Abstract
In vivo pretargeting stands as a promising approach to harnessing the exquisite tumor-targeting properties of antibodies for nuclear imaging and therapy while simultaneously skirting their pharmacokinetic limitations. The core premise of pretargeting lies in administering the targeting vector and radioisotope separately and having the 2 components combine within the body. In this manner, pretargeting strategies decrease the circulation time of the radioactivity, reduce the uptake of the radionuclide in healthy nontarget tissues, and facilitate the use of short-lived radionuclides that would otherwise be incompatible with antibody-based vectors. In this short review, we seek to provide a brief yet informative survey of the 4 preeminent mechanistic approaches to pretargeting, strategies predicated on streptavidin and biotin, bispecific antibodies, complementary oligonucleotides, and bioorthogonal click chemistry.
Keywords: pretargeting, multistep targeting, click chemistry, bispecific antibody, streptavidin, biotin
The specificity and affinity of antibodies have long made them enticing vectors for the delivery of diagnostic and therapeutic radionuclides to malignant tissue. Yet one of the fundamental traits that makes immunoglobulins effective as agents of the immune response can spell trouble in the context of nuclear medicine. Antibodies have evolved to possess long serum half-lives, undoubtedly a benefit in the context of detecting foreign antigens. However, this means that when antibodies are harnessed as biomedical vectors, they can take days or even weeks to reach their optimal biodistribution in vivo and must therefore be labeled with radionuclides with multiday physical half-lives. Although the use of isotopes such as 89Zr (half-life, 3.3 d) and 177Lu (half-life, 6.7 d) ensures that radioactivity remains after the antibody has reached its target, the choice of these nuclides is a double-edged sword. The prolonged circulation of radioimmunoconjugates bearing long-lived radionuclides creates significant clinical complications: supobtimal therapeutic indices for radioimmunotherapy and high radiation doses to healthy tissue for antibody-based PET.
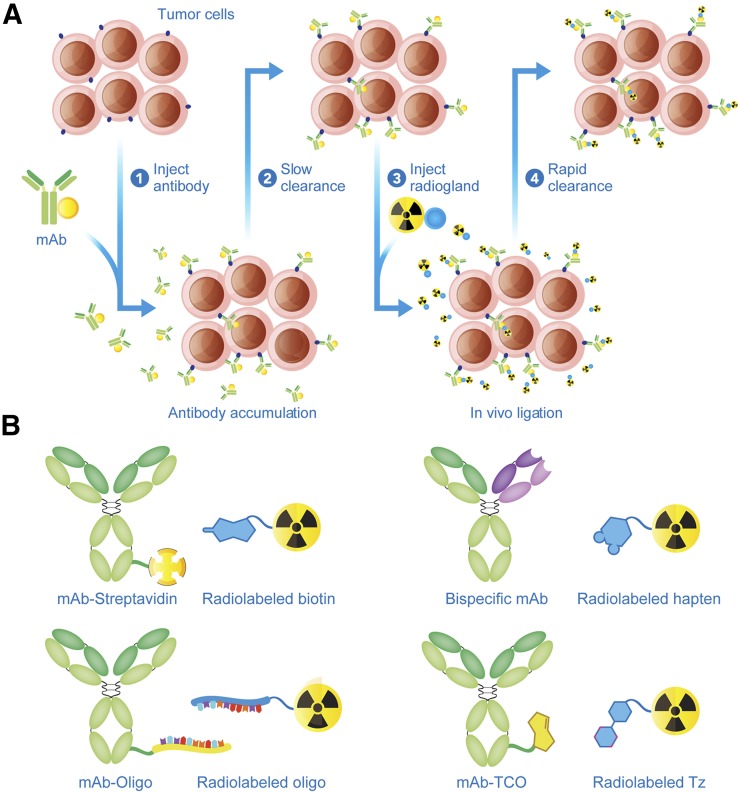
A tremendous amount of effort has been dedicated to circumventing these obstacles. One approach has centered on bioengineering lower-molecular-weight immunoglobulins with more rapid excretion rates. Yet despite the promise of this avenue, radiolabeled antibody fragments are often hampered by suboptimal tumor uptake and high retention in the kidneys. An alternative solution lies in the topic of this work: in vivo pretargeting. Conceived in 1985 and first executed 2 y later, pretargeting is founded on a simple yet radical premise: decoupling the antibody and the radioactivity (1,2). The 2 components are injected separately and combine within the body, in essence performing radiosynthesis at the tumor itself. A variety of mechanistic platforms for pretargeting have been developed, yet all share 2 common components: a radioligand and an antibody capable of binding both a tumor antigen and said radioligand. Generally speaking, pretargeting strategies have 4 steps: first, the injection of the antibody; second, the slow accumulation of the antibody at the tumor and its concomitant clearance from the blood; third, the injection of the radioligand; and fourth, the in vivo ligation of the antibody and the radioligand, followed by the rapid clearance of any excess radioligand (Fig. 1A). Some methodologies feature an additional step before the injection of the radioligand, the administration of a clearing agent designed to accelerate the removal of residual immunoconjugate from the bloodstream. Furthermore, conventional wisdom dictates that vectors for pretargeting should not be internalized upon binding their target, though a handful of recent investigations indicates that pretargeting is possible with internalizing systems. Details aside, injecting the radionuclide and immunoglobulin separately decreases the circulation time of radioactivity in the body, reduces the uptake of the radioisotope in healthy tissues, and facilitates the use of short-lived radionuclides (e.g., 68Ga) that would normally be incompatible with antibody-based vectors. Taken together, these traits translate to improved tumor-to-background activity concentration ratios and dramatically lowered radiation dose rates to healthy tissues.
FIGURE 1.
Schematic for in vivo pretargeting (A) and the 4 principal mechanisms of in vivo pretargeting (B).
The central problem at the core of any pretargeting strategy lies in how to recombine the antibody and radioligand in vivo. The interaction between the 2 components must be exquisitely selective, as each moiety has myriad other possible reaction partners within the body. Over the years, 4 major approaches have emerged. Each relies on a different in vivo ligation mechanism: the noncovalent interaction between streptavidin and biotin, the ability of bispecific antibodies (bsAbs) to bind both an antigen and a radiolabeled hapten, the hybridization of complementary oligonucleotides, and the bioorthogonal inverse electron demand Diels-Alder (IEDDA) click reaction (Fig. 1B). In this review, our goal is to provide a brief introduction to these approaches. Critically, we do not strive for an exhaustive treatment of any of these methods, as others have already done this with impressive depth and clarity (3,4). Rather, our mission is to provide a bird’s-eye view meant for experienced researchers and novices alike, in the hope that this primer will not only spur enthusiasm for pretargeting but also inspire innovation that will drive the field in the years to come.
APPROACH 1: STREPTAVIDIN AND BIOTIN
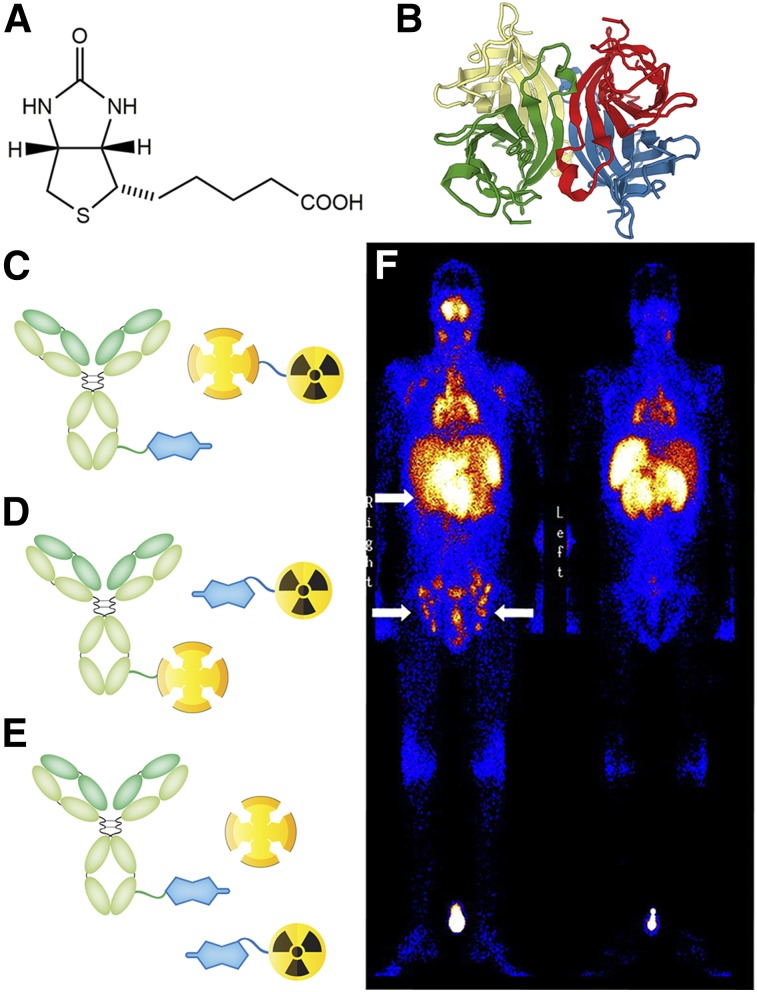
Pretargeting approaches based on the noncovalent interaction between biotin and streptavidin were among the earliest strategies to emerge and the first to appear in the clinic. Biotin is a 244-Da small molecule and an essential coenzyme for carboxylases (Fig. 2A). Streptavidin and avidin are tetrameric proteins composed of 4 monomers, each capable of binding a single biotin (Fig. 2B). The noncovalent interactions between biotin and these proteins are among the strongest observed in the natural world, with binding constants approaching 1015 M−1. Not surprisingly, both avidin and streptavidin have been leveraged for pretargeting; however, our discussion here will focus primarily on streptavidin, as it has been the subject of wider use in clinical trials (2,5).
FIGURE 2.
Structure of biotin (A); ribbon structure of the streptavidin tetramer (B); 3 different types of streptavidin-biotin pretargeting strategies (C–E); and γ-camera image of a patient with B-cell Hodgkin lymphoma injected with an anti-CD20-SA fusion protein and, 24 h later, 111In-DOTA-biotin (F). Sites of active tumor involvement are indicated by arrows. (Reprinted with permission of (10).)
Three different approaches to biotin and streptavidin pretargeting have been developed. In the first, a biotin-bearing antibody and a radiolabeled streptavidin are used (Fig. 2C) (6). This approach has been largely abandoned, however, as the protracted circulation times of the radiolabeled tetramer undermine the fundamental premise of pretargeting. The second strategy uses a biotin-based radioligand and a streptavidin-bearing immunoconjugate. In this case, the latter can be made via the creation of a fusion protein, the chemical conjugation of streptavidin to an antibody, or the noncovalent conjugation of streptavidin to a biotin-bearing antibody (Fig. 2D) (7). A significant obstacle to this approach is the presence of endogenous biotin in the blood. This biotin threatens to saturate the binding sites of the antibody-bound streptavidin, prompting the use of a clearing agent to remove excess biotin from circulation. A third technique—a 3-step strategy called avidin bridging—was also devised to combat natural biotin (Fig. 2E) (5). Here, a biotinylated antibody is administered first, followed later by avidin or streptavidin. The protein simultaneously serves 2 purposes: clearing the blood of endogenous biotin and binding to the monoclonal antibody–biotin construct at the target site. Once the free avidin or streptavidin clears, a biotin-based radioligand is injected that can then bind to 1 of the 3 remaining sites of the antibody-bound protein at the target site.
Streptavidin and biotin-based pretargeting systems have proven enormously successful in preclinical studies. These approaches have been used with radionuclides ranging from 90Y to 186Re, yielding impressive tumoral uptake while simultaneously lowering both activity concentrations in the blood and overall effective dose rates. In light of these data, several phase I and II clinical trials were initiated to probe efficacy in humans (7–9). Initial results using a monoclonal antibody–streptavidin/radiolabeled-biotin methodology proved promising. One phase II study of pretargeted radioimmunotherapy (PRIT) using 90Y showed that patients with non-Hodgkin lymphoma were able to withstand a higher 90Y dose during PRIT than during traditional radioimmunotherapy (8). Another trial in high-grade glioma patients demonstrated an increased median survival time for PRIT patients compared with untreated controls (9). Furthermore, Forero et al. demonstrated the clinical feasibility of pretargeted SPECT, using a monoclonal antibody–streptavidin conjugate and an 111In-biotin radioligand (Fig. 2F) (10).
Despite these successes, a critical observation in all of these studies was that patients administered streptavidin-based immunoconjugates exhibited increased levels of human anti-streptavidin antibody (7,8,10). Once a patient begins to express high enough levels of human antistreptavidin antibody, subsequent treatments can trigger an allergic reaction that leads to severe treatment-associated morbidity. This is particularly relevant in PRIT, as the administration of fractionated doses is common. Unfortunately, this immunogenicity has proven to be a nearly fatal flaw. Streptavidin-based pretargeting has received little attention for nearly a decade, and most of the field’s momentum has shifted elsewhere.
APPROACH 2: BISPECIFIC ANTIBODIES
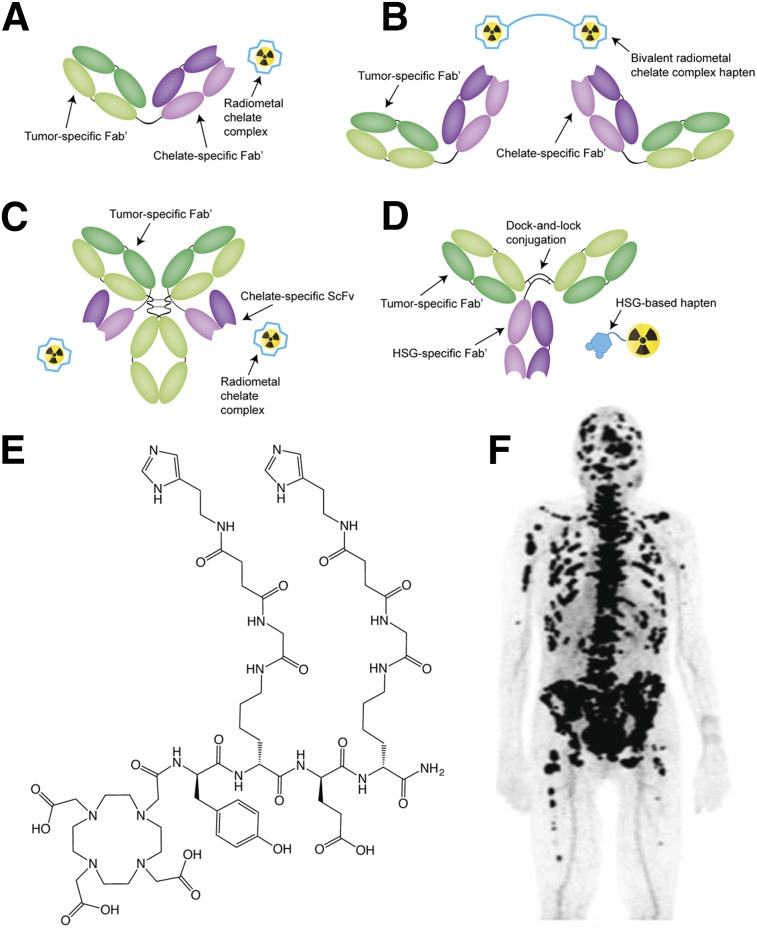
A second approach to in vivo pretargeting relies on bsAbs engineered to bind both a tumor-associated antigen and a radiolabeled hapten (4). Over the years, 3 different systems for pretargeting based on bsAbs have been developed. The first uses immunoconjugates bearing 2 different target-binding domains: one specific to a tumor-associated antigen and another specific to a radiolabeled hapten. These include conjugates in which an antigen-binding Fab′ is chemically coupled to a hapten-binding Fab′ as well as more sophisticated IgG-single-chain variable fragment (ScFv) fusion constructs bearing 2 antigen-binding Fab′ domains alongside a pair of hapten-binding ScFvs (Figs. 3A and 3C). Most of this work has used haptens based on radiometal chelate complexes, including a particularly interesting recent study demonstrating PRIT in mice (11). One concern with this approach, however, is that the affinity of the radioligand-specific Fab′ is highly sensitive to the coordination properties of the radiometal chelate, a trait that curtails the modularity of the system (12). This issue can be circumvented through the use of more modular peptide-based haptens such as histamine-succinyl-glycine (HSG) (13).
FIGURE 3.
Schematics of in vivo pretargeting strategies based on chemically linked Fab′ fragments (A), a divalent radiometal chelate hapten (B), an IgG–single-chain variable fragment (ScFv) construct (C), and an HSG-binding Tri-Fab (D); structure of the divalent IMP288 HSG hapten (E); pretargeted immuno-PET image of a patient with metastatic breast cancer recorded after the administration of 120 nmol of TF2 and, 30 h later, 3 nmol of 68Ga-IMP288 (F). (Adapted and reprinted with permission of (22).)
A second variant of this approach also uses bsAbs bearing 2 different Fab′ fragments but uses a bivalent radioligand to drive tumoral uptake (Fig. 3B) (14). Bivalent haptens can bind to 2 bsAbs occupying adjacent antigens on the tumor surface, thereby cross-linking the immunoconjugates and increasing the avidity of binding. This approach yields a marked improvement compared with systems using monovalent radioligands and, in most cases, has not required a clearing agent. Boerman et al., for example, used a bivalent radioligand approach to produce tumoral activity concentrations of approximately 90% of the injected dose per gram (%ID/g) and tumor-to-blood activity concentration ratios of approximately 148 in mice bearing CAIX-expressing xenografts (15). More recently, Kraeber-Bodéré et al. have demonstrated the clinical safety and efficacy of in vivo pretargeting using an anticarcinoembryonic antigen bsAb and a 131I-labeled bivalent hapten (14,16).
A third—and arguably most promising—approach uses bsAbs bearing 2 target-specific Fab′ fragments and 1 Fab′ capable of binding an HSG hapten (Fig. 3D). The 3 components of these immunoconjugates (dubbed Tri-Fab bsAbs) are assembled and locked into place via disulfide linkages (17). HSG-based haptens dramatically increase the modularity of the system. Not only can both mono- and divalent variants be synthesized, but also a wide array of chelators and prosthetic groups can be appended to the HSG core without abrogating binding to its Fab′. Over the last 5 y, a Tri-Fab bsAb system targeting the pancarcinoma glycoprotein antigen Trop-2 has been developed (18,19). Pretargeting using the Trop-2-targeting Tri-Fab—designated TF12—and an 111In-labeled variant of the divalent HSG hapten IMP-228 (111In-IMP228; Fig. 3E) produced tumoral activity concentrations of approximately 15 %ID/g and tumor-to-blood activity concentration ratios of more than 1,000 in mice bearing MDA-MB-468 xenografts (19). Subsequently, van Rij et al. demonstrated that PRIT using TF12 and 177Lu-IMP228 hapten significantly improved the median survival of mice bearing PC3 prostate cancer xenografts (18). The same laboratory also demonstrated that the TF12 platform can be leveraged for PET imaging with 68Ga-DOTA-IMP288 as well as multimodal imaging using a hapten bearing both 111In and a near-infrared fluorophore.
Clinical evaluation of PRIT using a carcinoembryonic antigen–targeting Tri-Fab bsAb (TF2) and 177Lu-IMP288 has been initiated in patients with metastatic colorectal and lung cancers (20). These studies demonstrate that this approach is feasible and safe. Infusion-related reactions were mild and could be reduced by the preadministration of prophylactic antihistamines and corticosteroids, and while human antibodies against TF2 were detected in 11 of 21 patients with colorectal cancer, immunogenicity in lung cancer was appreciably lower. Furthermore, prestudy imaging using 111In-IMP288 enabled accurate prediction of bone-marrow dose, making individualized activity dosing possible. More recently, the clinical feasibility and safety of pretargeted immuno-PET using TF2 and 68Ga-IMP288 was demonstrated in patients with metastatic medullary thyroid carcinoma (Fig. 3F) (21,22).
APPROACH 3: OLIGONUCLEOTIDES
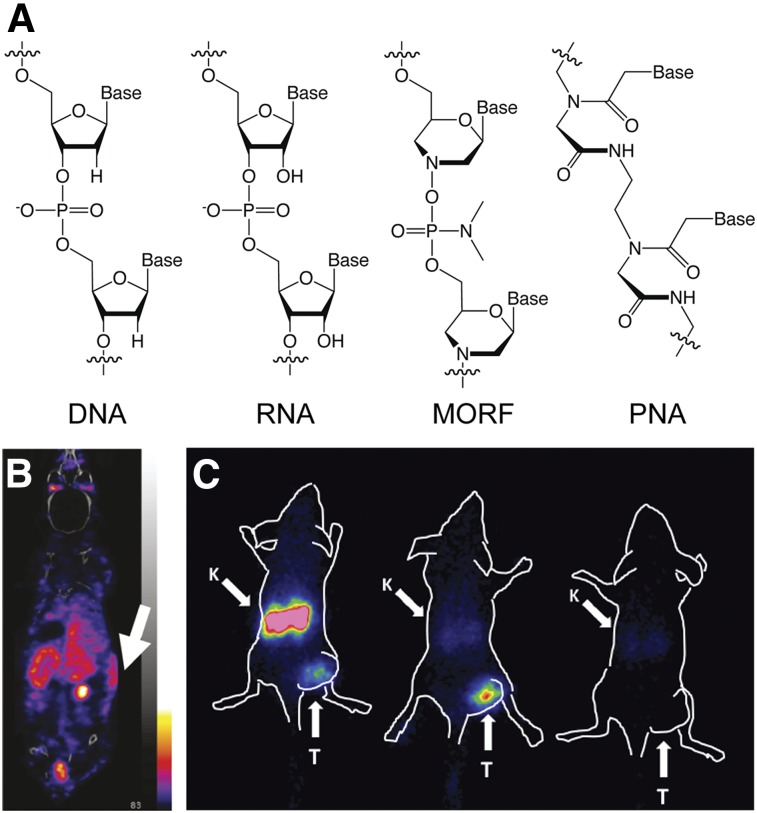
The next strategy is predicated on an interaction that is as specific and selective as it is ubiquitous: the hybridization of complementary oligonucleotides. Yet unmodified DNA and RNA oligomers are vulnerable to degradation by nucleases, which largely precludes their use as in vivo tools. To circumvent this issue, 2 types of nuclease-resistant DNA analogs have been exploited for in vivo pretargeting: phosphorodiamidate morpholino oligomers (MORFs) and peptide nucleic acids (PNAs).
MORFs are synthetic oligomers in which a phosphorodiamidate backbone replaces the (deoxy)ribofuranose-phosphodiester linkage (Fig. 4A). MORFs are water-soluble, are resistant to endo- and exonucleases, and retain specificity for Watson–Crick base-pairing. Much of the pioneering work on MORF-based pretargeting stems from the Hnatowich Laboratory, which first published the technique in 2002 (23). In 2011, this group reported that pretargeting using a MORF-bearing variant of the anti–tumor-associated glycoprotein 72 antibody CC49 and a 90Y-labeled complementary MORF produced tumoral activity concentrations of 7.2 ± 2.2 %ID/g and tumor-to-blood activity concentration ratios of more than 25 (24). This same work underscored the modularity of MORF-based pretargeting, as the complementary MORF could be labeled with 90Y-DOTA, 99mTc-mercaptoacetyltriglycine, or 188Re-mercaptoacetyltriglycine without interfering with hybridization or in vivo performance. Over the years, several promising variations on this theme have emerged, including the use of bivalent complementary MORFs, MORF-based clearing agents, and MORF-bearing dendrimers for signal amplification (25). Interestingly, Liu et al. have demonstrated the efficacy of MORF-based pretargeting for β-cell imaging, producing improved target–to–normal-organ activity concentration ratios compared to traditional immuno-SPECT (Fig. 4B) (26).
FIGURE 4.
Structure of DNA, RNA, MORF, and PNA oligonucleotides (A); a pretargeted SPECT image of subcutaneous transplanted human islet cells (white arrow) in a mouse administered a MORF-modified variant of the islet-cell–specific HPi1 antibody followed by a 99mTc-labeled complementary MORF radioligand (adapted and reprinted with permission from (26)) (B); and pretargeted SPECT images of mice bearing subcutaneous SKOV3 xenografts administered a directly radiolabeled 111In-ZHER2:K58 Affibody molecule (left), a PNA-modified ZHER2-HP1 Affibody molecule followed by complementary 111In-HP2 radioligand (center), and a 111In-HP2 radioligand alone (right) (C). K = kidney; T = tumor (adapted and reprinted with permission from (30)).
In PNAs, nitrogenous bases are conjugated to a pseudopeptide backbone made up of repetitive N-(2-aminoethyl)-glycine units connected through amide bonds (Fig. 4A). PNAs bind to complementary strands via Watson–Crick base-pairing and are thermally stable, chemically stable, nonimmunogenic, nontoxic, and resistant to digestion by both nucleases and proteases. The feasibility of PNA-mediated pretargeting was recently demonstrated using a variant of cetuximab bearing a pair of 17-mer PNAs (27). In this work, the complementary PNA strand was PEGylated, modified with a 2,2-dipicolylamine chelator, and labeled with 99mTc. Pretargeted SPECT experiments in mice bearing A431 xenografts revealed that tumor visualization was possible as early as 1 h after injection of the 99mTc-PNA. At 24 h after injection, the SUV for the tumor was 0.6 ± 0.3, whereas the tumor-to-blood and tumor-to-muscle activity concentration ratios were 0.5 ± 0.1 and 8 ± 1, respectively. At the same time point, however, elevated uptake was also observed in the kidneys, liver, and blood compared with control experiments, results attributed to the hybridization of the 99mTc-PNA with PNA-bearing immunoconjugate in the blood.
Affibody molecules (Affibody AB)—engineered proteins based on a 58-amino-acid (6.5 kDa) tri-helical scaffold—have also proven to be effective radiopharmaceutical vectors both in mice and in humans, producing high-contrast images only hours after administration (28). The high renal reabsorption of Affibody molecules, however, presents a substantial obstacle to using directly labeled variants for targeted radiotherapy. Not surprisingly, in vivo pretargeting offers an opportunity to remedy this issue. To this end, Westerlund et al. created an anti–human epidermal growth factor receptor 2 (HER2) Affibody molecule bearing a 15-mer PNA (ZHER2-HP1) as well as a complementary PEGylated 15-mer PNA (HP2) that demonstrated fast hybridization, slow dissociation, rapid clearance from the blood, and low retention in healthy tissues (29). Honarvar et al. demonstrated the efficacy of Affibody molecule–based pretargeting using ZHER2-HP1 and 111In-labeled HP2 in mice bearing HER2-expressing SKOV3 ovarian cancer xenografts (Fig. 4C) (30). The pretargeting strategy produced tumoral uptake of 19 ± 2 %ID/g 1 h after injection along with a 54 ± 19 tumor-to-blood activity concentration ratio at the same time point. Critically, activity concentrations in the kidneys were about 50 times lower than those observed in experiments using directly labeled Affibody molecules, a development that the authors believe will enable pretargeted radiotherapy.
APPROACH 4: CLICK CHEMISTRY
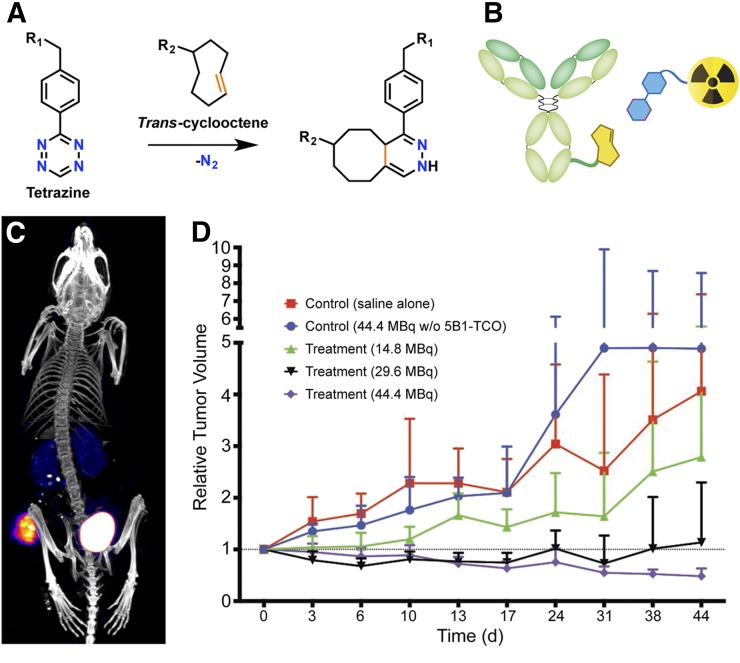
Since the advent of click chemistry over a decade and a half ago, the drive to apply these selective transformations to in vivo ligations has remained strong. In this regard, the development of bioorthogonal click reactions—most notably the Staudinger ligation and the strain-promoted azide-alkyne cycloaddition—proved critical, enabling selective chemistry within the complex environment of living systems. However, the sluggish kinetics of this first generation of bioorthogonal reactions made them unsuitable for in vivo pretargeting. This issue was largely solved in 2008 by the Fox Laboratory’s resurrection of the IEDDA cycloaddition reaction between tetrazine and trans-cyclooctene (TCO; Fig. 5A). The IEDDA reaction is selective, modular, and bioorthogonal, but what really sets it apart from other click ligations is its speed. Rate constants for the reaction between tetrazine dienes and TCO dienophiles can exceed 100,000 M−1s−1, orders of magnitude faster than either the Staudinger or strain-promoted azide-alkyne cycloaddition ligations. The potential of the IEDDA reaction as a tool for bioconjugation was recognized almost immediately, and the ligation quickly found wide-sweeping applications in a variety of fields, including radiopharmaceutical chemistry (31).
FIGURE 5.
The IEDDA ligation (A); the 2 components of IEDDA-based pretargeting system: a TCO-bearing immunoconjugate and a tetrazine-modified radioligand (B); SPECT/CT image of an LS174T tumor-bearing mouse pretargeted with CC49-TCO and 111In-DOTA-tetrazine (reprinted with permission of reference (34)) (C); and a longitudinal study of normalized tumor volume in mice bearing BxPC3 pancreatic ductal adenocarcinoma xenografts treated with a PRIT regimen composed of 5B1-TCO and 177Lu-DOTA-PEG7-tetrazine (reprinted with permission of (41)) (D).
The rapidity and bioorthogonality of the IEDDA reaction make it almost ideally suited for in vivo pretargeting (Fig. 5B), and perhaps not surprisingly, only 2 y passed before the first report of IEDDA-based pretargeting (32). In this pioneering work, the group of Rossin and Robillard used an anti–tumor-associated glycoprotein 72–targeting CC49-TCO immunoconjugate and an 111In-DOTA–labeled dipyridyltetrazine radioligand (Fig. 5C). SPECT imaging and biodistribution experiments revealed 4.2 %ID/g in the tumor and a 13.1 tumor-to-muscle activity concentration ratio at 24 h after the injection of the radioligand, modest yet promising results that the authors later improved on via the development of a tetrazine-bearing clearing agent (33). Notably, in this work—and almost all reports of IEDDA-based pretargeting—the TCO is attached to the antibody, and the tetrazine forms part of the radioligand, a choice that stems from the superior in vivo stability of the former. The group of Rossin and Robillard has since remained a leader in the field, extending its explorations to alternative TCO moieties and an IEDDA-activated therapeutic modality termed “click-to-release” (34).
Pretargeted PET was first reported by the laboratories of Weissleder and Lewis (35). Their approach used a colorectal cancer–targeting huA33-TCO immunoconjugate and a 64Cu-labeled tetrazine to produce high-contrast PET images at only a fraction of the radiation dose to healthy organs produced by directly labeled radioimmunoconjugates. The success of this work led others to investigate the use of even shorter-lived isotopes, including 18F, 68Ga, and 11C (36,37). In recent years, several laboratories have begun investigating pretargeting with more rapidly clearing vectors—including TCO-bearing diabodies, bisphosphonates, and Affibody molecules—a shift that may enable same-day procedures and further improve the dosimetric advantages of the approach (38–40).
PRIT using the tetrazine–TCO ligation has admittedly received less attention than imaging, yet it arguably holds even more promise. In 2013, Rossin et al. first provided biodistribution and dosimetry data to support the feasibility of tetrazine–TCO PRIT (33). However, it was not until last year that Houghton et al. first demonstrated the efficacy of this approach with a longitudinal therapy study (41). In this work, PRIT of mice bearing human pancreatic ductal adenocarcinoma xenografts using a CA19.9-targeting 5B1-TCO immunoconjugate and a 177Lu-DOTA–labeled tetrazine produced a dose-dependent therapeutic effect that all but completely eliminated tumor tissue at higher activities of the radioligand (Fig. 5D).
CONCLUSION
Reviews such as these often culminate in a critical comparison of the various strategies at hand. Unfortunately, however, contrasting the 4 approaches that we have discussed proves difficult for 2 reasons. First, the 4 methodologies have not been subject to a head-to-head competition using a single model system, rendering quantitative comparisons dubious at best. And second, each of the strategies stands at a different stage in its scientific development, a fact that inevitably muddies the waters of the conversation. Still, each approach clearly has its own set of advantages and disadvantages. Streptavidin-based strategies benefit from the multivalency of streptavidin as well as the remarkable affinity between the protein and biotin, yet both the immunogenicity of streptavidin and the presence of endogenous biotin have proven complicating factors. bsAbs have produced some of the finest clinical results to date, and the advent of HSG-based haptens eliminates some (but not all) of the concerns surrounding the modularity of these systems. Nonetheless, the complexity and expense of vector production still require attention. Oligonucleotide-centered approaches have produced some enticing preclinical results, but the sequence-dependent nature of hybridization rates and affinities as well as the hydrophobicity of PNAs must both be carefully considered as this technology evolves. Finally, strategies predicated on the IEDDA reaction have proven extremely effective; represent the only approaches that use covalent chemistry; and are modular, tunable, and completely bioorthogonal. However, some latent concerns remain surrounding the in vivo stability of the TCO and tetrazine moieties.
In the end, we believe that it is an incredibly exciting time for in vivo pretargeting, yet this excitement must be tempered somewhat by the exigencies of translational research. Three of the 4 strategies we have discussed face pivotal days in the near future. The approaches based on the IEDDA ligation and the hybridization of oligonucleotides both stand at a critical moment in their history: the move from the laboratory to the clinic. In contrast, pretargeting based on bsAbs has produced extremely promising clinical results; however, the modularity and complexity of these vectors remain concerns going forward. Moving beyond these established methodologies, there is equally fascinating work to be done unearthing the next generation of pretargeting strategies, whether they are predicated on host–guest relationships, enzymatic transformations, biologic binding partners, or yet-to-be-discovered bioorthogonal reactions. On all of these counts, we look forward with optimism to the data and developments of the years to come.
DISCLOSURE
This work is supported by the NIH (4R00CA178205-02), NIHMD (G12MD007599), TeamConnor Childhood Cancer Foundation, the Swedish Cancer Society (CAN 2015/350), Hunter College, and Uppsala University. No other potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Reardan DT, Meares CF, Goodwin DA, et al. Antibodies against metal chelates. Nature. 1985;316:265–268. [DOI] [PubMed] [Google Scholar]
- 2.Hnatowich DJ, Virzi F, Rusckowski M. Investigations of avidin and biotin for imaging applications. J Nucl Med. 1987;28:1294–1302. [PubMed] [Google Scholar]
- 3.Goldenberg DM, Chang CH, Rossi EA, McBride WJ, Sharkey RM. Pretargeted molecular imaging and radioimmunotherapy. Theranostics. 2012;2:523–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van de Watering FC, Rijpkema M, Robillard M, Oyen WJ, Boerman OC. Pretargeted imaging and radioimmunotherapy using antibodies and bioorthogonal chemistry. Front Med. 2014;1:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casalini P, Luison E, Menard S, Colnaghi MI, Paganelli G, Canevari S. Tumor pretargeting: role of avidin/streptavidin on monoclonal antibody internalization. J Nucl Med. 1997;38:1378–1381. [PubMed] [Google Scholar]
- 6.Sung C, van Osdol WW. Pharmacokinetic comparison of direct antibody targeting with pretargeting protocols based on streptavidin-biotin binding. J Nucl Med. 1995;36:867–876. [PubMed] [Google Scholar]
- 7.Kalofonos HP, Rusckowski M, Siebecker DA, et al. Imaging of tumor in patients with indium-111-labeled biotin and streptavidin-conjugated antibodies: preliminary communication. J Nucl Med. 1990;31:1791–1796. [PubMed] [Google Scholar]
- 8.Weiden PL, Breitz HB. Pretargeted radioimmunotherapy for treatment of non-Hodgkin’s lymphoma. Crit Rev Oncol Hematol. 2001;40:37–51. [DOI] [PubMed] [Google Scholar]
- 9.Grana C, Chinol M, Robertson C, et al. Pretargeted adjuvant radioimmunotherapy with yttrium-90-biotin in malignant glioma patients: a pilot study. Br J Cancer. 2002;86:207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forero A, Weiden PL, Vose JM, et al. Phase 1 trial of a novel anti-CD20 fusion protein in pretargeted radioimmunotherapy for B-cell non-Hodgkin lymphoma. Blood. 2004;104:227–236. [DOI] [PubMed] [Google Scholar]
- 11.Cheal SM, Xu H, Guo HF, Zanzonico PB, Larson SM, Cheung NK. Preclinical evaluation of multistep targeting of diasialoganglioside GD2 using an IgG-scFv bispecific antibody with high affinity for GD2 and DOTA metal complex. Mol Cancer Ther. 2014;13:1803–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orcutt KD, Slusarczyk AL, Cieslewicz M, et al. Engineering an antibody with picomolar affinity to DOTA chelates of multiple radionuclides for pretargeted radioimmunotherapy and imaging. Nucl Med Biol. 2011;38:223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Boisferon MH, Manetti C, Raguin O, et al. Pretargeted radioimmunotherapy using I-131-labelled bivalent hapten-bearing peptides. Lett Pept Sci. 1997;4:331–339. [Google Scholar]
- 14.Gautherot E, Rouvier E, Daniel L, et al. Pretargeted radioimmunotherapy of human colorectal xenografts with bispecific antibody and I-131-labeled bivalent hapten. J Nucl Med. 2000;41:480–487. [PubMed] [Google Scholar]
- 15.Boerman OC, Kranenborg MHGC, Oosterwijk E, et al. Pretargeting of renal cell carcinoma: improved tumor targeting with a bivalent chelate. Cancer Res. 1999;59:4400–4405. [PubMed] [Google Scholar]
- 16.Kraeber-Bodéré F, Rousseau C, Bodet-Milin C, et al. Targeting, toxicity, and efficacy of 2-step, pretargeted radioimmunotherapy using a chimeric bispecific antibody and I-131-labeled bivalent hapten in a phase I optimization clinical trial. J Nucl Med. 2006;47:247–255. [PubMed] [Google Scholar]
- 17.Rossi EA, Goldenberg DM, Cardillo TM, McBride WJ, Sharkey RM, Chang C-H. Stably tethered multifunctional structures of defined composition made by the dock and lock method for use in cancer imaging. Proc Natl Acad Sci USA. 2006;103:6841–6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Rij CM, Frielink C, Goldenberg DM, et al. Pretargeted radioimmunotherapy of prostate cancer with an anti-TROP-2xAnti-HSG bispecific antibody and a Lu-177-labeled peptide. Cancer Biother Radiopharm. 2014;29:323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharkey RM, van Rij CM, Karacay H, et al. A new Tri-Fab bispecific antibody for pretargeting Trop-2-expressing epithelial cancers. J Nucl Med. 2012;53:1625–1632. [DOI] [PubMed] [Google Scholar]
- 20.Schoffelen R, Boerman OC, Goldenberg DM, et al. Development of an imaging-guided CEA-pretargeted radionuclide treatment of advanced colorectal cancer: first clinical results. Br J Cancer. 2013;109:934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bodet-Milin C, Faivre-Chauvet A, Carlier T, et al. Immuno-PET using anticarcinoembryonic antigen bispecific antibody and Ga-68-labeled peptide in metastatic medullary thyroid carcinoma: clinical optimization of the pretargeting parameters in a first-in-human trial. J Nucl Med. 2016;57:1505–1511. [DOI] [PubMed] [Google Scholar]
- 22.Kraeber-Bodéré F, Rousseau C, Bodet-Milin C, et al. A pretargeting system for tumor PET imaging and radioimmunotherapy. Front Pharmacol. 2015;6:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu G, Mang’era K, Liu N, Gupta S, Rusckowski M, Hnatowich DJ. Tumor pretargeting in mice using Tc-99m-labeled morpholino, a DNA analog. J Nucl Med. 2002;43:384–391. [PubMed] [Google Scholar]
- 24.Liu G, Dou SP, Liu YX, Wang YZ, Rusckowski M, Hnatowich DJ. Y-90 labeled phosphorodiamidate morpholino oligomer for pretargeting radiotherapy. Bioconjug Chem. 2011;22:2539–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He J, Wang Y, Dou SP, et al. Affinity enhancement pretargeting: synthesis and testing of a Tc-99m-labeled bivalent MORF. Mol Pharm. 2010;7:1118–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu G, Dou S, Cheng D, et al. Human islet cell MORF/cMORF pretargeting in a xenogeneic murine transplant model. Mol Pharm. 2011;8:767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonidova A, Foerster C, Zarschler K, et al. In vivo demonstration of an active tumor pretargeting approach with peptide nucleic acid bioconjugates as complementary system. Chem Sci. 2015;6:5601–5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sörensen J, Velikyan I, Sandberg D, et al. Measuring HER2-receptor expression in metastatic breast cancer using Ga-68 ABY-025 affibody PET/CT. Theranostics. 2016;6:262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westerlund K, Honarvar H, Tolmachev V, Karlstrom AE. Design, preparation, and characterization of PNA-based hybridization probes for affibody-molecule-mediated pretargeting. Bioconjug Chem. 2015;26:1724–1736. [DOI] [PubMed] [Google Scholar]
- 30.Honarvar H, Westerlund K, Altai M, et al. Feasibility of affibody molecule-based PNA-mediated radionuclide pretargeting of malignant tumors. Theranostics. 2016;6:93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meyer J-P, Adumeau P, Lewis JS, Zeglis BM. Click chemistry and radiochemistry: the first 10 years. Bioconjug Chem. 2016;27:2791–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossin R, Renart Verkerk P, van den Bosch SM, et al. In vivo chemistry for pretargeted tumor imaging in live mice. Angew Chem Int Ed Engl. 2010;49:3375–3378. [DOI] [PubMed] [Google Scholar]
- 33.Rossin R, Lappchen T, van den Bosch SM, Laforest R, Robillard MS. Diels-Alder reaction for tumor pretargeting: in vivo chemistry can boost tumor radiation dose compared with directly labeled antibody. J Nucl Med. 2013;54:1989–1995. [DOI] [PubMed] [Google Scholar]
- 34.Rossin R, van Duijnhoven SMJ, Läppchen T, van den Bosch SM, Robillard MS. Trans-cyclooctene tag with improved properties for tumor pretargeting with the Diels-Alder reaction. Mol Pharm. 2014;11:3090–3096. [DOI] [PubMed] [Google Scholar]
- 35.Zeglis BM, Sevak KK, Reiner T, et al. A pretargeted PET imaging strategy based on bioorthogonal Diels-Alder click chemistry. J Nucl Med. 2013;54:1389–1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Denk C, Svatunek D, Mairinger S, et al. Design, synthesis, and evaluation of a low-molecular-weight 11C-labeled tetrazine for pretargeted PET imaging applying bioorthogonal in vivo click chemistry. Bioconjug Chem. 2016;27:1707–1712. [DOI] [PubMed] [Google Scholar]
- 37.Meyer J-P, Houghton JL, Kozlowski P, et al. 18F-based pretargeted PET imaging based on bioorthogonal Diels-Alder click chemistry. Bioconjug Chem. 2016;27:298–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yazdani A, Bilton H, Vito A, et al. A bone-seeking trans-cyclooctene for pretargeting and bioorthogonal chemistry: a proof of concept study using 99mTc- and 177Lu-labeled tetrazines. J Med Chem. 2016;59:9381–9389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altai M, Perols A, Tsourma M, et al. Feasibility of affibody-based bioorthogonal chemistry mediated radionuclide pretargeting. J Nucl Med. 2016;57:431–436. [DOI] [PubMed] [Google Scholar]
- 40.van Duijnhoven SMJ, Rossin R, van den Bosch SM, Wheatcroft MP, Hudson PJ, Robillard MS. Diabody pretargeting with click chemistry in vivo. J Nucl Med. 2015;56:1422–1428. [DOI] [PubMed] [Google Scholar]
- 41.Houghton JL, Membreno R, Abdel-Atti D, et al. Establishment of the in vivo efficacy of pretargeted radioimmunotherapy utilizing inverse electron demand Diels-Alder click chemistry. Mol Cancer Ther. 2017;16:124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]