Abstract
Background: Several efforts have been made to develop effective antipsychotic drugs. Currently, available antipsychotics are effective on positive symptoms, less on negative symptoms, but not on cognitive impairment, a clinically relevant dimension of schizophrenia. Drug repurposing offers great advantages over the long-lasting, risky and expensive, de novo drug discovery strategy. To our knowledge, the possible antipsychotic properties of buspirone, an azapirone anxiolytic drug marketed in 1986 as serotonin 5-HT1A receptor (5-HT1AR) partial agonist, have not been extensively investigated despite its intriguing pharmacodynamic profile, which includes dopamine D3 (D3R) and D4 receptor (D4R) antagonist activity. Multiple lines of evidence point to D3R as a valid therapeutic target for the treatment of several neuropsychiatric disorders including schizophrenia. In the present study, we tested the hypothesis that buspirone, behaving as dopamine D3R antagonist, may have antipsychotic-like activity.
Materials and Methods: Effects of acute administration of buspirone was assessed on a wide-range of schizophrenia-relevant abnormalities induced by a single administration of the non-competitive NMDAR antagonist MK-801, in both wild-type mice (WT) and D3R-null mutant mice (D3R-/-).
Results: Buspirone (3 mg⋅kg-1, i.p.) was devoid of cataleptogenic activity in itself, but resulted effective in counteracting disruption of prepulse inhibition (PPI), hyperlocomotion and deficit of temporal order recognition memory (TOR) induced by MK-801 (0.1 mg⋅kg-1, i.p.) in WT mice. Conversely, in D3R-/- mice, buspirone was ineffective in preventing MK-801-induced TOR deficit and it was only partially effective in blocking MK-801-stimulated hyperlocomotion.
Conclusion: Taken together, these results indicate, for the first time, that buspirone, might be a potential therapeutic medication for the treatment of schizophrenia. In particular, buspirone, through its D3R antagonist activity, may be a useful tool for improving the treatment of cognitive deficits in schizophrenia that still represents an unmet need of this disease.
Keywords: buspirone, dopamine D3 receptor, MK-801, schizophrenia, antipsychotics, prepulse inhibition, temporal order recognition
Introduction
Schizophrenia is a chronic and devastating multifactorial mental illness affecting approximately 0.7–1% of population worldwide (Landek-Salgado et al., 2016). The development of second-generation antipsychotics has yielded some advances in terms of efficacy, but only modest improvement in addressing the negative symptoms of schizophrenia. To date, no antipsychotics display robust effects on cognitive deficits or impaired social processing that are the most clinically relevant dimensions of the disease (Owen et al., 2016). Drug repositioning refers to the process of finding new uses for already approved and commercialized medications and it is thought to offer great advantages over the long-lasting, risky and expensive de novo drug discovery strategy. This is because the pharmacological and toxicological profiles of approved medications are well-characterized (Ashburn and Thor, 2004). It has been suggested that repositioned drugs may represent effective alternative compounds for the treatment of neuropsychiatric disorders for which the classical drug discovery process is hampered by the poor knowledge of the pathophysiological mechanisms (Lee and Kim, 2016). In this context, the azapirone anxiolytic drug buspirone (Buspar®), has been proposed for the treatment of substance use disorder (SUD; Leggio et al., 2016). Regarding schizophrenia, earlier clinical trials suggested that buspirone added to both typical and atypical antipsychotics ameliorates negative symptoms (Ghaleiha et al., 2010; Sheikhmoonesi et al., 2015), while other preclinical and clinical data showed buspirone as scarcely effective in improving cognitive dysfunction (Piskulić et al., 2009; Horiguchi and Meltzer, 2012; Maeda et al., 2014).
At a pharmacological level, buspirone, besides its claimed 5-HT1AR partial agonist activity, is endowed with D3R/D4R antagonist activity and binds to dopamine D2 receptor (D2R) with an affinity 5-fold lower than for D3R (Bergman et al., 2013). Available evidence indicates that D3R can be considered as a new validated pharmacological target for the treatment of several neuropsychiatric disorders, including SUD, Parkinson’s disease, depression and schizophrenia (Leggio et al., 2016; Maramai et al., 2016; Sokoloff and Le Foll, 2017). Published studies indicate that D3R play a key role in the pathophysiology of schizophrenia (Nunokawa et al., 2010; Gross et al., 2013). Moreover, D3R expression is increased in schizophrenics (Gurevich et al., 1997; Cui et al., 2015). The restricted localization of D3Rs in the limbic system, particularly in the nucleus accumbens (NAc), has attracted great interest especially for the development of safe and effective medications devoid of the classical side effects (extrapyramidal side effects and prolactin elevation) caused by D2R blockade (Gross and Drescher, 2012). In fact, antipsychotics targeting D3R, such as blonanserin and cariprazine, have been demonstrated effective in treating positive and negative symptoms with a good safety profile (Hori et al., 2014; Leggio et al., 2016; Earley et al., 2017). Beside the high expression in NAc, D3Rs are expressed specifically in the layer 5 pyramidal neurons of medial prefrontal cortex (mPFC, Lidow et al., 1998), where they control in a peculiar manner neuronal excitability (Clarkson et al., 2017). D3Rs play a fundamental role in physiological mechanisms underlying mPFC-dependent cognitive functions as well as in crucial pathophysiological processes subserving mPFC-dependent cognitive dysfunctions (Nakajima et al., 2013). In particular, it seems that selective antagonism on D3R improves cognitive functions while selective agonism exerts opposite, detrimental effects (Watson et al., 2012). Recently, it has been proposed that molecules joining 5HT1AR partial agonism and 5-HT2A antagonism to D3R antagonism may exhibit stronger antipsychotic effects (Brindisi et al., 2014). As aforementioned, the pharmacological profile of buspirone largely matches that of these potential antipsychotics. However, as far as we know, the antipsychotic properties of buspirone have not yet been extensively elucidated.
In the present study, we tested the hypothesis that buspirone, behaving as dopamine D3R antagonist, may exert antipsychotic-like properties in a preclinical model of schizophrenia, based on NMDAR hypofunction. This pharmacological model, as compared with dopamine-based models, appears to more efficiently recapitulate several symptoms of schizophrenia, particularly those related to cognitive dysfunction (Kantrowitz and Javitt, 2010). The effect of acute administration of buspirone was evaluated on hyperlocomotion, prepulse inhibition (PPI) disruption and temporal order recognition (TOR) memory impairment, elicited by acute administration of the non-competitive NMDAR antagonist MK-801 in WT mice. In order to assess the involvement of D3R on the effect of buspirone, the same behavioral paradigms, with or without buspirone, were applied to D3R-/- mice.
Materials and Methods
Animals and Housing
In these experiments, D3R-/- mice and their WT littermates (males, 8–12 weeks old), bred by a heterozygous (D3R+/- × D3R+/-) mating strategy, were tested. Animals were group-housed (2–5 mice per cage), with free access to chow and water, in an air-conditioned room, with a 12-h light–dark cycle. D3R mutant mice were 10th–12th generation of congenic C57BL/6J mice, generated by a back-crossing strategy (Accili et al., 1996). Genotypes were identified by PCR analysis of tail DNA as previously described (Leggio et al., 2011, 2015). The experimenters handled animals on alternate days during the week preceding the behavioral tests. Animals were acclimatized to the testing room at least 1 h before the beginning of the tests. Experiments were performed during the dark phase. All experimental procedures were carried out according to the Directive 2010/63/EU and were approved by the Institutional Animal Care and Use Committee of the Catania University.
Drugs
(+)MK-801 hydrogen maleate and buspirone hydrochloride (Sigma–Aldrich, St. Louis, MO, United States) were dissolved in saline. Clozapine and haloperidol (Sigma–Aldrich) were dissolved in few drops of 1 N HCl and further diluted with saline; the pH was adjusted to 7 with NaHCO3. All drug solutions were prepared daily and intraperitoneally (i.p.) administered by using an injection volume of 10 ml/kg.
Behavioral Testing
Temporal Order Recognition (TOR) Test
The TOR test was carried out as previously described (Barker and Warburton, 2011; Managò et al., 2016) with minor modifications. Animals explored in an evenly illuminated (9 ± 1 lux) square open field (40 × 40 × 40 cm, Ugo Basile, Gemonio, Italy) in which the floor was covered with sawdust. The behavior of animals was recorded using a video camera (Sony Videocam PJ330E) and then scored by an independent observer. The objects presented were made of plastic Duplo blocks (Lego®), different in shape, color, and size (9 × 8 × 7 cm to 12 × 11 × 10 cm) and too heavy to be moved by the mice. After 1 week of handling, a 4-day pretesting procedure was carried out. Mice were habituated to the empty arena for 10 min on the day 1 and 2. Afterward, on the day 3 and 4, mice were i.p. injected with saline 20 min before being placed in the arena containing two objects (different from those ones eventually used during the test) for 10 min. This pretesting procedure was performed in order to minimize stress-related behavior induced by injections as well as to prevent neophobia during the test. The objects were located in two corners of the arena, 10 cm far from the sidewalls. The test consisted of two sample phases and one test trial (Figure 1A). During the sample phases, animals were allowed to explore two copies of an identical object for a total of 5 min. Different objects were used for each sample phase, with a delay between the sample phases of 1 h. The test trial was performed 3 h after the sample phase 2. During the test trial (5 min duration), animals were exposed to a third copy of the objects from sample phase 1 and a third copy of the objects from sample phase 2. Objects were cleaned with a 10% ethanol solution in between each test in order to avoid olfactory cues. Any feces were removed and the sawdust was shaken in order to equally redistribute any odor cues. If the temporal order memory is intact, animals should spend more time exploring the object from sample 1, the less recently experienced object, compared with the object from sample 2, the more recently experienced object. The objects utilized in each sample phase as well as the positions of the objects during the test were counterbalanced between the animals. Exploratory behavior was defined as the animal directing its nose toward the object at a distance of <2 cm. Looking around while sitting, climbing or rearing against the objects were not considered as exploration. Animals that failed to complete a minimum of 2 seconds (sec) of exploration in each phase of the task were excluded from the analysis. Discrimination between the objects was calculated using a discrimination ratio (DR) that takes into account individual differences in the total amount of exploration. In particular, data are depicted as DR, calculated as [(less recently experienced object exploration time – more recently experienced object exploration time)/total exploration time]. The higher is the DR, the better is TOR memory.
FIGURE 1.
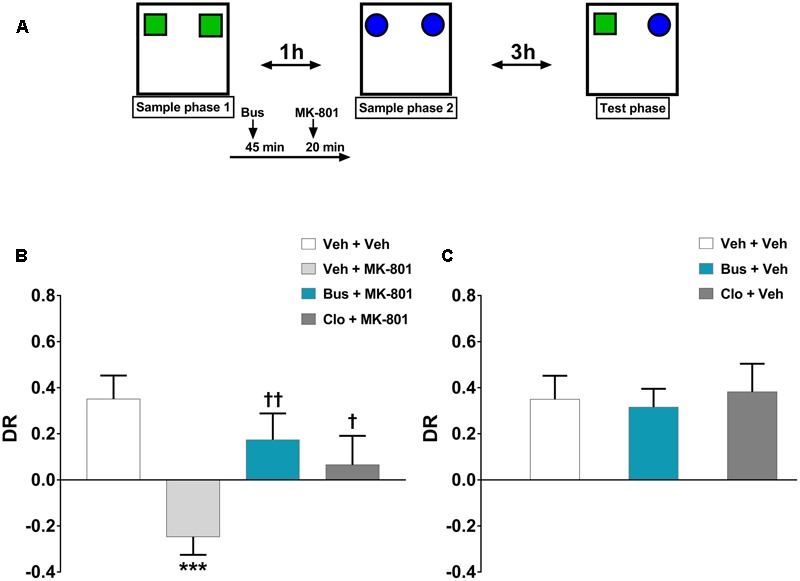
Buspirone counteracted MK-801-induced TOR memory impairment in WT mice. (A) Cartoon illustrating the TOR test and the schedule of treatment. Buspirone (Bus, 3 mg⋅kg-1, i.p.) or vehicle (Veh), clozapine (Clo, 1 mg⋅kg-1, i.p.) or Veh and MK-801 (0.1 mg⋅kg-1, i.p.) or Veh were injected 45, 30, and 20 min respectively, before the sample phase 2. (B,C) Discrimination ratio (DR) displayed by Veh + Veh (n = 10), Veh + MK-801 (n = 10), Bus + MK-801 (n = 9), Clo + MK-801 (n = 9), Bus + Veh (n = 8), Clo + Veh (n = 5) WT mice during the test phase. Data are shown as mean ± SEM. DR [(less recently experienced object exploration time - more recently experienced object exploration time)/total exploration time]. ∗∗∗p < 0.001 vs. Veh + Veh WT mice; ††p < 0.01 and †p < 0.05 vs. Veh + MK-801 WT mice (One-way ANOVA and Newman–Keuls post hoc test).
Acoustic Startle Response and Prepulse Inhibition (PPI) Test
Acoustic startle response and PPI were measured using four PPI sets from SR-Lab Systems (San Diego Instruments, San Diego, CA, United States). The experimental procedure was adapted from Papaleo et al. (2012). Animals were exposed to a short “matching” startle session before the PPI testing. They were placed in the startle chambers for a 5-min acclimation period with a 65 dB(A) background noise, and then exposed to a total of 17 acoustic startle stimulus (pulse) trials [40 ms — 120 dB (A) noise bursts] that were interspersed with 3 acoustic prepulse plus acoustic pulse trials in which the pulse was preceded 100 ms (onset-to-onset) by a 20 ms noise burst, 10 dB above background. Animals were assigned to each drug dose group based on average PPI% from the matching session to ensure similar baseline PPI levels between experimental groups. The PPI test session, with or without drug treatment, was carried out 5–7 days after the matching session. The animals were placed in the startle chambers for a 5-min acclimation period with a 65 dB(A) background noise. Animals were then exposed to a series of trial types, which were presented in pseudorandom order. The inter trial interval (ITI) was 5–60 s. One trial type measured the response to no stimulus (baseline movement), and another one measured the startle stimulus alone (acoustic amplitude), which was a 40 ms 120 dB sound burst. Other five trial types were acoustic prepulse plus acoustic startle stimulus trials. Prepulse tones were 20 ms at 70, 75, 80, 85, and 90 presented 100 ms before the startle stimulus. PPI was calculated by using the following formula: 100 ×{[pulse-only units - (prepulse + pulse units)]/(pulse-only units)}.
Open Field (OF) Test
Animals were tested in the same square open field mentioned above (divided into 16 quadrants by lines on the floor) over a 30 min-period. Locomotor activity was assessed during the first exposure to the empty open field arena. The apparatus was cleaned with a 10% ethanol solution in between each test to prevent olfactory cues. Locomotor activity was quantified by counting the numbers of lines crossed (crossings) with all four paws (Accili et al., 1996). The behavior of animals was recorded by using a video camera and eventually analyzed by one observer blinded to genotype/treatment.
Catalepsy Test
The catalepsy test was carried out as previously reported with minor changes (Fink-Jensen et al., 2011). The apparatus was made of 2 wooden supports linked by a steel bar (length: 7.5 cm; diameter 0.9 cm); The system was stabilized by another wooden support opposite to the steel bar. The catalepsy was evaluated by placing the animals with the forepaws on the horizontal steel bar positioned 4.5 cm above the floor. Animals were tested at different time points: 30, 60, 90, and 120 min after the pharmacological treatment. The latency (cut off time) was 600 s. The end point of the test was considered when both forepaws were removed from the bar or when the animal moved its head in exploratory manner. Each trial was repeated for three times and the highest time value was taken.
Experimental Design
The behavioral effects of a single injection of buspirone were evaluated on MK-801-induced schizophrenia-like phenotypes both in WT and in D3R-/- mice. These effects were compared to those of clozapine, the most effective commercially available antipsychotic (Owen et al., 2016), injected at a dose of 1 mg⋅kg-1. This dose has been revealed to be effective in ameliorating cognitive dysfunction (Mutlu et al., 2011; Park et al., 2014). The dose of buspirone (3 mg⋅kg-1) was selected based on our previous experience (Leggio et al., 2014) as well as according to a work by Di Ciano et al. (2017). To avoid effects of test-related anxiety, animals were divided into independent cohorts and subjected to the most stressful tests as the last. Animals were tested as follows: WT, cohort 1, open field test, catalepsy test; WT, cohort 2, TOR test, PPI test; D3R-/-, cohort 2, open field, TOR test. A washout period of at least 7 days was given between each experimental procedure.
Experiment 1 - Effect of Buspirone on MK-801-Induced TOR Memory Deficit in WT Mice
Administration of NMDAR antagonists before the sample phase 2 impairs TOR memory affecting both reconsolidation and consolidation mechanism (Warburton et al., 2013). Therefore, buspirone, clozapine and MK-801 were administered 45, 30, and 20 min, respectively, before the sample phase 2. The chosen dose of MK-801 (0.1 mg⋅kg-1) is able to produce cognitive impairment without inducing locomotor disturbance (stereotypies, ataxia; Blot et al., 2015).
Experiment 2 - Effect of Buspirone on MK-801-Stimulated Hyperlocomotion and Assessment of Catalepsy in WT Mice
Mice received injections of buspirone, clozapine and MK-801 with the same timing of treatment used for the TOR test and then placed into the empty open field. The dose of 0.1 mg⋅kg-1 MK-801 is effective in stimulating hyperlocomotion (Zhang et al., 2007). For the catalepsy test, animals were injected with buspirone, clozapine and haloperidol (1 mg⋅kg-1) and then tested at different time points (30, 60, 90, and 120 min). The haloperidol-induced catalepsy at the dose of 1 mg⋅kg-1 is a widely used model for the evaluation of extrapyramidal side effects induced by drugs (Pogorelov et al., 2017).
Experiment 3 - Effect of Buspirone on MK-801-Induced PPI Disruption in WT Mice
Mice were given injections of buspirone, clozapine and MK-801, 45, 30, and 20 min (including the 5-min acclimation period), respectively, before to be placed in the startle chambers for the PPI test. We chose the dose of 0.1 mg⋅kg-1 MK-801 because this dose is sufficient to disrupt PPI (Spooren et al., 2004; Zhang et al., 2007).
Experiment 4 - Effect of Buspirone on MK-801-Induced TOR Memory Deficit and Hyperlocomotion in D3R-/- Mice
To figure out whether or not the effects of buspirone were mainly mediated via the blockade of D3R, we tested D3R-/- mice (open field and TOR) treated with the same pharmacological treatment carried out in WT mice, both in terms of doses and timing of treatment. Unfortunately, we could not evaluate the effect of buspirone on PPI test because the vast majority of D3R-/- mice exhibited a very low acoustic startle reactivity during the startle matching session (data not shown). This made difficult the assembling of experimental groups with similar PPI%.
Statistics
Statistical analysis was performed by using graphpad prism 7 (graphpad software La Jolla, CA, United States). In the TOR experiments, one-way ANOVA with treatment as between-subject factor was used to determine the main effect. Acoustic startle reactivity was analyzed by performing a two-way ANOVA with acoustic startle stimulus as a within-subjects factor and treatment as a between-subjects factor. To analyze PPI%, a two-way repeated-measures ANOVA with prepulse intensity as a within-subjects factor and treatment as a between-subjects factor was carried out. Changes in locomotor activity (number of crossings for each time-point) as well as induction of catalepsy were assessed by performing a two-way repeated-measures ANOVA with time-point as a within-subjects factor and treatment as a between-subjects factor. A one-way ANOVA with treatment as between-subject factor was carried out for the assessment of the total number of crossings. For all data analyses, upon confirmation of significant main effects, differences among individual means were assessed using the Newman–Keuls’ post hoc test. For all analyses, significance was accepted with a p value less than 0.05. Standard error of the mean (SEM) and variance were found similar between groups. All data are presented as mean ± SEM.
Results
Buspirone Counteracted MK-801-Induced Memory Deficits in WT Mice Tested in the TOR Paradigm
The discrimination performance of WT mice was significantly affected by pharmacological treatments, during the test phase of the TOR test (main effect of treatment, F(5,45) = 5.374, P = 0.0006, n = 8/10 per group). MK-801 induced a strong TOR memory impairment. Indeed, veh + MK-801-treated WT mice exhibited a greater preference in exploring the more recently experienced object in comparison with veh + veh-treated WT mice, which, as expected, spent more time exploring the less recently experienced object (post hoc analysis: P < 0.001 vs. veh + veh group; Figure 1B). Worthy of note, bus + MK-801-treated WT mice explored significantly more the less recently experienced object than the more recently one in a similar manner as veh + veh-treated WT mice (post hoc analysis: P < 0.01 vs. veh + MK-801 group; P > 0.05 vs. veh + veh group; Figure 1B). Thus, buspirone efficiently prevented MK-801-induced TOR memory impairment. Clo + MK-801-treated WT mice did not show an optimal discrimination performance even though they performed significantly better than veh + MK-801-treated WT mice and not differently from the veh + veh-treated WT mice (post hoc analysis: P < 0.05 vs. veh + MK-801 group; P > 0.05 vs. veh + veh group; Figure 1B). Both buspirone and clozapine, when injected alone, had no effect on discrimination performance (post hoc analysis: P > 0.05 vs. veh + veh group; Figure 1C).
Buspirone Blocked MK-801-Stimulated Hyperactivity and Did Not Cause Catalepsy in WT Mice
The pharmacological treatments significantly modified the locomotor activity of WT mice during each 5-min time-point [main effects of treatment, F(5,54) = 10.42, P < 0.0001; time-point, F(5,270) = 4.274, P = 0.0009; treatment × time-point interaction, F(25,270) = 6.18, P < 0.0001; n = 9/11 per group]. In addition, ANOVA showed a significant main effect of treatment [F(5,54) = 10.42, P < 0.0001] on the total crossings over 30 min for WT mice. As expected, MK-801 produced a strong hyperlocomotion in WT mice. Indeed, veh + MK-801-treated WT mice performed a significant higher number of crossings compared to veh + veh-treated WT mice (post hoc analysis: 5-min: p < 0.01; from 10-min to 30-min p < 0.001 Figures 2A,C). Interestingly, buspirone did not alter per se the locomotor activity (post hoc analysis: all time-points p > 0.05 vs. veh + veh group; Figures 2B,D), but it completely blocked MK-801-induced hyperactivity. Bus + MK-801-treated WT mice performed a significant lower number of crossings compared to veh + MK-801-treated WT mice (post hoc analysis: all time-points: p < 0.001 vs. veh + MK-801 group; Figures 2A,C), displaying a locomotor activity similar to that of veh + veh-treated WT mice (post hoc analysis: All time-points: p > 0.05 vs. veh + veh group; Figures 2A,C). Clozapine did not modify per se the locomotor activity (post hoc analysis: all time-points: p > 0.05 vs. veh + veh group; Figures 2B,D), but it significantly prevented MK-801-induced hyperactivity only in the first 10 min, loosing progressively its efficacy from the 15-min time point to the end of the test (post hoc analysis: 5-min: p < 0.001; 10-min: p < 0.05 vs. veh + MK-801 group. 15-min: p < 0.01; from 20-min to 30-min p < 0.001 vs. veh + veh group; Figures 2A,C).
FIGURE 2.
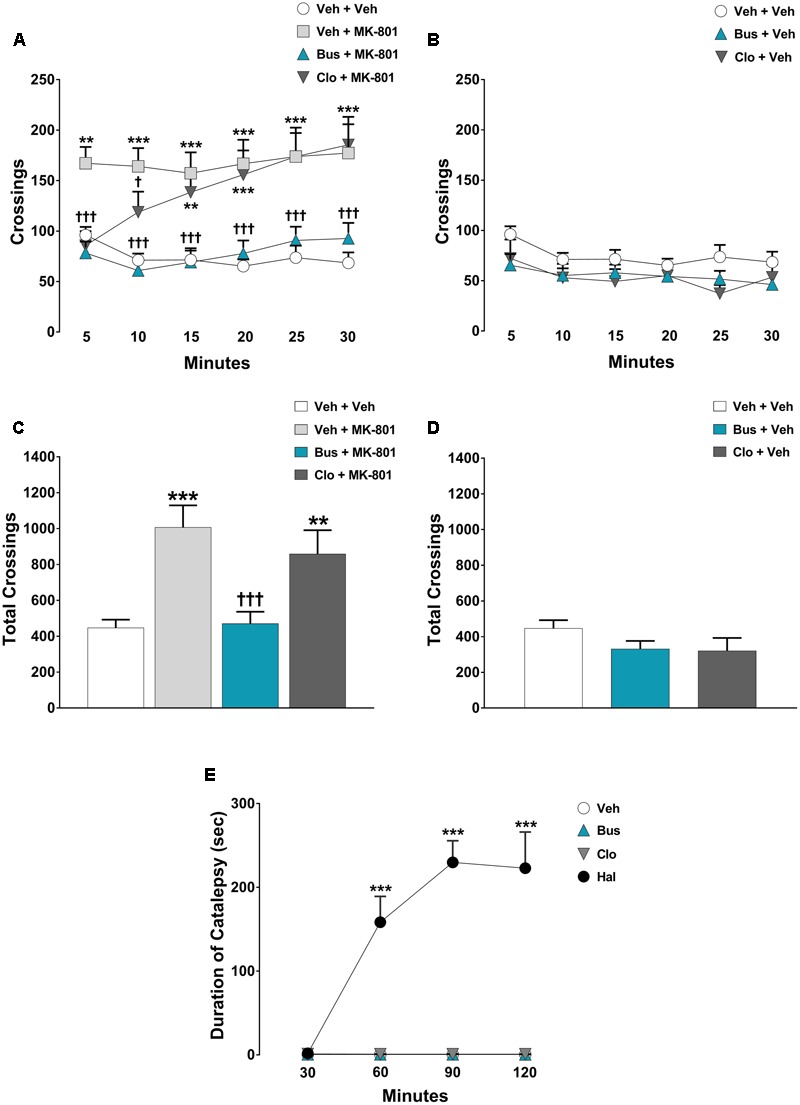
Buspirone blocked MK-801-stimulated hyperlocomotion, but did not cause catalepsy in WT mice. Buspirone (Bus, 3 mg⋅kg-1, i.p.) or vehicle (Veh), clozapine (Clo, 1 mg⋅kg-1, i.p.) or Veh and MK-801 (0.1 mg⋅kg-1, i.p.) or Veh were injected 45 min, 30 min and 20 min respectively, before the open field. (A,B) Locomotor activity (crossings) at each 5-min time point displayed by Veh + Veh (n = 11), Veh + MK-801 (n = 11), Bus + MK-801 (n = 10), Clo + MK-801 (n = 10), Bus + Veh (n = 9), Clo + Veh (n = 9) WT mice. (C,D) Locomotor activity (crossings) over a 30-min test period displayed by the same mice. (E) Duration of catalepsy state 30, 60, 90, and 120 min after drug injection (n = 6 animals/group). Haloperidol (Hal, 1 mg⋅kg-1) was used as positive control. Data are shown as mean ± SEM. ∗∗∗p < 0.001, ∗∗p < 0.01 vs. Veh + Veh WT mice; †††p < 0.001 and †p < 0.05 vs. Veh + MK-801 WT mice; ∗∗∗p < 0.001 vs. Veh (Two-way repeated-measures ANOVA and Newman–Keuls post hoc test).
Regarding the catalepsy test, significant main effects of treatment [F(3,20) = 48.11, P < 0.0001, n = 6 per group] and time-point [F(3,60) = 21.70, P < 0.0001], together with a significant treatment × time-point interaction [F(3,60) = 21.77, P < 0.0001] were found on the duration of catalepsy. As expected, haloperidol caused a severe catalepsy state (post-hoc analysis: all time-points: P < 0.001 vs. veh group; Figure 2E), an effect not induced by clozapine or buspirone (post hoc analysis: all time-points: P > 0.05 vs. veh group; Figure 2E).
Buspirone Blocked MK-801-Induced PPI Disruption in WT Mice
In the assessment of acoustic startle reactivity, ANOVA revealed a main effect of acoustic startle stimulus [F(1,110) = 76.42, P < 0.0001, n = 8/11 per group] but not a main effect of treatment [F(5,110) = 1.36, P = 0.2450] or a significant interaction between the factors [F(5,110) = 1.048, P = 0.3933]. Except for clozapine, which per se significantly decreased the acoustic startle reactivity at 120-dB (post hoc analysis at120 dB stimulus: P < 0.05 vs. veh + veh group; Figure 3B), all other experimental groups displayed similar acoustic startle reactivity (post hoc analysis: P > 0.05 vs. veh + veh group; Figures 3A,B). With regard to the PPI test, there were significant main effects of treatment [F(5,55) = 3.525, P = 0.0078] and prepulse intensity [F(4,220) = 49.16, P < 0.0001] but not a significant treatment × prepulse intensity interaction [F(20,220) = 1.4, P = 0.1238]. As expected, MK-801 significantly disrupted PPI; veh + MK-801-treated WT mice showed a progressively lower PPI% that reached statistical significance at 80 dB prepulse intensity, (post hoc analysis: P < 0.01 vs. veh + veh group; Figure 3C). Interestingly, buspirone, which had no effect on PPI when administered alone (post hoc analysis: P > 0.05 vs. veh + veh group; Figure 3D), completely blocked MK-801-induced PPI disruption. Bus + MK-801-treated WT mice exhibited PPI%, significantly greater than veh + MK-801-treated WT mice at 80 dB prepulse intensity, and similar to veh + veh-treated WT mice at all prepulse intensities (post hoc analysis: P < 0.01 vs. veh + MK-801 group, p > 0.05 vs. veh + veh group; Figure 3C). Noteworthy, clozapine per se disrupted PPI (post hoc analysis: 75 and 80 dB prepulse: p < 0.05 vs. veh + veh group; Figure 3D), but did not block MK-801-induced PPI disruption (post hoc analysis at 80 dB prepulse: p < 0.05 vs. veh + veh group and p > 0.05 vs. veh + MK-801 group; Figure 3C).
FIGURE 3.
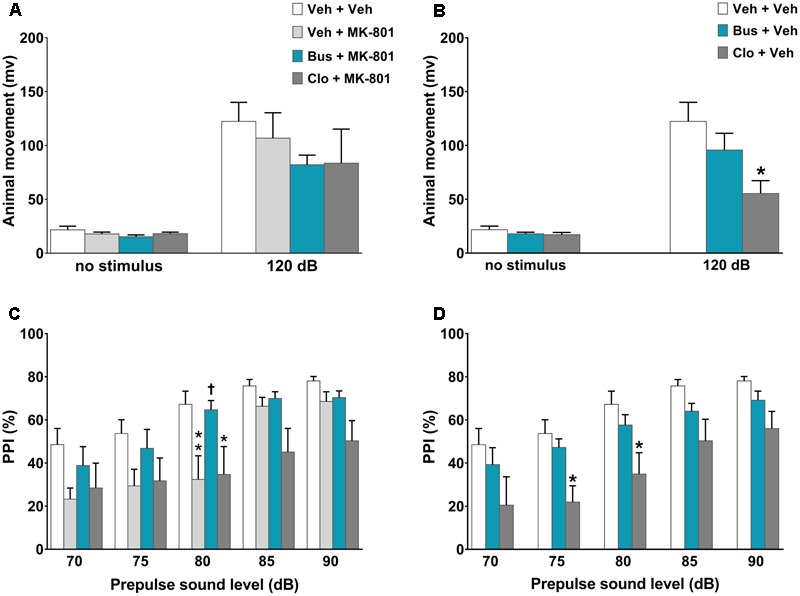
Buspirone blocked MK-801-induced PPI disruption in WT mice. Buspirone (Bus, 3 mg⋅kg-1, i.p.) or vehicle (Veh), clozapine (Clo, 1 mg⋅kg-1, i.p.) or Veh and MK-801 (0.1 mg⋅kg-1, i.p.) or Veh were injected 45, 30, and 20 min before the PPI test, respectively. (A,B) Animal movements displayed by Veh + Veh (n = 10), Veh + MK-801 (n = 10), Bus + MK-801 (n = 9), Clo + MK-801 (n = 8), Bus + Veh (n = 13), Clo + Veh (n = 8) WT mice. (C,D) PPI% displayed by the same WT mice. Data are shown as mean ± SEM. ∗∗p < 0.01,∗p < 0.05 vs. Veh + Veh WT mice; †p < 0.05 vs. Veh + MK-801 WT mice; (Two-way ANOVA with or without repeated-measures and Newman–Keuls post hoc test).
Buspirone Was Ineffective in Preventing MK-801-Induced TOR Memory Deficit and Scarcely Effective in Counteracting MK-801-Stimulated Hyperlocomotion in D3R-/- Mice
The memory of D3R-/- mice was significantly affected by pharmacological treatments, during the test phase of the TOR test [main effect of treatment F(3,18) = 7.478, P = 0.0019, n = 5/6 per group]. MK-801 produced a marked TOR memory deficit in D3R-/- mice, comparable to that observed in WT mice. In particularly, veh + MK-801-treated D3R-/- mice significantly preferred exploring the more recently experienced object than the less recently one in contrast with veh + veh-treated D3R-/- mice that displayed an intact TOR memory and behaved in the opposite way (post hoc analysis: P < 0.01 vs. veh + veh D3R-/- group; Figure 4A). Consistent with the hypothesis that buspirone acts on D3R receptors, bus + MK-801-treated D3R-/- mice behaved in a manner similar to veh + MK-801-treated D3R-/-, showing the same TOR memory impairment (post hoc analysis: P < 0.001 vs. veh + veh D3R-/- group, P > 0.05 vs. veh + MK-801 D3R-/-; Figure 4A), i.e., in D3R-/- buspirone was unable to prevent MK-801-induced TOR memory impairment as it did in WT mice. Notice that at variance with what observed in WT mice, in D3R-/- buspirone on its own disrupted the discrimination of the experienced objects, though not in a significant manner (post hoc analysis: P > 0.05 vs. veh + veh D3R-/- group; Figure 4B).
FIGURE 4.
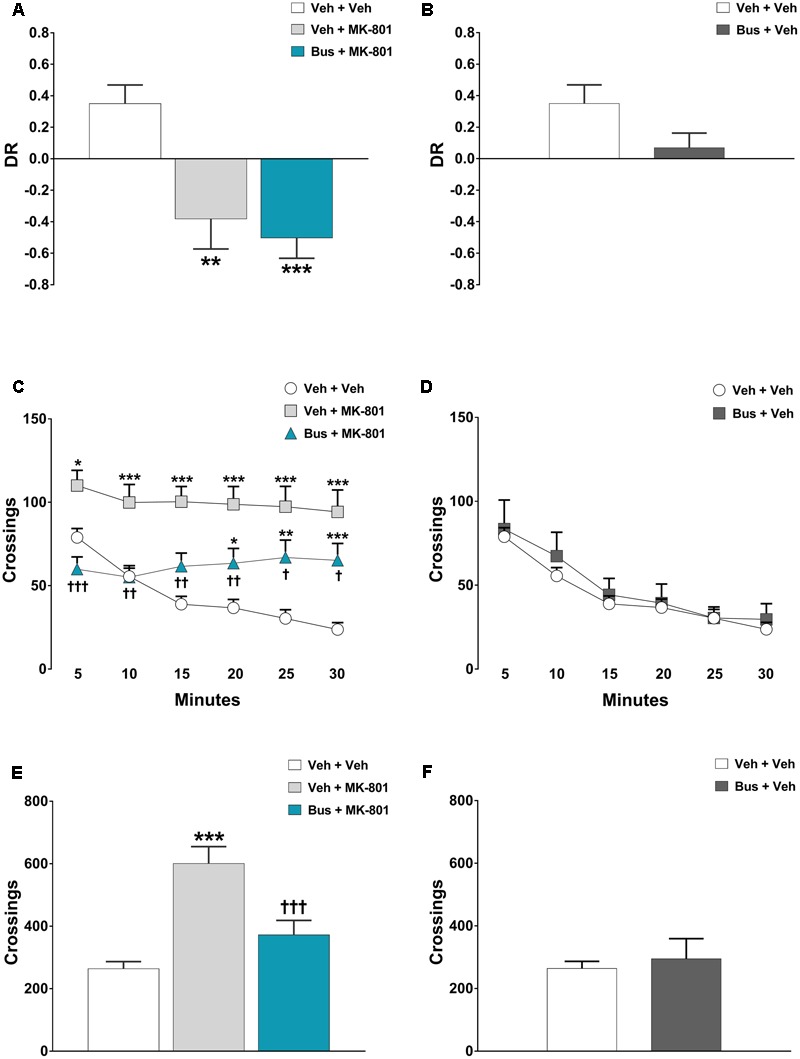
Buspirone was ineffective in preventing MK-801-induced TOR memory deficit and hyperlocomotion. Buspirone (Bus, 3 mg⋅kg-1, i.p.) or vehicle (Veh) and MK-801 (0.1 mg⋅kg-1, i.p.) or Veh were injected 45 min, and 20 min respectively, before the sample phase 2 or the open field test. (A,B) Discrimination ratio (DR) displayed by Veh + Veh (n = 5), Veh + MK-801 (n = 6), Bus + MK-801 (n = 5), Bus + Veh (n = 6), D3R-/- mice during the test phase. DR [(less recently experienced object exploration time - more recently experienced object exploration time)/total exploration time]. (C,D) Locomotor activity (crossings) at each 5-min time point displayed by Veh + Veh (n = 11), Veh + MK-801 (n = 11), Bus + MK-801 (n = 11), Bus + Veh (n = 6), D3R-/- mice. (E,F) Locomotor activity (crossings) over a 30-min test period displayed by the same mice. Data are shown as mean ± SEM. ∗∗∗p < 0.001, ∗∗p < 0.01, ∗p < 0.05 vs. Veh + Veh D3R-/- mice; †††p < 0.001, ††p < 0.01 and †p < 0.05 vs. Veh + MK-801 WT mice; (One-way or two-way repeated-measures ANOVA and Newman–Keuls post hoc test).
In the OF test, MK-801 produced also a robust and persistent hyperlocomotion in D3R-/- mice; significant main effects of treatment [F(3,35) = 11.74, P < 0.0001, n = 8/11 per group] and time-point [F(5,175) = 15.92, P < 0.0001], and a significant treatment x time-point interaction [F(15,175) = 5.123, P < 0.0001] were found on locomotor activity of D3R-/- mice during each 5-min time-point. Moreover, ANOVA revealed a significant main effect of treatment [F(3,35) = 11.34, P < 0.0001] on the total crossings that D3R-/- mice performed throughout the 30 min of the test. Veh + MK-801-treated D3R-/- mice, compared to veh + veh-treated D3R-/- mice, carried out a significant higher number of crossings (post hoc analysis at 5-min: p < 0.05; from 10-min to 30-min: p < 0.001; Figures 4C,E). Buspirone, which was devoid of effect when injected alone (post-hoc analysis: all time-points p > 0.05 vs. veh + veh D3R-/- group; Figures 4D,F), significantly attenuated MK-801-stimulated hyperlocomotion, but its effect diminished from the 10-min time point on. Indeed, Bus + MK-801-treated D3R-/- mice performed a number of crossings similar to that of veh + veh-treated D3R-/- mice but significantly lower compared to veh + MK-801-treated D3R-/- mice during the first 10 min (post hoc analysis at 5-min: p < 0.001; from 10-min to 20-min: p < 0.01; at 25-min and 30-min: p < 0.05 vs. veh + MK-801 D3R-/- group; Figures 4C,E).
Discussion
These results provide the first evidence that buspirone counteracts a wide-range of schizophrenia-relevant phenotypes through its antagonism at D3R.
To investigate the antipsychotic properties of buspirone, we chose a pharmacological model based on NMDAR hypofunction triggered by acute administration of the NMDAR antagonist MK-801. Although not devoid of limitations, this model is extensively employed for the assessment of potential antipsychotic activity of investigational compounds (Bubeníková-Valesová et al., 2008; Adell et al., 2012). Indeed, NMDAR dysfunction may recapitulate “core” symptoms of schizophrenia, particularly the multiplicity of cognitive deficits, more faithfully than dopamine-based models (Kantrowitz and Javitt, 2010).
Cognitive deficits observed in schizophrenic patients have been strongly associated with an abnormal PFC activity (Driesen et al., 2008). Earlier studies indicated that the cognitive impairment induced by MK-801 arises from an intensification of the discharge of mPFC pyramidal neurons, triggered via NMDAR blockade in inhibitory interneurons of mPFC and hippocampus (HP, Homayoun et al., 2005; Jodo et al., 2005; Homayoun and Moghaddam, 2007). D3Rs are expressed specifically in layer 5 pyramidal neurons of mPFC of both primate and rodents (Lidow et al., 1998) and uniquely modulate the neuronal excitability (Clarkson et al., 2017). Consequently, D3Rs play a fundamental role in prefrontal-dependent cognitive functions (Nakajima et al., 2013). Studies on dopamine receptor-specific reporter gene mice further revealed an abundant expression of D3Rs in HP1; furthermore, hippocampal lesions leave single item object recognition memory intact, while impair temporal order memory (Warburton and Brown, 2015). Based on these premises, we assessed the effect of buspirone in the TOR memory task. This behavioral task depends on interconnections among mPFC, perirhinal cortex (PRH) and HP (Barker and Warburton, 2011; Managò et al., 2016) and is used to measure recency discrimination, a cognitive function impaired in schizophrenic patients (Schwartz et al., 1991; Rizzo et al., 1996). To our knowledge, this is the first study demonstrating that acute systemic administration of MK-801 at the dose of 0.1 mg⋅kg-1, markedly impairs TOR memory in mice. Therefore, our results confirm the face validity of the pharmacological model based on NMDAR hypofunction triggered by acute administration of the NMDAR antagonist MK-801, being also consistent with earlier findings showing a disruption of TOR memory following intra-PRH or intra-mPFC infusion of the selective NMDAR antagonist AP5 (Warburton et al., 2013). We found that buspirone prevented MK-801-induced TOR memory impairment in WT mice even better than clozapine. Very interestingly, this effect was completely abolished in D3R-/- mice. Thus, these data provide the first evidence that buspirone may be effective in treating cognitive deficits in schizophrenia, and that its efficacy against MK-801-induced cognitive dysfunction relies exclusively on D3Rs blockade. These findings are particularly relevant, considering that cognitive dysfunction represents a major challenge in the pharmacological treatment of schizophrenic patients. Furthermore, our results are consistent with previous studies, reporting that some antipsychotics that behave as selective D3R antagonists or D3R preferring partial agonists enhance cognitive functions in schizophrenia (Nakajima et al., 2013; Zimnisky et al., 2013). None of the available antipsychotics is truly selective for D3R (Schotte et al., 1996; McCormick et al., 2010); as a result, current drug treatments generally improve positive symptoms (delusions, hallucinations), but poorly change negative symptoms (lack of motivation, social withdrawal, anhedonia) or cognitive dysfunction. The present data obtained with buspirone reinforce the view that blockade of D3R may improve cognition, which represents a translational potential for schizophrenia treatment.
Recently, Barker et al. (2017) discovered that the pharmacogenetic deactivation of a specific neuronal circuit originating in the dorsal CA1 region of HP and projecting to mPFC, selectively disrupts TOR memory in mice. Thus, we speculate that a glutamatergic/dopaminergic imbalance in specific neuronal circuits connecting HP and mPFC might disrupt the connection between these two brain areas, leading to memory impairment in mice tested in the TOR paradigm. In this context, D3R blockade, particularly in mPFC and HP, might prevent the hyperactivity of the dopaminergic system subsequent to NMDARs hypofunction (Snyder and Gao, 2013). However, because D3R-/- mice appeared to be as sensitive as WT mice to the cognitive effects of acute administration of MK-801, other neurotransmitters and/or dopamine receptor subtypes are likely to be involved, and may represent compensatory mechanisms that prevails over D3R control in D3R-/- mice.
Hyperactivity is a valuable correlate, easily modeled in rodents, widely associated with positive symptoms and psychomotor agitation in most schizophrenic patients (Jones et al., 2011). Here, we found that buspirone blocked MK-801-stimulated hyperactivity, but did not cause catalepsy in WT mice; moreover, because the preventing effect of buspirone on MK-801-stimulated hyperactivity was not very strong in D3R-/- mice, it must be, at least in part, attributable to D3R antagonism. This conclusion is consistent with earlier studies showing D3R antagonists as effective on MK-801-stimulated hyperactivity (Leriche et al., 2003; Brindisi et al., 2014; Sun et al., 2016). Considering that positive symptoms are not well-managed in a considerable number of patients suffering from schizophrenia (Miyamoto et al., 2012), our observation, together with other published reports, points to D3R as potential target to treat hyperactivity.
We cannot exclude a contribution of other receptors targeted by buspirone in mediating its antipsychotic-like effects in our experimental paradigms. Buspirone in fact, binds to 5-HT1AR, where it behaves as potent partial agonist (Bergman et al., 2013), and several studies have reported that 5-HT1AR antagonists or partial agonists attenuate psychotomimetic effects of MK-801 (Wedzony et al., 2000; Park et al., 2005; Bubenikova-Valesova et al., 2010). Furthermore, buspirone also binds to D4R with high affinity and behaves as antagonist (Bergman et al., 2013). A highly selective dopamine D4R antagonist was found to decrease amphetamine-induced hyperlocomotion (Boeckler et al., 2004). Consequently, we cannot exclude a contribute of D4R in the effects we reported here.
PPI is a valuable model to study the sensorimotor gating disruption classically observed in schizophrenia (Papaleo et al., 2012). Because animals and humans are tested in a similar way, this model has face, construct, and predictive validity and is widely employed to identify potential antipsychotic properties of recently developed drugs (Rigdon and Viik, 1991). Our findings demonstrated that buspirone, devoid of effect by itself, completely counteracted PPI disruption dependent on NMDAR hypofunction. These results are partially in agreement with previous studies showing that buspirone weakly counteracts apomorphine-induced PPI disruption (Rigdon and Viik, 1991) while it was without effect on its own (Van den Buuse and Gogos, 2007). The antipsychotic-like effect of buspirone on MK-801-induced PPI disruption might be mainly driven by its antagonist activity at D3R. Several reports proved that selective D3R antagonists improve PPI disruption in different preclinical models of schizophrenia (Zhang et al., 2006; Maramai et al., 2016; Sun et al., 2016). Unfortunately we could not directly address the D3R involvement on the buspirone’s effect in PPI by using D3R-/-, because these mice did not exhibit a robust acoustic startle reactivity, suitable for making reliable measurements. However, it is unlikely that the 5 HT1AR partial agonist activity of buspirone could contribute to its efficacy on MK-801-induced PPI disruption. Bubenikova-Valesova et al. (2010) found the selective 5-HT1AR partial agonist tandospirone exacerbates MK-801-induced PPI disruption and other groups reported a PPI disruption after 5-HT1AR stimulation (Rigdon and Weatherspoon, 1992; Gogos and Van den Buuse, 2003; Gogos et al., 2006). Again, we cannot exclude the possible involvement of the D4R blockade also in the effect of buspirone in MK-801-induced PPI disruption. However, contrasting results have shown positive/negative effects of D4R antagonists in ameliorating apomorphine-induced PPI disruption (Bristow et al., 1997; Mansbach et al., 1998; Boeckler et al., 2004). Worthy of note, clozapine did not prevent MK-801-induced PPI disruption or MK-801-stimulated hyperlocomotion. Considering that clozapine is one of the most effective antipsychotic drugs, the discrepancy with its poor efficacy in preclinical models point once more to the need for defining “gold pharmacological standards” preclinical models of schizophrenia (Jones et al., 2011), taking into account that doses, strains, behavioral paradigms, all affect the variability, reproducibility and translationality to clinical settings.
Conclusion
The present study demonstrates that buspirone, a drug currently approved for the treatment of anxious disorders, might be a potential antipsychotic medication and also that D3R represents a valuable pharmacological target especially for the treatment of cognitive deficits in schizophrenia. Anxious symptoms and cognitive impairment frequently co-occur especially in the prodromal phase of the disease, when the positive symptoms are below the threshold for psychosis (Corigliano et al., 2014). In this scenario, buspirone might represent a new pharmacological tool to treat the early phase of the disease. Indeed, the early intervention is the best way to prevent development of chronic disabilities. Finally, these findings are particularly relevant considering that a substantial number of pharmaceutical industries are turning away from developing antipsychotics for many reasons, including costs, unclear disease mechanisms and long-lasting developmental processes. Repositioning of buspirone and/or of other drugs endowed with D3R antagonist activity, could therefore open new avenues to foster schizophrenia drug treatments. However, further studies are needed to evaluate the efficacy of this drug after chronic treatment in an animal model provided with the three criteria of face, construct and predictive validity.
Author Contributions
ST, SS, and GL designed research; ST, FG, and GL performed research; FC and CB helped with some experiments; ST and GL analyzed data; FD, SS, and GL supervised the work; ST and GL wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by Finanziamento Ricerca di Ateneo – FIR Unict 7E646B. FG was supported by the Ph.D. program in Neuroscience, Catania University.
Footnotes
References
- Accili D., Fishburn C. S., Drago J., Steiner H., Lachowicz J. E., Park B. H., et al. (1996). A targeted mutation of the D3 dopamine receptor gene is associated with hyperactivity in mice. Proc. Natl. Acad. Sci. U.S.A. 93 1945–1949. 10.1073/pnas.93.5.1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adell A., Jiménez-Sánchez L., López-Gil X., Romón T. (2012). Is the acute NMDA receptor hypofunction a valid model of schizophrenia? Schizophr. Bull. 38 9–14. 10.1093/schbul/sbr133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburn T. T., Thor K. B. (2004). Drug repositioning: identifying and developing new uses for existing drugs. Nat. Rev. Drug Discov. 3 673–683. 10.1038/nrd1468 [DOI] [PubMed] [Google Scholar]
- Barker G. R., Banks P. J., Scott H., Ralph G. S., Mitrophanous K. A., Wong L. F., et al. (2017). Separate elements of episodic memory subserved by distinct hippocampal-prefrontal connections. Nat. Neurosci. 20 242–250. 10.1038/nn.4472 [DOI] [PubMed] [Google Scholar]
- Barker G. R., Warburton E. C. (2011). When is the hippocampus involved in recognition memory? J Neurosci. 31 10721–10731. 10.1523/JNEUROSCI.6413-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman J., Roof R. A., Furman C. A., Conroy J. L., Mello N. K., Sibley D. R., et al. (2013). Modification of cocaine self-administration by buspirone (buspar(R)): potential involvement of D3 and D4 dopamine receptors. Int. J. Neuropsychopharmacol. 16 445–458. 10.1017/S1461145712000661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blot K., Kimura S., Bai J., Kemp A., Manahan-Vaughan D., Giros B., et al. (2015). Modulation of hippocampus-prefrontal cortex synaptic transmission and disruption of executive cognitive functions by MK-801. Cereb. Cortex 25 1348–1361. 10.1093/cercor/bht329 [DOI] [PubMed] [Google Scholar]
- Boeckler F., Russig H., Zhang W., Löber S., Schetz J., Hübner H., et al. (2004). FAUC 213, a highly selective dopamine D4 receptor full antagonist, exhibits atypical antipsychotic properties in behavioural and neurochemical models of schizophrenia. Psychopharmacology (Berl). 175 7–17. 10.1007/s00213-004-1782-1 [DOI] [PubMed] [Google Scholar]
- Brindisi M., Butini S., Franceschini S., Brogi S., Trotta F., Ros S., et al. (2014). Targeting dopamine D3 and serotonin 5-HT1A and 5-HT2A receptors for developing effective antipsychotics: synthesis, biological characterization, and behavioral studies. J. Med. Chem. 57 9578–9597. 10.1021/jm501119j [DOI] [PubMed] [Google Scholar]
- Bristow L. J., Collinson N., Cook G. P., Curtis N., Freedman S. B., Kulagowski J. J., et al. (1997). L-745,870, a subtype selective dopamine D4 receptor antagonist, does not exhibit a neuroleptic-like profile in rodent behavioral tests. J. Pharmacol. Exp. Ther. 283 1256–1263. [PubMed] [Google Scholar]
- Bubeníková-Valesová V., Horácek J., Vrajová M., Höschl C. (2008). Models of schizophrenia in humans and animals based on inhibition of NMDA receptors. Neurosci. Biobehav. Rev. 32 1014–1023. 10.1016/j.neubiorev.2008.03.012 [DOI] [PubMed] [Google Scholar]
- Bubenikova-Valesova V., Svoboda J., Horacek J., Sumiyoshi T. (2010). Effect of tandospirone, a serotonin-1A receptor partial agonist, on information processing and locomotion in dizocilpine-treated rats. Psychopharmacology (Berl). 212 267–276. 10.1007/s00213-010-1951-3 [DOI] [PubMed] [Google Scholar]
- Clarkson R. L., Liptak A. T., Gee S. M., Sohal V. S., Bender K. J. (2017). D3 receptors regulate excitability in a unique class of prefrontal pyramidal cells. J. Neurosci. 37 5846–5860. 10.1523/JNEUROSCI.0310-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corigliano V., De Carolis A., Trovini G., Dehning J., Di Pietro S., Curto M., et al. (2014). Neurocognition in schizophrenia: from prodrome to multi-episode illness. Psychiatry Res. 220 129–134. 10.1016/j.psychres.2014.07.067 [DOI] [PubMed] [Google Scholar]
- Cui Y., Prabhu V., Nguyen T. B., Yadav B. K., Chung Y. C. (2015). The mRNA expression status of dopamine receptor D2, dopamine receptor D3 and DARPP-32 in T lymphocytes of patients with early psychosis. Int. J. Mol. Sci. 16 26677–26686. 10.3390/ijms161125983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Ciano P., Cormick P. M., Stefan C., Wong E., Kim A., Remington G., et al. (2017). The effects of buspirone on occupancy of dopamine receptors and the rat gambling task. Psychopharmacology (Berl) 10.1007/s00213-017-4715-5 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Driesen N. R., Leung H. C., Calhoun V. D., Constable R. T., Gueorguieva R., Hoffman R., et al. (2008). Impairment of working memory maintenance and response in schizophrenia: functional magnetic resonance imaging evidence. Biol. Psychiatry 64 1026–1034. 10.1016/j.biopsych.2008.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley W., Durgam S., Lu K., Laszlovszky I., Debelle M., Kane J. M. (2017). Safety and tolerability of cariprazine in patients with acute exacerbation of schizophrenia: a pooled analysis of four phase II/III randomized, double-blind, placebo-controlled studies. Int. Clin. Psychopharmacol. 10.1097/YIC.0000000000000187 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink-Jensen A., Schmidt L. S., Dencker D., Schülein C., Wess J., Wörtwein G., et al. (2011). Antipsychotic-induced catalepsy is attenuated in mice lacking the M4 muscarinic acetylcholine receptor. Eur. J. Pharmacol. 656 39–44. 10.1016/j.ejphar.2011.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaleiha A., Noorbala A. A., Farnaghi F., Hajiazim M., Akhondzadeh S. (2010). A double-blind, randomized, and placebo-controlled trial of buspirone added to risperidone in patients with chronic schizophrenia. J. Clin. Psychopharmacol. 30 678–682. 10.1097/JCP.0b013e3181fa8720 [DOI] [PubMed] [Google Scholar]
- Gogos A., Nathan P. J., Guille V., Croft R. J., van den Buuse M. (2006). Estrogen prevents 5-HT1A receptor-induced disruptions of prepulse inhibition in healthy women. Neuropsychopharmacology 31 885–889. 10.1038/sj.npp.1300933 [DOI] [PubMed] [Google Scholar]
- Gogos A., Van den Buuse M. (2003). Castration reduces the effect of serotonin-1A receptor stimulation on prepulse inhibition in rats. Behav. Neurosci. 117 1407–1415. 10.1037/0735-7044.117.6.1407 [DOI] [PubMed] [Google Scholar]
- Gross G., Drescher K. (2012). The role of dopamine D(3) receptors in antipsychotic activity and cognitive functions. Handb. Exp. Pharmacol. 213 167–210. 10.1007/978-3-642-25758-2_7 [DOI] [PubMed] [Google Scholar]
- Gross G., Wicke K., Drescher K. U. (2013). Dopamine D3 receptor antagonism–still a therapeutic option for the treatment of schizophrenia. Naunyn Schmiedebergs Arch. Pharmacol. 386 155–166. 10.1007/s00210-012-0806-3 [DOI] [PubMed] [Google Scholar]
- Gurevich E. V., Bordelon Y., Shapiro R. M., Arnold S. E., Gur R. E., Joyce J. N. (1997). Mesolimbic dopamine D3 receptors and use of antipsychotics in patients with schizophrenia. A postmortem study. Arch. Gen. Psychiatry. 54 225–232. 10.1001/archpsyc.1997.01830150047009 [DOI] [PubMed] [Google Scholar]
- Homayoun H., Jackson M. E., Moghaddam B. (2005). Activation of metabotropic glutamate 2/3 receptors reverses the effects of NMDA receptor hypofunction on prefrontal cortex unit activity in awake rats. J. Neurophysiol. 93 1989–2001. 10.1152/jn.00875.2004 [DOI] [PubMed] [Google Scholar]
- Homayoun H., Moghaddam B. (2007). NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J. Neurosci. 27 11496–11500. 10.1523/JNEUROSCI.2213-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori H., Yamada K., Kamada D., Shibata Y., Katsuki A., Yoshimura R., et al. (2014). Effect of blonanserin on cognitive and social function in acute phase Japanese schizophrenia compared with risperidone. Neuropsychiatr. Dis. Treat. 10 527–533. 10.2147/NDT.S59861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi M., Meltzer H. Y. (2012). The role of 5-HT1A receptors in phencyclidine (PCP)-induced novel object recognition (NOR) deficit in rats. Psychopharmacology (Berl.) 221 205–215. 10.1007/s00213-011-2561-4 [DOI] [PubMed] [Google Scholar]
- Jodo E., Suzuki Y., Katayama T., Hoshino K. Y., Takeuchi S., Niwa S., et al. (2005). Activation of medial prefrontal cortex by phencyclidine is mediated via a hippocampo-prefrontal pathway. Cereb. Cortex 15 663–669. 10.1093/cercor/bhh168 [DOI] [PubMed] [Google Scholar]
- Jones C. A., Watson D. J., Fone K. C. (2011). Animal models of schizophrenia. Br. J. Pharmacol. 164 1162–1194. 10.1111/j.1476-5381.2011.01386.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantrowitz J. T., Javitt D. C. (2010). N-methyl-D-aspartate (n.d.) receptor dysfunction or dysregulation: the final common pathway on the road to schizophrenia? Brain Res. Bull. 83 108–121. 10.1016/j.brainresbull.2010.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landek-Salgado M. A., Faust T. E., Sawa A. (2016). Molecular substrates of schizophrenia: homeostatic signaling to connectivity. Mol. Psychiatry 21 10–28. 10.1038/mp.2015.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. M., Kim Y. (2016). Drug repurposing is a new opportunity for developing drugs against neuropsychiatric disorders. Schizophr. Res. Treat. 2016:6378137 10.1155/2016/6378137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio G. M., Bucolo C., Platania C. B., Salomone S., Drago F. (2016). Current drug treatments targeting dopamine D3 receptor. Pharmacol. Ther. 165 164–177. 10.1016/j.pharmthera.2016.06.007 [DOI] [PubMed] [Google Scholar]
- Leggio G. M., Camillieri G., Platania C. B., Castorina A., Marrazzo G., Torrisi S. A., et al. (2014). Dopamine D3 receptor is necessary for ethanol consumption: an approach with buspirone. Neuropsychopharmacology 39 2017–2028. 10.1038/npp.2014.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio G. M., Micale V., Le Foll B., Mazzola C., Nobrega J. N., Drago F. (2011). Dopamine D3 receptor knock-out mice exhibit increased behavioral sensitivity to the anxiolytic drug diazepam. Eur. Neuropsychopharmacol. 21 325–332. 10.1016/j.euroneuro.2010.05.006 [DOI] [PubMed] [Google Scholar]
- Leggio G. M., Torrisi S. A., Castorina A., Platania C. B., Impellizzeri A. A., Fidilio A., et al. (2015). Dopamine D3 receptor-dependent changes in alpha6 GABAA subunit expression in striatum modulate anxiety-like behaviour: responsiveness and tolerance to diazepam. Eur. Neuropsychopharmacol. 25 1427–1436. 10.1016/j.euroneuro.2014.11.004 [DOI] [PubMed] [Google Scholar]
- Leriche L., Schwartz J. C., Sokoloff P. (2003). The dopamine D3 receptor mediates locomotor hyperactivity induced by NMDA receptor blockade. Neuropharmacology 45 174–181. 10.1016/S0028-3908(03)00145-X [DOI] [PubMed] [Google Scholar]
- Lidow M. S., Wang F., Cao Y., Goldman-Rakic P. S. (1998). Layer V neurons bear the majority of mRNAs encoding the five distinct dopamine receptor subtypes in the primate prefrontal cortex. Synapse 28 10–20. [DOI] [PubMed] [Google Scholar]
- Maeda K., Lerdrup L., Sugino H., Akazawa H., Amada N., McQuade R. D., et al. (2014). Brexpiprazole II: antipsychotic-like and procognitive effects of a novel serotonin-dopamine activity modulator. J. Pharmacol. Exp. Ther. 350 605–614. 10.1124/jpet.114.213819 [DOI] [PubMed] [Google Scholar]
- Managò F., Mereu M., Mastwal S., Mastrogiacomo R., Scheggia D., Emanuele M., et al. (2016). Genetic disruption of Arc/Arg3.1 in mice causes alterations in dopamine and neurobehavioral phenotypes related to Schizophrenia. Cell Rep. 16 2116–2128. 10.1016/j.celrep.2016.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansbach R. S., Brooks E. W., Sanner M. A., Zorn S. H. (1998). Selective dopamine D4 receptor antagonists reverse apomorphine-induced blockade of prepulse inhibition. Psychopharmacology (Berl). 135 194–200. 10.1007/s002130050501 [DOI] [PubMed] [Google Scholar]
- Maramai S., Gemma S., Brogi S., Campiani G., Butini S., Stark H., et al. (2016). Dopamine D3 receptor antagonists as potential therapeutics for the treatment of neurological diseases. Front. Neurosci. 10:451 10.3389/fnins.2016.00451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick P. N., Kapur S., Graff-Guerrero A., Raymond R., Nobrega J. N., Wilson A. A. (2010). The antipsychotics olanzapine, risperidone, clozapine, and haloperidol are D2-selective ex vivo but not in vitro. Neuropsychopharmacology 35 1826–1835. 10.1038/npp.2010.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S., Miyake N., Jarskog L. F., Fleischhacker W. W., Lieberman J. A. (2012). Pharmacological treatment of schizophrenia: a critical review of the pharmacology and clinical effects of current and future therapeutic agents. Mol. Psychiatry 17 1206–1227. 10.1038/mp.2012.47 [DOI] [PubMed] [Google Scholar]
- Mutlu O., Ulak G., Celikyurt I. K., Akar F. Y., Erden F., Tanyeri P. (2011). Effects of olanzapine, sertindole and clozapine on MK-801 induced visual memory deficits in mice. Pharmacol. Biochem. Behav. 99 557–565. 10.1016/j.pbb.2011.06.011 [DOI] [PubMed] [Google Scholar]
- Nakajima S., Gerretsen P., Takeuchi H., Caravaggio F., Chow T., Le Foll B., et al. (2013). The potential role of dopamine D3 receptor neurotransmission in cognition. Eur. Neuropsychopharmacol. 23 799–813. 10.1016/j.euroneuro.2013.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunokawa A., Watanabe Y., Kaneko N., Sugai T., Yazaki S., Arinami T., et al. (2010). The dopamine D3 receptor (DRD3) gene and risk of schizophrenia: case-control studies and an updated meta-analysis. Schizophr. Res. 116 61–67. 10.1016/j.schres.2009.10.016 [DOI] [PubMed] [Google Scholar]
- Owen M. J., Sawa A., Mortensen P. B. (2016). Schizophrenia. Lancet 388 86–97. 10.1016/S0140-6736(15)01121-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaleo F., Yang F., Garcia S., Chen J., Lu B., Crawley J. N., et al. (2012). Dysbindin-1 modulates prefrontal cortical activity and schizophrenia-like behaviors via dopamine/D2 pathways. Mol. Psychiatry 17 85–98. 10.1038/mp.2010.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. J., Lee Y., Oh H. K., Lee H. E., Lee Y., Ko S. Y., et al. (2014). Oleanolic acid attenuates MK-801-induced schizophrenia-like behaviors in mice. Neuropharmacology 86 49–56. 10.1016/j.neuropharm.2014.06.025 [DOI] [PubMed] [Google Scholar]
- Park W. K., Jeong D., Cho H., Lee S. J., Cha M. Y., Pae A. N., et al. (2005). KKHA-761, a potent D3 receptor antagonist with high 5-HT1A receptor affinity, exhibits antipsychotic properties in animal models of schizophrenia. Pharmacol. Biochem. Behav. 82 361–372. 10.1016/j.pbb.2005.09.006 [DOI] [PubMed] [Google Scholar]
- Piskulić D., Olver J. S., Maruff P., Norman T. R. (2009). Treatment of cognitive dysfunction in chronic schizophrenia by augmentation of atypical antipsychotics with buspirone, a partial 5-HT(1A) receptor agonist. Hum. Psychopharmacol. 24 437–446. 10.1002/hup.1046 [DOI] [PubMed] [Google Scholar]
- Pogorelov V. M., Rodriguiz R. M., Cheng J., Huang M., Schmerberg C. M., Meltzer H. Y., et al. (2017). 5-HT2C agonists modulate Schizophrenia-like behaviors in mice. Neuropsychopharmacology 42 2163–2177. 10.1038/npp.2017.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigdon G. C., Viik K. (1991). Prepulse inhibition as a screening test for potential antipsychotics. Drug Dev. Res. 23 91–99. 10.1002/ddr.430230110 [DOI] [Google Scholar]
- Rigdon G. C., Weatherspoon J. K. (1992). 5-Hydroxytryptamine1a receptor agonists block prepulse inhibition of acoustic startle reflex. J. Pharmacol. Exp. 263 486–493. [PubMed] [Google Scholar]
- Rizzo L., Danion J. M., van der Linden M., Grange D. (1996). Patients with schizophrenia remember that an event has occurred, but not when. Br. J. Psychiatry 168 427–431. 10.1192/bjp.168.4.427 [DOI] [PubMed] [Google Scholar]
- Schotte A., Janssen P. F., Gommeren W., Luyten W. H., Van Gompel P., Lesage A. S., et al. (1996). Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology 124 57–73. 10.1007/BF02245606 [DOI] [PubMed] [Google Scholar]
- Schwartz B. L., Deutsch L. H., Cohen C., Warden D., Deutsch S. I. (1991). Memory for temporal order in schizophrenia. Biol. Psychiatry 29 329–339. 10.1016/0006-3223(91)90218-B [DOI] [PubMed] [Google Scholar]
- Sheikhmoonesi F., Zarghami M., BahariSaravi S. F., Khalilian A., Ala S. (2015). A triple-blinded, randomized, placebo-controlled trial to examine the efficacy of buspirone added to typical antipsychotic drugs in patients with chronic schizophrenia. J. Res. Med. Sci. 20 140–145. [PMC free article] [PubMed] [Google Scholar]
- Snyder M. A., Gao W. J. (2013). NMDA hypofunction as a convergence point for progression and symptoms of schizophrenia. Front. Cell Neurosci. 7:31 10.3389/fncel.2013.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff P., Le Foll B. (2017). The dopamine D3 receptor, a quarter century later. Eur. J. Neurosci. 45 2–19. 10.1111/ejn.13390 [DOI] [PubMed] [Google Scholar]
- Spooren W., Mombereau C., Maco M., Gill R., Kemp J. A., Ozmen L., et al. (2004). Pharmacological and genetic evidence indicates that combined inhibition of NR2A and NR2B subunit containing NMDA receptors is required to disrupt prepulse inhibition. Psychopharmacology (Berl). 175 99–105. 10.1007/s00213-004-1785-y [DOI] [PubMed] [Google Scholar]
- Sun X., Gou H. Y., Li F., Lu G. Y., Song R., Yang R. F., et al. (2016). Y-QA31, a novel dopamine D3 receptor antagonist, exhibits antipsychotic-like properties in preclinical animal models of schizophrenia. Acta Pharmacol. Sin. 37 322–333. 10.1038/aps.2015.105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Buuse M., Gogos A. (2007). Differential effects of antipsychotic drugs on serotonin-1A receptor-mediated disruption of prepulse inhibition. J. Pharmacol. Exp. Ther. 320 1224–1236. 10.1124/jpet.106.113084 [DOI] [PubMed] [Google Scholar]
- Warburton E. C., Barker G. R., Brown M. W. (2013). Investigations into the involvement of NMDA mechanisms in recognition memory. Neuropharmacology 74 41–47. 10.1016/j.neuropharm.2013.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton E. C., Brown M. W. (2015). Neural circuitry for rat recognition memory. Behav. Brain Res. 285 131–139. 10.1016/j.bbr.2014.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D. J., Loiseau F., Ingallinesi M., Millan M. J., Marsden C. A., Fone K. C. (2012). Selective blockade of dopamine D3 receptors enhances while D2 receptor antagonism impairs social novelty discrimination and novel object recognition in rats: a key role for the prefrontal cortex. Neuropsychopharmacology 37 770–786. 10.1038/npp.2011.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedzony K., Maćkowiak M., Zajaczkowski W., Fijał K., Chocyk A., Czyrak A. (2000). WAY 100135, an antagonist of 5-HT1A serotonin receptors, attenuates psychotomimetic effects of MK-801. Neuropsychopharmacology 23 547–559. 10.1016/S0893-133X(00)00150-0 [DOI] [PubMed] [Google Scholar]
- Zhang L., Shirayama Y., Iyo M., Hashimoto K. (2007). Minocycline attenuates hyperlocomotion and prepulse inhibition deficits in mice after administration of the NMDA receptor antagonist dizocilpine. Neuropsychopharmacology 32 2004–2010. 10.1038/sj.npp.1301313 [DOI] [PubMed] [Google Scholar]
- Zhang M., Ballard M. E., Kohlhaas K. L., Browman K. E., Jongen-Rêlo A. L., Unger L. V., et al. (2006). Effect of dopamine D3 antagonists on PPI in DBA/2J mice or PPI deficit induced by neonatal ventral hippocampal lesions in rats. Neuropsychopharmacology 31 1382–1392. 10.1038/sj.npp.1300985 [DOI] [PubMed] [Google Scholar]
- Zimnisky R., Chang G., Gyertyán I., Kiss B., Adham N., Schmauss C. (2013). Cariprazine, a dopamine D(3)-receptor-preferring partial agonist, blocks phencyclidine-induced impairments of working memory, attention set-shifting, and recognition memory in the mouse. Psychopharmacology (Berl). 226 91–100. 10.1007/s00213-012-2896-5 [DOI] [PMC free article] [PubMed] [Google Scholar]


