westcott SL, Highlander SK. Sequencing 16S rRNA gene fragments using the PacBio SMRT DNA sequencing system. PeerJ. 2016;4:e1869. doi: 10.7717/peerj.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faino L, Seidl MF, Datema E, van den Berg GC, Janssen A, Wittenberg AH, et al. Single-Molecule Real-Time Sequencing Combined with Optical Mapping Yields Completely Finished Fungal Genome. MBio. 2015;6(4) doi: 10.1128/mBio.00936-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chapman MD, Wunschmann S, Pomes A. Proteases as Th2 adjuvants. Curr Allergy Asthma Rep. 2007;7(5):363–7. doi: 10.1007/s11882-007-0055-6. [DOI] [PubMed] [Google Scholar]
- 38.Lambrecht BN, Hammad H. Allergens and the airway epithelium response: gateway to allergic sensitization. J Allergy Clin Immunol. 2014;134(3):499–507. doi: 10.1016/j.jaci.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 39.Lambrecht BN, Hammad H. Asthma: the importance of dysregulated barrier immunity. Eur J Immunol. 2013;43(12):3125–37. doi: 10.1002/eji.201343730. [DOI] [PubMed] [Google Scholar]
- 40.Cayrol C, Girard JP. IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy. Curr Opin Immunol. 2014;31:31–7. doi: 10.1016/j.coi.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Trompette A, Divanovic S, Visintin A, Blanchard C, Hegde RS, Madan R, et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature. 2009;457(7229):585–8. doi: 10.1038/nature07548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karp CL. Guilt by intimate association: what makes an allergen an allergen? J Allergy Clin Immunol. 2010;125(5):955–60. doi: 10.1016/j.jaci.2010.03.002. quiz 61–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitehead GS, Thomas SY, Cook DN. Modulation of distinct asthmatic phenotypes in mice by dose-dependent inhalation of microbial products. Environ Health Perspect. 2014;122(1):34–42. doi: 10.1289/ehp.1307280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilson RH, Maruoka S, Whitehead GS, Foley JF, Flake GP, Sever ML, et al. The Toll-like receptor 5 ligand flagellin promotes asthma by priming allergic responses to indoor allergens. Nat Med. 2012;18(11):1705–10. doi: 10.1038/nm.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dickey BF. Exoskeletons and exhalation. N Engl J Med. 2007;357(20):2082–4. doi: 10.1056/NEJMe0706634. [DOI] [PubMed] [Google Scholar]
- 46.Mack I, Hector A, Ballbach M, Kohlhaufl J, Fuchs KJ, Weber A, et al. The role of chitin, chitinases, and chitinase-like proteins in pediatric lung diseases. Mol Cell Pediatr. 2015;2(1):3. doi: 10.1186/s40348-015-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eldridge MW, Peden DB. Allergen provocation augments endotoxin-induced nasal inflammation in subjects with atopic asthma. J Allergy Clin Immunol. 2000;105(3):475–81. doi: 10.1067/mai.2000.104552. [DOI] [PubMed] [Google Scholar]
- 48.Matsui EC, Hansel NN, Aloe C, Schiltz AM, Peng RD, Rabinovitch N, et al. Indoor pollutant exposures modify the effect of airborne endotoxin on asthma in urban children. Am J Respir Crit Care Med. 2013;188(10):1210–5. doi: 10.1164/rccm.201305-0889OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Da Silva CA, Pochard P, Lee CG, Elias JA. Chitin particles are multifaceted immune adjuvants. Am J Respir Crit Care Med. 2010;182(12):1482–91. doi: 10.1164/rccm.200912-1877OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang X, Lenburg ME, Spira A. Comparison of nasal epithelial smoking-induced gene expression on Affymetrix Exon 1.0 and Gene 1.0 ST arrays. Scientific World Journal. 2013;2013:951416. doi: 10.1155/2013/951416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lai PS, Liang L, Cibas ES, Liu AH, Gold DR, Baccarelli A, et al. Alternate methods of nasal epithelial cell sampling for airway genomic studies. J Allergy Clin Immunol. 2015;136(4):1120–3. e4. doi: 10.1016/j.jaci.2015.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang X, Sebastiani P, Liu G, Schembri F, Zhang X, Dumas YM, et al. Similarities and differences between smoking-related gene expression in nasal and bronchial epithelium. Physiol Genomics. 2010;41(1):1–8. doi: 10.1152/physiolgenomics.00167.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poole A, Urbanek C, Eng C, Schageman J, Jacobson S, O’Connor BP, et al. Dissecting childhood asthma with nasal transcriptomics distinguishes subphenotypes of disease. J Allergy Clin Immunol. 2014;133(3):670–8. e12. doi: 10.1016/j.jaci.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Salo PM, Arbes SJ, Jr, Crockett PW, Thorne PS, Cohn RD, Zeldin DC. Exposure to multiple indoor allergens in US homes and its relationship to asthma. J Allergy Clin Immunol. 2008;121(3):678–84. e2. doi: 10.1016/j.jaci.2007.12.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Salo PM, Arbes SJ, Jr, Jaramillo R, Calatroni A, Weir CH, Sever ML, et al. Prevalence of allergic sensitization in the United States: results from the National Health and Nutrition Examination Survey (NHANES) 2005–2006. J Allergy Clin Immunol. 2014;134(2):350–9. doi: 10.1016/j.jaci.2013.12.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Litonjua AA, Milton DK, Celedon JC, Ryan L, Weiss ST, Gold DR. A longitudinal analysis of wheezing in young children: the independent effects of early life exposure to house dust endotoxin, allergens, and pets. J Allergy Clin Immunol. 2002;110(5):736–42. doi: 10.1067/mai.2002.128948. [DOI] [PubMed] [Google Scholar]
- 57.Ownby DR, Johnson CC. Dogs, cats, and asthma: Will we ever really know the true risks and benefits? J Allergy Clin Immunol. 2016;138(6):1591–2. doi: 10.1016/j.jaci.2016.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ownby DR, Johnson CC, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. JAMA. 2002;288(8):963–72. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 59.Tovey ER, Almqvist C, Li Q, Crisafulli D, Marks GB. Nonlinear relationship of mite allergen exposure to mite sensitization and asthma in a birth cohort. J Allergy Clin Immunol. 2008;122(1):114–8. 8 e1–5. doi: 10.1016/j.jaci.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 60.Perzanowski MS, Chew GL, Divjan A, Jung KH, Ridder R, Tang D, et al. Early-life cockroach allergen and polycyclic aromatic hydrocarbon exposures predict cockroach sensitization among inner-city children. J Allergy Clin Immunol. 2013;131(3):886–93. doi: 10.1016/j.jaci.2012.12.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee WC, Catalano PJ, Yoo JY, Park CJ, Koutrakis P. Validation and Application of the Mass Balance Model To Determine the Effectiveness of Portable Air Purifiers in Removing Ultrafine and Submicrometer Particles in an Apartment. Environ Sci Technol. 2015;49(16):9592–9. doi: 10.1021/acs.est.5b03126. [DOI] [PubMed] [Google Scholar]
- 62.Paulin LM, Diette GB, Scott M, McCormack MC, Matsui EC, Curtin-Brosnan J, et al. Home interventions are effective at decreasing indoor nitrogen dioxide concentrations. Indoor Air. 2014;24(4):416–24. doi: 10.1111/ina.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoo JY, Park CJ, Kim KY, Son YS, Kang CM, Wolfson JM, et al. Development of an activated carbon filter to remove NO2 and HONO in indoor air. J Hazard Mater. 2015;289:184–9. doi: 10.1016/j.jhazmat.2015.02.038. [DOI] [PubMed] [Google Scholar]
- 64.Butz AM, Matsui EC, Breysse P, Curtin-Brosnan J, Eggleston P, Diette G, et al. A randomized trial of air cleaners and a health coach to improve indoor air quality for inner-city children with asthma and secondhand smoke exposure. Arch Pediatr Adolesc Med. 2011;165(8):741–8. doi: 10.1001/archpediatrics.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lanphear BP, Hornung RW, Khoury J, Yolton K, Lierl M, Kalkbrenner A. Effects of HEPA air cleaners on unscheduled asthma visits and asthma symptoms for children exposed to secondhand tobacco smoke. Pediatrics. 2011;127(1):93–101. doi: 10.1542/peds.2009-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jhun I, Gaffin JM, Coull BA, Huffaker MF, Petty CR, Sheehan WJ, et al. School Environmental Intervention to Reduce Particulate Pollutant Exposures for Children with Asthma. J Allergy Clin Immunol Pract. 2017;5(1):154–9. e3. doi: 10.1016/j.jaip.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Morgan WJ, Crain EF, Gruchalla RS, O’Connor GT, Kattan M, Evans R, 3rd, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068–80. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 68.Hanson B, Zhou Y, Bautista EJ, Urch B, Speck M, Silverman F, et al. Characterization of the bacterial and fungal microbiome in indoor dust and outdoor air samples: a pilot study. Environ Sci Process Impacts. 2016;18(6):713–24. doi: 10.1039/c5em00639b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sordillo JE, Alwis UK, Hoffman E, Gold DR, Milton DK. Home characteristics as predictors of bacterial and fungal microbial biomarkers in house dust. Environ Health Perspect. 2011;119(2):189–95. doi: 10.1289/ehp.1002004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baxi SN, Muilenberg ML, Rogers CA, Sheehan WJ, Gaffin J, Permaul P, et al. Exposures to molds in school classrooms of children with asthma. Pediatr Allergy Immunol. 2013;24(7):697–703. doi: 10.1111/pai.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pongracic JA, O’Connor GT, Muilenberg ML, Vaughn B, Gold DR, Kattan M, et al. Differential effects of outdoor versus indoor fungal spores on asthma morbidity in inner-city children. J Allergy Clin Immunol. 2010;125(3):593–9. doi: 10.1016/j.jaci.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Korkalainen M, Taubel M, Naarala J, Kirjavainen P, Koistinen A, Hyvarinen A, et al. Synergistic proinflammatory interactions of microbial toxins and structural components characteristic to moisture-damaged buildings. Indoor Air. 2017;27(1):13–23. doi: 10.1111/ina.12282. [DOI] [PubMed] [Google Scholar]
- 73.Behbod B, Sordillo JE, Hoffman EB, Datta S, Webb TE, Kwan DL, et al. Asthma and allergy development: contrasting influences of yeasts and other fungal exposures. Clin Exp Allergy. 2015;45(1):154–63. doi: 10.1111/cea.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kercsmar CM, Dearborn DG, Schluchter M, Xue L, Kirchner HL, Sobolewski J, et al. Reduction in asthma morbidity in children as a result of home remediation aimed at moisture sources. Environ Health Perspect. 2006;114(10):1574–80. doi: 10.1289/ehp.8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sauni R, Uitti J, Jauhiainen M, Kreiss K, Sigsgaard T, Verbeek JH. Remediating buildings damaged by dampness and mould for preventing or reducing respiratory tract symptoms, infections and asthma (Review) Evid Based Child Health. 2013;8(3):944–1000. doi: 10.1002/ebch.1914. [DOI] [PubMed] [Google Scholar]
- 76.Chew GL, Horner WE, Kennedy K, Grimes C, Barnes CS, Phipatanakul W, et al. Procedures to Assist Health Care Providers to Determine When Home Assessments for Potential Mold Exposure Are Warranted. J Allergy Clin Immunol Pract. 2016;4(3):417–22. e2. doi: 10.1016/j.jaip.2016.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lichtveld M, Kennedy S, Krouse RZ, Grimsley F, El-Dahr J, Bordelon K, et al. From Design to Dissemination: Implementing Community-Based Participatory Research in Postdisaster Communities. Am J Public Health. 2016;106(7):1235–42. doi: 10.2105/AJPH.2016.303169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krieger J. Home is Where the Triggers Are: Increasing Asthma Control by Improving the Home Environment. Pediatr Allergy Immunol Pulmonol. 2010;23(2):139–45. doi: 10.1089/ped.2010.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matsui EC. Environmental exposures and asthma morbidity in children living in urban neighborhoods. Allergy. 2014;69(5):553–8. doi: 10.1111/all.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Colton MD, Laurent JG, MacNaughton P, Kane J, Bennett-Fripp M, Spengler J, et al. Health Benefits of Green Public Housing: Associations With Asthma Morbidity and Building-Related Symptoms. Am J Public Health. 2015;105(12):2482–9. doi: 10.2105/AJPH.2015.302793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garland E, Steenburgh ET, Sanchez SH, Geevarughese A, Bluestone L, Rothenberg L, et al. Impact of LEED-certified affordable housing on asthma in the South Bronx. Prog Community Health Partnersh. 2013;7(1):29–37. doi: 10.1353/cpr.2013.0010. [DOI] [PubMed] [Google Scholar]
- 82.Jacobs DE, Ahonen E, Dixon SL, Dorevitch S, Breysse J, Smith J, et al. Moving Into Green Healthy Housing. J Public Health Manag Pract. 2014 doi: 10.1097/PHH.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 83.Carter MC, Perzanowski MS, Raymond A, Platts-Mills TA. Home intervention in the treatment of asthma among inner-city children. J Allergy Clin Immunol. 2001;108(5):732–7. doi: 10.1067/mai.2001.119155. [DOI] [PubMed] [Google Scholar]
- 84.Morgan WJ, Crain EF, Gruchalla RS, O’Connor GT, Kattan M, Evans R, III, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;351(11):1068–80. doi: 10.1056/NEJMoa032097. [DOI] [PubMed] [Google Scholar]
- 85.Phipatanakul W, Cronin B, Wood RA, Eggleston PA, Shih MC, Song L, et al. Effect of environmental intervention on mouse allergen levels in homes of inner-city Boston children with asthma. Ann Allergy Asthma Immunol. 2004;92(4):420–5. doi: 10.1016/S1081-1206(10)61777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Krieger JW, Takaro TK, Song L, Weaver M. The Seattle-King County Healthy Homes Project: a randomized, controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. Am J Public Health. 2005;95(4):652–9. doi: 10.2105/AJPH.2004.042994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eggleston PA, Butz A, Rand C, Curtin-Brosnan J, Kanchanaraksa S, Swartz L, et al. Home environmental intervention in inner-city asthma: a randomized controlled clinical trial. AnnAllergy Asthma Immunol. 2005;95(6):518–24. doi: 10.1016/S1081-1206(10)61012-5. [DOI] [PubMed] [Google Scholar]
- 88.Chew GL, Wilson J, Rabito FA, Grimsley F, Iqbal S, Reponen T, et al. Mold and endotoxin levels in the aftermath of Hurricane Katrina: a pilot project of homes in New Orleans undergoing renovation. Environ Health Perspect. 2006;114(12):1883–9. doi: 10.1289/ehp.9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sever ML, Arbes SJ, Jr, Gore JC, Santangelo RG, Vaughn B, Mitchell H, et al. Cockroach allergen reduction by cockroach control alone in low-income urban homes: a randomized control trial. J Allergy Clin Immunol. 2007;120(4):849–55. doi: 10.1016/j.jaci.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pongracic JA, Visness CM, Gruchalla RS, Evans R, 3rd, Mitchell HE. Effect of mouse allergen and rodent environmental intervention on asthma in inner-city children. Ann Allergy Asthma Immunol. 2008;101(1):35–41. doi: 10.1016/S1081-1206(10)60832-0. [DOI] [PubMed] [Google Scholar]
- 91.Howden-Chapman P, Pierse N, Nicholls S, Gillespie-Bennett J, Viggers H, Cunningham M, et al. Effects of improved home heating on asthma in community dwelling children: randomised controlled trial. BMJ. 2008;337:a1411. doi: 10.1136/bmj.a1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bryant-Stephens T, Kurian C, Guo R, Zhao H. Impact of a household environmental intervention delivered by lay health workers on asthma symptom control in urban, disadvantaged children with asthma. Am J Public Health. 2009;99(Suppl 3):S657–65. doi: 10.2105/AJPH.2009.165423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Breysse J, Jacobs DE, Weber W, Dixon S, Kawecki C, Aceti S, et al. Health outcomes and green renovation of affordable housing. Public Health Rep. 2011;126(Suppl 1):64–75. doi: 10.1177/00333549111260S110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Enterprise. Green Communities. [cited 2017 24 Feb]. Available from: http://www.enterprisecommunity.org/solutions-and-innovation/green-communities.
- 95.Mitchell H, Cohn RD, Wildfire J, Thornton E, Kennedy S, El-Dahr JM, et al. Implementation of evidence-based asthma interventions in post-Katrina New Orleans: the Head-off Environmental Asthma in Louisiana (HEAL) study. Environ Health Perspect. 2012;120(11):1607–12. doi: 10.1289/ehp.1104242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hoppe KA, Metwali N, Perry SS, Hart T, Kostle PA, Thorne PS. Assessment of airborne exposures and health in flooded homes undergoing renovation. Indoor Air. 2012;22(6):446–56. doi: 10.1111/j.1600-0668.2012.00785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Turyk M, Banda E, Chisum G, Weems D, Jr, Liu Y, Damitz M, et al. A multifaceted community-based asthma intervention in Chicago: effects of trigger reduction and self-management education on asthma morbidity. J Asthma. 2013;50(7):729–36. doi: 10.3109/02770903.2013.796971. [DOI] [PubMed] [Google Scholar]
- 98.Breysse J, Dixon S, Gregory J, Philby M, Jacobs DE, Krieger J. Effect of weatherization combined with community health worker in-home education on asthma control. Am J Public Health. 2014;104(1):e57–64. doi: 10.2105/AJPH.2013.301402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Colton MD, MacNaughton P, Vallarino J, Kane J, Bennett-Fripp M, Spengler JD, et al. Indoor air quality in green vs conventional multifamily low-income housing. Environ Sci Technol. 2014;48(14):7833–41. doi: 10.1021/es501489u. [DOI] [PubMed] [Google Scholar]
- 100.DiMango E, Serebrisky D, Narula S, Shim C, Keating C, Sheares B, et al. Individualized Household Allergen Intervention Lowers Allergen Level But Not Asthma Medication Use: A Randomized Controlled Trial. J Allergy Clin Immunol Pract. 2016;4(4):671–9. e4. doi: 10.1016/j.jaip.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Matsui E, Perzanowski M, Peng R, Wise R, Balcer-Whaley S, Newman M, et al. Effect of an Integrated Pest Management Intervention on Asthma Symptoms among Mouse-sensitized Children and Adolescents with Asthma: A Randomized Clinical Trial. JAMA. 2017 doi: 10.1001/jama.2016.21048. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rabito FA, Carlson JC, He H, Werthmann D, Schal C. A single intervention for cockroach control reduces cockroach exposure and asthma morbidity in children. J Allergy Clin Immunol. 2017 doi: 10.1016/j.jaci.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 103.Murray CS, Foden P, Sumner H, Shepley E, Custovic A, Simpson A. Preventing Severe Asthma Exacerbations in Children: A Randomised Trial of Mite Impermeable Bedcovers. Am J Respir Crit Care Med. 2017 doi: 10.1164/rccm.201609-1966OC. [DOI] [PubMed] [Google Scholar]
- 104.Bousquet J, Gern JE, Martinez FD, Anto JM, Johnson CC, Holt PG, et al. Birth cohorts in asthma and allergic diseases: report of a NIAID/NHLBI/MeDALL joint workshop. J Allergy Clin Immunol. 2014;133(6):1535–46. doi: 10.1016/j.jaci.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Platts-Mills TA, Rakes G, Heymann PW. The relevance of allergen exposure to the development of asthma in childhood. J Allergy Clin Immunol. 2000;105(2 Pt 2):S503–8. doi: 10.1016/S0091-6749(00)90051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peat JK, Tovey E, Toelle BG, Haby MM, Gray EJ, Mahmic A, et al. House dust mite allergens. A major risk factor for childhood asthma in Australia. Am J Respir Crit Care Med. 1996;153(1):141–6. doi: 10.1164/ajrccm.153.1.8542107. [DOI] [PubMed] [Google Scholar]
- 107.Sly PD, Boner AL, Bjorksten B, Bush A, Custovic A, Eigenmann PA, et al. Early identification of atopy in the prediction of persistent asthma in children. Lancet. 2008;372(9643):1100–6. doi: 10.1016/S0140-6736(08)61451-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bufford JD, Reardon CL, Li Z, Roberg KA, DaSilva D, Eggleston PA, et al. Effects of dog ownership in early childhood on immune development and atopic diseases. Clin Exp Allergy. 2008;38(10):1635–43. doi: 10.1111/j.1365-2222.2008.03018.x. [DOI] [PubMed] [Google Scholar]
- 109.Simpson A, Custovic A. Prevention of allergic sensitization by environmental control. Curr Allergy Asthma Rep. 2009;9(5):363–9. doi: 10.1007/s11882-009-0053-y. [DOI] [PubMed] [Google Scholar]
- 110.Arshad SH, Matthews S, Gant C, Hide DW. Effect of allergen avoidance on development of allergic disorders in infancy. Lancet. 1992;339(8808):1493–7. doi: 10.1016/0140-6736(92)91260-f. [DOI] [PubMed] [Google Scholar]
- 111.Arshad SH, Bateman B, Sadeghnejad A, Gant C, Matthews SM. Prevention of allergic disease during childhood by allergen avoidance: the Isle of Wight prevention study. J Allergy Clin Immunol. 2007;119(2):307–13. doi: 10.1016/j.jaci.2006.12.621. [DOI] [PubMed] [Google Scholar]
- 112.Scott M, Roberts G, Kurukulaaratchy RJ, Matthews S, Nove A, Arshad SH. Multifaceted allergen avoidance during infancy reduces asthma during childhood with the effect persisting until age 18 years. Thorax. 2012;67(12):1046–51. doi: 10.1136/thoraxjnl-2012-202150. [DOI] [PubMed] [Google Scholar]
- 113.Chan-Yeung M, Manfreda J, Dimich-Ward H, Ferguson A, Watson W, Becker A. A randomized controlled study on the effectiveness of a multifaceted intervention program in the primary prevention of asthma in high-risk infants. Arch Pediatr Adolesc Med. 2000;154(7):657–63. doi: 10.1001/archpedi.154.7.657. [DOI] [PubMed] [Google Scholar]
- 114.Chan-Yeung M, Ferguson A, Watson W, Dimich-Ward H, Rousseau R, Lilley M, et al. The Canadian Childhood Asthma Primary Prevention Study: outcomes at 7 years of age. J Allergy Clin Immunol. 2005;116(1):49–55. doi: 10.1016/j.jaci.2005.03.029. [DOI] [PubMed] [Google Scholar]
- 115.Custovic A, Simpson BM, Simpson A, Hallam C, Craven M, Brutsche M, et al. Manchester Asthma and Allergy Study: low-allergen environment can be achieved and maintained during pregnancy and in early life. J Allergy Clin Immunol. 2000;105(2 Pt 1):252–8. doi: 10.1016/s0091-6749(00)90073-3. [DOI] [PubMed] [Google Scholar]
- 116.Custovic A, Simpson BM, Simpson A, Kissen P, Woodcock A. Effect of environmental manipulation in pregnancy and early life on respiratory symptoms and atopy during first year of life: a randomised trial. Lancet. 2001;358(9277):188–93. doi: 10.1016/S0140-6736(01)05406-X. [DOI] [PubMed] [Google Scholar]
- 117.Woodcock A, Lowe LA, Murray CS, Simpson BM, Pipis SD, Kissen P, et al. Early life environmental control: effect on symptoms, sensitization, and lung function at age 3 years. Am J Respir Crit Care Med. 2004;170(4):433–9. doi: 10.1164/rccm.200401-083OC. [DOI] [PubMed] [Google Scholar]
- 118.Koopman LP, van Strien RT, Kerkhof M, Wijga A, Smit HA, de Jongste JC, et al. Placebo-controlled trial of house dust mite-impermeable mattress covers: effect on symptoms in early childhood. Am J Respir Crit Care Med. 2002;166(3):307–13. doi: 10.1164/rccm.2106026. [DOI] [PubMed] [Google Scholar]
- 119.Corver K, Kerkhof M, Brussee JE, Brunekreef B, van Strien RT, Vos AP, et al. House dust mite allergen reduction and allergy at 4 yr: follow up of the PIAMA-study. Pediatr Allergy Immunol. 2006;17(5):329–36. doi: 10.1111/j.1399-3038.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 120.Gehring U, de Jongste JC, Kerkhof M, Oldewening M, Postma D, van Strien RT, et al. The 8-year follow-up of the PIAMA intervention study assessing the effect of mite-impermeable mattress covers. Allergy. 2012;67(2):248–56. doi: 10.1111/j.1398-9995.2011.02739.x. [DOI] [PubMed] [Google Scholar]
- 121.Marks GB, Mihrshahi S, Kemp AS, Tovey ER, Webb K, Almqvist C, et al. Prevention of asthma during the first 5 years of life: a randomized controlled trial. J Allergy Clin Immunol. 2006;118(1):53–61. doi: 10.1016/j.jaci.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 122.Maas T, Kaper J, Sheikh A, Knottnerus JA, Wesseling G, Dompeling E, et al. Mono and multifaceted inhalant and/or food allergen reduction interventions for preventing asthma in children at high risk of developing asthma. Cochrane Database Syst Rev. 2009;(3):Cd006480. doi: 10.1002/14651858.CD006480.pub2. [DOI] [PubMed] [Google Scholar]
- 123.van Strien RT, Koopman LP, Kerkhof M, Oldenwening M, de Jongste JC, Gerritsen J, et al. Mattress encasings and mite allergen levels in the Prevention and Incidence of Asthma and Mite Allergy study. Clin Exp Allergy. 2003;33(4):490–5. doi: 10.1046/j.1365-2222.2003.01626.x. [DOI] [PubMed] [Google Scholar]
- 124.Simpson A, John SL, Jury F, Niven R, Woodcock A, Ollier WE, et al. Endotoxin exposure, CD14, and allergic disease: an interaction between genes and the environment. Am J Respir Crit Care Med. 2006;174(4):386–92. doi: 10.1164/rccm.200509-1380OC. [DOI] [PubMed] [Google Scholar]
- 125.Fujimura KE, Johnson CC, Ownby DR, Cox MJ, Brodie EL, Havstad SL, et al. Man’s best friend? The effect of pet ownership on house dust microbial communities. J Allergy Clin Immunol. 2010;126(2):410–2. 2.e1–3. doi: 10.1016/j.jaci.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Sears MR, et al. Infant gut microbiota and the hygiene hypothesis of allergic disease: impact of household pets and siblings on microbiota composition and diversity. Allergy Asthma Clin Immunol. 2013;9(1):15. doi: 10.1186/1710-1492-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Levin AM, Sitarik AR, Havstad SL, Fujimura KE, Wegienka G, Cassidy-Bushrow AE, et al. Joint effects of pregnancy, sociocultural, and environmental factors on early life gut microbiome structure and diversity. Sci Rep. 2016;6:31775. doi: 10.1038/srep31775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE, et al. Innate Immunity and Asthma Risk in Amish and Hutterite Farm Children. N Engl J Med. 2016;375(5):411–21. doi: 10.1056/NEJMoa1508749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016;22(10):1187–91. doi: 10.1038/nm.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Celedon JC, Roman J, Schraufnagel DE, Thomas A, Samet J. Respiratory health equality in the United States. The American Thoracic Society perspective. Ann Am Thorac Soc. 2014;11(4):473–9. doi: 10.1513/AnnalsATS.201402-059PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Leaderer BP, Belanger K, Triche E, Holford T, Gold DR, Kim Y, et al. Dust mite, cockroach, cat, and dog allergen concentrations in homes of asthmatic children in the northeastern United States: impact of socioeconomic factors and population density. Environ Health Perspect. 2002;110(4):419–25. doi: 10.1289/ehp.02110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Olmedo O, Goldstein IF, Acosta L, Divjan A, Rundle AG, Chew GL, et al. Neighborhood differences in exposure and sensitization to cockroach, mouse, dust mite, cat, and dog allergens in New York City. J Allergy Clin Immunol. 2011;128(2):284–92. e7. doi: 10.1016/j.jaci.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Suglia SF, Enlow MB, Kullowatz A, Wright RJ. Maternal intimate partner violence and increased asthma incidence in children: buffering effects of supportive caregiving. Arch Pediatr Adolesc Med. 2009;163(3):244–50. doi: 10.1001/archpediatrics.2008.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen W, Boutaoui N, Brehm JM, Han YY, Schmitz C, Cressley A, et al. ADCYAP1R1 and asthma in Puerto Rican children. Am J Respir Crit Care Med. 2013;187(6):584–8. doi: 10.1164/rccm.201210-1789OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sternthal MJ, Jun HJ, Earls F, Wright RJ. Community violence and urban childhood asthma: a multilevel analysis. The European respiratory journal. 2010;36(6):1400–9. doi: 10.1183/09031936.00003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cacioppo JT, Berntson GG, Malarkey WB, Kiecolt-Glaser JK, Sheridan JF, Poehlmann KM, et al. Autonomic, neuroendocrine, and immune responses to psychological stress: the reactivity hypothesis. Ann N Y Acad Sci. 1998;840:664–73. doi: 10.1111/j.1749-6632.1998.tb09605.x. [DOI] [PubMed] [Google Scholar]
- 137.Szentpetery SE, Forno E, Canino G, Celedon JC. Asthma in Puerto Ricans: Lessons from a high-risk population. J Allergy Clin Immunol. 2016;138(6):1556–8. doi: 10.1016/j.jaci.2016.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nurmatov U, Devereux G, Sheikh A. Nutrients and foods for the primary prevention of asthma and allergy: systematic review and meta-analysis. J Allergy Clin Immunol. 2011;127(3):724–33. e1–30. doi: 10.1016/j.jaci.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 139.Litonjua AA, Lange NE, Carey VJ, Brown S, Laranjo N, Harshfield BJ, et al. The Vitamin D Antenatal Asthma Reduction Trial (VDAART): rationale, design, and methods of a randomized, controlled trial of vitamin D supplementation in pregnancy for the primary prevention of asthma and allergies in children. Contemp Clin Trials. 2014;38(1):37–50. doi: 10.1016/j.cct.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sewell DA, Hammersley VS, Devereux G, Robertson A, Stoddart A, Weir C, et al. Investigating the effectiveness of the Mediterranean diet in pregnant women for the primary prevention of asthma and allergy in high-risk infants: protocol for a pilot randomised controlled trial. Trials. 2013;14:173. doi: 10.1186/1745-6215-14-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.McEvoy CT, Schilling D, Clay N, Jackson K, Go MD, Spitale P, et al. Vitamin C supplementation for pregnant smoking women and pulmonary function in their newborn infants: a randomized clinical trial. JAMA. 2014;311(20):2074–82. doi: 10.1001/jama.2014.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Moreno-Macias H, Dockery DW, Schwartz J, Gold DR, Laird NM, Sienra-Monge JJ, et al. Ozone exposure, vitamin C intake, and genetic susceptibility of asthmatic children in Mexico City: a cohort study. Respir Res. 2013;14:14. doi: 10.1186/1465-9921-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Moreno-Macias H, Romieu I. Effects of antioxidant supplements and nutrients on patients with asthma and allergies. J Allergy Clin Immunol. 2014;133(5):1237–44. doi: 10.1016/j.jaci.2014.03.020. quiz 45. [DOI] [PubMed] [Google Scholar]
- 144.Keet CA, Shreffler WG, Peng RD, Matsui W, Matsui EC. Associations between serum folate and vitamin D levels and incident mouse sensitization in adults. The Journal of allergy and clinical immunology. 2014;133(2):399–404. doi: 10.1016/j.jaci.2013.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Okupa AY, Lemanske RF, Jr, Jackson DJ, Evans MD, Wood RA, Matsui EC. Early-life folate levels are associated with incident allergic sensitization. The Journal of allergy and clinical immunology. 2013;131(1):226–8. e1–2. doi: 10.1016/j.jaci.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Litonjua AA, Carey VJ, Laranjo N, Harshfield BJ, McElrath TF, O’Connor GT, et al. Effect of Prenatal Supplementation With Vitamin D on Asthma or Recurrent Wheezing in Offspring by Age 3 Years: The VDAART Randomized Clinical Trial. JAMA. 2016;315(4):362–70. doi: 10.1001/jama.2015.18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fuchs O, Bahmer T, Rabe KF, von Mutius E. Asthma transition from childhood into adulthood. Lancet Respir Med. 2016 doi: 10.1016/S2213-2600(16)30187-4. [DOI] [PubMed] [Google Scholar]
- 148.National Institutes of Health. Environmental Influence on Child Health Outcomes (ECHO) Program. [cited 2017 24 Feb]. Available from: https://www.nih.gov/ECHO.
- 149.Chan-Yeung M, Dimich-Ward H, Becker A. Atopy in early life and effect of a primary prevention program for asthma in a high-risk cohort. J Allergy Clin Immunol. 2007;120(5):1221–3. doi: 10.1016/j.jaci.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 150.Crocker DD, Kinyota S, Dumitru GG, Ligon CB, Herman EJ, Ferdinands JM, et al. Effectiveness of home-based, multi-trigger, multicomponent interventions with an environmental focus for reducing asthma morbidity: a community guide systematic review. Am J Prev Med. 2011;41(2 Suppl 1):S5–32. doi: 10.1016/j.amepre.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 151.Woodcock A, Forster L, Matthews E, Martin J, Letley L, Vickers M, et al. Control of exposure to mite allergen and allergen-impermeable bed covers for adults with asthma. N Engl J Med. 2003;349(3):225–36. doi: 10.1056/NEJMoa023175. [DOI] [PubMed] [Google Scholar]
- 152.Kattan M, Stearns SC, Crain EF, Stout JW, Gergen PJ, Evans R, 3rd, et al. Cost-effectiveness of a home-based environmental intervention for inner-city children with asthma. J Allergy Clin Immunol. 2005;116(5):1058–63. doi: 10.1016/j.jaci.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 153.Johnson CC, Ownby DR. The infant gut bacterial microbiota and risk of pediatric asthma and allergic diseases. Transl Res. 2017;179:60–70. doi: 10.1016/j.trsl.2016.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]