Abstract
Recently published reports have suggested that antiangiogenic drugs such as sunitinib could potentiate the osteonecrosis of the jaw (ONJ) induced by bisphosphonates (BPs) and even induce this adverse effect per se. We reported a case of ONJ with renal cell carcinoma under sunitinib medication and history of BPs therapy. A 53-year-old man was referred to the oral surgery clinic complaining of painful exposed oral lesion and bone extraction from right lower jaw in the mouth. He underwent nephrectomy followed by 5 months treatment with cycles of 50 mg sunitinib (Sutent®) once a day for 4 weeks followed by 2 weeks drug free before lesion exposure in October 2016. However, the patient has encounter to intermittent mucositis and gingivitis in oral cavity several times. Our patient had a history of zoledronic acid (4 mg intravenously two times) administration due to primary cancer misdiagnosis. In our case, no dental procedure contributed to the occurrence of ONJ. The lesion was improved by sunitinib cessation and administration of antibiotics through 2 weeks. Mucosal injury induction as well as inhibition of angiogenic signaling pathways by sunitinib administration may have precipitated the occurrence of ONJ. In addition, a possible synergistic effect by previously BP treatment is another accused.
KEYWORDS: Bisphosphonate, osteonecrosis of the jaw, renal cell carcinoma, Sunitinib, tyrosine kinase inhibitors
INTRODUCTION
Osteonecrosis of the jaw (ONJ) is described as a bone infarction due to ischemia. It is a clinically devastating condition that can affect the cancer patients' quality of life.[1] The main predisposing factors for ONJ are surgical intervention and oral mucosa breakdown in patients encountering to radiotherapy and chemotherapy agents.[2] Primarily, it assumes as a bisphosphonate (BP)-related adverse effect but has been reported in patients receiving other anticancer treatment such as antiangiogenic and targeted therapy agents with and without BPs.[1] Furthermore, the medication-related osteonecrosis of jaw has been recently named in medical literature and is matter of concern in novel drug therapy in cancer patients. Tyrosine kinase inhibitors (TKIs) are drugs with differentiated effects including tumor growth blockage and angiogenesis inhibition through multi-target mechanisms.[3] Among TKIs, sunitinib is mostly linked to TKI-related ONJ cases that were treated for renal cell carcinoma (RCC) in accompany with or without BP therapy.[4,5,6] We will describe a patient who was treated with sunitinib for RCC management and developed ONJ with a history of BP administration separately.
CASE REPORT
A 53-year-old man was referred to the oral surgery clinic affiliated to Isfahan University of Medical Sciences complaining of painful exposed oral lesion and bone extraction from right lower jaw in the mouth. He has declined any surgical or other dentistry procedure on his mouth recently. In his medical history, RCC with lung metastasis has been a diagnosis for him in March 2016, and he underwent a left total nephrectomy on April 2016. For the previous 5 months, he has been treated with cycles of 50 mg sunitinib (Sutent®) once a day for 4 weeks followed by 2 weeks drug free. In fact, he had received almost 4 cycles of sunitinib before lesion exposure in October 2016. Neither mandibular pain nor bone or mucosal lesions were present at the time of sunitinib initiation. During the past 5-month sunitinib therapy, the patient has encounter to intermittent mucositis and gingivitis in oral cavity several times. He had no other positive history for any chronic conditions such as hepatic or renal failure. He declined using any other medications such as glucocorticoid's drugs. Unfortunately, due to complex condition at the time of cancer presentation and misdiagnosis with multiple myeloma, our patient had received zoledronic acid (4 mg intravenously 2 times) on March 2016, and after diagnosis confirmation, it was discontinued quickly. Hence, more than 7 months before lesion exposure, our patient had a history of BP administration. After, 4 course of chemotherapy with sunitinib, oral lesion with persistent pain and progressive mandibular bone exposure has been emerged. In following, patient complained of chewing problem led to our patient refer to oral surgery clinic at the end of October 2016.
Clinical examination revealed a painful exposed bone (around 10 mm) at the left hemimandible [Figure 1a] on the contralateral side. The panoramic radiograph [Figure 1b] showed irregular sclerotic bone at these regions, and the fusion of computerized tomography (CT) scans and bone scans demonstrated locally elevated nucleotide activity there. Axial CT scan showed a single osteonecrotic process involving the mandible, associated with increased bone marrow density and initial signs of bone sequestration and periosteal reaction.
Figure 1.
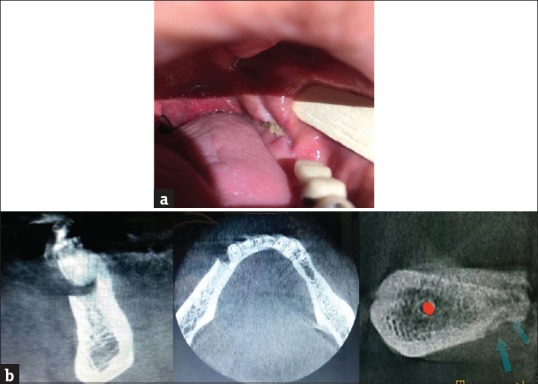
(a) Osteonecrosis of the jaw. (b) Osteonecrosis of the jaw in cone beam computed tomography, cone beam computed tomography cross section related to posterior part of mandibular ridge in the left side showed the necrotic bone which got separated from normal bone
Finally, Grade 1 of ONJ was diagnosed. In this grade, there is a necrotic or exposed bone in patients while the patients are asymptomatic and have no evidence of infection. It supposed to be happened in the patient who received sunitinib therapy while he had a history of BP administration. As the main accused reason was sunitinib therapy, the treatment was withheld and unnecessarily oral amoxicillin (500 mg tid) and metronidazole (500 mg tid) for 7 days were prescribed for the patient. He also was recommended to use chlorhexidine mouthwash twice daily. We followed him for more than 4 weeks, his lesion was Grade 1 of ONJ and the symptom recovery occurred after 2 weeks without any further complication. After 1 month, therapy with sunitinib was resumed for other remained courses. He is taking his medication under the supervision of our team, and the teams frequently monitor him for any other predicament.
DISCUSSION
Sunitinib is an orally administrated small molecule that inhibits tyrosine kinase as well as vascular endothelial growth factor receptors, and platelet-derived growth factor receptors which are overexpressed in the cancer cells. TKIs impair the function of cell surface receptors that initiate growth, differentiation, and survival of cancer cell.[7] Sunitinib is approved for advanced RCC. The most common sunitinib treatment protocol is 6-week cycles of 50 mg/day for 4 weeks followed by 2 weeks drug holiday. In contrast to other conventional chemotherapy, which is given in courses during a defined period, sunitinib therapy will last a longer period.[1,8]
Adverse effects such as diarrhea, mucositis, altered taste, skin abnormalities, and hypertension were reported repeatedly. The majorities of side effects are reversible and would not result in drug withholding. ONJ is often reported during BP therapy; however, the role of infection in pathogenesis is unclear.[8]
There are some reports of ONJ development when BP and antiangiogenic drugs have been combined.[5,7,9] The estimated incidence is 0.9%–2.4%.[10] Even some case reports noted that sunitinib specifically may increase the risk of BP-induced ONJ or worsen it.[7] It was suggested that the antiangiogenic drugs result in ONJ due to mucosal breakdown, angiogenesis disturbance and bone remodeling especially in patients who had been received BP.[8] There is a lack of report around the cases who has affected with ONJ only by sunitinib therapy without any previous exposure to BPs.
Fleissig et al. reported a 58-year-old woman under sunitinib treatment who developed ONJ. She had never been received any BP or corticosteroids. She is one of the best examples of cases who developed ONJ just by sunitinib administration. She was treated and fully recovered with antibiotics and physiotherapy for 12 weeks.[8]
Our case had a short-term history of BP consumption, and we cannot be sure about the diagnosis of ONJ due to BP treatment. Probably, sunitinib as an antiangiogenic drug could potentiate the effect of BP or maybe induce the adverse effect lonely.
Brunello et al. reported a 59-year-old patient with RCC and history of zoledronic acid administration for 6 months as well as sunitinib treatment separately who developed ONJ. The authors hypothesized that the potent antiangiogenic activity of sunitinib may amplify the inhibition of bone remodeling by zoledronic acid.[7]
Hoefertand Eufingernoted 3 patients with RCC under BP medication who developed ONJ during and after sunitinib treatment. They thought that ONJ linked to the occurrence of oral mucositis due to chemotherapy with sunitinib.[11]
It is clear that the mechanism involved in ONJ induced by antiangiogenic drugs is inhibition in jawbone remodeling and wound repairing. Our case also encountered to mucositis during treatment and confirmed the previous events. This supports the emerging role of soft-tissue damage in the pathogenesis of ONJ.
Other antiangiogenic drugs such as bevacizumab (avastin) have already been suspected of having a similar effect. ONJ, with a calculated risk of up to 0.2% was reported by bevacizumab administration.[8,12]
In most of reported data, patients had a history of dental extraction or surgical procedure before ONJ development, but in our case, he had not accomplished any dental procedure before ONJ development. It can be concluded that the potential risk factors for ONJ development in the era of antiangiogenic therapy or nonantiangiogenic therapy have yet to be uncovered.
In the era of differential diagnosis, since, our patient had no history of taking other medications such as glucocorticoids or radiotherapy to the mandibular, secondary osteonecrosis due to other issue except than the previous history of sunitinib or BP treatment is ruled out. The other diagnosis issue such as osteomyelitis is unlikely due to lack of infectious evidences and the trend of improvement proceeding. Laboratory findings did not show any increase in leukocytosis and serum inflammatory markers, however, as we thought that the patients had not any risk factor such as open fractures or recent surgery for developing osteomyelitis, microbial culture was not considered.
On the other hand, the patient had no evidence of disease progression. The recent CT performed for bone metastases have not shown any evidence of metastases in jaw. Hence, developing metastases were excluded from the differential diagnosis.
Another distinct diagnosis is osteosarcoma of the jaw which occurs in older patients rarely. Diagnosis is based on bone biopsy and present of feature such as sarcomatous stroma and production of tumor osteoid and bone.[13] Our radiography findings by cone beam CT (CBCT), were in favor of sclerotic lesions, osteolysis, and bony fractures which were not according to osteosarcoma feature. Our patient had no evidence of developing second malignancy, and his cancer was completely in our control. Furthermore, although the bone biopsy is the gold standard of diagnoses, diagnoses based on imaging were so strong for oncologist to prevent any other invasive modality for the patient.
Taken together, we mostly suggest that our case was affected more from sunitinib therapy than BP. The duration of treatment, pattern of lesion (persisted for <8 weeks) and the process of healing after sunitinib discontinuing were matched more with sunitinib administration.
When considering Naranjo adverse drug reaction probability scale,[14] our score will be calculated 5 points. It means probable adverse drug reaction due to sunitinib therapy. Our score has been gotten from these items:
There are some previous conclusive reports on this reaction (+1); The adverse event appeared after the suspected drug was administered (+2); The adverse reaction improved when the drug was discontinued (+1); The adverse event was confirmed by objective evidence such as CBCT (+1).
In conclusion, our reported case was in favor of sunitinib-related ONJ, and that discontinuation may lead to clinical improvement; however, more research is needed to support these findings. Because long-term side effects of sunitinib are still not known, oncologists, as well as dentists and maxillofacial surgeons, should be aware of ONJ as a recently frequent adverse effect of antiangiogenic therapy. They should be aware of the potential for development of ONJ as a result of surgical intervention or even without any intervention in sunitinib therapy. A more understanding of this adverse effect will allow the clinicians to make a more accurate judgment about risk, prognosis, treatment, and patients' outcome.
AUTHORS' CONTRIBUTION
FA and AD are the responsible physicions of patient's managment who identified and reported the case. BM are the dentist who managed the oral complication. The manuscript was written and finallized by AM.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Fusco V, Santini D, Armento G, Tonini G, Campisi G. Osteonecrosis of jaw beyond antiresorptive (bone-targeted) agents: New horizons in oncology. Expert Opin Drug Saf. 2016;15:925–35. doi: 10.1080/14740338.2016.1177021. [DOI] [PubMed] [Google Scholar]
- 2.Hoff AO, Toth BB, Altundag K, Johnson MM, Warneke CL, Hu M, et al. Frequency and risk factors associated with osteonecrosis of the jaw in cancer patients treated with intravenous bisphosphonates. J Bone Miner Res. 2008;23:826–36. doi: 10.1359/JBMR.080205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patyna S, Laird AD, Mendel DB, O'farrell AM, Liang C, Guan H, et al. SU14813: A novel multiple receptor tyrosine kinase inhibitor with potent antiangiogenic and antitumor activity. Mol Cancer Ther. 2006;5:1774–82. doi: 10.1158/1535-7163.MCT-05-0333. [DOI] [PubMed] [Google Scholar]
- 4.Smidt-Hansen T, Folkmar TB, Fode K, Agerbaek M, Donskov F. Combination of zoledronic acid and targeted therapy is active but may induce osteonecrosis of the jaw in patients with metastatic renal cell carcinoma. J Oral Maxillofac Surg. 2013;71:1532–40. doi: 10.1016/j.joms.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Bozas G, Roy A, Ramasamy V, Maraveyas A. Osteonecrosis of the jaw after a single bisphosphonate infusion in a patient with metastatic renal cancer treated with sunitinib. Oncol Res Treat. 2010;33:321–3. doi: 10.1159/000313680. [DOI] [PubMed] [Google Scholar]
- 6.Beuselinck B, Wolter P, Karadimou A, Elaidi R, Dumez H, Rogiers A, et al. Concomitant oral tyrosine kinase inhibitors and bisphosphonates in advanced renal cell carcinoma with bone metastases. Br J Cancer. 2012;107:1665–71. doi: 10.1038/bjc.2012.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunello A, Saia G, Bedogni A, Scaglione D, Basso U. Worsening of osteonecrosis of the jaw during treatment with sunitinib in a patient with metastatic renal cell carcinoma. Bone. 2009;44:173–5. doi: 10.1016/j.bone.2008.08.132. [DOI] [PubMed] [Google Scholar]
- 8.Fleissig Y, Regev E, Lehman H. Sunitinib related osteonecrosis of jaw: A case report. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;113:e1–3. doi: 10.1016/j.tripleo.2011.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Christodoulou C, Pervena A, Klouvas G, Galani E, Falagas ME, Tsakalos G, et al. Combination of bisphosphonates and antiangiogenic factors induces osteonecrosis of the jaw more frequently than bisphosphonates alone. Oncology. 2009;76:209–11. doi: 10.1159/000201931. [DOI] [PubMed] [Google Scholar]
- 10.Guarneri V, Miles D, Robert N, Diéras V, Glaspy J, Smith I, et al. Bevacizumab and osteonecrosis of the jaw: Incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Res Treat. 2010;122:181–8. doi: 10.1007/s10549-010-0866-3. [DOI] [PubMed] [Google Scholar]
- 11.Hoefert S, Eufinger H. Sunitinib may raise the risk of bisphosphonate-related osteonecrosis of the jaw: Presentation of three cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:463–9. doi: 10.1016/j.tripleo.2010.04.049. [DOI] [PubMed] [Google Scholar]
- 12.Estilo CL, Fornier M, Farooki A, Carlson D, Bohle G, 3rd, Huryn JM. Osteonecrosis of the jaw related to bevacizumab. J Clin Oncol. 2008;26:4037–8. doi: 10.1200/JCO.2007.15.5424. [DOI] [PubMed] [Google Scholar]
- 13.Slootweg PJ, Miiller H. Osteosarcoma of the jaw bones. J Maxillofac Surg. 1985;13:158–66. doi: 10.1016/s0301-0503(85)80040-0. [DOI] [PubMed] [Google Scholar]
- 14.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. Amethod for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]


