Abstract
Objectives
To evaluate the significance of serum chromogranin A (CgA) status in patients with and without different neuroendocrine tumors (NETs) by conducting a retrospective assessment of the diagnostic utility and limitations of CgA as a biomarker for NETs in a tertiary care hospital in Oman.
Methods
We conducted a retrospective analysis of CgA requests referred to the Clinical Biochemistry Laboratory, Royal Hospital, Oman over a 24-month period (April 2012 to March 2014). During this time, 302 CgA tests for 270 patients (119 males and 151 females; age range 11–86 years and mean±standard deviation (SD) 44.0±18.0 years), were requested. Of these CgA tests, 245 tests were performed for 245 patients investigated for the diagnosis of NETs, and 57 CgA tests were performed for 25 patients with diagnosed NETs who were undergoing follow-up. Serum CgA levels were analyzed using the enzyme-linked immunosorbent assay based on a cut-off value of 22 IU/L.
Results
Of the 302 CgA tests reviewed, 197 (65.2%) were within the quoted normal range; however, 105 (34.8%) had CgA > 22 IU/L. Of the 245 patients with first-line CgA, 38 patients (15.5%) had NET that included carcinoid, pheochromocytoma, pancreatic NET, adrenal adenoma, prostatic adenocarcinoma, gastrointestinal NET, medullary thyroid carcinoma, Schwannoma, lung small cell carcinoma, parathyroid adenoma, and pituitary macroadenoma. The mean±SD of CgA in these patients with NETs was 205.0±172.0 IU/L. Meanwhile, there were 45 (18.3%) patients with CgA > 22 IU/L (83.0±116.0 IU/L) who did not have NETs. The conditions/diseases included: essential hypertension, chronic kidney disease, heart failure, peptic ulcer, chronic diarrhea, use of proton pump inhibitors, and other chronic diseases (hypothyroidism, asthma, diabetes mellitus). Of the 25 patients with known NET who were followed-up, there were 57 CgA results (29 with CgA ≤ 22 IU/L and 28 with CgA > 22 IU/L). The overall clinical sensitivity of CgA in the diagnosis of NETs was 84.2%, overall specificity was 78.2%, positive predictive value was 41.5%, negative predictive value was 96.4%, and overall efficiency was 79.2%. In patients with individual NET, a good reflection in CgA was noticed in the follow-up period following surgery or therapy.
Conclusions
Serum CgA is a sensitive and effective noninvasive laboratory test for the clinical detection and management of NETs. Awareness of the pitfalls of the tests in patients with non-NET conditions, particularly chronic diseases and use of certain drugs, is important to be considered during the interpretation of the CgA levels.
Keywords: Chromogranin A; Neuroendocrine Tumor; Pheochromocytoma; Carcinoid Tumor; Markers, Tumor
Introduction
Neuroendocrine tumors (NETs) are a heterogeneous group of tumors that are often clinically silent. Diagnosis is usually delayed, and the majority of cases present late with metastases.1 Recently, there has been a growing interest in the use of circulating biomarkers (which are also often used in immunohistochemistry) for the diagnosis and follow-up of patients with NETs.2 Measurement of these markers has been contributing in the workup plan towards improving the management outcome of patients with NETs.
Chromogranins, including chromogranin A (CgA), are members of the granin family of neuroendocrine secretory proteins located in the secretory vesicles of neurons and endocrine cells.3 In addition to the primary function chromogranins in granulogenesis of vesicle formation, CgA is the precursor for many functional peptides such as vasostatin, pancreastatin, catestatin, parastatin, serpenin, and other peptides that have many physiological functions (including autocrine, paracrine, and endocrine) in the corresponding neuroendocrine physiology.4,5
The most studied chromogranin is CgA, which was first isolated from chromaffin cells of the adrenal medulla as a single polypeptide chain of 439 amino acids and 10 dibasic cleavage sites. Although CgA is most abundant in chromaffin cells of the adrenal medulla, it was then found to be present in a variety of neuroendocrine tissues including the gastrointestinal tract (GIT), pancreas, parathyroid, reproductive system, adipocytes, cardiovascular, and immune system.4,5 CgA is often secreted by NETs, and its concentration in the circulation can be measured to provide information for the diagnosis, prognosis, and monitoring of patients with these tumors if other non-NET physiological, pathological, and pharmacological causes are excluded or considered in the interpretation.
The objectives of this study were to evaluate the significance of serum CgA status in patients with and without different NETs. We conducted a retrospective assessment of the diagnostic utility and limitations of CgA as a biomarker for NETs in a tertiary care hospital.
Methods
We conducted a retrospective analysis of CgA requests referred to the Clinical Biochemistry Laboratory, Royal Hospital, Oman during a two-year period (April 2012 to March 2014). Ethical approval for conducting this work was obtained from the Research and Ethical Review Committee, Directorate of Research and Studies, Royal Hospital (MESRC#9/2016 on 5 January 2016).
Requests for serum CgA that were made as a part of workup investigations for patients with suspected NETs either as an initial screening, work-up, or follow-up were reviewed. The requests involved those referred to the Royal Hospital from all regional hospitals of the Ministry of Health as the CgA test is only provided at the Royal Hospital Biochemistry Laboratory. We excluded from the review those requests with no clinical details and only the initial request was considered for any duplicate requests.
Serum CgA levels were assayed by a commercially-available enzyme-linked immunosorbent assay (ELISA)(Dako, Glostrup, Denmark) based on a cut-off value of 22 IU/L. The results are reported using Al-Shifa information system, and data analysis for this study was done using Microsoft Excel (2010).
During the two-year period, there were 302 CgA tests for 270 patients (119 males and 151 females; aged 11–86 years, mean±standard deviation (SD) 44.0±18.0 years). Of these, 245 tests were done for 245 patients who were investigated for the diagnosis of NETs, and 57 CgA tests for 25 patients with already diagnosed NETs who were undergoing follow-up.
Results
Of the 302 CgA tests reviewed, 197 (65.2%) results were within the quoted normal range. However, 105 (34.8%) results had CgA > 22 IU/L. Of the 245 patients having first-line CgA, 38 patients (15.5%) had newly diagnosed NET or a related tumor (5 carcinoid, 3 pheochromocytoma, 5 pancreatic NET, 7 adrenal adenoma, 2 prostatic adenocarcinoma, 2 GIT NET, 1 medullary thyroid carcinoma, 1 Schwannoma, 2 lung small cell carcinoma, 2 parathyroid adenoma, and 8 pituitary macroadenoma). The mean±SD of CgA in these patients was 205.0±172.0 IU/L. Of the 25 patients with known NET who were followed-up, there were 57 CgA results (29 with CgA ≤ 22 IU/L and 28 with CgA > 22 IU/L). The mean±SD, median (range), and proportion of abnormal results of CgA in patients with different NET are shown in Table 1.
Table 1. Serum chromogranin A (CgA, IU/L) and proportion of abnormal results in patients with different neuroendocrine tumors (NETs) and related tumors.
| NET | n | Newly diagnosed | n | Follow-up | ||
|---|---|---|---|---|---|---|
| Mean ± SD Median (range) |
Proportion of abnormal | Mean ± SD Median (range) |
Proportion of abnormal | |||
| Carcinoid | 5 | 260.0 ± 237.9 262 (50–466) |
5/5 | 24 | 100.0 ± 140.9 14 (2–466) |
12/24 |
| Pheochromocytoma | 3 | 266.0 ± 80.6 266 (209–323) |
3/3 | 9 | 96.0 ± 114.0 44 (8–300) |
6/9 |
| Paraganglioma | 0 | - | - | 7 | 165.0 ± 338.0 21 (2–466) |
2/7 |
| Pancreatic NET | 5 | 360.0 ± 183.2 443 (87–466) |
5/5 | 3 | 159.0 ± 153.0 67 (26.9–435) |
3/3 |
| Adrenal adenoma | 7 | 106.0 ± 119.0 106 (27–309) |
7/7 | 2 | 74.0 ± 110.0 111 (10–201) |
1/2 |
| Prostate carcinoma | 2 | 34.0 ± 8.5 34 (28–40) |
2/2 | 0 | - | - |
| GI NET | 2 | 262.5 ± 287.8 262 (59–466) |
2/2 | 0 | - | - |
| Medullary thyroid Carcinoma | 1 | 51 | 1/1 | 1 | 89 | 1/1 |
| Shwannoma | 1 | 153 | 1/1 | 0 | - | - |
| Small cell carcinoma | 2 | 60.0 ± 18.3 60 (47–73) |
2/2 | 3 | 15.0 ± 11.0 16 (4–27) |
1/3 |
| Parathyroid adenoma | 2 | 569.0 ± 243.9 285 (112–457) |
2/2 | 5 | 28.7 ± 4.5 29 (24–33) |
2/5 |
| Pituitary macroadenoma | 8 | 13.0 ± 11.0 14 (2–32) |
2/8 | 2 | 13.5 ± 12.0 13.5 (5–22) |
0/2 |
| Inulinoma | 0 | - | - | 1 | 17 | 0/1 |
| Total | 38 | - | 32/38 | 57 | - | 28/57 |
Data presented as mean±standard deviation (SD), median (range), and proportion of change compared with assay cut-off of 22 IU/L. GI: gastrointestinal.
There were 45 (18.%) patients with CgA > 22 IU/L (83.0±116.0 IU/L) who did not have NETs. The conditions/diseases noted in these patients included essential hypertension, chronic kidney disease (CKD), heart failure, peptic ulcer, chronic diarrhea, use of proton pump inhibitors (PPI) and other chronic diseases (hypothyroidism, asthma, and diabetes mellitus) [Table 2].
Table 2. Clinical characteristics of patients with non-NET conditions who had raised chromogranin A (CgA > 22 IU/L).
| Clinical condition/disease | n | Median (range) | Proportion of increase (X) |
|---|---|---|---|
| CKD (eGFR< 60 mL/min/1.73 m2) | 10 | 256 (23–466) | 11.6 |
| Heart failure | 2 | 204 (151–258) | 9.3 |
| Chronic diarrhea | 1 | 146 | 6.6 |
| Peptic ulcer | 2 | 67 (60–74) | 3.0 |
| Hypertension | 16 | 39 (25–97) | 1.8 |
| Other diseases (asthma, hypothyroidism, diabetes mellitus) | 11 | 34 (29–36) | 1.5 |
| Proton pump inhibitors | 3 | 70 (59–77) | 3.2 |
Data presented as median (range), and proportion of increase in CgA compared with assay cut-off of 22 IU/L.
NET: neuroendocrine tumor; CKD: chronic kidney disease.
In the 245 patients with and without NETs (excluding the 25 patients with established NETs who were on follow-up), the overall clinical sensitivity of CgA in the diagnosis of NETs was 84.2%. The overall specificity was 78.2%, positive and negative predictive values were 41.5% and 96.4%, respectively, and overall efficiency was 79.2% [Table 3]. All patients with carcinoid, pheochromocytoma, pancreatic NET, prostatic adenocarcinoma, GIT NET, medullary thyroid carcinoma, schwannoma, lung small cell carcinoma, adrenal adenoma, and parathyroid adenoma had raised CgA values. However, only two of six patients with pituitary macroadenoma had raised CgA values > 22 IU/L.
Table 3. Contingency data for the overall validity indicators of serum chromogranin A (CgA) in the diagnosis of overall neuroendocrine tumors (NETs) the study population.
| CgA result | NETs present | ||
|---|---|---|---|
| Yes | No | Total | |
| Raised | 32 | 45 | 77 |
| Normal | 6 | 162 | 168 |
| Total | 38 | 207 | 245 |
*38 patients with newly diagnosed NET were included: pheochromocytoma, carcinoid, parathyroid adenoma, pancreatic NET, shwannoma, medullary thyroid cancer, GIT NET, adrenal adenoma, prostate cancer, small cell lung cancer, and pituitary macroadenoma. **57 patients with established NET who were on follow up treatment were excluded from validity study. ***Overall validity indicators: sensitivity 84.2%, specificity 78.2%, positive predictive value 41.5%, negative predictive value 96.4%, and efficiency 79.2%.
In patients with individual NET, a good response in CgA was noticed during the follow-up period following surgery or therapy. The CgA results in the follow-up of four patients with different NETs (paraganglioma, parathyroid adenoma, pheochromocytoma, and carcinoid) are presented [Figure 1].
Figure 1.
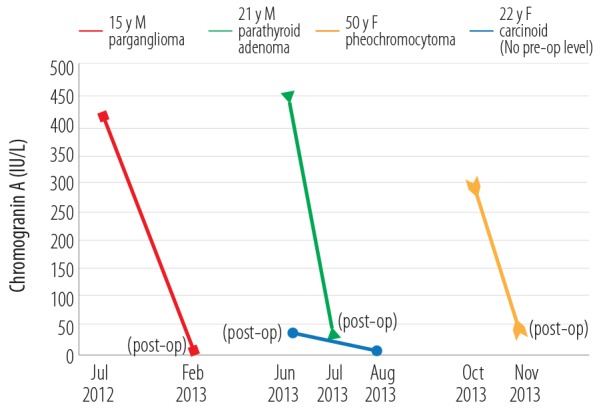
Follow-up of serum chromogranin A (IU/L) levels in four patients with neuroendocrine tumors
Discussion
Using a cohort of patients with and without different NETs, we evaluated the use of serum CgA levels in different patients investigated at the Royal Hospital. We also assessed the relevance of the presence of NET or non-NET conditions in patients with raised serum CgA levels. The overall diagnostic sensitivity and other validity indicators for CgA in the different NETs were also assessed. The pattern of CgA change in the follow-up of patients undergoing treatment for different NETs was evaluated.
In the present study, serum CgA levels were clearly and markedly raised in all patients with newly diagnosed NETs: carcinoid (5/5), pheochromocytoma (3/3), pancreatic (5/5), GIT NET (2/2), and parathyroid adenoma (2/2). CgA was moderately raised in all patients with adrenal adenomas (7/7), prostatic adenocarcinoma (2/2), medullary thyroid carcinoma (1/1), Schwannoma (1/1), and small cell lung carcinoma (2/2). However, in only two of eight patients with pituitary adenoma CgA level was mildly raised, which is expected since these tumors are not classically considered NETs. Because CgA is present in NE tissues, this peptide will be produced and released into the circulation of patients with NETs.4,5 The highest mean values were observed in patients with carcinoid, pheochromocytoma, pancreatic/GIT NET, and parathyroid adenoma. The mean values were more than 10-fold the upper limit of normal range.
Our data indicate that serum CgA measurement may be a useful laboratory tool for the diagnosis and follow-up of patients with these NETs. In the follow-up patients series, CgA levels were raised in 28/57 (49.1%) results. Also, in four patients with paraganglioma, pheochromocytoma, parathyroid adenoma, and carcinoid tumors, who were monitored throughout their treatment course, CgA dropped sharply in the post-treatment phase within days, weeks or months, depending on the tumor.
Based on CgA cut-off value of 22 IU/L, we observed an overall diagnostic sensitivity of 84.2% for serum CgA test in diagnosing NETs in our patients cohort. However, the sensitivity may be higher (> 95%) in the diagnosis of individual NETs particularly pheochromocytoma, paraganglioma, pancreatic/GI NET, parathyroid adenoma, and carcinoid in which all patients in our cohort had raised CgA. False-negative results were not obtained in patients with these specified NETs; only 6/8 false-negative results were noted in patients with pituitary adenoma. The overall negative predictive value (NPV) was 96.4%.
On the other hand, the positive predictive value (PPV) was 41.5%, which is explained by the high rate of false-positive results in patients with end-stage organ dysfunction (e.g., CKD, heart failure) where a moderate increase in CgA was reported. Drug use (particularly PPI and possibly anti-hypertension drugs) and chronic diseases (such as diabetes mellitus, hypothyroidism, asthma, and GI diseases) exhibited a mild increase in CgA. This increase in CgA in diseases/conditions of non-NETs nature has limited the overall specificity of CgA in our cohort to 78.2%. These data demonstrated that serum CgA has reasonable sensitivity and specificity that makes the test valuable in the diagnosis and management of NETs. Patients with established NETs who were monitored by serum CgA showed a good response parallel to clinical improvement following surgery or therapy. Serum CgA can be used as a screening or monitoring test for NET in combination with the specific metabolic test for that particular NET (e.g., plasma fractionated metanephrines for pheochromocytoma, plasma 5-hydroxyindoleacetic acid for carcinoid, plasma gastrin for gastrinomas, plasma glucagon for glucagonomas) and necessary radiological investigation(s), all of which will help to diagnose the burden and progress of the tumor.
The common nature of many chronic diseases, particularly cardiac and renal dysfunction or failure and use of drugs particularly PPIs, has limited the diagnostic yield of CgA as a biomarker for NETs. Many have considered its clinical utility in cardiovascular diseases particularly for identifying those at risk of developing short- and long-term mortality using CgA alone or in combination with other markers such as the natriuretic peptide or the use of a multianalyte algorithm.6-8 One has to be aware of pitfalls in the interpretation of the CgA test, which also raises the notion towards the importance of the negative test result which will point more strongly to the exclusion of NET. Other researchers have reported comparable reports of false-positive and false-negative results of CgA when used to diagnose NETs.9,10
In comparison with other studies, Yang et al,11 in their 13 studies search that included 1 260 patients with NETs and 967 healthy subjects reported an overall sensitivity of 73%, specificity of 95% and diagnostic odds ratio of 56.3, with positive and negative likelihood ratios of 14.56 and 0.26, respectively. They confirmed CgA as an efficient marker for the clinical management of NETs.Lyubimova et al,12 in their study of 227 patients with NETs of various locations and 66 normal subjects reported an overall diagnostic sensitivity of 85.8% and specificity of 98.5% confirming the high efficiency of CgA as a marker of NET. Maximum CgA concentrations were detected in patients with NETs of the stomach and lung, and the highest median CgA values were found in patients with tumors of the small intestine, large intestine, and pancreas, particularly in patients with liver metastases and carcinoid syndrome. Chou et al,13 in their study of 44 gastroenteropancreatic-NET and 26 healthy subjects reported a sensitivity of 86% and a specificity of 88%. CgA levels at 110 U/L differentiated patients without recurrence from those with recurrence with a sensitivity of 100% and a specificity of 80%. All five patients with stable disease showed partial response after treatment with a more than 20% decrease in CgA levels compared with baseline values, and all six patients with progressive disease showed more than a 20% increase in CgA levels.
Finally, it is worth mentioning that this is the first study which evaluated the overall validity of CgA in the diagnosis of different NETs in a tertiary care hospital in Oman. The results of this study support the usefulness of this non-invasive laboratory tool as a biomarker for work-up investigations of patients with NETs. However, the limitations of this study were mainly related to its retrospective nature and the relatively small sample size for patients with specified NETs as per their locations. Our study needs to be substantiated in a large group of patients and over a longer time to have more data for each location-derived NET including their classification according to tumor burden, progression, and fate.
Conclusion
We have shown that measurement of serum CgA is a sensitive and effective non-invasive laboratory test for the clinical detection and management of NETs. Awareness of the pitfalls of the test in patients with non-NET conditions, particularly chronic diseases such as heart failure or renal failure, and use of certain drugs, especially proton pump inhibitors, is important to consider when interpreting CgA levels.
Disclosure
The authors declared no conflicts of interest. No funding was received for this study.
Acknowledgements
The authors want to thank Novartis Pharma AG for their support in providing Chromogranin A assay kits throughout the study.
References
- 1.Pape UF, Berndt U, Müller-Nordhorn J, Böhmig M, Roll S, Koch M, et al. Prognostic factors of long-term outcome in gastroenteropancreatic neuroendocrine tumours. Endocr Relat Cancer 2008. Dec;15(4):1083-1097. 10.1677/ERC-08-0017 [DOI] [PubMed] [Google Scholar]
- 2.O’Toole D, Grossman A, Gross D, Delle Fave G, Barkmanova J, O’Connor J, et al. Mallorca Consensus Conference participants. European Neuroendocrine Tumor Society Mallorca Consensus Conference participants; European Neuroendocrine Tumor Society. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: Biochemical markers. Neuroendocrinology 2009;90(2):194-202. 10.1159/000225948 [DOI] [PubMed] [Google Scholar]
- 3.Helman LJ, Ahn TG, Levine MA, Allison A, Cohen PS, Cooper MJ, et al. Molecular cloning and primary structure of human chromogranin A (secretory protein I) cDNA. J Biol Chem 1988. Aug;263(23):11559-11563. [PubMed] [Google Scholar]
- 4.Eskeland NL, Zhou A, Dinh TQ, Wu H, Parmer RJ, Mains RE, et al. Chromogranin A processing and secretion: specific role of endogenous and exogenous prohormone convertases in the regulated secretory pathway. J Clin Invest 1996. Jul;98(1):148-156. 10.1172/JCI118760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’amico MA, Ghinassi B, Izzicupo P, Manzoli L, Di Baldassarre A. Biological function and clinical relevance of chromogranin A and derived peptides. Endocr Connect 2014. Apr;3(2):R45-R54. 10.1530/EC-14-0027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kidd M, Bodei L, Modlin IM. Chromogranin A: any relevance in neuroendocrine tumors? Curr Opin Endocrinol Diabetes Obes 2016. Feb;23(1):28-37. 10.1097/MED.0000000000000215 [DOI] [PubMed] [Google Scholar]
- 7.Goetze JP, Hilsted LM, Rehfeld JF, Alehagen U. Plasma chromogranin A is a marker of death in elderly patients presenting with symptoms of heart failure. Endocr Connect 2014. Mar;3(1):47-56. 10.1530/EC-14-0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Penna C, Tullio F, Perrelli MG, Mancardi D, Pagliaro P. Cardioprotection against ischemia/reperfusion injury and chromogranin A-derived peptides. Curr Med Chem 2012;19(24):4074-4085. 10.2174/092986712802429966 [DOI] [PubMed] [Google Scholar]
- 9.Sanduleanu S, De Bruïne A, Stridsberg M, Jonkers D, Biemond I, Hameeteman W, et al. Serum chromogranin A as a screening test for gastric enterochromaffin-like cell hyperplasia during acid-suppressive therapy. Eur J Clin Invest 2001. Sep;31(9):802-811. 10.1046/j.1365-2362.2001.00890.x [DOI] [PubMed] [Google Scholar]
- 10.Spadaro A, Ajello A, Morace C, Zirilli A, D’arrigo G, Luigiano C, et al. Serum chromogranin-A in hepatocellular carcinoma: diagnostic utility and limits. World J Gastroenterol 2005. Apr;11(13):1987-1990. 10.3748/wjg.v11.i13.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang X, Yang Y, Li Z, Cheng C, Yang T, Wang C, et al. Diagnostic value of circulating chromogranin a for neuroendocrine tumors: a systematic review and meta-analysis. PLoS One 2015. Apr;10(4):e0124884. 10.1371/journal.pone.0124884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lyubimova NV, Churikova TK, Kushlinskii NE. Chromogranin As a Biochemical Marker of Neuroendocrine Tumors. Bull Exp Biol Med 2016. Mar;160(5):702-704. 10.1007/s10517-016-3254-0 [DOI] [PubMed] [Google Scholar]
- 13.Chou WC, Hung YS, Hsu JT, Chen JS, Lu CH, Hwang TL, et al. Chromogranin A is a reliable biomarker for gastroenteropancreatic neuroendocrine tumors in an Asian population of patients. Neuroendocrinology 2012;95(4):344-350. 10.1159/000333853 [DOI] [PubMed] [Google Scholar]


