Abstract
Aims/hypothesis
The aims of the study were to evaluate the association between type 2 diabetes and the risk of death from any cancer and specific cancers in East and South Asians.
Methods
Pooled analyses were conducted of 19 prospective population-based cohorts included in the Asia Cohort Consortium, comprising data from 658,611 East Asians and 112,686 South Asians. HRs were used to compare individuals with diabetes at baseline with those without diabetes for the risk of death from any cancer and from site-specific cancers, including cancers of the oesophagus, stomach, colorectum, colon, rectum, liver, bile duct, pancreas, lung, breast, endometrium, cervix, ovary, prostate, bladder, kidney and thyroid, as well as lymphoma and leukaemia.
Results
During a mean follow-up of 12.7 years, 37,343 cancer deaths (36,667 in East Asians and 676 in South Asians) were identified. Baseline diabetes status was statistically significantly associated with an increased risk of death from any cancer (HR 1.26;95% CI1.21, 1.31). Significant positive associations with diabetes were observed for cancers of the colorectum(HR 1.41;95% CI 1.26, 1.57), liver (HR 2.05;95% CI 1.77, 2.38), bile duct (HR 1.41;95% CI 1.04, 1.92), gallbladder (HR 1.33; 95% CI1.10, 1.61), pancreas (HR 1.53; 95% CI1.32, 1.77), breast (HR 1.72; 95% CI1.34, 2.19), endometrium (HR 2.73; 95% CI1.53, 4.85), ovary (HR 1.60; 95% CI1.06, 2.42), prostate (HR 1.41; 95% CI1.09, 1.82), kidney (HR 1.84; 95% CI1.28, 2.64) and thyroid (HR 1.99; 95% CI1.03, 3.86), as well as lymphoma (HR 1.39; 95% CI1.04, 1.86). Diabetes was not statistically significantly associated with the risk of death from leukaemia and cancers of the bladder, cervix, oesophagus, stomach and lung.
Conclusions/interpretation
Diabetes was associated with a 26% increased risk of death from any cancer in Asians. The pattern of associations with specific cancers suggests the need for better control (prevention, detection, management) of the growing epidemic of diabetes (as well as obesity), in order to reduce cancer mortality.
Keywords: Asia Cohort Consortium, Asians, Cancer mortality, Meta-analysis, Type 2 diabetes
Introduction
There is emerging evidence of a link between type 2 diabetes and an increased risk of developing cancer and death from cancer. A meta-analysis estimated that diabetes was associated with a 17% and 21% increased risk of developing any cancer and death from any cancer, respectively [1]. However, most studies of the association between diabetes and cancer risk have been conducted in Western populations [2]. Epidemiological studies have documented consistent increases in the prevalence of diabetes across Asia [3, 4]. Some evidence suggests that, at any given BMI, Asians are more susceptible to insulin resistance and have a higher prevalence of diabetes in comparison with whites [5]. The risk of diabetes-related complications and comorbidities may be different in Asians and whites. Some evidence also suggests that cancer risk associated with diabetes is higher in Asians than in non-Asians [1]; however, the data are inconclusive. Given the increasing prevalence of diabetes and diabetes risk factors in Asian populations [3, 4], it is important to understand the effect of diabetes on cancer in Asians.
Several prospective studies of diabetes and site-specific cancer risk have been conducted in individual cohorts in East Asians. However, the majority of the studies have examined risk for one or only a few cancer sites [6–10], included a small number of patients with diabetes [11, 12], or failed to control for important potential confounding variables such as BMI or obesity [6–8, 12, 13]. Asia is heterogeneous, with higher cancer mortality found in East Asian countries such as China, Japan and South Korea (125–164 per 100,000 in men and 65–82 per 100,000 in women) than in South Asian countries such as Bangladesh and India (70–90 per 100,000 in men and 60–72 per 100,000 in women) [14]. However, no prospective cohort studies have investigated the influence of diabetes on cancer in South Asians. Large prospective studies on the association between diabetes and cancer at specific organ sites in Asian populations are therefore needed.
We conducted a pooled analysis to evaluate the association between baseline diabetes and risk of death from all cancers and site -specific cancers, in 19 Asian prospective cohorts in the Asia Cohort Consortium (ACC).
Methods
ACC
Details of the ACC, an international collaboration committed to the study of the aetiology of diseases in Asian populations, have been presented elsewhere [15, 16]. Cohorts were identified through a systematic search of the literature in early 2008, followed by a survey that was sent to each cohort to assess data availability [16]. The ACC includes more than 20 cohorts representing Japan, China, South Korea, India, Taiwan, Bangladesh and Singapore. Cohort studies that were included in the present study are listed in Table 1. Several of these cohorts have examined the association between diabetes and cancer risk [9, 17, 18]. Our study was approved by the institutional review board of the Fred Hutchinson Cancer Research Center (Seattle, WA, USA) and by the ethics committees of each individual cohortstudy.
Table 1.
Selected characteristics of participating cohorts
| Cohort | No. of participants | Enrolment period | Mean follow-up (years) | Mean age at baseline (years) | Male sex (%) | Ever smokers (%) | Diabetes at baseline (%) | No. of cancer deaths | |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Men | Women | ||||||||
| East Asians | 658,611 | 1984–2006 | 13.9 | 54.5 | 48.2 | 72.5 | 7.1 | 5.1 | 36,667 |
| China (mainland) | |||||||||
| Linxian | 29,464 | 1984–1987 | 18.5 | 51.9 | 44.6 | 67.1 | 0.2 | 0.1 | 2087 |
| SCS | 18,092 | 1986–1989 | 16.3 | 55.3 | 100.0 | 57.3 | – | 1.3 | 1974 |
| SMHS | 61,466 | 2001–2006 | 8.3 | 55.3 | 100.0 | 69.6 | – | 6.3 | 1977 |
| SWHS | 74,935 | 1996–2000 | 13.8 | 52.6 | 0.0 | – | 2.8 | 4.4 | 2682 |
| Taiwan | |||||||||
| CBCSP | 23,755 | 1991–1992 | 15.2 | 47.3 | 50.3 | 56.5 | 1.0 | 2.5 | 1012 |
| CVDFACTS | 5156 | 1990–1993 | 14.9 | 47.6 | 44.1 | 54.2 | 1.7 | 3.5 | 219 |
| Singapore | |||||||||
| SCHS | 63,257 | 1993–1999 | 11.5 | 56.5 | 44.2 | 58.0 | 8.8 | 9.0 | 3887 |
| South Korea | |||||||||
| KMCC | 13,143 | 1993–2004 | 13.5 | 54.9 | 40.0 | 79.6 | 9.3 | 4.7 | 833 |
| Seoul Male | 13,833 | 1992–1993 | 15.6 | 49.2 | 100.0 | 77.3 | – | 2.9 | 495 |
| Japan | |||||||||
| 3 Pref. Aichi | 23,111 | 1985 | 11.7 | 54.8 | 47.0 | 83.3 | 15.0 | 4.7 | 1119 |
| JPHC1 | 43,051 | 1990–1992 | 21.0 | 49.6 | 47.9 | 75.9 | 7.5 | 4.0 | 2933 |
| JPHC2 | 56,535 | 1992–1995 | 17.7 | 54.3 | 47.4 | 75.8 | 7.8 | 5.7 | 4737 |
| 3 Pref. Miyagi | 31,345 | 1984 | 11.5 | 57.3 | 44.6 | 77.4 | 12.6 | 5.7 | 1884 |
| Miyagi | 47,604 | 1990 | 16.2 | 52.1 | 48.0 | 81.7 | 11.3 | 4.3 | 2367 |
| Ohsaki | 51,250 | 1995 | 10.7 | 60.5 | 47.9 | 81.6 | 11.2 | 6.7 | 2886 |
| JACC | 75,485 | 1988–1990 | 12.8 | 57.0 | 42.0 | 78.9 | 6.3 | 5.5 | 3988 |
| Takayama | 27,129 | 1992 | 13.5 | 56.1 | 45.3 | 83.4 | 17.5 | 5.3 | 1587 |
| South Asians | 112,686 | 1991–2002 | 5.8 | 50.3 | 65.7 | 35.3 | 1.4 | 2.4 | 676 |
| India | |||||||||
| MCS | 101,262 | 1991–1997 | 5.0 | 51.8 | 68.3 | 32.5 | 0.4 | 2.4 | 552 |
| Bangladesh | |||||||||
| HEALS | 11,424 | 2000–2002 | 12.4 | 37.0 | 42.5 | 74.2 | 6.2 | 2.1 | 124 |
| Total | 771,297 | 1984–2006 | 12.7 | 53.9 | 50.8 | 65.3 | 6.5 | 4.7 | 37,343 |
3 Pref. Aichi, Three-Prefecture Cohort Study Aichi; 3 Pref. Miyagi, Three-Prefecture Cohort Study Miyagi; CBCSP, Community-Based Cancer Screening Program; CVDFACTS, Cardiovascular Disease Risk Factors Two-Township Study; HEALS, Health Effects of Arsenic Longitudinal Study; JACC, Japan Collaborative Cohort Study; JPHC, Japan Public Health Center-Based Prospective Study; KMCC, Korea Multicenter Cancer Cohort Study; Seoul Male, Seoul Male Cohort Study; Linxian, Linxian General Population Trial Cohort Study; MCS, Mumbai Cohort Study; Miyagi, Miyagi Cohort Study; Ohsaki, Ohsaki National Health Insurance Beneficiaries Cohort Study; SCHS, Singapore Chinese Health Study; SCS, Shanghai Cohort Study; SMHS, Shanghai Men’s Health Study; SWHS, Shanghai Women’s Health Study; Takayama, Takayama Study
Data manipulation
Relevant data from each of the participating cohorts were transferred and harmonised at the ACC coordinating centre at the Fred Hutchinson Cancer Research Center. Harmonisation involved several rounds of discussions to ensure that variables were correctly interpreted and extracted. Data were checked for illogical or missing values, and queries were sent back for clarification. The distributions of individual variables were explored to identify false or implausible values. All personal identifiers were removed, but study-specific ID numbers were retained to facilitate referral of all queries back to the individual cohort.
Diabetes and outcome data
The 19 participating cohorts included a total of 847,009 individuals. Self-reported data on diabetes at baseline were collected from all cohorts. Available data on other indicators of diabetes in some of the cohorts support the overall validity of these self-reported data. For instance, in the Bangladeshi cohort, glycosuria was measured in 11,122 (95%) members, and 62% of participants who tested positive for glycosuria also self-reported as having been diagnosed with diabetes by a physician, while 99.1% of those who tested negative reported no such history [19]. The result is comparable with that from high-income countries such as Japan, where the sensitivity and specificity of the questionnaire for diabetic hyperglycaemia were 46% and 98%, respectively [15, 20].
All the participating cohorts were required to have available baseline data on diabetes status (self-reported), BMI (measured or self-reported), cigarette-smoking status, age, sex, follow-up time, vital status and cause of death (ICD-9 [www.icd9data.com/2007/Volume1] or ICD-10 [www.who.int/classifications/icd/en/]). In the present analysis, we excluded participants with data missing on age (n=528), baseline diabetes (n=73,178)and vital status (n=2775), and those for whom data on follow-up duration were invalid or missing (n=2947). After these exclusions, 771,297 participants remained (391,619 men and 379,678 women). Information on the types of diabetes (i.e. type 1 or type 2) was not available; however, given that type 1 diabetes accounts for less than 3% of total diabetes in Asia [21]and that we included adult cohorts only, we assume the large majority would have had type 2 diabetes. Additional data were available from selected cohorts on alcohol drinking (17 cohorts), education (14 cohorts), urban/rural residence (18 cohorts), duration of diabetes (six cohorts), and diabetes treatment (four cohorts).
Cancer death was ascertained from the underlying causes of death recorded on death certificates or death records in East Asian cohorts and in the Mumbai cohort. A validated verbal autopsy questionnaire was used in the Bangladesh cohort [22]. Outcomes of interest included: death from any cancer and cancers of the oesophagus, stomach, colorectum, colon, rectum, liver, bile duct, pancreas, lung, breast, endometrium, cervix, ovary, prostate, bladder, kidney and thyroid, as well as lymphoma and leukaemia (electronic supplementary material [ESM] Table 1). Cancers of interest were selected based on prior literature on the association between diabetes and the risk of death from cancer [2, 23].
Statistical analyses
To examine the relationship between diabetes and the risk of death from cancer, we estimated HRs and 95% CIs based on Cox proportional hazards regression models that combined studies in ameta -analysis of individual participant data [24, 25]. The assumption of proportional hazards was examined by testing the cross-product terms between covariate variables and log function of survival time within individual cohorts; p values for all the terms were >0.10. In all analyses, the effect of diabetes on the risk of death from cancer was taken to be cohort-specific, assuming that the log-HR associated with diabetes had a fixed-effect component in each cohort that was common to all cohorts and a cohort-specific random effect that was normally distributed with mean zero.
Potential confounders included baseline age, sex, cigarette smoking (never/ever), BMI, alcohol consumption (never/ever), educational attainment (no formal education, primary, secondary, trade/technical, university degree, postgraduate degree, other)and urban residence. Likelihood-based methods were used to test for heterogeneity across studies or geographic regions by comparing models with and without interactions between cohort or geographic region and baseline diabetes status. Stratified analyses were conducted by sex. To evaluate the potential effect of reverse causation, analyses excluded person-years and deaths with fewer than 3 years of follow-up.
For any cancer and cancers with sufficient number of deaths among those with diabetes (n>70) that were also associated with diabetes status in the main analyses, including colorectal cancer, liver cancer, gallbladder cancer, pancreatic cancer and breast cancer, we further performed stratified analyses among East Asians (China, Taiwan, Singapore, South Korea and Japan) and South Asians (Bangladesh and India); by baseline age (<60 or ≥60 years, the median among cancer deaths in the combined East and South Asians); by baseline smoking status (never/ever); by baseline alcohol consumption status (never/ever); and by baseline BMI (22.8 kg/m2, the median among cancer deaths), to evaluate whether the influence of diabetes was similar in different subgroups. In cohorts with the available information, sensitivity analyses were also conducted to: (1) further control for baseline status of hypertension or cardiovascular disease (CVD); (2) further control for baseline status of cancer; (3) exclude studies that were initially clinical trials; and (4) exclude prevalent cases of CVD and cancer at baseline. In a subgroup of six cohorts (n=243,214) with data on age at first diagnosis of diabetes, diabetes duration at baseline was calculated as the difference between age at first diagnosis and age at enrolment. We conducted analyses to estimate HRs, comparing those with different diabetes duration (<5 or ≥5 years) with those without diabetes at baseline. A separate model was also conducted including only the diabetes cases to compare the risk of death from cancer by diabetes duration (<5 vs ≥5 years) and to estimate the p values of the comparisons. Similar analyses were conducted in a subgroup of four cohorts (n=198,440) with data on current treatment of diabetes (yes/no). All statistical analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, NC, USA) or Stata, version 14.0 (StataCorp LP, College Station, TX, USA). All tests were two-sided and p<0.05 was considered statistically significant.
Results
After exclusions, the study comprised a total of 771,297 participants from 19 cohorts (Table 1), including 658,611 East Asians and 112,686 South Asians. The participants were, on average, 53.9 years old at baseline and had a mean follow-up of 12.7 years. Across all studies, 50.8% were men and 65.3% of men were ever smokers. The diabetes prevalence was lowest in the Linxian General Population Trial Cohort Study in China (0.1%) and highest in the Singapore Chinese Health Study (9.0%). Overall, diabetes was statistically significantly associated with a higher risk of death from any cancer and from cancers of the colorectum, colon and rectum separately, liver, bile duct, gallbladder, pancreas, breast, endometrium, ovary, prostate, kidney and thyroid, as well as lymphoma (Table 2). The association was strongest for cancers of the liver, endometrium and thyroid. After excluding the first 3 years of follow-up, the associations between diabetes and the risk of death from any cancer and site-specific cancers were not materially changed.
Table 2.
Association between diabetes at baseline and site-specific cancer mortality in Asia
| Type of cancer | All participants
|
Excluding first 3 years of follow-up
|
||||
|---|---|---|---|---|---|---|
| Deaths (n) with diabetes | Deaths (n) without diabetes | HR (95% CI)a | Deaths (n) with diabetes | Deaths (n) without diabetes | HR (95% CI)a | |
| Any cancer | 2694 | 34,649 | 1.26 (1.21, 1.31) | 2229 | 30,023 | 1.25 (1.20, 1.31) |
| Oesophagus | 71 | 1302 | 1.09 (0.85, 1.38) | 57 | 1134 | 1.07 (0.81, 1.42) |
| Stomach | 342 | 5974 | 1.08 (0.95, 1.23) | 285 | 5126 | 1.11 (0.96, 1.29) |
| Colorectum | 360 | 3834 | 1.41 (1.26, 1.57) | 294 | 3372 | 1.36 (1.20, 1.53) |
| Colon | 248 | 2433 | 1.50 (1.30, 1.73) | 205 | 2147 | 1.47 (1.25, 1.72) |
| Rectum | 112 | 1401 | 1.30 (1.07, 1.58) | 89 | 1225 | 1.23 (0.99, 1.54) |
| Liver | 434 | 3318 | 2.05 (1.77, 2.38) | 347 | 2812 | 2.02 (1.74, 2.35) |
| Bile ductb | 45 | 528 | 1.41 (1.04, 1.92) | 40 | 487 | 1.42 (1.02, 1.96) |
| Gallbladder | 121 | 1493 | 1.33 (1.10, 1.61) | 102 | 1305 | 1.35 (1.10, 1.66) |
| Pancreas | 208 | 2225 | 1.53 (1.32, 1.77) | 178 | 1951 | 1.57 (1.34, 1.83) |
| Lung | 463 | 7401 | 0.98 (0.89, 1.09) | 381 | 6510 | 0.96 (0.86, 1.07) |
| Breastc | 75 | 950 | 1.72 (1.34, 2.19) | 62 | 786 | 1.95 (1.49, 2.56) |
| Endometriumc | 15 | 143 | 2.73 (1.53, 4.85) | 10 | 132 | 2.09 (1.05–4.12) |
| Cervixc | 17 | 257 | 1.70 (0.90, 3.23) | 12 | 214 | 1.82 (0.80, 4.13) |
| Ovaryc | 26 | 396 | 1.60 (1.06, 2.42) | 23 | 323 | 1.85 (1.19, 2.88) |
| Prostated | 66 | 692 | 1.41 (1.09, 1.82) | 57 | 630 | 1.37 (1.03, 1.82) |
| Bladder | 38 | 468 | 1.38 (0.90, 2.10) | 32 | 427 | 1.45 (0.90, 2.34) |
| Kidney | 51 | 514 | 1.84 (1.28, 2.64) | 44 | 450 | 1.89 (1.34, 2.68) |
| Thyroid | 11 | 137 | 1.99 (1.03, 3.86) | 10 | 124 | 1.97 (1.00, 3.90) |
| Lymphoma | 69 | 841 | 1.39 (1.04, 1.86) | 62 | 729 | 1.51 (1.11, 2.04) |
| Leukaemia | 52 | 790 | 1.30 (0.90, 1.88) | 47 | 666 | 1.47 (1.00, 2.16) |
HRs were adjusted for sex, baseline age, BMI, cigarette smoking, alcohol consumption, educational attainment and urban residence
Data were available only from the Seoul Male Cohort Study, Three-Prefecture Cohort Study Aichi, Japan Public Health Center-Based Prospective Study 1 and 2, Miyagi Cohort Study, Three-Prefecture Cohort Study Miyagi and the Ohsaki National Health Insurance Beneficiaries Cohort Study
Among women only
Among men only
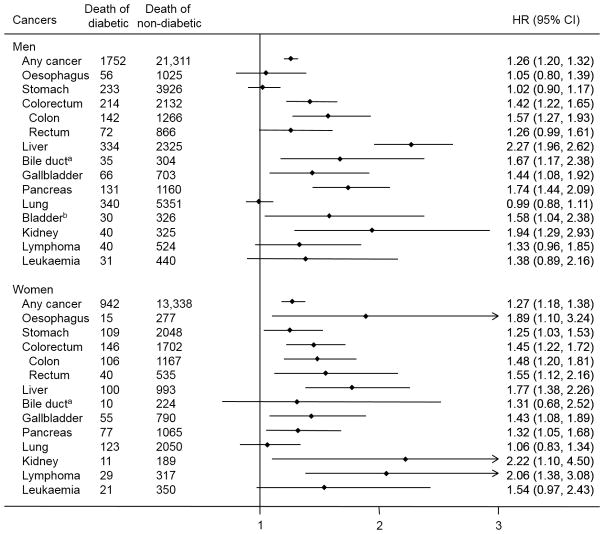
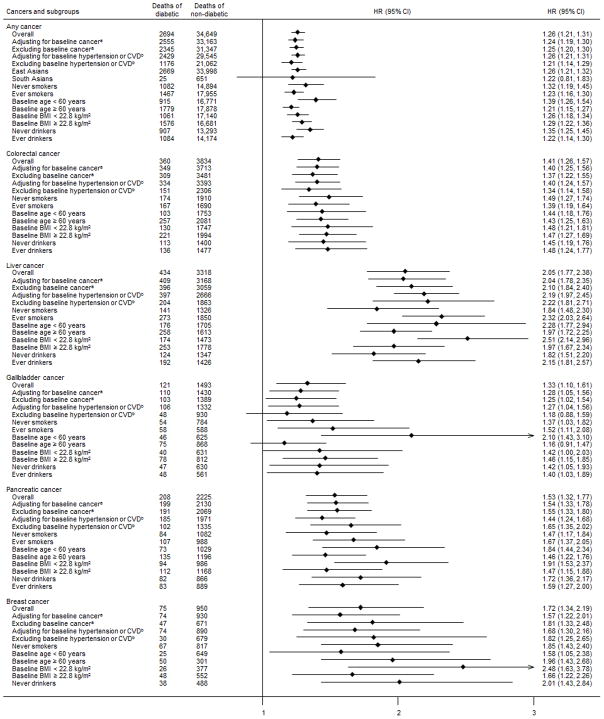
The pattern of the associations between diabetes and cancer mortality in men was similar to that seen overall (Fig. 1). The risk of death from lymphoma, oesophageal cancer and stomach cancer associated with diabetes was greater in women than in men. There was evidence of effect modification by sex for lymphoma (pinteraction<0.01) and stomach cancer (pinteraction=0.02). The positive association between diabetes and any cancer was similar in East and South Asians, although the estimate in South Asians did not reach statistical significance, possibly owing to the small number of deaths in participants with diabetes (Fig. 2). Excluding participants with a history of cancer, hypertension or CVD, and additional adjustment for these conditions did not change the strength of the association appreciably. A greater risk of death from any cancer was associated with diabetes among individuals who were <60 years at baseline than among individuals ≥60 years (pinteraction<0.01). For colorectal cancer, the HRs were similar across all subgroups (Fig. 2). Similarly, a stronger association between diabetes and the risk of death from liver cancer and gallbladder cancer was observed in individuals <60 years compared with those ≥60 years (pinteraction<0.01). For pancreatic cancer, the risk of death associated with diabetes was stronger in individuals <60 years and in those with a BMI <22.8 kg/m2compared with the HRs observed in the respective counterparts; the interaction of diabetes with age and BMI was statistically significant (pinteraction=0.02 and 0.03, respectively). The positive association between diabetes and death from breast cancer remained robust with additional control for the influence of baseline cancer, hypertension or CVD, and among never smokers and never drinkers. The association was stronger in those with a lower BMI compared with those with a higher BMI, although the interaction was not statistically significant.
Fig. 1.
Association between diabetes at baseline and site-specific cancer mortality in Asia by sex. HRs were adjusted for sex, baseline age, BMI, cigarette smoking, alcohol consumption, educational attainment and urban residence. aData were available only from the Seoul Male Cohort Study, Three-Prefecture Cohort Study Aichi, Japan Public Health Center-Based Prospective Study 1 and 2, Miyagi Cohort Study, Three-Prefecture Cohort Study Miyagi and Ohsaki National Health Insurance Beneficiaries Cohort Study. bResults are not shown among women because there were fewer than ten cancer deaths among participants with diabetes at baseline
Fig. 2.
Subgroup analyses for the association between diabetes at baseline and mortality from any cancer, colorectal cancer, liver cancer, gallbladder cancer, pancreatic cancer and breast cancer. HRs were adjusted for sex, baseline age, BMI, cigarette smoking, alcohol consumption, educational attainment and urban residence. Results for breast cancer among ever smokers and ever drinkers are not presented because there were fewer than ten deaths among participants with diabetes at baseline. aAnalyses excluded people with missing information on history of cancer and data from the Seoul Male Cohort Study and Mumbai Cohort Study, which did not have data on previous diagnoses of cancer. bAnalyses excluded people with missing information on history of CVD or hypertension and data from the Mumbai Cohort Study
We further explored the relationship of duration and treatment of diabetes with the risk of death from cancer in cohorts with available data. Analyses were done for cancers with ten or more deaths among diabetic participants. With the exception of breast cancer, among diabetic participants the associations between diabetes of ≥5 years ’duration at baseline and the risk of death from any cancer and colorectal cancer, liver cancer or pancreatic cancer were weaker than those related to diabetes of <5 years’ duration; however, none of the differences in estimates was statistically significant (ESM Table 2). In cohorts with data on current treatment, participants currently treated for diabetes, compared with those not currently treated, tended to have a greater risk of death from any cancer and cancers of the liver, gallbladder and pancreas; however, the risk difference reached statistical significance for death from any cancer only (p=0.03; ESM Table 3). Results from sensitivity analyses excluding studies that were initially clinical trials (Linxian General Population Trial Cohort Study) were essentially the same (data not shown).
Discussion
In this pooled analysis of 19 Asian cohorts including more than 771,000 participants followed for upto 21 years, diabetes was associated with a 26% increase in the risk of death from any cancer. Specifically, diabetes was related to an increased risk of death from colorectal cancer, liver cancer, bile duct cancer, gallbladder cancer, pancreatic cancer, breast cancer, endometrial cancer, ovarian cancer, prostate cancer, kidney cancer, thyroid cancer and lymphoma. The positive associations for any cancer, colorectal cancer, liver cancer, gallbladder cancer, pancreatic cancer and breast cancer remained a cross different strata of BMI, smoking status and alcohol drinking status. Consistently stronger associations were seen in individuals <60 years at enrolment than in their older counterparts for liver cancer, gallbladder cancer and pancreatic cancer.
Several studies have addressed the association of diabetes with the risk of death from any cancer. A pooled analysis of 97 prospective cohorts in 820,900 whites from the Emerging Risk Factors Collaboration (ERFC) reported an HR of 1.25 (95% CI 1.19, 1.31) for death from any cancer in people with diabetes compared with those without diabetes [26]. In a prospective cohort of one million US adults in Cancer Prevention Study II (CPSII), diabetes was associated with increases in the risk of death from any cancer of 7% and 11% in men and women, respectively [27]. A meta-analysis reported a summary relative risk of death from any cancer associated with diabetes of 1.32 (95% CI 1.20, 1.45) based on three studies in Asians, and 1.16 (95% CI 1.01, 1.34) based on 14 studies in non-Asians [1]. In the present pooled analysis of Asians, we report an HR of 1.26 (95% CI 1.21, 1.31) for death from any cancer associated with diabetes. In CPS II, diabetes was positively related to death from cancers of the colon, liver or intrahepatic bile duct, pancreas, breast, endometrium, prostate (reduced risk) and bladder (in men only) [27]. The ERFC study reported that diabetes was moderately associated with death from cancers of the colorectum, liver, pancreas, breast, ovary, lung and bladder [26]. Our observed cancer-specific associations are largely consistent with these reports from two large studies in cohorts from Western countries on digestive cancers (including cancers of the colorectum, liver, bile duct, gallbladder and pancreas), as well as breast cancer. These data together suggest that the influence of diabetes on the risk of death from overall cancer, digestive cancers and breast cancer is largely similar in Asians and in developed Western countries.
The moderate associations between diabetes and the risk of death from liver and pancreatic cancer observed in our analyses are consistent with previous studies [2, 28]. A recent meta-analysis of 25 prospective studies of Asians and whites reported a twofold higher risk of liver cancer associated with diabetes, after controlling for alcohol intake, cirrhosis and viral hepatitis B or C infection [29]. However, reverse causation has been suspected, as mechanistic studies found that pancreatic cancer causes paraneoplastic beta cell dysfunction by shedding exosomes containing adrenomedullin and CA19-9 into circulation that inhibit insulin secretion[30]. In our subgroup analyses, although we observed that diabetes of short duration was more strongly related to the risk of cancer compared with diabetes of long duration, the difference was not statistically significant and the risk associated with diabetes of long duration remained elevated. A recent meta-analysis of 36 cohort studies reported that summary relative risks of pancreatic cancer remained elevated when excluding diabetes of short duration [31], suggesting that reverse causation does not account for all the association. The higher risk of liver cancer and pancreatic cancer in diabetes could potentially be related to exposure of these organs to particularly elevated insulin in people with diabetes, particularly if the tumour cells remain sensitive to insulin [23, 32]. In the present study, a stronger association seen in younger participants for liver, gallbladder and pancreatic cancer also supports metabolic dysfunction (more predominant in the early years of diabetes because of insulin resistance [33]) as being involved in the association between diabetes and these cancers. Taken together, given the magnitude of the associations and the consistent data across studies and different populations, diabetes control should be considered in cancer prevention for these cancers in Asians, especially for liver cancer, which has a high incidence in Asians.
Available data on the association between diabetes and colorectal cancer mortality are limited but are generally consistent with our findings [26, 34]. In addition to hyperinsulinaemia, hypothesised mechanisms include the slower bowel transit time and the elevated faecal bile acid concentrations related to diabetes [32]. We also found that diabetes was related to a 30–40% increased risk of death from gallbladder cancer and bile duct cancer, consistent with previous meta-analyses in whites and Asians [35, 36]. Aside from hyperinsulinaemia and resultant high levels of insulin-like growth factors, possible explanations for the increased risk include the propensity of diabetic individuals to develop gallstones [37], and increased production of biliary cholesterol and lithogenic bile salts as a result of insulin resistance, which also directly promotes the formation of gallstones [38].
We observed positive associations of diabetes with the risk of death from breast cancer, endometrial cancer, ovarian cancer, prostate cancer, kidney cancer, thyroid cancer and lymphoma. Hormone-related mechanisms, such as increases in bioavailable oestrogen, stimulation of endometrial stromal cells, increases in androgen synthesis and increases in endogenous sex steroids[23] due to increased circulating insulin, may underlie the associations for breast and other female cancers. Cumulative epidemiological evidence suggests an association between diabetes and breast cancer [34]. Genetic factors have emerged that potentially contribute to the associations with some female cancers [39, 40]. Data on ovarian cancer are less well established, possibly due to small sample sizes in existing studies, including the present analysis. Further large studies are needed to confirm and investigate effect modifiers for the influence of diabetes on less common hormone-related female cancers such as ovarian cancer in Asians.
Consistent with other prospective studies of cancer mortality in both whites [27, 41]and Asians [42], we did not observe an association between diabetes and the risk of lung cancer mortality. The association with mortality from oesophageal cancer and stomach cancer in whites and Asians has been generally null [26, 27, 42], except for a South Korean study, which found a positive association between diabetes and the risk of death from both cancers in men [28]. By contrast, we observed an increased risk of death from these two cancers that was confined to women. The lack of consistency may be caused by the heterogeneous nature of these cancers (each has subtypes with different aetiologies). Future studies are needed to investigate associations by subtypes of cancer and to explore whether other sex-specific risk differences, such as in the pattern and extent of alcohol drinking, can explain the difference by sex. Evidence of the association between diabetes and kidney cancer, thyroid cancer and lymphoma has also been inconsistent, although meta-analyses have suggested positive associations between diabetes and these cancers [43–45]. Two cohort studies in Asians reported a statistically significant increase in the risk of thyroid cancer incidence or mortality among diabetic patients [10, 13]. We observed moderate associations for kidney (HR 1.84;95% CI 1.28, 2.64) and thyroid (HR 1.99; 95% CI 1.03, 3.86) cancer in Asians, suggesting that the effects of diabetes on these cancers may be greater than those observed in whites. However, future larger studies that include information on subtypes of these cancers are needed to confirm the associations in Asians.
For prostate cancer, most of the studies that found an inverse association were conducted in whites [27, 46]. Studies in Asians generated inconsistent findings, and a meta-analysis of studies in Asians concluded that there was a positive association [47]. The influence of diabetes on prostate cancer in Asians and whites appears different, possibly due to differences in incidence rates, screening, diagnosis and treatment in the two populations. In addition, because the outcomes in this study are based on mortality data, they reflect the combined influence of diabetes on disease incidence and survival. Additional studies with incidence data in Asians are warranted, as such data are critical for prostate cancer, for which there is a greater difference between cancer incidence and cancer mortality, compared with other cancers such as liver and pancreatic cancers, which have a shorter survival time.
Major strengths of the present study included a large sample size of diverse Asian populations, allowing us to conduct several sensitivity analyses and stratified analyses by sex, age, smoking status and alcohol consumption. In addition, we had detailed data at the individual level from all cohorts for covariate adjustments and exploration of heterogeneity, avoiding some drawbacks of meta-analysis based on aggregate data from published results. In addition, we included a comprehensive list of causes of cancer death. A limitation of our study is the use of self-reported diabetes status and the lack of update on diabetes status during follow-up. This might have resulted in non-differential misclassification. In a subgroup analysis, we found that the association between treated diabetes and cancer was stronger than that between untreated diabetes and cancer, suggesting that the use of a more stringent definition of diabetes may reduce this misclassification. The use of self-reported information prevented us from distinguishing between type 1 and type 2 diabetes; however, type 2 diabetes accounts for more than 90% of diabetes [16, 48]. Certain diabetes treatments have been reported to increase (e.g. exogenous insulin) or decrease (e.g. metformin) the risk of cancer [23, 49, 50], but our pooled analyses cannot address the influence of specific treatments, due to the lack of data. We had data on the absence and presence of current treatment only in a small subset of cohorts. Finally, we have limited sample sizes to evaluate the influence of diabetes on site-specific cancers in South Asians. Given that South Asian populations are undergoing an epidemiological transition, there is an emerging need for cohort studies or studies to be conducted for cancer risk factors.
Conclusion
We observed that diabetes was related to an increased risk of death from any cancer, colorectal cancer, liver cancer, bile duct cancer, gallbladder cancer, pancreatic cancer, breast cancer, endometrial cancer, ovarian cancer, prostate cancer, kidney cancer, thyroid cancer and lymphomain Asians. The findings indicate a potential need for appropriate cancer screening among individuals with diabetes and a greater emphasis on lifestyle modifications related to cancer mortality not only in Western populations but also in Asians. We also found an increased risk associated with diabetes from other cancers, which requires future confirmation.
Supplementary Material
Acknowledgments
The authors would like to thank all research team members and participants of each cohort study for their contribution to this pooled analysis.
Funding This work was supported by the US National Cancer Institute at the National Institutes of Health (R03CA150038) and by the Fred Hutchinson Cancer Research Center. The cohorts participating in the pooled analysis were supported by the following grants: Three-Prefecture Cohort Study Aichi (Japanese Ministry of the Environment); Japan Collaborative Cohort Study (National Cancer Center Research and Development Fund, Grant-in-Aid for Cancer Research; Grant for Health Services and Grant for Comprehensive Research on Cardiovascular and Life-Style Related Diseases from the Ministry of Health, Labour and Welfare, Japan; Grant for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan); Japan Public Health Center-Based Study 1and 2 (National Cancer Center Research and Development Fund [23-A-31 (toku) and 26-A-2 (since 2011)]; Grant-in-Aid for Cancer Research from the Ministry of Health, Labour and Welfare, Japan [from 1989 to 2010]); Three -Prefecture Cohort Study Miyagi, Miyagi Cohort Study and Ohsaki National Health Insurance Beneficiaries Cohort Study (Grants-in-Aid for Cancer Research and for the Third Term Comprehensive Ten-Year Strategy for Cancer Control [H21-3jigan-ippan-003], Ministry of Health, Labour and Welfare, Japan); Takayama Study (National Cancer Center Research and Development Fund; National Heart, Lung, and Blood Institute [U01HL072507]; Chinese Academy of Medical Sciences); Shanghai Cohort Study (National Institutes of Health [R01CA144034, U01CA182876]); Shanghai Men’s Health Study and Shanghai Women’s Health Study (National Institutes of Health [R37CA070867, UM1CA182910, R01CA82729, UM1CA173640]); Community-Based Cancer Screening Program (National Science Council and Department of Health, Taiwan); Cardiovascular Disease Risk Factors Two-Township Study (Department of Health, Taiwan [DOH80-27, DOH81-021, DOH8202-1027, DOH83-TD-015 and DOH84-TD-006]); Korea Multicenter Cancer Cohort Study (Ministry of Education, Science and Technology, South Korea [2009-0087452]; National Research Foundation of Korea [2009-0087452]); Singapore Chinese Health Study (National Institutes of Health [R01CA144034, U01CA182876]); Health Effects of Arsenic Longitudinal Study (National Institutes of Health [P42ES010349, R01CA102484, R01CA107431]); Mumbai Cohort Study (International Agency for Research on Cancer, France; Clinical Trial Service Unit, UK; World Health Organization). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- ACC
Asia Cohort Consortium
- CPS II
Cancer Prevention Study II
- CVD
Cardiovascular disease
- ERFC
Emerging Risk Factors Collaboration
Footnotes
Data availability The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
Duality of interest The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement YC conceived and designed the study. FW and ES analysed the data. PCG, NS, AT, X-OS, W-PK, Y-BX, YT, KS, SKP, KM, CN, YS, Y-LQ, S-LY, RW, M-HS, W-HP, MSP, ST, HC, J-MY, Y-TG, IT, SK, HI, KW, Y-OA, K-YY, HA, KSC, WZ, MI and DK contributed to acquisition of data. YC and FW wrote the manuscript. YL, MS, HNL, PB, WZ, X-OS and JDP revised the manuscript for important intellectual content. YC is the study guarantor. All authors contributed to the interpretation of data and approved the final version of the manuscript. YC had full access to all the data in the study and takes responsibility for t he integrity of the data and the accuracy of the data analysis.
References
- 1.Noto H, Tsujimoto T, Noda M. Significantly increased risk of cancer in diabetes mellitus patients: a meta-analysis of epidemiological evidence in Asians and non-Asians. J Diabetes Investig. 2012;3:24–33. doi: 10.1111/j.2040-1124.2011.00183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JP. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. Bmj. 2015;350:g7607. doi: 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- 3.Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet. 2010;375:408–418. doi: 10.1016/S0140-6736(09)60937-5. [DOI] [PubMed] [Google Scholar]
- 4.Chan JC, Malik V, Jia W, et al. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–2140. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 5.Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64–91. doi: 10.1111/nyas.12098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen HF, Chen P, Li CY. Risk of malignant neoplasms of liver and biliary tract in diabetic patients with different age and sex stratifications. Hepatology. 2010;52:155–163. doi: 10.1002/hep.23641. [DOI] [PubMed] [Google Scholar]
- 7.Lee MY, Lin KD, Hsiao PJ, Shin SJ. The association of diabetes mellitus with liver, colon, lung, and prostate cancer is independent of hypertension, hyperlipidemia, and gout in Taiwanese patients. Metabolism: clinical and experimental. 2012;61:242–249. doi: 10.1016/j.metabol.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Lin Y, Tamakoshi A, Kawamura T, et al. Risk of pancreatic cancer in relation to alcohol drinking, coffee consumption and medical history: findings from the Japan collaborative cohort study for evaluation of cancer risk. International journal of cancer. 2002;99:742–746. doi: 10.1002/ijc.10402. [DOI] [PubMed] [Google Scholar]
- 9.Seow A, Yuan JM, Koh WP, Lee HP, Yu MC. Diabetes mellitus and risk of colorectal cancer in the Singapore Chinese Health Study. Journal of the National Cancer Institute. 2006;98:135–138. doi: 10.1093/jnci/djj015. [DOI] [PubMed] [Google Scholar]
- 10.Tseng CH. Diabetes and thyroid cancer mortality: a 12-year prospective follow-up of Taiwanese. Eur J Clin Invest. 2013;43:595–601. doi: 10.1111/eci.12086. [DOI] [PubMed] [Google Scholar]
- 11.Inoue M, Iwasaki M, Otani T, Sasazuki S, Noda M, Tsugane S. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med. 2006;166:1871–1877. doi: 10.1001/archinte.166.17.1871. [DOI] [PubMed] [Google Scholar]
- 12.Khan M, Mori M, Fujino Y, et al. Site-specific cancer risk due to diabetes mellitus history: evidence from the Japan Collaborative Cohort (JACC) Study. Asian Pac J Cancer Prev. 2006;7:253–259. [PubMed] [Google Scholar]
- 13.Lo SF, Chang SN, Muo CH, et al. Modest increase in risk of specific types of cancer types in type 2 diabetes mellitus patients. International journal of cancer. 2013;132:182–188. doi: 10.1002/ijc.27597. [DOI] [PubMed] [Google Scholar]
- 14.Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008 v1.2: Cancer incidence and mortality worldwide: IARC Cancer Base No. 10. Lyon (France): IARC Press; 2013. [accessed 5 January 2010]. [Google Scholar]
- 15.Boffetta P, McLerran D, Chen Y, et al. Body mass index and diabetes in Asia: a cross-sectional pooled analysis of 900,000 individuals in the Asia cohort consortium. PLoS One. 2011;6:e19930. doi: 10.1371/journal.pone.0019930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Copeland WK, Vedanthan R, et al. Association between bodymass index and cardiovascular disease mortality in east Asians and south Asians: pooled analysis of prospective data from the Asia Cohort Consortium. Bmj. 2013;347:f5446. doi: 10.1136/bmj.f5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sasazuki S, Charvat H, Hara A, et al. Diabetes mellitus and cancer risk: pooled analysis of eight cohort studies in Japan. Cancer Sci. 2013;104:1499–1507. doi: 10.1111/cas.12241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang WS, Li HL, Xu HL, et al. Type 2 diabetes and the risk of non-Hodgkin’s lymphoma: a report from two population-based cohort studies in China. Eur J Cancer Prev. 2016;25:149–154. doi: 10.1097/CEJ.0000000000000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Ahsan H, Slavkovich V, et al. No association between arsenic exposure from drinking water and diabetes mellitus: a cross-sectional study in Bangladesh. Environmental health perspectives. 2010;118:1299–1305. doi: 10.1289/ehp.0901559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waki K, Noda M, Sasaki S, et al. Alcohol consumption and other risk factors for self-reported diabetes among middle-aged Japanese: a population-based prospective study in the JPHC study cohort I. Diabetic medicine. 2005;22:323–331. doi: 10.1111/j.1464-5491.2004.01403.x. [DOI] [PubMed] [Google Scholar]
- 21.Cockram CS. The epidemiology of diabetes mellitus in the Asia-Pacific region. Hong Kong Med J. 2000;6:43–52. [PubMed] [Google Scholar]
- 22.Argos M, Parvez F, Rahman M, et al. Arsenic and lung disease mortality in Bangladeshi adults. Epidemiology. 2014;25:536–543. doi: 10.1097/EDE.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith C, Williamson PR, Marson AG. An overview of methods and empirical comparison of aggregate data and individual patient data results for investigating heterogeneity in meta-anlaysis of time-to-event outcomes. J Eval Clin Pract. 2005;11:468–478. doi: 10.1111/j.1365-2753.2005.00559.x. [DOI] [PubMed] [Google Scholar]
- 25.Riley R, Simmonds MC, Look MP. Evidence synthesis combining individual patient data and aggregate data: a systematic review indentified current practice and possible methods. J Clin Epidemiol. 2007;60:431–439. doi: 10.1016/j.jclinepi.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Emerging Risk Factors Collaboration. Seshasai SR, Kaptoge S, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campbell PT, Newton CC, Patel AV, Jacobs EJ, Gapstur SM. Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care. 2012;35:1835–1844. doi: 10.2337/dc12-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jee SH, Ohrr H, Sull JW, Yun JE, Ji M, Samet JM. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293:194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 29.Wang C, Wang X, Gong G, et al. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. International journal of cancer. 2012;130:1639–1648. doi: 10.1002/ijc.26165. [DOI] [PubMed] [Google Scholar]
- 30.Javeed N, Sagar G, Dutta SK, et al. Pancreatic cancer-derived exosomes cause paraneoplastic beta-cell dysfunction. Clin Cancer Res. 2015;21:1722–1733. doi: 10.1158/1078-0432.CCR-14-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huxley R, Ansary-Moghaddam A, Berrington de Gonzalez A, Barzi F, Woodward M. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. Br J Cancer. 2005;92:2076–2083. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103–1123. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 33.Satija A, Spiegelman D, Giovannucci E, Hu FB. Type 2 diabetes and risk of cancer. Bmj. 2015;350:g7707. doi: 10.1136/bmj.g7707. [DOI] [PubMed] [Google Scholar]
- 34.De Bruijn KM, Arends LR, Hansen BE, Leeflang S, Ruiter R, van Eijck CH. Systematic review and meta-analysis of the association between diabetes mellitus and incidence and mortality in breast and colorectal cancer. Br J Surg. 2013;100:1421–1429. doi: 10.1002/bjs.9229. [DOI] [PubMed] [Google Scholar]
- 35.Jing W, Jin G, Zhou X, et al. Diabetes mellitus and increased risk of cholangiocarcinoma: a meta-analysis. Eur J Cancer Prev. 2012;21:24–31. doi: 10.1097/CEJ.0b013e3283481d89. [DOI] [PubMed] [Google Scholar]
- 36.Ren HB, Yu T, Liu C, Li YQ. Diabetes mellitus and increased risk of biliary tract cancer: systematic review and meta-analysis. Cancer Causes Control. 2011;22:837–847. doi: 10.1007/s10552-011-9754-3. [DOI] [PubMed] [Google Scholar]
- 37.Shebl FM, Andreotti G, Rashid A, et al. Diabetes in relation to biliary tract cancer and stones: a population-based study in Shanghai, China. Br J Cancer. 2010;103:115–119. doi: 10.1038/sj.bjc.6605706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biddinger SB, Haas JT, Yu BB, et al. Hepatic insulin resistance directly promotes formation of cholesterol gallstones. Nature medicine. 2008;14:778–782. doi: 10.1038/nm1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhao Z, Wen W, Michailidou K, et al. Association of genetic susceptibility variants for type 2 diabetes with breast cancer risk in women of European ancestry. Cancer Causes Control. 2016;27:679–693. doi: 10.1007/s10552-016-0741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nead KT, Sharp SJ, Thompson DJ, et al. Evidence of a causal association between insulinemia and endometrial cancer: a Mendelian randomization analysis. Journal of the National Cancer Institute. 2015:107. doi: 10.1093/jnci/djv178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou XH, Qiao Q, Zethelius B, et al. Diabetes, prediabetes and cancer mortality. Diabetologia. 2010;53:1867–1876. doi: 10.1007/s00125-010-1796-7. [DOI] [PubMed] [Google Scholar]
- 42.Lam EK, Batty GD, Huxley RR, et al. Associations of diabetes mellitus with site-specific cancer mortality in the Asia-Pacific region. Ann Oncol. 2011;22:730–738. doi: 10.1093/annonc/mdq405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bao C, Yang X, Xu W, et al. Diabetes mellitus and incidence and mortality of kidney cancer: a meta-analysis. J Diabetes Complications. 2013;27:357–364. doi: 10.1016/j.jdiacomp.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Mitri J, Castillo J, Pittas AG. Diabetes and risk of Non-Hodgkin’s lymphoma: a meta-analysis of observational studies. Diabetes Care. 2008;31:2391–2397. doi: 10.2337/dc08-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmid D, Behrens G, Jochem C, Keimling M, Leitzmann M. Physical activity, diabetes, and risk of thyroid cancer: a systematic review and meta-analysis. Eur J Epidemiol. 2013;28:945–958. doi: 10.1007/s10654-013-9865-0. [DOI] [PubMed] [Google Scholar]
- 46.Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer epidemiology, biomarkers & prevention. 2006;15:2056–2062. doi: 10.1158/1055-9965.EPI-06-0410. [DOI] [PubMed] [Google Scholar]
- 47.Long XJ, Lin S, Sun YN, Zheng ZF. Diabetes mellitus and prostate cancer risk in Asian countries: a meta-analysis. Asian Pac J Cancer Prev. 2012;13:4097–4100. doi: 10.7314/apjcp.2012.13.8.4097. [DOI] [PubMed] [Google Scholar]
- 48.Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88:1254–1264. doi: 10.2522/ptj.20080020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hemkens LG, Grouven U, Bender R, et al. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009;52:1732–1744. doi: 10.1007/s00125-009-1418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Landman GW, Kleefstra N, van Hateren KJ, Groenier KH, Gans RO, Bilo HJ. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care. 2010;33:322–326. doi: 10.2337/dc09-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.