Abstract
Objective:
To evaluate whether metformin's cancer-related benefits reported in patients with solid tumors (ST) are also present in acute myeloid leukemia (AML) patients.
Methods:
Baseline demographic and clinical history for all diabetes mellitus patients newly diagnosed with AML or cancer of the breast, ovary, prostate, gastrointestinal tract, lung, or kidney at Roswell Park Cancer Institute in Buffalo, NY (January 2003–December 2010, n = 924) was collected. Overall survival (OS) and disease-free survival (DFS) were assessed by Kaplan–Meier (KM) analysis and Cox proportional hazards regression (hazard ratio [HR]).
Findings:
Baseline metformin use provided significant OS and DFS benefit in ST but not in AML (KM: PST-OS= 0.003; PST-DFS= 0.002; PAML-OS= 0.961; PAML-DFS= 0.943). AML median survival was slightly better with metformin use, but users derived no relapse benefit. In ST, metformin nonusers had shorter median survival, 57.7 versus 86 months, and poorer outcomes (HRST-OS= 1.33; PST-OS= 0.002; HRST-DFS= 1.32; PST-DFS= 0.002). These findings remained significant in age-adjusted models (HRST-OS= 1.21; PST-OS= 0.039; HRST-DFS= 1.23; PST-DFS= 0.02) but not fully adjusted models (HRST-OS= 0.96; PST-OS= 0.688; HRST-DFS= 1.0; PST-DFS= 0.94). Higher mortality was noted in AML patients taking insulin versus oral diabetes pharmacotherapy at baseline (HRAML-OS= 2.03; PAML-OS= 0.04).
Conclusion:
Lack of metformin benefit in AML could be due to advanced age at cancer diagnosis. Metformin substitution with insulin before computed tomography scans with contrast – a frequent AML assessment practice – may also explain the lack of subsequent benefit despite taking metformin at baseline. A temporary metformin substitution is recommended by the package insert due to a possible drug interaction with the contrast dye. Our data suggest that metformin substitution was permanent in many patients. Nonetheless, the observed benefit in other malignancies warrants further investigation of metformin use in AML.
KEYWORDS: Acute myeloid leukemia, disease-free survival, Metformin, overall survival, solid tumors
INTRODUCTION
Type 2 diabetes mellitus (T2DM) patients using metformin have a lower cancer risk compared to users of other T2DM therapies .[1,2] If diagnosed with cancer, metformin users develop tumors with a more treatable phenotype including hormone receptor-positive breast cancer, have fewer recurrences, and extended survival.[3,4,5,6] However, metformin's benefit has been observed mainly in patients diagnosed with solid tumors (STs) and – although benefits may be extrapolated to hematologic malignancies[7] – evidence is lacking.
Acute myeloid leukemia (AML) is the most common form of adult leukemia, 2016 estimates of the American Cancer Society forecast approximately 21380 new diagnoses in 2017 in the United States alone .[8] With nearly unchanged death rates between 2004 and 2013 ,[9] AML remains a complex group of diseases with “an urgent unmet need for therapeutic improvements,” as described in a recent editorial .[10] A further complication is that patients presenting with AML also have more comorbidities, including approximately a 3-fold higher prevalence of T2DM than the general population.[11] If metformin use in these patients presents similar survival benefits as reported in solid malignancies, then more AML patients could potentially achieve durable remission. However, despite the value of such a research undertaking, a significant challenge distinguishes the investigation of metformin use in patients with AML from those with STs.
In AML, metformin is often substituted with insulin by default due to the anticipated use of iodinated contrast media for computed tomography (CT) scans. CTs are routinely performed during AML treatment for necessary clinical assessment of the disease. This is not the case with solid malignancies where CTs are not routinely performed. This substitution is made in AML patients to avoid a drug-drug interaction between iodinated contrast media and metformin; contrast media temporarily inhibits the renal clearance of metformin resulting in elevated metformin blood concentrations. This elevation is perceived to pose a higher risk of renal failure and lactic acidosis. Although the package inserts for both drugs provide recommendations for reinitiating metformin 48 h after contrast media discontinuation, metformin is often never reinitiated. Such a decision is of particular concern as it may not only be denying AML patients' potential metformin-related benefits but also replacing it with insulin, which is known to exacerbate hematologic malignancies.[12]
In addition, concerns regarding unforeseen disease complications requiring tight glucose control have added to the trend of managing T2DM with insulin in AML patients. Together, these clinical factors have contributed to the paucity of studies investigating metformin in AML and bolstered an opinion that metformin therapy should be ruled out as an option in these patients. Interestingly, a recent study reported that intensive insulin use increased mortality in patients with acute lymphoblastic leukemia .[12] These data suggested that insulin substitution for metformin may, in fact, lead to poorer outcomes. Furthermore, the Food and Drug Administration mandated a drug label change indicating that metformin is safe to use in cases with mild and moderate kidney function impairment .[13] This regulatory update reopened the interest in the relationship between metformin use and AML outcomes. This survey of existing evidence aimed at evaluating if baseline utilization of specific T2DM pharmacotherapy was associated with cancer outcomes.
METHODS
This retrospective hospital cohort study was approved by the Institutional Review Board (EDR193511) and conducted in accordance with the ethical standards of the Helsinki Declaration of 1975 as revised in 2013. Adults with preexisting T2DM and emergent AML or malignancy of the breast, ovary, prostate, gastrointestinal (GI) tract, lung, or kidney diagnosed between January 2003 and December 2010 were included in the study. Cancer diagnosis was determined based on tumor registry records encoded by the International Classification of Diseases, Ninth Revision. Nearly 1178 new cancer patients were identified. Of them, 254 patients were excluded due to unknown diabetes type, diabetes Type 1, gestational or diabetes diagnosed after cancer (n1 =76), incomplete records (n2 =41), or previous cancer diagnosis (n3 =137). The remaining 924 cases were included in the final analyses. Clinical and treatment history along with demographic information and outcomes was documented. T2DM treatment groups were defined using self-reported pharmacotherapy at baseline; concomitant therapy was also recorded. Metformin use alone or in combination at the time of diagnosis was considered “metformin users,” all others were considered “metformin nonusers” (similarly for “insulin users” and “insulin nonusers”). Outcomes of interest were cancer recurrence and/or death. Follow-up began at diagnosis lasted through the last day of contact or vital status update, whichever was more recent. Overall survival (OS) was defined as the time from cancer diagnosis to death with patients alive at last follow-up treated as censored. Disease-free survival (DFS) was defined as the time from cancer diagnosis to recurrence or death with, patients alive without recurrence were treated as censored. Cases lost to follow-up were censored at the date of the last contact. Event documentation was limited to data collected through to June 30, 2016. Accuracy was confirmed by double or triple review where needed.
Associations between categorical variables were assessed by Fisher's exact, Chi-square, or likelihood-ratio tests where appropriate. Survival testing was performed by Kaplan–Meier analysis with log-rank statistics and Bonferroni-corrected intra-strata comparisons as well as hazard ratio (HR) analysis by Cox proportional hazards regressions. Akaike's information criterion was used for covariate selection. Included covariates in all HRs were age (continuous), body mass index (BMI) (underweight, BMI <18.5; healthy, BMI: 18.5–24.9; overweight, BMI 25.0–29.9; obese, BMI 30–39.9; morbidly obese, BMI >40; and unknown), tumor site (blood, breast, kidney, lower GI tract, lung, ovary, prostate, and upper GI tract), cardiovascular comorbidity score (0, 1, 2, 3, 4, 5+) – defined as the number of all cardiovascular comorbidities at baseline, and presence or absence of baseline metastatic disease. The model accounted for “good prognosis” – early stage per American Joint Committee on Cancer (AJCC) Stages 0, I, and II – and “poor prognosis” (AJCC Stages III and IV). For the AML group, subtypes M1, M2, M4, and M5 were considered “good prognosis” while M0, M6, and M7 were considered “poor prognosis” based on previous observations .[14,15,16] Instances of unstaged disease were categorized as unknown. HR 95% confidence intervals (CIs) were computed by the Wald method. A nominal significance threshold of 0.05 was used in all testing and all analyses, and plots were computed using SAS, version 9.4 (SAS Institute Inc., Cary, North Carolina, USA).
RESULTS
The study population included 397 (43%) males and 527 (57%) females. Median OS was 63.3 months (95% CI: 55.7–74.5), and follow-up time ranged from 0 to 152.1 months. Median DFS was 45.3 months (95% CI: 38.6–52.5). At baseline, 752 (81.4%) participants self-identified as Caucasian non-Hispanic, 121 (13.1%) as African American, and 51 (5.5%) as other ethnicity. No significant difference in gender distribution was observed. Complete information on included participants by disease group is depicted in Figure 1.
Figure 1.
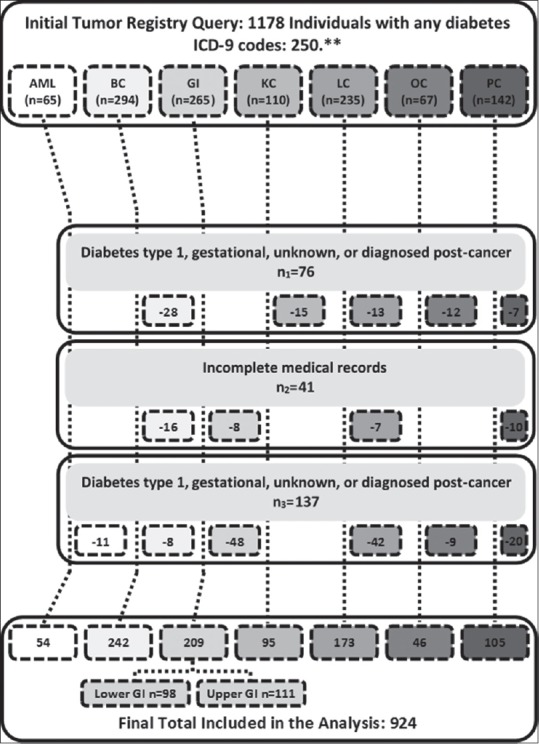
Inclusion and exclusions criteria. AML=Acute myeloid leukemia, BC=Breast cancer, GI=Gastrointestinal (upper gastrointestinal, esophagus, stomach cancer; lower gastrointestinal, small intestine, colon, and rectum cancer), KC=Kidney cancer, LC=Lung cancer, OC=Ovarian cancer, PC=Prostate cancer
Age at diagnosis varied from 20 to 91 years of age, having a median of 66 and a mode of 63 years old in solid malignancies. The AML group remarked by more individuals >60 years old as compared to solid malignancies group. Receiving metformin was associated with a younger age in both AML and solid malignancies, P < 0.001. In the overall dataset, significantly more females used insulin alone or in combination than oral monotherapy, P = 0.007. Fewer women used metformin in the AML group (42.1%) as compared to the solid malignancies group (58.8%); however, this difference was not significant. The number of African-American insulin users at baseline was higher than expected by Fisher's exact test as compared to insulin nonusers, P = 0.004. The BMI distribution was significantly different between metformin users and nonusers, more obese cases than expected being observed among metformin users, P = 0.027. By contrast, no difference in the BMI distribution was noted between insulin users and nonusers (P = 0.577). Documented BMIs in the AML group were consistently over 30 (P < 0.001). However, an overwhelming majority of the cases diagnosed with AML presented with two or fewer baseline cardiovascular comorbidities while less than two-thirds of the solid malignancies group presented with a similar extent of cardiovascular comorbidity, P < 0.001.
Individuals treated with oral T2DM pharmacotherapies had fewer cardiovascular comorbidities as compared to insulin users, P = 0.008. Among solid malignancies, more metformin users than nonusers presented with an early disease stage at the time of diagnosis, P < 0.001. In the AML group, metformin users had a higher than expected proportion of patients with advanced disease (80% vs. 58%) at baseline. Overall, significantly more metformin users were diagnosed with an early stage and more nonusers remained unstaged (P = 0.017). Metformin users also presented with metastatic disease less than nonusers (P < 0.001).
Within the AML group, the relationship between iodinated contrast media use and baseline metformin or insulin use was evaluated to assess whether switching all metformin users to insulin is necessary. More metformin users required a CT with contrast than nonusers (P = 0.054); 26.3% of metformin users required six or more CTs with contrast, nearly half received five or less CT scans with contrast, and the remaining quarter did not undergo any CT with contrast. No differences were observed in relationship with the CT scan without contrast.
The univariate analysis stratified by cancer disease site revealed a significant difference in OS and DFS (χ2OS= 435.15, P < 0.0001, χ2DFS= 415.61, P < 0.0001). AML was associated with the poorest outcomes (OSmedian= 6.95 months, DFSmedian= 1.5 months). Among metformin users, a lower proportion of overall deaths (244/486 vs. 18/19) and DFS events (271/486 vs. 18/19) were reported in solid malignancies versus those with AML, indicating a lack of survival benefit provided by metformin in AML as compared to solid malignancies, Figure 2. There was no significant difference between the number of AML deaths and DFS events in metformin users (18/19) versus nonusers (32/35). However, a significant difference was noted in the AML group between the number of deaths and DFS events in insulin users (10/10) versus nonusers (40/44), Figure 2. We also observed a lower proportion of deaths (244/486 vs. 225/384) and DFS events (271/486 vs. 250/384) in metformin users versus nonusers diagnosed with solid malignancies.
Figure 2.
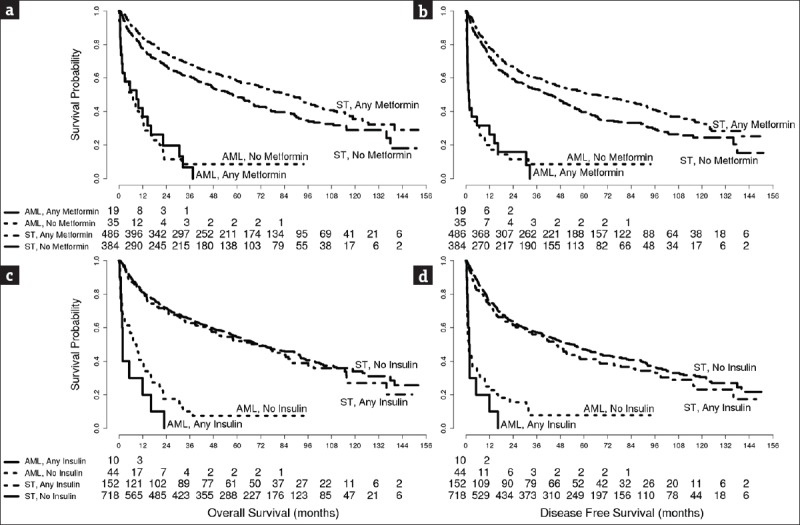
Overall survival (panels a and c) and disease-free survival (panels b and d) by metformin (panels a and b) or insulin (panels c and d) use in individuals with preexisting Type 2 diabetes mellitus diagnosed with solid tumors or acute myeloid leukemia. AML=Acute myeloid leukemia, T2DM=Type 2 diabetes mellitus, ST=Solid tumor
The median survival of the metformin users diagnosed with a solid malignancy was significantly longer than that of nonusers, 86 versus 57.7 months, respectively (P = 0.004). Metformin use was associated with better outcomes in the ST group (χ2OS= 9.55, POS= 0.004; χ2DFS= 10.34, PDFS< 0.003) but not in the AML group (POS= 0.961, PDFS= 0.943), Figure 2, panels a and b. Although we did not see a significant mortality difference between users and nonusers of insulin in the ST group, the insulin users in the AML group remarked by poorer outcomes, Figure 2, panels c and d.
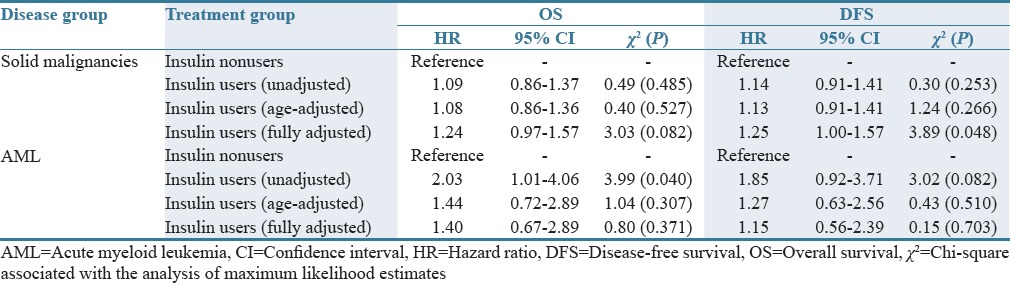
Our unadjusted and age-adjusted Cox proportional hazards model demonstrates a survival and recurrence advantage associated with taking metformin if diagnosed with a solid malignancy [Table 1]. In the unadjusted analysis, metformin nonusers diagnosed with a solid malignancy had roughly 30% higher mortality (χ2 = 9.34, P = 0.002) or recurrence (χ2 = 10.00, P = 0.002) as compared to metformin users. The risk was slightly lower in the age-adjusted analysis [Table 1]. No relationship between metformin use and OS or DFS was observed in the AML group. Insulin use in individuals with solid malignancies was associated with a nearly 25% higher risk of death and recurrence in the fully adjusted model (P = 0.082 and P = 0.048), Table 2. In the AML group, insulin use provided double the risk of death (HR = 2.03, χ2 = 3.99, P = 0.040) and roughly 85% greater risk of relapse (HR = 1.85, χ2 = 3.02, P = 0.082), thus confirming the trends observed in our univariate analysis [Figure 2 and Table 2].
Table 1.
Association of baseline metformin use and cancer outcomes: A proportional hazards model
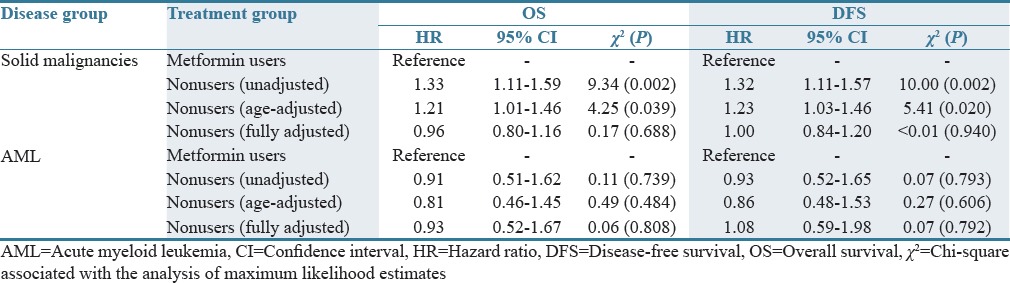
Table 2.
Association of baseline insulin use and cancer outcomes: A proportional hazards model
DISCUSSION
This paper represents the first report regarding the relationship between preexisting T2DM pharmacotherapy use and AML versus ST outcomes. The solid malignancies included in this study represent some of the most often diagnosed cancer diseases in association with which metformin use was shown to improve outcomes. In order to control for any T2DM confounding effects, all the individuals enrolled in this study had a confirmed T2DM diagnosis at the time of inclusion in the study. Being a single site allowed for exhaustive and highly accurate data collection, as well as relatively homogeneous treatment approaches, but restricted the inclusion of additional malignancies. Malignancies with insufficient representation could not reach power as covariates in the HR models, and patients newly diagnosed with AML receiving metformin were, as is common practice, immediately switched to insulin while those with solid malignancies were not. Furthermore, the advanced age of the AML population taking metformin presents a naturally higher risk of death, making the age-adjusted models particularly important. Ethnicity and gender did not meet the criteria for inclusion in the fully adjusted HR model, and although the BMI category was included, it may have introduced a considerable bias in the analysis of the AML group since nearly half of them had unknown BMI. Thus, specifically for the AML group analysis, the age-adjusted model may reveal a more realistic representation of the associations observed.
In light of metformin's cancer benefit in many cancers, future studies should be initiated to explore the potential benefits of metformin could afford AML patients with T2DM, should it be reinitiated after CT as recommended by the package inserts. The present study confirms that metformin is of wide clinical benefit for T2DM patients with many solid malignancies and highlights an urgent need to better evaluate whether benefit may also exist in AML as the current practice of switching to insulin for T2DM management in AML is, in fact, harmful and unnecessary in at least 25% of cases despite the previously assumed benefit of tighter glucose control.
AUTHORS' CONTRIBUTION
Alice C. Ceacareanu was responsible for the study concept, design, definition of intellectual content, data acquisition and analysis supervision, and manuscript preparation; George K. Nimako was responsible for data acquisition literature search, manuscript editing, and review; Zachary A. P. Wintrob was responsible for statistical analysis, manuscript editing, and review and has contributed to the study design. Alice C. Ceacareanu takes full responsibility for the integrity of the work from the inception to the published article and is designated as “Guarantor.”
Financial support and sponsorship
Wadsworth Foundation Peter Rowley Breast Cancer Grant awarded to Alice C. Ceacareanu (Grant C026588), Health Workforce Retraining Initiative, New York State Department of Health Grant awarded to Alice C. Ceacareanu (Grant C029525), the New York State Council of Health-system Pharmacists, Research and Education Foundation Oncology Leadership Grant awarded to Alice C. Ceacareanu (Grant 50151), and the New York State Council of Health-system Pharmacists, Research and Education Foundation Clinical Pharmacy awarded to Alice C. Ceacareanu, Grant 53967.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Authors acknowledge the support of Dr. Meir Wetzler, Chief of Leukemia Section (Roswell Park Cancer Institute), and the assistance of Mrs. Laurie-Ann Ford, Clinical Research Coordinator, in retrieving the information related to leukemia subgroups, relapse and OS. We are grateful to Dr. Adekunle Odunsi for the support extended in accessing the staging and tumor phenotype information pertaining to the ovarian cancer population. Authors also acknowledge the help of our interns Dimitra Bitikofer, Caitlin Jackowiak, Heather Rodman, Thang Bui, Dustyn Miller, Michelle Amsler and Caitlin Frohnapple with data collection, as well as the assistance of Ms. Nana Wang with manuscript editing.
REFERENCES
- 1.Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–77. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 2.Libby G, Donnelly LA, Donnan PT, Alessi DR, Morris AD, Evans JM. New users of metformin are at low risk of incident cancer: A cohort study among people with type 2 diabetes. Diabetes Care. 2009;32:1620–5. doi: 10.2337/dc08-2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiralerspong S, Gonzalez-Angulo AM, Hung MC. Expanding the arsenal: Metformin for the treatment of triple-negative breast cancer? Cell Cycle. 2009;8:2681. doi: 10.4161/cc.8.17.9502. [DOI] [PubMed] [Google Scholar]
- 4.Berstein LM, Boyarkina MP, Tsyrlina EV, Turkevich EA, Semiglazov VF. More favorable progesterone receptor phenotype of breast cancer in diabetics treated with metformin. Med Oncol. 2011;28:1260–3. doi: 10.1007/s12032-010-9572-6. [DOI] [PubMed] [Google Scholar]
- 5.Bowker SL, Majumdar SR, Veugelers P, Johnson JA. Increased cancer-related mortality for patients with type 2 diabetes who use sulfonylureas or insulin. Diabetes Care. 2006;29:254–8. doi: 10.2337/diacare.29.02.06.dc05-1558. [DOI] [PubMed] [Google Scholar]
- 6.Jiralerspong S, Palla SL, Giordano SH, Meric-Bernstam F, Liedtke C, Barnett CM, et al. Metformin and pathologic complete responses to neoadjuvant chemotherapy in diabetic patients with breast cancer. J Clin Oncol. 2009;27:3297–302. doi: 10.1200/JCO.2009.19.6410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosilio C, Ben-Sahra I, Bost F, Peyron JF. Metformin: A metabolic disruptor and anti-diabetic drug to target human leukemia. Cancer Lett. 2014;346:188–96. doi: 10.1016/j.canlet.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 8.ACS. Cancer Facts and Figures. Atlanta: American Cancer Society; 2016. [Google Scholar]
- 9.SEER Stat Fact Sheets: Acute Myeloid Leukemia (AML) Surveillance, Epidemiology, and End Results (SEER) Program. 2016. [Last accessed on 2017 Apr 30]. Available from: http://www.seer.cancer.gov .
- 10.Löwenberg B, Rowe JM. Introduction to the review series on advances in acute myeloid leukemia (AML) Blood. 2016;127:1. doi: 10.1182/blood-2015-10-662684. [DOI] [PubMed] [Google Scholar]
- 11.Chang KH, Hwang WL, Muo CH, Hsu CY, Teng CJ. Outcome and late effects among acute myeloid leukemia survivors: A nationwide population-based study. Support Care Cancer. 2016;24:4993–5000. doi: 10.1007/s00520-016-3361-5. [DOI] [PubMed] [Google Scholar]
- 12.Vu K, Busaidy N, Cabanillas ME, Konopleva M, Faderl S, Thomas DA, et al. Arandomized controlled trial of an intensive insulin regimen in patients with hyperglycemic acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk. 2012;12:355–62. doi: 10.1016/j.clml.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.FDA. Safety announcement. Washington, DC: Food and Drug Administration; 2016. [Last accessed on 2017 Apr 30]. Drug safety communication: FDA revises warnings regarding use of the diabetes medicine metformin in certain patients with reduced kidney function. Available from: https://www.fda.gov/downloads/Drugs/DrugSafety/UCM494140.pdf . [Google Scholar]
- 14.Walter RB, Othus M, Burnett AK, Löwenberg B, Kantarjian HM, Ossenkoppele GJ, et al. Significance of FAB subclassification of “acute myeloid leukemia, NOS” in the 2008 WHO classification: Analysis of 5848 newly diagnosed patients. Blood. 2013;121:2424–31. doi: 10.1182/blood-2012-10-462440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tallman MS, Kim HT, Paietta E, Bennett JM, Dewald G, Cassileth PA, et al. Acute monocytic leukemia (French-American-British classification M5) does not have a worse prognosis than other subtypes of acute myeloid leukemia: A report from the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22:1276–86. doi: 10.1200/JCO.2004.08.060. [DOI] [PubMed] [Google Scholar]
- 16.Pulsoni A, Iacobelli S, Bernardi M, Borgia M, Camera A, Cantore N, et al. M4 acute myeloid leukemia: The role of eosinophilia and cytogenetics in treatment response and survival. The GIMEMA experience. Haematologica. 2008;93:1025–32. doi: 10.3324/haematol.11889. [DOI] [PubMed] [Google Scholar]


