Abstract
Advanced glycation end products (AGEs) may induce cardiac remodeling in kidney disease by promoting fibroblast growth factor 23 (FGF-23) expression. Since AGEs are increased in diabetes mellitus (DM), our first aim was to evaluate the existence of any potential association between AGEs, FGF-23, inflammation, and increased cardiovascular risk in DM patients on dialysis (CKD-G5D). Secondarily, we explored the potential role of the soluble receptor for AGEs (sRAGE) as a marker of heart failure. Levels of glycated albumin (GA), sRAGE, c-terminal FGF-23 (cFGF-23), brain natriuretic peptide (BNP), and inflammatory mediators were compared between DM and non-DM CKD-G5D patients. The levels of sRAGE, cFGF-23, BNP, and proinflammatory markers were over the ranges of normality in both DM and non-DM groups. Only GA and sRAGE levels were increased in DM compared to non-DM patients. Plasma levels of sRAGE and CRP were the only independent predictors of BNP concentration. In conclusion, in DM CKD-G5D patients, sRAGE appeared to be a marker of cardiac remodeling. Indeed, its increase could be a potential protective mechanism against the increased risk of cardiovascular complications related to AGEs and inflammation. The causal relationship between sRAGE and cardiovascular risk in these patients needs to be further confirmed by mechanistic studies.
1. Introduction
End-stage renal disease (ESRD) in patients with chronic kidney disease (CKD) is a condition characterized by including volume overload, hyperkalemia, metabolic acidosis, hypertension, anemia, and mineral and bone disorders (MBDs), and it is considered a clinical model of premature aging. ESRD patients have an increased risk of different diseases, mainly at cardiovascular and cerebrovascular level, and have a mortality rate at least 20–30 times higher than what their healthy age-matched patients have [1]. ESRD is stricly related to cardiovascular diseaes (CVDs) through several mechanisms, which include the inflammatory response, the production of reactive oxygen species, the phosphate toxicity, and the activation of different endocrine pathways, such as the fibroblast growth factor 23 system (FGF-23) [1]. Also, MBDs represent a severe complication and an important mortality risk factor in CKD patients on dialysis (CKD-G5D) [2].
FGF-23 is a 32 kDa glycoprotein secreted by osteocytes which has been receiving great interest as a new risk factor for CVDs and death both in individuals with CKD [3, 4] and in adults with preserved kidney function [5, 6]. In particular, increased FGF-23 levels have been associated with vascular dysfunction, left ventricular hypertrophy, and the risk of heart failure, stroke, and death [3, 7, 8]. In CKD, FGF-23 levels increase as a compensatory mechanism to keep normal phosphate levels by inhibiting renal phosphate reabsorption and 1-alpha-hydroxilase activity, the key enzyme for calcitriol production [9]. Anyway, although this increase is acknowledged as a physiological protective mechanism, it could directly contribute to the onset and progression of inflammation and CVDs [7, 8, 10].
It has been recently observed that FGF-23 expression may be promoted in vitro by advanced glycation end products (AGEs) through the upregulation of NF-κB [11]. Indeed, in a mouse model of renal failure, the activation of the cell-surface receptor for AGEs (RAGE) induced FGF-23 expression in cardiac fibroblasts and promoted cardiac remodeling [12].
Metabolic disorders including diabetes mellitus (DM) are characterized by high levels of AGEs that are key mediators of DM-related complications, inflammation, and aging. These products, generated by nonenzymatic reactions between reducing sugars and protein or lipids, mainly promote reactive oxygen species generation and a proinflammatory response through RAGE activation. Besides the cell membrane form, RAGE also exists as a soluble circulating molecule, sRAGE. This form, by binding the circulating AGEs and preventing their activation of RAGE, plays a role as an important protective agent [13, 14].
In renal diseases, AGEs and sRAGE may accumulate due to their increased formation and reduced elimination [15–19]. Indeed, the RAGE pathway has been suggested as a causal risk factor for both atherosclerosis [20] and left ventricular hypertrophy [21] in these patients.
Although the potential role of sRAGE as a marker for CVDs has been pointed out in different previous studies [22–27], its role in ESRD is less characterized.
To better evaluate the role of the AGEs/sRAGE pathway in ESRD, we firstly evaluated the existence of any potential association between AGEs, FGF-23, inflammation, and increased risk of CVDs in DM CKD-G5D patients. Secondarily, we explored the potential role of sRAGE as a marker of heart failure in CKD-G5D.
2. Materials and Methods
2.1. Source Population
We performed a cross-sectional study in patients on CKD-G5D. We enrolled patients who underwent hemodialysis (HD) or peritoneal dialysis (PD) treatment for at least 3 months with age ≥ 18 years and agreement to participate in the study. We excluded patients with missing or incomplete clinical history, incapacity to cooperate to the study, and hepatic encephalopathy. This study was performed in accordance with the ethical principles of the Declaration of Helsinki, as revised in 2013. The protocol was approved by the Ethics Committee of San Bortolo Hospital (N.41/14). All participants were informed of the objectives of the study and signed the informed consent.
2.2. Measurements
2.2.1. Data Collection
Demographic, anthropometric, and clinical data (i.e., age, gender, smoking status, alcohol consumption, hypertension, DM, cardiovascular disease, and cerebrovascular disease) were collected. Screening and diagnosis of DM were performed according to the American Diabetes Association guidelines [28]. Hypertension was defined as values ≥ 140 mmHg systolic blood pressure and/or ≥90 mmHg diastolic blood pressure [29].
Blood samples in EDTA were collected during outpatient visits in PD patients or prior to dialysis treatment after long interdialytic intervals in HD patients. Samples for nonroutine assays were immediately frozen and stored at −80°C until measurements.
Concerning routine biochemical assays, total bilirubin (reference value (RV): male 0.3–1.5 mg/dL, female 0.2–1.2 mg/dL), calcium (RV: 8.5–10.5 mg/dL), phosphorous (RV: 2.2–4.2 mg/dL), LDL cholesterol (RV: <115 mg/dL), HDL cholesterol (RV: male > 40 mg/dL, female > 45 mg/dL), and total protein (RV: 6.4–8.7 g/dL) were quantified using colorimetric methods on Dimension Vista® 1500 Intelligent Lab System (Siemens, Milan, Italy). The same laboratory equipment was used for urea (RV: 15–50 mg/dL under 70 years old, 19–65 mg/dL over 70 years old), creatinine (RV: male up to 1.3 mg/dL, female up to 0.9 mg/dL under 70 years old and 1.2 mg/dL over 70 years old), uric acid (RV: male 3–8 mg/dL, female 2.4–6.6 mg/dL under 70 years old and 3–8 mg/dL over 70 years old), alanine aminotransferase (ALT) (RV: female < 31 U/L, male < 53 U/L under 70 years old and <34 U/L over 70 years old), and aspartate aminotransferase (AST) (RV: <37 U/L), which were all quantified by enzymatic methods, for total cholesterol (RV: <190 mg/dL) and triglycerides (RV: <150 mg/dL), both measured by kinetic enzyme assays, then for brain natriuretic peptide (BNP) (RV: <50 ng/L under 70 years old, <300 ng/L in the age range 51–75, and <600 ng/L over 70 years old) and C-reactive protein (CRP) (RV: <0.5 mg/dL), which were quantified by an immunochemiluminescent and a turbidimetric method, respectively. Sodium (RV: 35–145 mmol/L), potassium (3.3–5.0 mmol/L), and chloride (95–110 mmol/L) were measured on Dimension Vista System using ion-selective electrodes. Glucose (RV: <100 ng/mL) and albumin (RV: 2.1–4.5 g/dL) were quantified on the ILab650 system (Instrumentation Laboratory, A Werfen Company, Milan, Italy) using an enzymatic and a colorimetric method, respectively. Parathyroid hormone (intact PTH) (RV: 5–35 ng/L), 25-hydroxy vitamin D (25-(OH)D3) (RV: 30–100 μg/L), and β2-microglobulin (β2-microglobulin: 0.8–2.5 mg/L) were measured using the Liaison XL system (DiaSorin, Vercelli, Italy) by immunochemiluminescent methods. The acid-base equilibrium (pH, HCO3−) (RV: 7.32–7.42 for pH, 22–29 mmol/L for HCO3−) was quantified by the Rapidpoint 405 Blood Gas Analyzer (Siemens).
2.2.2. FGF-23 Quantification
The carboxyl-terminal (C-terminal) portion of FGF-23 (cFGF-23) levels was determined in plasma by two-site enzyme-linked immunosorbent assay (ELISA), according to the manufacturer's protocol (Immutopics Inc., San Clemente, CA). Two hundred microliters of plasma was used to assay the sample in duplicate. Samples with values greater than the highest standard were diluted 1 : 10 or greater with the 0 RU/mL standard or optional sample diluent reagent and reassayed. The lowest concentration of cFGF-23 measurable is 1.5 RU/mL, and the maximum intra- and interassay coefficients of variations were 2.4% and 4.7%, respectively.
2.2.3. Glycated Albumin Quantification
The glycated albumin (GA) and the percentage of glycated albumin (GA%) were determined in plasma by the enzymatic QuantiLab® glycated albumin assay (Instrumentation Laboratory) using the ILab650 system (Instrumentation Laboratory). The ILab analyzer automatically calculates the results of each sample. The GA% is calculated by the GA/albumin ratio and corrected by arithmetic algorithm defined to align the GA% levels to the HPLC method [30–32]. The minimum detectable concentration of GA measurable is 1.15 g/L. The maximum intra- and interassay coefficient of variations were 2.1% and 1.3% for GA and 1.2% and 1.0% for GA%, respectively.
2.2.4. sRAGE and Inflammatory Cytokine Quantification
The quantitative determinations of sRAGE, pentraxin-3 (PTX3), and tumor necrosis factor alpha (TNFα) concentrations were performed by commercial human ELISA kits (R&D System, Minneapolis, MN, USA) according to the manufacturer's instructions. The minimum detectable dose ranged from 1.23 to 16.14 pg/mL for sRAGE, 0.007 to 0.116 ng/mL for PTX3, and 0.5 to 5.5 pg/mL for TNFα. The maximum intra- and interassay coefficients of variations were, respectively, 4.8% and 8.3% for sRAGE, 4.4% and 6.2% for PTX3, and 5.2% and 7.4% for TNFα. The GloMax®-Multi Microplate Multimode Reader was used for photometric measurements (Promega, Milan, Italy).
2.3. Statistical Analysis
Qualitative variables are summarized as numbers and percentages; quantitative variables are expressed as mean with standard deviation (SD) or median and interquartile range (IQR). The normality of data distribution was assessed by the Kolmogorov-Smirnoff test. t-test and Mann–Whitney U test were used for group comparison. To test the univariate association between variables, Pearson (for normal-distributed data) or Spearman (for non-normal distributed data) correlation tests were used, as appropriate. Stepwise regression analysis was performed to evaluate the independent correlates of BNP in CKD-G5D patients. All statistical analyses were performed using STATISTIX 7.0 (Analytical Software, Tallahassee, FL, USA) and GraphPAd Prism 5.0 biochemical statistical package (GraphPad Software, San Diego, CA, USA). A p value < 0.05 was considered significant.
3. Results
3.1. Patient Characteristics
We enrolled a total of 76 CKD-G5D patients (32 HD, 44 PD, median age 62.41 (IQR: 52.02–72.05) years, 55M) of which 24 were with DM (type 2 DM: 22; type 1 DM: 2) (mean age 61.01 (50.94–72.83) years, 35M) and 54 were without DM (65.42 (54.83–70.94) years, 20M). Demographic and anthropometrical data are presented in Table 1. Sixty-seven patients (87%) were under treatment with vitamin D or its synthetic analog. The active vitamin D therapy, which included cholecalciferol and calcitriol, was used in 45 (59.21%) patients. Twenty-two (28.95%) patients were treated with paricalcitol and cinacalcet.
Table 1.
Demographic, anthropometric, and clinical characteristics of CKD patients included in the study.
| All CKD (n = 76) | Non-DM CKD (n = 52) | DM CKD (n = 24) | p | |
|---|---|---|---|---|
| Age (years) | 62.41 (52.02–72.05) | 61.21 ± 13.94 | 65.42 (54.83–70.94) | 0.80 |
| Male gender (n, %) | 55, 72.37% | 35, 67.31% | 20, 83.33% | 0.18 |
| BMI | 27.20 ± 5.56 | 27.38 ± 5.28 | 24.14 (22.10–31.35) | 0.50 |
|
| ||||
| HD (n, %) | 32, 42.11% | 20, 38.46% | 12, 50% | 0.45 |
|
| ||||
| Smoking (n, %) | 9, 11.84% | 4, 7.69% | 5, 20.83% | 0.13 |
| Ex-smoking (n, %) | 29, 38.16% | 20, 38.46% | 9, 37.50% | 1.00 |
| Alcohol consumption (n, %) | 2, 2.63% | 2, 3.85% | 0, 0% | 1.00 |
| Hypertension (n, %) | 48, 63.16% | 35, 67.30% | 13, 54.17% | 0.31 |
| Cardiovascular diseases (n, %) | 22, 28.95% | 12, 23.08% | 10, 41.67% | 0.11 |
| Cerebrovascular diseases (n, %) | 4, 5.26% | 2, 3.85% | 2, 8.33% | 0.59 |
|
| ||||
| Therapy with activated vitamin D | 45, 59.21% | 31, 59.62% | 14, 58.33% | 1.00 |
| Therapy with paricalcitol | 22, 28.95% | 17, 32.69% | 5, 20.83% | 0.05 |
Data are expressed as median (25th–75th percentiles) or number and proportions. BMI: body mass index; HD: hemodialysis. Comparison between groups was performed by Mann–Whitney U test or Fisher exact test.
Biochemical characteristics of patients included in the study are shown in Table 2.
Table 2.
Biochemical characteristics of CKD-G5D patients included in the study.
| All CKD-G5D (n = 76) | Non-DM CKD-G5D (n = 52) | DM CKD-G5D (n = 24) | p | |
|---|---|---|---|---|
| Creatinine (mg/dL) | 9.40 ± 3.09 | 9.71 ± 3.39 | 8.71 ± 2.19 | 0.13 |
| Uric acid (mg/dL) | 5.70 ± 1.29 | 5.67 ± 1.24 | 5.76 ± 1.41 | 0.78 |
| Urea (mg/dL) | 124.60 ± 31.41 | 122.10 ± 31.60 | 129.90 ± 30.97 | 0.32 |
| Total bilirubin (g/dL) | 0.40 (0–30-0.50) | 0.40 (0.30–0.50) | 0.42 ± 0.16 | 0.56 |
|
| ||||
| pH venous | 7.35 (7.32–7.38) | 7.35 (7.32–7.38) | 7.36 (7.33–7.38) | 0.47 |
| HCO3 venous (mmol/L) | 25.18 ± 4.29 | 25.02 ± 4.54 | 25.53 ± 3.76 | 0.63 |
| K—potassium (mmol/L) | 4.46 ± 0.72 | 4.46 ± 0.71 | 4.48 ± 0.75 | 0.91 |
| Na—sodium (mmol/L) | 140.00 (138.00–142.00) | 140.00 (138.30–142.80) | 139.50 ± 3.02 | 0.33 |
| Cl—chloride (mmol/L) | 101.50 (97.00–105.00) | 101.10 ± 4.28 | 101.20 ± 5.74 | 0.95 |
| Ca—calcium (mg/dL) | 8.95 ± 0.47 | 8.98 ± 0.48 | 8.88 ± 0.44 | 0.38 |
| P—phosphorus (mg/dL) | 5.15 ± 1.36 | 5.14 ± 1.34 | 5.20 ± 1.44 | 0.86 |
|
| ||||
| Total cholesterol (mg/dL) | 157.10 ± 40.66 | 163.90 ± 35.42 | 142.50 ± 47.76 | 0.032 |
| LDL cholesterol (mg/dL) | 80.82 ± 35.92 | 85.65 ± 32.32 | 70.33 ± 43.17 | 0.016 |
| HDL cholesterol (mg/dL) | 44.00 (38.00–54.00) | 45.50 (40.00–60.25) | 42.42 ± 11.81 | 0.030 |
| Triglycerides (mg/dL) | 131.50 (86.25–201.80) | 117.50 (82.75–204.30) | 154.00 ± 74.15 | 0.55 |
| ALT (UI/L) | 19.00 (15.00–24.00) | 20.12 ± 7.79 | 20.00 (15.25–31.25) | 0.30 |
| AST (UI/L) | 11.00 (6.00–15.75) | 11.00 (6.00–14.75) | 11.42 ± 6.79 | 0.93 |
| Glucose (mg/dL) | 103.50 (92.00–138.50) | 99.00 (91.00–110.00) | 165.00 (131.80–220.80) | <0.0001 |
| Total protein (g/dL) | 7.00 ± 0.57 | 7.02 ± 0.63 | 6.96 ± 0.41 | 0.64 |
| Albumin (g/dL) | 35.03 ± 4.82 | 35.01 ± 4.81 | 34.70 (31.35–39.23) | 0.75 |
| GA% | 14.00 (12.03–17.15) | 13.09 ± 2.05 | 18.30 (17.13–23.20) | <0.0001 |
|
| ||||
| iPTH (pg/mL) | 103.50 (53.25–211.00) | 102.00 (55.00–254.30) | 134.30 ± 119.90 | 0.46 |
| 25-(OH)D3 (ng/mL) | 17.90 (10.93–23.28) | 17.95 (11.45–25.15) | 16.32 ± 6.77 | 0.30 |
|
| ||||
| CRP (mg/L) | 0.34 (0.29–0.78) | 0.31 (0.29–0.77) | 0.41 (0.29–1.04) | 0.39 |
| β2-microglobulin (ng/mL) | 22.80 (16.66–28.98) | 22.96 (16.88–29.61) | 21.08 ± 7.66 | 0.24 |
| BNP (pg/mL) | 2542.00 (1511.00–10762.00) | 2265.00 (1108.00–8272.00) | 3064.00 (1795.00–18558.00) | 0.148 |
|
| ||||
| cFGF-23 (RU/mL) | 1441.00 (759.00–3614.00) | 1345.00 (508.10–3087.00) | 1707.00 (1183.00–4016.00) | 0.142 |
| sRAGE (pg/mL) | 3089.30 ± 1339.74 | 2838.00 ± 1163.75 | 3633.80 ± 1548.45 | 0.015 |
| PTX3 (ng/mL) | 1.57 (0.76–2.94) | 1.57 (0.75–3.23) | 1.89 ± 1.48 | 0.65 |
| TNFα (pg/mL) | 10.71 (4.85–21.34) | 12.15 (5.60–24.19) | 11.66 ± 10.28 | 0.134 |
Data are expressed as mean ± SD or median (25th–75th percentiles). ALT: alanine transaminase; AST: aspartate transaminase; BNP: brain natriuretic peptide; CRP: C-reactive protein; GA: glycated albumin; cFGF-23: c-terminal portion of fibroblast growth factor-23; iPTH: intact parathyroid hormone; 25-(OH)D3: 25-hydroxy vitamin D; PTX3: pentraxin-related protein PTX3; sRAGE: soluble receptor for advanced glycation end products; TNFα: tumor necrosis factor alpha. Comparison between groups was performed by unpaired t-test or Mann–Whitney U test. p values less than 0.05 are indicated in bold.
3.2. Plasma Levels of GA, FGF-23, sRAGE, and Inflammatory Markers
CKD-G5D patients were classified according to the presence of DM, and the two groups were compared to explore potential differences in the levels of GA, as a marker of protein glycation, sRAGE, cFGF-23, and the proinflammatory molecules CRP, PTX-3, and TNFα.
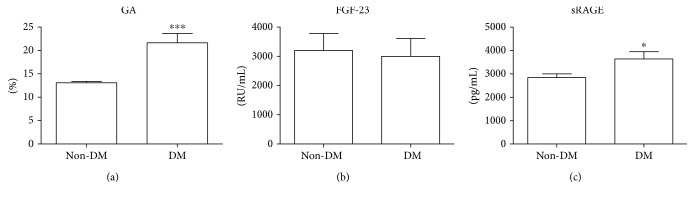
According to the reference limits of GA [33], which have been very recently documented also in Caucasians [34, 35] (upper reference limit: 14.5% (95% CI: 14.3–14.7) [34]; range: 9.0% (90% CI: 8.7–9.5) to 16.0% (90% CI: 15.6–16.4) [35]), in non-DM CKD-G5D patients, GA (95% CI: 12.52–13.66) was within the ranges of normality (Table 1 and Figure 1()). Differently, in the DM CKD-G5D group, it reached pathological levels and it was statistically significantly higher than in the non-DM CKD-G5D patients (p < 0.001) (Table 1 and Figure 1()). According to our previous results on sRAGE concentrations in healthy subjects (mean value 1363.0 ± 693.2 ng/mL) [36], sRAGE levels were above the normal values both in non-DM CKD-G5D and DM CKD-G5D patients and resulted statistically significantly higher in the DM CKD-G5D compared to the non-DM CKD-G5D group (p < 0.05) (Table 1 and Figure 1()). cFGF-23 levels were higher than the reference value (<180 RU/mL), but we did not find any significant difference between groups (non-DM CKD-G5D: median value, 1345.00, 25th–75th percentiles (508.10–3087.00) RU/mL; DM CKD-G5D: 1707.00, (1183.00–4016.00 RU/mL) (Table 2 and Figure 1())).
Figure 1.
Evaluation of GA, FGF-23, and sRAGE levels in CKD-G5D patients. GA levels (a) and sRAGE (c) were higher in DM CKD-G5D patients than in non-DM CKD-G5D patients (∗∗∗p < 0.001 and ∗p < 0.05, resp.). FGF-23 levels (b) were the same in the two groups.
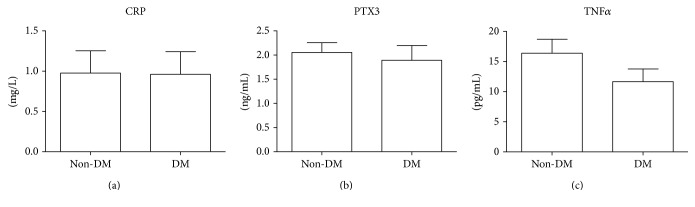
As markers of inflammation, we evaluated CRP, PTX-3, and TNFα. According to the existing reference values for healthy subjects (<0.5 mg/L for CRP, <1.18 ng/mL for PTX-3, and 1.12 pg/mL for TNFα, resp.), all the proinflammatory markers evaluated were greatly upregulated in both groups but without significant differences between them (Table 2 and Figure 2()).
Figure 2.
Evaluation of inflammation-related molecules in CKD-G5D patients. CKD-G5D patients were classified into two groups according to the presence of diabetes mellitus (DM). CRP (a), PTX3 (b), and TNFα (c) levels were compared between DM and non-DM groups. No statistically significant differences were observed between the two groups.
A univariate association analysis was then performed in CKD-G5D patients to explore potential correlations between the markers previously studied. We did not find any significant correlation between GA and cFGF-23 (r = 0.073, p = 0.529), between sRAGE and cFGF-23 (r = −0.056; p = 0.633), and then between GA and sRAGE (r = 0.29, p = 0.061).
3.3. Relationships between CVD Risk Factors and BNP
The potential correlations between BNP, a marker used for screening and prognosis of heart failure, and clinical variables in CKD-G5D patients were explored. BNP was significantly positively correlated with creatinine (r = 0.27; p = 0.017), potassium (r = 0.247; p = 0.031), CRP (r = 0.260; p = 0.023), sRAGE (r = 0.314; p = 0.006), and β2-microglobulin (r = 0.407; p < 0.001) and significantly negatively correlated with sodium (r = −0.341; p = 0.003).
In a multivariate stepwise regression model, plasma sRAGE and CRP levels were the only independent predictors of BNP (Table 3). All the other parameters did not enter the model.
Table 3.
Stepwise regression analysis (t value) of the association between some independent variables and BNP in CKD-G5D patients.
| BNP (pg/mL) | Independent variables | Model R2 | ||||
|---|---|---|---|---|---|---|
| CRP (mg/L) | Creatinin (mg/dL) | Na (mmol/L) | K (mmol/L) | sRAGE (pg/mL) | ||
| 2.44 | 0.84 | −0.81 | 1.40 | 2.72 | 0.20 | |
| p value | 0.017 | 0.40 | 0.42 | 0.17 | 0.008 | |
| Constant value | ||||||
| Regression coefficient | 1652.06 | 2.49 | ||||
| SE regression coefficient | 676.00 | 0.92 | ||||
CRP: C-reactive protein; sRAGE: soluble receptor of advanced glycation end products; SE: standard error.
4. Discussion
CKD-G5D patients are an interesting model of premature aging. These patients, due to the lack of renal function, show a uremic milieu in which phosphate retention and uremic toxin accumulation, including AGEs, promote oxidative stress and inflammation. These conditions may in turn activate specific cellular mechanisms, such as telomere attrition, DNA damage, and mitochondrial dysfunction, which affect cellular homeostasis, promote premature cellular senescence, and increase the risk of death mainly due to cerebrovascular and cardiovascular complications [37].
AGEs are recognized as important damaging molecules for the cardiovascular system due to their ability to promote endothelial dysfunction, arterial stiffness, atherosclerosis, immune system alteration, and cardiac fibrosis and remodeling [38–41]. It is known that the generation of AGEs is strongly increased in DM, being AGEs by-products of hyperglycemia. Recent preclinical studies [11, 12] suggested that these molecules, in addition to their known role as proinflammatory agents, are able to increase the production of FGF-23, a key molecule involved in the crosstalk between kidney function, bone metabolism, and the cardiovascular system [7, 8, 42]. To our knowledge, this is the first study investigating in human any potential association between AGEs, sRAGE, cFGF-23, and cardiovascular complications in CKD-G5D patients with DM. Our results indicated that both GA and sRAGE levels were increased in DM CKD-G5D compared to non-DM CKD-G5D patients, but the levels of cFGF-23 did not differ between the two groups. Similarly, the concentrations of the proinflammatory molecules evaluated were almost the same in the two groups, although we expected to observe higher levels in DM CKD-G5D patients, as a consequence of the increased glycated milieu. To our opinion, one possible explanation just deals with the upregulation of sRAGE. Different studies have shown that sRAGE levels are increased in DM as a counteract system against glycated products [26, 43–46]. Assuming the activation of the same mechanism also in our DM CKD-G5D patients, sRAGE, by blocking glycated products, could reduce the activation of various damaging cellular mechanisms, including the stimulation of cFGF-23 and other proinflammatory molecules. Indeed, since AGE accumulation has been associated with the development and progression of heart failure [47, 48], the lack of difference also in BNP levels between the two groups reinforces the idea of a protective role of sRAGE in DM CKD-G5D patients. A further explanation could arise by considering that cFGF-23, which starts to rise early in CKD, in CKD-G5D is up to thousandfold higher than the normal levels [49]. For this reason, we could not exclude the possibility that in DM CKD-G5D patients, a further stimulation of the FGF-23 system by potential activators, like AGEs [11, 12], is not possible or may not be appreciated.
Concerning AGEs, we focused our attention on GA. As for other AGEs, we expected to observe that GA levels were over the ranges of normality not only in DM CKD-G5D group but also in non-DM CKD-G5D patients, due to the increased oxidative stress and the reduced kidney clearance typical of the disease [15, 17, 18, 50]. Of course, the upregulation of sRAGE at levels above controls in both groups and its further increase in DM seem to suggest the existence of a glycated milieu in all CKD-G5D patients, regardless of the presence of DM. According to these data, the observation that GA levels were over the range of normality only in DM CKD-G5D group strongly reinforces the utility of GA as a useful glycation marker for DM monitoring in CKD-G5D patients in which HbA1c does not work well due to just kidney-related anemia [51, 52].
sRAGE has been regarded as a diagnostic and prognostic marker of cardiovascular outcome in various pathological conditions, that is, obesity, DM, metabolic syndrome, chronic heart failure, and also CKD [20–22, 24, 26]. Concerning heart failure, conflicting results on the relationship between sRAGE and heart failure risk exist. Both lower and higher circulating levels of sRAGE were described as valuable predictors of heart failure, its severity, and mortality, and some studies suggested the existence of a robust association between NT-pro BNP levels, as a diagnostic and prognostic marker of heart failure and sRAGE [27, 48, 53–56]. Also, in our study, we observed a positive correlation between sRAGE and BNP. Indeed, sRAGE emerged as an independent predictor of BNP levels, thus suggesting its potential role as a marker of cardiac remodeling in CKD-G5D patients.
Leonardis et al. [21] studied the relationships between sRAGE and left ventricular hypertrophy in CKD, not in CKD-G5D. They showed that sRAGE levels were increased compared to those of controls but, unlike us, were inversely correlated with functional parameters of cardiac function. Probably, since the two studies have been performed on different populations, they are not easily comparable and further studies in ESRD are therefore necessary to support data herein presented.
The study of Kim et al. [20] has been performed on PD patients but explored sRAGE correlation with carotid atherosclerosis, not parameters of heart failure. Although different in its aim, some data of this study could be useful for a better comprehension also of our results. They observed that CKD-G5D patients had increased sRAGE levels compared to controls but, differently from our results, the subgroup of DM patients had lower sRAGE and higher IL-6 levels, a marker of inflammation, than the non-DM group. To our opinion, this observation seems to reinforce our hypothesis of a protective role of sRAGE against a further increase of the inflammatory status in DM patients. Anyway, the reasons of the different results are not clear but could deal with a different regulation of sRAGE expression at cellular level, the duration of disease, and features of patients included in the study.
In conclusion, in DM CKD-G5D patients, sRAGE appeared to be a marker of cardiac remodeling. Indeed, its increase could be a potential protective mechanism against the increased risk of cardiovascular complications related to AGEs and inflammation. The causal relationship between sRAGE and cardiovascular risk in these patients needs to be further confirmed by mechanistic studies. Also, the evaluation of additional glycated products, the quantification of sRAGE, secreted form of the receptor, and a comparison between HD and PD could help to improve the knowledge of the role of glycated pathways in these patients.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Authors' Contributions
Elena Dozio and Valentina Corradi equally contributed to the manuscript.
References
- 1.Collins A. J., Foley R. N., Herzog C., et al. United States Renal Data System 2008 annual data report. American Journal of Kidney Diseases. 2009;53(1 Supplement):S1–374. doi: 10.1053/j.ajkd.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 2.Moe S., Drüeke T., Cunningham J., et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney International. 2006;69(11):1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 3.Fujii H., Joki N. Mineral metabolism and cardiovascular disease in CKD. Clinical and Experimental Nephrology. 2017;21(Supplement 1):53–63. doi: 10.1007/s10157-016-1363-8. [DOI] [PubMed] [Google Scholar]
- 4.Seiler S., Reichart B., Roth D., Seibert E., Fliser D., Heine G. H. FGF-23 and future cardiovascular events in patients with chronic kidney disease before initiation of dialysis treatment. Nephrology, Dialysis, Transplantation. 2010;25(12):3983–3989. doi: 10.1093/ndt/gfq309. [DOI] [PubMed] [Google Scholar]
- 5.Parker B. D., Schurgers L. J., Brandenburg V. M., et al. The associations of fibroblast growth factor 23 and uncarboxylated matrix Gla protein with mortality in coronary artery disease: the Heart and Soul Study. Annals of Internal Medicine. 2010;152(10):640–648. doi: 10.7326/0003-4819-152-10-201005180-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gutierrez O. M., Wolf M., Taylor E. N. Fibroblast growth factor 23, cardiovascular disease risk factors, and phosphorus intake in the Health Professionals Follow-up Study. Clinical Journal of the American Society Nephrology. 2011;6(12):2871–2878. doi: 10.2215/CJN.02740311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yilmaz M. I., Sonmez A., Saglam M., et al. FGF-23 and vascular dysfunction in patients with stage 3 and 4 chronic kidney disease. Kidney International. 2010;78(7):679–685. doi: 10.1038/ki.2010.194. [DOI] [PubMed] [Google Scholar]
- 8.Faul C., Amaral A. P., Oskouei B., et al. FGF23 induces left ventricular hypertrophy. Journal of Clinical Investigation. 2011;121(11):4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Isakova T., Wahl P., Vargas G. S., et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney International. 2011;79(12):1370–1378. doi: 10.1038/ki.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gutiérrez O. M., Januzzi J. L., Isakova T., et al. Fibroblast growth factor 23 and left ventricular hypertrophy in chronic kidney disease. Circulation. 2009;119(19):2545–2552. doi: 10.1161/CIRCULATIONAHA.108.844506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bar L., Wachter K., Wege N., Navarrete Santos A., Simm A., Foller M. Advanced glycation end products stimulate gene expression of fibroblast growth factor 23. Molecular Nutrition & Food Research. 2017;61(8) doi: 10.1002/mnfr.201601019. [DOI] [PubMed] [Google Scholar]
- 12.Yan L., Bowman M. A. Chronic sustained inflammation links to left ventricular hypertrophy and aortic valve sclerosis: a new link between S100/RAGE and FGF23. Inflammation and Cell Signaling. 2014;1(5) doi: 10.14800/ics.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kisugi R., Kouzuma T., Yamamoto T., et al. Structural and glycation site changes of albumin in diabetic patient with very high glycated albumin. Clinica Chimica Acta. 2007;382(1-2):59–64. doi: 10.1016/j.cca.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 14.Dozio E., Vianello E., Briganti S., et al. Expression of the receptor for advanced glycation end products in epicardial fat: link with tissue thickness and local insulin resistance in coronary artery disease. Journal of Diabetes Research. 2016;2016:8. doi: 10.1155/2016/2327341.2327341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diepeveen S. H., Verhoeven G. H., van der Palen J., et al. Oxidative stress in patients with end-stage renal disease prior to the start of renal replacement therapy. Nephron. Clinical Practice. 2004;98(1):c3–c7. doi: 10.1159/000079921. [DOI] [PubMed] [Google Scholar]
- 16.Kalousová M., Hodková M., Kazderová M., et al. Soluble receptor for advanced glycation end products in patients with decreased renal function. American Journal of Kidney Diseases. 2006;47(3):406–411. doi: 10.1053/j.ajkd.2005.12.028. [DOI] [PubMed] [Google Scholar]
- 17.Thornalley P. J. Advanced glycation end products in renal failure. Journal of Renal Nutrition. 2006;16(3):178–184. doi: 10.1053/j.jrn.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Meerwaldt R., Zeebregts C. J., Navis G., Hillebrands J. L., Lefrandt J. D., Smit A. J. Accumulation of advanced glycation end products and chronic complications in ESRD treated by dialysis. American Journal of Kidney Diseases. 2009;53(1):138–150. doi: 10.1053/j.ajkd.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 19.Basta G., Leonardis D., Mallamaci F., et al. Circulating soluble receptor of advanced glycation end product inversely correlates with atherosclerosis in patients with chronic kidney disease. Kidney International. 2010;77(3):225–231. doi: 10.1038/ki.2009.419. [DOI] [PubMed] [Google Scholar]
- 20.Kim J. K., Park S., Lee M. J., et al. Plasma levels of soluble receptor for advanced glycation end products (sRAGE) and proinflammatory ligand for RAGE (EN-RAGE) are associated with carotid atherosclerosis in patients with peritoneal dialysis. Atherosclerosis. 2012;220(1):208–214. doi: 10.1016/j.atherosclerosis.2011.07.115. [DOI] [PubMed] [Google Scholar]
- 21.Leonardis D., Basta G., Mallamaci F., et al. Circulating soluble receptor for advanced glycation end product (sRAGE) and left ventricular hypertrophy in patients with chronic kidney disease (CKD) Nutrition, Metabolism, and Cardiovascular Diseases. 2012;22(9):748–755. doi: 10.1016/j.numecd.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Koyama H., Shoji T., Yokoyama H., et al. Plasma level of endogenous secretory RAGE is associated with components of the metabolic syndrome and atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25(12):2587–2593. doi: 10.1161/01.ATV.0000190660.32863.cd. [DOI] [PubMed] [Google Scholar]
- 23.Basta G. Receptor for advanced glycation endproducts and atherosclerosis: from basic mechanisms to clinical implications. Atherosclerosis. 2008;196(1):9–21. doi: 10.1016/j.atherosclerosis.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 24.Choi K. M., Yoo H. J., Kim H. Y., et al. Association between endogenous secretory RAGE, inflammatory markers and arterial stiffness. International Journal of Cardiology. 2009;132(1):96–101. doi: 10.1016/j.ijcard.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 25.Vazzana N., Santilli F., Cuccurullo C., Davi G. Soluble forms of RAGE in internal medicine. Internal and Emergency Medicine. 2009;4(5):389–401. doi: 10.1007/s11739-009-0300-1. [DOI] [PubMed] [Google Scholar]
- 26.Colhoun H. M., Betteridge D. J., Durrington P., et al. Total soluble and endogenous secretory receptor for advanced glycation end products as predictive biomarkers of coronary heart disease risk in patients with type 2 diabetes: an analysis from the CARDS trial. Diabetes. 2011;60(9):2379–2385. doi: 10.2337/db11-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boschetto P., Campo I., Stendardo M., et al. Plasma sRAGE and N-(carboxymethyl) lysine in patients with CHF and/or COPD. European Journal of Clinical Investigation. 2013;43(6):562–569. doi: 10.1111/eci.12079. [DOI] [PubMed] [Google Scholar]
- 28.American Diabetes A. Standards of medical care in diabetes-2015 abridged for primary care providers. Clinical Diabetes. 2015;33(2):97–111. doi: 10.2337/diaclin.33.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.ESH/ESC Task Force for the Management of Arterial Hypertension. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. Journal of Hypertension. 2013;31(10):1925–1938. doi: 10.1097/HJH.0b013e328364ca4c. [DOI] [PubMed] [Google Scholar]
- 30.Kouzuma T., Usami T., Yamakoshi M., Takahashi M., Imamura S. An enzymatic method for the measurement of glycated albumin in biological samples. Clinica Chimica Acta. 2002;324(1-2):61–71. doi: 10.1016/s0009-8981(02)00207-3. [DOI] [PubMed] [Google Scholar]
- 31.Kouzuma T., Uemastu Y., Usami T., Imamura S. Study of glycated amino acid elimination reaction for an improved enzymatic glycated albumin measurement method. Clinica Chimica Acta. 2004;346(2):135–143. doi: 10.1016/j.cccn.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 32.Kohzuma T., Yamamoto T., Uematsu Y., Shihabi Z. K., Freedman B. I. Basic performance of an enzymatic method for glycated albumin and reference range determination. Journal of Diabetes Science and Technology. 2011;5(6):1455–1462. doi: 10.1177/193229681100500619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dozio E., Di Gaetano N., Findeisen P., Corsi Romanelli M. M. Glycated albumin: from biochemistry and laboratory medicine to clinical practice. Endocrine. 2017;55(3):682–690. doi: 10.1007/s12020-016-1091-6. [DOI] [PubMed] [Google Scholar]
- 34.Bellia C., Zaninotto M., Cosma C., et al. Definition of the upper reference limit of glycated albumin in blood donors from Italy. Clinical Chemistry and Laboratory Medicine. 2017 doi: 10.1515/cclm-2017-0179. [DOI] [PubMed] [Google Scholar]
- 35.Testa R., Ceriotti F., Guerra E., et al. Glycated albumin: correlation to HbA1c and preliminary reference interval evaluation. Clinical Chemistry and Laboratory Medicine. 2017;55(2):e31–ee3. doi: 10.1515/cclm-2016-0512. [DOI] [PubMed] [Google Scholar]
- 36.Dozio E., Briganti S., Delnevo A., et al. Relationship between soluble receptor for advanced glycation end products (sRAGE), body composition and fat distribution in healthy women. European Journal of Nutrition. 2016 doi: 10.1007/s00394-016-1291-0. [DOI] [PubMed] [Google Scholar]
- 37.Stenvinkel P., Larsson T. E. Chronic kidney disease: a clinical model of premature aging. American Journal of Kidney Diseases. 2013;62(2):339–351. doi: 10.1053/j.ajkd.2012.11.051. [DOI] [PubMed] [Google Scholar]
- 38.Ramasamy R., Yan S. F., Herold K., Clynes R., Schmidt A. M. Receptor for advanced glycation end products: fundamental roles in the inflammatory response: winding the way to the pathogenesis of endothelial dysfunction and atherosclerosis. Annals of the New York Academy of Sciences. 2008;1126:7–13. doi: 10.1196/annals.1433.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van Heerebeek L., Hamdani N., Handoko M. L., et al. Diastolic stiffness of the failing diabetic heart: importance of fibrosis, advanced glycation end products, and myocyte resting tension. Circulation. 2008;117(1):43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 40.Wu F., Feng J. Z., Qiu Y. H., et al. Activation of receptor for advanced glycation end products contributes to aortic remodeling and endothelial dysfunction in sinoaortic denervated rats. Atherosclerosis. 2013;229(2):287–294. doi: 10.1016/j.atherosclerosis.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 41.Stinghen A. E., Massy Z. A., Vlassara H., Striker G. E., Boullier A. Uremic toxicity of advanced glycation end products in CKD. Journal of the American Society of Nephrology. 2016;27(2):354–370. doi: 10.1681/ASN.2014101047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gutierrez O. M. Fibroblast growth factor 23 and disordered vitamin D metabolism in chronic kidney disease: updating the “trade-off” hypothesis. Clinical Journal of the American Society of Nephrology. 2010;5(9):1710–1716. doi: 10.2215/CJN.02640310. [DOI] [PubMed] [Google Scholar]
- 43.Challier M., Jacqueminet S., Benabdesselam O., Grimaldi A., Beaudeux J. L. Increased serum concentrations of soluble receptor for advanced glycation endproducts in patients with type 1 diabetes. Clinical Chemistry. 2005;51(9):1749–1750. doi: 10.1373/clinchem.2005.051961. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura K., Yamagishi S., Adachi H., et al. Elevation of soluble form of receptor for advanced glycation end products (sRAGE) in diabetic subjects with coronary artery disease. Diabetes/Metabolism Research and Reviews. 2007;23(5):368–371. doi: 10.1002/dmrr.690. [DOI] [PubMed] [Google Scholar]
- 45.Nakamura K., Yamagishi S., Adachi H., et al. Serum levels of soluble form of receptor for advanced glycation end products (sRAGE) are positively associated with circulating AGEs and soluble form of VCAM-1 in patients with type 2 diabetes. Microvascular Research. 2008;76(1):52–56. doi: 10.1016/j.mvr.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 46.Thomas M. C., Woodward M., Neal B., et al. Relationship between levels of advanced glycation end products and their soluble receptor and adverse outcomes in adults with type 2 diabetes. Diabetes Care. 2015;38(10):1891–1897. doi: 10.2337/dc15-0925. [DOI] [PubMed] [Google Scholar]
- 47.Hartog J. W., Voors A. A., Bakker S. J., Smit A. J., van Veldhuisen D. J. Advanced glycation end-products (AGEs) and heart failure: pathophysiology and clinical implications. European Journal of Heart Failure. 2007;9(12):1146–1155. doi: 10.1016/j.ejheart.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 48.Raposeiras-Roubín S., Rodiño-Janeiro B. K., Grigorian-Shamagian L., et al. Soluble receptor of advanced glycation end products levels are related to ischaemic aetiology and extent of coronary disease in chronic heart failure patients, independent of advanced glycation end products levels: new roles for soluble RAGE. European Journal of Heart Failure Eur J Heart Fail. 2010;12(10):1092–1100. doi: 10.1093/eurjhf/hfq117. [DOI] [PubMed] [Google Scholar]
- 49.Viaene L., Bammens B., Meijers B. K., Vanrenterghem Y., Vanderschueren D., Evenepoel P. Residual renal function is an independent determinant of serum FGF-23 levels in dialysis patients. Nephrology, Dialysis, Transplantation. 2012;27(5):2017–2022. doi: 10.1093/ndt/gfr596. [DOI] [PubMed] [Google Scholar]
- 50.Diepeveen S. H., Verhoeven G. H., van der Palen J., et al. The effect of the initiation of renal replacement therapy on lipid profile and oxidative stress during the first 6 months of treatment. Clinica Chimica Acta. 2005;361(1-2):112–118. doi: 10.1016/j.cccn.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 51.Inoue K., Goto A., Kishimoto M., et al. Possible discrepancy of HbA1c values and its assessment among patients with chronic renal failure, hemodialysis and other diseases. Clinical and Experimental Nephrology. 2015;19(6):1179–1183. doi: 10.1007/s10157-015-1110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yazdanpanah S., Rabiee M., Tahriri M., et al. Evaluation of glycated albumin (GA) and GA/HbA1c ratio for diagnosis of diabetes and glycemic control: a comprehensive review. Critical Reviews in Clinical Laboratory Sciences. 2017;54(4):219–232. doi: 10.1080/10408363.2017.1299684. [DOI] [PubMed] [Google Scholar]
- 53.Koyama Y., Takeishi Y., Niizeki T., et al. Soluble receptor for advanced glycation end products (RAGE) is a prognostic factor for heart failure. Journal of Cardiac Failure. 2008;14(2):133–139. doi: 10.1016/j.cardfail.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 54.Raposeiras-Roubín S., Rodiño-Janeiro B. K., Grigorian-Shamagian L., et al. Relation of soluble receptor for advanced glycation end products to predict mortality in patients with chronic heart failure independently of Seattle heart failure score. The American Journal of Cardiology. 2011;107(6):938–944. doi: 10.1016/j.amjcard.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 55.Wang L. J., Lu L., Zhang F. R., Chen Q. J., De Caterina R., Shen W. F. Increased serum high-mobility group box-1 and cleaved receptor for advanced glycation endproducts levels and decreased endogenous secretory receptor for advanced glycation endproducts levels in diabetic and non-diabetic patients with heart failure. European Journal of Heart Failure. 2011;13(4):440–449. doi: 10.1093/eurjhf/hfq231. [DOI] [PubMed] [Google Scholar]
- 56.Lazo M., Halushka M. K., Shen L., et al. Soluble receptor for advanced glycation end products and the risk for incident heart failure: the Atherosclerosis Risk in Communities study. American Heart Journal. 2015;170(5):961–967. doi: 10.1016/j.ahj.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]