Abstract
Livestock grazing affects over 60% of the world's agricultural lands and can influence rangeland ecosystem services and the quantity and quality of wildlife habitat, resulting in changes in biodiversity. Concomitantly, livestock grazing has the potential to be detrimental to some wildlife species while benefiting other rangeland organisms. Many imperiled grouse species require rangeland landscapes that exhibit diverse vegetation structure and composition to complete their life cycle. However, because of declining populations and reduced distributions, grouse are increasingly becoming a worldwide conservation concern. Grouse, as a suite of upland gamebirds, are often considered an umbrella species for other wildlife and thus used as indicators of rangeland health. With a projected increase in demand for livestock products, better information will be required to mitigate the anthropogenic effects of livestock grazing on rangeland biodiversity. To address this need, we completed a data‐driven and systematic review of the peer‐reviewed literature to determine the current knowledge of the effects of livestock grazing on grouse populations (i.e., chick production and population indices) worldwide. Our meta‐analysis revealed an overall negative effect of livestock grazing on grouse populations. Perhaps more importantly, we identified an information void regarding the effects of livestock grazing on the majority of grouse species. Additionally, the reported indirect effects of livestock grazing on grouse species were inconclusive and more reflective of differences in the experimental design of the available studies. Future studies designed to evaluate the direct and indirect effects of livestock grazing on wildlife should document (i) livestock type, (ii) timing and frequency of grazing, (iii) duration, and (iv) stocking rate. Much of this information was lacking in the available published studies we reviewed, but is essential when making comparisons between different livestock grazing management practices and their potential impacts on rangeland biodiversity.
Keywords: conservation, effect size, grassland, grouse, Hedges' g, herbivory
1. INTRODUCTION
A recent assessment of vertebrates found one‐fifth classified as Threatened on the International Union for Conservation of Nature (IUCN) Red List (“The IUCN Red List of Species. Version 2015‐04”, 2015). On average, 52 species move one category closer to extinction each year. In 2010, most indicators of the state of biodiversity (i.e., population trends, extinction risk, habitat extent and quality, and community composition) declined, whereas the indicators of pressures on biodiversity increased (Butchart et al., 2010). Increased anthropogenic land use is implicated as a major factor in decreased biodiversity (de Baan, Alkemade, & Koellner, 2012; Jetz, Wilcove, & Dobson, 2007; Sala et al., 2000; Sisk, Launer, Switky, & Ehrlich, 1994).
Globally, livestock grazing is the predominant anthropogenic land use (Alkemade, Reid, van den Berg, de Leeuw, & Jeuken, 2013). Livestock grazing occurs on approximately 60% of the world's agricultural land and supports approximately 1.5 billion cattle and buffalo (Bovinae) and 1.9 billion sheep (Ovis spp.) and goats (Capra spp. and related species) (Alexandratos & Bruinsma, 2012). Global production of livestock for human consumption has more than doubled since the 1960s (Speedy, 2003). Concomitantly, the demand for livestock products is projected to increase 70% by 2050 in response to human population growth, increased discretionary income, and urbanization (Alexandratos & Bruinsma, 2012; Thornton, 2010).
Rangelands (i.e., grasslands, shrublands, woodlands, and tundra) are estimated to provide over 70% of the forage consumed by livestock worldwide (Lund, 2007). Rangelands also provide habitat for a diversity of wildlife species (Krausman et al., 2009). Thus, how these areas are managed can have important consequences for wildlife worldwide (Alkemade et al., 2013; Bock, Saab, Rich, & Dobkin, 1993; Jankowski et al., 2014; Kantrud & Kologiski, 1982; Krausman et al., 2009; Owens & Myres, 1973). Of particular concern, are ground nesting birds, such as grouse species (Tetraonidae), whose habitats are often associated with livestock grazing throughout the northern hemisphere. Livestock grazing has been implicated as both a source of mortality and an indirect driver of declines in habitat and populations in rangeland environments (Baines, 1996; Boyd, Beck, & Tanaka, 2014; Calladine, Baines, & Warren, 2002; Jenkins & Watson, 2001; Warren & Baines, 2004). Additionally, many of these grouse species depend on disturbances such as grazing or grazing in combination with fire during some or all of their life history, underscoring the importance of informed grazing practices (Hovick, Elmore, Fuhlendorf, & Dahlgren, 2015; McNew, Winder, Pitman, & Sandercock, 2015).
There are 20 species in the Tetraonidae family worldwide (Storch, 2007, 2015), 13 of which have been red listed by the IUCN (Table 1). In addition, populations for 18 of these species are declining (Storch, 2007, 2015). Habitat loss and degradation have been identified as the primary threat to grouse (Storch, 2007, 2015) and intense livestock grazing has been implicated as a conservation threat for six of the seven grouse species that occupy rangeland habitats (“The IUCN Red List of Species. Version 2015‐04”, 2015).
Table 1.
Twenty recognized grouse species, their population estimate, population status, and population trend
| Common name | Scientific name | Pop. estimatea | Statusb | Trendb |
|---|---|---|---|---|
| Black Grousec | Lyrurus tetrix | 27,500,000 | Least concern | Decreasing |
| Black‐billed Capercaillie | Tetrao urogalloides | <550,000 | Least concern | Decreasing |
| Western Capercaillie | Tetrao urogallus | 7,500,000 | Least concern | Decreasing |
| Caucasian Black Grouse | Lyrurus mlokosiewiczi | <46,600 | Near threatened | Decreasing |
| Chinese Grouse | Bonasa sewerzowi | Not quantified | Near threatened | Decreasing |
| Hazel Grouse | Bonasa bonasia | 27,500,000 | Least concern | Decreasing |
| Ruffed Grouse | Bonasa umbellus | Not quantified | Least concern | Decreasing |
| Dusky Grouse | Dendragapus obscurus | 3,000,000 | Least concern | Decreasing |
| Sooty Grouse | Dendragapus fuliginosus | Not quantified | Least concern | Decreasing |
| Greater Prairie‐Chickenc | Tympanuchus cupido | <700,000 | Vulnerable | Decreasing |
| Lesser Prairie‐Chickenc | Tympanuchus pallidicinctus | 30,000 | Vulnerable | Decreasing |
| Sharp‐tailed Grousec | Tympanuchus phasianellus | Not quantified | Least concern | Decreasing |
| Greater Sage‐Grousec | Centrocercus urophasianus | <150,000 | Near threatened | Decreasing |
| Gunnison Sage‐Grousec | Centrocercus minimus | <2,500 | Endangered | Decreasing |
| White‐tailed Ptarmigan | Lagopus leucura | Not quantified | Least concern | Decreasing |
| Willow Ptarmiganc | Lagopus lagopus | >40,000,000 | Least concern | Decreasing |
| Rock Ptarmigan | Lagopus muta | >8,000,000 | Least concern | Decreasing |
| Siberian Grouse | Falcipennis falcipennis | Not quantified | Near threatened | Decreasing |
| Spruce Grouse | Falcipennis canadensis | Not quantified | Least concern | Stable |
| Franklin's Grouse | Falcipennis franklinii | Not quantified | Least concern | Stable |
We report the mid‐point of population estimates.
All status, trend, and population estimates were gathered from BirdLife International 2016.
Species that inhabit rangelands.
As an example, the prairie grouse species that inhabit rangelands of North America are considered some of the most imperiled and at the greatest risk to improper livestock grazing practices (Silvy & Hagen, 2004). The Gunnison sage‐grouse (Centrocercus minimus) in North America (NA) was listed as a threatened species by the US Fish and Wildlife Service (USFWS) under the Endangered Species Act (ESA) and Endangered by the IUCN because of low population sizes, restricted range, and ongoing population decline (“The IUCN Red List of Species. Version 2015‐04”, 2015; U.S. Fish and Wildlife Service 2014). Similarly, greater and lesser prairie‐chickens (Tympanuchus cupido and T. pallidicinctus, respectively) are listed as Vulnerable. The sharp‐tailed grouse (T. phasianellus), once considered to have the most extensive range in NA, has declined markedly (Connelly, Gratson, & Reese, 1998; Johnsgard, 1983). Moreover, the greater sage‐grouse (C. urophasianus; hereafter sage‐grouse) which is listed by the IUCN as near threatened (Storch, 2015) was also considered by the USFWS for ESA protection (U.S. Fish and Wildlife Service 2015). Grazing by livestock is the predominant land use within the current sage‐grouse range and a paucity of information exists on the direct effects of grazing on these populations (Beck & Mitchell, 2000; Knick et al., 2011).
Given the projected global increase in demand for livestock production (Thornton, 2010), better information will be needed to mitigate the potential for increased impacts on rangeland ecosystems and associated wildlife species. However, our collective understanding of how grazing influences grouse species, which are often considered indicators for their ecosystems, is poorly understood despite the volumes of research that has been published about the ecology of these species (Haukos & Boal, 2016; Knick & Connelly, 2011). Therefore, a data‐driven and systematic review of the influence of grazing on grouse populations across the northern hemisphere is warranted to inform future conservation actions for these highly imperiled species.
We completed a data‐driven and systematic review of the peer‐reviewed literature to determine the current knowledge of the effect of livestock grazing on grouse populations (i.e., population indices represented by adult counts and chick production) worldwide. We used meta‐analytical methods to calculate unbiased estimates of Hedges' g (Hedges, 1981) as a measure of the direct effect of livestock grazing on grouse populations in addition to a categorical model meta‐analytic technique to quantify overall effects. We highlight knowledge gaps and research needs related to the effects of livestock grazing, the broadest anthropogenic land use on rangelands, on grouse populations.
2. MATERIALS AND METHODS
We conducted a literature search in May 2017 using the ISI Web of Science and Scopus databases. Searches were limited to peer‐reviewed journals or edited book series (e.g., Studies in Avian Biology). We developed keyword combinations to identify papers that included livestock, grazing, and grouse (Table 2). We used all terms for both title and topic searches to ensure returning the greatest number of papers possible. Common names of grouse species were included to capture studies that examined other grouse species absent from searches using the generic term “grouse.” As part of our search strategy, we included literature cited from the papers used in our analysis. No temporal or language restrictions were applied to our searches.
Table 2.
Search terms and resulting number of publications using the ISI Web of Science and Scopus databases to locate peer‐reviewed literature assessing the effects of livestock grazing on grouse populations
| Search results (number of publications) | ||
|---|---|---|
| Search term(s) | ISI web of science | Scopus |
| grouse* | 3,083 | 2,554 |
| (grouse* and livestock*) | 64 | 49 |
| (grouse* and grazing*) | 107 | 98 |
| (grouse* and habitat* and grazing*) | 76 | 65 |
| (prairie‐chicken* and livestock*) | 8 | 9 |
| (prairie‐chicken* and grazing*) | 23 | 21 |
| (prairie‐chicken* and habitat* and grazing*) | 20 | 17 |
| (capercaillie* and livestock*) | 5 | 3 |
| (capercaillie* and grazing*) | 8 | 3 |
| (capercaillie* and habitat* and grazing*) | 6 | 1 |
| (ptarmigan* and livestock*) | 3 | 3 |
| (ptarmigan* and grazing*) | 6 | 8 |
| (ptarmigan* and habitat* and grazing*) | 4 | 5 |
In cases of irregular plurals, “*” allows search engines to retrieve all forms of the root word.
2.1. Study inclusion criteria
To refine our search, we removed papers that lacked our specific search terms within the title, abstract, or keywords. We then reviewed the remaining papers to determine whether they quantified and reported the effects of livestock grazing on grouse populations. Finally, we only retained papers that compared grouse population metrics within ≥2 grazing intensities (e.g., heavy grazing, reduced grazing, or no grazing) for the meta‐analysis. Of the initial 5,637 topic search results, only four studies met our inclusion criteria (Figure 1).
Figure 1.
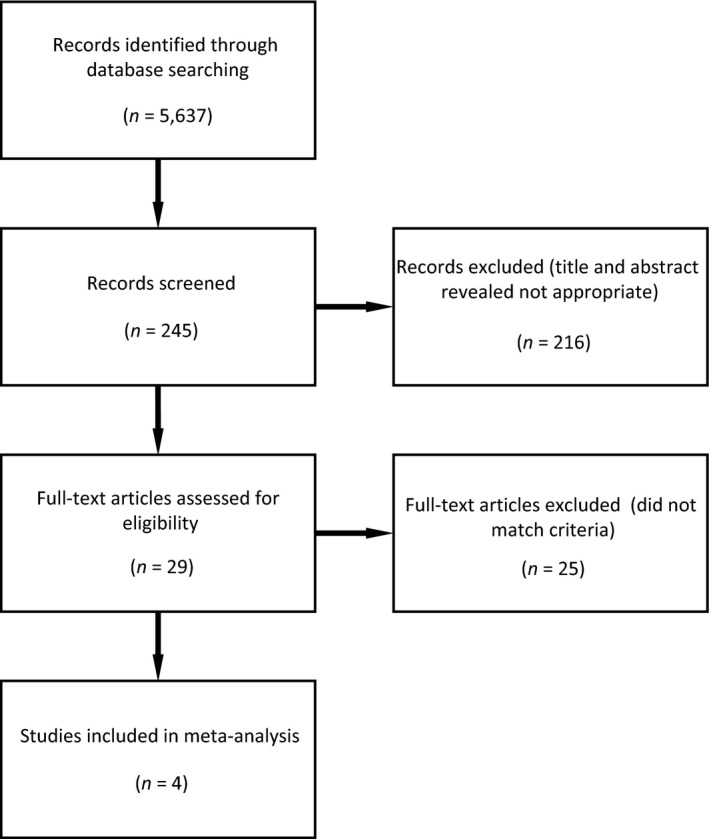
Preferred Reporting Items for Systematic reviews and Meta‐analyses (PRISMA) diagram illustrating study selection process
2.2. Data extraction
Because of the limited number of published papers that met our search criteria, we maximized the number of metrics obtained from each study. For example, Baines (1996) and Calladine et al. (2002) each reported grazing effects on both adult counts (a population indices comprised of the total males counted on leks) and chick production (chicks per female). In each study, direct effects were independently determined and analyzed separately in the meta‐analysis. Finally, one study (Jenkins & Watson, 2001) involved two species of grouse and were separated in the analysis.
2.3. Meta‐analysis
We quantified the direct effects of livestock grazing on grouse populations using calculated effect sizes with analyses similar to Hovick, Elmore, Dahlgren, Fuhlendorf, and Engle (2014). We standardized the reported results from each study by estimating effect sizes using the means, standard deviation, and sample sizes. To control for small sample size bias, we used Hedges' g effect sizes (Hedges, 1981) calculated using “compute.es” package (Del Re, 2013) in the R 3.2.3 programming environment (R Development Core Team 2015). Because field studies often lack true treatment and control levels (Hovick et al., 2014) and quantifiable grazing intensities, we categorized groups of grouse from each study into either higher‐intensity grazing sites or reduced or absent grazing sites. All meta‐analytic models were calculated using MetaWin 2.1.5 (Rosenberg, Adams, & Gurevitch, 2000). Generally, effect sizes are interpreted as <|0.2| low, |0.5| moderate, and >|0.8| high (Cohen, 1988).
Because our meta‐analysis relied on small sample sizes, we ran bootstrapping replications with replacement to improve approximations of the confidence intervals (Efron & Tibshirani, 1986). We analyzed these data using a categorical random‐effects model in Meta‐Win 2.1.5. We selected a categorical model based on the separation of our data into two distinct population measurement groups, adult counts (population indices) and chick production. Because studies differed spatially, temporally, by grazing system, and level of grazing pressure, there may be different effect sizes underlying each (Borenstein, Hedges, Higgins, & Rothstein, 2010). To address variation in the true effect size of livestock grazing based on the unique environmental and temporal factors of each study, we selected a random‐effects model. Weighted averages were used in the models to estimate the cumulative effect size by calculating the reciprocal of each studies' sampling variance, w i = 1/v i. Because individual studies within a meta‐analysis often vary in sample size, weighting becomes necessary (Rosenberg et al., 2000). We calculated the percentage of total variation across studies that is due to heterogeneity using the I 2 statistic (Borenstein, Hedges, Higgins, & Rothstein, 2009).
We tested for publication bias, or the “file drawer problem” (i.e., when only studies reporting significant results are published) using the approaches developed by Egger, Smith, Schneider, and Minder (1997). Egger's test uses linear regression in which the standardized effect estimate z i is regressed against its precision preci (Rothstein, Sutton, & Borenstein, 2006):
3. RESULTS
We analyzed six measurements of grazing's effect on adult grouse numbers and three on chick production. Our results demonstrated that livestock grazing had a negative impact on adult grouse numbers (random effects = −1.28, df = 5, 95% CI: −2.02, −0.85). Additionally, we estimated a negative effect of livestock grazing on grouse chick production (random effects = −0.84, df = 2, 95% CI: −1.34, −0.59). Based on these studies, there is evidence supporting an overall moderate to high negative effect of livestock grazing on adult grouse numbers and chick production (random effects = −1.12, df = 8, 95% CI: −1.63, −0.59) (Figure 2).
Figure 2.
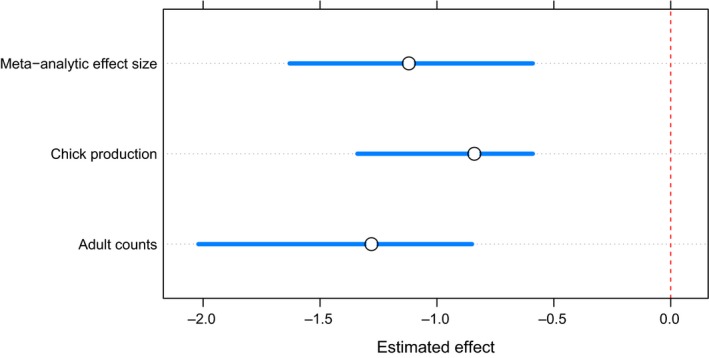
Livestock grazing had a negative effect on Lagopus lagopus scotica and Lyrurus tetrix adult counts and chick production. Estimated effect sizes (circle) and 95% confidence interval (line) of mixed‐effects model results for adult counts, chick production, and pooled mean effect size
We tested total proportion of variance owing to heterogeneity (I 2 = 12.5%, df = 8) for both adult counts and chick production. Our results indicate that the variance among effect sizes were within expected sampling error (Cooper, 1998) and that grazing level is a valid explanatory variable for the model. However, results of Egger's test (z = −3.62, p = .0003) showed that publication bias was an issue within our meta‐analysis (Figure 3).
Figure 3.
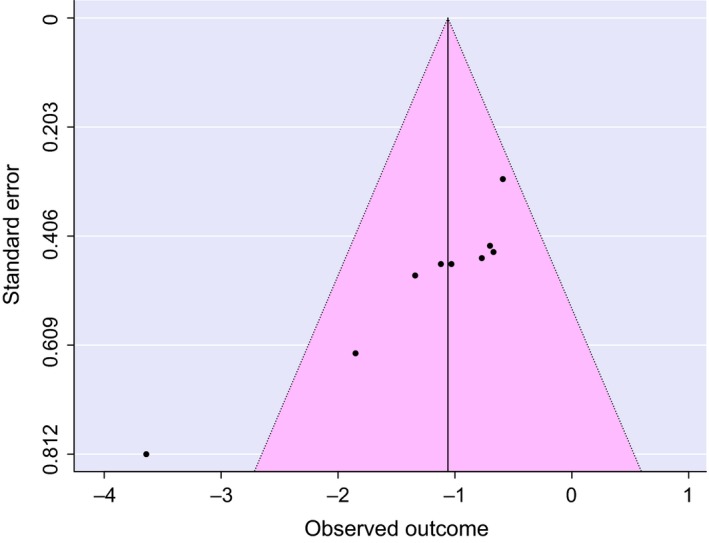
Studies meeting selection criteria demonstrate potential publication bias. Funnel plot of reported effect sizes against precision illustrates the asymmetry and potential bias of study results
4. DISCUSSION
Rangelands provide habitat for a diversity of wildlife and grouse species (Krausman et al., 2009). Livestock grazing is not only the predominant use of rangelands (Alkemade et al., 2013), but has been implicated in declines of grouse populations (Baines, 1996; Boyd et al., 2014; Calladine et al., 2002; Jenkins & Watson, 2001; Warren & Baines, 2004). Our investigation of the influence of grazing on grouse found an overall negative effect on both adult counts and chick production for two populations of European grouse species that are in decline (Baines, 1996; Calladine et al., 2002; Jenkins & Watson, 2001; Jouglet, Ellison, & Léonard, 1999; Storch, 2015). The largest reported individual effect was on adult numbers that resulted from the introduction of heavy sheep grazing into a previously ungrazed area which negatively altered the native vegetation composition (Jenkins & Watson, 2001). This review of the effects of grazing on wildlife suggests that grazing has a general negative effect on the studied grouse populations, and presents some concern for grazing in areas where grouse conservation is a main objective. However, the number of studies that reported a measurable effect of grazing on adult counts and production was limited and many considerations of grazing management warrant discussion.
These studies lend support to concerns that livestock grazing management focused on maximizing meat production through high stocking rates can negatively impact grouse populations (Beck & Mitchell, 2000; Boyd et al., 2011; Silvy & Hagen, 2004) and other wildlife species (Krausman et al., 2009). Our analysis was limited to studies of black (Lyrurus tetrix) and red (Lagopus lagopus scotica) grouse (Figure 4) and lacked studies for NA prairie grouse, Arctic species of ptarmigan, and the forest species of Eurasia. Also, the total number of papers meeting our criterion were limited. There was much specific information on grouse ecology that was lacking from our dataset. This paucity of information highlights a need for more research that directly measures the effects of livestock grazing on grouse. Also, despite efforts to limit issues of publication bias within our meta‐analysis, we could not overcome the scarcity of appropriate studies in the published literature.
Figure 4.

Often considered a subspecies of the willow grouse (Lagopus l. lagopus), red grouse (Lagopus lagopus scotica) are endemic to the heather moorlands of Great Britain
There was consensus in the published literature that overgrazing of rangelands by livestock has predominately negative effects on wildlife and their habitats (Boyd et al., 2011; Krausman et al., 2009; Silvy & Hagen, 2004). However, our meta‐analysis highlighted the general lack of knowledge of the direct effects of livestock grazing needed to develop best management practices (BMPs) for grouse in general and individual species specifically. With so few published studies, it is inappropriate to make broad general statements regarding the impact of livestock grazing on grouse and the BMPs for the conservation of rangelands and grouse populations without further research (Boyd et al., 2011).
The studies we analyzed were missing specific information regarding grazing management practices. They also lacked consistency in the reporting of quantifiable stocking rates for both the treatment and control groups (Baines, 1996; Jenkins & Watson, 2001). Although Calladine et al. (2002) and Jouglet et al. (1999) provided stocking rates for both the treatment and reference sites, this information was not included in their analysis. Additionally, stocking rates were not comparable across biomes. Understanding the effects of stocking rates in similar vegetation communities can help inform land‐use management decisions regarding the effect of grazing management on wildlife (Dahlgren et al., 2015; Krausman et al., 2009).
Livestock grazing systems are a complex combination of factors that include animal type, stocking rate, animal distribution, timing, duration, frequency, and many more (Briske et al., 2008; Heitschmidt & Walker, 1996; Teague et al., 2008; Veblen, Nehring, McGlone, & Ritchie, 2015; Veblen & Young, 2010). Livestock grazing may not be invariably “good” or “bad” for wildlife—rather, there can be positive, negative, or benign effects dependent on aforementioned factors in combination with soil conditions, precipitation, plant community, and the organism of concern (Krausman et al., 2009). Livestock grazing can have direct negative effects on grouse including destruction of habitat, trampling eggs, nest abandonment, and reducing food availability (Beck & Mitchell, 2000). While direct effects are often infrequent (Hovick et al., 2012), indirect effects can be more common and include conversion of habitat to forage, introduction of invasive plant species (Beck & Mitchell, 2000), and subsidizing increased predator densities (Coates et al., 2016).
The role of human dimensions in grazing systems can indirectly contribute to the ecological outcome of grazing systems (Briske et al., 2011). The manner in which livestock grazing is managed affects the structure of rangeland ecosystems, which in turn influences the flows of other ecosystem goods and services from rangelands and ultimately affects wildlife populations (Dahlgren et al., 2015; Heitschmidt & Walker, 1996; Veblen et al., 2015). While grazing has been a part of many researched systems, its effects on wildlife populations are rarely investigated in an explicit and rigorous scientific manner. The effects of livestock grazing are generally diffuse across large landscapes and research of these effects will need to occur on scales that encompass those vast landscapes (Knick et al., 2011).
Future research investigating the effects of livestock grazing on wildlife populations should account for the complex ecological landscape of rangelands. For future research, we provide the following recommendations. Studies should document the (i) livestock type, (ii) timing and frequency of grazing, (iii) duration, and (iv) stocking rate. For example, livestock type has been demonstrated to differentially affect plant composition (Rook et al., 2004) while timing and duration affect vegetation structure (Fischer et al., 2009; Hockett, 2002). These habitat changes have been demonstrated to ultimately affect wildlife biodiversity on rangelands (Alkemade et al., 2013; Krausman et al., 2009). The implementation of standardized measures of vegetation composition cover and height across all studies would help in quantifying the effects on wildlife habitats. Additionally, researchers may need to account and control for other drivers of population and habitat change such as climate and predators (Fuhlendorf, Briske, & Smeins, 2001; Guttery et al., 2013).
CONFLICT OF INTEREST
None declared.
AUTHORS' CONTRIBUTIONS
Seth Dettenmaier, Terry Messmer, Torre Hovick, and Dave Dahlgren conceived the ideas and designed the methodology; Seth Dettenmaier collected and analyzed the data; and Seth Dettenmaier, Terry Messmer, Torre Hovick, and Dave Dahlgren contributed critically to the drafting and revision of the manuscript and gave final approval for publication.
DATA ACCESSIBILITY
All data used in this study were sourced from published studies.
ACKNOWLEDGMENTS
Funding support provided by the USDA‐NRCS Sage‐grouse Initiative, Utah State University Extension, the Quinney Professorship for Wildlife Conflict Management, Jack H. Berryman Institute, Pheasants Forever LLC, Deseret Land and Livestock, USDI Bureau of Land Management, and Utah Department of Wildlife Resources.
Dettenmaier SJ, Messmer TA, Hovick TJ, Dahlgren DK. Effects of livestock grazing on rangeland biodiversity: A meta‐analysis of grouse populations. Ecol Evol. 2017;7:7620–7627. https://doi.org/10.1002/ece3.3287
REFERENCES
- Alexandratos, N. , & Bruinsma, J. (2012). World Agriculture towards 2030/2050: The 2012 revision. Rome: UN Food and Agricultural Organization (FAO). [Google Scholar]
- Alkemade, R. , Reid, R. S. , van den Berg, M. , de Leeuw, J. , & Jeuken, M. (2013). Assessing the impacts of livestock production on biodiversity in rangeland ecosystems. Proceedings of the National Academy of Sciences, 110, 20900–20905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Baan, L. , Alkemade, R. , & Koellner, T. (2012). Land use impacts on biodiversity in LCA: A global approach. The International Journal of Life Cycle Assessment, 18, 1216–1230. [Google Scholar]
- Baines, D. (1996). The implications of grazing and predator management on the habitats and breeding success of black grouse Tetrao tetrix. Journal of Applied Ecology, 33, 54–62. [Google Scholar]
- Beck, J. L. , & Mitchell, D. L. (2000). Influences of livestock grazing on sage grouse habitat. Wildlife Society Bulletin, 28, 993–1002. [Google Scholar]
- Bock, C. E. , Saab, V. A. , Rich, T. D. , & Dobkin, D. S. (1993). Effects of livestock grazing on Neotropical migratory landbirds in western North America, pp. 296–309. US Dept. of Agriculture, Forest Service, Rocky Mountain Research Station. [Google Scholar]
- Borenstein, M. , Hedges, L. V. , Higgins, J. P. T. , & Rothstein, H. R. (2009). Introduction to meta‐analysis. Chichester, UK: John Wiley & Sons Ltd. [Google Scholar]
- Borenstein, M. , Hedges, L. V. , Higgins, J. P. T. , & Rothstein, H. R. (2010). A basic introduction to fixed‐effect and random‐effects models for meta‐analysis. Research Synthesis Methods, 1, 97–111. [DOI] [PubMed] [Google Scholar]
- Boyd, C. S. , Beck, J. L. , & Tanaka, J. A. (2014). Livestock grazing and sage‐grouse habitat: Impacts and opportunities. Journal of Rangeland Applications, 1, 58–77. [Google Scholar]
- Boyd, C. , Petersen, S. , Gilgert, W. , Rodgers, R. , Fuhlendorf, S. , Larsen, R. , … Messmer, T. (2011). Looking toward a brighter future for lekking grouse. Rangelands, 33, 2–11. [Google Scholar]
- Briske, D. D. , Derner, J. D. , Brown, J. R. , Fuhlendorf, S. D. , Teague, W. R. , Havstad, K. M. , … Willms, W. D. (2008). Rotational grazing on rangelands: Reconciliation of perception and experimental evidence. Rangeland Ecology & Management, 61, 3–17. [Google Scholar]
- Briske, D. D. , Sayre, N. F. , Huntsinger, L. , Fernandez‐Gimenez, M. , Budd, B. , & Derner, J. D. (2011). Origin, persistence, and resolution of the rotational grazing debate: Integrating human dimensions into rangeland research. Rangeland Ecology & Management, 64, 325–334. [Google Scholar]
- Butchart, S. H. M. , Walpole, M. , Collen, B. , van Strien, A. , Scharlemann, J. P. W. , Almond, R. E. A. , … Watson, R. (2010). Global biodiversity: Indicators of recent declines. Science, 328, 1164–1168. [DOI] [PubMed] [Google Scholar]
- Calladine, J. , Baines, D. , & Warren, P. (2002). Effects of reduced grazing on population density and breeding success of black grouse in Northern England. Journal of Applied Ecology, 39, 772–780. [Google Scholar]
- Coates, P. S. , Brussee, B. E. , Howe, K. B. , Gustafson, K. B. , Casazza, M. L. , & Delehanty, D. J. (2016). Landscape characteristics and livestock presence influence common ravens: Relevance to greater sage‐grouse conservation. Ecosphere, 7(2), e01203. [Google Scholar]
- Cohen, J. (1988). Statistical power analysis for the behavioral sciences, 2nd ed. Hillsdale, N.J.: Routledge. [Google Scholar]
- Connelly, J. W. , Gratson, M. W. , & Reese, K. P. (1998). Sharp‐tailed grouse (Tympanuchus phasianellus).
- Cooper, H. M. (1998). Synthesizing research: A guide for literature reviews, 3rd ed. Thousand Oaks, CA: Sage Publications. [Google Scholar]
- Dahlgren, D. K. , Larsen, R. T. , Danvir, R. , Wilson, G. , Thacker, E. T. , Black, T. A. , … Messmer, T. A. (2015). Greater sage‐grouse and range management: Insights from a 25‐year case study in Utah and Wyoming. Rangeland Ecology & Management, 68, 375–382. [Google Scholar]
- Del Re, A. C. (2013). Compute.es: Compute effect sizes.
- Efron, B. , & Tibshirani, R. (1986). Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Statistical Science, 1, 54–75. [Google Scholar]
- Egger, M. , Smith, G. D. , Schneider, M. , & Minder, C. (1997). Bias in meta‐analysis detected by a simple, graphical test. BMJ, 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, J. , Stott, J. , Zerger, A. , Warren, G. , Sherren, K. , & Forrester, R. I. (2009). Reversing a tree regeneration crisis in an endangered ecoregion. Proceedings of the National Academy of Sciences, 106, 10386–10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhlendorf, S. D. , Briske, D. D. , & Smeins, F. E. (2001). Herbaceous vegetation change in variable rangeland environments: The relative contribution of grazing and climatic variability. Applied Vegetation Science, 4, 177–188. [Google Scholar]
- Guttery, M. R. , Dahlgren, D. K. , Messmer, T. A. , Connelly, J. W. , Reese, K. P. , Terletzky, P. A. , … Koons, D. N. (2013). Effects of landscape‐scale environmental variation on greater sage‐grouse chick survival. PLoS One, 8(6), e65582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haukos D. A., & Boal C. W. (Eds.) (2016). Ecology and conservation of lesser prairie‐chickens, 1st ed. Boca Raton, FL: Studies in Avian Biology. [Google Scholar]
- Hedges, L. V. (1981). Distribution theory for Glass's estimator of effect size and related estimators. Journal of Educational and Behavioral Statistics, 6, 107–128. [Google Scholar]
- Heitschmidt, R. , & Walker, J. (1996). Grazing management: Technology for sustaining rangeland ecosystems? The Rangeland Journal, 18, 194–215. [Google Scholar]
- Hockett, G. A. (2002). Livestock impacts on the herbaceous components of sage grouse habitat: A review. Intermountain Journal of Sciences, 8, 105–114. [Google Scholar]
- Hovick, T. J. , Elmore, R. D. , Dahlgren, D. K. , Fuhlendorf, S. D. , & Engle, D. M. (2014). Review: Evidence of negative effects of anthropogenic structures on wildlife: A review of grouse survival and behaviour ed C. Elphick. Journal of Applied Ecology, 51, 1680–1689. [Google Scholar]
- Hovick, T. J. , Elmore, R. D. , Fuhlendorf, S. D. , & Dahlgren, D. K. (2015). Weather constrains the influence of fire and grazing on nesting greater prairie‐chickens. Rangeland Ecology & Management, 68, 186–193. [Google Scholar]
- Hovick, T. J. , Miller, J. R. , Dinsmore, S. J. , Engle, D. M. , Debinski, D. M. , & Fuhlendorf, S. D. (2012). Effects of fire and grazing on grasshopper sparrow nest survival. The Journal of Wildlife Management, 76, 19–27. [Google Scholar]
- Jankowski, M. D. , Russell, R. E. , Franson, J. C. , Dusek, R. J. , Hines, M. K. , Gregg, M. , & Hofmeister, E. K. (2014). Corticosterone metabolite concentrations in greater sage‐grouse are positively associated with the presence of cattle grazing. Rangeland Ecology & Management, 67, 237–246. [Google Scholar]
- Jenkins, D. , & Watson, A. (2001). Bird numbers in relation to grazing on a grouse moor from 1957–61 to 1988–98. Bird Study, 48, 18–22. [Google Scholar]
- Jetz, W. , Wilcove, D. S. , & Dobson, A. P. (2007). Projected impacts of climate and land‐use change on the global diversity of birds. PLoS Biol., 5(6), e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnsgard, P. A. (1983). The grouse of the world, 1st ed. Lincoln: University of Nebraska Press. [Google Scholar]
- Jouglet, J. P. , Ellison, L. , & Léonard, P. (1999). Impact du pâturage ovin estival sur l'habitat et les effectifs du tétras lyre (Tetrao tetrix) dans les Hautes‐Alpes. Gibier Faune Sauvage, 16, 289–316. [Google Scholar]
- Kantrud, H. A. , & Kologiski, R. L. (1982). Effects of soils and grazing on breeding birds of uncultivated upland grasslands of the northern great plains. Washington, DC: Federal Government Series, U.S. Fish and Wildlife Service. [Google Scholar]
- Knick S. T., & Connelly J. W. (Eds.) (2011). Greater sage‐grouse: Ecology and conservation of a landscape species and its habitats, 1st ed. Berkeley, CA: University of California Press. [Google Scholar]
- Knick, S. T. , Hanser, S. E. , Miller, R. F. , Pyke, D. A. , Wisdom, M. J. , Finn, S. P. , … Henny, C. J. (2011). Ecological influence and pathways of land use in sagebrush. Studies in Avian Biology, 38, 203–251. [Google Scholar]
- Krausman, P. R. , Naugle, D. E. , Frisina, M. R. , Northrup, R. , Bleich, V. C. , Block, W. M. , … Wright, J. D. (2009). Livestock grazing, wildlife habitat, and rangeland values. Rangelands, 31, 15–19. [Google Scholar]
- Lund, H. G. (2007). Accounting for the world's rangelands. Rangelands, 29, 3–10. [Google Scholar]
- McNew, L. B. , Winder, V. L. , Pitman, J. C. , & Sandercock, B. K. (2015). Alternative rangeland management strategies and the nesting ecology of greater prairie‐chickens. Rangeland Ecology & Management, 68, 298–304. [Google Scholar]
- Owens, R. A. , & Myres, M. T. (1973). Effects of agriculture upon populations of native passerine birds of an Alberta fescue grassland. Canadian Journal of Zoology, 51, 697–713. [Google Scholar]
- R Development Core Team . (2015). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- Rook, A. J. , Dumont, B. , Isselstein, J. , Osoro, K. , WallisDeVries, M. F. , Parente, G. , & Mills, J. (2004). Matching type of livestock to desired biodiversity outcomes in pastures—a review. Biological Conservation, 119, 137–150. [Google Scholar]
- Rosenberg, M. S. , Adams, D. C. , & Gurevitch, J. (2000). Metawin: Statistical software for meta‐analysis. Sunderland, MA, USA: Sinauer Associates. [Google Scholar]
- Rothstein, H. R. , Sutton, A. J. , & Borenstein, M. (2006). Publication bias in meta‐analysis: Prevention, assessment and adjustments. New York: John Wiley & Sons. [Google Scholar]
- Sala, O. E. , Chapin, F. S. III , Armesto, J. J. , Berlow, E. , Bloomfield, J. , Dirzo, R. , … Wall, D. H. (2000). Global biodiversity scenarios for the year 2100. Science, 287, 1770–1774. [DOI] [PubMed] [Google Scholar]
- Silvy, N. J. , & Hagen, C. A. (2004). Introduction: Management of imperiled prairie grouse species and their habitat. Wildlife Society Bulletin, 32, 2–5. [Google Scholar]
- Sisk, T. D. , Launer, A. E. , Switky, K. R. , & Ehrlich, P. R. (1994). Identifying extinction threats. BioScience, 44, 592–604. [Google Scholar]
- Speedy, A. W. (2003). Global production and consumption of animal source foods. The Journal of Nutrition, 133, 4048S–4053S. [DOI] [PubMed] [Google Scholar]
- Storch I. (Ed.) (2007). Grouse: Status survey and conservation action plan 2006–2010. IUCN. Gland, Switzerlandand: IUCN; and Fordingbridge, UK: World Pheasant Association. [Google Scholar]
- Storch, I. (2015). Conservation status and threats to grouse worldwide: An update.
- Teague, W. R. , Provenza, F. D. , Norton, B. , Steffens, T. , Barnes, M. , Kothmann, M. , & Roath, R. (2008). Benefits of multi‐paddock grazing management on rangelands: Limitations of experimental grazing research and knowledge gaps In H. G Schröder. (Ed.), Grasslands: Ecology, Management and Restoration (pp. 41–80). New York: Nova Science Pub Inc. [Google Scholar]
- The IUCN Red List of Species. Version 2015‐04 . (2015) http://www.iucnredlist.org/
- Thornton, P. K. (2010). Livestock production: Recent trends, future prospects. Philosophical Transactions of the Royal Society B: Biological Sciences, 365, 2853–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Fish and Wildlife Service . (2014). Endangered and threatened wildlife and plants; threatened status for Gunnison sage‐grouse. Federal Register, 79, 69192–69310. [Google Scholar]
- U.S. Fish and Wildlife Service . (2015). Endangered and threatened wildlife and plants; 12‐month finding on a petition to list greater sage‐grouse (Centrocercus urophasianus) as an endangered or threatened species. Federal Register, 80, 59858–59942. [Google Scholar]
- Veblen, K. E. , Nehring, K. C. , McGlone, C. M. , & Ritchie, M. E. (2015). Contrasting effects of different mammalian herbivores on sagebrush plant communities. PLoS ONE, 10(2), e0118016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veblen, K. E. , & Young, T. P. (2010). Contrasting effects of cattle and wildlife on the vegetation development of a savanna landscape mosaic. Journal of Ecology, 98, 993–1001. [Google Scholar]
- Warren, P. , & Baines, D. (2004). Black grouse in Northern England: Stemming the decline. British Birds, 97, 183–189. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this study were sourced from published studies.


